Abstract
The outcomes of cord blood transplantation with non‐irradiated reduced‐intensity conditioning for hematological malignancies need to be improved because of graft failure and delayed engraftment. Intrabone infusion of cord blood cells has the potential to resolve the problems. In this phase II study, 21 adult patients with hematological malignancy received intrabone transplantation of serological HLA‐A, B, and DR ≥4/6 matched single cord blood with a median number of cryopreserved total nucleated cells of 2.7 × 107/kg (range, 2.0–4.9 × 107/kg) following non‐irradiated fludarabine‐based reduced‐intensity conditioning. Short‐term methotrexate and tacrolimus were given as graft‐versus‐host disease prophylaxis, and granulocyte colony‐stimulating factor was given after transplantation. No severe adverse events related to intrabone injection were observed. The cumulative incidences of neutrophils ≥0.5 × 109/L, reticulocytes ≥1%, and platelets ≥20 × 109/L recoveries were 76.2%, 71.4%, and 76.2%, respectively, with median time to recoveries of 17, 28, and 32 days after transplantation, respectively. The probability of survival with neutrophil engraftment on day 60 was 71.4%, and overall survival at 1 year after transplantation was 52.4%. The incidences of grade II–IV and III–IV acute graft‐versus‐host disease were 44% and 19%, respectively, with no cases of chronic graft‐versus‐host disease. The present study showed the safety of direct intrabone infusion of cord blood. Further analysis is required to confirm the efficacy of intrabone single cord blood transplantation with non‐irradiated reduced‐intensity conditioning for adult patients with hematological malignancy. This study was registered with UMIN‐CTR, number 000000865.
Keywords: Cord blood transplantation, engraftment, hematological malignancy, intrabone, non‐myeloablative conditioning
Cord blood transplantation is a treatment option for patients with hematological malignancy.1 The comparison of outcomes of CBT and HLA‐A, B, C and DRB1 allele‐matched unrelated bone marrow transplantation for adult patients with acute leukemia showed no significant difference in overall survival between these groups.2 Additionally, the recent development of RIC allows elderly patients and patients with comorbidities to benefit from CBT.3 However, CBT with RIC, especially a non‐irradiated conditioning regimen, has yet to be optimized, due in part to graft failure or delayed engraftment.4, 5, 6, 7
In terms of successful engraftment, higher cell dose and better HLA‐matching have an advantage.8 Donor‐specific anti‐HLA antibodies should also be considered.9 Double‐unit CBT was established in the early 2000s.10 However, a recent prospective randomized study comparing single‐unit and double‐unit CBT after myeloablative conditioning for children and adolescents indicated that neutrophil recoveries were similar, and double‐unit CBT was associated with lower and slower platelet recovery and a higher incidence of GVHD.11 Expansion of cord blood cells ex vivo before transplantation has been developed,12, 13 and further clinical studies to confirm efficacy for reduction of transplant‐related mortality are in progress. However, these strategies require specialized techniques, drugs, or cells, and cannot be adopted everywhere. Direct intrabone transplantation of cord blood cells, established by Frassoni et al.,14 potentially ensures engraftment and shortens the time for hematological recovery in single CBT. Neither special devices nor particular skills are required for this technique. Fortunately, no severe intrabone injection‐related complications have been reported so far.
Based on these data, a phase II study was undertaken to assess the efficacy of intrabone transplantation of a single cord blood unit following non‐irradiated RIC for adult patients with hematological malignancy.
Materials and Methods
Patients
Patients were eligible for the study if they had hematological malignancy, needed CBT, were ≥55 years or 16–54 years old with a hematopoietic stem cell transplantation‐specific comorbidity index ≥1,15 and had available cord blood with serological HLA‐A, B, and DR ≥4/6 matched and with cryopreserved TNCs at least 2 × 107/kg. Cord blood was obtained from the Japanese Red Cross Cord Blood Banks (Hokkaido, Kanto‐Koshinetsu, Kinki, and Kyushu), Chubu Cord Blood Bank, Hyogo Cord Blood Bank, and Japan Cord Blood Bank Network (defunct) in Japan. This study was approved by the ethics committees of the following institutes: Nagoya University, Okayama University, Niigata University, Tohoku University, and Hokkaido University (all Japan). Patients were enrolled at these institutes between September 2007 and July 2015.
Transplantation
All patients received non‐irradiated fludarabine‐based RIC on the basis of patient characteristics and pretransplant therapies. All patients received tacrolimus (initial dose 0.02–0.03 mg/kg) and short‐term methotrexate (15 mg/m2 on day 1, and 10 mg/m2 on days 3 and 6; or 10 mg/m2 on day 1, and 7 mg/m2 on days 3 and 6) as GVHD prophylaxis. All patients received granulocyte colony‐stimulating factor starting from 5–7 days after transplantation until neutrophil engraftment.
