Key Points
Question
What is the nature of the infiltrating cells in histiocytoid Sweet syndrome?
Findings
In this case series study, double immunostaining in 33 cases of histiocytoid Sweet syndrome demonstrated that the infiltrate is mostly composed of immature myeloid cells.
Meaning
The dermal infiltrate of cutaneous lesions of histiocytoid Sweet syndrome is composed mostly of immature cells of myeloid lineage, but not of histiocytes, and it should not be interpreted as leukemia cutis; this histopathologic variant of Sweet syndrome is associated with hematologic malignancy with the same frequency as classic neutrophilic Sweet syndrome.
Abstract
Importance
Histiocytoid Sweet syndrome is a rare histopathologic variant of Sweet syndrome. The nature of the histiocytoid infiltrate has generated considerable controversy in the literature.
Objective
The main goal of this study was to conduct a comprehensive overview of the immunohistochemical phenotype of the infiltrate in histiocytoid Sweet syndrome. We also analyze whether this variant of Sweet syndrome is more frequently associated with hematologic malignancies than classic Sweet syndrome.
Design
This is a retrospective case series study of the clinicopathologic, immunohistochemical, and molecular features of 33 patients with a clinicopathologic diagnosis of histiocytoid Sweet syndrome was conducted in the dermatology departments of 5 university hospitals and a private laboratory of dermatopathology.
Main Outcome and Measures
The clinical, histopathological, immunohistochemical, and follow-up features of 33 patients with histiocytoid Sweet syndrome were analyzed. In some cases, cytogenetic studies of the dermal infiltrate were also performed. We compare our findings with those of the literature.
Results
The dermal infiltrate from the 33 study patients (20 female; median age, 49 years; age range, 5-93 years; and 13 male; median age, 42 years; age range, 4-76 years) was mainly composed of myeloperoxidase-positive immature myelomonocytic cells with histiocytoid morphology. No cytogenetic anomalies were found in the infiltrate except in 1 case in which neoplastic cells of chronic myelogenous leukemia were intermingled with the cells of histiocytoid Sweet syndrome. Authentic histiocytes were also found in most cases, with a mature immunoprofile, but they appeared to be a minor component of the infiltrate. Histiocytoid Sweet syndrome was not more frequently related with hematologic malignancies than classic neutrophilic Sweet syndrome.
Conclusions and Relevance
The dermal infiltrate of cutaneous lesions of histiocytoid Sweet syndrome is composed mostly of immature cells of myeloid lineage. This infiltrate should not be interpreted as leukemia cutis.
This case series study characterizes the immunohistochemical phenotype of the infiltrate in histiocytoid Sweet syndrome and evaluates whether this variant of Sweet syndrome is more frequently associated with hematologic malignancies than classic Sweet syndrome.
Introduction
Histiocytoid Sweet syndrome (HSS) was first described by our research group in 2005 in a study of 41 patients with clinical features of Sweet syndrome (SS) and a dermal infiltrate composed mostly of mononuclear cells, which were interpreted as immature myeloid cells. In the following years, many case reports and some small case series were published mainly highlighting the frequent association of this rare variant of SS with hematologic malignant processes. More recently, the myeloid nature of the infiltrate has been questioned. In this study we try to dispel doubts about the true nature of the predominant cells infiltrating the dermis in HSS, and we analyze the frequency of associated hematologic malignancies in patients with HSS.
Methods
