Key Points
Question
Can microneedle pretreatment expedite photodynamic therapy (PDT) for field treatment of actinic keratoses (AKs)?
Findings
In this randomized clinical trial, pretreatment with microneedles before a 20-minute incubation of aminolevulinic acid (ALA) resulted in AK clearance of 76%, which is similar in efficacy to conventional PDT with 1-hour ALA incubation time. In addition to expediting the protocol, microneedle pretreatment resulted in virtually painless PDT.
Meaning
The findings are promising for shortening treatment time and achieving greater patient satisfaction for PDT without compromising efficacy.
This randomized clinical trial evaluates efficacy of and pain associated with microneedle-assisted photodynamic therapy for actinic keratoses.
Abstract
Importance
Photodynamic therapy (PDT) is an effective and cosmetically favorable treatment modality for actinic keratoses (AKs). However, prolonged incubation times and pain associated with treatment are burdensome to the patient and a hindrance to widespread use of PDT as standard field therapy for AK.
Objective
To evaluate efficacy and pain associated with microneedle expedited PDT.
Design, Setting, and Participants
The Microneedle Photodynamic Therapy II (MNPDT-II) study was a randomized, single-blinded, split-face controlled, 2-arm clinical trial. Thirty-three participants with AK on the face were recruited in a university dermatology outpatient clinic from 2015 to 2016, and 32 participants completed the study.
Interventions
Participants were randomized into 2 incubations arms, either 10-minute or 20-minute aminolevulinic acid (ALA) incubation times, after pretreatment with a microneedle roller (200 um) vs a sham roller. They were blinded to the laterality of microneedle and sham roller assignments. After incubation, they were exposed to blue light (Blu-U, Dusa Pharmaceuticals) for 1000 seconds for a total fluence of 10 J/cm2.
Main Outcomes and Measures
The primary outcome was to quantitatively measure AK resolution, and the secondary outcome was to assess pain associated with microneedle pretreatment.
Results
Thirty-three individuals were recruited and randomized to either the 20-minute or the 10-minute incubation arm. Thirty-two participants completed the study with a mean follow-up time of 34.5 days in the 20-minute group, and 30.2 days in the 10-minute group. For the 20-minute incubation arm, average AK clearance was 76% vs 58% on the sham side (P < .01), including 3 patients with complete clearance, although not statistically significant (P = .25). Pain assessment on the visual analog scale (VAS) during blue light illumination was not significantly different between the microneedle and sham sides (0.7 and 0.4; P = .28), respectively. For the 10-minute incubation arm AK clearance for the microneedle pretreated side was 43% compared with 38% on the sham side (P = .66). Pain during the blue light exposure was not significantly different between the microneedle and sham sides, 4.5 mm and 3.4 mm (P = .21), respectively.
Conclusions and Relevance
Photodynamic therapy with microneedle pretreatment at a 20-minute ALA incubation time significantly improved AK clearance with efficacy similar to that of a conventional 1-hour ALA incubation time. The additional advantage to expedited treatment was that the procedure was virtually painless. However, expedited exposure of a 10-minute ALA incubation time did not reach significantly different AK clearance from the sham control.
Trial Registration
clinicaltrials.gov Identifier: NCT02594644
Introduction
Actinic keratoses (AKs) are considered to be a premalignant manifestation in the continuum of transformation to cutaneous squamous cell carcinoma (SCC). Chronic cumulative sun exposure, fair complexion, immunosuppression, and increased age contribute to increased risk of AK development. The prevalence of AKs in the United States was estimated at 11% to 25% in 2008 with associated annual costs of greater than $1 billion. The risk of progression of AK to SCC is considered low (0.025%-20% per year), but variable, since up to 65% of SCCs are documented to have arisen from lesions previously diagnosed as AKs. Given the high prevalence, economic burden, and possible morbidly and mortality associated with malignant transformation to SCC, standard of care recommendations support treatment for AK on diagnosis.
Treatment options for AKs are numerous and depend on several factors, including severity of disease, distribution of lesions, associated costs, and patient preference. While lesion-directed therapy aims to treat clinically identifiable AKs (including cryotherapy, laser therapy, curettage, or dermabrasion), field-directed therapy aims to also treat subclinical lesions in the surrounding treatment area. Common field therapy options include photodynamic therapy (PDT), topical 5-fluorouracil, imiquimod, ingenol mebutate, diclofenac, chemical peels, and ablative laser resurfacing. These treatments show variable long-term efficacy with recurrence in 28% to 96% of patients at the 1-year follow-up, even after complete short-term clearance; therefore, observation and maintenance for AK as a chronic disease are required. The development of new or improved treatment modalities for AKs thus mandates considerations in addition to efficacy, such as duration, convenience, and patient adherence to the treatment.
