Abstract
Background
In 2000, American Samoa had 16.5% prevalence of lymphatic filariasis (LF) antigenemia. Annual mass drug administration (MDA) was conducted using single-dose albendazole plus diethylcarbamazine from 2000 to 2006. This study presents the results of a 2007 population-based PacELF C-survey in all ages and compares the adult filarial antigenemia results of this survey to those of a subsequent 2010 survey in adults with the aim of improving understanding of LF transmission after MDA.
Results
The 2007 C-survey used simple random sampling of households from a geolocated list. In 2007, the overall LF antigen prevalence by immunochromatographic card test (ICT) for all ages was 2.29% (95% CI 1.66–3.07). Microfilaremia prevalence was 0.27% (95% CI 0.09–0.62). Increasing age (OR 1.04 per year, 95% CI 1.02–1.05) was significantly associated with ICT positivity on multivariate analysis, while having ever taking MDA was protective (OR 0.39, 95% CI 0.16–0.96). The 2010 survey used a similar spatial sampling design.
The overall adult filarial antigenemia prevalence remained relatively stable between the surveys at 3.32% (95% CI 2.44–4.51) by ICT in 2007 and 3.23 (95% CI 2.21–4.69) by Og4C3 antigen in 2010. However, there were changes in village-level prevalence. Eight village/village groupings had antigen-positive individuals identified in 2007 but not in 2010, while three villages/village groupings that had no antigen-positive individuals identified in 2007 had positive individuals identified in 2010.
Conclusions
After 7 years of MDA, with four rounds achieving effective coverage, a representative household survey in 2007 showed a decline in prevalence from 16.5 to 2.3% in all ages. However, lack of further decline in adult prevalence by 2010 and fluctuation at the village level showed that overall antigenemia prevalence at a broader scale may not provide an accurate reflection of ongoing transmission at the village level.
Keywords: Lymphatic filariasis, American Samoa, Prevalence, MDA, Epidemiology, Wuchereria bancrofti, PacELF
Background
Lymphatic filariasis (LF) is a neglected mosquito-borne parasitic disease caused by three species of filarial parasites—Wuchereria bancrofti, Brugia malayi and Brugia timori [1]. In May 1997, the World Health Assembly passed a resolution calling for the global elimination of LF as a public health problem by the year 2020. The World Health Organisation (WHO) subsequently launched the Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000 to facilitate this aim [2]. In 1999, the Pacific Programme for the Elimination of Lymphatic Filariasis (PacELF) was formed to coordinate regional efforts toward elimination in the 22 Pacific Island countries and territories (PICTs) by 2020, utilising a strategy of annual mass drug administration (MDA) of a single dose of diethylcarbamazine (DEC) plus albendazole for the entire at-risk population [3].
The WHO criteria for ceasing MDA after a minimum of five effective annual rounds (coverage exceeding 65% of the total population) in areas where Aedes mosquitos are the primary vector is <1% antigenemia in a transmission assessment survey (TAS) of 6- to 7-year-old children. Critical cut-off values are calculated based on sample sizes designed so that a TAS evaluation unit (EU) has at least a 75% chance of passing if antigenemia is 0.5% and no more than 5% chance of passing if antigenemia is ≥1% [4]. Prior to the development of the TAS, PacELF had established separate criteria for ceasing MDA if <1% filarial antigenemia (<2% upper 95% CI) across all age groups, based on the results of a population-based cluster survey (C-survey).
American Samoa is an unincorporated territory of the USA. The population of 54,454 (in 2015) inhabits the islands of Tutuila, Aunu’u, Ofu-Olosega and Ta’u. The capital, Pago Pago, is located on Tutuila, the largest island, where >95% of the population reside. The territory was partitioned from neighbouring Samoa in 1899: however, the two communities continue to share strong family, cultural, linguistic and economic bonds. LF in American Samoa is caused by the diurnally sub-periodic Wuchereria bancrofti, transmitted predominantly by the day-biting mosquito Aedes polynesiensis, with the night-biting Aedes samoanus as a secondary vector [5–7].
LF prevalence surveys have been conducted in American Samoa since 1923, with microfilaremia (Mf) prevalence in pre-PacELF surveys as high as 21% in 1962 (n = 1000). MDA with DEC (6 mg/kg monthly for a total of 72 mg/kg over a year) was undertaken in 1963 and 1965. A follow-up survey in 1968 found Mf prevalence had decreased to 0.3% (n = 1053 across 13 villages) [3]. No further MDA interventions were recorded until the commencement of PacELF and the national elimination programme in 1999, when a nationwide convenience survey of 18 villages established a baseline prevalence of 16.5% (n = 3018) filarial antigenemia by immunochromatographic card test (ICT) [3]. Seven rounds of annual MDA followed during 2000–2006, targeting the whole population except pregnant women, children less than 2 years old and the severely ill [8].