Skin biopsy specimens from 33 patients with HSS were analyzed. The cases were collected from the files of the authors from 2011 through 2015, inclusive. All specimens were from typical cutaneous lesions of SS found on patients with fever and abnormal laboratory findings at presentation (eg, leukocytosis) and a histopathologic study demonstrating a dermal infiltrate composed mostly of mononuclear cells. Because this was a retrospective study using material from the files of dermatopathology units, no institutional review board or human participant approval was requested.
Results
Clinical Data
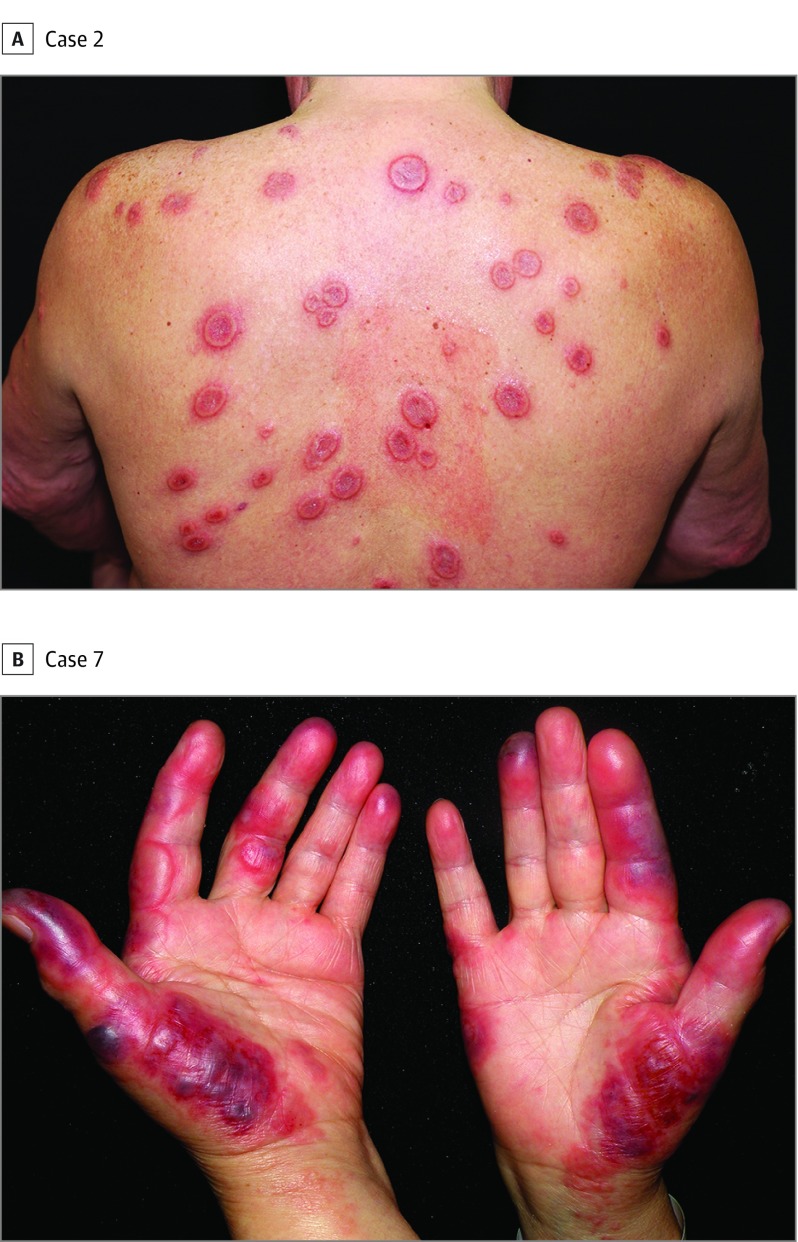
Clinical features of each patient are listed in eTable 2 in the Supplement. Briefly, a total of 33 patients were enrolled in this study. Twenty female patients ranged in age from 5 to 93 years (median, 49 years), and 13 male patients ranged in age from 4 to 76 years (median, 42 years). The most common cutaneous presentation consisted of tender erythematous plaques (Figure 1) and nodules on the extremities and trunk accompanied by systemic symptoms such as fever and arthralgia. Hematologic malignancies were present in 8 patients (24%). In all cases except cases 3, 12, and 24, hematologic malignancies had been diagnosed before the patients developed HSS. The most common hematologic malignancy was myelodysplastic syndrome (MDS), found in 3 patients (1 refractory anemia with excessive blasts, case 27; 1 refractory cytopenia with multilineage dysplasia, case 15; and 1 unspecified type, case 11). Acute myelogenous leukemia (cases 4 and 8) and chronic myelomonocytic leukemia (cases 12 and 13) were present in 2 patients each. Chronic myelogenous leukemia occurred in 1 patient (case 1). There were 3 patients with associated solid cancers: 1 breast carcinoma (case 8, the same patient has also acute myelogenous leukemia), 1 lung cancer (case 16), and 1 concurrent bladder and lung cancer (case 29). In 1 patient, a non-Hodgkin lymphoma was diagnosed 5 years after the occurrence of a single episode of HSS (case 24).
Figure 1. Clinical Features of Histiocytoid Sweet Syndrome.
A, Tender erythematous plaques on the back, some of them with annular morphology showing a raised erythematous border and a slightly depressed violaceous center. B, Painful erythemato-edematous plaques on the palms.
Histopathologic Findings
Specific histopathologic features of each patient are listed in the Table. Of the 33 cases included in this study, the majority presented with what we called typical pattern of HSS under conventional light microscopy. That consisted of spared epidermis, moderate to intense edema in the papillary dermis, no evidence of vasculitis, and an underlying dense, bandlike, inflammatory infiltrate involving the superficial and mid dermis predominantly composed of mononuclear cells (Figure 2) (eFigure 1 in the Supplement). In 2 cases (cases 12 and 13), the infiltrate extended into the subcutis (eFigure 2 in the Supplement). These mononuclear cells showed large, elongated, twisted or kidney-shaped vesicular nuclei, inconspicuous nucleoli, and scant eosinophilic cytoplasm mimicking small histiocytes.
Table. Immunohistochemical Results in This Series of Histiocytoid Sweet Syndrome.
| Case | Histopathology | CD163/ MPOa |
CD163 | MPO | MNDA/ MPOa |
MNDA | MPO | MNDA/ CD163a |
MNDA | CD163 | MNDA/ CD14 |
MNDA | CD14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HSS typical pattern. Mitoses | − | + | +++ | +++ | + | + | +/− | +++ | + | + | +++ | ++ |
| 2 | Scant edema | − | + | +++ | ++ | ++ | + | + | +++ | + | ++ | +++ | + |
| 3 | HSS typical pattern | − | + | +++ | +++ | +/− | +/− | +/− | +++ | +/− | +/− | +++ | + |
| 4 | Scant edema. Perivascular and interstitial infiltrate | − | +/− | +++ | ++ | + | + | + | +++ | +/− | ++ | +++ | + |
| 5 | Prominent edema. Interstitial infiltrate | − | +/− | +++ | +++ | + | +/− | +++ | +/− | +/− | +++ | + | +/− |
| 6 | Scant edema. Interstitial infiltrate | − | +/− | +++ | +++ | + | +/− | + | +++ | + | +++ | +/− | +/− |
| 7 | Focal edema. Superficial and deep infiltrate | − | + | ++ | +++ | + | +/− | + | +++ | + | + | +++ | + |
