Abstract
Osteoarthritis is amongst the top five most disabling conditions affecting Americans over 65 years of age and imposes an annual economic burden estimated at $ 89.1 billion. Nearly half of the cost of care of Osteoarthritis is attributable to hospitalizations for total knee arthroplasties (TKA) and total hip arthroplasties (THA). The current clinical practice relies predominantly on subjective assessment of physical function and pain via patient reported outcome measures (PROM) that have proven inadequate for providing a validated, reliable and responsive measure of TKA outcomes. Wearable activity monitors, which produce a trace of regularly monitored physical activity derived from accelerometer measurements, provide a novel opportunity to objectively assess physical functional status in Osteoarthritis patients. Using data from the Osteoarthritis Initiative (OAI), we demonstrate the feasibility of quantifying the relative change in physical activity patterns in Osteoarthritis subjects using accelerometer based measurements of daily physical activity.
Introduction
The prevalence of lifestyle and demographic risk factors associated with Osteoarthritis [1,2], combined with expanding indications of TKA have resulted in a dramatic increase in TKA rates over the years. Based on current trends, nearly 3.5 million TKAs will be performed in 2030 [3]. TKA is widely regarded as a cost effective and efficacious treatment for end stage knee Osteoarthritis [4] and is associated with better pain and function related outcomes compared to non-surgical interventions [5]. An examination of the direct and indirect costs associated with TKAs has shown a lifetime societal net benefit from the procedure [6]. Paradoxically, numerous studies suggest that a significant proportion of patients remain dissatisfied with the outcome of their primary TKAs [7,8,9–10]. Given the rising demand for prosthetic replacements and the high absolute cost associated with the procedure, an individualized cost-benefit assessment of its value is essential, based on eligibility, stage and outcomes with alternative treatment pathways. Unfortunately though, patient reported measures of physical function and pain (PROM), which are the primary instruments for outcomes measurement in Osteoarthritis clinical practice, are subjective assessments that lack precision due to limitations stemming from their programmed content and responder bias. As a result, no PROM has demonstrated sufficient validity, reliability and responsiveness in clinical studies of TKA patients; nor has any PROOM provided a clear definition of treatment success. [11,12].
The International Classification of Functioning, Disability and Health (ICF) groups the functioning levels of an individual into two broad categories: capacity (ability to perform standardized tasks) and performance (ability to carry out tasks in the individual’s usual environment). Traditionally, clinical measures of functional disability have focused on capacity. Wearable activity monitors (WAM) provide a way to objectively measure the performance dimension of physical function. Given the interplay between physical activity, patient satisfaction and overall health, assessment of TKA outcomes through free-living physical activity may enable patient-centric decision making. Equipped with accelerometers, WAMs are able to provide continuous samples of measured activity intensity, duration and frequency.
Accelerometer based physical activity assessment has been shown to be superior to patient reported measures, with reference to the doubly labeled water method – the current gold standard in objective measurement of free-living physical activity [13]. The doubly labeled water method quantifies physical activity via the equivalent energy expenditure, whereas WAMs record activity directly. Such direct measurement capability can provide an advantage over energy based measurement methods—particularly for detecting latent activity patterns in subjects with mobility limiting diseases. In subjects with physical disabilities, the daily activity profile consists primarily of sedentary and light activities [14,15–16]. Specific patterns of differences in energy expenditure in such low intensity activities can be hard to detect, whereas patterns in directly sampled activity traces may be detected using appropriate statistical methods, as we demonstrate in our study.
Studies comparing accelerometer based, pre and post TKA, measurements differ in their study design, metric used in the comparison, and the method of analysis. Walker et al. studied the ‘total ambulatory activity’ in 19 subjects before and after TKA and found a significant increase 6 months post TKA, compared with pre TKA measurements obtained within a month of surgery [17]. Brandes et al. compared the ‘change in gait cycles’ in 44 subjects 3 weeks prior to TKA and at 6 months follow up (insignificant) 12 months follow up (increase) [18]. However Harding et al. compared ‘mean activity counts’ in 25 subjects prior to TKA (median 58 days) and at 6 month follow up and reported no significant difference [19]. A similar result was reported by Kahn et al. who compared ‘mean minutes’ of light, moderate and vigorous activity as well as average daily activity counts in two independent groups of subjects one of whom had undergone TKA and the other was scheduled to undergo TKA [20]. On the other hand, the study by Lutzner et al. reported a significant increase in ‘total number of steps’ as well as in in the number of moderate and vigorous steps in 97 subjects who were assed prior to TKA and at 1 year follow up [21]. Such variety in methods and results is common in an emerging area of enquiry; however, such diversity also makes the interpretation and the clinical translation of the results challenging. In addition, accelerometry studies of free-living activity have other challenges; for example, activity patterns of a subject may differ substantially from day to day. Over long time intervals (years), such studies require appropriate handling of confounding factors such as age, comorbidity as well as the calendar month of measurement (to account for weather related changes).
