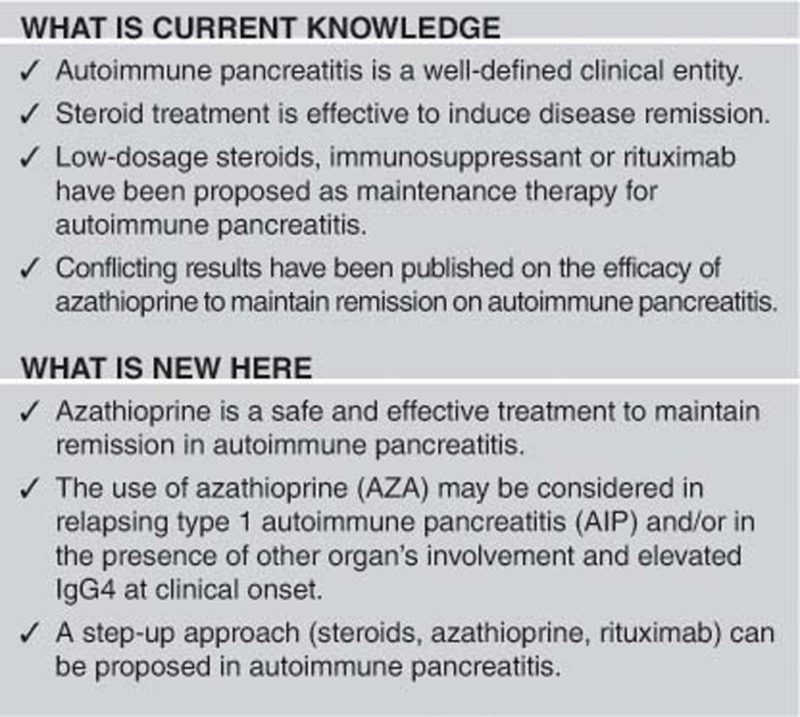
Abstract
Objectives:
Steroids are used to induce remission in autoimmune pancreatitis (AIP). Low-dosage steroid therapy or immunosuppressant (IMs) has been proposed as maintenance therapy to prevent AIP relapse. Few and conflicting data have been published on the efficacy of azathioprine (AZA) in preventing AIP relapse. The aim of this study was to evaluate the indication and efficacy of AZA as maintenance therapy to prevent disease relapse in AIP.
Methods:
Patients suffering from AIP diagnosed according to the ICDC in type 1, type 2, and not otherwise specified (NOS) were divided in those treated with AZA (AZA+ group) as maintenance therapy and not treated with maintenance therapy (AZA− group). Exclusion criteria were: previous pancreatic surgery, other autoimmune diseases as indication for AZA treatment, and use of IMs different from AZA. Drug safety, clinical and instrumental outcome of AZA+ patients were evaluated.
Results:
A total of 23 patients (18 Males and 5 Females, mean age 54±11 years) in AZA+ group and 97 (58 Males and 39 Females, mean age 45±18 years) in AZA− group were compared. In AZA+ group, patients were significantly older (P=0.043), type 1 AIP was more frequently diagnosed (87 vs. 51%, P=0.006), sIgG4 higher (758±625 vs. 311±409 mg/dl, P<0.001), other organ involvement (OOI) more frequently observed (83 vs. 48%, P=0.002), with higher frequency of relapse before AZA treatment (78 vs. 14%, P<0.001). Three patients in AZA+ group required drug discontinuation because of adverse events. Twenty patients were therefore evaluated for outcome. Six out of 20 patients (30%) relapsed after 24±15 months (5 in pancreas and 1 on biliary tract). They were retreated with steroids and continued AZA. Two out of 6 patients (33%) had a second relapse,after respectively 11 months (in pancreas and kidney) and 22 months (in kidney).
Conclusions:
AZA is an effective and safe treatment to prevent AIP relapses.
Introduction
Autoimmune pancreatitis (AIP) is a particular form of pancreatitis with unique histological features.1, 2 Two histological subtypes in AIP have been recognized with different clinical profiles.2, 3, 4, 5 Type 1 AIP is characterized by periductal infiltration of lymphocytes, abundant IgG4-positive plasma cells in pancreatic parenchyma, storiform fibrosis and obliterative phlebitis. Patients suffering from type 1 AIP are elderly, with a prevalence of male sex, with high IgG4 serum levels, extra pancreatic involvement (biliary tree, salivary glands, kidney, retroperitoneum), and frequent relapses. Type 2 AIP is characterized by the presence of granulocytic epithelial lesions, whereas IgG4-positive plasma cells are rare or absent in pancreatic parenchyma. Patients suffering from type 2 AIP are younger, inflammatory bowel disease is commonly observed, and relapses after steroids are rare.
