Summary
In humans, a complex interaction between the host immune system and commensal microbiota is required to maintain gut homeostasis. In this symbiotic relationship, the microbiota provides carbohydrate fermentation and digestion, vitamin synthesis and gut‐associated lymphoid tissue development, as well as preventing colonization by pathobionts, whereas the host offers a niche and nutrients for the survival of the microbiota. However, when this mutualistic relationship is compromised and an altered interaction between immune cells and microorganisms occurs, the gut microbiota may cause or contribute to the establishment of infectious diseases and trigger autoimmune diseases. Researchers have made efforts to clarify the role of the microbiota in autoimmune disease development and find new therapeutic approaches to treat immune‐mediated diseases. However, the exact mechanisms involved in the dysbiosis and breakdown of the gut epithelial barrier are currently unknown. Here, we provide a general overview of studies describing gut microbiota perturbations in animal models of autoimmune diseases, such as type 1 diabetes, multiple sclerosis, rheumatoid arthritis and systemic lupus erythematosus. Moreover, we include the main studies concerning dysbiosis in humans and a critical discussion of the existing data on the use of probiotics in these autoimmune diseases.
Keywords: autoimmunity, dysbiosis, gut barrier, inflammation, probiotics
Abbreviations
- CIA
collagen‐induced arthritic mice
- CNS
central nervous system
- DAS28
disease activity score calculator for rheumatoid arthritis
- EAE
experimental autoimmune encephalomyelitis
- GF
germ‐free mice
- IFN‐γ
interferon‐γ
- IL‐17
interleukin‐17
- MS
multiple sclerosis
- NOD
non‐obese diabetic mice
- NZB
New Zealand black mice
- RA
rheumatoid arthritis
- RRMS
relapsing–remitting multiple sclerosis
- SFB
segmented filamentous bacteria
- SLE
systemic lupus erythematosus
- T1D
type 1 diabetes
- Th1
T helper type 1
- TLR
Toll‐like receptors
- Treg
regulatory T
Introduction
The skin surface and mucous membranes in vertebrates are colonized by a high number of microorganisms, mainly bacteria, representing commensal microbiota.1, 2, 3 In humans, approximately 30–400 trillion microorganisms (3 × 1013 to 40 × 1013) colonize the healthy human intestinal tract, and the vast majority reside in the distal portion.4, 5, 6 The gut bacteria have co‐evolved in a symbiotic relationship with the human host, and their composition depends on immunogenetic and environmental factors.7, 8
From birth, gut microbiota colonization depends on several factors, including age, mode of delivery (vaginal or caesarean section), mothers' microbiota composition, early use of antibiotics and feeding regimen (breastfeeding or formula).9, 10 The mode of delivery and mode of feeding in the first years of life strongly influence the establishment of gut microbiota composition and may affect the development of autoimmune diseases.10 Additionally, caesarean delivery and formula use have been associated with higher incidence of infectious diseases and increased susceptibility to allergic diseases.10, 11
In humans, the intestinal microbiota tends to stabilize and reaches greater diversity at approximately 3 years of age, and these microorganisms collaborate with many host physiological processes; in turn, the host contributes to their growth and survival by offering a niche, substrates and nutrients.10, 11, 12, 13 The main contributions of the microbiota to the host include carbohydrate fermentation and digestion, vitamin synthesis, gut‐associated lymphoid tissue development, the polarization of specific immune responses, and the prevention of colonization by pathobionts.14, 15, 16, 17, 18
The two predominant bacterial phyla in the healthy human gut are Firmicutes and Bacteroidetes. Also present are Proteobacteria, Actinobacteria, Fusobacteria and Verrucomicrobia phyla but to a lesser extent.19, 20 The most prevalent genera in the adult human gut are represented by Gram‐positive bacteria, such as Clostridium, Bifidobacterium, Lactobacillus, Ruminococcus, Streptococcus, and Gram‐negative bacteria, such as Bacteroides and Escherichia.21
A complex interaction between the host immune system and the microbiota is required to maintain gut homeostasis.14, 15, 16, 17, 18 However, when this mutualistic relationship is compromised, with alterations in bacterial function and diversity, a process called dysbiosis, the gut microbiota may cause or contribute to the establishment of infectious diseases and to autoimmune disease development.22
For several decades, researchers interested in enteroinfections and gut‐associated diseases have studied the gut microbiota. It has been evident that the microbiota plays an important role in immune homeostasis in the gut mucosa, and researchers are extensively investigating microbiota–immune system interactions and their role in the susceptibility or resistance to gut infections and in the triggering of autoimmune, allergic and chronic inflammatory diseases.23
Gut microbiota and immune system
Studies in germ‐free (GF) mice showed that the gut microbiota is required for normal immune system maturation, including gut‐associated lymphoid tissue development, which plays important roles in tolerance induction to autoantigens in the gut mucosa.24, 25, 26 These GF mice showed decreased numbers of CD4+ T cells, secreting IgA plasma cells and antimicrobial peptides, a thinner mucus layer and Peyer's patches.23 The spleen and lymph nodes are abnormally developed in GF mice, with decreased numbers of B and T cells in the germinal centres and parafollicular region, respectively.27, 28
In mice and humans, during equilibrium, intestinal dendritic cells and macrophages are hyporesponsive to pathogen‐associated molecular patterns.1, 29 However, when epithelial barrier breakdown occurs, the pattern recognition receptors, which are present in innate immune cells, recognize gut microbiota or pathobionts through toll‐like receptors, NOD‐like receptors, soluble retinoic acid‐inducible gene I, or melanoma differentiation‐associated protein 5, which triggers an inflammatory cascade, pro‐inflammatory cytokine secretion, and the activation of adaptive immune responses.29, 30
Gut microbiota and T cells
The resident microbiota regulates the development of specific subsets of lymphocytes in the gut. T helper type 17 (Th17) lymphocytes are essential in defence against bacterial and fungal infections and play roles in autoimmune disease development by producing pro‐inflammatory cytokines, such as interleukin‐17 (IL‐17) and IL‐22, and by the recruitment of neutrophils.31 Th17 cells accumulate in the intestine, suggesting that intrinsic processes in the gut mucosa could regulate the development of these cells. Moreover, studies have shown that the number of Th17 cells are reduced in GF mice or antibiotic‐treated adult mice, and it was observed that some particular species of Clostridia, called segmented filamentous bacteria (SFB), promote the generation of Th17 cells in the gut32, 33, 34, 35, 36 (Fig. 1).