Our comparison of pre and post TKA physical activity levels is based on the time spent in different activity levels that were determined by thresholds calibrated in earlier studies [22,23]. We first present a method to detect differences in minutes of light and moderate physical activity levels per day. Our method accounts for the inherent complexities in physical activity data through an interpretable statistical approach. We then explore the choice of an alternative thresholding scheme to quantify activity-intensity. We show that the activity-intensity thresholds derived from an analysis of free-living physical activity (in musculo-skeletal pain and mobility-limited populations) permit a more detailed characterization of pre and post TKA activity levels, as opposed the traditional, energy based thresholds.
Data
Our analysis is based on data from the Osteoarthritis Initiative (http://www.oai.ucsf.edu), a federally funded initiative for studying a cohort consisting of subjects with clinical grade Osteoarthritis (progression), subjects at risk of developing Osteoarthritis (incidence), and subjects who do not have Osteoarthritis nor are at risk (controls). The data from annual follow up visits from all 4796 subjects in the study consists of clinical, radiographic, and biomarker data. Additionally, at the 48 and 72 month follow up visits, an invited subset of the subjects participated in a physical activity study that recorded daily physical activity of the subjects via a ActiGraph GT1M uniaxial accelerometer (ActiGraph; Pensacola, Florida). The Actigraph GT1M is a compact, hip-worn device (3.8 cm X 3.7 cm X 1.8 cm, 27 g) that measures dynamic acceleration in the range of 0.05g – 2.0 g, whose validity and reliability in physical activity research has been reported in a number of studies [24,25,26,27–28] and that has been used in large scale studies on physical activity in health research [29].The participants in the physical activity studies wore the accelerometer upon arising in the morning and continuously until retiring at night, except during water activities, for seven consecutive days. The participants were also asked to maintain a daily log recording time spent in water and cycling activities as the accelerometer may not have been able to capture these activities accurately. Post facto analysis revealed that participants spent little time in water and cycling activities (median 0 minutes/day, interquartile range = 0.0 to 3.4 minutes/day), indicating that little activity was missed by accelerometer monitoring. The key attributes of the 48 month and 72-month PA study-data are summarized in Table Table 1.
Table 1.
Key attributes of the physical activity data
| Physical activity (PA) study | ||
|---|---|---|
| 48 month | 72 month | |
| Cohort size | ||
| Incidence | 1490 | 1080 |
| Progression | 505 | 340 |
| Control | 6 | 6 |
| Total | 2001 | 1426 |
| TKA date | ||
| Before PA study | 63 | 74 |
| After PA study | 115 | 45 |
| Age (mean, sd) | (65.08, 9.09) | (66.73, 9.04) |
| Gender (% male) | 44.53 | 45.37 |
| BMI (mean, sd) | (28.52, 4.87) | (28.22, 4.88) |
| Mean Comorbidity Index | 0.52 | 0.56 |
| Median PA days | 7 | 7 |
Method
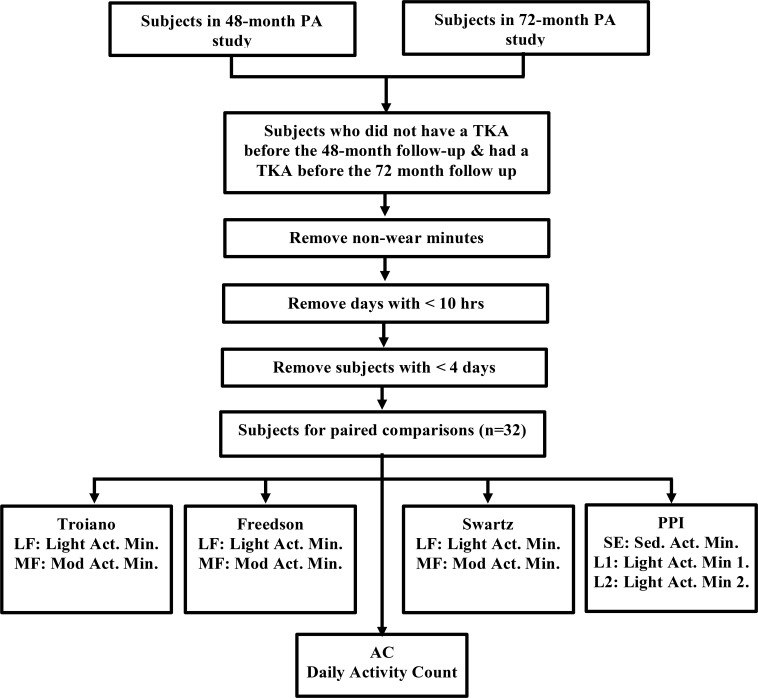
In order to make paired comparisons in physical activity before and after TKA, we selected those subjects from the PA studies who underwent a TKA after the first PA study (48 month follow up) but before the second PA study (72 month follow up). The accelerometer data obtained from the PA studies consists of “activity counts” per minute. Activity counts are a weighted sum of the discretely sampled values of the one dimensional acceleration. Since zero or low values of activity counts could arise from non-wear time, it is desirable to filter out non-wear periods from the data to avoid overestimating the proportion of sedentary or inactive periods. Following a methodology validated in adults with Osteoarthritis, continuous periods that were more than 90 minutes long and zero activity counts (allowing for interruptions of up to 2 consecutive minutes with fewer than 100 counts) were discarded as non-wear periods [30]. A day with a wear time of 10 hours or more was considered as valid, and only subjects with 4 or more valid days were considered in the analysis. For the the 32 subjects that met this criterion (key attributes are summarized in Table Table 2) we compared the pre and post TKA physical activity based on daily minutes spent in the different intensity strata, defined according to different thresholding schemes. Figure 1 describes the key steps in our analysis.
