Abstract
Objectives:
The analysis of secretin-stimulated pancreatic fluid is being evaluated as an approach to improve the early detection of pancreatic cancer and pancreatic precursor neoplasms. The method of pancreatic fluid sampling may have a significant impact on tumor marker measurements. The aim of this study was to compare concentrations of mutant DNA in pancreatic fluid from patients who had samples collected from both the pancreatic duct and duodenal lumen.
Methods:
Thirty-six participants enrolled in the Cancer of the Pancreas Screening studies at Johns Hopkins Hospital who had secretin-stimulated pancreatic fluid collected from the duodenum during endoscopic ultrasound (EUS) and from the pancreatic duct during subsequent endoscopic retrograde cholangiopancreatography. Mutant KRAS and GNAS DNA concentrations were measured in pancreatic fluid samples using digital high-resolution melt-curve analysis and pyrosequencing and were related total DNA concentrations in these samples.
Results:
Thirty-four patients had subtle parenchymal abnormalities by EUS; seven had small pancreatic cysts; none had pancreatic cancer. KRAS mutations were detected in 29 of 36 (80.6%) pancreatic duct fluid samples. Of these 29 patients, 23 had mutations detected in their duodenal fluid (79.3%). Patients with detectable pancreatic fluid but not duodenal fluid KRAS mutations had lower average pancreatic duct fluid KRAS mutation concentrations (P=0.01). Patients with KRAS or GNAS mutations detected in pancreatic fluid but not duodenal fluid had higher total DNA concentrations in their duodenal compared with pancreatic fluid (P=0.03). KRAS and GNAS mutation concentrations were higher in pancreatic duct fluid samples than in matching duodenal fluid samples (for KRAS: 2.62 vs. 0.39%, P<0.0001).
Conclusions:
KRAS and GNAS mutation concentrations are significantly lower in secretin-stimulated pancreatic fluid samples collected from the duodenum compared with samples collected from the pancreatic duct. Efforts to improve the purity of pancreatic fluid collections from the duodenum could improve the detection of mutations arising from the pancreas.
Introduction
Pancreatic cancer is characterized by a late clinical presentation and high mortality and its incidence in the United States is increasing.1 Only a minority of patients who present with symptoms of pancreatic cancer have resectable disease, and of these, <10% present with stage 1 disease.2 In an effort to improve the early detection of pancreatic cancer, pancreatic screening has been offered to asymptomatic individuals whose family history or germline gene mutations represent a sufficiently increased risk of developing pancreatic cancer.3, 4, 5, 6, 7, 8, 9, 10, 11 The diagnostic yield of pancreatic screening tests depends on many factors including the age and family history of patients who undergo screening and the accuracy of the tests used for screening.12 Endoscopic ultrasound (EUS) and magnetic resonance cholangiopancreatography have been the primary tools used for pancreatic screening12 and are often used in the surveillance of incidentally identified pancreatic cysts,13, 14 because they can accurately detect pancreatic cysts without the radiation exposure of repeated computerized tomography scanning. Small pancreatic cysts and subtle parenchymal abnormalities are commonly identified by EUS in patients undergoing studies of pancreatic cancer screening,3, 4, 5 but imaging tests are unable to detect microscopic pancreatic intraepithelial neoplasia (PanIN). Patients with a familial susceptibility to pancreatic cancer often harbor widespread PanIN, including PanIN-3.15 PanIN lesions can harbor the driver mutations of pancreatic ductal adenocarcinoma. For example, over 90% of PanIN-1 lesions harbor KRAS mutations and higher grade PanINs can harbor other mutations in other driver genes mutated in pancreatic ductal adenocarcinomas, including CDKN2A, TP53, and SMAD4.16, 17 Most of the pancreatic cystic lesions identified in patients undergoing pancreatic screening are thought to be intraductal papillary mucinous neoplasms (IPMNs).4, 18 Most IPMNs harbor GNAS and KRAS mutations19 and some harbor mutations in other genes, particularly TP53 and RNF43.18 In an attempt to develop better tests that indicate the presence of pancreatic neoplasia, particularly PanIN and small pancreatic cancers, secretin-stimulated pancreatic fluid samples have been collected from subjects enrolled in the Cancer of the Pancreas Screening (CAPS) trials and analyzed to identify accurate markers of pancreatic neoplasia.3, 4, 5 Secretin-stimulated pancreatic fluid analysis serves as a standard pancreatic function test to evaluate patients for the presence of pancreatic insufficiency.20 Studies using these samples revealed that TP53 and GNAS mutations detected in pancreatic fluid samples collected from the duodenum are highly correlated with the presence of high-grade dysplasia/pancreatic cancer and pancreatic cysts/IPMNs in the corresponding pancreas, respectively.18, 21 Pancreatic fluid is normally collected from the duodenal lumen unless patients are undergoing endoscopic retrograde cholangiopancreatography (ERCP), but pancreatic fluid samples from the pancreatic duct are purer, whereas pancreatic fluid collected in the duodenal lumen is diluted by duodenal contents, potentially obscuring the presence of markers of pancreatic neoplasia. The magnitude of this dilution is not clear.
