Abstract
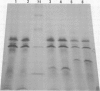
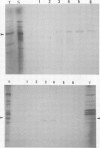
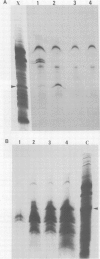
We have cloned the X gene (HBx) and the HBc antigen (HBc Ag) gene of human hepatitis B virus (HBV) in Escherichia coli as fusion products with beta-galactosidase. Both HBV genes are expressed in E. coli strain CSR 603. Expression is detected by u.v. irradiation of the bacteria, metabolic labelling and electrophoresis of the labelled extracts on SDS-polyacrylamide gels. The HBc Ag protein produced in bacteria can be recognised by anti-HBc sera and peptides derived from the protein are also recognised by anti-HBe sera. The HBx protein is recognised by some, but not all, sera which are anti-HBe positive. HBx Ag is also recognised by a woodchuck antibody similar to anti-HBe (anti-WHe). These results constitute the first proof that the open reading frame X is a true viral gene and is expressed during HBV (and WHV) infection and that an HBx/anti-HBx system, which may have important biological implications, can exist in parallel with the classic HBe/anti-HBe system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Charnay P., Mandart E., Hampe A., Fitoussi F., Tiollais P., Galibert F. Localization on the viral genome and nucleotide sequence of the gene coding for the two major polypeptides of the hepatitis B surface antigen (HBs Ag). Nucleic Acids Res. 1979 Sep 25;7(2):335–346. doi: 10.1093/nar/7.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Perricaudet M., Galibert F., Tiollais P. Bacteriophage lambda and plasmid vectors, allowing fusion of cloned genes in each of the three translational phases. Nucleic Acids Res. 1978 Dec;5(12):4479–4494. doi: 10.1093/nar/5.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Pourcel C., Louise A., Fritsch A., Tiollais P. Cloning in Escherichia coli and physical structure of hepatitis B virion DNA. Proc Natl Acad Sci U S A. 1979 May;76(5):2222–2226. doi: 10.1073/pnas.76.5.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Hadziyannis S. J., Lieberman H. M., Karvountzis G. G., Shafritz D. A. Analysis of liver disease, nuclear HBcAg, viral replication, and hepatitis B virus DNA in liver and serum of HBeAg Vs. anti-HBe positive carriers of hepatitis B virus. Hepatology. 1983 Sep-Oct;3(5):656–662. doi: 10.1002/hep.1840030505. [DOI] [PubMed] [Google Scholar]
- Hantz O., Pichoud C., Vitvitski L., Trepo C. Use of the cross-reactivity with hepatitis B virus antigens and antibodies for the demonstration of a woodchuck hepatitis virus 'e' antigen-antibody system. J Virol Methods. 1983 Jul;7(1):45–55. doi: 10.1016/0166-0934(83)90022-8. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hoofnagle J. H. Serologic markers of hepatitis B virus infection. Annu Rev Med. 1981;32:1–11. doi: 10.1146/annurev.me.32.020181.000245. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacKay P., Lees J., Murray K. The conversion of hepatitis B core antigen synthesized in E coli into e antigen. J Med Virol. 1981;8(4):237–243. doi: 10.1002/jmv.1890080404. [DOI] [PubMed] [Google Scholar]
- Magnius L. O., Espmark J. A. New specificities in Australia antigen positive sera distinct from the Le Bouvier determinants. J Immunol. 1972 Nov;109(5):1017–1021. [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Molnar-Kimber K. L., Summers J. W., Mason W. S. Mapping of the cohesive overlap of duck hepatitis B virus DNA and of the site of initiation of reverse transcription. J Virol. 1984 Jul;51(1):181–191. doi: 10.1128/jvi.51.1.181-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Kimber K. L., Summers J., Taylor J. M., Mason W. S. Protein covalently bound to minus-strand DNA intermediates of duck hepatitis B virus. J Virol. 1983 Jan;45(1):165–172. doi: 10.1128/jvi.45.1.165-172.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohori H., Yamaki M., Onodera S., Yamada E., Ishida N. Antigenic conversion from HBcAg to HBeAg by degradation of hepatitis B core particles. Intervirology. 1980;13(2):74–82. doi: 10.1159/000149110. [DOI] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E., Sninsky J. J. Predicted secondary structure similarity in the absence of primary amino acid sequence homology: hepatitis B virus open reading frames. Proc Natl Acad Sci U S A. 1984 May;81(9):2902–2906. doi: 10.1073/pnas.81.9.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan W., Prince A. M., Brotman B. Modulation of hepatitis B infection by intravenous application of an immunoglobulin preparation that contains antibodies to hepatitis B e and core antigens but not to hepatitis B surface antigen. J Virol. 1984 Aug;51(2):420–424. doi: 10.1128/jvi.51.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. E., Neurath R. A., Beasley R. P., Szmuness W. HBeAg and anti-HBe detection by radioimmunoassay: correlation with vertical transmission of hepatitis B virus in Taiwan. J Med Virol. 1979;3(3):237–241. doi: 10.1002/jmv.1890030310. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Machida A., Funatsu G., Nomura M., Usuda S., Aoyagi S., Tachibana K., Miyamoto H., Imai M., Nakamura T. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983 Jun;130(6):2903–2907. [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Williams A., Le Bouvier G. Heterogeneity and thermolability of 'e'. Bibl Haematol. 1976;42:71–75. doi: 10.1159/000398996. [DOI] [PubMed] [Google Scholar]