In the United States, the US Food and Drug Administration (FDA) has approved PDT for lesion-directed treatment of minimally to moderately thick AKs of the face or scalp with 14- to 18-hour incubation with 20% topical solution of aminolevulinic acid (ALA) and activation by 10 J/cm2 of blue light for 1000 seconds. ALA is preferentially absorbed by dysplastic cells and metabolized to protoporphyrin IX (PpIX). When PpIX is activated by a specific wavelength of light in the presence of oxygen, the resultant reactive oxygen species lead to a cytotoxic cascade and destruction of affected cells with minimal damage to surrounding unaffected skin. Cutaneous photosensitivity is transient owing to metabolic conversion of the remaining PpIX to heme within 48 hours. Off-label use of ALA PDT for field therapy with shorter incubation times is common. and several investigator-initiated studies have supported efficacy with sustained clearance at a 1-year follow-up time in up to 59% of patients.
However, the shallow penetration depth (<2 mm) of ALA, prolonged incubation time, and pain experienced during light exposure are major drawbacks to PDT field treatment. The hydrophilic nature of ALA limits its permeation through the hydrophobic stratum corneum. Innovations for improving ALA penetration or for enhanced conversion to PpIX include incubating ALA under occlusion, methylation of ALA for a more lipophilic substrate, and thermal enhancement of PDT. To specifically address delivery of ALA through the stratum corneum and epidermis, several clinical studies have demonstrated augmentation of PDT by application of metal microneedles to the skin prior to ALA incubation for enhanced mechanical penetration.
Microneedle rollers are devices that can pierce transient aqueous microchannels in the superficial layers of the skin, thus bypassing the stratum corneum for drug delivery. Microneedles have been studied for safety and efficacy in other applications, such as topical anesthetics. They are promising for improving efficacy of PDT because the devices are easy to handle, fast, low cost, and relatively painless, while not increasing erythema, bleeding, or pain during PDT light exposure. In fact, adequate photosensitizer was detected by fluorescence dosimetry in as little as 30 minutes after application of methyl aminolevulinic acid after microneedle pretreatment.
Our previous investigation demonstrated equivalent efficacy of complete AK clearance on the forehead (71.4% vs 68.3%; P > .05) in expedited microneedle PDT with 20-minute ALA incubation vs the standard 1-hour incubation with sham microneedle pretreatment. The current study aimed to expand on these findings and investigate the efficacy of expedited ALA PDT with microneedle pretreatment at short ALA incubation times in a randomized clinical trial.
The primary tested hypothesis was that pretreatment with microneedles can enhance penetration of topical ALA and improve efficacy of PDT for AKs on the face at shorter incubation times of 10 or 20 minutes. The secondary hypothesis was that shorter incubation times are associated with less pain during blue light illumination.
Methods
Study Population
Participants were recruited from the University of California–Davis Dermatology Clinic. To be included in the study, patients (1) had to be at least 18 years of age, (2) had to have at least 3 distinct grade II AKs on the face, (3) had to have been referred for PDT field therapy by a board-certified dermatologist, and (4) did not meet any of the exclusion criteria. Exclusion criteria consisted of (1) active smoking; (2) photosensitizing condition, such as lupus or porphyria; (3) established allergy to ALA; or (4) nonmelanoma skin cancer on the face documented within the past 6 months.
This study was conducted in accordance with the Declaration of Helsinki principles. All participants gave written informed consent and were compensated. The study was approved by the University of California–Davis institutional review board. The trial protocol is provided in Supplement 1.
Study Design
The Microneedle Photodynamic Therapy II (MNPDT-II) was a randomized, single-blinded, split-face controlled, 2-arm clinical trial comparing the efficacy of AK clearance after microneedle pretreatment. Microneedle rollers were applied to half of each participant’s face vs a sham roller on the control side. The 2 arms included a 10-minute and a 20-minute ALA incubation time.
