Abstract
Clinicians and clinical decision-support systems often follow pharmacotherapy recommendations for patients based on clinical practice guidelines (CPGs). In multimorbid patients, these recommendations can potentially have clinically significant drug–drug interactions (DDIs). In this study, we describe and validate a method for programmatically detecting DDIs among CPG recommendations. The system extracts pharmacotherapy intervention recommendations from narrative CPGs, normalizes the terms, creates a mapping of drugs and drug classes, and then identifies occurrences of DDIs between CPG pairs. We used this system to analyze 75 CPGs written by authoring entities in the United States that discuss outpatient management of common chronic diseases. Using a reference list of high-risk DDIs, we identified 2198 of these DDIs in 638 CPG pairs (46 unique CPGs). Only 9 high-risk DDIs were discussed by both CPGs in a pairing. In 69 of the pairings, neither CPG had a pharmacologic reference or a warning of the possibility of a DDI.
Introduction
Patients with multiple, co-existing chronic conditions commonly have medication regimens with interacting drugs1. In the United States, more than 67% of adults of age 65 years or older have 3 or more chronic conditions, and 50% take 4 or more prescribed drugs2, 3. The incidence of adverse drug events (ADE) is positively associated with an increase in the number of medications4, and older adults are often prescribed medications with potentially severe drug–drug interactions5. With multimorbidity and polypharmacy increasing in the US, there will likely be a higher prevalence of patients at risk for ADEs from drug–drug interactions (DDI).
Clinical decision support (CDS) systems have been shown to improve prescriber behavior and reduce the prescription of interacting medications6. Despite the increased prevalence of CDS implementations for drug–drug interactions, there have been several factors hindering their efficacy. One complicating factor is that CDS tools often use drug compendiums that list numerous DDIs with unclear clinical risk7,8-9. In addition, the CDS usually provides recommendations in the form of alerts generated during processing of prescription orders. As a result of these factors, the alert notifications are often overridden due to the large number of recommendations generated, unclear clinical relevance, and resistance from cognitive inertia10,11-12. Clinicians may also not have enough knowledge about the DDIs to appropriately alter management13.
Providing information about possible interactions earlier in the decision making process has been supported as one method to improve the quality of DDI CDS7. However, CDS systems are often based on clinical practice guidelines (CPGs) known for a lack of multimorbidity and polypharmacy discussion14,15,16-17. To set a standard for guideline development, the Institute of Medicine included a benefits and harms assessment as an essential characteristic of CPGs in its March 2011 report18. While not intended to be comprehensive references for all possible scenarios in clinical practice, improvements in CPG discussion of drug–drug interactions have been recommended for their potential in reducing adverse polypharmacy in multimorbidity management19,20.
In this study, we describe and validate a method for programmatically detecting high-risk DDIs that could occur based on CPGs written for clinicians in the United States. We use the method to examine CPGs for outpatient management of chronic conditions in adults to determine if they are addressing high-risk DDIs.
Methods
Possible DDIs between CPGs were identified by an automated system that processes the treatment recommendations in a corpus of CPG text and that cross-matches drug-treatment recommendations to a DDI database. This process is described in detail below with an overview diagram shown in Figure 1.
Figure 1.
Overview flow diagram of DDI detection process
Clinical practice guideline corpus
Clinical practice guideline summaries were selected from the repository maintained by the National Guideline Clearinghouse (NGC), which includes only guidelines that “contain an assessment of the benefits and harms of recommended care and alternative care options”. From the guidelines available in the NGC repository in December 2015, we selected XML guideline summaries written by a national physician organization based in the United States. Each guideline XML followed a standardized schema of named elements containing HTML content. For each CPG, we extracted the “Guideline Category”, “Intended Users”, “Clinical Specialty”, “Target Population”, and “Disease/Condition(s)” XML elements. For inclusion in this study, we selected clinical practice guidelines categorized for “Treatment” and “Management” that were written for “Physicians” in the “Internal Medicine” or “Family Practice” clinical specialties. From the remaining corpus, we selected guidelines discussing chronic “Disease/Condition(s)” as identified by a physician. CPGs were excluded if the guidelines primarily discussed inpatient, pediatric, surgical, or obstetric management.
Pharmacotherapy identification.