The International Association of Pancreatology in 2011 defined the International Consensus Diagnostic Criteria (ICDC) based on five cardinal features (parenchymal and pancreatic duct imaging, serology, other organ involvement, histology, and response to steroid) categorized as level 1 and 2 of evidence according to their reliability to diagnose AIP.6 These criteria are able to diagnose AIP subtypes also in the absence of histology, and introduced criteria for AIP not otherwise specified (NOS), if type 1 and type 2 AIP cannot be diagnosed.
A dramatic response to steroid therapy has been reported in AIP, independently from the subtype.7, 8, 9 However, a significant proportion of patients (15–60%) develop disease relapse after steroids, more frequently those suffering from type 1 and NOS AIP compared to type 2 AIP.7, 8, 9, 10 There is no consensus on indications, type and duration of maintenance therapy because no prospective randomized controlled trials have been published yet. To prevent recurrences low-dosage long-term steroid therapy (that is, prednisone 2.5–10 mg up to 3 years) after induction of remission has been proposed mainly by Japanese authors,11 whereas immunosuppressant drugs (azathioprine [AZA], 6-mercaptopurine, mycophenolate mofetil) have been suggested in Western countries to avoid the side effects of steroids.12, 13 Few studies report the efficacy of immunosuppressants as maintenance therapy for AIP.8, 14, 15, 16, 17 A study from Mayo Clinic shows no differences in relapse-free survival in patients treated with immunosuppressants and steroids compared to steroids alone.17 In the same study, rituximab (RTX), an anti-CD20 antibody, seems to be effective in 12 patients in steroid-dependent or intolerant, and resistant to immunosuppressants. A recent UK multicenter study in patients suffering from IgG4-related disease involving the pancreas (92%) shows that in 41 patients treated by AZA, 13 were intolerant and only eight of the remaining (28%) had a disease relapse.18 Furthermore, none of AZA treated patients need to be retreated with steroids or RTX.
The aim of this study was therefore to evaluate indication, efficacy and safety of AZA as maintenance therapy in patients suffering from AIP.
Methods
We retrospectively reviewed the AIP database in our Department on January 2014. We included patients with a diagnosis of AIP based on ICDC6 and patients were therefore classified in type 1, 2, and NOS. Exclusion criteria were: (1) pancreatic surgery; (2) use of an immunosuppressant different from azathioprine; (3) indication for the use of azathioprine different from AIP. Patients were then divided into those treated with AZA as maintenance therapy (AZA+ group) and those not treated (AZA− group).
The demographic data (sex, age at presentation, alcoholic consumption, smoking habits), symptoms and signs at clinical onset (weight loss, pancreatitis, jaundice, steatorrhea, diabetes), radiological (CT, MRI) findings (focal and diffuse involvement of the pancreas, abdominal extra pancreatic involvement), serum IgG4 (sIgG4) levels, fecal elastase 1, steroid treatment were evaluated. Salivary gland involvement was based on clinical examination.
The upper normal limit of sIgG4 levels was considered 135 mg/dl, as previously reported by Hamano et al.19
Prednisone was administered at initial dose of 1 mg/kg of body weight per day for 2–4 weeks and then tapered by 5 mg every week up to suspension.
Relapsing AIP was defined as development of pancreatic and/or extra pancreatic alterations at imaging.20, 21 In particular, sIgG4 elevation alone was not considered disease relapse.
AZA is generally used in our Department after a first or second relapse in AIP patients or in AIP patients with a high risk of relapse (other organ’s involvement and/or high IgG4 levels). After recurrence, patients were re-treated with another course of prednisone. In those treated with AZA, the immunosuppressant was added after starting the steroid course, and maintained after steroids withdrawal.
Tuberculosis, cytomegalovirus, Epstein–Barr, hepatitis B and C screening prior to treatment with AZA was evaluated in all patients. AZA was started at 25–50 mg per day and increased by 25 mg every 3–7 days after regular monitoring the biochemical data (peripheral blood counts, pancreatic, kidney, and liver serum tests). The target dose was 2–2.5 mg/kg per day, and monthly laboratory test were routinely performed. AZA withdrawal was decided by physician when adverse drug reactions occurred, or in the presence of altered laboratory tests after an attempt of dose reduction. All patients underwent CT or MRI imaging 3–6 months after steroid and AZA introduction and then yearly, as protocol in our Institution. Further imaging assessments were performed depending on clinical and biochemical evaluation.