MDA coverage in American Samoa was initially poor, ranging from 19% in 2000 to 49% in 2002. From 2002 to 2005, the national programme was independently evaluated using a variety of formative research methods including focus groups with drug distributors (programme directors, nurses, health assistants and volunteers); a multi-stage household cluster survey of community knowledge, attitudes and practices; and key informant interviews with church leaders. The programme evaluation resulted in significant changes to community mobilisation, behaviour change communication and drug delivery strategies. Notably, the evaluation identified the involvement of churches as a key driver of improved programme coverage, with over half of the population receiving treatment in conjunction with church attendance [9]. Coverage increased to 71% in 2003 and was sustained at a relatively high level in 2004 (65%), 2005 (67%) and 2006 (70%) [9].
A significant decrease in LF antigen prevalence was observed in sentinel villages between 2001 and 2006, coinciding with the improvements in MDA participation and coverage [10]. Two spot check village surveys in 2006 (a total of four villages) found higher prevalence outside of sentinel villages [8, 10, 11].
This paper reports on the results of a 2007 population-based PacELF C-survey and compares the proportion of antigen-positive adults in 2007 with those found in a 2010 study for corresponding villages. We aimed to identify potential risk factors for infection in 2007 and examine small-scale changes in antigenemia over time by comparing village-level adult seroprevalence between the 2007 and 2010 surveys. Furthermore, we aimed to reflect on how these small-scale variations in disease transmission may affect post-MDA surveillance practices and strategies.
Methods
Data sources
The 2007 C-survey data were collected by American Samoa Department of Health (AS DOH) staff with the support of staff from the US Centers for Disease Control and Prevention (US CDC). The survey team used geographic information systems (GIS) data from the American Samoa Department of Commerce to identify buildings in villages on the populated islands of Tutuila, Aunu’u, Ofu-Olosega and Tau. Under the assumption of an average household size of five individuals, a simple random sample of 540 buildings was taken (500 in Tutuila and Aunu’u, 20 in Ofu-Olosega, 20 in Tau), with a target sample size of 2700 individuals. GIS software (ArcGIS, Environmental Systems Research Institute, Redlands, CA) was used to identify and highlight selected buildings and generate printed maps for use by survey teams to identify these households on-ground. All individuals 2 years of age and older residing in selected households were invited to participate and tested for filarial antigenemia using ICT (BinaxNOW Filariasis ICT Alere, Scarborough, USA). ICT-positive individuals were tested for Mf by microscopic examination of thick blood smears (20 μL of blood, Giemsa-stained) and treated with DEC and albendazole [12]. Where possible, those who were absent at the time of visit were tracked down and tested according to the same procedure. A standard questionnaire was administered at the time of testing to collect data on demographics and MDA compliance history. All individuals involved in the survey provided verbal consent prior to participation.
The 2010 survey data were collected as part of a leptospirosis seroprevalence study, using a spatially representative household sampling design similar to the C-survey. The study was designed to include a representative sample of the adult population (≥18 years old), and methods and sampling designs have been previously described [13, 14]. The serum bank was subsequently used for a study of the seroprevalence and spatial epidemiology of LF in American Samoa after MDA, which identified possible residual foci of antigen-positive adults [15]. Filarial antigenemia in stored venous serum samples was measured through the detection of circulating filarial antigen (CFA) using the Og4C3 antigen ELISA test (cut-off value of >32 units was considered seropositive).
Data analysis
The 2007 survey data were entered into Microsoft Excel before being exported into STATA 13.1 (College Station, TX), which was used for subsequent analyses. Descriptive statistics were derived from the data to examine the characteristics of the sample. Univariate logistic regression analysis was performed on independent variables (sex, age, years lived in American Samoa, having ever taken MDA, number of years having taken MDA, island of residence) to assess association with the dependent variable (ICT positivity). Years lived in American Samoa was categorised into <7 and ≥7 years in order to assess possible differences in risk between individuals who had lived in American Samoa prior to MDA and those who had lived in American Samoa only during MDA (<7 years). All independent variables with a P value of <0.25 on univariate analysis were considered for inclusion in a full multivariate logistic regression model. Starting with all potentially significant independent variables from the univariate analysis, a backward stepwise regression procedure (P ≤ 0.05) was performed to refine and select the final set of variables for inclusion in the multivariate model.
For comparison of village-level adult filarial antigenemia prevalence between the 2007 and 2010 surveys, villages in which five or more individuals were sampled in each survey were included for analysis. Some small adjacent villages that did not meet this cut-off were combined into village groupings for this analysis if the groupings were considered to be ecologically and geographically appropriate. Villages which could not be grouped and did not meet the cut-off were excluded from the comparison. As the 2010 survey only sampled adults, individuals under 18 years of age were excluded from the 2007 data for the purposes of the comparison.
Results
2007 survey
From a total of 540 buildings selected, 2216 individuals from 394 buildings were identified as being eligible for inclusion in the survey. Of these individuals, 1881 were available for testing and enrolled in the survey. The most common reasons for an individual being missed by the survey (n = 335) were not being home at the time (59.9%), followed by being at work (22.3%), being off-island (13.3%) and refusing to participate (4.6%). The sample population is described in Table 1.
Table 1.