Table 2.
Key attributes of the 32 subjects in the paired comparison across the 48th month and the 72nd month visits.
| 48 month | 72 month | |
| Age (mean, sd) | (70.03, 8.30) | (72.03, 8.30) |
| BMI (mean, sd) | (28.95, 4.53) | (28.74, 4.54) |
| Mean Comorbidity Index | 0.75 | 0.78 |
| Median days to/from TKA | 284.56 | 448.63 |
| Gender (% male) | 53.13% | |
Figure 1.
Key steps in the analysis
Statistical Analysis
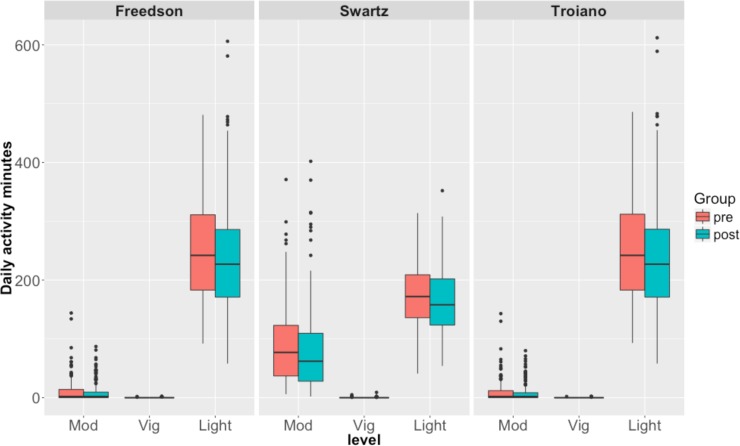
Our metric for comparing the relative change in physical activity is the time spent (minutes per day) in different activity levels, which correspond to different levels of energy expenditure. A 3-way grouping defined by commonly cited activity level thresholds provides light, moderate and vigorous levels of activity. For the 32 subjects in our paired comparisons, the distributions of light, moderate and vigorous activity minutes (per day), based on three different thresholding schemes [22,31,32] are shown in Figure 2 . It is evident from the box plots that the measured effect of TKA depends on the thresholding scheme used to define activity levels. There is very little activity in our data at the vigorous activity levels for a meaningful comparison.
Figure 2.
Distribution of moderate, vigorous and light activity minutes before and after TKA. The headers of the plots show the specific thresholding scheme used.
We used mixed effects models to perform between group comparisons taking into account repeated measures from each subject before and after TKA. Such paired comparisons allow for effective self-control, however effects due to age, body-mass-index (BMI), comorbidity index and calendar month of measurement still need to be adjusted for. Concomitant illnesses are known to modulate daily physical activity, particularly in the setting of chronic diseases [33,34]. We used the Modified Charleson Index score (MCIS) [35] to adjust for comorbidities at the time of follow up closest to the PA studies. There is also weak evidence that weather influences the severity of rheumatic diseases [36,37–38]. We adjust for weather effects by using a cyclic representation of the month computed by taking the cosine of the proportional angle, which assigns each month a numeric value given by Cos(𝑖× 2𝜋/12) where i is the month index 1..12. This has the advantage of preserving the seasonal proximity between months; therefore, in this representation the values for January and June are farther apart compared to the values for January and December.