Figure 1.
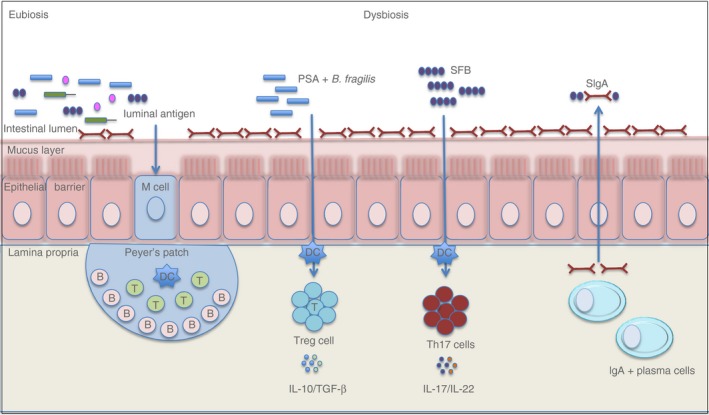
A schematic representation of the interaction between commensal microbiota and the immune system. Under eubiosis, there are microbiota diversity and immune homeostasis in the gut mucosa. Commensal microorganisms instruct dendritic cells to induce IgA‐secreting cell differentiation, and in turn, IgA regulates the composition of the gut microbiota. During dysbiosis, there are decreased diversity in commensal microbiota and deregulated interactions between immune cells and these microorganisms. Some specific bacteria, such as Bacteroides fragilis, induce regulatory T cell differentiation and the secretion of anti‐inflammatory cytokines, whereas segmented filamentous bacteria (SFB) promote T helper type 17 (Th17) cell differentiation and the secretion of pro‐inflammatory cytokines, which play roles in several autoimmune diseases.
Regulatory T (Treg) cells aggregate in the intestine and help with the maintenance of homeostasis.37, 38 Treg cell depletion induces an abnormal expansion of CD4+ T cells expressing T‐cell receptors against commensal microbiota, resulting in gut inflammation.1 Studies have shown that the Treg cell numbers were reduced in the lamina propria of GF mice and particular species of Clostridium are involved in the Treg induction in the gut.38, 39 In addition, the polysaccharide A, which is secreted by Bacteroides fragilis, can induce CD4+ T cells into Foxp3+ Treg cells, which secretes IL‐10, an anti‐inflammatory cytokine, and mediates mucosal tolerance40 (Fig. 1).
Gut microbiota and B cells
An important relationship exists between intestinal microbiota and the humoral immune responses. Secreted IgA protects us against enteroinfections and coats commensal bacteria by inhibiting their binding to the intestinal epithelium and the lamina propria invasion.41 Secreted IgA has barrier functions and shapes the microbiota composition through antibody‐mediated immunoselection.27
B cells differentiate into plasma cells by T‐helper‐dependent or T‐helper‐independent mechanisms. In Peyer's patches, T‐helper‐dependent responses occur with isotype switching, somatic hypermutation and affinity maturation, which induce the differentiation of IgA‐secreting plasma cells with a high affinity for antigens.41 T‐helper‐independent responses are mainly mediated by B1 cells that differentiate into polyclonal IgA‐secreting plasma cells with a low affinity for antigens.27 The T‐helper‐dependent and T‐helper‐independent immune responses in the gut have been extensively reviewed in the literature.41, 42, 43, 44, 45, 46
The gut microbiota also induces the expression of factors involved in the induction of IgA+ B cells, such as B‐cell activating factor and a proliferation‐inducing ligand (known as APRIL) in dendritic cells in the lamina propria.46 Therefore, the microbiota instructs dendritic cells and follicular dendritic cells to induce IgA‐secreting plasma cells, and in turn, IgA regulates the composition and function of the gut microbiota (Fig. 1).46 To confirm the essential role of this mutualistic relationship, studies have shown that GF mice and mice deficient in activation‐induced cytidine deaminase have decreased plasma cells and alterations in the gut microbiota composition, respectively.42, 43, 44, 45, 46
Gut microbiota and autoimmune diseases
Evidence from animal models has implied the direct involvement of gut microbiota in disease development, and some intestinal microbiota are associated with autoimmune diseases.24, 25, 26
Intestinal dysbiosis observed in autoimmune diseases is associated with decreased bacterial function and diversity, impaired gut barrier function, increased inflammation and decreased Treg cells in the gut.47, 48 Additionally, the hypotheses proposed to link intestinal dysbiosis with autoimmune diseases include molecular mimicry, bystander T‐cell activation, and the amplification of autoimmunity by pro‐inflammatory milieu, which is elicited by altered gut microbiota.49 Finally, a more recent hypothesis proposed by Lerner et al. implicated the post‐translational modification of luminal proteins, promoted by enzymes from dysbiotic microbiota, which modify substrates in a different way from that performed under eubiotic conditions.49 The defective post‐translational modification of luminal proteins may induce neo‐epitope generation that could become immunogenic and may induce systemic autoimmunity and trigger autoimmune diseases.49
Here, we describe several reports concerning alterations in the intestinal microbiota in some autoimmune diseases, such as type 1 diabetes, multiple sclerosis, rheumatoid arthritis and systemic lupus erythematosus. Additionally, we provide a critical discussion concerning the data from the use of probiotics in autoimmune diseases.