Characteristics of the eligible sampled and missed population in the 2007 lymphatic filariasis C-survey in American Samoa
| Eligible (%) | Sampled (%) | Missed (%) | |
|---|---|---|---|
| Total | 2216 | 1881 | 335 |
| Gender | |||
| Female | 1150 (51.9) | 1000 (53.2) | 150 (48.8) |
| Male | 1066 (48.1) | 881 (46.8) | 185 (52.2) |
| Age group (years) | |||
| 2–9 | 293 (13.2) | 269 (14.3) | 24 (7.2) |
| 10–19 | 601 (27.1) | 512 (27.2) | 89 (26.6) |
| 20–29 | 325 (14.7) | 266 (14.1) | 59 (17.6) |
| 30–39 | 330 (14.9) | 273 (14.5) | 57 (17.0) |
| 40–49 | 296 (13.4) | 235 (12.5) | 61 (18.2) |
| 50–59 | 186 (8.4) | 169 (9.0) | 17 (5.1) |
| 60 + | 185 (8.3) | 157 (8.3) | 28 (8.4) |
| Island of residence | |||
| Tutuila/Aunu’u | 2094 (94.5) | 1773 (94.3) | 321 (95.8) |
| Ofu-Olosega | 57 (2.6) | 53 (2.8) | 4 (1.2) |
| Ta’u | 65 (2.9) | 55 (2.9) | 10 (3.0) |
The overall LF antigenemia prevalence for all ages by ICT was 2.29% (43/1881, 95% CI 1.66–3.07). Table 2 summarises LF antigenemia prevalence by island group. Variations in village-level LF antigenemia prevalence are described in Figs. 1 and 2. Four ICT-positive children aged <10 years were identified, all residents on the island of Tutuila. Age-specific LF antigenemia prevalence is shown in Fig. 3.
Table 2.
W. bancrofti antigenemia prevalence by island group in the 2007 lymphatic filariasis C-survey in American Samoa
| Island | No. of participants | No. of ICT positive | Prevalence (%) |
|---|---|---|---|
| Tutuila/Aunu’u | 1773 | 40 | 2.26 |
| Ofu-Olosega | 53 | 2 | 3.77 |
| Ta’u | 55 | 1 | 1.82 |
Fig. 1.
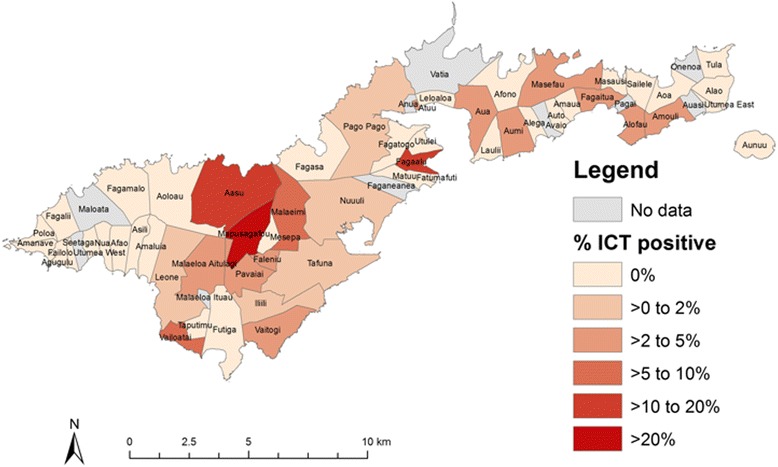
Village-level filarial antigenemia prevalence in the 2007 lymphatic filariasis C-survey in American Samoa—Tutuila and Aunu’u
Fig. 2.
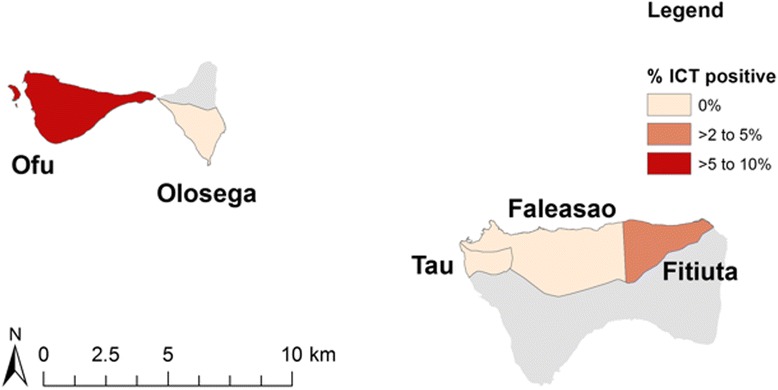
2007 village-level filarial antigenemia prevalence in the 2007 lymphatic filariasis C-survey in American Samoa—Ofu-Olosega and Tau
Fig. 3.
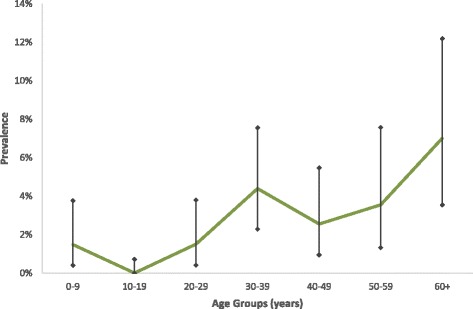
Age-specific prevalence for W. bancrofti antigenemia in the 2007 lymphatic filariasis C-survey in American Samoa. Bars indicate 95% confidence intervals
Microfilaremia prevalence was 0.27% (5/1881, 95% CI 0.09–0.62). Of the five microfilaremic individuals identified, all resided in different villages on the island of Tutuila. Three were aged 30–39 and two were aged ≥60 years.