To identify pharmacologic treatment interventions recommended for a chronic condition, we examined the text of the “Interventions” element in the CPG summaries. Pharmacologic treatments were identified through a longest- phrase matching algorithm using SNOMED_CT as the compendium for drugs and drug classes. Negation detection logic was developed to process the mix of HTML and text phrases consistently used in the “Interventions” element. Recommended treatments were then normalized to their preferred name in SNOMED_CT. Evaluation of the identification algorithm was performed against a reference standard that was established through physician annotation of the guideline summaries identifying recommended pharmacologic treatments.
Drug list preparation
For each pharmacologic intervention recommended by a CPG, we determined if the intervention represented a specific medication or a class of drugs. This was accomplished by cross-referencing the intervention names with medications in DrugBank, a comprehensive drug compendium. Intervention entities that were not identified in DrugBank were treated as potential drug classes. These drug classes were then expanded to their children in the SNOMED_CT hierarchy and filtered for medication products. Intervention entities that were not identified in DrugBank and did not have specific drug treatments as children in SNOMED_CT were excluded in the analysis and reviewed separately. Drug lists for each CPG were stored in a MySQL database for further analysis.
Drug–drug interaction data source
For this study, we used the set of DDIs identified by Phansalkar et al. as our data source for high-risk and absolutely contraindicated drug pairings. In their report, a panel of experts identified 15 high-risk DDI subgroups that should be included in any EHR DDI CDS system21. For 14 of the subgroups of drug pairings identified, Phansalkar et al. list 86 unique drugs. For the last subgroup, QT-prolonging drugs, the report referenced a list of QT-prolonging agents listed by CredibleMeds22. For this analysis, we used 52 drugs identified in the CredibleMeds database as having a clear association with torsades de pointes, a potentially fatal arrhythmia, as of June 19, 2016. All drug pairings between the 138 drugs were stored in the database.
DDI identification
For each CPG, individual drugs that were recommended in the CPG were paired with the drugs of the other CPGs in the analysis. Pairings were then matched against the high-risk DDIs in the data repository. Matched pairings were identified as potential DDIs between the CPGs. Extraneous entries were removed from the list of potential DDIs through two main filtering criteria (Figure 2). First, for guidelines that recommend a drug class without naming specific drugs, only one DDI was counted even if multiple child drugs were implicated from that class (Figure 2). In addition, DDIs were excluded if both drugs of a known DDI pair appeared in one of the CPG pairs (Figure 2). These pairings were excluded, since many drugs recommended in a CPG are alternatives to one another. In addition, if a guideline did recommend the use of multiple drugs at the same time, their interactions were usually well discussed and unlikely to be absolutely contraindicated pairings.
Figure 2.
Logic for counting DDIs: (A) Each drug specified in CPG 1 that interacts with a different drug in CPG 2 is counted as one DDI. (B) If a drug class is specified in CPG 1, then only one DDI is counted even if multiple drugs from that class interact with a drug in CPG 2. (C) If a drug specified in CPG 1 is also specified in CPG 2, no interactions are counted involving that drug.
Review of Guidelines
After compiling the list of potential DDIs, the CPGs were examined to see if the potential DDI was mentioned in the text. For each CPG that was identified in a potential high-risk drug, the hypertext link provided in the NGC’s CPG summary was used to download the full-text version including supplemental materials. A physician expert then manually reviewed the CPG text to identify a warning or discussion of the possible DDI.
Results
Guideline Inclusion
From the 2238 guidelines in the NGC in December 2015, 924 were written by a US-based organization with an intended audience of a physician (Figure 3). Of those guidelines, 75 were written for the outpatient management of an adult with a chronic condition in the primary-care setting.
Figure 3.
Inclusion and exclusion of clinical practice guidelines
In the 75 guidelines included in the study, a total of 181 chronic diseases were listed as topics for management (Table Table 1). Of the chronic diseases discussed, 144 were unique; 33 different authoring entities were identified with 14 entities being comprised of multiple collaborating national organizations.
Table 1.
Characteristics of guidelines and chronic condition
| Characteristic | Total |
|---|---|
| Guidelines | 75 |
| Guideline authoring entities | 33 |
| Total chronic conditions discussed | 181 |
| Unique chronic conditions | 144 |
Drug Treatment Identification
From the guidelines, our system identified 451 interventions (270 drugs, 181 drug classes) as recommended pharmacologic treatment interventions. 8 of the identified interventions could not be matched to a specific drug entity in DrugBank and were not included in the DDI analysis. Of these 8 text entities, 6 (“aldosterone”, “angiotensin”, “cystain”, “fat”, “ferritin”, “octanoate”) were incorrectly identified as a possible pharmacologic intervention. The other 2 entities, “tenofovir disoproxil fumarate” and “fondaparinux”, were medications that did not match their actual references in DrugBank, “tenofovir” and “fondaparinux sodium” respectively. The two text entities also did not exactly match any of the synonyms listed in DrugBank.