Type 1 diabetes
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by the immune destruction of insulin‐secreting pancreatic β‐cells, resulting in exogenous insulin dependence to control blood glucose levels.50 The aetiopathogenesis may involve the interaction of predisposing HLA genes and environmental factors, such as viral enteroinfections and intestinal dysbiosis.51 According to the International Diabetes Federation, 79 100 children under the age of 15 will develop T1D annually worldwide.52
The role of the gut microbiota in T1D aetiology has been the subject of research over the last decade to clarify its role in disease development and determine preventive approaches, such as diet manipulation and probiotic administration.51
One of the first studies in non‐obese diabetic mice (NOD) showed that the composition of the intestinal microbiota modulates innate and adaptive immune functions and enhances the disease in MyD88−/− NOD mice. However, protection against diabetes in these mice is abrogated by the administration of antibiotics and GF conditions, suggesting that commensal bacteria may be important to reduce disease susceptibility in these mice.53 Additionally, Emani et al. reported that NOD mice treated with a conventional diet presented impaired tolerance to gut microbes, an altered barrier permeability, an increased number of peritoneum macrophages and a decreased abundance of Firmicutes.54
An imbalance among Th1, Th17 and Treg cell differentiation in the gut was reported in GF NOD mice and is associated with insulitis and pancreas inflammation.55 Interestingly, the SFB, which are associated with the exacerbation of the Th17 responses in other autoimmune diseases, are involved in protection against diabetes in NOD mice.56
In biobreeding diabetes‐prone rats, increased percentages of Bacteroides, Ruminococcus and Eubacterium reads in stool samples were observed, and there was a higher abundance of Bifidobacterium and Lactobacillus in stool samples from biobreeding diabetes‐resistant rats.57 The altered gut microbiota, such as the increased abundance of Bacteroidetes, could promote increased intestinal permeability and precede the clinical onset of T1D in animal models and pre‐diabetic and diabetic patients.58, 59, 60, 61 In fact, the role of the gut microbiota in T1D has been suggested since 1987, and the first study in humans was performed in Finland by using stool samples from four T1D children and four matched controls.62 They observed that children with T1D had decreased microbiota diversity compared with controls and had a reduction in the Firmicutes : Bacteroidetes ratio62, 63 (Fig. 2). Additionally, recent studies showed that the composition of the intestinal microbiota is altered in children with pre‐diabetes with genetic susceptibility and autoantibodies against β‐cells.64, 65, 66
Figure 2.
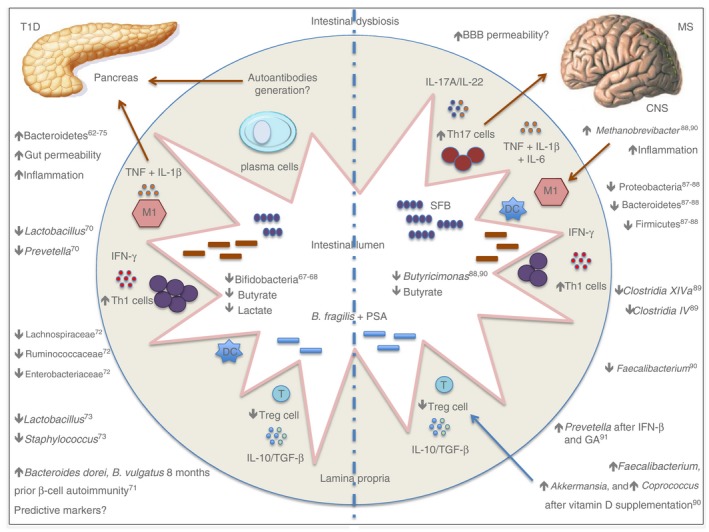
A schematic representation of the intestinal dysbiosis in organ‐specific autoimmune diseases in humans.
Studies from the De Goffau group showed that children with autoantibodies against β‐cells exhibit an increased number of Bacteroidetes and decreased abundance of lactate and butyrate‐producing bacteria in faeces.63, 65 In agreement with this study, Brown et al. showed a decreased number of mucin‐degrading and butyrate‐producing bacteria in T1D patients compared with healthy controls.67 Butyrate has anti‐inflammatory activity, induces Treg cell differentiation in the gut and enhances the gut barrier via tight junctions.64
Endesfelder et al. did not observe significant differences in gut microbiota diversity in 22 children with positive‐islet autoantibodies compared with 22 children with negative ones.68 Additionally, children with T1D have decreased numbers of lactate‐producing bacteria, such as Bifidobacterium longum, subspecies infantis. Bifidobacteria members promote carbohydrate fermentation, generate acetate and lactate, release polyphenols and linoleic acids, and have antioxidant activities.68 They also play a role in gut‐associated lymphoid tissue development maturation during early life and guarantee protection against pathobionts by bacteriocin release, causing decreases in the luminal pH and the inhibition of adhesion to epithelial cells.69 In addition, Bifidobacteria species synthesize B‐group vitamins, which can induce Treg cells in the gut mucosa70, 71 (Fig. 2).
