Abstract
Angiosarcomas are rare, malignant neoplasms of vascular origin that account for less than 1% of all soft tissue tumors. Angiosarcomas of the oral cavity are especially rare, and brachytherapy may be prescribed as a localized treatment to manage these malignancies. Intraoral brachytherapy requires collaboration between the radiation oncologist and a dental professional for the fabrication of the brachytherapy delivery prosthesis. The present clinical report describes an intraoral angiosarcoma and the fabrication of an intraoral brachytherapy prosthesis to manage this malignancy.
INTRODUCTION
Angiosarcomas are rare, malignant neoplasms of vascular origin that account for less than 1% of all soft tissue tumors. The presentation and behavior of angiosarcomas differ depending on location; consequently, angiosarcomas can be divided into several clinical groups.1 Although angiosarcomas can arise anywhere in the body, 60% arise in skin or superficial soft tissue.2 Cutaneous angiosarcomas, the most common form, primarily affect elderly men and are typically located in the head and neck, particularly the scalp.1,2 However, angiosarcomas can arise extremely rarely in the oral and salivary glands. A recent series on oral and salivary gland angiosarcomas found that they constitute 1% of all cases of angiosarcoma.3 In general, the prognosis for angiosarcoma is poor: overall survival has been reported to be between 10% and 34%, with most patients dying within 2 to 3 years of metastases to the lung, liver, or lymph node.2-4 Comparatively, the case series on oral and salivary gland angiosarcomas found that, during a mean follow-up of 8.6 years, most situations were without recurrence or metastasis, suggesting that biological behavior of angiosarcomas in these locations is more favorable.3 Surgery is the preferred treatment, and in most patients, surgery is followed by radiation therapy, as it has been shown to prolong survival.1,5 Whether adjuvant chemotherapy prolongs overall survival, however, is unclear.1,6
CLINICAL REPORT
In August 2013, a 76-year-old white man was referred to the Dental Service by the Radiation Oncology Department at Memorial Sloan Kettering with a diagnosis of metastatic angiosarcoma. The patient was first diagnosed with an angiosarcoma of the right submandibular gland in 1996 while undergoing a nasal septoplasty and has had multiple recurrences in the head and neck. In 2012, a positron emission tomography (PET) scan confirmed the presence of hypermetabolic masses in the lung and liver which were later confirmed, by biopsy to be metastatic angiosarcoma. The patient’s condition has been managed with a combination of chemotherapy (liposal doxorubicin and paclitaxel), radiotherapy, and surgery. He was referred to the Dental Service for collaboration in the management of a vascular, raised midline lesion measuring 2.3×2.3 cm (infiltrating 3 mm) and extending from the hard palate to the border of the soft palate (Fig. 1). The patient had a full range of motion in the head and neck and was without extraoral swelling or palpable cervical lymphadenopathy. On intraoral examination, the patient presented with a normal interincisal opening of approximately 35 mm and could protrude the tongue without deviation. A brachytherapy approach was prescribed by the Radiation Oncology Service for delivery of radiation treatment, and the Dental Service was requested to fabricate the brachytherapy delivery prosthesis. An alternative treatment option for this malignancy could have been external beam radiation; however, the sequelae for external beam radiation would have included a much larger field of mucositis as compared to the selected brachytherapy approach.
Fig. 1.
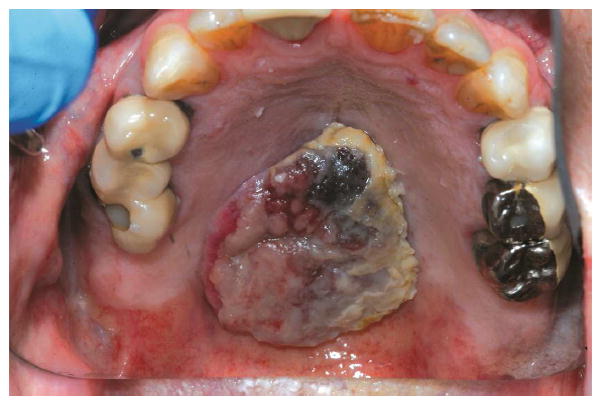
Intraoral malignancy (angiosarcoma), 2.3×2.3 cm, on hard palate.