| 8 | HSS typical pattern | − | + | +++ | +++ | + | +/− | − | +++ | +/− | + | ++ | − |
| 9 | Scant edema. Superficial and deep perivascular infiltrate | − | + | +++ | +++ | +/− | +/− | +/− | +++ | +/− | ++ | + | +/− |
| 10 | HSS typical pattern | − | + | +++ | +++ | +/− | +/− | +/− | +++ | + | + | ++ | +/− |
| 11 | HSS typical pattern | − | + | +++ | +++ | +/− | +/− | +/− | +++ | +/− | +/− | +++ | +/− |
| 12 | Diffuse interstitial infiltrate with septal involvement of the subcutis. Mucin deposits | − | +/− | +++ | +++ | +/− | + | +++ | + | +/− | +/− | +++ | +/− |
| 13 | Diffuse interstitial infiltrate with septal involvement of the subcutis. Mucin deposits | − | +/− | +++ | +++ | +/− | +/− | +++ | +/− | +/− | +/− | +++ | + |
| 14 | HSS typical pattern | − | + | +++ | +++ | + | +/− | + | +++ | + | + | +++ | +/− |
| 15 | HSS typical pattern | +/− | + | +++ | +++ | +/− | − | +/− | +++ | + | + | +++ | − |
| 16 | HSS typical pattern | +/− | + | +++ | +++ | +/− | +/− | +/− | +++ | + | + | +++ | + |
| 17 | HSS typical pattern | − | + | +++ | +++ | + | +/− | +/− | +++ | + | + | +++ | − |
| 18 | HSS typical pattern and neutrophils | +/− | + | +++ | +++ | + | + | +/− | +++ | +/− | +/− | +++ | +/− |
| 19 | Interstitial pattern | − | ++ | + | +++ | + | + | + | ++ | +++ | +++ | ++ | +/− |
| 20 | HSS typical pattern | − | + | +++ | +++ | + | +/− | +/− | +++ | + | + | +++ | +/− |
| 21 | HSS typical pattern | +/− | + | +++ | +++ | ++ | − | +/− | +++ | +/− | +/− | +++ | +/− |
| 22 | Focal edema. Scattered neutrophils | +/− | + | +++ | +++ | + | − | − | +++ | +/− | +/− | +++ | − |
| 23 | HSS typical pattern | +/− | +/− | +++ | +++ | +/− | + | − | +++ | +/− | +/− | +++ | − |
| 24 | HSS typical pattern | − | +/− | +++ | +++ | +/− | + | − | +++ | +/− | +/− | +++ | − |
| 25 | HSS typical pattern | − | + | +++ | +++ | + | +/− | + | +++ | +/− | +/− | ++ | +/− |
| 26 | HSS typical pattern | − | +/− | +++ | +++ | +/− | +/− | +/− | +++ | + | + | +++ | +/− |
| 27 | HSS typical pattern | − | +/− | +++ | +++ | + | +/− | + | +++ | +/− | +/− | +++ | +/− |
| 28 | HSS typical pattern | − | + | +++ | +++ | +/− | +/− | +++ | +/− | +/− | +/− | +++ | +/− |
| 29 | HSS typical pattern | − | + | +++ | +++ | + | +/− | + | +++ | +/− | +/− | +++ | +/− |
| 30 | HSS typical pattern | − | + | +++ | +++ | + | +/− | − | +++ | + | + | +++ | +/− |
| 31 | HSS typical pattern | − | +/− | +++ | +++ | +/− | +/− | +/− | +++ | +/− | +/− | +++ | + |
| 32 | HSS typical pattern | − | +/− | +++ | +++ | +/− | +/− | +/− | +++ | +/− | +/− | +++ | + |
| 33 | HSS typical pattern | − | + | +++ | +++ | +/− | +/− | +/− | +++ | +/− | +/− | +++ | + |
Abbreviations: HSS, histiocytoid Sweet syndrome; MNDA, myeloid nuclear differentiation antigen; MPO, myeloperoxidase.
One of the most important double immunostains.
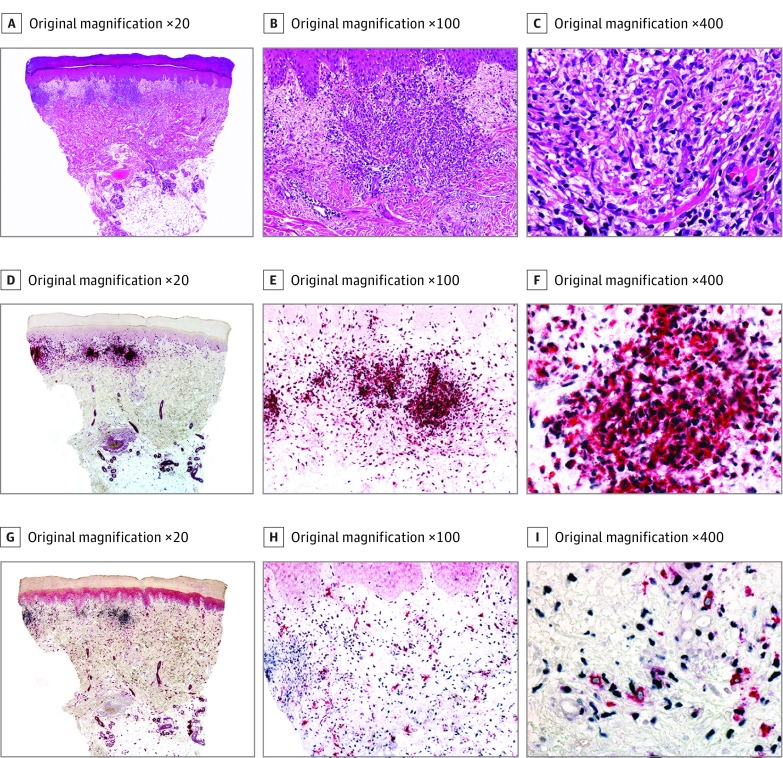
Figure 2. Histopathologic and Immunohistochemical Features of Histiocytoid Sweet Syndrome (Case 8).
A-C, Hematoxylin-eosin–stained specimens. A, Scanning power shows edema and nodular infiltrates in the superficial dermis. B, The infiltrate is close to the epidermis, but there is no exocytosis. C, The nodules are mostly composed of mononuclear cells with twisted vesicular nuclei and scant eosinophilic cytoplasm. D-F, Double immunostained specimens (myeloid nuclear differentiation antigen [MNDA, black] and myeloperoxidase [MPO, red]). D, Scanning power. E, With this double immunostain, exocytosis of single cells is more evident than with hematoxylin-eosin. F, Most of the cells of the infiltrate coexpress MNDA in their nuclei and MPO in their cytoplasm. G-I, Double immunostained specimens (MNDA [black] and CD163 [red]). G, Scanning power. H, With this double immunostain, most of the cells express MNDA in their nuclei, while less scattered cells express CD163 in their cytoplasm. I, Higher magnification demonstrates that cells expressing CD163 in their cytoplasm do not express MNDA in their nuclei.