Outcome of patients treated with AZA was then assessed. Pancreatic exocrine function was also evaluated in AZA+ group before AZA initiation and after at least 6 months of immunosuppressant therapy by fecal elastase 1.
Patients without a radiological detection in the last 3 years were considered drop-out. The end of follow-up was January 2014.
Kruskal–Wallis test and Mann–Whitney U-test were used to analyze the non parametric data.
Fisher’s exact test and χ2 test were used for discrete variables as appropriate. Survival curve was used to evaluate relapses during AZA therapy.
Spearman’s rank-order correlation was used to measure the strength of association between serum IgG4 levels before and after steroids, and after steroids and after AZA.
A P-value <0.05 was considered significant. Mean and s.d. are reported. Statistic was processed using the SPSS 17 statistical program (SPSS Inc., Chicago, IL, USA).
Results
A total of 165 patients were in our database of AIP on January 2014. Forty-five patients were excluded from this study (13 could not be classified according to the ICDC, 21 underwent pancreatic surgery, eight had other autoimmune diseases as indication for AZA treatment and three were treated with other immunosuppressants). A total of 120 patients were therefore included in the present study and divided in those treated with AZA (AZA+ group) as maintenance therapy and those not treated (AZA− group). Twenty-three patients in AZA+ group (18 Males and 5 Females, mean age 54±11 years, 20 type 1 AIP and 3 AIP-NOS) and 97 in AZA− group (58 Males and 39 Females, mean age 45±18 years, 50 type 1 AIP, 19 type 2 AIP and 28 AIP-NOS) were compared. The characteristics of these two groups are summarized in Table 1.
Table 1. Characteristics of AZA+ group and AZA− group.
| Parameter | AZA + | AZA − | P |
|---|---|---|---|
| N | 23 | 97 | – |
| Male sex | 18 (78%) | 58 (60%) | ns |
| Age at onset (years) | 54±11 | 45±18 | 0.043 |
| Follow-up (years) | 4.9±4.1 | 2.7±3 | 0.005 |
| Drop-out | 0% | 15% | 0.07 |
| Drinkers | 4/21 (19%) | 27/93 (29%) | ns |
| Smokers | 5/21 (24%) | 24/92 (24%) | ns |
| AIP type (according to the ICDC) | |||
| Type 1 AIP | 20 (87%) | 50 (51%) | 0.006 |
| Type 2 AIP | 0 | 19 (20%) | |
| AIP-NOS | 3 (13%) | 28 (29%) | |
| Enlargement of pancreas | |||
| Focal | 11 (48%) | 53 (55%) | ns |
| Diffuse | 12 (52%) | 44 (45%) | |
| Symptom at clinical onset | |||
| Acute pancreatitis | 3 (13%) | 27 (28%) | ns |
| Jaundice | 18 (78%) | 35 (36%) | <0.0001 |
| Body weight loss | 20/22 (91%) | 63/92 (68%) | 0.035 |
| Diabetes | 4 (17%) | 10 (10%) | ns |
| Steatorrhoea | 1 (4%) | 5 (5%) | ns |
| Elevated sIgG4 at onset (>135 mg/dl) | 17/21 (74%) | 42/84 (50%) | 0.013 |
| sIgG4 at onset (mg/dl) | 758±625 | 311±409 | <0.001 |
| Other organ involvement (OOI) | 19 (83%) | 47 (48%) | 0.002 |
| Bile duct | 12 (52%) | 17 (17%) | <0.0001 |
| Kidney | 8 (35%) | 4 (4%) | <0.0001 |
| Salivary glands | 4 (17%) | 3 (3%) | 0.025 |
| Colon | 1 (4%) | 26 (27%) | 0.024 |
| Retroperitoneal fibrosis | 0 | 4 (4%) | ns |
| >1 | 5 (22%) | 7 (7%) | 0.052 |
| Steroid treatment at clinical onset | 23 (100%) | 91 (94%) | ns |
| Recurrences a | |||
| 0 | 5 (22%) | 83 (86%) | <0.0001 |
| 1 | 13 (56%) | 11 (11%) | |
| >1 | 5 (22%) | 3 (3%) | |
AIP, autoimmune pancreatitis; AZA, azathioprine; ICDC, International Consensus Diagnostic Criteria; NOS, not otherwise specified; ns, not specofied.
For AZA+ group only recurrences before immunosuppressant treatment were considered.