Several techniques for the fabrication of brachytherapy prostheses have been described.7,8 The prosthesis fabricated for this palatal lesion includes a lead shield to protect the intraoral structures from additional and unnecessary exposure to radiotherapy. Maxillary and mandibular irreversible hydrocolloid impressions were made, and special care was taken to reproduce the area of the lesion in the impression material. The treating radiation oncology team was then consulted to determine the prescribed arrangement and proximity of the brachytherapy catheters (two-stage interstitial HDR catheters; Mick Radio Nuclear Instruments) within the prosthesis (5 mm between catheters and 5 mm from the intaglio surface of the prosthesis). A heat-polymerized polymethyl methacrylate (Type 1 Hygenic Denture Resin; Coltène/Whaledent) maxillary prosthesis was then fabricated. Brachytherapy catheters were embedded in the maxillary prosthesis as prescribed by the radiation oncologist (Figs. 2, 3). The processed prosthesis was then evaluated in the patient’s mouth and adjusted for comfort. The mandibular teeth contacted the intaglio of the prosthesis to provide additional stability.
Fig. 2.
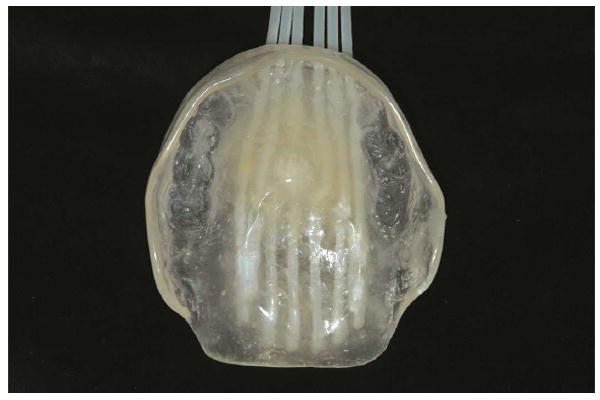
Intaglio view of brachytherapy catheters embedded in brachytherapy prosthesis, as prescribed by radiation oncology team.
Fig. 3.
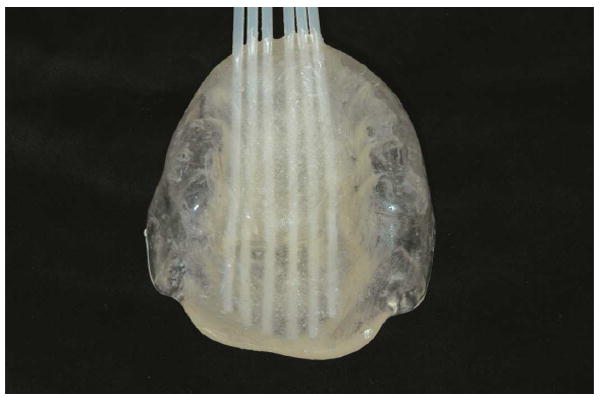
Cameo view of brachytherapy catheters embedded in brachytherapy prosthesis, as prescribed by radiation oncology team.
By using the treatment planning and dose delivery software (BrachyVision; Varian Brachytherapy), the brachytherapy treatment was simulated with the prosthesis, without the patient present (Fig. 4). The treatment volume was visually available to confirm that the prosthesis adequately facilitated the radiotherapy treatment. After successful simulation, a 7×70×10 mm sheet of lead was integrated into the palatal aspect of the prosthesis with autopolymerizing polymethyl methacrylate clear resin (Caulk Orthodontic Resin; Dentsply Intl) (Fig. 5). The lead design, as calculated by the treatment planning and dose delivery software, was effective in blocking 94% of the radiation dose inferior to the tumor volume. The completed prosthesis was finally evaluated in the patient, polished, and delivered (Fig. 6). The patient’s vertical dimension of occlusion was increased to comfortably allow maximum distance between the maxillary arch and inferior structures of the oral cavity.
Fig. 4.