FISH Analysis Results
Seven patients underwent fluorescence in situ hybridization (FISH) analysis of their cutaneous biopsy specimens. In all of these cases, FISH analysis had previously been performed on bone marrow. In case 1, chronic myelogenous leukemia was diagnosed with a 9;22 translocation resulting in bcr/abl fusion in bone marrow. The same translocation was found in some scattered cells within the infiltrate involving the dermis (Figure 3). In case 33, acute myelogenous leukemia was diagnosed with monosomy of chromosome 5 in bone marrow, but no chromosome aberration was found in the cutaneous biopsy specimen. In cases 7, 9, 10, 11, and 32, no chromosome aberrations were found in either the cutaneous specimen or the bone marrow.
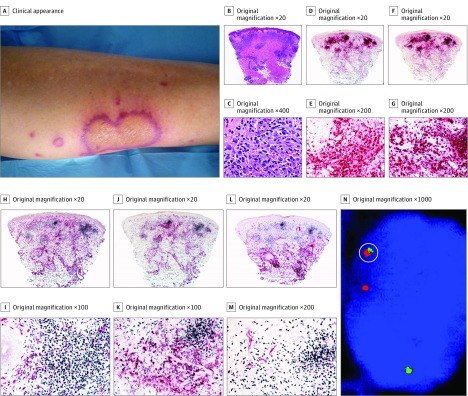
Figure 3. Histiocytoid Sweet Syndrome in a Patient With Chronic Myelogenous Leukemia (Case 1).
A, Clinically, the lesions occurred on the lower extremities and were annular with an erythematous border. B and C, Hematoxylin-eosin–stained specimens. B, Histopathologic features consist of edema in the papillary dermis and a bandlike infiltrate in the superficial dermis; there are also perivascular infiltrates around deep dermal vascular plexus. C, At higher magnification, the predominant cells of the infiltrate are mononuclear cells with vesicular twisted nuclei and scant eosinophilic cytoplasm; nuclei are slightly pleomorphic, and there are some mitotic figures. D and E, Double immunostained specimens (myeloperoxidase [MPO, red] and CD163 [black]). D, Scanning power. E, At higher magnification, most cells express MPO, and very few cells at the periphery express CD163. F and G, Double immunostained specimens (MPO [red] and myeloid nuclear differentiation antigen [MNDA, black]). F, Scanning power. G, At higher magnification, most cells of the infiltrate coexpressed MNDA in their nuclei and MPO in their cytoplasm. H and I, Double immunostained specimens (MNDA [black] and CD163 [red]). H, Scanning power. I, At higher magnification, most cells of the infiltrate expressed MNDA in their nuclei, whereas some cells at the periphery expressed CD163 in their cytoplasm; no cells coexpress both markers. J and K, Double immunostained specimens(MNDA [black] and CD14 [red]). J, Scanning power. K, At higher magnification, the cells of the nodules express MNDA in their nuclei, whereas cells expressing CD14 in their cytoplasm are at the periphery of the nodules. L and M, Double immunostained specimens (MNDA [black] and CD34 [red]). L, Scanning power. M, At higher magnification, most cells of the infiltrate express MNDA in their nuclei, whereas only endothelial cells of the blood capillaries express CD34 in their cytoplasm. N, By fluorescent in situ hybridization, single scattered cells show red-green bcr/abl fusion signal (circled); these cells are interpreted as leukemia cells intermingled with nonneoplastic cells of histiocytoid Sweet syndrome.
Immunohistochemical Findings
Immunohistochemical findings are listed in the Table. Briefly, coexpression of both myeloperoxidase (MPO) and CD163+ was not found except in 6 samples in which this double positivity was seen in less than 10% of the whole cell population of the infiltrate. The isolated MPO+ expression was higher than 75% of the cells of the infiltrate in all cases except in 2 cases in which it was about 50% and 25%, respectively (cases 2 and 19). Isolated CD163+ cells were found in 10% to 25% of the infiltrate in all but 1 case (case 19), with an isolated CD163+ population of approximately 50% of the cells. Curiously, the distribution of CD163+ cells exhibited a marked peripheral arrangement, and it consisted of scattered cells, never grouped in clusters, around the main bulk of the infiltrate (Figure 2) (eFigure 1 in the Supplement).
Strong and diffuse coexpression of myeloid nuclear differentiation antigen (MNDA) and MPO+ was the rule in all cases except case 19, where weak positivity was found. Coexpression of MNDA+/CD163+ was found in less of the 25% of the cell population in 29 of our cases (88%). In case 19, the majority of the infiltrate was composed of isolated CD163+ cells. The MNDA+/CD14+ population was quite similar to the MNDA+/CD163+ population, perhaps with a slightly higher number of positive cells. Coexpression of MNDA+/CD34+ and MNDA+/CD117+ was found in only 1 case (case 28).