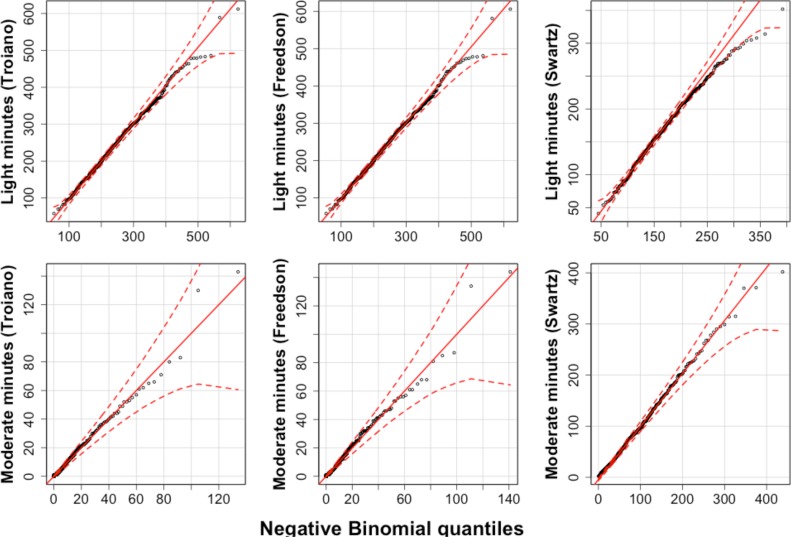
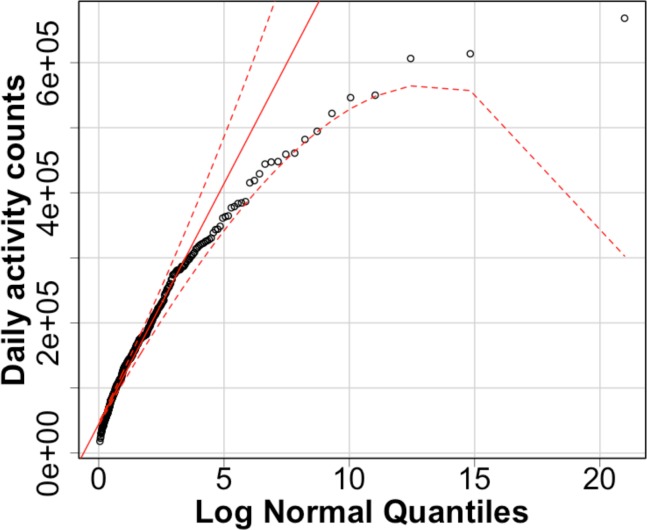
There are two parts to our analysis. First, we use two kinds of thresholding schemes for defining the light and moderate activity levels – and compute activity minutes in each level of interest for each thresholding scheme. Second, we fit generalized linear mixed models (GLMM) [39] for the activity minutes (light and moderate) as well as the daily activity counts, with TKA status, age, BMI, comorbidity index and month as fixed effects and subject ID a random effect. GLMMs are an extension of Generalized Linear Models [40] in that they allow the errors in the response to be modelled via a suitable exponential family distribution, and allow for the relationship with certain predictors to be described as random effects. Since the activity minutes lie within bounds denoting the 95% confidence envelope of the simulated distributions (as seen in the quantile plots in Figure 3a and Figure 3b), the Negative Binomial distribution is an appropriate choice for modelling the discrete daily minute counts and the Log Normal distribution for modelling the activity counts.
Figure 3a.
QQ plots for light and moderate activity minutes
Figure 3b.
QQ plot - activity counts
Inference in mixed effects models is difficult because the cumulative distribution function of the estimated coefficients (under the null hypotheses) is not known in most hierarchical designs. Typically, inference in mixed effects models is done via a likelihood ratio test (LRT) that tests the asymptotic log likelihood ratio against a Chi- Squared distribution. However, the approach assumes a quadratic log likelihood surface – an assumption easily violated by many nesting designs. Therefore, we obtained significance estimates of the fixed effects in our models through the likelihood ratio-test approach, as well as through bootstrap confidence intervals as recommended in current literature [41].
Physical performance intervals
Our prior work has focused on the relationship between pain and patterns of natural physical activity (performance) using accelerometry data [42]. Based on a cluster analysis of the observed activity counts per minute, the prior work proposed a stratification scheme, called physical performance intervals (PPI), for minute-wise activity counts that can describe perturbations in movement related to a variety of pain conditions. Unlike the activity thresholds calibrated against the estimated energy expenditure, PPI are based on activity strata that differentiated between various types of pain in a population-based sample who reported pain in different regions of the body. PPI are defined as: Performance Sedentary (PSE) = 1-100, Performance Light 1 (PL1) = 101-350, Performance Light 2 (PL2) = 351-800, Performance Light 3 (PL3) = 801-2500, and Performance Moderate and Vigorous (PMV) = 2501-30000. We repeated the pre and post TKA activity-level comparisons by using the activity minutes computed for the PPI and fitting GLMMs as described earlier.
Results
We present results corresponding to the two parts of our analysis. First, using different thresholding schemes for defining light and moderate activity, we compute activity minutes in each level of interest. Second, we fit generalized linear mixed models (GLMM) [39] for the activity minutes (light and moderate) as well as the daily activity counts, accounting for TKA status, age, BMI, comorbidity index and month as fixed effects and the subject ID as a random effect. The fixed effects for our models relating PA to TKA status (TKA), age (Age), calendar month of measurement (Month), BMI and the modified Charleson comorbidity index score (MCIS) are presented in Tables Table 3 and Table 4 below. Table Table 3 summarizes the parameters for our models that are based on daily activity minutes computed from three different energy-expenditure based intensity thresholds. Table Table 4 summarizes the parameters for models that use daily activity minutes computed from PPIs (based on observed activity patterns in patients with mobility-limiting disease). Since our GLMMs were based on a log link function, the estimated coefficient for a predictor represents the log of the change in the response variable, due to a unit change in the predictor after adjusting for other predictors. The shaded cells in Table Table 3 represent significant effect (p < 0.05) estimates for each of the models, identified by their respective labels (LT: Light Activity minutes - Troiano, MT: Moderate Activity Minutes – Troiano, LF: Light Activity minutes- Freedson, MF: Moderate Activity Minutes – Freedson, LS: Light Activity minutes - Swartz, MS: Moderate Activity Minutes – Swartz, AC: Daily Activity Counts).