The purpose of this study was to compare KRAS and GNAS mutation concentrations in secretin-stimulated pancreatic fluid samples collected from the pancreatic duct and from the duodenal lumen in patients who underwent pancreatic EUS followed by ERCP to investigate abnormalities identified by EUS.
Methods
Ethical approval
All elements of this study were approved by the Johns Hopkins institutional review board and written informed consent was provided from all patients.
Patients and specimens
This single-center study is part of the ongoing Cancer of the Pancreas Screening (CAPS) studies to evaluate the utility of pancreatic screening and to evaluate markers of pancreatic neoplasia. Specimens and clinical information were obtained from participants enrolled in the CAPS2, CAPS3, and CAPS4 studies (clinicaltrials.gov NCT00438906, NCT00714701).3, 4 For this study, we identified all subjects enrolled in the CAPS studies at Johns Hopkins Hospital (JHH, Baltimore, Maryland) who had undergone both EUS and ERCP (within a few days or weeks of each other) and had sufficient sample available for analysis. Thirty-six subjects were included (31 from CAPS2, 1 from CAPS3, and 4 from CAPS4). All but two CAPS study subjects were asymptomatic subjects undergoing pancreatic screening for their family history of pancreatic cancer, or inherited predisposition to pancreatic cancer. The remaining two patients were enrolled as disease control subjects. A summary of their demographic and diagnostic characteristics is provided in Table 1. Patients enrolled in the CAPS2 study (2002–2004)4 underwent ERCP to evaluate pancreatic parenchymal abnormalities or pancreatic cysts identified by EUS. At that time, ERCP was used as part of the study protocol to evaluate pancreatic lesions in the familial pancreatic cancer relatives, including parenchymal changes that were thought to be the result of pancreatic neoplasia.10, 22 Patients in the CAPS3 and CAPS4 studies only underwent ERCP for lesions that remained poorly defined after EUS and magnetic resonance cholangiopancreatography (such as suspected pancreatic duct strictures and main duct dilation).3, 4, 5
Table 1. Patient characteristics and diagnosis.
| Patient | Sex | Age | Diagnosis |
|---|---|---|---|
| 1 | Female | 42 | Familial, parenchymal changesa |
| 2 | Male | 53 | Familial, parenchymal changesa |
| 3 | Male | 48 | Familial, parenchymal changesa |
| 4 | Male | 32 | Familial, parenchymal changesa |
| 5 | Female | 58 | Familial, parenchymal changesa |
| 6 | Female | 47 | PJS, parenchymal changesa |
| 7 | Male | 54 | Chronic pancreatitis |
| 8 | Male | 52 | Familial, parenchymal changesa |
| 9 | Female | 58 | Familial, parenchymal changesa |
| 10 | Male | 42 | Familial, parenchymal changesa |
| 11 | Female | 65 | Familial, parenchymal changesa, small cyst |
| 12 | Male | 39 | Familial, parenchymal changesa (resection; PanIN 2) |
| 13 | Female | 46 | Familial, parenchymal changesa |
| 14 | Male | 53 | Familial, parenchymal changesa |
| 15 | Male | 62 | Familial, parenchymal changesa |
| 16 | Male | 59 | Familial, parenchymal changesa, small cyst |
| 17 | Female | 48 | Familial, parenchymal changesa |
| 18 | Female | 62 | Small cyst |
| 19 | Male | 53 | Familial, parenchymal changesa |
| 20 | Female | 57 | Familial, parenchymal changesa (small cyst in the future) |
| 21 | Female | 53 | Familial, small cyst |
| 22 | Female | 58 | Familial, parenchymal changesa, small cyst |
| 23 | Male | 42 | Familial, parenchymal changesa |
| 24 | Male | 58 | Familial, parenchymal changesa |
| 25 | Female | 77 | Familial, parenchymal changesa, 18mm cyst (resection: PanIN3 & IPMN) |
| 26 | Male | 47 | Familial, parenchymal changesa (resection; endocrine tumor, PanIN2) |
| 27 | Female | 56 | Familial, parenchymal changesa |
| 28 | Female | 50 | Familial, parenchymal changesa |
| 29 | Female | 63 | Familial, BRCA2 mutation |
| 30 | Female | 47 | Familial, parenchymal changesa |
| 31 | Female | 53 | PJS, parenchymal changesa |
| 32 | Male | 56 | Familial, BRCA2 mutation, parenchymal changesa(1 year later, IPMN resected) |
| 33 | Female | 75 | Familial, parenchymal changesa |
| 34 | Male | 62 | Familial, 3 small cysts (8 months later, resection; PanIN2 & IPMN adenoma) |
| 35 | Female | 50 | Familial, parenchymal changesa |
| 36 | Female | 72 | Familial, parenchymal changesa (resection; IPMN adenoma, PanIN3) |
IPMN, intraductal papillary mucinous neoplasm; PanIN, pancreatic intraepithelial neoplasia; PJS, Peutz-Jeghers syndrome.
