Abstract
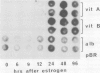
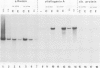
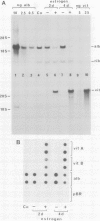
To clarify on what level of gene expression estrogen represses albumin synthesis in Xenopus hepatocytes, we have analyzed nuclear RNAs and the transcriptional rates in isolated nuclei. Since in nuclear RNA the quantity of albumin mRNA and its precursors does not change and the transcription remains constant during estrogen treatment, we conclude that a posttranscriptional control, possibly involving destabilization of cytoplasmic mRNA, is responsible for the repression of albumin synthesis by estrogen. This post-transcriptional control is in contrast to the well-known transcriptional induction of vitellogenin gene activity. The results can be reproduced in liver cube cultures thereby establishing that estrogen interferes directly with hepatic albumin synthesis. In these liver cube cultures albumin mRNA levels are reduced compared with the liver used to set up the culture whereas the transcription of the albumin genes is not influenced. This reveals another post-transcriptional control of hepatic albumin synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozzoni I., Beccari E., Luo Z. X., Amaldi F. Xenopus laevis ribosomal protein genes: isolation of recombinant cDNA clones and study of the genomic organization. Nucleic Acids Res. 1981 Mar 11;9(5):1069–1086. doi: 10.1093/nar/9.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen regulates the absolute rate of transcription of the Xenopus laevis vitellogenin genes. J Biol Chem. 1983 May 10;258(9):5449–5455. [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Ryffel G. U., Weber R. Estradiol-induced accumulation of vitellogenin mRNA and secretion of vitellogenin in liver cultures of Xenopus. Mol Cell Endocrinol. 1978 Nov;12(2):151–166. doi: 10.1016/0303-7207(78)90111-9. [DOI] [PubMed] [Google Scholar]
- Gerber-Huber S., Felber B. K., Weber R., Ryffel G. U. Estrogen induces tissue specific changes in the chromatin conformation of the vitellogenin genes in Xenopus. Nucleic Acids Res. 1981 Jun 11;9(11):2475–2494. doi: 10.1093/nar/9.11.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber-Huber S., May F. E., Westley B. R., Felber B. K., Hosbach H. A., Andres A. C., Ryffel G. U. In contrast to other Xenopus genes the estrogen-inducible vitellogenin genes are expressed when totally methylated. Cell. 1983 May;33(1):43–51. doi: 10.1016/0092-8674(83)90333-1. [DOI] [PubMed] [Google Scholar]
- Gorski J., Welshons W., Sakai D. Remodeling the estrogen receptor model. Mol Cell Endocrinol. 1984 Jun;36(1-2):11–15. doi: 10.1016/0303-7207(84)90079-0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M. A., Brock M. L., Shapiro D. J. The role of estrogen receptor in Xenopus laevis vitellogenin gene expression. Am J Physiol. 1982 Jul;243(1):C1–C6. doi: 10.1152/ajpendo.1982.243.1.E1. [DOI] [PubMed] [Google Scholar]
- Hayward M. A., Mitchell T. A., Shapiro D. J. Induction of estrogen receptor and reversal of the nuclear/cytoplasmic receptor ratio during vitellogenin synthesis and withdrawal in Xenopus laevis. J Biol Chem. 1980 Dec 10;255(23):11308–11312. [PubMed] [Google Scholar]
- Hayward M. A., Shapiro D. J. A middle-affinity estrogen-specific binding protein in livers of vitellogenic and nonvitellogenic Xenopus laevis. Dev Biol. 1981 Dec;88(2):333–340. doi: 10.1016/0012-1606(81)90177-9. [DOI] [PubMed] [Google Scholar]
- Landes G. M., Villeponteau B., Pribyl T. M., Martinson H. G. Hemoglobin switching in chickens. Is the switch initiated post-transcriptionally? J Biol Chem. 1982 Sep 25;257(18):11008–11014. [PubMed] [Google Scholar]