After expansion of the drug classes, the guidelines recommended 2511 drugs as possible treatments. From the review, 763 were unique drugs in the guideline corpus. Evaluation of the drug identification from 30 expert- annotated CPGs showed a precision of 0.92 and recall of 0.81. The f-measure was 0.86.
High-risk DDIs between CPGs
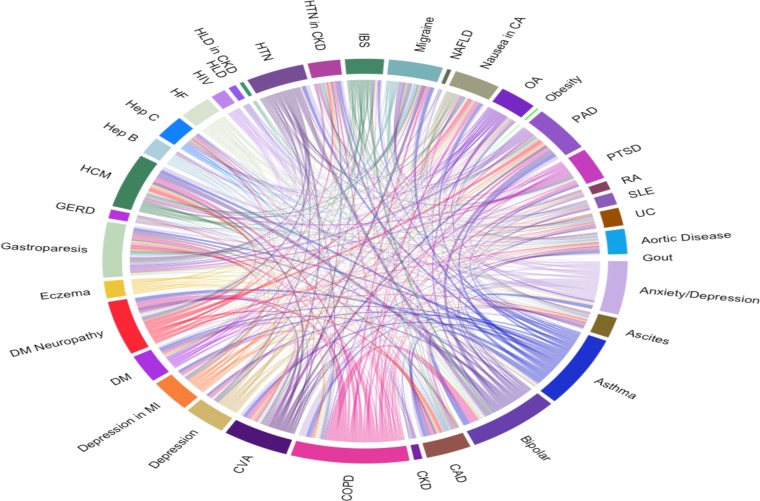
We identified 46 CPGs that recommended drugs that are listed in the high-risk DDI set. Each of these CPGs recommended an average of 7.5±6.7 high-risk drugs. When cross matched, each of these CPGs had possible interactions with an average of 29 other CPGs for a total of 638 interacting CPG pairs with at least one possible high-risk DDI. There were 2198 possible DDIs between the CPGs (visually represented in Figure 4). 1914 (87.1%) of these DDIs were between QT-prolonging drugs (Table Table 2). Excluding QT-prolonging agent interactions, 284 possible DDIs were identified between CPGs from the other high-risk DDI groups (Figure 5).
Figure 4.
Total interactions between CPGs
Table 2.
Possible high-risk DDIs identified by group
| DDI group | Total DDIs | Percentage of Total DDIs |
|---|---|---|
| QT-prolonging agents – QT-prolonging agents | 1914 | 87.08% |
| Statins - CYP3A4 inhibitors | 204 | 9.28% |
| Tizanidine - CYP1A2 inhibitors | 22 | 1.00% |
| Amphetamines derivatives - MAO inhibitors | 16 | 0.73% |
| Narcotics - MAO inhibitors | 16 | 0.73% |
| Ramelteon - Specific CYP1A2 inhibitors | 12 | 0.55% |
| Strong CYP3A4 inducers - Protease inhibitors | 12 | 0.55% |
| Atazanavir - Proton pump inhibitors | 2 | 0.05% |
| Total | 2198 | 100.00% |
Figure 5.
Total interactions between CPGs excluding QT prolonging agents
Guideline Discussion of DDIs
Out of the 2198 possible high risk DDIs identified, 49.8% were not discussed in the text of either of the associated CPGs (Table Table 3). Excluding QT-prolonging drug interactions, 26.1% of the remaining DDIs were not discussed in either CPG text. Discussion by both CPGs was found in only 1.0% of the DDIs.
Table 3.