Endoscopic ultrasound changes similar to those found in patients with chronic pancreatitis.
Pancreatic fluid samples were collected from all participants after infusion of intravenous human synthetic secretin (0.2 μg/kg infused over 1 min). Pancreatic fluid samples (~5–15 ml) were collected from the duodenal lumen (“duodenal fluid”) as it was secreted from the papilla by suctioning the fluid repeatedly through the endoscopic channel over ~5–10 min with the linear array echoendoscope with the tip positioned near the Ampulla of Vater. ERCP and pancreatic fluid collection were performed separately after EUS and duodenal fluid collections and usually at a later date. The interval between the EUS and ERCP is provided in Table 2. Pancreatic duct fluid (~2–5 ml) was collected through the ERCP catheter over ~5–10 min. This relatively short duration of pancreatic fluid collection is standard for all pancreatic fluid collections for pancreatic screening as the goal of these collections was to obtain a lavage of the pancreatic ductal system. The normal positioning of the patient during EUS (left lateral) and ERCP (prone) was not changed to facilitate the collection of pancreatic fluid. Secretin was provided for CAPS3 and CAPS4 by ChiRhoClin (Burtonsville, MD).3, 18, 21 Collected fluid (“juice”) samples were stored at −80 °C before use. Further details of the CAPS studies and study subjects are provided elsewhere.3, 4, 5
Table 2. KRAS and GNAS mutation analysis for pancreatic duct fluid and duodenal fluid.
|
KRAS |
GNAS |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pancreatic duct fluid | Duodenal fluid | Pancreatic duct fluid |
Duodenal fluid |
|||||||||
| Patient | CAPS | Interval | Color of duodenal fluid | Ratio of duodenal fluid to pancreatic duct fluid DNA concentration | Mutation (%) | Mutation type (estimated %) | Mutation (%) | Mutation type (estimated %) | Mutation (%) | Mutation type (estimated %) | Mutation (%) | Mutation type (estimated %) |
| 1 | 2 | 3 days | Serous-pink | 25.4 | 0 | 0 | 0 | |||||
| 2 | 2 | 3 days | Green | 1.4 | 0 | 0 | 0 | |||||
| 3 | 2 | 3 days | Serous-pink | 1.0 | 0 | 0 | 0 | |||||
| 4 | 2 | 3 days | Green | 0.4 | 0 | 0 | 0.22 | R201C (50%), R201H (50%) | 0 | |||
| 5 | 2 | 3 days | Serous | 0.3 | 0 | 0 | 0 | |||||
| 6 | 2 | 3 days | Serous-pink | 0.8 | 0 | 0 | 0 | |||||
| 7 | 3 | Same day | Green | 1.1 | 0 | 0 | 0 | |||||
| 8 | 2 | 3 days | Serous-pink | 7.0 | 0.22 | G12R (100%) | 0 | 0 | ||||
| 9 | 2 | 3 days | Serous | 0.2 | 0.22 | G12D (100%) | 0.11 | G12D (100%) | 0 | |||
| 10 | 2 | 3 days | Serous | 5.7 | 0.22 | G12D (50%), G12R (50%) | 0 | 0.11 | R201H (100%) | 0 | ||
| 11 | 4 | Same day | Serous | 0.5 | 0.22 | G13D (100%) | 0 | 0 | ||||
| 12 | 2 | 3 days | Pink | 8.2 | 0.33 | G12D (66%), G12V (33%) | 0 | 0 | ||||
| 13 | 2 | 3 days | Green | 2.0 | 0.33 | G12S (33%), G12R (66%) | 0.11 | G12R (100%) | 0 | |||
| 14 | 2 | 3 days | Serous-pink | 3.9 | 0.33 | G12D (66%), G12V (33%) | 0.11 | G12V (100%) | 0 | |||
| 15 | 2 | 3 days | Serous-pink | 21.7 | 0.56 | G12D (40%), G12V (40%),G12C (20%) | 0.17 | G12C (100%) | 0 | |||
| 16 | 2 | 3 days | Green | 1.1 | 0.67 | G12D (83%), G12R (17%) | 0.11 | G12D (100%) | 0.33 | R201C (100%) | 0 | |
| 17 | 2 | 3 days | Serous-pink | 2.1 | 0.89 | G12D (100%) | 0 | 0.33 | R201H (100%) | 0 | ||
| 18 | 4 | Same day | Green | 2.0 | 0.89 | G12V (56%), G12A (22%), G12R (22%) | 0.11 | G12R (100%) | 0 | |||
| 19 | 2 | 3 days | Serous-pink | 7.8 | 1.00 | G12D (36%), G12V (27%), G12R (36%) | 0 | 0 | ||||
| 20 | 2 | 2 months | Serous | 1.4 | 1.22 | G12D (100%) | 0.11 | G12D (100%) | 0.11 | R201H (100%) | 0.11 | R201H (100%) |
| 21 | 2 | 3 days | Green | 0.8 | 1.44 | G12D (81%), G12R (18%) | 0.22 | G12R (100%) | 0.78 | R201C (100%) | 0.06 | R201C (100%) |