Intrabone injection
Intrabone injection was carried out as described previously.14 Cord blood was thawed, washed with a saline solution plus dextran and human albumin,16 resuspended in approximately 10 mL of the solution, and aliquoted in two to four syringes. After local anesthesia, standard bone marrow aspiration needles were inserted into iliac bone. Aspiration of <0.5 mL bone marrow was done to assess that the needle was securely inserted into the bone marrow cavity. Then approximately 5 mL cord blood cell suspension was gently infused. This procedure was repeated for all remaining aliquots across the iliac crest.
Definitions
Neutrophil engraftment was defined as neutrophil recovery ≥0.5 × 109/L before day 60 with donor chimerism ≥90%. Donor chimerism was determined by quantitative PCR for informative short tandem repeats using DNA extracted from peripheral blood T cells.17 The time to neutrophil recovery was defined as the first of 3 consecutive days of absolute neutrophil count ≥0.5 × 109/L. The time to reticulocyte and platelet recoveries was defined as the first of 3 consecutive days of reticulocytes ≥1%, platelet count ≥20 × 109/L, and platelet count ≥50 × 109/L without transfusion support. Acute GVHD and chronic GVHD were diagnosed and graded according to established criteria.18, 19
Statistical analysis
The primary end‐point was the probability of survival with neutrophil engraftment on day 60 after transplantation. Twenty‐one patients were required to provide at least 80% power to differentiate the primary end‐point of 80% with the width of the 95% CI being 25%. Assuming a drop‐out rate of one patient, 22 patients were required. The probabilities of hematopoietic recovery and relapse were estimated on the basis of cumulative incidence curves. Overall survival after transplantation was estimated according to the Kaplan–Meier method. The groups were compared using the log–rank test. All tests were two‐sided, and P < 0.05 was considered significant. The data were analyzed by stata statistical software (StataCorp, College Station, TX, USA).
Results
Patient and transplantation characteristics
Twenty‐two patients with hematological malignancy were enrolled in this study, but 1 patient did not receive intrabone CBT due to general deterioration after enrollment. The characteristics of 21 patients who received intrabone CBT are summarized in Table 1. The median age was 57 years (range, 38–66 years). Fourteen patients (66%) had leukemia (six in first complete remission, three in third complete remission, and five in non‐complete remission), six (29%) had malignant lymphoma (two in first complete remission, one in second complete remission, and two in non‐complete remission), and one (5%) had myelodysplastic syndromes in refractory anemia with excess blasts‐1. The median number of cryopreserved TNCs in a cord blood unit was 2.7 × 107/kg (range, 2.0–4.9 × 107/kg), and the median number of cryopreserved CD34+ cells in it was 0.92 × 105/kg (range, 0.44–3.14 × 105/kg). Serological HLA‐A, B, and DR 6/6, 5/6, and 4/6 matched cord blood in the host‐versus‐graft direction were used for two (10%), six (29%), and 13 (61%) patients, respectively. In one patient (no. 1), HLA‐antibody against cord blood HLA was detected. All patients received fludarabine 150–180 mg/m2 (150 mg/m2 for one patient and 180 mg/m2 for 19 patients) and cyclophosphamide 60–120 mg/kg (60 mg/kg for one patient and 120 mg/kg for 19 patients), with the exception of one patient who received fludarabine 125 mg/m2 and melphalan 140 mg/m2. No patient received TBI.
Table 1.