Table 3.
Fixed effect estimates for models of daily activity minutes and daily activity counts
| Models | TKA | Age | Month | BMI | MCIS | |
|---|---|---|---|---|---|---|
| LT | Estimate | -0.0665 | -0.1166 | -0.1153 | -0.0754 | -0.0228 |
| p (LRT) | 0.0199 | 0.0214 | 0.0149 | 0.0901 | 0.3734 | |
| 95% low | -0.1189 | -0.2236 | -0.2059 | -0.1617 | -0.0742 | |
| 95% high | -0.0119 | -0.0132 | -0.0205 | 0.002 | 0.0296 | |
| MT | Estimate | 0.0356 | -1.0787 | -0.1302 | 0.1 | -0.0823 |
| p (LRT) | 0.8138 | 0 | 0.59 | 0.6287 | 0.5546 | |
| 95% low | -0.2236 | -1.4689 | -0.6073 | -0.2574 | -0.3122 | |
| 95% high | 0.2929 | -0.664 | 0.3422 | 0.5062 | 0.1813 | |
| LF | Estimate | -0.0671 | -0.1143 | -0.1146 | -0.0758 | -0.0231 |
| p (LRT) | 0.0187 | 0.0234 | 0.0153 | 0.0872 | 0.3652 | |
| 95% low | -0.1274 | -0.2185 | -0.1971 | -0.1638 | -0.0732 | |
| 95% high | -0.0125 | -0.0115 | -0.0165 | 0 | 0.033 | |
| MF | Estimate | 0.0129 | -1.0431 | -0.1308 | 0.1359 | -0.0756 |
| p (LRT) | 0.9283 | 0 | 0.569 | 0.4968 | 0.5722 | |
| 95% low | -0.2886 | -1.4736 | -0.5688 | -0.2416 | -0.3307 | |
| 95% high | 0.2755 | -0.5799 | 0.3012 | 0.5319 | 0.1836 | |
| LS | Estimate | -0.0724 | -0.0026 | -0.1102 | -0.0936 | -0.027 |
| p (LRT) | 0.0081 | 0.948 | 0.0104 | 0.0089 | 0.2531 | |
| 95% low | -0.1233 | -0.0815 | -0.1876 | -0.1593 | -0.0728 | |
| 95% high | -0.0179 | 0.0726 | -0.027 | -0.0244 | 0.0203 | |
| MS | Estimate | -0.0942 | -0.4389 | -0.1276 | -0.004 | 0.0344 |
| p (LRT) | 0.0643 | 2e-04 | 0.1294 | 0.9641 | 0.4467 | |
| 95% low | -0.1875 | -0.6684 | -0.292 | -0.1749 | -0.0458 | |
| 95% high | 0.005 | -0.2057 | 0.0397 | 0.1777 | 0.1229 | |
| AC | Estimate | 0.049 | -0.3795 | -0.1281 | -0.1438 | 0.0108 |
| p (LRT) | 0.2386 | 0 | 0.0347 | 0.0346 | 0.795 | |
| 95% low | -0.0629 | -0.4668 | -0.3295 | -0.3858 | -0.0747 | |
| 95% high | 0.1514 | -0.3165 | 0.0843 | -0.0073 | 0.0951 | |
Table 4.
Fixed effect estimates for models of daily activity minutes (Physical Performance Intervals)
| Models | TKA | Age | Month | BMI | MCIS | |
|---|---|---|---|---|---|---|
| SE | Estimate | -0.0102 | 0.0292 | -0.0086 | -0.0404 | -0.0168 |
| p (LRT) | 0.6972 | 0.5386 | 0.8422 | 0.3214 | 0.4817 | |
| 95% low | -0.059 | -0.0576 | -0.0958 | -0.1267 | -0.0651 | |
| 95% high | 0.0463 | 0.1235 | 0.0752 | 0.0446 | 0.0249 | |
| L1 | Estimate | -0.0763 | 0.0412 | -0.1017 | -0.092 | -0.028 |
| p (LRT) | 0.0059 | 0.2903 | 0.0186 | 0.0096 | 0.244 | |
| 95% low | -0.1299 | -0.0376 | -0.1895 | -0.1509 | -0.0743 | |
| 95% high | -0.0182 | 0.1127 | -0.0143 | -0.0301 | 0.0253 | |
| L2 | Estimate | -0.0917 | -0.1588 | -0.1412 | -0.1226 | -0.0111 |
| p (LRT) | 0.0156 | 0.0232 | 0.0265 | 0.0457 | 0.7464 | |
| 95% low | -0.1643 | -0.2931 | -0.2586 | -0.2345 | -0.0743 | |
| 95% high | -0.0174 | -0.0264 | -0.0223 | -0.0091 | 0.0643 | |
| L3 | Estimate | -0.1089 | -0.5211 | -0.0642 | 0.0628 | 0.0519 |
| p (LRT) | 0.0737 | 3e-04 | 0.5162 | 0.5613 | 0.3447 | |
| 95% low | -0.2314 | -0.801 | -0.2401 | -0.141 | -0.0481 | |
| 95% high | 0.0155 | -0.2447 | 0.1441 | 0.2628 | 0.1616 | |
| MV | Estimate | -0.0256 | -1.132 | -0.0813 | -0.1074 | -0.2104 |
| p (LRT) | 0.8955 | 0 | 0.7714 | 0.6076 | 0.2025 | |
| 95% low | -0.369 | -1.6587 | -0.663 | -0.4689 | -0.4931 | |
| 95% high | 0.3662 | -0.5464 | 0.4459 | 0.3131 | 0.2451 | |
TKA status was significant in all the light activity minute models (LT, LF and LS). The TKA effect on light activity minutes (Troiano) may be interpreted as a reduction by a factor of exp(-0.0665), or equivalently a reduction of about 6.4%. Month effects were significant only in the light activity models a 10.9% reduction in the daily light activity time (in the Troiano thresholding scheme) corresponding to a unit change in the predictor (approximately over a quarter in the cyclic coding scheme). Both age and BMI are well known risk factors for Osteoarthritis progression.