A number of independent variables were significantly associated with being ICT positive (P < 0.25) on univariate logistic regression analysis and included in the full multivariate model. Table 3 provides a summary of associations between independent variables and ICT positivity on univariate logistic regression analysis.
Table 3.
Association between variables and immunochromatographic card test (ICT) positivity on univariate logistic regression analysis for the 2007 lymphatic filariasis C-survey in American Samoa
| No. of participants | ICT positive | Prevalence (%) | Univariate OR (95% CI) | P value | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 1000 | 18 | 1.80 | Reference | |
| Male | 881 | 25 | 2.84 | 1.59 (0.86–2.94) | 0.14 |
| Age | |||||
| 1-year increments | 1881 | 43 | 2.29 | 1.04 (1.02–1.05) | <0.001 |
| Years lived in AS | |||||
| <7 years | 303 | 9 | 2.97 | 1.39 (0.66–2.92) | 0.39 |
| ≥7 years | 1575 | 34 | 2.16 | Reference | |
| Ever taken MDA | |||||
| No | 109 | 6 | 5.50 | Reference | |
| Yes | 1763 | 37 | 2.10 | 0.37 (0.15–0.89) | 0.03 |
| Years taken MDA | |||||
| 0 | 115 | 6 | 5.22 | Reference | |
| 1 | 134 | 6 | 4.48 | 0.85 (0.27–2.72) | 0.79 |
| 2 | 183 | 3 | 1.64 | 0.3 (0.07–1.24) | 0.10 |
| 3 | 218 | 2 | 0.92 | 0.17 (0.03–0.85) | 0.03 |
| 4 | 256 | 7 | 2.73 | 0.51 (0.17–1.55) | 0.24 |
| 5 | 647 | 13 | 2.01 | 0.37 (0.14–1.00) | 0.05 |
| 6 | 299 | 6 | 2.01 | 0.37 (0.12–1.18) | 0.09 |
| 7 | 9 | 0 | 0.00 | – | – |
| Island of residence | |||||
| Tutuila | 1773 | 40 | 2.26 | Reference | |
| Ofu-Olosega | 53 | 2 | 3.77 | 1.7 (0.4–7.22) | 0.47 |
| Ta’u | 55 | 1 | 1.82 | 0.8 (0.11–5.94) | 0.83 |
Italicized type indicates significance for inclusion in the full multivariate model
Increasing age (OR 1.04 per year, 95% CI 1.02–1.05) remained significantly associated with ICT positivity in the final multivariate logistic regression model, while having ever taken part in an MDA round was protective (OR 0.39, 95% CI 0.16–0.96). Table 4 provides a summary of the final multivariate logistic regression model.
Table 4.
Variables significantly associated with immunochromatographic card test (ICT) positivity on multivariate logistic regression analysis in the 2007 lymphatic filariasis C-survey in American Samoa
| Variable | Adjusted odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age (1-year increments) | 1.04 | 1.02–1.05 | <0.001 |
| Ever taken MDA | 0.39 | 0.16–0.96 | 0.04 |
Adult filarial antigenemia—comparison between the 2007 and 2010 surveys
The adult filarial antigenemia was 3.32% (95% CI 2.44–4.51) by ICT in 2007 and 3.23% (95% CI 2.21–4.69) by Og4C3 in 2010. The overall adult filarial antigenemia prevalence remained relatively stable between the surveys.
Forty-four villages were included for village-level comparison, consisting of 23 individual villages and eight village groupings (of between two to four villages). Antigen-positive individuals were identified in both the 2007 and 2010 surveys in nine (29.0%) of the 31 villages/village groupings. Eight (25.8%) village/village groupings had antigen-positive individuals identified in 2007 but not in 2010, while three (9.6%) villages/village groupings that had no antigen-positive individuals identified in 2007 had positive individuals identified in 2010. Table 5 describes village-level filarial antigenemia in the 2007 and 2010 surveys.
Table 5.