Murri et al. evaluated the gut microbiota in a cohort of 16 children with T1D and 16 controls and showed an increased abundance of Clostridium, Bacteroides and Veillonella in patients compared with controls. Furthermore, the Firmicutes : Bacteroidetes ratio and levels of Lactobacillus, Bifidobacterium and Prevotella were decreased in these patients.72
Davis‐Richardson et al. evaluated stool samples isolated from 29 patients with seroconverted T1D disease and 47 healthy controls and observed a high abundance of Bacteroides dorei and Bacteroides vulgatus in seroconverted patients with T1D 8 months before β‐cell autoimmunity. These data suggest that early dysbiosis may be relevant to predict T1D in genetically predisposed individuals.73
Kostic et al. evaluated stool samples from 33 children genetically predisposed to T1D and reported alterations in microbiota α‐diversity between and within the children over time. They also observed a decrease in the number of Gram‐negative members and an inverse correlation between Lachnospiraceae and Ruminococcaceae with Enterobacteriaceae, a Gram‐negative aerobe. In addition, they reported that early commensals are aerobic, whereas later microbes appear to be anaerobic.74
A more complex study with 35 patients newly diagnosed with T1D, 21 first‐degree relatives with β‐cell autoimmunity, 32 relatives with negative autoantibodies, and 23 controls showed that the numbers of Lactobacillus and Staphylococcus reads were decreased in patients and in first‐degree relatives when compared with controls. Furthermore, the gut microbiota from subjects with negative and positive autoantibodies grouped together but in a different cluster from newly diagnosed patients.75 This study and the work performed by Meiía‐León et al. support the hypothesis that there is an intestinal microbiota signature associated with T1D development in seropositive children.76
Even though studies have shown that intestinal dysbiosis can affect gut permeability via their metabolites and play a role in T1D development, there is no evidence for the real role of intestinal microbiota in the development of autoimmunity to β‐cells and in tissue damage in humans. Additional studies are needed to find the specific microbial ligands that signal through immune cells in the gut and might be involved in the autoreactivity to β‐cells.77
Multiple sclerosis
Multiple sclerosis (MS) is a chronic and inflammatory disease that affects the central nervous system (CNS) and is characterized by autoimmune reactions against myelin proteins. Susceptible HLA alleles and environmental factors, such as virus infection, a hypercaloric diet, vitamin D deficiency and dysbiosis, have been implicated in triggering MS.78 MS promotes disability in young adults and affects twice as many women as men. According to the Multiple Sclerosis International Federation and World Health Organization (WHO), the prevalence of MS increased from 2·1 million in 2008 to 2·3 million in 2013.79
Studies have shown that gut microbiota can affect the development of MS, and these works implicated intestinal dysbiosis as one of the possible causes of extraintestinal disease development.80 The colonization of GF mice with SFB promotes an increase in the number of Th17 cells in the lamina propria and CNS, worsening disease severity in experimental autoimmune encephalomyelitis (EAE), an MS animal model.81 Likewise, the colonization of the same mice with Bacteroides fragilis and polysaccharide A, which induces Foxp3+ Treg cell differentiation, decreases symptoms in EAE mice.82
The immune response in EAE is mediated mainly by Th1 and Th17 cells. The gut SFB induces Th17 cells in EAE mice. To validate the influence of the intestinal microbiota in the development of EAE, mice were treated with antibiotics, and they exhibited a reduction in clinical score, suggesting the role of the microbiota in the induction of inflammatory cells in these models. The attenuation of the clinical score was accompanied by decreased interferon‐γ (IFN‐γ), macrophage inflammatory protein 1α, monocyte chemoattractant protein 1, IL‐17 and IL‐6 and increased IL‐10 and IL‐13 secretion.83
The colonization of EAE mice with Bacteroides fragilis and polysaccharide S resulted in decreased clinical scores and the protection of mice from disease.84 This effect was achieved because of the induction of Treg cells, increased IL‐10 and reduced IL‐17, suggesting the use of these microorganisms as probiotics in humans.85 Some of the tested probiotics seemed to have an effect on the autoimmunity of EAE mice. Oral administration of Lactobacillus spp. and Bifidobacterium bifidum showed a significant decrease in the clinical score and increase in the Treg cell number in treated mice.86
Recently, it was also demonstrated that the gut microbiota could affect the permeability of the blood–brain barrier.86 The defective tight‐junctions at the blood–brain barrier of GF mice are restored after they are colonized with conventional microbiota.86 The use of GF mice also demonstrated the importance of intestinal microbiota in the development of EAE. The induction of EAE in GF mice resulted in the reduction of IFN‐γ and IL‐17A in the CNS, accompanied by an increase in the number of Treg cells in the gut.81
In patients, recent studies have evaluated seven individuals with relapsing–remitting MS (RRMS) and found a reduction in Firmicutes, Bacteroidetes and Proteobacteria members.87, 88 Jhangi et al. evaluated 22 untreated MS patients and observed an increase in Methanobrevibacter smithii and reduction in Firmicutes, as well as Butyricimonas, which are butyrate‐producing members of the microbiota.88 A Japanese study found altered intestinal microbiota in RRMS patients, with decreased Clostridia XIVa and IV groups and Bacteroidetes members.89 Another study, including 15 RRMS patients with an Expanded Disability Status Score ≤ 3·0 showed a decreased amount of Faecalibacterium and increased amounts of Akkermansia, Coprococcus and Faecalibacterium after vitamin D supplementation90 (Fig. 2).
A recent study investigated 60 RRMS patients, 28 untreated patients, and 43 healthy controls and showed increased amounts of Methanobrevibacter and Akkermansia and decreased amounts of Butyricimonas in untreated patients.90 Methanobrevibacter is involved in inflammatory conditions by recruiting macrophages and activating dendritic cells.91 Akkermansia species have immunoregulatory effects by converting mucin into short‐chain fatty acids; however, they could play a role in degrading the mucus layer and promoting inflammation.92, 93 Butyricimonas species are butyrate‐producing bacteria and have immunomodulatory properties by inducing Treg cells in the gut.71 In treated patients (IFN‐β and glatiramer acetate), there was an increased number of Prevotella compared with untreated patients.94 This genus is associated with high‐fibre ingestion and has regulatory roles via butyrate generation95 (Fig. 2).