| 22 | 4 | Same day | Serous | 10.3 | 1.67 | G12D (10%), G12V (80%), G12R (10%) | 0.22 | G12D (50%), G12R (50%) | 0.11 | R201C (100%) | 0 | |
| 23 | 2 | 3 days | Serous-pink | 2.4 | 1.89 | G12D (40%), G12R (60%) | 0.11 | G12D (100%) | 0.11 | R201H (100%) | 0 | |
| 24 | 2 | 3 days | Serous | 2.1 | 1.89 | G12D (10%), G12V (50%), G12R (40%) | 0.11 | G12R (100%) | 0 | |||
| 25 | 2 | 3 days | Pink | 1.0 | 2.00 | G12D (50%), G12V (10%), G12R (40%) | 0.11 | G12R (100%) | 0.33 | R201H (100%) | 0 | |
| 26 | 2 | 3 days | Serous-pink | 0.3 | 2.44 | G12V (30%), G12R (70%) | 0.67 | G12R (100%) | 0 | |||
| 27 | 2 | 3 days | Serous | 1.2 | 2.56 | G12D (10%), G12V (70%), G12R (10%), G12A (10%) | 0.22 | G12V (100%) | 0 | |||
| 28 | 2 | 3 days | Serous-pink | 3.2 | 2.56 | G12D (20%), G12V (80%) | 0.06 | G12V (100%) | 0 | |||
| 29 | 2 | 3 days | Serous-pink | 1.4 | 4.11 | G12D (43%), G12V (14%), G12R (43%) | 0.22 | G12D (100%) | 0 | |||
| 30 | 2 | 3 days | Green | 1.7 | 4.78 | G12D (38%), G12V (42%), G12R (20%) | 0.11 | G12R (100%) | 0.11 | R201C (100%) | 0 | |
| 31 | 2 | 3 months | Green | 13.5 | 5.56 | G12D (29%), G12V (29%), G12R (41%) | 0.22 | G12D (50%), G12R (50%) | 0 | |||
| 32 | 2 | 3 days | Green | 5.8 | 6.67 | G12D (47%), G12V (42%), G12R (11%) | 0.11 | G12D (100%) | 3.67 | R201C (67%), R201H (33%) | 0.22 | R201C (100%) |
| 33 | 2 | 3 days | Serous | 6.1 | 7.33 | G12D (8%), G12V (8%), G12R (83%) | 0.11 | G12R (100%) | 0.11 | R201H (100%) | 0 | |
| 34 | 4 | Same day | Serous | 0.1 | 7.44 | G12D (24%), G12V (18%), G12R (58%) | 7.11 | G12D (30%), G12V (12%), G12R (58%) | 0.11 | R201C (100%) | 0.22 | R201C (100%) |
| 35 | 2 | 4 months | Serous | 0.8 | 8.00 | G12D (47%), G12V (53%) | 0.11 | G12D (100%) | 0 | |||
| 36 | 2 | 3 days | Unknown | 6.9 | 8.56 | G12D (90%), G12V (9%) | 0.22 | G12D (50%), G12R (50%) | 0 | |||
Bold and underlined mutation types matched pancreatic duct fluid with duodenal fluid.
Digital-high-resolution melt (HRM)-curve analysis and pyrosequencing
Digital-HRM analysis and pyrosequencing were used to evaluate KRAS and GNAS mutation concentrations in pancreatic fluid samples as described previously.18 All samples were analyzed for KRAS mutations in the same manner and blinded to sample origin. GNAS mutation analysis was first determined in pancreatic duct fluid samples and then in the duodenal fluid in samples from patients with GNAS detected in their pancreatic duct sample. For digital-HRM analysis, 900 genome equivalents of DNA were dispensed into 90 wells of each 96-well plate of pancreatic fluid DNA analyzed (10 genome equivalents per well), five wells had wild-type DNA and one well had water.17, 18 The primers used for digital-HRM included for KRAS (forward 5′-AGGCCTGCTGAAAATGACTG-3′, reverse 5′TTGTTGGATCATATTCGTCCAC-3′) and for GNAS (forward 5′CGGTTGGCTTTGGTGAGATC-3′ and reverse 5′-CAGTTGGCTTACTGGAAGTTG-3′). Using these assay conditions, the concentration of mutations in a juice sample is represented by the number of wells having a mutation. If the first 96-well plate failed to detect mutations, additional 96-well plates of pancreatic fluid DNA were analyzed for mutations using digital-HRM pyrosequencing analysis. When KRAS and GNAS mutations were not detected in duodenal fluids but detected in pancreatic duct fluid, deeper sampling was performed by performing digital-HRM. There were no samples excluded for technical reasons such as poor DNA quality. To confirm the digital-HRM results, pyrosequencing was performed on PCR products from representative HRM-positive (up to 10 or more positive wells) and one HRM-negative well and one wild-type well for each plate.16 The primers used for pyrosequencing were for KRAS (5′-GTGGTAGTTGGAGCT-3′) and for GNAS (5′- AGGACCTGCTTCGCTG-3′).