Village-level adult filarial antigenemia prevalence in the 2007 and 2010 lymphatic filariasis surveys in American Samoa
| Village/village grouping | 2007 Tested |
2007 ICT positive |
2007 % positive |
95% CI | 2010 Tested |
Og4C3 Positive |
2010 % positive |
95% CI |
|---|---|---|---|---|---|---|---|---|
| Aasu Fou/Aoloau Fou | 22 | 1 | 4.55 | 0.12–22.84 | 35 | 1 | 2.86 | 0.07–4.92 |
| Afao/Amaluia/Asili | 7 | 0 | 0 | 0–40.96 | 50 | 2 | 4.00 | 0.49–13.71 |
| Agugulu/Nua/Seetaga | 29 | 0 | 0 | 0–11.94 | 28 | 0 | 0 | 0–12.34 |
| Alao | 13 | 0 | 0 | 0–24.71 | 19 | 0 | 0 | 0–17.65 |
| Alega/Amaua/Auto/Avaio | 21 | 0 | 0 | 0–16.11 | 23 | 2 | 8.70 | 1.07–28.04 |
| Amouli | 17 | 1 | 5.88 | 0.15–28.69 | 18 | 1 | 5.56 | 0.14–27.29 |
| Aoa | 7 | 0 | 0 | 0–40.96 | 15 | 0 | 0 | 0–21.80 |
| Aua | 39 | 3 | 7.69 | 1.62–20.87 | 22 | 1 | 4.55 | 0.12–22.84 |
| Auasi/Utumea East | 12 | 0 | 0 | 0–26.46 | 13 | 0 | 0 | 0–24.71 |
| Aumi/Laulii | 22 | 1 | 4.55 | 0.12–22.84 | 23 | 0 | 0 | 0–14.82 |
| Aunuu | 10 | 0 | 0 | 0–30.85 | 16 | 0 | 0 | 0–20.59 |
| Fagaalu | 22 | 3 | 13.64 | 2.91–34.91 | 17 | 0 | 0 | 0–19.51 |
| Fagaitua/Pagai | 13 | 1 | 7.69 | 0.19–36.03 | 19 | 0 | 0 | 0–17.65 |
| Fagali’i | 8 | 0 | 0 | 0–36.94 | 13 | 4 | 30.77 | 9.09–61.43 |
| Fagasa | 10 | 0 | 0 | 0–30.85 | 17 | 0 | 0 | 0–19.51 |
| Fagatogo | 27 | 0 | 0 | 0–12.77 | 21 | 0 | 0 | 0–16.11 |
| Faleasao/Fitiuta/Tau | 31 | 1 | 3.23 | 0.08–16.70 | 43 | 0 | 0 | 0–8.22 |
| Faleniu | 22 | 2 | 9.09 | 1.12–29.16 | 18 | 0 | 0 | 0–18.53 |
| Ili’ili | 39 | 1 | 2.56 | 0.06–13.48 | 23 | 4 | 17.39 | 4.95–38.78 |
| Leone | 89 | 1 | 1.12 | 0.03–6.10 | 20 | 0 | 0 | 0–16.84 |
| Malaeimi | 39 | 2 | 5.13 | 0.77–20.81 | 28 | 1 | 3.57 | 0.09–18.35 |
| Malaeloa | 17 | 1 | 5.88 | 0.15–28.69 | 30 | 3 | 10.00 | 2.11–26.53 |
| Mesepa | 16 | 0 | 0 | 0–20.59 | 7 | 0 | 0 | 0–40.96 |
| Ofu | 19 | 2 | 10.53 | 1.30–33.14 | 11 | 0 | 0 | 0–28.49 |
| Olosega | 10 | 0 | 0 | 0–30.85 | 14 | 0 | 0 | 0–23.16 |
| Pago Pago | 88 | 2 | 2.27 | 0.28–7.97 | 73 | 2 | 2.74 | 0.33–9.55 |
| Pavaiai | 70 | 2 | 2.86 | 0.35–9.94 | 16 | 1 | 6.25 | 0.16–30.23 |
| Tafuna | 135 | 3 | 2.22 | 0.46–6.36 | 21 | 0 | 0 | 0–16.11 |
| Taputimu | 10 | 0 | 0 | 0–30.85 | 14 | 0 | 0 | 0–23.16 |
| Tula | 7 | 0 | 0 | 0–40.96 | 26 | 0 | 0 | 0–13.23 |
| Vaitogi | 30 | 2 | 6.67 | 0.82–22.07 | 7 | 1 | 14.29 | 0.36–57.87 |
Italicized type indicates statistical significance at the .05 level
The largest discrepancies in proportions of antigen-positive adults between 2007 and 2010 were in the villages of Fagali’i (0 to 33.77%, P = 0.08), Ili’ili (2.56 to 17.39%, P = 0.04), Faga’alu (13.64 to 0%, P = 0.11) and Ofu (10.53 to 0%, P = 0.27). Figure 4 illustrates the changes in filarial antigenemia prevalence in villages/village groupings between 2007 and 2010.
Fig. 4.
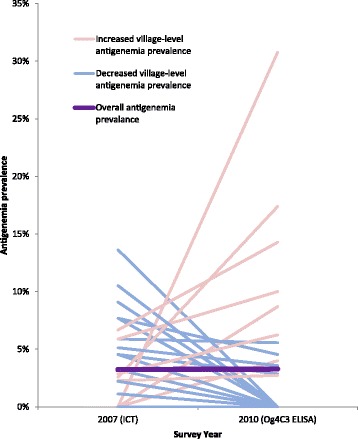
Comparison of overall and village-level adult filarial antigenemia prevalence between the 2007 and 2010 lymphatic filariasis surveys in American Samoa
Discussion
Since the commencement of PacELF in 1999, when a nationwide mapping survey established a baseline prevalence of 16.5% antigenemia, American Samoa has made significant progress toward the elimination of LF. A timeline of LF programme activities in American Samoa is presented in Table 6. Antigenemia declined to 2.29% in all ages by 2007 after four effective rounds of annual MDA; however, overall antigen prevalence in adults stayed relatively constant over the subsequent 3 years (3.32% in 2007 (ICT) and 3.23% in 2010 (Og4C3 antigen)). Despite remaining at an overall low level, village-based data from the two independent surveys suggest fluctuation in antigen prevalence among adults in some villages.