Chen et al. also reported dysbiosis in RRMS patients after comparing stool samples from 31 patients and 36 controls. They observed an increased abundance of the Pseudomonas, Mycoplasma, Haemophilus, Blautia and Dorei genera in patients compared with healthy counterparts, which showed a prevalence of Prevotella and Parabacteroides.96
Available findings on dysbiosis in animal models and patients with MS point to the gut–brain axis connection. The relationship between immunity in the gastrointestinal mucosa and commensal bacteria seems to promote important physiological homeostasis for the host.97 However, future studies are required to determine the real role of the gut microbiota in CNS demyelinating diseases.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic autoimmune disorder characterized by chronic inflammation of multiple joints, bone erosion and cartilage destruction. Moreover, RA can affect internal organs such as the lungs, heart and kidneys. Anti‐cyclic citrullinated peptide and/or rheumatoid factor are the most important autoantibodies in RA and can be found before disease onset.98 The disease is three times more common in women, and according to WHO, the worldwide prevalence, which is between 0·3 and 1%, ranks the disease among the most common autoimmune disorders. The triggering of RA involves the interaction of HLA genes and environmental factors, such as smoking and infections.99 Among environmental factors, dysbiosis has been identified as a possible trigger factor for autoimmunity and RA development.100
Experiments in animal models suggest that the gut microbiota influences local and systemic immunity and might trigger joint inflammation.100, 101 Studies in mice with collagen‐induced arthritis (CIA) showed that the administration of antibiotics exacerbates the disease and increases the level of IL‐6, IFN‐γ and IL‐17 pro‐inflammatory cytokines.102 Further study showed differences in the gut microbiota composition between CIA‐susceptible and CIA‐resistant mice, with a prevalence of Desulfovibrio, Prevotella, Parabacteroides, Odoribacter, Acetatifactor, Blautia, Coprococcus and Ruminococcus genera in arthritic mice, in addition to increased levels of serum IL‐17 and CD4 Th17 cells in the spleen.102
Mice deficient in IL‐1RA signalling spontaneously develop arthritis; however, under GF conditions, RA is attenuated because of decreases in IL‐17 and IL‐1β secretion and decreased Toll‐like receptor 2 and Toll‐like receptor 4 stimulation.103, 104, 105 On the other hand, when these IL‐RA−/− GF mice were colonized with Bifidobacterium bifidum, there was an increase in clinical scores compared with mice that were conventionally housed.104
Recent studies have investigated the gut microbiota of the genetically arthritis‐susceptible transgenic mice *0401 and in the genetically resistant transgenic mice *0402. Clostridia were prevalent in susceptible mice, whereas the Porphyromonadaceae and Bifidobacteriaceae families were dominant in resistant mice. Moreover, the authors observed increased intestinal permeability and a Th17 profile in susceptible mice, suggesting that genetic background influences the individual's microbiota profile.106
Similar to that observed in EAE mice, Th17 cells induced by SFB in the lamina propria induced autoantibodies involved in RA development in animal models.107 The correlation between intestinal dysbiosis and RA aetiology is not a new concept. The ‘toxaemic factor’ hypothesis was proposed in the twentieth century when it was suggested that the increase in the level of Gram‐negative bacteria in the intestinal lumen could induce an increase in toxic molecules/metabolites that enter the bloodstream, promoting systemic inflammation.100 Recent studies have shown that the intestinal microbiota of newly diagnosed RA patients was dominated by Gram‐negative Prevotella members, especially Prevotella copri, compared with healthy individuals.108, 109 In the work of Maeda et al., the gut microbiota transplant from RA patients to GF arthritis‐prone SKG mice induced an increase in the number of Th17 cells in the gut and severe arthritis. Additionally, the co‐culture of SKG dendritic cells with Prevotella copri increased IL‐17 secretion in response to RA autoantigens, suggesting that RA gut microbiota may induce autoreactive cells in the gut and promote joint inflammation109 (Fig. 3).
Figure 3.
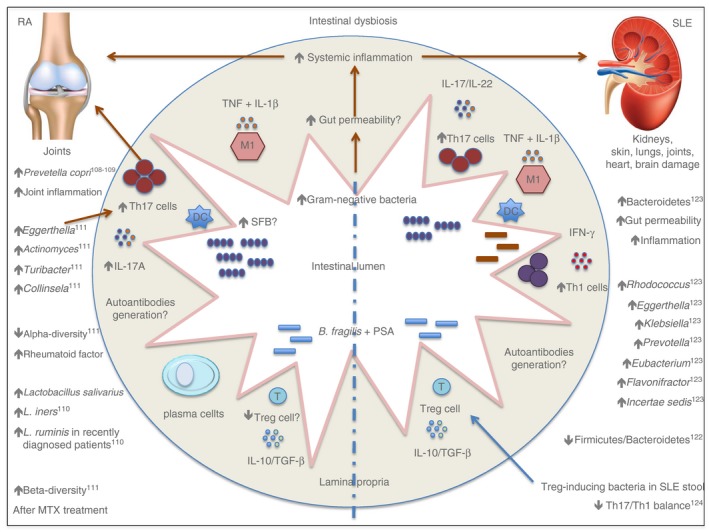
A schematic representation of intestinal dysbiosis in rheumatic autoimmune diseases in humans.