Characteristics of patients with hematological malignancies and outcomes of intrabone single unit cord blood transplant
| Pt no. | Age, years | Diagnosis | Disease stage | Sex Pt/Do | ABO Pt/Do | DSA | Preconditioning | Cord blood | Time to recovery, days | Acute GVHD, grade | Chronic GVHD | Status at day 365 | Cause of death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC, × 107/kg | CD34+ cells, × 105/kg | HLA‐m | N | R | P2 | P5 | |||||||||||||
| GVH | HVG | ||||||||||||||||||
| 1 | 53 | AML | Non‐CR | Ma/Ma | O/AB | Pos | F (125) + M (140) | 2.7 | 0.69 | 4/6 | 4/6 | NE | NE | NE | NE | NE | NE | Dead (72)† | Graft failure |
| 2 | 38 | AML | 3CR | Fe/Ma | B/O | Neg | F (180) + C (120) | 4.9 | 3.14 | 4/6 | 4/6 | 15 | 34 | 32 | 34 | II | No | Dead (244) | VAHS |
| 3 | 56 | ATLL | Non‐CR | Fe/Fe | B/O | NA | F (180) + C (120) | 2.2 | 0.92 | 4/6 | 5/6 | 19 | 41 | 46 | 77 | 0 | No | Dead (168) | RMF |
| 4 | 62 | AML | Non‐CR | Fe/Fe | A/B | Neg | F (180) + C (120) | 2.3 | 0.83 | 5/6 | 4/6 | 18 | 27 | 25 | 41 | 0 | No | Alive | |
| 5 | 59 | AML | Non‐CR | Ma/Ma | A/A | Neg | F (180) + C (120) | 2.4 | 0.70 | 6/6 | 6/6 | 17 | 29 | 29 | 38 | 0 | No | Alive | |
| 6 | 55 | DLBCL | Non‐CR | Ma/Fe | O/O | NA | F (180) + C (120) | 3.4 | 0.75 | 6/6 | 6/6 | 18 | 30 | 84 | 112 | 0 | No | Dead (311) | Relapse (170)‡ |
| 7 | 59 | NK/T Leu | 1CR | Ma/Fe | AB/O | Neg | F (180) + C (120) | 2.9 | 0.95 | 5/6 | 5/6 | 16 | 28 | 32 | 32 | III | No | Dead (159) | Relapse (78) |
| 8 | 64 | NK/T Lym | 1CR | Ma/Fe | AB/A | Neg | F (180) + C (120) | 3.1 | 1.29 | 4/6 | 5/6 | 16 | 29 | 23 | 31 | IV | No | Dead (95) | Relapse (76) |
| 9 | 56 | Ph+ ALL | 1CR | Ma/Ma | B/B | Neg | F (180) + C (120) | 2.8 | 2.05 | 4/6 | 4/6 | 14 | 17 | 26 | 28 | II | No | Alive | |
| 10 | 60 | T‐LBL | 1CR | Ma/Fe | A/A | Neg | F (180) + C (120) | 2.4 | 0.73 | 4/6 | 4/6 | 16 | 26 | 26 | 34 | II | No | Alive | |
| 11 | 51 | ATLL | 1CR | Ma/Fe | B/O | NA | F (180) + C (120) | 2.9 | 0.96 | 5/6 | 5/6 | 19 | 28 | 26 | 35 | 0 | No | Alive | |
| 12 | 61 | ALL | 1CR | Fe/Fe | AB/A | Neg | F (180) + C (120) | 3.7 | 0.63 | 5/6 | 4/6 | 19 | 28 | 40 | 58 | I | No | Alive | |
| 13 | 55 | AML | 3CR | Ma/Ma | AB/A | Neg | F (180) + C (120) | 3.8 | 1.35 | 6/6 | 5/6 | 14 | 26 | 38 | 38 | I | No | Alive | |
| 14 | 66 | MDS | RAEB1 | Ma/Ma | O/O | Neg | F (180) + C (120) | 4.8 | 2.60 | 4/6 | 4/6 | 17 | 25 | 30 | 36 | III | No | Dead (79) | PML |
| 15 | 41 | NK/T Lym | 2CR | Fe/Ma | O/A | Neg | F (180) + C (120) | 2.0 | 1.10 | 4/6 | 4/6 | NE | NE | NE | NE | NE | NE | Alive | |
| 16 | 60 | AML | Non‐CR | Ma/Fe | O/B | Neg | F (180) + C (120) | 2.6 | 0.54 | 4/6 | 4/6 | 18 | NE | 36 | NE | 0 | NE | Dead (42) | VOD |
| 17 | 63 | DLBCL | Non‐CR | Ma/Fe | O/AB | Neg | F (180) + C (120) | 2.9 | 0.51 | 4/6 | 4/6 | 24 | 33 | 42 | 47 | II | No | Dead (186) | Relapse (111) |
| 18 | 47 | AML | 3CR | Ma/Fe | B/A | Neg | F (180) + C (120) | 2.3 | 0.44 | 5/6 | 4/6 | 25 | 32 | 53 | 64 | I | No | Alive | |
| 19 | 60 | Ph+ ALL | 1CR | Fe/Ma | AB/O | Neg | F (180) + C (120) | 2.5 | 1.60 | 4/6 | 4/6 | NE | NE | NE | NE | NE | NE | Alive | |
| 20 | 57 | T‐LBL | Non‐CR | Ma/Fe | O/A | Neg | F (150) + C (120) | 2.1 | 0.72 | 4/6 | 5/6 | NE | NE | NE | NE | NE | NE | Dead (249) | Relapse (169) |