In AZA+ group, patients were significantly older (P=0.043), type 1 AIP was more frequently diagnosed (87 vs. 51%, P=0.006), sIgG4 higher (758±625 vs. 311±409 mg/dl, P<0.001), other organ involvement (OOI) more frequently observed (83 vs. 48%, P=0.002). AIP relapse was observed in 18 patients (78%) before AZA treatment in AZA+ group and in 14 patients (14%) in AZA− group (P<0.001).
The indications for the maintenance therapy with AZA were relapse of AIP in 18 patients (78%), extra pancreatic involvement in 3 (13%, 1 proximal bile duct stricture and 2 renal lesions) and markedly increase of serum IgG4 after steroid treatment in 2 (9%).
Three patients (13%) required AZA discontinuation within two months after starting because of adverse events: hepatitis (1 patient, then treated with RTX), anaphylactic shock (1 patient, then treated with maintenance dose of 5 mg per day of oral prednisone), nausea and body weight loss (1 patient who then refused AZA dose reduction and re-challenge).
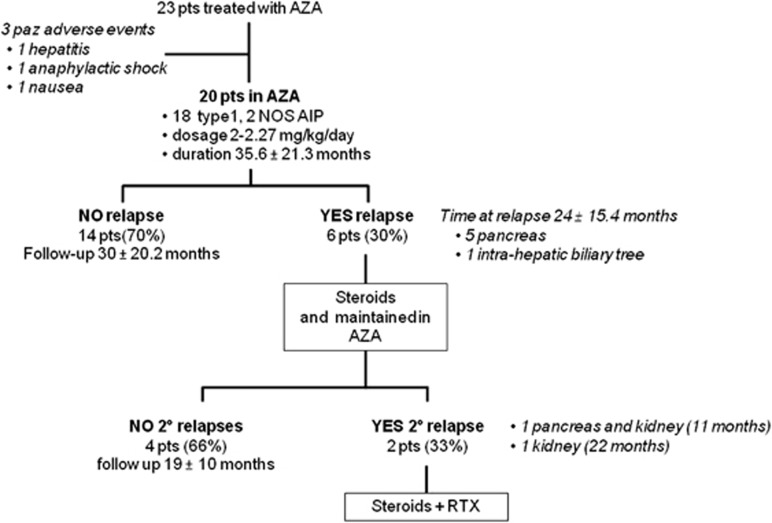
Twenty patients were therefore evaluated for outcome (15 Males and 5 Females mean age 53±10.8 years, 18 type 1 AIP and 2 AIP-NOS). Mean duration of treatment was 35.6±21.3 months and AZA dose was between 2 and 2.27 mg/kg per day. Figure 1 summarized the results of AZA treatment.
Figure 1.
Summary of the outcome in 23 patients treated with AZA.
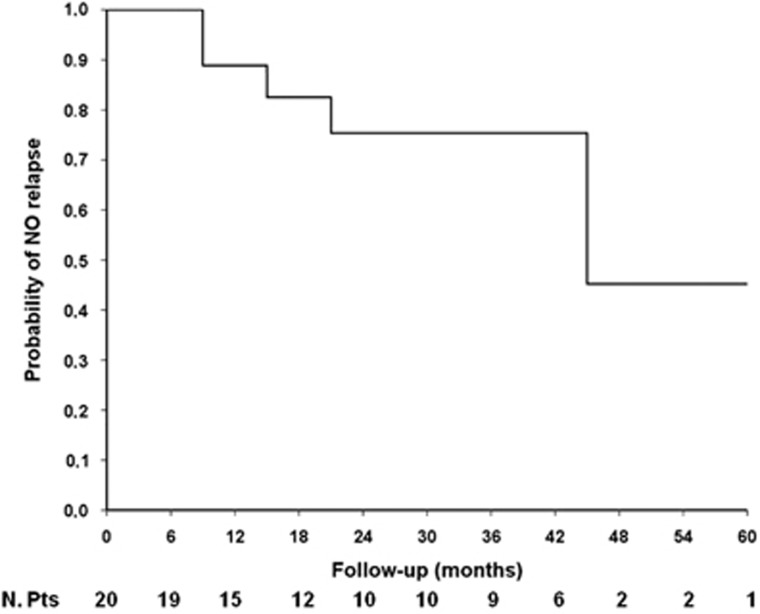
Fourteen out of 20 patients (70%) reached and maintained complete disease remission during follow-up (30±20.2months), while 6 out of 20 (30%) relapsed after 24±15.4 months. Five patients had disease relapse in pancreas and 1 in biliary tract. Relapse-free survival from the beginning of AZA treatment to the first relapse under immunosuppressant therapy is shown in Figure 2. Median relapse-free survival time was 47 months.