Cases 5, 12, and 13 were very similar in their immunochemical profile. In those cases, it was observed that, while a CD163+/MPO+ population of cells was absent, double immunostaining for MNDA+/MPO+ and MNDA+/CD163+ was intensely positive. In case 5, lesions disappeared after treatment with low doses of oral corticosteroids, and follow-up for 3 years failed to demonstrate any associated hematologic malignancy. In cases 12 and 13, myelomonocytic leukemia was diagnosed. In case 12, HSS appeared approximately 5 years before the leukemia diagnosis, while in case 13 HSS developed approximately 2 years before the diagnosis of leukemia. Lesions disappeared after treatment with low doses of oral corticosteroids, and no subsequent recurrent lesions were reported. Case 28 also showed coexistence of MNDA+/MPO+ (+++) and MNDA+/CD163+ (++) cells, and the presence of MNDA+/CD34+ (++) and MNDA+/CD117+ (++) cells was also seen. However, follow-up of this patient during 10 years failed to demonstrate any associated leukemia or other hematologic malignancy. Case 19 showed, as in most other cases, a complete absence of a CD163+/MPO+ cell population, but isolated CD163+ cells were seen in large number in the CD163+/MPO+ and in the MNDA+/CD163+ double immunostains. In contrast with the findings in the rest of the series, case 19 showed a very sparse MNDA+/MPO+ population. Thus, this case probably corresponded to a true histiocytic SS, that is, a late-stage lesion of conventional SS in which authentic histiocytes replaced the previous neutrophilic infiltrate.
Discussion
Our research group originally described HSS in 2005 after studying 41 patients with clinical features of SS and a histopathologic picture of dermal infiltrate composed mostly of mononuclear cells, which, because their MPO positivity, were interpreted as immature myeloid cells. After the original description of HSS, some publications reported association of this process with different clinical scenarios, including the administration of drugs and the presence of autoimmune diseases, inflammatory bowel disease, malignant processes, rheumatoid arthritis and pleuritis, relapsing polychondritis, parotiditis, and uveitis.
Regarding comorbidities, many patients in the present study had associated disorders. Approximately 30% (11 of 33) had an associated malignancy, 80% of which (8 of the 11) were hematological malignancies. The most common associated disorder was MDS, representing 35% of the hematological malignancies (3 of 8) or 9% of patients overall (3 of the 33 cases). While our group’s original data on HSS have been misquoted by some authors, who have reported that only 1 case showed associated hematological malignancy, in fact 6 (15%) of 41 cases in our earlier study had had some underlying hematological disorder. Even at that, this proportion of associated hematologic processes is substantially lower than that reported in other publications about HSS, where hematologic malignancies have been as high as 61% of the cases in a series of 13 patients. Our results are similar to those described in classic neutrophilic SS, in which approximately 21% of patients have been linked with malignancy; and of these, 85% had hematological disorders, most frequently acute myelogenous leukemia. Therefore, in contrast with what has been previously suggested, we found that HSS is not more frequently associated with hematologic malignancy than classic SS. According to the literature, there seems to be a specific and particularly frequent association between HSS and MDS. In the present study, 3 of the 8 patients with hematologic malignancies had MDS, the most common hematologic disorder. However this association was not strong enough to suggest that in patients with HSS, MDS must be ruled out.
Regarding the nature of the mononuclear cells of the dermal infiltrate of HSS, both myeloid and histiocytic origins have been proposed, and it has even been proposed that HSS is nothing more than skin involvement by a myeloproliferative disorder, ie, leukemia cutis. Remarkably, without accompanying clinical information, even expert dermatopathologists are tempted to interpret HSS as a morphological variant of leukemia cutis. Of course, cutaneous lesions initially suggestive of neutrophilic dermatosis may actually represent skin involvement by leukemia cutis, even in the absence of peripheral blood involvement, the so-called aleukemic leukemia cutis. It is also possible that patients with leukemia cutis show scattered leukemia cells intermingled with neutrophils in cutaneous lesions of neutrophilic dermatosis (case 1 of the present series). In our opinion, however, those cases described as clonal neutrophilic skin proliferation in the setting of an underlying myeloproliferative disease and as clonal neutrophilic skin proliferation with no findings, still, of hematologic neoplasia are better interpreted as leukemia cutis and aleukemic leukemia cutis, respectively.
In a recent report, Chavan et al discuss the usefulness of performing FISH analysis on skin biopsy specimens of patients with a diagnosis of SS and concomitant hematologic malignancy. Of the 22 patients recruited, the study was performed in 5 patients with an identifiable chromosomal abnormality in the bone marrow. In 4 of the 5 patients, leukemic cells were detected in the cutaneous infiltrate using—and this is a very important point—the appropriate malignancy-specific FISH probes. In the present study, we used FISH analysis on the cutaneous specimens of 7 patients, and of those, only 1 (case 1) had the same recognizable chromosomal anomaly (t 9:22) in both bone marrow and skin lesions. However, the fusion gene was found only in a minority of the cell population infiltrating the skin. After having performed a thorough study of the clinical, histopathological, immunohistochemical, and molecular data, we believe that the most plausible interpretation of this unusual case 1 is the coexistence of leukemic cells and nonleukemic myeloid cells in the dermal infiltrate, ie, coexisting leukemia cutis and HSS. The limitation of the FISH analysis performed in the skin is that it is only useful where there is a known hematologic malignancy with an identifiable chromosomal abnormality. Therefore, regardless of immunohistochemical, molecular, and genetic techniques, in most cases only the clinical follow-up of the patient may determine with confidence whether a skin infiltration may be diagnosed as leukemia cutis.