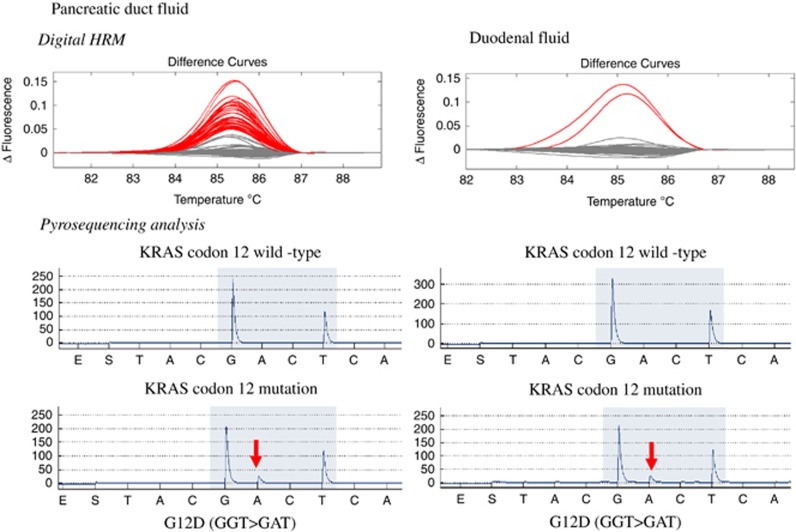
Figure 1 contains representative pancreatic fluid and duodenal fluid digital-HRM and pyrosequencing results from the same individual. The mutation score was determined by the number of HRM-positive wells with mutations confirmed by pyrosequencing. The mutation concentration (the percentage of wells with mutations) was calculated for each juice sample. A mutation percentage was determined for each type of mutations.
Figure 1.
Representative examples of melt-curve analysis and pyrosequencing of pancreatic duct fluid (left) and duodenal fluid (right). The curves in a represent the melt curves generated from one 96-well plate of PCR products amplified from pancreatic duct juice DNA. b is the same, except that they are generated from duodenal fluid DNA. The gray curves are wild-type; these same curves are generated from melting PCR products amplified from wild-type DNA. The red curves are scored as mutant because similar curves are generated when melting PCR products amplified from DNA samples with KRAS codon 12 mutations. There are many more red curves in a than b because there were more KRAS-mutant DNA molecules in the duct juice sample compared with the duodenal fluid sample. c and d are representative pyrosequencing results from PCR wells with normal melt curves (i.e., wild-type for KRAS). e and f are pyrosequencing results from PCR wells that had mutant melt curves.
Statistical analysis
Mutation concentrations in pancreatic duct fluid and duodenal fluid samples were compared by Wilcoxon matched-pairs signed rank test. Spearman's rank correlation coefficient was used to evaluate the correlation of the mutation score of paired pancreatic ductal and duodenal fluid samples. Statistical analysis was performed using JMP Pro 11.1.1 software (SAS, Cary, NC). P<0.05 was considered statistically significant.
Results
DNA concentrations of pancreatic duct fluid samples and duodenal fluid samples
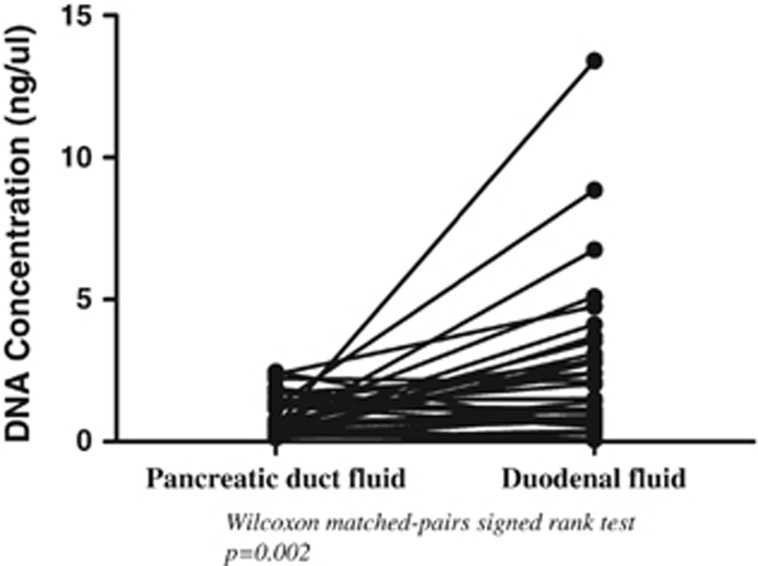
Mean total DNA concentrations were significantly higher in duodenal fluid samples (2.39±2.70 ng/μl) than in pancreatic duct fluid samples (0.92±0.65 ng/μl) from the same subjects (Figure 2, P=0.002). Some duodenal fluid samples were bile colored but there was no correlation between DNA concentrations and the color of duodenal fluid samples.