The primary outcome was to quantitatively measure AK resolution with pretreatment and posttreatment AK counts on the microneedle-treated side compared with the sham treatment side of the face. The secondary outcome was to assess the participants’ pain during microneedle and sham pretreatment and during blue light illumination by participants’ ratings on a visual analog scale (VAS).
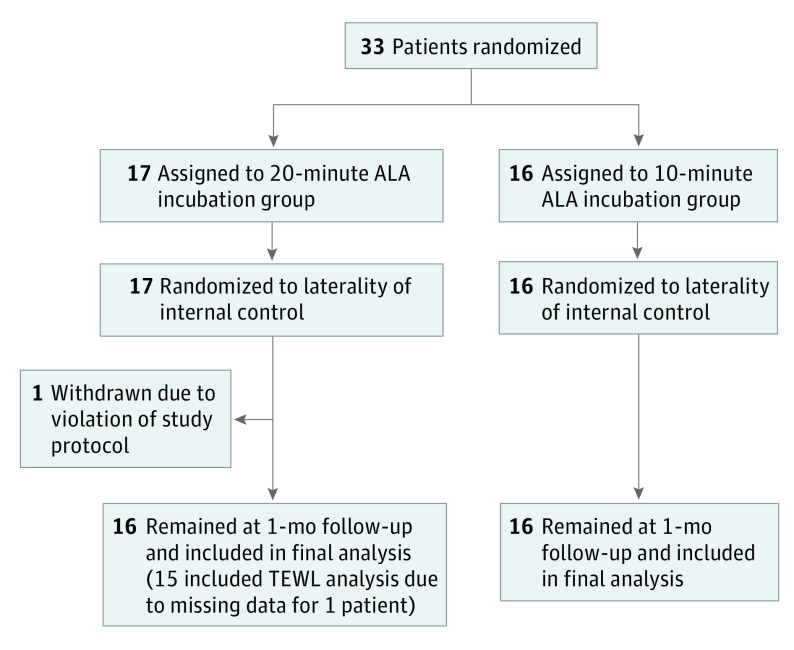
Recruitment occurred between March 2015 and May 2016, and follow-up was concluded in June 2016. Using a binary randomization scheme, participants were assigned in a 1:1 ratio to either the 10-minute or the 20-minute incubation time arm. A second binary randomization scheme was used to assign each participant to receive microneedle roller or sham roller administration prior to ALA application on the either the right or the left side of the face. The participants were blinded to the laterality of microneedle vs sham roller assignments (Figure 1).
Figure 1. CONSORT Study Flow Diagram.
ALA indicates aminolevulinic acid; TEWL, transepidermal water loss.
Treatment Protocol
Each participant’s face was cleansed with isopropyl alcohol followed by high-resolution photographic imaging of the face with the Brigh-Tex Bio-Photonics Research 3-D System.
Baseline demographic data were collected for each patient. Prior to intervention, each participant was evaluated on clinical examination for extent of disease by a trained blinded investigator (T.A.P. or R.K.S.). Grade II AKs (moderately thick and visible) were mapped on a transparent overlay for both sides of the face (including forehead, nose, upper and lower cutaneous lips, cheeks down to jaw line, and chin). Lip and eyelid keratoses were not included). Transepidermal water loss (TEWL) measurements were recorded with either Tewameter TM 300 (Courage and Khazaka) or Vapometer (Delfin Technologies Ltd) at 4 points of the patients’ forehead and cheeks.
According to the binary randomization scheme, each patient had either a microneedle or a sham roller applied to each respective half of the face for a total of 8 forward and backward passes in 4 directions (vertical, horizontal, and each diagonal). The patients were blinded as to which side of the face would receive the microneedle intervention (Video). The microneedle device (MTS Roller MR2 Clinical Resolution Laboratory Inc) consisted of a single-use sterile array of microneedles 200 µm in length. The sham treatment consisted of the applicator roller without actual microneedles. Immediately after sham and microneedle pretreatment, each participant rated their pain on a 100-mm VAS for each respective side of the face. The TEWL measurements were repeated at the 4 points of the patients’ forehead and cheeks.
Video. Video Illustration Depicting the Microneedle Roller and Application Technique.
Videography by Buno Photography and Video, Sacramento, California.