Human neutrophilic myeloid cells contain 2 types of cytoplasmic granules, the primary or azurophilic granules and the secondary or specific granules. The primary granules are identified early in differentiation (eg, in myeloblasts and promyeloblasts) and contain MPO, neutrophil elastase, unique hydrolytic enzymes, and part of the cytoplasmic lysozyme. The secondary granules are found later in maturation (myelocytic stage) and apparently lack hydrolytic enzymes but contain alkaline phosphatase, lactoferrin, and most of the lysozyme. In the study that has best characterized the expression, sensitivity, specificity, and practical usefulness of MPO, Pinkus and Pinkus studied 161 bone marrow biopsy specimens, 5 lymph nodes, and 1 spleen sample from patients with and without myeloproliferative disorders. They found that immature myeloid cells, including blast forms, expressed strong cytoplasmic MPO positivity in nearly all cases, and they concluded that MPO is a much more specific marker than lysozyme, neutrophil elastase, and chloracetate esterase for myeloid cells, and therefore, immunohistochemical detection of MPO is a highly effective and specific method for the identification of mature and immature myeloid cells in paraffin sections. When evaluating cases of acute monocytic leukemia, they found that monoblasts could appear to be MPO+ in low titers of MPO dilution (1:100), but findings would be very weakly positive or even negative at higher dilutions of the antibody. According to the authors, MPO positivity in monocytes was variable, but mostly negative, while histiocytes were a nonreactive population for MPO. In the cases of HSS detailed in the present study, most of the mononuclear cells of the dermal infiltrate showed strong cytoplasmic expression of MPO, with a granular pattern, supporting their myeloid nature.
Expression of CD163 is restricted to the monocyte-macrophage lineage cells. Monocytes have a modest immunoreactivity, and weak or absent CD163 expression is seen in other monocyte-derived cells, such as dendritic cells, Langerhans cells, and white pulp macrophages in the spleen. Macrophage plasticity is demonstrated by acquisition of distinct morphological and functional properties in response to specific immunological microenvironments in different tissues. At both ends of the spectrum of the well-known macrophage polarization, M1 and M2 types have been identified. Type M1 macrophages are characterized by a proinflammatory phenotype, promotion of helper T cell, type 1 (TH1) immune response and tumoricidal activity, while M2 macrophages display regulatory functions in tissue repair, remodeling and promotion of TH2 immune response. Types M1 and M2 macrophages have distinct chemokine profiles, with M1 macrophages expressing TH1-attracting chemokines such as CXCL9 and CXCL10, and M2 macrophages expressing the chemokines CCL17, CCL22 and CCL24. They also have distinct features in terms of the metabolism of iron, folate, and glucose. None of these chemokines is suitable for identification by immunohistochemical analysis with commercially available antibodies. For some time, CD163 had falsely been considered an M2-like macrophage marker. However, Barros et al demonstrated that CD163 is not an M2-specific marker but rather a histiocytic lineage indicator. Regardless, the cases in the present study showed only few and scattered mononuclear CD163+ cells, both with double immunostain positivity (MNDA+/CD163+) as well as cells expressing only cytoplasmic CD163 positivity, and these few CD163+ cells were seen mostly at the periphery of the main bulk of the infiltrate, which tested almost entirely CD163 negative.
Myeloid nuclear differentiation antigen is the only immunohistochemically demonstrable nuclear antigen expressed specifically in nonblastic myelomonocytic cell lines. In a comprehensive study published some years ago, Miranda et al studied the MNDA immunoreactivity patterns of different cell lines in different lymphoid and hematopoietic organs at baseline and in several pathologic conditions. This study demonstrated that cell lines with relatively mature myelomonocytic features were strongly MNDA positive. The specific nuclear expression of MNDA makes its interpretation much easier than markers with cytoplasmic or membrane localization, where staining usually obscures cell morphology, and it is especially helpful for double immunostaining combined with other cytoplasmic markers. In the present study, the strong double expression for MNDA+/MPO+ in most of the cells of the infiltrate of these cases of HSS demonstrated their myelomonocytic rather than histiocytic lineage.
In our group’s original description of HSS, the immunohistochemical phenotype of most of the cells of the infiltrate led us to conclude that the main population constituting the infiltrate of this new variant of SS was of myeloid lineage. The decisive marker for this interpretation was MPO, which was strongly expressed in all cases. Subsequently, this interpretation generated some controversy. Peroni et al reported 12 cases of HSS in which dermal infiltrating histiocytoid cells expressed CD68 (PG-M1), CD163, MPO, and MNDA, and tested negative for CD15, CD34, and CD117. A few months later, Magro et al reported a limited series of 13 patients with very similar immunophenotypic profile, ie, CD163+, CD68+, and MPO+. Because CD163 was considered at that time to be a specific marker for M2-like macrophages, both groups of authors interpreted their findings in HSS as expression of a cutaneous infiltration of M2 macrophages, with a peculiar concomitant MPO+ expression. Other authors reached similar conclusions. In our opinion, to assert with confidence that a particular cell coexpresses 2 markers, double immunostaining is mandatory; and serial sections are not enough because different cells may be stained in 2 consecutive sections.
In the present study double immunostaining was performed in all cases. As summarized in the Table, coexpression of MPO+/CD163+ was negative in all but 6 samples. In these 6 samples, the positivity represented less than 10% of the whole cell population, while the isolated MPO+ expression was higher than 75% in almost all 6 samples, and isolated CD163+ was only found in 10% to 25% of the infiltrate in all but 1 case, and that in a marked peripheral arrangement. In our view, these findings rule out the possibility that (1) highly differentiated histiocytic cells were the predominant ones and (2) the same unique cell was expressing both MPO+ and CD163+. The isolated and scattered CD163+ cells might correspond to authentic M2 macrophages, but this cannot be asserted with confidence by immunohistochemical analysis alone. In any case, the few CD163+ cells should be interpreted as accompanying cells and not as the principal component of the infiltrate. We believe that of all double immunostainings that we performed, the most important and informative for determining the true nature of the infiltrating cells in HSS is MPO/CD163. The MNDA+/MPO+ double immunostaining demonstrated that the cells expressing MPO reactivity in their cytoplasm were also MNDA+ mononuclear cells, but not polymorphonuclear cells, and therefore, they were not mature neutrophils.