Table Table 4 shows the fixed effects (shading indicates a significant effect) of the models for activity times based on the Physical Performance Intervals, and labeled accordingly (SE: sedentary, L1-L3: light activity 1-3, MV: moderate to vigorous). Post TKA, the light activity time in level 1 and 2 is reduced by a factor of exp(-0.0763) and exp(-0.0917), equivalent to 7.3% and 8.8% respectively. Month effects for the activity times in level 1 and 2 accounted for 9.7% and 13.2% reductions respectively.
In all the activity time models, Month and BMI effects were significant only at the low activity levels. The effect of comorbidities was not significant in any model. We tested our counts response models for over-dispersion and examined the residual-fit plots from all models to check for potential fitting issues.
Discussion
An emerging view of knee Osteoarthritis etiology believes it to be a cascade of maladaptations, occurring in response to altered dynamics of the knee joint components [43]. Therefore one could expect that a biomechanical intervention aimed at restoring the kinematic alignment of the knee joint [44] must bring about significant changes in the physical function phenotype associated with end stage Osteoarthritis. As a result, many of the clinical measures of TKA outcome focus on functional capability with regard to standardized tests such as the stand up and go test, 6 minute walk, stair climbing and isometric quadriceps strength measurement, to name a few [45].
Less is known about how TKA affects the levels of routine physical activity, which is a better barometer for patient- satisfaction and quality of life, and is also correlated with long term health and mortality. Given that the means of objectively measuring routine physical activity are now available, establishing systematic patterns in routine physical activity data is a worthwhile endeavor. Since free-living physical activity occurs outside the constraints of a pre- ascertained protocol, the data it generates is vulnerable to many sources of confounding. Within the setting of a TKA, factors such as the type and duration of post-operative care, adherence to the prescribed physiotherapeutic regimen, concomitant illnesses and medication, dependence on care givers, time after surgery and type of prosthesis may all affect the intensity and duration of the observed physical activity.
In this work, we retrospectively analyzed physical activity data, taking the approach used in observational studies to adjust for several confounding factors—which has not been done in the physical activity analysis work so far. The reduction in light activities post TKAs can be meaningfully interpreted with reference to a compendium of routinely performed activities, classified on the basis of type, purpose and energy expenditure [46]. For instance, typical light activities (with energy expenditure between 1 – 3 METS) include light yard work. stretching, sitting and casual walking. The quantified reduction post TKA (at a median duration of 448 days) for the end stage Osteoarthritis population maybe understood as the proportional reduction in similar routine activities. The observed reduction in light activity levels post TKA may well be on account of improved joint function that allows a more efficient allocation of energy – a plausible explanation that follows from the positive (though statistically insignificant) effect sizes for moderate activity minutes in the Troiano and Freedson schemes as well as for the overall activity counts. In order to capture the subtle changes occurring in magnitude of low intensity activities, a thresholding scheme such as PPI, that emphasizes the lower intensity bands is required.
The patient population undergoing TKA is diverse, and as a consequence of the high rate of success as well as the cost effectiveness of the procedure, will likely consist of younger patients in the future. A limitation of our analysis is its applicability to a much narrower population, as described in Table Table 2. Also, the relatively small size of the sample does not allow to adjust for variables such as medication usage and duration after surgery, that may potentially affect physical activity level. Our analysis, if repeated on accelerometry data from a larger TKA population, would allow us to control for factors that affect physical activity without compromising statistical power.