Figure 2.
Matching total DNA concentrations in pancreatic duct fluid and duodenal fluid (mean; 0.92±0.65 ng/μl and 2.39±2.70 ng/μl, respectively) (P=0.002). HRM, high-resolution melt-curve analysis.
KRAS mutations concentrations in pancreatic duct fluid and duodenal fluid samples
Thirty-six individuals had paired samples of secretin-stimulated pancreatic fluid collected from the duodenum during EUS and from the pancreatic duct subsequent ERCP. The indication for EUS was pancreatic screening for 34 participants, 30 for their family history of pancreatic cancer alone, 2 for Peutz-Jeghers syndrome, and 2 with germline BRCA2 mutations. For one patient, the indication for EUS and ERCP was suspected chronic pancreatitis and for another, it was to evaluate a pancreatic cyst in a patient without a family history of pancreatic cancer. EUS detected parenchymal abnormalities similar to those found in subjects with chronic pancreatitis in 31 of the 34 screened patients (91.2%, Table 2). Seven patients had pancreatic cysts detected by EUS (average cyst size; 7.6±2.8 mm).
When analyzing 900 g.e. (i.e., one 96-well plate), KRAS mutations (almost all codon 12 mutations) were detected in 29 of 36 pancreatic duct fluid samples (80.6%) and in 17 of the 29 corresponding duodenal fluid samples (58.6%). To increase the rate of detection of KRAS mutations in the 12 duodenal fluid samples in which mutations were not detected with this initial analysis, we performed additional digital-HRM and pyrosequencing of another aliquot of DNA from these same duodenal fluid samples. With this additional analysis, KRAS mutations were detected in six additional duodenal fluid samples so that overall KRAS mutations were detected in 23 of the 29 duodenal fluid samples that had KRAS mutations in their pancreatic duct fluid sample (79.3%). KRAS mutations were not detected in any duodenal fluid samples from patients that did not have KRAS mutations detected in their pancreatic duct fluid sample. Most pancreatic fluid samples with mutations had multiple different KRAS mutations (24 of 29) (Table 2). Most of the patients with KRAS mutations detected in their pancreatic fluid did not have pancreatic cysts, and although some had EUS abnormalities that met criteria for parenchymal changes like those of chronic pancreatitis, others had no such changes. The KRAS mutation spectrum observed in pancreatic fluid samples was the same as has been observed previously in pancreatic ductal adenocarcinomas, PanINs, and IPMNs with the most common KRAS gene mutations being G12D (43%), G12V (28%), and G12R (27%)(Table 2).23, 24, 25
The mean KRAS mutation concentrations were significantly higher in pancreatic duct fluid than in duodenal fluid samples (2.62 vs. 0.39%, P<0.0001). Despite the low concentration of KRAS mutations in duodenal fluid, there was a significant correlation between the mutation concentrations in pancreatic duct fluid and duodenal fluid from the same patient (P=0.617, P=0.0004). Perhaps not surprisingly, KRAS mutations were more likely to be detected in duodenal fluid in patients with higher pancreatic duct fluid KRAS mutation concentrations. KRAS mutations were detected in 17 of 18 (94.4%) duodenal fluid samples when the patient’s corresponding pancreatic duct fluid KRAS mutation concentration was over 1.0% of total DNA, but in only 6 of 11 duodenal fluid samples (54.6%) when the pancreatic duct fluid KRAS concentration was <1.0% (P=0.01).
GNAS mutations analysis in pancreatic fluid samples and duodenal fluid samples
GNAS mutations were detected in 12 of the 36 pancreatic duct samples by sampling 900 g.e of pancreatic duct fluid DNA. Initial analysis of the same amount of duodenal fluid DNA identified GNAS mutations in three samples. Deeper digital-HRM analysis identified GNAS mutations in one additional sample. GNAS mutation concentrations in pancreatic duct samples were lower than KRAS mutation concentrations (P=0.001), which might explain the lower rate of detection of GNAS mutations in duodenal fluid. Indeed, only one patient had pancreatic duct fluid GNAS mutation concentrations above 1% (Table 2). Pancreatic cysts were identified at the time of their EUS or during a subsequent EUS in 7 of the 13 patients who had GNAS mutations in the pancreatic duct samples including the 4 patients who had GNAS mutations detected in their duodenal fluid. All six patients with pancreatic duct GNAS mutations but no detectable pancreatic cyst had low concentrations of mutant GNAS in their pancreatic fluid sample (<1% of total DNA) and did not have GNAS mutations detected in their duodenal fluid sample. All of the patients who had GNAS mutations detected in their duodenal fluid also had KRAS mutations detected in their duodenal fluid. The average GNAS mutation concentrations in pancreatic duct samples was 10-fold higher than that of duodenal fluid samples (0.17 vs. 0.017%) (P=0.01).