Figure 2.
Relapse-free survival curve for AIP patients treated with AZA.
The proportion of subjects having a relapse was similar in those with and without biliary involvement (22 vs. 36%, respectively, P=ns). Disease relapse was not correlated with focal or diffuse pancreatic involvement at diagnosis (33 vs. 27%, respectively, P=ns). None of the four patients with only pancreatic involvement had a relapse during AZA treatment compared with 6 out of 16 with other organ involvement (38%), but this difference did not reach the statistical significance. Moreover, none of the four patients with normal serum IgG4 at onset but 6 out of 14 (43%) with abnormal serum IgG4 levels at onset had a relapse, but also this difference was not significant.
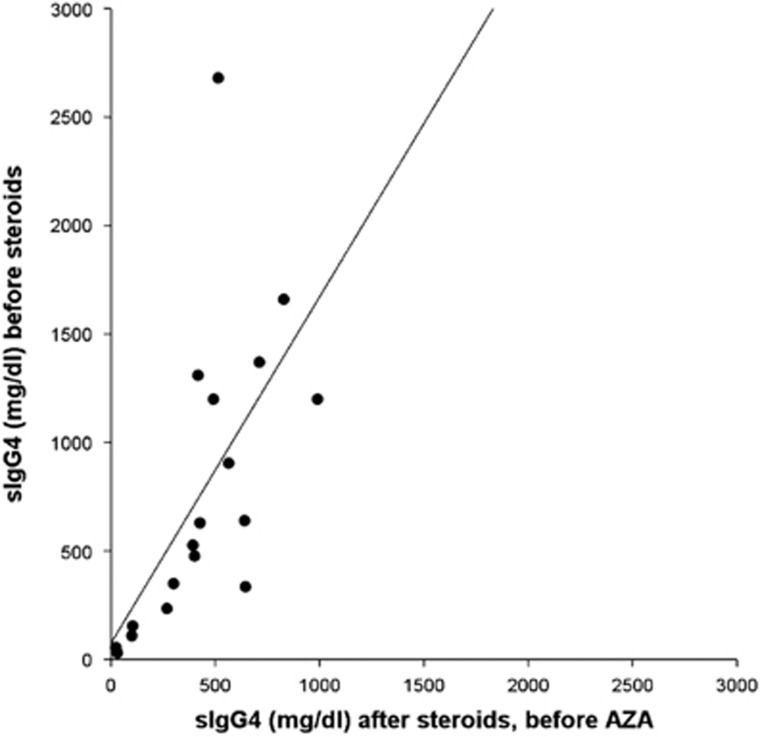
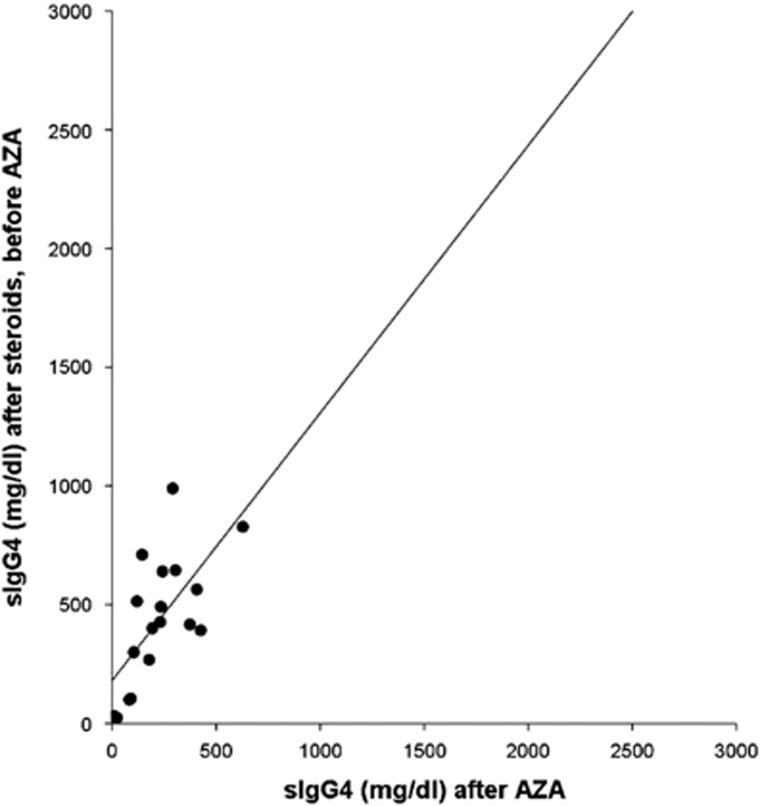
Patients who presented recurrences were all retreated with another course of steroids followed by complete tapering and continued AZA therapy. Two out of six patients (33%) had a second relapse after respectively 11 months (in pancreas and kidney) and 22 months (in kidney) and are in screening for RTX treatment. The other four patients did not have a second relapse after a mean follow-up time of 19±10 months. Serum IgG4 levels in AZA+ group were higher at onset compared with those detected after steroids and before the AZA treatment (764±675 mg/dl vs. 435±272 mg/dl, respectively, P=0.006). We found a correlation between sIgG4 measured at onset and before AZA treatment (R=0.797, P<0.0001) (Figure 3). Serum IgG4 levels were significantly higher in patients before AZA treatment compared with those detected almost 6 months after AZA treatment (435±272 vs. 225±155 mg/dl, respectively, P=0.003). sIgG4 measured before and after AZA treatment were significantly correlated (R=0.684, P=0.002) (Figure 4).