Another study, which was performed by Liu et al., investigated the Lactobacillus community by quantitative real‐time PCR in faecal samples from 15 patients with RA and 15 healthy controls and reported increased absolute copy numbers of Lactobacillus salivarius, Lactobacillus iners and Lactobacillus ruminis in untreated RA patients that were recently diagnosed.110
Chen et al. identified the gut microbiota profile in patients with RA and found decreased species richness (α‐diversity) that positively correlated with increased rheumatoid factor levels and disease progression.111 The authors evaluated 40 patients with RA and 32 healthy controls and found increased Eggerthella, Actinomyces, Turibacter, Streptococcus and Collinsela reads in the gut microbiota of people with RA, with positive correlations with the pro‐inflammatory cytokine IL‐17. The rheumatoid factor, C‐reactive protein, disease progression and methotrexate treatment correlated with β‐diversity found in the gut microbiota of patients with RA, suggesting that these clinical data might a play role in gut microbiota modulation111 (Fig. 3).
Increasing evidence suggests an association between intestinal dysbiosis and rheumatic diseases and their role in disease progression and the inflammatory microenvironment. Future studies should demonstrate the role of gut inflammation as a trigger for the development of autoimmunity and RA. The determination of an intestinal dysbiotic signature will provide us with the means to develop therapeutic tools for the adjuvant treatment of immune‐mediated diseases.112, 113
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune and heterogeneous disease characterized by damage to the skin, kidneys, lungs, joints, heart and brain.114 The disease affects mainly females, and its worldwide prevalence varies from 30 to 60 per 100 000 in the UK and the USA.115 The pathogenesis of SLE may involve genetic and environmental factors, such as viral infections, defective apoptosis and solar exposure to ultraviolet‐B waves. Regarding immune response, it is known that autoantibodies bind mainly with nuclear and cytoplasmic antigens.116 Moreover, increased evidence has emerged that suggests the role of intestinal dysbiosis in SLE development.117
In female lupus‐prone mice, Zhang et al. reported a decrease in the relative abundance of Lactobacillus spp. and an increase in Lachnospiraceae members when compared with controls. Early disease onset and severe symptoms correlated with increased Lachnospiraceae reads in female lupus‐prone mice. Additionally, the number of Clostridiaceae and Lachnospiraceae reads increased at specific time‐points during disease progression.118 Another study reported that dietary intervention, such as caloric restriction, in NZB/W F1 mice promoted changes in the gut microbiota and avoided disease progression in this animal model.119
Similarly, Johnson et al. reported that (SWR × NZB) F1 mice given drinking water with a low pH have altered gut microbiota and decreased antinuclear antibodies, and develop nephritis more slowly, suggesting that gut microbiota modulation might influence disease progression.120 In addition, when examining GF lymphotoxin‐deficient mice, Van Praet et al. reported that the intestinal microbiota could play a role in antinuclear antibody induction and could be associated with SFB colonization and IL‐17 receptor signalling.121
Hevia et al. analysed human stool samples from 20 patients with SLE and 20 healthy controls, and the results showed decreased Firmicutes : Bacteroidetes ratios in the patients with SLE. Additionally, in silico analysis suggested that the intestinal dysbiosis observed in patients with SLE could be linked to an increase in oxidative phosphorylation and the glycan metabolism pathways induced by patients' intestinal microbiota.122 In this manner, another study evaluated stool samples from 45 patients with SLE and 48 control subjects and reported a decreased amount of Firmicutes members, an increased level of Bacteroidetes members, and a prevalence of Rhodococcus, Eggerthella, Klebsiella, Prevotella, Eubacterium, Flavonifractor and Incertae sedis genera in patients with SLE, suggesting a gut microbiota profile for patients with SLE123 (Fig. 3).
Based on these reports showing intestinal dysbiosis in patients with SLE, López et al. evaluated the role of microbes derived from stool samples from patients with SLE and controls in the in vitro differentiation of Treg, Th1 and Th17 cells. The authors showed that patients' samples induced Th17 differentiation and supplementation with Treg‐inducing bacteria significantly decreased the Th17/Th1 balance, supporting the use of these strains as therapeutic probiotics for autoimmune diseases.124
Probiotic applications in autoimmune diseases
According to the WHO, a probiotic is ‘a live organism, which provides a benefit to the host when provided in adequate quantities'.125 Studies suggest that probiotics influence systemic immune responses, ensure the homeostasis of the healthy microbiota in the intestinal mucosa and could, therefore, be used as adjuvant therapy to treat immune‐mediated diseases.125 The mechanisms proposed to achieve this include mucus secretion, antimicrobial peptide production, the maintenance of the function of the gastrointestinal–epithelial barrier, ensuring adequate interactions between the gut microbiota and the mucosal immune cells, and finally, helping the activation of the host immune system in response to pathobionts.126 Here, we reported the results of the main clinical trials concerning the applicability of probiotics in autoimmune diseases.