The role of total DNA concentrations in pancreatic and duodenal fluid in mutation detection
We compared concentrations of total DNA in duodenal fluid relative to pancreatic duct fluid (DNA concentration ratio: DNA concentration of duodenal fluid (ng/μl)/DNA concentration of pancreatic fluid (ng/μl)). Eleven of the 13 patients who had KRAS or GNAS mutations (or both) detected in their pancreatic fluid but not in their duodenal fluid had higher total DNA concentrations in their duodenal fluid relative to total pancreatic fluid DNA (concentration ratio >1) compared with only 11 of the 23 cases in which mutations were detected in both samples (P=0.03).
Discussion
In this study, we report three main findings. First, we find that the average concentration of KRAS mutations and GNAS mutations in a patient’s secretin-stimulated duodenal fluid sample are considerably lower (7–10-fold) than in their corresponding pancreatic duct fluid sample (P<0.001). Second, we find that KRAS mutations are more likely to be detected in duodenal fluid samples when patients have relatively high concentrations of mutations (>1%) in their pancreatic duct fluid sample. Third, we find that mutations detected in pancreatic duct fluid were less likely to be detected in duodenal fluid when the concentration of total DNA in these samples is higher than in pancreatic duct fluid suggesting that high DNA concentrations in the duodenal lumen could obscure the detection of low concentrations of mutant DNA in secretin-stimulated pancreatic fluid.
The lower concentration of mutations arising from the pancreas in pancreatic fluid samples collected from the duodenal lumen necessitates these samples undergoing deeper sampling with more sensitive, specific, and expensive assays to detect mutations. An alternative to employing more sensitive mutation detection assays to detect mutations in duodenal fluid collections would be to collect purer samples of pancreatic fluid. The contaminating effect of duodenal contents is not due simply to fluid pooling in the duodenum; there is normally little or no fluid in the duodenal lumen to aspirate prior to secretin-stimulated pancreatic fluid collection. Most of the DNA in the duodenal lumen is probably shed from cells lining the duodenum.26 As using ERCP to sample the pancreatic duct is not appropriate in the pancreatic screening setting, other approaches are needed. Alternative endoscopic approaches to pancreatic fluid collection at the papilla that did not involve cannulating the papilla of Vater could be very helpful, particularly if secretin-stimulated pancreatic fluid samples proved to be a clinically useful source of markers of pancreatic neoplasia.
We found a high prevalence of KRAS mutations (~80% of pancreatic duct fluid samples) in our study population, which consisted mostly of patients undergoing pancreatic screening. We have also observed a similarly high prevalence of KRAS mutations in the duodenal fluid samples of other patients undergoing pancreatic screening as part of the CAPS studies (Goggins M, Eshleman J et al., unpublished data), often in patients without any diagnostic pancreatic abnormalities by imaging. Although these results suggest that the detection of KRAS mutations in pancreatic fluid is not a useful test for the diagnosis of pancreatic cancer, these results do not rule out the possibility that quantifying KRAS mutations in pancreatic fluid samples might have diagnostic utility. It should be noted that our study was limited to patients who had undergone EUS and ERCP and was not designed to evaluate the diagnostic utility of KRAS mutations. We have maintained long-term follow-up of the patients undergoing pancreatic screening in our study and although several had cystic lesions that required surgery, none developed pancreatic cancer (Table 2). In a separate study, we have found that patients with pancreatic ductal adenocarcinoma have significantly higher concentrations of KRAS mutations in their duodenal collections of pancreatic fluid than patients undergoing pancreatic screening and those without evidence of pancreatic disease, but such a test does not reliably distinguish cancer cases from controls (Goggins M, Eshleman J et al., unpublished data). Patients with an extensive family history of pancreatic cancer who undergo pancreatic screening commonly have numerous PanINs and IPMNs.15, 27 For example, 39% of patients enrolled in the CAPS3 pancreatic screening study were found to have small, mostly subcentimeter, pancreatic cysts.3 Most patients who undergo pancreatic resection for enlarging or concerning cystic lesions detected by screening also have PanIN lesions identified in their resection specimen. These PanIN lesions are generally more abundant in the resection specimen than their IPMNs.15, 27 It is also known that most middle-aged and older individuals without any family history of pancreatic cancer have some PanIN-1 in their pancreata.28, 29 For these reasons, it is likely that a lot of the mutant KRAS detected in the pancreatic fluid of patients undergoing pancreatic screening arise from multifocal low-grade PanIN lesions. The presence of microscopic PanIN explains why we often detected KRAS mutations in pancreatic fluid samples of patients who did not have pancreatic cysts or GNAS mutations and why among patients harboring both KRAS and GNAS mutations in their pancreatic fluid the KRAS mutation concentrations are generally higher than GNAS mutations.