Figure 3.
Correlation between sIgG4 before steroids and after steroids/before AZA in 18 patients of AZA+ group.
Figure 4.
Correlation between sIgG4 after steroids/before AZA and after AZA (at least 6 months) in 18 patients of AZA+ group.
Sixteen out of 20 patients underwent determination of fecal elastase 1 before AZA treatment and after almost 6 months later. Fecal elastase 1 was significantly lower in these 16 patients before AZA treatment (109.2±98.8 mg/dl) than after (270.8±156.7 mg/dl) (P=0.002). No modification on diabetic status was observed before and after AZA treatment.
No malignancy has been observed in AZA treated patients.
Among the 97 AZA- patients, 14 experienced a relapse of the disease. Among these, 5 (including 3 patients with >1 relapse) refused AZA, one had a recent breast cancer treated with chemo- and radio-therapy, and eight were retreated with steroids after the first relapse without experiencing additional relapses.
Discussion
The results of this study seem to show the efficacy and safety of AZA maintenance therapy in AIP.
The conflicting and retrospective data have been published on the efficacy of AZA in the treatment of AIP14, 15, 16, 17 depending on limited number of patients, indications, inclusion/exclusion criteria, dose and duration of therapy, definition of disease relapse.
In the current study, we excluded patients who underwent pancreatic surgery because partial pancreatectomy has been suggested as an effective therapeutic approach for AIP.22, 23, 24 We excluded also subjects with other autoimmune diseases different from AIP as indication for AZA treatment, and those treated with other immunosuppressants (methotrexate, cyclosporine and mycophenolate mofetil), suggested or used in previous studies.25, 26 Indeed, the main end point of this study was to clarify the efficacy of AZA for AIP and not immunosuppressant at all.
We retrospectively studied AZA+ group patients compared with AZA− group to evaluate the indications for AZA in our Center. The results show that patients with type 1 (n=20) and NOS (n=3) AIP, but not with type 2 AIP, were treated with AZA, according to previous studies showing a higher disease relapse rate after steroids in type 1 and NOS AIP.3, 4, 7, 10, 17 All the other features differentiating the two groups are related to this data. Indeed, patients treated with AZA were older, showed more often an elevation of serum IgG4, had more frequently disease relapses, and presented more often extra pancreatic involvement. Therefore, type 1 AIP patients with extra pancreatic involvement and serum IgG4 elevation, have a higher probability to be treated with AZA. This clinical profile seem to identify patients with an IgG4-related disease.27
AZA treatment seems to be effective as maintenance therapy of AIP since 70% of patients treated with AZA did not show disease recurrence in a mean follow-up of 30 months. Survival curve for disease relapse shows that 75% of treated patients maintained remission up to 36 months. This evidence is even more significant since patients treated have a more aggressive disease and AZA can keep in remission a noteworthy proportion of them for a long time. We observed also a decrease of sIgG4 levels after steroid treatment and a further decrease after AZA treatment in AZA+ group, as well as a significant improvement of the exocrine pancreatic function evaluated with fecal elastase 1 after AZA treatment. These surrogate laboratory data support the efficacy of AZA as maintenance therapy in AIP.