In a recent study in NOD mice, the oral administration of a Lactobacillaceae‐enriched probiotic protects mice from T1D by suppressing IL‐1β expression and the release of immunomodulatory indoleamine 2,3‐dioxygenase and by promoting the differentiation of CD103+ tolerogenic dendritic cells in the gut.127
In humans, a TEDDY study group evaluated probiotic supplementation for children with genetic risk for T1D during their first year of life. This multicentre prospective cohort study (USA, Finland, Germany and Sweden) investigated 7473 children ranging from 4 to 10 years in age. Early probiotic administration was correlated with a decreased risk of islet autoimmunity when compared with the group that received probiotics after 27 days of life or no supplementation.128
Several studies in EAE mice demonstrated the immunoregulatory functions of probiotic administration. Treatment with Lactobacillus spp., Pediococcus acidolactici, Bifidobacterium bifidum, Bifidobacterium animalis and Bacteroides fragilis improved CNS inflammation through the induction of Treg cells in the gut mucosa by promoting the secretion of IL‐10 and transforming growth factor‐β,and inducing decreased Th1/Th17 inflammatory subsets.86, 129, 130, 131, 132, 133
In humans, Kouchaki et al. reported improved Expanded Disability Status Score, insulin resistance and a decrease in inflammatory markers in MS patients treated with probiotic supplementation containing Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus fermentum and Bifidobacterium bifidum. This randomized double‐blind placebo‐controlled clinical trial analysed probiotic intake for 12 weeks in 60 MS patients.134
In a recent study, the oral administration of Bacillus coagulans, which has an anti‐inflammatory effect, promoted a decrease in the level of serum amyloid A protein and decreased tumour necrosis factor serum levels in rat models of RA.134
Some performed studies evaluating the effect of probiotics as an adjuvant therapy for RA treatment have shown no significant results.135 Some of these conducted studies have smaller numbers of patients and a short period of treatment.136 In a double‐blind, placebo‐controlled trial, the oral administration of Lactobacillus rhamnosus and Lactobacillus reuteri for 3 months to 29 RA patients did not improve the disease, measured by the American College of Rheumatology criteria (ACR20). However, the authors reported functional improvement within the probiotic supplementation group compared with the placebo group.136
Vaghef‐Mehrabany et al. investigated the role of Lactobacillus casei intake in 46 RA patients for 8 weeks. This randomized, double‐blind placebo‐controlled trial showed improvement in disease activity score, increased levels of serum IL‐10, and decreased levels of tumour necrosis factor, IL‐6 and IL‐12 in treated patients.137 Another clinical trial with the same study design evaluated the administration of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum for 8 weeks in 60 RA patients. Probiotic intake improved DAS28, decreased the level of serum C‐reactive protein, and promoted a decrease in the insulin levels.138
In a lupus‐like animal model, the administration of retinoic acid restored Lactobacillus spp. and improved lupus symptoms, suggesting the use of these species as a probiotic to diminish inflammation in patients with SLE.118 Some Lactobacillus species have been demonstrated to have immunomodulatory properties in the host gut mucosa, such as inhibiting neutrophil extracellular trap formation, improving antioxidant status and increasing the expression of adhesion molecules in the gut.139, 140 However, currently, there are no clinical trials reported at clinicaltrials.gov investigating the role of probiotics as an adjuvant therapy in the treatment of patients with SLE.
Future studies with higher numbers of patients and longer evaluation times are necessary to corroborate these results. Such confirmation may lead to the routine use of probiotics as an adjunctive therapy in the treatment of immune‐mediated diseases. However, these studies should take into consideration the patient's immunological status before probiotic introduction.
Conclusions
Emerging findings associate intestinal dysbiosis with autoimmune disease pathogenesis. Mucosal surfaces with impaired microbiota function and diversity, such as in the gut, could represent a trigger site of autoimmunity by neo‐antigen generation under dysbiotic conditions. If this hypothesis is validated, all the generated data could collaborate in the discovery of new probiotics, predictive biomarkers and therapeutic approaches for use in clinical settings for the adjuvant treatment of autoimmune diseases.
Disclosures
The authors report no conflict of interest.
References
- 1. Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 2013; 13:321–35. [DOI] [PubMed] [Google Scholar]
- 2. Karczewski J, Poniedzialek B, Adamski Z, Rzymski P. The effects of the microbiota on the host immune system. Autoimmunity 2014; 47:494–504. [DOI] [PubMed] [Google Scholar]
- 3. Peterson CT, Sharma V, Elmén L, Peterson SN. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol 2015; 179:363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD et al Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol 1999; 65:4799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez‐Lopez A, Behnsen J, Nuccio SP, Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol 2016; 16:135–48. [DOI] [PubMed] [Google Scholar]
- 6. Webb CR, Koboziev I, Furr KL, Grisham MB. Protective and pro‐inflammatory roles of intestinal bacteria. Pathophysiology 2016; 23:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marietta E, Rishi A, Taneja V. Immunogenetic control of the intestinal microbiota. Immunology 2015; 145:313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonnenbrug JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016; 535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin R, Nauta AJ, Ben Amor K, Knippels LM, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes 2010; 1:367–82. [DOI] [PubMed] [Google Scholar]
- 10. Dominguez‐Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010; 107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez‐Bello MG, Contreras M et al Human gut microbiome viewed across age and geography. Nature 2012; 486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dore J, Blotière H. The influence of diet on the gut microbiota and its consequences for health. Curr Opin Biotechnol 2015; 32:195–9. [DOI] [PubMed] [Google Scholar]
- 13. Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010; 10:159–69. [DOI] [PubMed] [Google Scholar]
- 14. Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 2011; 10:311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol 2011; 12:9–23. [DOI] [PubMed] [Google Scholar]
- 16. Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol 2015; 172:R167–77. [DOI] [PubMed] [Google Scholar]
- 17. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 2011; 474:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012; 3:289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M et al Diversity of the human intestinal microbial flora. Science 2005; 308:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2016; 14:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014; 7:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol 2012; 30:759–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gill N, Finlay BB. The gut microbiota: challenging immunology. Nat Rev Immunol 2011; 11:636–7. [DOI] [PubMed] [Google Scholar]
