Abstract
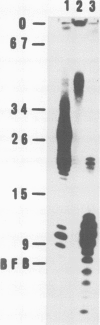
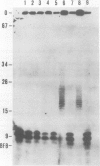
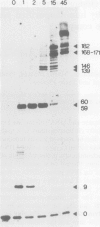
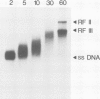
Yeast DNA primase and DNA polymerase I can be purified by immunoaffinity chromatography as a multipeptide complex which can then be resolved into its functional components and further reassembled in vitro. Isolated DNA primase synthesizes oligonucleotides of a preferred length of 9-10 nucleotides and multiples thereof on a poly(dT) template. In vitro reconstitution of the DNA primase:DNA polymerase complex allows the synthesis of long DNA chains covalently linked to RNA initiators shorter than those synthesized by DNA primase alone. The SS (single-stranded) circular DNA of phage M13mp9 can also be replicated by the DNA primase:DNA polymerase complex. Priming by DNA primase occurs at multiple sites and the initiators are utilized by the DNA polymerase moiety of the complex, so that almost all the SS template is converted into duplex form. The rate of DNA synthesis catalyzed by isolated yeast DNA polymerase I on the M13mp9 template is not constant and is characterized by distinct pausing sites, which partly correlate with secondary structures on the template DNA. Thus, replication of M13mp9 SS DNA with the native primase:polymerase complex gives rise to a series of DNA chains with significantly uniform termini specified by the primase start sites and the polymerase stop sites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conaway R. C., Lehman I. R. Synthesis by the DNA primase of Drosophila melanogaster of a primer with a unique chain length. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4585–4588. doi: 10.1073/pnas.79.15.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse F., Krauss G. Replication of M13mp7 single-stranded DNA in vitro by the 9-S DNA polymerase alpha from calf thymus. Eur J Biochem. 1984 May 15;141(1):109–114. doi: 10.1111/j.1432-1033.1984.tb08164.x. [DOI] [PubMed] [Google Scholar]
- Hübscher U. The mammalian primase is part of a high molecular weight DNA polymerase alpha polypeptide. EMBO J. 1983;2(1):133–136. doi: 10.1002/j.1460-2075.1983.tb01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearsey S. Structural requirements for the function of a yeast chromosomal replicator. Cell. 1984 May;37(1):299–307. doi: 10.1016/0092-8674(84)90326-x. [DOI] [PubMed] [Google Scholar]
- Kitani T., Yoda K., Okazaki T. Discontinuous DNA replication of Drosophila melanogaster is primed by octaribonucleotide primer. Mol Cell Biol. 1984 Aug;4(8):1591–1596. doi: 10.1128/mcb.4.8.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevani P., Badaracco G., Augl C., Chang L. M. DNA polymerase I and DNA primase complex in yeast. J Biol Chem. 1984 Jun 25;259(12):7532–7539. [PubMed] [Google Scholar]
- Reichard P., Eliasson R. Synthesis and function of polyoma initiator RNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):271–277. doi: 10.1101/sqb.1979.043.01.033. [DOI] [PubMed] [Google Scholar]
- Riedel H. D., König H., Stahl H., Knippers R. Circular single stranded phage M13-DNA as a template for DNA synthesis in protein extracts from Xenopus laevis eggs: evidence for a eukaryotic DNA priming activity. Nucleic Acids Res. 1982 Sep 25;10(18):5621–5635. doi: 10.1093/nar/10.18.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Shioda M., Nelson E. M., Bayne M. L., Benbow R. M. DNA primase activity associated with DNA polymerase alpha from Xenopus laevis ovaries. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7209–7213. doi: 10.1073/pnas.79.23.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Dumas L. B. A DNA primase that copurifies with the major DNA polymerase from the yeast Saccharomyces cerevisiae. J Biol Chem. 1984 Jun 25;259(12):7936–7940. [PubMed] [Google Scholar]
- Singh H., Dumas L. B. A DNA primase that copurifies with the major DNA polymerase from the yeast Saccharomyces cerevisiae. J Biol Chem. 1984 Jun 25;259(12):7936–7940. [PubMed] [Google Scholar]
- Tseng B. Y., Ahlem C. N. Mouse primase initiation sites in the origin region of simian virus 40. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2342–2346. doi: 10.1073/pnas.81.8.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Erickson J. M., Goulian M. Initiator RNA of nascent DNA from animal cells. J Mol Biol. 1979 Apr 25;129(4):531–545. doi: 10.1016/0022-2836(79)90467-4. [DOI] [PubMed] [Google Scholar]
- Weaver D. T., DePamphilis M. L. Specific sequences in native DNA that arrest synthesis by DNA polymerase alpha. J Biol Chem. 1982 Feb 25;257(4):2075–2086. [PubMed] [Google Scholar]
- Yagura T., Kozu T., Seno T. Mouse DNA replicase. DNA polymerase associated with a novel RNA polymerase activity to synthesize initiator RNA of strict size. J Biol Chem. 1982 Sep 25;257(18):11121–11127. [PubMed] [Google Scholar]