These results are in accordance with a recent multicenter study from UK in the use of AZA in IgG4-related disease, involving in 92% of case the pancreas, where AZA was able to maintain 20 out of 28 patients (71%) in remission, excluding those intolerant to the drug.18
Disease relapses during AZA treatment were observed in all but one patient mainly in the pancreas. We therefore suggest to schedule an imaging follow-up in patients with AIP treated with AZA. We did not find predictive factors of disease relapse, probably due to the small sample size (n=20). However, extra pancreatic organ involvement and high sIgG4 before may be predictor factors of disease relapse during AZA treatment, but other studies are needed to confirm this data.
We observed three adverse effects (13%), leading to drug discontinuation. This rate is similar to that observed in the AZA treatment of other gastrointestinal autoimmune diseases.28 Furthermore, no acute pancreatitis has been observed in our cohort. Therefore, despite acute pancreatitis is described in AZA-treated patients,29 these results seems to suggest the safety of AZA therapy in AIP.
Recently, some studies suggested a risk to develop extra pancreatic neoplasia in AIP17, 30, 31 (10% within the first 5 years from AIP diagnosis), and it is still debated if it is increased compared with the general population or only age-related. In any case, AZA treatment may further increase this risk, as suggested in other gastrointestinal inflammatory disease (that is, IBD). However, a maintenance therapy with low dose of steroids leads to adverse effects, particularly in the elderly (osteoporosis, diabetes). Therefore, a maintenance therapy should be evaluated considering the risk-benefit balance of these different approaches, and the previous diagnosis of any cancer.
Patients who presented a second relapse during AZA maintenance therapy were re-treated with steroids, continuing the immunosuppressant, and only two patients had a further relapse. This approach was forced since biologics (RTX) were not available. Indeed, the use of RTX in AIP has not been yet approved by the Italian Drug Agency (AIFA) and it must be justified as off-label for single patient on the basis of a recent study in 12 patients by Mayo Clinic. The possible use of biologics will introduce also in AIP the possibility of a step-up vs. a top down approach, similarly to other inflammatory chronic disease (that is, Crohn disease). Applying forcedly a step-up approach (steroid→AZA→RTX), only a limited proportion of them may have an indication for RTX (three AZA intolerant and six relapsing in AZA). Considering this low number of patients, the high cost of RTX and the absence of randomized controlled trial, we believe that a step-up approach should be preferred in presence of a 30% relapse rate observed in our AIP patients.
The immediate administration of AZA concomitant to the steroid therapy and prolonged after steroid withdrawal (“primary maintenance therapy”) may be considered in untreated patients with a high risk of recurrence such as patients with high serum IgG4 and patients with other organ’s involvement (especially biliary tree). However, the definitive data supporting this strategy are lacking.
A critical issue not yet clarified is how long we can treat AIP patients with AZA. Our behavior is to try to stop AZA after a 5-year treatment, similar to that suggested in Crohn’s disease, even considering that this approach exposes AIP patients to disease relapse. However, this approach is only theoretical, and need to be confirmed by future studies.
The main limitation of the present study is the lack of an appropriate relapsing type 1 AIP control group. This is consequence of the retrospective nature of the study. However, future prospective studies focused mainly on relapsing type 1 AIP patients might investigate this aspect.
In conclusion, AZA seems to be effective to prevent disease relapse and safe as maintenance therapy in patients suffering from AIP. Prospective randomized trials are necessary to confirm these retrospective data.
Study Highlights
Footnotes
Guarantor of the article: Luca Frulloni, MD, PhD.
Specific author contributions: Conception or design of the work: Luca Frulloni and Armando Gabbrielli. Acquisition of the data: Nicolò de Pretis, Antonio Amodio, Laura Bernardoni, Pietro Campagnola, Fabiana Capuano, Stefano Crinò, Arianna Massella. Analysis or interpretation of data: Nicolò de Pretis, Antonio Amodio and Luca Frulloni. Drafting the work: Nicolò de Pretis and Luca Frulloni. Revising it critically for important intellectual content: Suresh Chari, Mark Topazian and Luca Frulloni. Final approval of the version published: all authors.
Potential competing interests: None.
Financial support: None.