Topical ALA (ALA, Levulan Kerastick, DUSA Pharmaceuticals) was applied to the entire face, and time for incubation was based on the previously determined randomization protocol of either the 10-minute or 20-minute study arm. At the end of the incubation period, participants were exposed to blue light (Blu-U, Dusa Pharmaceuticals) with a mean (SD) wavelength of 417 (5) nm, for 1000 seconds for a total fluence of 10 J/cm2. Immediately after blue light exposure, participants again rated their pain on a 10-cm VAS for each respective side of the face.
The participants were instructed to avoid sun exposure for 36 hours after treatment and returned for follow-up approximately 1 month after treatment. At that time their face was photographed, and remaining AKs were counted and mapped. The participants were also asked about any adverse events they may have experienced as result of PDT (eFigure 1 in Supplement 2).
Statistical Analysis
Based on pilot data for the primary outcome, at least 12 patients were required in each group to detect a 25% difference in the clearance of AKs between the split-face treatment groups with 95% power with α = .05. Data are presented as mean ± standard error of the mean. t Test was used to compare the significance between data points. Analysis for complete clearance was calculated with McNemar test for paired proportions. All analyses were conducted using Excel (Microsoft Corp) or GraphPad Software (GraphPad Software Inc). P <.05 was taken to represent a statistically significant difference.
Results
Thirty-three individuals were recruited and randomized to either the 20-minute or the 10-minute incubation arm. One patient in the 20-minute group was excluded from the study after violating protocol and experiencing an adverse event of excessive pain, erythema, and peeling owing to prolonged sun exposure immediately after treatment on the first study visit. Thirty-two participants completed the study with a mean follow-up time of 34.5 days in the 20-minute group, and 30.2 days in the 10-minute group. There were no significant differences in the baseline demographics of age and sex between the 2 study arms (Table 1).
Table 1. Baseline Characteristics of Participantsa.
| Characteristic | ALA Incubation Time, Mean (SEM) | P Value | |
|---|---|---|---|
| 20 min | 10 min | ||
| Total participants, No. | 16 | 16 | >.99 |
| Male sex, No. (%) | 11 (69) | 11 (69) | >.99 |
| Age, mean (SD), y | 62.8 (2.1) | 65.4 (2.5) | .41 |
| Baseline | |||
| AK count | |||
| Microneedle side | 11.8 (1.8) | 16.1 (3.3) | .24 |
| Sham side | 13.3 (2.3) | 14.7 (2.8) | .65 |
| TEWL, g/h/m2 | |||
| Microneedle side | 33.0 (2.3) | 30.8 (2.6) | .75 |
| Sham side | 33.6 (2.2) | 31.5 (2.7) | .72 |
| Days to follow-up | 34.1 (1.9) | 30.2 (1.1) | .15 |
Abbreviations: AK, actinic keratosis; ALA, aminolevulinic acid; SEM, standard error of the mean; TEWL, transepidermal water loss.
P > .05 for all baseline characteristic comparisons between the 2 randomized incubation groups.
The mean total grade II AK counts on the face at enrollment were 25 for the 20-minute group and 31 for the 10-minute group. All participants had at least 8 AKs on the face. There were no significant differences in total AKs between the 2 groups, or between the microneedle and the sham side of the face within each group.
Arm 1: 20-Minute Incubation
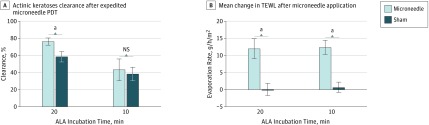
In the microneedle pretreated side there was an average of 76% AK clearance, compared with 58% on the sham side (P < .01), including 3 patients with complete clearance, although this was not statistically significant (P = .25). TEWL measurement increased on average by 36% for the microneedle side vs 0% for the sham side (P < .001), confirming adequate production of pores through the stratum corneum to facilitate improved drug delivery. One participant was not included in the TEWL final analysis owing to missing data from equipment malfunction. Pain assessment on the 10-point VAS for the microneedle-pretreated side averaged 1.3 and statistically different from the sham side of 0.3 (P = .02). However, pain during the blue light exposure was not significantly different between the microneedle and sham side (0.7 and 0.4, respectively; P = .28). See Table 2, Figure 2, Figure 3, and eFigure 2 in Supplement 2.