Conclusions
The present study demonstrates that most of the cells of the infiltrate in cutaneous lesions of HSS belong to the myeloid lineage, being mainly immature nonblastic cells, that is, promyelocytes and myelocytes. The few scattered and predominantly periphery macrophages encountered are better interpreted by us as accompanying inflammatory cells that never are the predominant cell.
eAppendix. Material and methods
eFigure 1. (Case 23) Histopathologic and immunohistochemical features of histiocytoid Sweet syndrome
eFigure 2. (Case 12) Histopathologic and immunohistochemical features of histiocytoid Sweet syndrome with subcutaneous involvement.
eFigure 1 and 2. Legends
eTable 1. Immunohistochemical markers used in this study
eTable 2. Clinical characteristics of this series of histiocytoid Sweet syndrome
References
- 1.Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141(7):834-842. [DOI] [PubMed] [Google Scholar]
- 2.Kim JS, Roh HS, Lee JW, Lee MW, Yu HJ. Distinct variant of Sweet’s syndrome: bortezomib-induced histiocytoid Sweet’s syndrome in a patient with multiple myeloma. Int J Dermatol. 2012;51(12):1491-1493. [DOI] [PubMed] [Google Scholar]
- 3.Wu AJ, Rodgers T, Fullen DR. Drug-associated histiocytoid Sweet’s syndrome: a true neutrophilic maturation arrest variant. J Cutan Pathol. 2008;35(2):220-224. [PubMed] [Google Scholar]
- 4.Murase JE, Wu JJ, Theate I, Cole GW, Barr RJ, Dyson SW. Bortezomib-induced histiocytoid Sweet syndrome. J Am Acad Dermatol. 2009;60(3):496-497. [DOI] [PubMed] [Google Scholar]
- 5.Bonazza S, Dalton B, Hardin J, Metelitsa A. Histiocytoid variant of Sweet syndrome associated with azacitidine and recurrence upon rechallenge. Can J Hosp Pharm. 2015;68(4):339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llamas-Velasco M, Concha-Garzón MJ, Fraga J, Aragüés M. Histiocytoid sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015;81(3):305-306. [DOI] [PubMed] [Google Scholar]
- 7.Hau E, Vignon Pennamen MD, Battistella M, et al. Neutrophilic skin lesions in autoimmune connective tissue diseases: nine cases and a literature review. Medicine (Baltimore). 2014;93(29):e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camarillo D, McCalmont TH, Frieden IJ, Gilliam AE. Two pediatric cases of nonbullous histiocytoid neutrophilic dermatitis presenting as a cutaneous manifestation of lupus erythematosus. Arch Dermatol. 2008;144(11):1495-1498. [DOI] [PubMed] [Google Scholar]
- 9.Spencer B, Nanavati A, Greene J, Butler DF. Dapsone-responsive histiocytoid Sweet’s syndrome associated with Crohn’s disease. J Am Acad Dermatol. 2008;59(2)(suppl 1):S58-S60. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Torres RM, Castro S, Moreno A, Alvarez R, Fonseca E. Subcutaneous histiocytoid sweet syndrome associated with Crohn disease in an adolescent. Case Rep Dermatol Med. 2014;2014:954254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser R, Connolly K, Linker C, Maldonado J, Fye K. Stem cell transplant for myelodysplastic syndrome-associated histiocytoid Sweet’s syndrome in a patient with arthritis and myalgias. Arthritis Rheum. 2008;59(12):1832-1834. [DOI] [PubMed] [Google Scholar]
- 12.Hünermund A, Wendel AM, Geissinger E, Bröcker EB, Stoevesandt J. Typically atypical: histiocytoid Sweet syndrome, associated with malignancy. J Dtsch Dermatol Ges. 2011;9(9):666-669. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Liu Y, Zheng H. Histiocytoid Sweet’s syndrome associated with rheumatoid arthritis and pleuritis. Chin Med J (Engl). 2014;127(7):1396. [PubMed] [Google Scholar]
- 14.Arima Y, Namiki T, Ueno M, et al. Histiocytoid Sweet syndrome: a novel association with relapsing polychondritis. Br J Dermatol. 2016;174(3):691-694. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Namiki T, Tokoro S, Miura K, Yokozeki H. Histiocytoid Sweet syndrome with ophthalmologic involvements: a novel association with uveitis [published online May 14, 2016]. J Dermatol. 2016. doi: 10.1111/1346-8138.13453 [DOI] [PubMed] [Google Scholar]
- 16.Jo MS, Lim YB, Shin HK, Choe J, Seul JH, Jang TJ. A case report of Sweet’s syndrome with parotitis. Arch Plast Surg. 2012;39(1):59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magro CM, Momtahen S, Nguyen GH, Wang X. Histiocytoid Sweet’s syndrome: a localized cutaneous proliferation of macrophages frequently associated with chronic myeloproliferative disease. Eur J Dermatol. 2015;25(4):335-341. [DOI] [PubMed] [Google Scholar]
- 18.Cohen PR. Sweet’s syndrome: a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107(5):369-378. [DOI] [PubMed] [Google Scholar]
- 20.Ghoufi L, Ortonne N, Ingen-Housz-Oro S, et al. Histiocytoid Sweet syndrome is more frequently associated with myelodysplastic syndromes than the classical neutrophilic variant: a comparative series of 62 patients. Medicine (Baltimore). 2016;95(15):e3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalaby MM, Riahi RR, Rosen LB, Soine EJ. Histiocytoid Sweet’s syndrome in a patient with myelodysplastic syndrome: report and review of the literature. Dermatol Pract Concept. 2016;6(1):9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush JW, Wick MR. Cutaneous histiocytoid Sweet syndrome and its relationship to hematological diseases. J Cutan Pathol. 2016;43(4):394-399. [DOI] [PubMed] [Google Scholar]