| 21 | 50 | ALL | 1CR | Ma/Fe | A/A | Neg | F (180) + C (60) | 2.2 | 1.02 | 4/6 | 4/6 | NE | NE | NE | NE | NE | NE | Alive | |
†Number in parentheses after “dead” indicates survival days after transplantation.‡Number in parentheses after “relapse” indicates the day when the patient had a relapse. 1CR, first CR; 2CR, second CR; 3CR, third CR; ABO, ABO blood type; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATLL, adult T‐cell leukemia/lymphoma; C, cyclophosphamide (mg/kg); CR, complete remission; DLBCL, diffuse large B‐cell lymphoma; Do, donor; DSA, donor HLA‐specific antigen; F, fludarabine (mg/m2); Fe, female; GVH, graft‐versus‐host direction; GVHD, graft‐versus‐host disease; HLA‐m, human leukocyte antigen‐matching; HVG, host‐versus‐graft direction; M, melphalan (mg/m2); Ma, male; MDS, myelodysplastic syndromes; N, neutrophils ≥0.5 × 109/L; NA, not available; NE, not evaluable; Neg, negative; NK/T Leu, natural killer/T‐cell leukemia; NK/T Lym, extranodal natural killer/T‐cell lymphoma, nasal type; P2, platelets ≥20 × 109/L; P5, platelets ≥50 × 109/L; Ph+, Philadelphia chromosome‐positive; PML, progressive multifocal leukoencephalopathy; Pos, positive; Pt, patient; R, reticulocytes ≥1%; RAEB1, refractory anemia with excess blasts‐1; RMF, remission failure; T‐LBL, T lymphoblastic lymphoma; TNC, total nuclear cells; VAHS, virus‐associated hemophagocytic syndrome; VOD, veno‐occlusive disease.
Intrabone injection
Intrabone injection of cord blood was carried out with local anesthesia in the patient's room. No patient required i.v. sedative drugs, such as propofol. No severe adverse events were observed. Mild swelling of the skin at the injection site was observed in one patient, but it resolved spontaneously.
Hematopoietic recovery
Patient no. 1 showed no signs of any hematopoietic recovery and received transplantation with other cord blood on day 42, but died of multiorgan failure without a sign of engraftment of the second cord blood. Four patients (nos. 15, 19, 20, and 21) showed autologous hematopoietic recovery. Patient no. 20 received 150 mg/m2 fludarabine and 120 mg/kg cyclophosphamide as preconditioning, and patient no. 21 received 180 mg/m2 fludarabine and 60 mg/kg cyclophosphamide, whereas patients nos. 15 and 19 received 180 mg/m2 fludarabine and 120 mg/kg cyclophosphamide, which was the preconditioning used in patients with successful engraftment (Table 1). Donor HLA‐specific antigen was not detected in these four patients. Sex mismatch in host‐versus‐graft direction (combination of female patient and male donor) was not associated with a higher incidence of autologous hematopoietic recovery. The cumulative incidences of neutrophils ≥0.5 × 109/L on day 60, reticulocytes ≥1% on day 60, platelets ≥20 × 109/L on day 100, and platelets ≥50 × 109/L on day 100 recoveries were 76.2% (95% CI, 56.9–91.3%), 71.4% (51.8–88.3%), 76.2% (56.9–91.3%), and 66.7% (46.9–85.1%), respectively (Fig. 1). For those who achieved hematopoietic recovery, the median time to neutrophils, reticulocytes, platelets ≥20 × 109/L, and platelets ≥50 × 109/L recoveries were 17, 28, 32, and 38 days, respectively (Table 1). ABO major and/or minor mismatch did not affect reticulocyte recovery.
Figure 1.