Table 2. Results of Measured Characteristics With Expedited Microneedle PDT.
| Measurement | ALA Incubation Time, Mean (SEM) | |||||
|---|---|---|---|---|---|---|
| 20 min Arm | 10 min Arm | |||||
| MN | Sham | P Value | MN | Sham | P Value | |
| AK | ||||||
| Count after PDT | 2.4 (0.6) | 3.8 (1.0) | .02 | 6.6 (1.7) | 4.8 (1.2) | .89 |
| Clearance per participant, % | 76 (3.9) | 58 (5.7) | .01 | 43 (13.0) | 38 (8.1) | .66 |
| Patients with complete AK clearance, No. | 3 | 0 | .25 | 1 | 0 | >.99 |
| TEWL change, g/h/m2 | 11.8 (3.0) | 0.0 (1.8) | <.001 | 12.3 (1.9) | 0.7 (1.5) | <.001 |
| Pain VAS | ||||||
| After microneedle application | 1.3 (0.4) | 0.3 (0.2) | .02 | 1.4 (0.5) | 0.3 (0.2) | .004 |
| After blue light exposure | 0.7 (0.2) | 0.4 (0.1) | .28 | 0.5 (0.2) | 0.3 (0.2) | .21 |
Abbreviations: AK, actinic keratoses; ALA, aminolevulinic acid; MN, microneedle; PDT, photodynamic therapy; SEM, standard error of the mean; TEWL, transepidermal water loss; VAS, visual analog scale.
Figure 2. Clearance of Actinic Keratoses (AKs) After Microneedle Photodynamic Therapy.
A, In the 20-minute aminolevulinic acid (ALA) incubation arm (n = 16 patients), the microneedle-pretreated side had a mean AK clearance of 76% vs 58% for the sham side (P < .01). In the 10-minute ALA incubation arm (n = 16 patients), the microneedle-pretreated side had a mean AK clearance of 43% vs 38% for the sham side (P = .66). B, In the 20-minute ALA incubation arm (n = 15 patients), the mean change in transepidermal water loss (TEWL) after microneedle pretreatment was 36% vs 0% for the sham side (P < .001). In the 10-minute ALA incubation arm (n = 16), the mean change in TEWL after microneedle pretreatment was 40% vs 2% for the sham side (P < .001). Error bars indicate the standard error of the mean; NS, not significant.
aStatistically significant.
Figure 3. Pain Associated with Microneedle Pretreatment and Blue Light illumination.
A, In the 20-minute aminolevulinic acid (ALA) incubation arm (n = 16 patients), the mean pain assessment on the 10-point visual analog scale (VAS) for the microneedle-pretreated side was 1.3 vs 0.3 for the sham side (P = .02). In the 10-minute ALA incubation arm (n = 16 patients), the mean pain assessment on the microneedle-pretreated side was 1.4 vs 0.3 for the sham side (P = .004). B, In the 20-minute ALA incubation arm (n = 16 patients), the mean pain assessment during blue light illumination for the microneedle-pretreated side was 0.7 vs 0.4 for the sham side (P = .28). In the 10-minute ALA incubation arm (n = 16 patients), the mean pain assessment for the microneedle-pretreated side was 0.5 vs 0.3 for the sham side (P = .21). Error bars indicate the standard error of the mean; NS, not significant.
aStatistically significant.
Arm 2: 10-Minute Incubation
In the microneedle-pretreated side there was an average of 43% AK clearance compared with 38% on the sham side (P = .66), including 1 patient with complete clearance (P > .99), neither significantly different. The TEWL measurement increased on average by 40% for the microneedle side vs 2% for the sham side (P < .001). Pain assessment on the VAS for the microneedle-pretreated side averaged 1.4 and was statistically different from the assessment on sham side, 0.3 (P = .004). However, pain during the blue light exposure was not significantly different between the microneedle and sham side, with VAS assessments of 0.5 and 0.3 (P = .21), respectively. See Table 2, Figure 2, and Figure 3.
Discussion
The MNPDT-II trial aimed to evaluate efficacy of AK clearance with microneedle-assisted PDT prior to ultra-short ALA incubation times of 10 and 20 minutes. Participants experienced significantly superior AK lesion clearance (76% vs 58%) at 20-minute incubation times. While the 10-minute group also experienced improvement in AK counts, the clearance rates between the microneedle side and the sham side were not significantly different (43% vs 38%). Interestingly, the secondary outcome of pain associated with blue light exposure during PDT was nominal and not significantly different from the sham side (0.7 vs 0.4 on a 10-point VAS for the 20-minute group). The study confirms and expands on the MNPDT-I pilot study that showed equivalent complete AK clearance rates between 20-minute ALA incubation with microneedle pretreatment vs traditional PDT with 1-hour incubation.