- 23.Srisuttiyakorn C, Reeve J, Reddy S, Imaeda S, Lazova R. Subcutaneous histiocytoid Sweet’s syndrome in a patient with myelodysplastic syndrome and acute myeloblastic leukemia. J Cutan Pathol. 2014;41(5):475-479. [DOI] [PubMed] [Google Scholar]
- 24.Pinal-Fernandez I, Ferrer Fabrega B, Ramentol Sintas M, Solans Laque R. Histiocytoid Sweet syndrome and cutaneous polyarteritis nodosa secondary to myelodysplastic syndrome. Int J Rheum Dis. 2013;16(6):777-779. [DOI] [PubMed] [Google Scholar]
- 25.Park JY, Park JS, Kim YC. Histiocytoid Sweet’s syndrome potentially related to decitabine in a patient with myelodysplastic syndrome. Eur J Dermatol. 2012;22(6):811-812. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Zhang Q, Chen M. Subcutaneous histiocytoid Sweet’s syndrome in a patient associated with myelodysplastic syndrome-refractory anemia. J Dermatol. 2012;39(1):99-101. [DOI] [PubMed] [Google Scholar]
- 27.Ten Oever J, Kuijper PH, Kuijpers AL, Dercksen MW, Vreugdenhil G. Complete remission of MDS RAEB following immunosuppressive treatment in a patient with Sweet’s syndrome. Neth J Med. 2009;67(8):347-350. [PubMed] [Google Scholar]
- 28.Kulasekararaj AG, Kordasti S, Basu T, Salisbury JR, Mufti GJ, du Vivier AW. Chronic relapsing remitting Sweet syndrome: a harbinger of myelodysplastic syndrome. Br J Haematol. 2015;170(5):649-656. [DOI] [PubMed] [Google Scholar]
- 29.Chow S, Pasternak S, Green P, et al. Histiocytoid neutrophilic dermatoses and panniculitides: variations on a theme. Am J Dermatopathol. 2007;29(4):334-341. [DOI] [PubMed] [Google Scholar]
- 30.Liu CI, Hsiao CH, Wu JT, Tsai TF. Sweet syndrome with histiocytoid infiltrate and neutropenia: a rare combination. J Am Acad Dermatol. 2009;61(5):882-884. [DOI] [PubMed] [Google Scholar]
- 31.Heymann WR. Histiocytoid Sweet syndrome. J Am Acad Dermatol. 2009;61(4):693-694. [DOI] [PubMed] [Google Scholar]
- 32.Peroni A, Colato C, Schena D, Rongioletti F, Girolomoni G. Histiocytoid Sweet syndrome is infiltrated predominantly by M2-like macrophages. J Am Acad Dermatol. 2015;72(1):131-139. [DOI] [PubMed] [Google Scholar]
- 33.Miller J, Lee N, Sami N. Histiocytoid Sweet syndrome treated with azathioprine: a case report. Dermatol Online J. 2015;21(7):13030/qt0cg9m1h9. [PubMed] [Google Scholar]
- 34.Zhenying Z, Xiaoming L, Yongjun P, Shixin H. A case of leukemia cutis presenting as histiocytoid Sweet’s syndrome. Int J Dermatol. 2013;52(11):1338-1341. [DOI] [PubMed] [Google Scholar]
- 35.Chavan RN, Cappel MA, Ketterling RP, et al. Histiocytoid Sweet syndrome may indicate leukemia cutis: a novel application of fluorescence in situ hybridization. J Am Acad Dermatol. 2014;70(6):1021-1027. [DOI] [PubMed] [Google Scholar]
- 36.Narváez-Moreno B, Pereyra-Rodríguez JJ, Pulpillo-Ruiz A, Cabrera-Pérez R, Espigado-Tocino I, Conejo-Mir J. Acute myeloid leukemia 7 years after aleukemic leukemia cutis. Int J Dermatol. 2015;54(4):459-461. [DOI] [PubMed] [Google Scholar]
- 37.Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132(1):101-110. [DOI] [PubMed] [Google Scholar]
- 38.Magro CM, De Moraes E, Burns F. Sweet’s syndrome in the setting of CD34-positive acute myelogenous leukemia treated with granulocyte colony stimulating factor: evidence for a clonal neutrophilic dermatosis. J Cutan Pathol. 2001;28(2):90-96. [DOI] [PubMed] [Google Scholar]
- 39.Magro CM, Kiani B, Li J, Crowson AN. Clonality in the setting of Sweet’s syndrome and pyoderma gangrenosum is not limited to underlying myeloproliferative disease. J Cutan Pathol. 2007;34(7):526-534. [DOI] [PubMed] [Google Scholar]
- 40.Pinkus GS, Pinkus JL. Myeloperoxidase: a specific marker for myeloid cells in paraffin sections. Mod Pathol. 1991;4(6):733-741. [PubMed] [Google Scholar]
- 41.Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013;18(17):2352-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowal K, Silver R, Sławińska E, Bielecki M, Chyczewski L, Kowal-Bielecka O. CD163 and its role in inflammation. Folia Histochem Cytobiol. 2011;49(3):365-374. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677-686. [DOI] [PubMed] [Google Scholar]
- 44.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344-346. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176-185. [DOI] [PubMed] [Google Scholar]
- 47.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8(11):e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda RN, Briggs RC, Shults K, Kinney MC, Jensen RA, Cousar JB. Immunocytochemical analysis of MNDA in tissue sections and sorted normal bone marrow cells documents expression only in maturing normal and neoplastic myelomonocytic cells and a subset of normal and neoplastic B lymphocytes. Hum Pathol. 1999;30(9):1040-1049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Material and methods
eFigure 1. (Case 23) Histopathologic and immunohistochemical features of histiocytoid Sweet syndrome
eFigure 2. (Case 12) Histopathologic and immunohistochemical features of histiocytoid Sweet syndrome with subcutaneous involvement.
eFigure 1 and 2. Legends
eTable 1. Immunohistochemical markers used in this study
eTable 2. Clinical characteristics of this series of histiocytoid Sweet syndrome