References
- Zamboni G, Luttges J, Capelli P et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch 2004; 445: 552–563. [DOI] [PubMed] [Google Scholar]
- Chari ST, Kloeppel G, Zhang L et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas 2010; 39: 549–554. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chari S, Smyrk TC et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas 2011; 40: 1172–1179. [DOI] [PubMed] [Google Scholar]
- Sah RP, Chari ST, Pannala R et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology 2010; 139: 140–148. [DOI] [PubMed] [Google Scholar]
- Detlefsen S, Zamboni G, Frulloni L et al. Clinical features and relapse rates after surgery in type 1 autoimmune pancreatitis differ from type 2: a study of 114 surgically treated European patients. Pancreatology 2012; 12: 276–283. [DOI] [PubMed] [Google Scholar]
- Shimosegawa T, Chari ST, Frulloni L et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 2011; 40: 352–358. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Chari ST, Giday SA et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas 2011; 40: 809–814. [DOI] [PubMed] [Google Scholar]
- Hart PA, Kamisawa T, Brugge WR et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013; 62: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frulloni L, Scattolini C, Falconi M et al. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol 2009; 104: 2288–2294. [DOI] [PubMed] [Google Scholar]
- Ikeura T, Manfredi R, Zamboni G et al. Application of international consensus diagnostic criteria to an Italian series of autoimmune pancreatitis. United European Gastroenterol J 2013; 1: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nishimori I, Inoue N et al. Treatment for autoimmune pancreatitis: consensus on the treatment for patients with autoimmune pancreatitis in Japan. J Gastroenterol 2007; 42 (Suppl 18): 50–58. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis E, Webster GJ. Review article: autoimmune pancreatitis—management of an emerging disease. Aliment Pharmacol Ther 2011; 33: 291–303. [DOI] [PubMed] [Google Scholar]
- Pannala R, Chari ST. Corticosteroid treatment for autoimmune pancreatitis. Gut 2009; 58: 1438–1439. [DOI] [PubMed] [Google Scholar]
- Maire F, Le Baleur Y, Rebours V et al. Outcome of patients with type 1 or 2 autoimmune pancreatitis. Am J Gastroenterol 2011; 106: 151–156. [DOI] [PubMed] [Google Scholar]
- Church NI, Pereira SP, Deheragoda MG et al. Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol 2007; 102: 2417–2425. [DOI] [PubMed] [Google Scholar]
- Sandanayake NS, Church NI, Chapman MH et al. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol 2009; 7: 1089–1096. [DOI] [PubMed] [Google Scholar]
- Hart PA, Topazian MD, Witzig TE et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut 2013; 62: 1607–1615. [DOI] [PubMed] [Google Scholar]
- Huggett MT, Culver EL, Kumar M et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol 2014; 109: 1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano H, Kawa S, Horiuchi A et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344: 732–738. [DOI] [PubMed] [Google Scholar]
- Manfredi R, Frulloni L, Mantovani W et al. Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology 2011; 260: 428–436. [DOI] [PubMed] [Google Scholar]
- Manfredi R, Graziani R, Cicero C et al. Autoimmune pancreatitis: CT patterns and their changes after steroid treatment. Radiology 2008; 247: 435–443. [DOI] [PubMed] [Google Scholar]
- Hardacre JM, Iacobuzio-Donahue CA, Sohn TA et al. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg 2003; 237: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnelldorfer T, Lewin DN, Adams DB. Long-term results after surgery for autoimmune sclerosing pancreatitis. J Gastrointest Surg 2007; 11: 56–58. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Morales-Oyarvide V, Zaydfudim V et al. Short-term and long-term outcomes for patients with autoimmune pancreatitis after pancreatectomy: a multi-institutional study. J Gastrointest Surg 2013; 17: 899–906. [DOI] [PubMed] [Google Scholar]
- Seleznik G, Graf R. Alternatives to steroids?! Beneficial effects of immunosuppressant drugs in autoimmune pancreatitis. Gut 2014; 63: 376–377. [DOI] [PubMed] [Google Scholar]
- Raina A, Yadav D, Krasinskas AM et al. Evaluation and management of autoimmune pancreatitis: experience at a large US center. Am J Gastroenterol 2009; 104: 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012; 366: 539–551. [DOI] [PubMed] [Google Scholar]
- Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol 2004; 2: 731–743. [DOI] [PubMed] [Google Scholar]
- Weersma RK, Peters FT, Oostenbrug LE et al. Increased incidence of azathioprine-induced pancreatitis in Crohn's disease compared with other diseases. Aliment Pharmacol Ther 2004; 20: 843–850. [DOI] [PubMed] [Google Scholar]
- Hart PA, Law RJ, Dierkhising RA et al. Risk of cancer in autoimmune pancreatitis: a case-control study and review of the literature. Pancreas 2014; 43: 417–421. [DOI] [PubMed] [Google Scholar]
- Shiokawa M, Kodama Y, Yoshimura K et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 2013; 108: 610–617. [DOI] [PubMed] [Google Scholar]