Table 6.
Timeline of lymphatic filariasis programme activities in American Samoa, 1999–2011
| Year | Activity | Survey population size | Age group | Prevalence (% antigenemia, ICT) | MDA coverage (% total population treated) |
|---|---|---|---|---|---|
| 1999 | Nationwide mapping (convenience survey) [3] | 3018 (18 villages) | All | 16.5 | |
| 2000 | MDA [9] | 19 | |||
| 2001 | MDA [9] | 51 | |||
| 2001 | Sentinel survey [10] | 1024 (4 villages) | All | 11.52 | |
| 2002 | MDA [9] | 49 | |||
| 2003 | Drug distributor evaluation Community KAP survey Programme modifications [9] |
||||
| 2003 | MDA [9] | 71 | |||
| 2003 | Drug distributor evaluation Church leader survey Programme modifications [9] |
||||
| 2003 | Sentinel survey [10] | 917 (4 villages) | All | 13.74 | |
| 2004 | MDA [9] | 65 | |||
| 2004 | Drug coverage survey [9] | 86 | |||
| 2005 | MDA [9] | 67 | |||
| 2006 | MDA [9] | 70 | |||
| 2006 | Sentinel survey [10] | 1371 (4 villages) | All | 0.95 | |
| 2006 | Purposeful survey [11] | 569 (3 villages) | >5 years | 4.22 | |
| 2007 | Random household C-survey (spatial sampling design) | 1881 (national) | All | 2.29 | |
| 2010 | Random household survey (spatial sampling design) [15] | 807 (national) | ≥18 years | 3.2 (Og4C3 antigen ELISA) | |
| 2011 | TAS [18] | 949 | 6–7 years | 0.21 |
ELISA enzyme-linked immunosorbent assay, ICT immunochromatographic card test, KAP knowledge, attitudes, practices, MDA mass drug administration, TAS transmission assessment survey
Both the 2007 and 2010 studies showed a broad distribution of a few infected individuals with some clustering within villages, demonstrating that significant geographic heterogeneity can exist even in such a small island setting. This widespread, low-level distribution has also been demonstrated in a 2011 molecular xenomonitoring study [16]. A subsequent study that compared results of the 2010 human seroprevalence study and the 2011 entomology study provided evidence for good concordance between results from serology and molecular xenomonitoring during post-MDA surveillance in American Samoa [17].
The 2007 survey results did not meet the PacELF criterion of filarial antigenemia <1% (upper CI <2%) for ceasing MDA. The PacELF technical working group recommended that American Samoa undertake an additional round of MDA in 2008; however, this was not accomplished because of limited resources. Even in the absence of additional MDA, American Samoa conducted and passed a TAS in 2011, with a school-based survey of 1st and 2nd graders on Tutuila (n = 949, approximating to the 6- to 7-year-old age group) identifying two ICT-positive individuals (0.21%), below the critical cut-off value of six individuals. The two ICT-positive individuals attended the same school on Tutuila, but lived in different villages [18]. A subsequent 2010 seroepidemiological study of adults (using samples originally collected for a leptospirosis study) found evidence of spatial clustering of antigen-positive individuals in two villages on Tutuila (Fagali’i and Ili’ili), suggesting possible residual foci of transmission post-MDA. One of the villages included the school where the two antigen-positive children were identified in the 2011 TAS. Analysis of the demographic data indicated that adult males and recent migrants were at higher risk of LF antigenemia and demonstrated antibody positivity. This association suggests adults may serve as the primary reservoirs for continued transmission, despite low prevalence in younger age groups [15].
TAS and the PacELF C-survey are not designed to provide village-level prevalence estimates, but rather estimates of mean prevalence across an evaluation unit of one or more districts, which was a territory-wide estimate in this island setting. The comparisons between surveys confirm that these cross-sectional survey methods may not always provide an accurate reflection of antigenemia at the smaller-scale village level as expected, given the limited number of persons selected per village. Foci of residual transmission may not be identified which could then serve as areas of potential resurgence. Discrepancies in filarial antigenemia prevalence within some villages between 2007 and 2010 may suggest that foci of local transmission have developed in some villages and declined in others over a relatively short time period. However, these discrepancies could also be explained by limitations in the sampling method, sample sizes and tests used for the comparison.
The findings should be considered in light of the study’s limitations. Although both surveys utilised a similar random spatial sampling method, the 2007 study included multiple adults from the majority of households, while the 2010 study only included one person from the majority of households. No adjustment for clustering within households (which would have widened the confidence intervals around the prevalence estimate) was made for the 2007 dataset, although at the observed level of prevalence it would be expected to have had negligible effect. A further limitation is that the 2007 survey used ICT, while in 2010, stored serum samples collected for a different survey were tested for Og4C3 antigen [15]. Both tests measure circulating filarial antigen, but comparison is hampered by lack of a true gold standard. A multi-country comparison of ICT and Og4C3 antigen in multiple field laboratories concluded that although positive concordance was unexpectedly low at individual level, there was no difference in the prevalence estimates given by the two tests at a population level [19]. More recent studies have shown that concordance between the two tests is much greater when using serum/plasma (as done in this study) rather than dried blood spots for Og4C3 antigen testing [20].