The AK clearance rate in the MNPDT-II trial is similar to those in previous investigations of traditional PDT with ALA incubation times of 1 hour (78.6%), 2 hours (76.5%), and 3 hours (80.0%) at the 12-week follow up. An even shorter incubation time was investigated under occlusion of an ALA patch for 30 minutes but showed AK clearance rates of only 47% at the 4-week follow up.
While microneedle pretreatment improved efficacy of PDT at the 20-minute ALA incubation, it did not show significant difference in AK clearance at the 10-minute incubation time compared with the sham control (43% vs 38%). While assisting drug delivery through the stratum corneum and epidermis with microneedles may expedite ALA incubation, it is likely that the additional steps required for downstream conversion of ALA to activated PpIX, production of the resultant reactive oxygen species, and apoptosis require more time. Future studies aimed at expediting the downstream biochemical cascade, such as with thermal PDT, may be warranted to improve efficacy of AK clearance at even shorter ALA incubation times.
Interestingly, in both arms of the trial, the sham side of the face had rates of AK clearance (58% for 20-minute ALA incubation and 38% for 10-minute incubation) higher than previously reported vehicle placebo PDT (7.1%). Similarly, in another study, AK clearance after 15 minutes of ALA incubation time but a prolonged PDT light exposure of 1 hour also revealed AK clearance rates of 52%.
An additional benefit identified in the MNPDT-II trial is the relatively painless illumination phase (0.7 for the 20-minute group and 0.5 for the 10-minute group on the 10-point VAS) compared with reported scores of 4.6 for traditional PDT after 3 hours of ALA incubation. In fact, initial PDT trials of 14- to 18-hour ALA incubation had 90% of patients reporting moderate to severe pain. The rates were reduced for shorter incubation times of 1 to 3 hours, for which 60% of patients reported moderate to severe pain. No participants in the MNPDT-II trial reported moderate or severe pain (6-10 on the VAS). Pain associated with PDT is the most severe adverse effect and may lead to interruption or discontinuation of treatment, resulting in refusal to repeat the process at a future date owing to unbearable discomfort.
In addition, most patients reported minimal erythema and peeling and virtually no edema or pain after treatment. Corroborating similar results have been obtained previously where greater incidence of post-PDT severity of erythema and crusting was associated with greater ALA incubation time, but without significant improvement in efficacy of AK clearance.
Limitations
A limitation of the current study is the short follow-up time of 4 weeks after treatment. Because actinic damage is cumulative, there is potential for thicker AKs to recur or for new lesions to develop during a longer follow-up period. However, a previous study has reported that prolonged lesion clearance may be achieved by having the patient undergo a second PDT treatment after 8 weeks, with similar efficacy of AK clearance between the 1-hour (68%-79%) and the 18-hour (83.4%) FDA-approved incubation time with the 2-treatment approach. In practice, PDT treatments are typically delivered 2 or 3 times over the period of 2 to 3 months. Future studies should assess AK clearance over a longer period of time, and the results of this pilot study warrant further study of 20-minute incubation PDT, especially after microneedle pretreatment. Another limitation is that our study focused on the face, and further studies are needed before the results reported herein can be extrapolated to the arms.
Conclusions
The results of the MNPDT-II trial support efficacy of microneedle pretreatment for short ALA incubation time of 20 minutes, similar to conventional ALA PDT of 1-hour incubation. In addition, patients felt little to no pain while achieving AK clearance rates of 76%. Limitations of the trial are small number of participants and a short follow-up period. Large-scale randomized clinical trials evaluating microneedle augmentation for shorter PDT incubation times are needed to further validate the observed efficacy of treatment and reduction in patient discomfort. Overall, the findings are promising for shortening treatment time and achieving greater patient satisfaction for PDT without compromising efficacy.