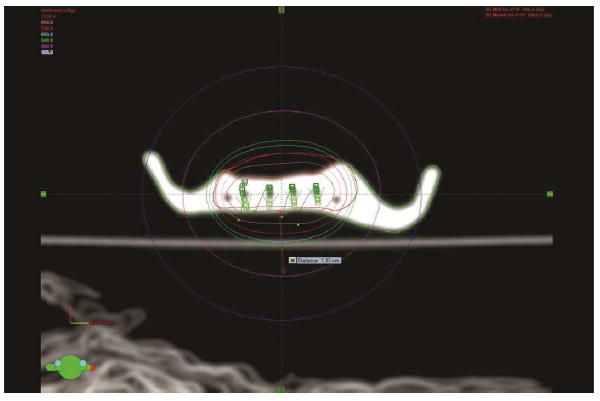
Brachytherapy simulation with brachytherapy prosthesis, using BrachyVision software.
Fig. 5.

Integration of 7×70 mm lead sheet into palatal aspect of prosthesis with autopolymerizing polymethyl methacrylate clear resin.
Fig. 6.
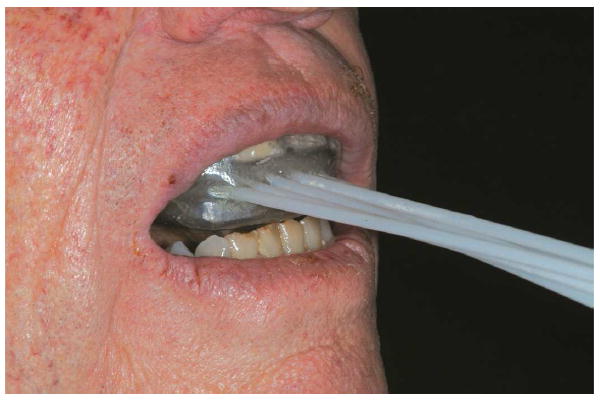
Completed prosthesis delivered to patient.
The patient and the prosthesis were then escorted to the Radiation Oncology Department for brachytherapy treatment. The patient was prescribed and completed a 5-day, 30 Gy course of brachytherapy. The treatment was delivered once daily on 5 consecutive days for approximately 8 minutes per day. Each dose was 6 Gy. The treatment volume included the area of the gross tumor and extended 5 mm laterally and 2 mm inferiorly to the margins of the lesion. The sequelae of treatment included mild mucositis, which presented after 1 week of treatment. The mucositis was managed with a mouthwash suspension of diphenhydramine hydrochloride, lidocaine, and nystatin every 2 to 3 hours or at a longer interval between doses as needed. The mucositis completely resolved within 3 weeks of initial presentation.
Approximately 3 months after treatment, the lesion completely resolved. In February 2014, a nodularity was identified on the right hard palate (Fig. 7), as well as subcutaneous nodules in the right thigh, right axilla, and right periscapular area, consistent with metastatic disease. The recurrence on the palate was biopsy proven angiosarcoma and was outside of the field (posterior) of the area treated with brachytherapy. The patient is no longer on a chemotherapy regimen and continues to receive palliative radiotherapy for local control of his systemic disease as indicated by the medical oncology team.
Fig 7.
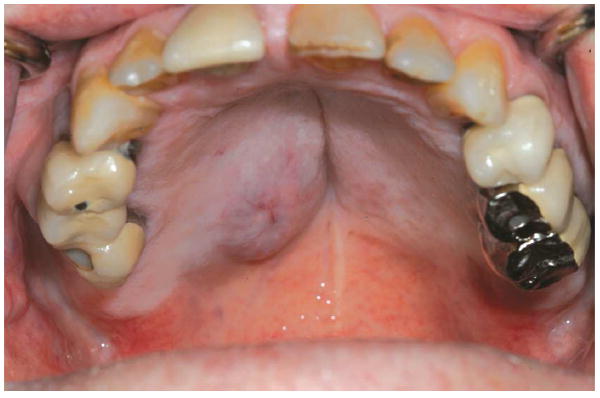
Recurrent nodularity on right hard palate identified in February 2014.
DISCUSSION
This patient presents an example of an intraoral angiosarcoma and its management. As this patient had metastatic disease, brachytherapy was not intended to be curative; however, the presenting lesion responded within 1 month to the palliative treatment. The patient experienced minimal postradiotherapy mucositis, which presented immediately after completing radiotherapy,
The primary advantage of brachytherapy is that it is a personalized treatment delivery system that allows highly specific radiotherapy exposure, thereby minimizing the postradiotherapy sequelae of xerostomia, mucositis, dysphagia, and dysgeusia. It is also a treatment with limited duration, which can be an important quality of life consideration in patients with limited life expectancy.