Conclusions
The variability in village-level antigenemia within the 2007 and 2010 surveys suggests that there remains a significant gap in knowledge regarding LF transmission dynamics at small spatial scales. Further research and a greater understanding of these transmission dynamics, their impact on LF elimination efforts and how to accurately assess significant residual infection may become increasingly important as more countries move into the post-MDA phase and continue to work toward elimination. This research should include further longitudinal studies in villages or areas in which increases or a lack of decline in prevalence has been observed.
In American Samoa specifically, programmatic priorities to support the elimination of LF should include ongoing investigation and surveillance of hotspots identified during surveys, targeted testing and treatment of known high-risk groups and the comparison of TAS results in children against prevalence in older age groups.
Acknowledgements
While this manuscript was in preparation, we lost our beloved colleague and friend, Ms. Molisamoa Pa’au. She was an adopted mother to us, a wonderful person and compassionate nurse who inspired respect. We also acknowledge the American Samoa Department of Health, Lyndon B. Johnson Tropical Medical Center, American Samoa Community College Department of Nursing, Patricia Brooks, John Chase, Troy Curry, Eric Ottesen, Mark Schmaedick, Kimberly Won and Peter Wood.
Funding
This publication was supported by a grant from the United States Agency for International Development (USAID) through NTD SC, a program of The Task Force for Global Health, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the views of The Task Force for Global Health, Inc., NTD SC or the USAID
Funding for the 2007 survey was provided by the US Centers for Disease Prevention and Control. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding for the 2010 serology was provided by James Cook University and Glaxo Smith Kline to the JCU WHO Collaborating Centre for Control of Lymphatic Filariasis, Soil-transmitted Helminths and other NTDs. CLL was supported by an Australian National Health and Medical Research Council (NHMRC) Fellowship (1109035). The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The 2007 and 2010 datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The authors confirm that for approved reasons, some access restrictions apply to the data underlying the findings. We are unable to provide individual-level serological and demographic data because of the potential for breaching participant confidentiality. The communities in American Samoa are very small, and individual-level data such as age, sex, occupation and village of residence could potentially be used to identify specific persons.
Entomological data used in this paper have previously been published and are available from http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0003087.
Results of the human seroprevalence and demographic data used in this study have also previously been published: http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0003297.
Additional results of the human data used in this study analysed in different ways have previously been published:
http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005108
Authors’ contributions
JDK and JR designed the 2007 survey. JDK, MP, SF and MRK collected the 2007 data. CLL and PMG provided the 2010 data and assisted with the methodology. SPC analysed and interpreted the data and drafted the manuscript. CLL assisted with the visualisation of results. JDK, PJL, CLL and PMG critically reviewed and contributed to the final manuscript. All authors read and approved the final manuscript.
Author’s information
Not applicable.
Ethics approval and consent to participate
The 2007 survey was conducted under routine monitoring and evaluation activities for the LF programme of the American Samoa Department of Health. This study was reviewed by the CDC/OPHPR human subjects official, and it was determined to be public health research not involving human subjects.
For the original 2010 leptospirosis study, ethics approvals were obtained from the American Samoa Institutional Review Board, the Medical Research Ethics Committee of the University of Queensland (2010000114, approved 25 February 2010, amended 16 October 2012 to allow LF testing) and Queensland Health Forensic and Scientific Services Human Ethics Committee (HREC/10/QFSS/1). Permission was also sought from the Department of Samoan Affairs and village chiefs before village visits.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shaun P. Coutts, Email: shaun.coutts@my.jcu.edu.au
Jonathan D. King, Email: kingj@who.int
Saipale Fuimaono, Email: sfuima6@yahoo.com.
Joseph Roth, Email: jroth@cdc.gov.
Mary Rose King, Email: andres_mrc@yahoo.com.
Patrick J. Lammie, Email: plammie@taskforce.org
Colleen L. Lau, Email: colleen.lau@anu.edu.au
Patricia M. Graves, Email: patricia.graves@jcu.edu.au
References
- 1.Heymann DL, American Public Health Association . Control of Communicable Diseases Manual. Washington, DC: American Public Health Association; 2004. [Google Scholar]
- 2.Ichimori K, King JD, Engels D, Yajima A, Mikhailov A, Lammie P, Ottesen EA. Global programme to eliminate lymphatic filariasis: the processes underlying programme success. PLoS Negl Trop Dis. 2014;8:e3328. doi: 10.1371/journal.pntd.0003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Regional Office for the Western Pacific . The PacELF way: towards the elimination of lymphatic filariasis from the Pacific, 1999-2005. Manila: WHO Regional Office for the Western Pacific; 2006. [Google Scholar]
- 4.World Health Organization. Lymphatic filariasis: monitoring and epidemiological assessment of mass drug administration. In: Ichimori K, editor. A manual for national elimination programmes. Geneva: World Health Organization; 2011. p. 1–78.