- May F. E., Ryffel G. U., Weber R., Westley B. R. Estrogen dramatically decreases albumin mRNA levels and albumin synthesis in Xenopus laevis liver. J Biol Chem. 1982 Dec 10;257(23):13919–13923. [PubMed] [Google Scholar]
- May F. E., Westley B. R., Wyler T., Weber R. Structure and evolution of the Xenopus laevis albumin genes. J Mol Biol. 1983 Aug 5;168(2):229–249. doi: 10.1016/s0022-2836(83)80016-3. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Roop D. R., Lai E. C., Nordstrom J. L., Catterall J. F., Swaneck G. E., Colbert D. A., Tsai M. J., Dugaiczyk A., Woo S. L. The ovalbumin gene: organization, structure, transcription, and regulation. Recent Prog Horm Res. 1979;35:1–46. doi: 10.1016/b978-0-12-571135-7.50005-9. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W. Steroid hormone action in eucaryotic cells. J Clin Invest. 1984 Aug;74(2):307–312. doi: 10.1172/JCI111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel G. U., Wahli W., Weber R. Quantitation of vitellogenin messenger RNA in the liver of male Xenopus toads during primary and secondary stimulation by estrogen. Cell. 1977 May;11(1):213–221. doi: 10.1016/0092-8674(77)90332-4. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Wyler T., Muellener D. B., Weber R. Identification, organization and processing intermediates of the putative precursors of Xenopus vitellogenin messenger RNA. Cell. 1980 Jan;19(1):53–61. doi: 10.1016/0092-8674(80)90387-6. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Tata J. R. Vitellogenin gene expression in male Xenopus hepatocytes during primary and secondary stimulation with estrogen in cell cultures. Cell. 1981 Mar;23(3):741–746. doi: 10.1016/0092-8674(81)90437-2. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Selective steroid hormonal regulation of gene expression in multigene families. J Steroid Biochem. 1981 Dec;15:87–97. doi: 10.1016/0022-4731(81)90262-4. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Ryffel G. U., Weber R. Vitellogenesis and the vitellogenin gene family. Science. 1981 Apr 17;212(4492):298–304. doi: 10.1126/science.7209528. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Jaggi R. B., Weber R., Ryffel G. U. Vitellogenin in Xenopus laevis is encoded in a small family of genes. Cell. 1979 Mar;16(3):535–549. doi: 10.1016/0092-8674(79)90028-x. [DOI] [PubMed] [Google Scholar]
- Wangh L. J. Glucocorticoids act together with estrogens and thyroid hormones in regulating the synthesis and secretion of Xenopus vitellogenin, serum albumin, and fibrinogen. Dev Biol. 1982 Feb;89(2):294–298. doi: 10.1016/0012-1606(82)90318-9. [DOI] [PubMed] [Google Scholar]
- Wangh L. J., Osborne J. A., Hentschel C. C., Tilly R. Parenchymal cells purified from Xenopus liver and maintained in primary culture synthesize vitellogenin in response to estradiol-17 beta and serum albumin in response to dexamethasone. Dev Biol. 1979 Jun;70(2):479–499. doi: 10.1016/0012-1606(79)90040-x. [DOI] [PubMed] [Google Scholar]
- Westley B., Wyler T., Ryffel G., Weber R. Xenopus laevis serum albumins are encoded in two closely related genes. Nucleic Acids Res. 1981 Aug 11;9(15):3557–3574. doi: 10.1093/nar/9.15.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. L., Tata J. R. Simultaneous analysis of conformation and transcription of A and B groups of vitellogenin genes in male and female Xenopus during primary and secondary activation by estrogen. Nucleic Acids Res. 1983 Feb 25;11(4):1151–1166. doi: 10.1093/nar/11.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. V., Wright S. C., Knowland J. Partial purification of estradiol receptor from Xenopus laevis liver and levels of receptor in relation to estradiol concentration. EMBO J. 1983;2(6):973–977. doi: 10.1002/j.1460-2075.1983.tb01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]