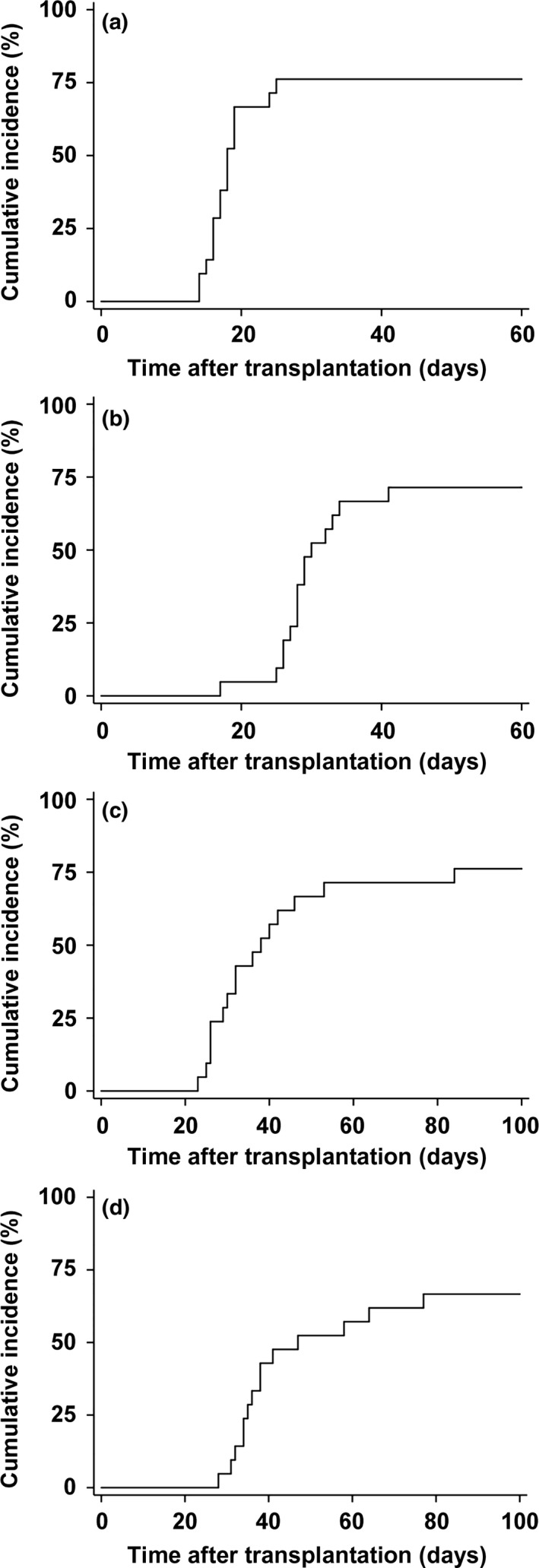
Hematopoietic recoveries after intrabone single cord blood transplantation. Cumulative incidences of neutrophil recovery ≥0.5×109/L (a), reticulocyte recovery ≥1% (b), platelet recovery ≥20×109/L (c), and platelet recovery ≥50×109/L (d) after cord blood transplantation with non‐irradiated reduced‐intensity conditioning are shown.
Neutrophil recovery was evaluated according to the numbers of TNCs and CD34+ cells, and HLA matching in the host‐versus‐graft direction (Table 2). There was a significant difference in the incidence of neutrophil recovery between patients receiving ≤2.7 × 107/kg TNCs (n = 11) and >2.7 × 107/kg TNCs (n = 10) (54.5% vs 100%, P = 0.0046).
Table 2.
Neutrophil recovery according to the numbers of total nucleated cells and CD34+ cells, and HLA matching in the host‐versus‐graft (HVG) direction
| Neutrophil recovery, % | P‐value | |
|---|---|---|
| Total nucleated cells | ||
| ≤2.7 × 107/kg (n = 11) | 54.5 | 0.0046 |
| >2.7 × 107/kg (n = 10) | 100 | |
| CD34+ cells | ||
| ≤0.92×105/kg (n = 11) | 81.8 | 0.6850 |
| >0.92×105/kg (n = 10) | 70.0 | |
| HLA‐matching in HVG direction | ||
| 4/6 (n = 13) | 69.2 | 0.2720 |
| 5/6 or 6/6 (n = 8) | 87.5 | |
Other outcomes
Other outcomes are summarized in Table 1. Of 16 patients who showed neutrophil recovery, seven (43.8%) and three (18.8%) developed grade II–IV and III–IV acute GVHD, respectively. No patients developed chronic GVHD. Thirteen (81.3%) patients developed cytomegalovirus antigenemia within 1 year of transplantation (data not shown). The probability of relapse was 75.0% (95% CI, 12.5–91.2%). Patient no. 16 obtained neutrophil recovery with full donor chimera, but died of veno‐occlusive disease on day 42. The probability of survival with neutrophil engraftment on day 60 after transplantation was 71.4% (95% CI, 51.8–88.3%). Overall survival at 1 year after transplantation was 52.4% (95% CI, 29.7–70.9%). Six patients died of their original hematological malignancies. Four other patients died of graft failure, virus‐associated hemophagocytic syndrome, leukoencephalopathy, and veno‐occlusive disease, respectively. Patient no.16, who developed veno‐occlusive disease, had advanced disease (acute myeloid leukemia with 33% blasts in the bone marrow just prior to preconditioning) and iron overload (red blood cell transfusion ≥30 times before transplantation, with approximately 1000 ng/mL ferritin) as risk factors for this condition.