In conclusion, we find that the concentrations of KRAS and GNAS mutations are considerably higher in secretin-stimulated pancreatic fluid samples collected from the pancreatic duct than when pancreatic fluid is collected from the duodenal lumen. The dilution of pancreatic fluid by duodenal lumen contents can limit the detection of pancreatic fluid mutations. Improvements in the endoscopic collection of pancreatic fluid from the duodenum may improve the detection of mutations arising from the pancreas during EUS screening for pancreatic cancer in high risk individuals.
Study Highlights
Acknowledgments
We acknowledge Hilary Cosby, RN, and Kieran Brune,MB for their assistance in the CAPS studies.
Footnotes
Guarantor of the article: Michael Goggins, MB, MD.
Specific author contributions: Yoshi Sadakari, Mitsuro Kanda, Kosuke Maitani, Michael Borges: acquisition of data, analysis and interpretation of data, revision of the manuscript. Marcia Irene Canto: material support, acquisition and interpretation of data, revision of the manuscript, obtained funding. Michael Goggins: study concept and design, study supervision, interpretation of data, drafting and revision of the manuscript, obtained funding. Each author approved the final draft of the paper.
Financial support: The sponsors did not have any role in the study design, collection, analysis, interpretation of the data, or in the writing of the paper. This work was supported by NIH grants (CA62924, and R01CA176828), the American Association for Cancer Research (RAN grant to MG and MIC), Susan Wojcicki and Dennis Troper, the Lustgarten Foundation for Pancreatic Cancer Research, the Jimmy V Foundation, the Michael Rolfe Foundation, Michael Hooven and Susan Spies, Hugh and Rachel Victor.
Potential competing interests: There are no conflicts of interest for any of the authors. Recombinant secretin was provided for this study by ChiRhoClin. The company did not have any part in the design of this study, analysis or interpretation of data, or in the writing of this manuscript. The corresponding authors had full access to all of the data and takes full responsibility for the veracity of the data and statistical analysis.
References
- Rahib L, Smith BD, Aizenberg R et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- Ferrone CR, Pieretti-Vanmarcke R, Bloom JP et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery 2012; 152: S43–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto MI, Hruban RH, Fishman EK et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012; 142: 796–804 PMCID3321068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto MI, Goggins M, Hruban RH et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006; 4: 766–781. [DOI] [PubMed] [Google Scholar]
- Canto MI, Goggins M, Yeo CJ et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004; 2: 606–621. [DOI] [PubMed] [Google Scholar]
- Ludwig E, Olson SH, Bayuga S et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol 2011; 106: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna EC, Hwang C, Stevens PD et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010; 16: 5028–5037. [DOI] [PubMed] [Google Scholar]
- Poley JW, Kluijt I, Gouma DJ et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009; 104: 2175–2181. [DOI] [PubMed] [Google Scholar]
- Langer P, Kann PH, Fendrich V et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009; 58: 1410–1418. [DOI] [PubMed] [Google Scholar]
- Brentnall TA, Bronner MP, Byrd DR et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med 1999; 131: 247–255. [DOI] [PubMed] [Google Scholar]
- Al-Sukhni W, Borgida A, Rothenmund H et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012; 16: 771–783. [DOI] [PubMed] [Google Scholar]
- Canto MI, Harinck F, Hruban RH et alInternational Cancer of Pancreas Screening (CAPS) Consortium. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013; 62: 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Fernandez-Del Castillo C, Adsay V et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chari S, Adsay V et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006; 6: 17–32. [DOI] [PubMed] [Google Scholar]
- Brune K, Abe T, Canto M et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 2006; 30: 1067–1076. [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Matthaei H, Wu J et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012; 142: 730–733, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Sadakari Y, Borges M et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013; 11: 719–730, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Knight S, Topazian M et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 2013; 62: 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Matthaei H, Maitra A et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011; 3: 92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T, Dumot JA, Zuccaro G Jr. et al. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol 2009; 7: 114–119. [DOI] [PubMed] [Google Scholar]
- Kanda M, Sadakari Y, Borges M et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013; 11: 719–730, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmey MB, Bronner MP, Byrd DR et al. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc 2002; 56: S82–S86. [DOI] [PubMed] [Google Scholar]
- Vasan N, Boyer J, Herbst RS. A RAS renaissance: emerging targeted therapies for KRAS-mutated non-small cell lung cancer. Clin Cancer Res 2014; 20: 3921–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova MN, Khalid A, Fasanella KE et al. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Mod Pathol 2013; 26: 1478–1487. [DOI] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321: 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H, Sato N, Brune K et al. Age- and disease-related methylation of multiple genes in nonneoplastic duodenum and in duodenal juice. Clin Cancer Res 2005; 11: 573–583. [PubMed] [Google Scholar]
- Shi C, Klein AP, Goggins M et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res 2009; 15: 7737–7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol 2003; 16: 996–1006. [DOI] [PubMed] [Google Scholar]
- Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res 1976; 36: 2690–2698. [PubMed] [Google Scholar]