Discussion of DDIs in CPG pairs
| Discussion of high risk DDI in CPG pairs | All DDIs (2198) | QT-prolonging drug pairs (1914) | Excluding QT-prolonging drug pairs (284) |
|---|---|---|---|
| No discussion in either CPG | 1094 (49.8%) | 1020 (53.3%) | 74 (26.1%) |
| Specific discussion in at least one CPG | 474 (21.6%) | 365 (19.1%) | 109 (38.4%) |
| Specific discussion in both CPGs | 23 (1.0%) | 12 (0.6%) | 11 (3.9%) |
| Reference to drug literature or acknowledgement of possible drug interactions in at least one CPG | 724 (32.9%) | 596 (31.3%) | 128 (45.1%) |
| Reference to drug literature or acknowledgement of possible drug interactions in both CPGs | 61 (2.8%) | 43 (2.2%) | 18 (6.3%) |
Among the 638 interacting CPG pairs, 16 of the pairs contained high-risk DDI discussion in both of the associated CPGs. 198 of the pairs had high-risk DDI discussion by at least one of the CPGs in the pair. 383 of the pairs had at least one CPG that discussed or referred the possibility of drug interactions.
Excluding QT-prolonging drug pairs, discussion of a high-risk DDI was examined by both CPGs in 9 of out of the 224 CPG pairs. 84 of the possible pairings had specific discussion of the high risk DDI by at least one CPG in the pairing. 156 of the pairs had at least one CPG in the pairing that provided an external pharmacologic resource or a warning of the general possibility of drug–drug interactions. In 69 of the pairings, neither CPG had a pharmacologic reference or a warning of the possibility of a DDI.
Discussion
We developed a programmatic method to determine possible drug regimen combinations that could result based on CPG recommendations in a multimborbid patient. By applying the method to a set of recent CPGs and cross- referencing with a list of DDIs, we identified high-risk DDIs that could occur in the management of multimorbidity. Many of these DDIs are not discussed or referenced by the CPGs, verifying the continuation of this known gap in multimorbidity support. With the prevalence of multimorbidity increasing in the United States, this study supports the need for interventions to improve the simultaneous management of multiple chronic conditions and prevent clinically significant DDIs.
While many of the drugs listed in the set of high-risk DDIs are not necessarily recommended as first-line treatment interventions by the CPGs, their potential use in an interacting regimen is significant in clinical practice. Most healthcare providers usually have good knowledge of first-line interventions for common chronic conditions. However, they are often most in need of medical reference and decision support when first-line treatments cannot be used. Our analysis identifies potential harms that could arise from these less common treatment pathways. High-risk DDIs identified in this manner should be considered for increased attention in CDS systems and CPG discussion.
QT-prolonging drugs, which can induce a fatal cardiac arrhythmia, made up the longest list of medications in the high risk DDI set. Accordingly, QT-prolonging drugs also represented the largest proportion of DDIs found in our analysis. These drugs were also discussed the least in the CPG text. This discrepancy might be due to the differences in knowledge and opinion on the clinical significance of different QT prolonging agents. The difficulty in determining clinically significant QT prolongation is well known, and the unclear relevance of some DDIs results in low levels of awareness among clinicians23. This likely contributes to the variability of discussion of QT-prolonging agents in CPGs. For example, it would not be surprising if a clinician overrides a QT-prolongation alert when prescribing a medication such as azithromycin, a commonly prescribed antibiotic which has clinically significant QT prolongation in only certain contexts24. In order to improve usability and effectiveness, highlighting contextual risk of a DDI in CDS is critical in providing the necessary information to the clinician 7.
Our results are similar to those in a recent study by Dumbreck et al., in which the authors manually examined possible DDIs in 12 common chronic disease management guidelines written by the United Kingdom’s National Institute for Health and Care Excellence (NICE)25. Their study also showed that potential high-risk DDIs were common between the NICE CPGs and that many of these DDIs occurred with second-line interventions. In addition, they also noted that there was limited discussion of these DDIs in the CPGs. Our study expands on their findings by automating the laborious process of analyzing CPGs, allowing us to analyze the possible high-risk DDIs that could occur in a larger set of chronic conditions. Our results demonstrate that this issue
Limitations
Our methodology makes the assumption that the CPG summary written by actual CPG authors accurately reflects the recommendations provided in the full-text version. This assumption is not true for all CPGs, especially for those that provide recommendations for a wide range of conditions or with a wide range of possible interventions. For some of these CPGs, the guideline summaries did not always include second or third-line interventions in their interventions section.
In addition, there were CPG summaries that listed unspecific drug classes rather than providing a specific list of recommended drugs. For some of the CPGs, the full-text version was more specific; however, there are many instances where the full-text recommendations also recommend a drug class as an intervention. This ambiguity is compounded in our system as drug databases often classify drugs in more categories than are clinically relevant. As a result, our methodology attributes more drugs as recommendations of a CPG than the authors likely intended. It is important to note that this misattribution could also occur in clinical practice. For example, a clinician could choose a medication from a recommended drug class that has not been proven to be clinically beneficial. In our review of the DDIs, however, only a small number of drugs that were inadvertently attributed to a CPG’s recommendations were also listed in a potential DDI.