Discussion
The present study indicated the feasibility of intrabone single CBT with non‐TBI RIC regimens for adult patients with hematological malignancy. Since Rizzieri et al.20 reported successful engraftment in two cases, several studies have confirmed the feasibility of CBT with TBI‐containing RIC. Barker et al.21 reported that the cumulative incidence of neutrophil recovery was 76% for adult patients with hematological malignancy preconditioned with fludarabine + busulfan + TBI 2Gy and 94% for adult patients with fludarabine + cyclophosphamide + TBI 2Gy. Miyakoshi et al.22 reported that the cumulative incidence of neutrophil recovery was 93% for adult patients with advanced hematological malignancy preconditioned with fludarabine + melphalan + TBI 4 Gy. The most recently published paper from the Japanese Society for Hematopoietic Cell Transplantation reported that TBI regimens were significantly associated with a higher rate of neutrophil engraftment in CBT with RIC.23 However, non‐TBI RIC regimens in CBT often result in dismal outcomes. In a study from Duke University (Durham, NC, USA), in which 10 patients received fludarabine and cyclophosphamide without TBI followed by single CBT, only three (30%) engrafted with donor hematopoietic cells.4 Another study from Duke University, in which 10 patients received fludarabine and busulfan without TBI followed by double CBT, showed that only two (20%) achieved donor engraftment.6 A study from Japan, in which fludarabine and busulfan were used as the preconditioning for single CBT, reported a cumulative incidence of engraftment on day 60 of 53%.5 Given this result, we regarded as clinically relevant if at least 55% of patients achieved a primary end‐point. We also reported that only four (40%) of 10 patients were alive with donor engraftment on day 60 after preconditioning consisting of fludarabine + melphalan.7 In the present study, the cumulative incidence of neutrophil recovery was 76.2%, suggesting that intrabone transplantation can be an option to improve engraftment in CBT with non‐TBI RIC regimens.
Nonetheless, it is not clear whether direct intrabone infusion of cord blood can overcome engraftment failure in CBT. Frassoni et al.14 undertook a phase I/II study in which 32 adult patients were treated by intrabone single CBT, mostly with myeloablative preconditioning, and the cumulative incidence of neutrophil recovery on day 44 was 85%. Okada et al.24 conducted a phase I study in which 10 adult patients were treated by intrabone transplantation of unwashed cord blood with TBI‐containing RIC, and nine (90%) patients successfully achieved neutrophil recovery. These results are better than previous studies of i.v. CBT for adult patients,23, 25 but they may be inconsistent with animal experiments showing that intrabone transplantation is 10–15 times more efficient in the engraftment of cord blood cells than i.v. transplantation.26 In fact, a retrospective analysis comparing outcomes of intrabone single CBT with i.v. double CBT after myeloablative preconditioning did not show a significant difference in the incidence of neutrophil recovery on day 60 (84% vs 91%, P = 0.62).27 Interestingly, the present study showed that there was a correlation between the number of TNCs and the engraftment rate, suggesting that the success or failure of neutrophil engraftment after intrabone CBT might also depend on the number of TNCs. Further study is required to determine whether intrabone injection truly improves the engraftment rate in CBT for adult patients.
In the present study, four patients showed autologous hematopoietic recovery. Two patients (nos. 20 and 21) received less intensive preconditioning than that used in patients with successful engraftment. This could be one of the causes of their autologous hematopoietic recovery. However, more importantly, another two patients (nos. 15 and 19) received the same preconditioning as patients with successful engraftment. Thus, the preconditioning regimen in intrabone CBT needs to be much optimized, and it seems interesting to consider whether the combination of intrabone infusion with more intensive non‐TBI or TBI‐containing regimens could result in much better engraftment rates in CBT.
The fact common to the present study and previous reports,24, 28, 29 except for one,30 is rapid engraftment in intrabone CBT. The median time to neutrophil recovery was 17–23 days, and platelet recovery was 32–41 days, which are much faster than i.v. CBT.23, 25 A retrospective comparative study showed that neutrophil recovery was significantly faster in intrabone single CBT than in i.v. double CBT (23 vs 28 days, P = 0.001).27 This was the same for platelet recovery (36 vs 49 days, P = 0.003). Rapid engraftment may be the greatest benefit of intrabone transplantation because it may contribute to reducing infection, use of antibacterial drugs, blood transfusion, and medical costs.