Trial protocol
eFigure 1. Study protocol schematic
eFigure 2. Clinical photograph of a patient
References
- 1.Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review [published online August 8, 2016]. Br J Dermatol. doi: 10.1111/bjd.14852 [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg G, Perl M. Actinic keratosis: update on field therapy. J Clin Aesthet Dermatol. 2014;7(10):28-31. [PMC free article] [PubMed] [Google Scholar]
- 3.Pariser DM, Houlihan A, Ferdon MB, Berg JE; PDT-AK Investigational Group . Randomized vehicle-controlled study of short drug incubation aminolevulinic acid photodynamic therapy for actinic keratoses of the face or scalp. Dermatol Surg. 2016;42(3):296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol. 2014;150(12):1281-1288. [DOI] [PubMed] [Google Scholar]
- 6.Mikolajewska P, Donnelly RF, Garland MJ, et al. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm Res. 2010;27(10):2213-2220. [DOI] [PubMed] [Google Scholar]
- 7.Sivamani RK, Stoeber B, Liepmann D, Maibach HI. Microneedle penetration and injection past the stratum corneum in humans. J Dermatolog Treat. 2009;20(3):156-159. [DOI] [PubMed] [Google Scholar]
- 8.Bahadoran P, Le Duff F, Pascual T, et al. Comparative methods for improving transepidermal methylaminolevulinate delivery: a randomized clinical trial. JAMA Dermatol. 2015;151(12):1371-1373. [DOI] [PubMed] [Google Scholar]
- 9.Ko DY, Kim KH, Song KH. Comparative study of photodynamic therapy with topical methyl aminolevulinate versus 5-aminolevulinic acid for facial actinic keratosis with long-term follow-up. Ann Dermatol. 2014;26(3):321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40(10):1094-1102. [DOI] [PubMed] [Google Scholar]
- 11.Kearney M-C, Brown S, McCrudden MT, Brady AJ, Donnelly RF. Potential of microneedles in enhancing delivery of photosensitising agents for photodynamic therapy. Photodiagnosis Photodyn Ther. 2014;11(4):459-466. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly RF, Morrow DIJ, Fay F, et al. Microneedle-mediated intradermal nanoparticle delivery: potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagnosis Photodyn Ther. 2010;7(4):222-231. [DOI] [PubMed] [Google Scholar]
- 13.Lev-Tov H, Larsen L, Zackria R, Chahal H, Eisen DB, Sivamani RK. Microneedle-assisted incubation during aminolaevulinic acid photodynamic therapy of actinic keratoses: a randomized controlled evaluator-blind trial. Br J Dermatol. 2017;176(2):543-545. doi: 10.1111/bjd.15116 [DOI] [PubMed] [Google Scholar]
- 14.Ornelas J, Foolad N, Shi V, Burney W, Sivamani RK. Effect of microneedle pretreatment on topical anesthesia: a randomized clinical trial. JAMA Dermatol. 2016;152(4):476-477. [DOI] [PubMed] [Google Scholar]
- 15.Sivamani RK, Liepmann D, Maibach HI. Microneedles and transdermal applications. Expert Opin Drug Deliv. 2007;4(1):19-25. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues PGS, Campos de Menezes PF, Fujita AK, et al. Assessment of ALA-induced PpIX production in porcine skin pretreated with microneedles. J Biophotonics. 2015;8(9):723-729. [DOI] [PubMed] [Google Scholar]
- 17.Upadhyay C, Cameron K, Murphy L, Battistella M. Measuring pain in patients undergoing hemodialysis: a review of pain assessment tools. Clin Kidney J. 2014;7(4):367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauschild A, Popp G, Stockfleth E, et al. Effective photodynamic therapy of actinic keratoses on the head and face with a novel, self-adhesive 5-aminolaevulinic acid patch. Exp Dermatol. 2009;18(2):116-121. [DOI] [PubMed] [Google Scholar]
- 19.Mamalis A, Koo E, Sckisel GD, Siegel DM, Jagdeo J. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175(3):512-519. [DOI] [PubMed] [Google Scholar]
- 20.Martin GM. In-office painless aminolevulinic acid photodynamic therapy: a proof of concept study and clinical experience in more than 100 patients. J Clin Aesthet Dermatol. 2016;9(2):19-26. [PMC free article] [PubMed] [Google Scholar]
- 21.Sandberg C, Stenquist B, Rosdahl I, et al. Important factors for pain during photodynamic therapy for actinic keratosis. Acta Derm Venereol. 2006;86(5):404-408. [DOI] [PubMed] [Google Scholar]
- 22.Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140(1):41-46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eFigure 1. Study protocol schematic
eFigure 2. Clinical photograph of a patient