Conclusion
Efforts to optimize TKA utilization must balance eligibility, disease stage and outcomes from alternative treatment pathways. A quantitative assessment of the treatment outcome is a pre-requisite for appropriate stratification of the patient population. Given sufficient data, after correcting for non-wear time and other invalid observations, treatment effect of TKA can be quantified by fitting a hierarchical model that adjusts for confounders. Our analysis reveals a reduction in light activity time at a median 448 days after TKA. While follow-up analysis based on larger sample sizes would be required to confirm the estimated change after TKA, the difference in pre and post TKA activity levels depends on the thresholding scheme for activity intensity. Performance based thresholds provide an improvement in resolution over energy-expenditure based thresholds.
Acknowledgements
Vibhu Agarwal was supported by the Stanford Center for Biomedical Informatics Research. NHS also acknowledges support from Janssen R&D for effort on the Observational Health Data Science and Informatics research network.
References
- 1.Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, et al. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;1(11) doi: 10.1186/1478-7954-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leigh JP, Seavey W, Leistikow B. Estimating the costs of job related arthritis. J Rheumatol. 2001;28(7):1647–54. [PubMed] [Google Scholar]
- 3.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. The American Orthopedic Association. 2007;89(4):780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. Lancet (London, England). 2012;379(9823):1331–40. doi: 10.1016/S0140-6736(11)60752-6. [DOI] [PubMed] [Google Scholar]
- 5.Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, et al. A Randomized, Controlled Trial of Total Knee Replacement. N Engl J Med. Massachusetts Medical Society. 2015;373(17):1597–1606.. doi: 10.1056/NEJMoa1505467. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz D, Koenig L, Dall TM, Gallo P, Narzikul A, Parvizi J, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473–80. doi: 10.2106/JBJS.L.01488. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JG, Wixson RL, Tsai D, Stulberg SD, Chang RW. Functional outcome and patient satisfaction in total knee patients over the age of 75. J Arthroplasty. 1996;11(7):831–40. doi: 10.1016/s0883-5403(96)80183-5. [DOI] [PubMed] [Google Scholar]
- 8.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. Springer. 2010;468(1):57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunbar MJ, Robertsson O, Ryd L, Lidgren L. Appropriate questionnaires for knee arthroplasty. Results of a survey of 3600 patients from The Swedish Knee Arthroplasty Registry. J Bone Joint Surg Br. 2001;83(3):339–44. doi: 10.1302/0301-620x.83b3.11134. [DOI] [PubMed] [Google Scholar]
- 10.Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27, 372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71(3):262–7. doi: 10.1080/000164700317411852. [DOI] [PubMed] [Google Scholar]
- 11.Ramkumar PN, Harris JD, Noble PC. Patient-reported outcome measures after total knee arthroplasty: a systematic review. Bone Joint Res. British Editorial Society of Bone and Joint Surgery. 2015;4(7):120–7. doi: 10.1302/2046-3758.47.2000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terwee CB, Bouwmeester W, van Elsland SL, de Vet HCW, Dekker J. Instruments to assess physical activity in patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Osteoarthritis Cartilage. 2011;19(6):620–33. doi: 10.1016/j.joca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Westerterp KR. Reliable assessment of physical activity in disease: an update on activity monitors. Curr Opin Clin Nutr Metab Care. 2014;17(5):401–6. doi: 10.1097/MCO.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 14.Ezeugwu V, Klaren RE, Hubbard E A., Manns P (Trish), Motl RW. Mobility disability and the pattern of accelerometer-derived sedentary and physical activity behaviors in people with multiple sclerosis. Prev Med Reports. 2015;2:241–246. doi: 10.1016/j.pmedr.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fjeldstad C, Fjeldstad AS, Pardo G. Use of Accelerometers to Measure Real-Life Physical Activity in Ambulatory Individuals with Multiple Sclerosis: A Pilot Study. Int J MS Care. The Consortium of Multiple Sclerosis Centers; 2015;17(5):215–20. doi: 10.7224/1537-2073.2014-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Hernandez V, Ferraz-Amaro I, Diaz-Gonzalez F. Rheumatology. 4. Vol. 53. Oxford University Press;; 2014. Influence of disease activity on the physical activity of rheumatoid arthritis patients; pp. 722–731. [DOI] [PubMed] [Google Scholar]
- 17.Walker DJ, Heslop PS, Chandler C, Pinder IM. Measured ambulation and self-reported health status following total joint replacement for the osteoarthritic knee. Rheumatology (Oxford). 2002;41(7):755–8. doi: 10.1093/rheumatology/41.7.755. [DOI] [PubMed] [Google Scholar]
- 18.Brandes M, Ringling M, Winter C, Hillmann A, Rosenbaum D. Changes in physical activity and health- related quality of life during the first year after total knee arthroplasty. Arthritis Care Res (Hoboken). 2011;63(3):328–34. doi: 10.1002/acr.20384. [DOI] [PubMed] [Google Scholar]