A similar methodology to performing CPG text-processing and drug-extraction has been demonstrated by Leung et al.26. Our approach differs in that we use DrugBank and SNOMED_CT instead of the Anatomical Therapeutic Chemical (ATC) classification system to determine drug classifications. The differences between the three drug references have not been established formally to our knowledge, and comparison would help guide future research. Another major difference in methodologies was our use of the “Interventions” rather than the “Major Recommendations” section of the CPG summary. As discussed, the “Interventions” section can have possible issues with completeness and ambiguity as compared to the free-text of the “Major Recommendations”. However, the simplicity of the “Interventions” section also contributed to our higher precision and recall in determining whether a drug reference constituted an actual drug recommendation.
Future Work
Combined with data on frequently co-occurring chronic diseases, our results could highlight drug combinations that are likely to be prescribed together and whose interactions need to be highlighted for clinicians. While there has some work to identify common co-occurring combinations, more extensive work is needed to fully understand the context of these high risk DDIs27,28,29-30. In addition, it is possible to extend our data-mining process to provide further insight on challenges in multimorbidity, such as an analysis of drug-disease interactions between the chronic condition management.
Conclusion
We identified possible high-risk DDIs that could occur in multimorbid patients based on the recommendations of commonly used CPGs. We found that many recent CPGs do not discuss the possibility of these high risk DDIs. Guideline authoring entities should deliberately consider potential high-risk DDIs. Health informatics can continue to identify and highlight clinically significant DDIs to improve clinical decision support for multimorbidity.
Acknowledgements
This work was supported in part by VA Health Services Research and Development (HSR&D) grant IIR 11-071-1 (PI: Goldstein). An Advanced Fellowship in Medical Informatics that is funded by the VA Office of Academic Affiliations, HSR&D Service, and Office of Informatics and Analytics supported Dr. Tso’s time. Views expressed are those of the authors and not necessarily those of the Department of VA or other affiliated institutions.
References
- 1.Field TS, Gurwitz JH, Harrold LR, Rothschild J, DeBellis KR, Seger AC, et al. Risk factors for adverse drug events among older adults in the ambulatory setting. Journal of the American Geriatrics Society. 2004;52(8):1349–54. doi: 10.1111/j.1532-5415.2004.52367.x. [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth CJ, Smit E, Lee DS, Alramadhan F, Odden MC. Polypharmacy among adults aged 65 years and older in the United States: 1988–2010. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015. p. glv013. [DOI] [PMC free article] [PubMed]
- 3.Salive ME. Multimorbidity in older adults. Epidemiologic reviews. 2013. p. mxs009. [DOI] [PubMed]
- 4.Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11‐year national analysis. Pharmacoepidemiology and drug safety. 2010;19(9):901–10. doi: 10.1002/pds.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasina L, Djade CD, Nobili A, Tettamanti M, Franchi C, Salerno F, et al. Drug–drug interactions in a cohort of hospitalized elderly patients. pharmacoepidemiology and drug safety. 2013;22(10):1054–60. doi: 10.1002/pds.3510. [DOI] [PubMed] [Google Scholar]
- 6.Jaspers MW, Smeulers M, Vermeulen H, Peute LW. Effects of clinical decision-support systems on practitioner performance and patient outcomes: a synthesis of high-quality systematic review findings. Journal of the American Medical Informatics Association. 2011;18(3):327–34. doi: 10.1136/amiajnl-2011-000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne TH, Hines LE, Chan RC, Hartman S, Kapusnik-Uner J, Russ AL, et al. Recommendations to improve the usability of drug-drug interaction clinical decision support alerts. Journal of the American Medical Informatics Association. 2015. p. ocv011. [DOI] [PMC free article] [PubMed]
- 8.Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, Van Bergen RC, et al. Concordance of severity ratings provided in four drug interaction compendia. Journal of the American Pharmacists Association: JAPhA. 2004;44(2) doi: 10.1331/154434504773062582. [DOI] [PubMed] [Google Scholar]