Although a low incidence of acute GVHD may be a feature of intrabone transplantation,27, 29 the present study demonstrated incidences of grade II–IV and III–IV acute GVHD of 43.8% and 18.8%, respectively, which are comparable to those in i.v. CBT for Japanese patients.31 Although a low incidence of relapse after intrabone transplantation is reported,27 the present study showed that the probability of relapse was 75.0%, which may be due to inclusion of many cases with advanced or high‐risk hematological malignancy, or it may be due to weakness of conditioning regimens.
In conclusion, intrabone transplantation of a single cord blood unit using a non‐TBI RIC regimen provides an opportunity for CBT to patients with hematological malignancy who are unable to be exposed to irradiation at the time of preconditioning for several reasons, such as preservation of fertility, second transplantation following first transplantation with high‐dose TBI, and lack of availability of TBI equipment in the hospital. Even in hospitals where TBI is available, if a non‐TBI regimen is selectable, a transplant schedule can be made at the best time for patients, without dependence on the radiation schedule. In addition, non‐TBI RIC may be especially useful for patients receiving urgent CBT for engraftment failure. Prospective randomized studies with homogeneous preconditioning and GVHD prophylaxis regimens are required to determine whether intrabone CBT provides definitively better outcomes for patients with hematological malignancy than i.v. CBT.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- CBT
cord blood transplantation
- CI
confidence interval
- GVHD
graft‐versus‐host disease
- RIC
reduced‐intensity conditioning
- TBI
total body irradiation
- TNC
total nuclear cell
Acknowledgments
The authors would like to thank the medical staff at each transplantation center. This study was supported in part by a Practical Research Project for Allergic Diseases and Immunology (15ek0510010h0003 to M. Murata) from the Japan Agency for Medical Research and Development and a Grant‐in‐Aid for Scientific Research (KAKENHI 15K09498 to M. Murata) from the Japan Society for the Promotion of Science.
Cancer Sci 108 (2017) 1634–1639
Funding information
Practical Research Project for Allergic Diseases and Immunology (15ek0510010h0003); Japan Agency for Medical Research and Development and a Grant‐in‐Aid for Scientific Research (KAKENHI 15K09498); Japan Society for the Promotion of Science.
References
- 1. Utsunomiya A, Choi I, Chihara D, Seto M. Recent advances in the treatment of adult T‐cell leukemia‐lymphomas. Cancer Sci 2015; 106: 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Terakura S, Atsuta Y, Tsukada N et al Comparison of outcomes of 8/8 and 7/8 allele‐matched unrelated bone marrow transplantation and single‐unit cord blood transplantation in adults with acute leukemia. Biol Blood Marrow Transplant 2016; 22: 330–8. [DOI] [PubMed] [Google Scholar]
- 3. Cutler C, Ballen K. Reduced‐intensity conditioning and umbilical cord blood transplantation in adults. Bone Marrow Transplant 2009; 44: 667–71. [DOI] [PubMed] [Google Scholar]
- 4. Chao NJ, Koh LP, Long GD et al Adult recipients of umbilical cord blood transplants after nonmyeloablative preparative regimens. Biol Blood Marrow Transplant 2004; 10: 569–75. [DOI] [PubMed] [Google Scholar]
- 5. Komatsu T, Narimatsu H, Yoshimi A et al Successful engraftment of mismatched unrelated cord blood transplantation following reduced intensity preparative regimen using fludarabine and busulfan. Ann Hematol 2007; 86: 49–54. [DOI] [PubMed] [Google Scholar]
- 6. Horwitz ME, Morris A, Gasparetto C et al Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transplant 2008; 14: 591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narimatsu H, Watanabe M, Kohno A et al High incidence of graft failure in unrelated cord blood transplantation using a reduced‐intensity preparative regimen consisting of fludarabine and melphalan. Bone Marrow Transplant 2008; 41: 753–6. [DOI] [PubMed] [Google Scholar]
- 8. Terakura S, Azuma E, Murata M et al Hematopoietic engraftment in recipients of unrelated donor umbilical cord blood is affected by the CD34+ and CD8+ cell doses. Biol Blood Marrow Transplant 2007; 13: 822–30. [DOI] [PubMed] [Google Scholar]
- 9. Hanajiri R, Murata M, Sugimoto K et al Integration of humoral and cellular HLA‐specific immune responses in cord blood allograft rejection. Bone Marrow Transplant 2015; 50: 1187–94. [DOI] [PubMed] [Google Scholar]
- 10. Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical‐cord blood from two partially matched unrelated donors. N Engl J Med 2001; 344: 1870–71. [DOI] [PubMed] [Google Scholar]