- 5.Lambdin BH, Schmaedick MA, McClintock S, Roberts J, Gurr NE, Marcos K, Waller L, Burkot TR. Dry season production of filariasis and dengue vectors in American Samoa and comparison with wet season production. Am J Trop Med Hyg. 2009;81:1013–1019. doi: 10.4269/ajtmh.2009.09-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramalingam S, Belkin JN. Vectors of sub-periodic Bancroftian filariasis in the Samoa-Tonga area. Nature. 1964;201:105–106. doi: 10.1038/201105b0. [DOI] [PubMed] [Google Scholar]
- 7.Ichimori K. Entomology of the filariasis control programme in Samoa, Aedes polynesiensis and Ae. samoanus. Med Entomol Zool. 2001;52:11–21. doi: 10.7601/mez.52.11_1. [DOI] [Google Scholar]
- 8.Pacific Programme to Eliminate Lymphatic Filariasis, Country Programmes, American Samoa, National activities carried out to date [http://www.wpro.who.int/southpacific/pacelf/countries/ams/activities/en/]. Accessed 22 June 2016.
- 9.King JD, Zielinski-Gutierrez E, Pa'au M, Lammie P. Improving community participation to eliminate lymphatic filariasis in American Samoa. Acta Trop. 2011;120:S48–S54. doi: 10.1016/j.actatropica.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Liang JL, King JD, Ichimori K, Handzel T, Pa'au M, Lammie PJ. Impact of five annual rounds of mass drug administration with diethylcarbamazine and albendazole on Wuchereria bancrofti infection in American Samoa. Am J Trop Med Hyg. 2008;78:924–928. [PubMed] [Google Scholar]
- 11.Mladonicky JM, King JD, Liang JL, Chambers E, Pa'au M, Schmaedick MA, Burkot TR, Bradley M, Lammie PJ. Assessing transmission of lymphatic filariasis using parasitologic, serologic, and entomologic tools after mass drug administration in American Samoa. Am J Trop Med Hyg. 2009;80:769–773. [PubMed] [Google Scholar]
- 12.World Health Organization . Bench aids for the diagnosis of filarial infections. Geneva: World Health Organization; 1997. [Google Scholar]
- 13.Lau CL, Clements ACA, Skelly C, Dobson AJ, Smythe LD, Weinstein P. Leptospirosis in American Samoa—estimating and mapping risk using environmental data. PLoS Negl Trop Dis. 2012;6:e1669. doi: 10.1371/journal.pntd.0001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau CL, Dobson AJ, Smythe LD, Fearnley EJ, Skelly C, Clements AC, Craig SB, Fuimaono SD, Weinstein P. Leptospirosis in American Samoa 2010: epidemiology, environmental drivers, and the management of emergence. Am J Trop Med Hyg. 2012;86:309–319. doi: 10.4269/ajtmh.2012.11-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau CL, Won KY, Becker L, Soares Magalhaes RJ, Fuimaono S, Melrose W, Lammie PJ, Graves PM. Seroprevalence and spatial epidemiology of lymphatic filariasis in American Samoa after successful mass drug administration. PLoS Negl Trop Dis. 2014;8:e3297. doi: 10.1371/journal.pntd.0003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmaedick MA, Koppel AL, Pilotte N, Torres M, Williams SA, Dobson SL, Lammie PJ, Won KY. Molecular xenomonitoring using mosquitoes to map lymphatic filariasis after mass drug administration in American Samoa. PLoS Negl Trop Dis. 2014;8:e3087. doi: 10.1371/journal.pntd.0003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau CL, Won KY, Lammie PJ, Graves PM. Lymphatic filariasis elimination in American Samoa: evaluation of molecular xenomonitoring as a surveillance tool in the endgame. PLoS Negl Trop Dis. 2016;10:e0005108. doi: 10.1371/journal.pntd.0005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu BK, Deming M, Biritwum NK, Bougma WR, Dorkenoo AM, El-Setouhy M, Fischer PU, Gass K, Gonzalez de Pena M, Mercado-Hernandez L, et al. Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl Trop Dis. 2013;7:e2584. doi: 10.1371/journal.pntd.0002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gass K, de Rochars MVE B, Boakye D, Bradley M, Fischer PU, Gyapong J, Itoh M, Ituaso-Conway N, Joseph H, Kyelem D, et al. A multicenter evaluation of diagnostic tools to define endpoints for programs to eliminate Bancroftian Filariasis. PLoS Negl Trop Dis. 2012;6:e1479. doi: 10.1371/journal.pntd.0001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masson J, Douglass J, Roineau M, Aye K, Htwe K, Warner J, Graves P. Relative performance and predictive values of plasma and dried blood spots with filter paper sampling techniques and dilutions of the lymphatic filariasis Og4C3 antigen ELISA for samples from Myanmar. Trop Med Infect Dis. 2017;2:7. doi: 10.3390/tropicalmed2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The 2007 and 2010 datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. The authors confirm that for approved reasons, some access restrictions apply to the data underlying the findings. We are unable to provide individual-level serological and demographic data because of the potential for breaching participant confidentiality. The communities in American Samoa are very small, and individual-level data such as age, sex, occupation and village of residence could potentially be used to identify specific persons.
Entomological data used in this paper have previously been published and are available from http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0003087.
Results of the human seroprevalence and demographic data used in this study have also previously been published: http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0003297.
Additional results of the human data used in this study analysed in different ways have previously been published:
http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005108