Brachytherapy is a treatment modality in which the dose is primarily dependent upon the distance from the site of interest. In contrast to external beam radiotherapy in which megavoltage photons may originate almost a meter away from the site of interest, brachytherapy uses short range radioisotopes adjacent to or directly within the site of interest. The inverse square law states that the radiation intensity is inversely proportional to the square of the distance of the source. This is especially important in brachytherapy because the position of the radioisotope will determine the dose to the site of interest and surrounding tissues. With the administration of photon beams, a minimum distance of 3 mm created by a stent can effectively shield backscatter.9,10 In the administration of brachytherapy, the absorbed dose of radioisotope can also be reduced to surrounding tissues as the thickness of lead increases.11 The intraoral thickness of lead is limited by the patient’s maximum interocclusal distance. The previously described brachytherapy prosthesis maximizes the distance between the brachytherapy target site and the surrounding structures to minimize unwanted radiotherapy exposure. The primary disadvantages to this technique are that brachytherapy may not be ideal for larger tumor volumes and that it requires the fabrication of a delivery prosthesis to plan and adequately deliver the desired treatment dose.
SUMMARY
Angiosarcomas of the oral cavity are extremely rare malignancies that require collaborative treatment. The brachytherapy prosthesis fabricated in this situation was designed after the dental team evaluated the patient and consulted with the radiation oncology team. The use of a brachytherapy prosthesis can be appropriate for the management of an intraoral malignancy with minimal patient morbidity.
Footnotes
Presented at the 60th Meeting of the American Academy of Maxillofacial Prosthetics, Albuquerque, New Mexico, October 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss S. Enzinger and Weiss’s soft tissue tumors. 5. Philadelphia: Mosby Elsevier; 2008. pp. 917–938. [Google Scholar]
- 2.Mark RJ. Angiosarcoma: A report of 67 patients and a review of the literature. Cancer. 1996;77:2400–6. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Fanburg-Smith JC. Oral and salivary gland angiosarcoma: A clinicopathologic study of 29 cases. Mod Pathol. 2003;16:263–71. doi: 10.1097/01.MP.0000056986.08999.FD. [DOI] [PubMed] [Google Scholar]
- 4.Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046–57. doi: 10.1002/1097-0142(19870301)59:5<1046::aid-cncr2820590533>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Paulino AF, McGinn CJ, Baker LH, Cohen DS, Morris JS, et al. Cutaneous angiosarcoma of the scalp. Cancer. 2003;98:1716–26. doi: 10.1002/cncr.11667. [DOI] [PubMed] [Google Scholar]
- 6.Fury MG. A 14-year retrospective review of angiosarcoma: Clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–7. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Jolly DE, Nag S. Technique for construction of dental molds for high-dose-rate remote brachytherapy. Spec Care Dentist. 1992;12:219–24. doi: 10.1111/j.1754-4505.1992.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi H. Radiotherapy prostheses. J Med Dent Sci. 2000;47:12–26. [PubMed] [Google Scholar]
- 9.Reitemeier B, Reitemeier G, Schmidt A, Schaal W, Blochberger P, Lehmann D, Herrmann T. Evaluation of a device for attenuation of electron release from dental restorations in a therapeutic radiation field. J Prosthet Dent. 2002;87:323–7. doi: 10.1067/mpr.2002.122506. [DOI] [PubMed] [Google Scholar]
- 10.Chin DW, Treister N, Friedland B, Cormack RA, Tishler RB, Makrigiorgos GM, et al. Effect of dental restorations and prostheses on radiotherapy dose distribution: a Monte Carlo study. J Appl Clin Med Phys. 2009;10:2853. doi: 10.1120/jacmp.v10i1.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudoh T, Ikushima H, Honda E. Shielding effect of a customized intraoral mold including lead material in high-dose-rate 192-Ir brachytherapy for oral cavity cancer. J Radiat Res. 2012;53:130–7. doi: 10.1269/jrr.11102. [DOI] [PubMed] [Google Scholar]