- 11. Wagner JE Jr, Eapen M, Carter S et al One‐unit versus two‐unit cord‐blood transplantation for hematologic cancers. N Engl J Med 2014; 371: 1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Lima M, McNiece I, Robinson SN et al Cord‐blood engraftment with ex vivo mesenchymal‐cell coculture. N Engl J Med 2012; 367: 2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wagner JE Jr, Brunstein CG, Boitano AE et al Phase I/II trial of StemRegenin‐1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand‐alone graft. Cell Stem Cell 2016; 18: 144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frassoni F, Gualandi F, Podestà M et al Direct intrabone transplant of unrelated cord‐blood cells in acute leukaemia: a phase I/II study. Lancet Oncol 2008; 9: 831–9. [DOI] [PubMed] [Google Scholar]
- 15. Sorror ML, Giralt S, Sandmaier BM et al Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood 2007; 110: 4606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubinstein P, Dobrila L, Rosenfield RE et al Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A 1995; 92: 10119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imahashi N, Ohashi H, Terakura S et al Chimerism status after unrelated donor bone marrow transplantation with fludarabine‐melphalan conditioning is affected by the melphalan dose and is predictive of relapse. Ann Hematol 2015; 94: 1139–48. [DOI] [PubMed] [Google Scholar]
- 18. Przepiorka D, Weisdorf D, Martin P et al 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–8. [PubMed] [Google Scholar]
- 19. Filipovich AH, Weisdorf D, Pavletic S et al National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–56. [DOI] [PubMed] [Google Scholar]
- 20. Rizzieri DA, Long GD, Vredenburgh JJ et al Successful allogeneic engraftment of mismatched unrelated cord blood following a nonmyeloablative preparative regimen. Blood 2001; 98: 3486–8. [DOI] [PubMed] [Google Scholar]
- 21. Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced‐intensity conditioning. Blood 2003; 102: 1915–9. [DOI] [PubMed] [Google Scholar]
- 22. Miyakoshi S, Yuji K, Kami M et al Successful engraftment after reduced‐intensity umbilical cord blood transplantation for adult patients with advanced hematological diseases. Clin Cancer Res 2004; 10: 3586–92. [DOI] [PubMed] [Google Scholar]
- 23. Nakasone H, Fuji S, Yakushijin K et al Impact of total body irradiation on successful neutrophil engraftment in unrelated bone marrow or cord blood transplantation. Am J Hematol 2017; 92: 171–8. [DOI] [PubMed] [Google Scholar]
- 24. Okada M, Yoshihara S, Taniguchi K et al Intrabone marrow transplantation of unwashed cord blood using reduced‐intensity conditioning treatment: a phase I study. Biol Blood Marrow Transplant 2012; 18: 633–9. [DOI] [PubMed] [Google Scholar]
- 25. Terakura S, Wake A, Inamoto Y et al Exploratory research for optimal GvHD prophylaxis after single unit CBT in adults: short‐term methotrexate reduced the incidence of severe GvHD more than mycophenolate mofetil. Bone Marrow Transplant 2017; 52: 423–30. [DOI] [PubMed] [Google Scholar]
- 26. Castello S, Podestà M, Menditto VG et al Intra‐bone marrow injection of bone marrow and cord blood cells: an alternative way of transplantation associated with a higher seeding efficiency. Exp Hematol 2004; 32: 782–7. [DOI] [PubMed] [Google Scholar]
- 27. Rocha V, Labopin M, Ruggeri A et al Unrelated cord blood transplantation: outcomes after single‐unit intrabone injection compared with double‐unit intravenous injection in patients with hematological malignancies. Transplantation 2013; 95: 1284–91. [DOI] [PubMed] [Google Scholar]
- 28. Saglio F, Berger M, Vassallo E et al Intrabone cord blood hematopoietic stem cell transplantation in a subset of very high‐risk pediatric patients: a safety and feasibility pilot study. J Pediatr Hematol Oncol 2012; 34: 359–63. [DOI] [PubMed] [Google Scholar]
- 29. Frassoni F, Varaldo R, Gualandi F et al The intra‐bone marrow injection of cord blood cells extends the possibility of transplantation to the majority of patients with malignant hematopoietic diseases. Best Pract Res Clin Haematol 2010; 23: 237–44. [DOI] [PubMed] [Google Scholar]
- 30. Brunstein CG, Barker JN, Weisdorf DJ et al Intra‐BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA‐matched units. Bone Marrow Transplant 2009; 43: 935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanda J, Morishima Y, Terakura S et al Impact of graft‐versus‐host disease on outcomes after unrelated cord blood transplantation. Leukemia 2017; 31: 663–8. [DOI] [PubMed] [Google Scholar]