- 19.Harding P, Holland AE, Delany C, Hinman RS. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop Relat Res. 2014;472(5):1502–11. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn TL, Schwarzkopf R. Does Total Knee Arthroplasty Affect Physical Activity Levels? Data from the Osteoarthritis Initiative. J Arthroplasty. Elsevier Inc. 2015;30(9):1521–1525. doi: 10.1016/j.arth.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Lützner C, Kirschner S, Lützner J. Patient activity after TKA depends on patient-specific parameters. Clin Orthop Relat Res. 2014;472(12):3933–40. doi: 10.1007/s11999-014-3813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Freedson P, Bowles HR, Troiano R, Haskell W. Assessment of physical activity using wearable monitors: recommendations for monitor calibration and use in the field. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S1–4. doi: 10.1249/MSS.0b013e3182399b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eston RG, Rowlands A V, Ingledew DK. Validity of heart rate, pedometry, and accelerometry for predicting the energy cost of children’s activities. J Appl Physiol. 1998;84(1):362–71. doi: 10.1152/jappl.1998.84.1.362. [DOI] [PubMed] [Google Scholar]
- 25.Janz KF. Validation of the CSA accelerometer for assessing children’s physical activity. Med Sci Sports Exerc. 1994;26(3):369–75. [PubMed] [Google Scholar]
- 26.Janz KF, Witt J, Mahoney LT. The stability of children’s physical activity as measured by accelerometry and self-report. Med Sci Sports Exerc. 1995;27(9):1326–32. [PubMed] [Google Scholar]
- 27.Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sports Exerc. 2000;32(2):426–31. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Trost SG, Ward DS, Moorehead SM, Watson PD, Riner W, Burke JR. Validity of the computer science and applications (CSA) activity monitor in children. Med Sci Sports Exerc. 1998;30(4):629–33. doi: 10.1097/00005768-199804000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Lee I-M, Shiroma EJ. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br J Sports Med. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2014;48(3):197–201. doi: 10.1136/bjsports-2013-093154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song J, Semanik P, Sharma L, Chang RW, Hochberg MC, Mysiw WJ, et al. Assessing physical activity in persons with knee osteoarthritis using accelerometers: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2010;62(12):1724–32. doi: 10.1002/acr.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troiano RP, Berrigan D, Dodd KW, M??sse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 32.Swartz AM, Strath SJ, Bassett DR, O'Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450–6. doi: 10.1097/00005768-200009001-00003. [DOI] [PubMed] [Google Scholar]
- 33.Rosa CSC, Bueno DR, Souza GD, Gobbo LA, Freitas IF, Sakkas GK, et al. Factors associated with leisure-time physical activity among patients undergoing hemodialysis. BMC Nephrol. BioMed Central. 2015;15(1):192. doi: 10.1186/s12882-015-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sievi NA, Senn O, Brack T, Brutsche MH, Frey M, Irani S, et al. Impact of comorbidities on physical activity in COPD. Respirology. 2015;20(3):413–8. doi: 10.1111/resp.12456. [DOI] [PubMed] [Google Scholar]
- 35.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Hawley DJ, Wolfe F, Lue FA, Moldofsky H. Seasonal symptom severity in patients with rheumatic diseases: a study of 1, 424 patients. J Rheumatol. 2001;28(8):1900–9. [PubMed] [Google Scholar]
- 37.Guedj D, Weinberger A. Effect of weather conditions on rheumatic patients. Ann Rheum Dis. BMJ Group; 1990;49(3):158–9. doi: 10.1136/ard.49.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergés J, Montell E, Tomàs E, Cumelles G, Castañeda G, Marti N, et al. Weather conditions can influence rheumatic diseases. Proc West Pharmacol Soc. 2004;4(7):134–6. [PubMed] [Google Scholar]
- 39.McCullagh P, Nelder JA. Gen Linear Model Second Ed. Generalized Linear Models, Second Edition. p. 1989. [Google Scholar]
- 40.Nelder JA, Wedderburn RWM. Generalized Linear Models. J R Stat Soc Ser A. 1972;135(3):370. [Google Scholar]
- 41.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. Elsevier; 2009;24(3):127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Kao M-CJ, Jarosz R, Mackey S, Smuck M, Tomkins-Lane C. Poster 392 Physical Activity Intensity Signatures (PAIS) of Pain: Large-Scale Study Reveals Novel Cut-Points for Accelerometry Analysis in Regional Body Pain. PM&R. Elsevier. 2012;4(10):S324. [Google Scholar]
- 43.Teichtahl AJ, Wluka AE, Wijethilake P, Wang Y, Ghasem-Zadeh A, Cicuttini FM, et al. Wolff’s law in action: a mechanism for early knee osteoarthritis. Arthritis Res Ther. BioMed Central; 2015;17(1):207. doi: 10.1186/s13075-015-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiraldi M, Bonzanini G, Chirillo D, de Tullio V. Mechanical and kinematic alignment in total knee arthroplasty. Ann Transl Med. AME Publications; 2016;4(7):130. doi: 10.21037/atm.2016.03.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizner RL, Petterson SC, Clements KE, Zeni JA, Irrgang JJ, Snyder-Mackler L, et al. Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: a longitudinal analysis of outcomes. J Arthroplasty. NIH Public Access; 2011;26(5):728–37. doi: 10.1016/j.arth.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]