- 9.Vitry AI. Comparative assessment of four drug interaction compendia. British journal of clinical pharmacology. 2007;63(6):709–14.. doi: 10.1111/j.1365-2125.2006.02809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phansalkar S, Van der Sijs H, Tucker AD, Desai AA, Bell DS, Teich JM, et al. Drug—drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. Journal of the American Medical Informatics Association. 2013;20(3):489–93. doi: 10.1136/amiajnl-2012-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenes RA. Clinical decision support: the road ahead. Amsterdaml Boston: Elsevier Academic Press; 2007. p. 581. xv. [Google Scholar]
- 12.Forseen SE, Corey AS. Clinical decision support and acute low back pain: evidence-based order sets. Journal of the American College of Radiology. 2012;9(10):704–12. doi: 10.1016/j.jacr.2012.02.014. e4. [DOI] [PubMed] [Google Scholar]
- 13.Chhina HK, Bhole VM, Goldsmith C, Hall W, Kaczorowski J, Lacaille D. Effectiveness of academic detailing to optimize medication prescribing behaviour of family physicians. Journal of Pharmacy & Pharmaceutical Sciences. 2013;16(4):511–29. doi: 10.18433/j3kk6c. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein M, Hoffman B, Coleman R, Musen MA, Tu S, Advani A, et al. Proceedings of the AMIA Symposium; editors. American Medical Informatics Association; 2000. Implementing clinical practice guidelines while taking account of changing evidence: ATHENA DSS, an easily modifiable decision- support system for managing hypertension in primary care. [PMC free article] [PubMed] [Google Scholar]
- 15.Musen MA, Middleton B, Greenes RA. Clinical decision-support systems. Biomedical informatics: Springer;; 2014. pp. 643–74. [Google Scholar]
- 16.Sittig DF, Wright A, Osheroff JA, Middleton B, Teich JM, Ash JS, et al. Grand challenges in clinical decision support. Journal of biomedical informatics. 2008;41(2):387–92. doi: 10.1016/j.jbi.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zulman DM, Asch SM, Martins SB, Kerr EA, Hoffman BB, Goldstein MK. Quality of care for patients with multiple chronic conditions: the role of comorbidity interrelatedness. Journal of general internal medicine. 2014;29(3):529–37. doi: 10.1007/s11606-013-2616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E. Clinical practice guidelines we can trust. National Academies Press; 2011. [PubMed] [Google Scholar]
- 19.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. Jama. 2005;294(6):716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 20.Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PloS one. 2011;6(10):e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phansalkar S, Desai AA, Bell D, Yoshida E, Doole J, Czochanski M, et al. High-priority drug–drug interactions for use in electronic health records. Journal of the American Medical Informatics Association. 2012;19(5):735–43. doi: 10.1136/amiajnl-2011-000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.QTDrug Lists: Arizona Center for Education and Research on Therapeutics; 2016. [Available from: http://www.crediblemeds.org.
- 23.Heist EK, Ruskin JN. Drug-induced arrhythmia. Circulation. 2010;122(14):1426–35. doi: 10.1161/CIRCULATIONAHA.109.894725. [DOI] [PubMed] [Google Scholar]
- 24.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. New England Journal of Medicine. 2012;366(20):1881–90.. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. bmj. 2015. pp. 350–h949. [DOI] [PMC free article] [PubMed]
- 26.Leung TI, Dumontier M. Overlap in drug-disease associations between clinical practice guidelines and drug structured product label indications. Journal of Biomedical Semantics. 2016;7(1):1. doi: 10.1186/s13326-016-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung TI, Jalal H, Zulman DM, Dumontier M, Owens DK, Musen MA, et al. Automating identification of multiple chronic conditions in clinical practice guidelines. AMIA Summits on Translational Science Proceedings. 2015;201(5):456. [PMC free article] [PubMed] [Google Scholar]
- 28.Ward BW. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Preventing chronic disease. 2013. p. 10. [DOI] [PMC free article] [PubMed]
- 29.Britt HC, Harrison CM, Miller GC, Knox SA. Prevalence and patterns of multimorbidity in Australia. Med J Aust. 2008;189(2):72–7. doi: 10.5694/j.1326-5377.2008.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 30.Zulman DM, Martins SB, Liu Y, Tu SW, Hoffman BB, Asch SM, et al. Using a Clinical Knowledge Base to Assess Comorbidity Interrelatedness Among Patients with Multiple Chronic Conditions. AMIA Annu Symp Proc. 2015;201(5):1381–9. [PMC free article] [PubMed] [Google Scholar]