- 24. Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S et al Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol 2002; 168:57–64. [DOI] [PubMed] [Google Scholar]
- 25. Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol 2013; 6:451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi Y, Mu L. An expanding stage for commensal microbes in host immune regulation. Cell Mol Immunol 2017; 14:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kubinak JL, Round JL. Do antibodies select a healthy microbiota? Nat Rev Immunol 2016; 26:767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomkovich S, Jobin C. Microbiota and host immune responses: a love–hate relationship. Immunology 2016; 147:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamada N, Múñez G. Role of the gut microbiota in the development and function of lymphoid cells. J Immunol 2013; 190:1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011; 9:356–68. [DOI] [PubMed] [Google Scholar]
- 31. Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010; 140:845–58. [DOI] [PubMed] [Google Scholar]
- 32. Ivanov II, Frutos RL, Manel N, Yoshinaga K, Rifkin D, Sartor RB et al Specific microbiota direct the differentiation of IL‐17‐producing T‐helper cells in the mucosa of the small intestine. Cell Host Microbe 2008; 4:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaboriau‐Routhiau V, Rakotobe S, Lécuver E, Mulder I, Lan A, Bridonneau C et al The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009; 31:677–89. [DOI] [PubMed] [Google Scholar]
- 34. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U et al Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw MH, Kamada N, Kim YG, Núñez G. Microbiota‐induced IL‐1β, but not IL‐6, is critical for the development of steady‐state TH17 cells in the intestine. J Exp Med 2012; 209:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan TG, Sefik E, Geva‐Zatorsky N, Kua L, Naskar D, Teng F et al Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci USA 2016; 113:E8141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y et al Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H et al Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500:232–6. [DOI] [PubMed] [Google Scholar]
- 39. Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S et al Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011; 34:794–806. [DOI] [PubMed] [Google Scholar]
- 40. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T‐cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA 2010; 107:12204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell‐dependent and T cell‐independent IgA synthesis. Annu Rev Immunol 2010; 28:243–73. [DOI] [PubMed] [Google Scholar]
- 42. Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honio T. Critical roles of activation‐induced cytidine deaminase in the homeostasis of gut flora. Science 2002; 298:1424–7. [DOI] [PubMed] [Google Scholar]
- 43. Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol 2010; 8:656–67. [DOI] [PubMed] [Google Scholar]
- 44. Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004; 303:1662–5. [DOI] [PubMed] [Google Scholar]
- 45. Macpherson AJ, Geuking MB, McCoy KD. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol 2012; 33:160–7. [DOI] [PubMed] [Google Scholar]
- 46. Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M et al Prominent role for plasmacytoid dendritic cells in mucosal T cell‐independent IgA induction. Immunity 2011; 34:247–57. [DOI] [PubMed] [Google Scholar]
- 47. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012; 3:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosser EC, Mauri C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J Autoimmun 2016; 74:85–93. [DOI] [PubMed] [Google Scholar]
- 49. Lerner A, Aminov R, Matthias T. Dysbiosis may trigger autoimmune diseases via inappropriate post‐translational modification of host proteins. Front Microbiol 2016; 7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hara N, Alkanani AK, Ir D, Robertson CE, Wagner BD, Frank DN et al The role of the intestinal microbiota in type 1 diabetes. Clin Immunol 2013; 146:112–9. [DOI] [PubMed] [Google Scholar]
- 51. Atkinson MA, Chervonsky A. Does the gut microbiota have a role in type 1 diabetes? Early evidence from humans and animal models of the disease. Diabetologia 2012; 55:2868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. International Diabetes Federation . International Diabetes Federation Atlas, 7th edn Karakas Print: Brussels, 2015. [Google Scholar]
- 53. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesvan L, Stonebraker AC et al Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008; 455:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Emani R, Alam C, Pekkala S, Zafar S, Emani MR, Hänninen A. Peritoneal cavity is a route for gut‐derived microbial signals to promote autoimmunity in non‐obese diabetic mice. Scand J Immunol 2015; 81:102–9. [DOI] [PubMed] [Google Scholar]
- 55. Alam C, Bittoun E, Bhagwat D, Valkonen S, Saari A, Jaakkola U et al Effects of a germ‐free environment on gut immune regulation and diabetes progression in non‐obese diabetic (NOD) mice. Diabetologia 2011; 54:1398–406. [DOI] [PubMed] [Google Scholar]
- 56. Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA 2011; 108:11548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roesch LF, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM et al Culture‐independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J 2009; 3:536–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek‐Tenace LM, Hatch M et al Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr 2005; 40:589–95. [DOI] [PubMed] [Google Scholar]
- 59. Bosei E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E et al Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia 2006; 49:2824–7. [DOI] [PubMed] [Google Scholar]
- 60. Sapone A, De Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F et al Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006; 55:1443–9. [DOI] [PubMed] [Google Scholar]
- 61. Vaarala O. Human intestinal microbiota and type 1 diabetes. Curr Diab Rep 2013; 13:601–7. [DOI] [PubMed] [Google Scholar]
- 62. Hu C, Wong FS, Wen L. Type 1 diabetes and gut microbiota: friends or foe? Pharmacol Res 2015; 98:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G et al Toward defining the autoimmune microbiome for type 1 diabetes. ISME J 2011; 5:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Goffau MC, Fuentes S, Van Den Bogert B, Honkanen H, de Vos WM, Welling GW et al Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 2014; 57:1569–77. [DOI] [PubMed] [Google Scholar]
- 65. Li X, Atkinson MA. The role for gut permeability in the pathogenesis of type 1 diabetes – a solid or leaky concept? Pediatr Diabetes 2015; 16:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. De Goffau MC, Luopaiärvi K, Knip M, Ilonen J, Ruohtula T, Härkönen T et al Fecal microbiota composition differs between children with β‐cell autoimmunity and those without. Diabetes 2013; 62:1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brown CT, Davis‐Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N et al Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One 2011; 6:e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Endesfelder D, Zu Castell W, Ardissone A, Davis‐Richardson AG, Achenbach P, Hagen M et al Compromised gut microbiota networks in children with anti‐islet cell autoimmunity. Diabetes 2014; 63:2006–14. [DOI] [PubMed] [Google Scholar]
- 69. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate‐producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 2016; 7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Arpaia N, Campbell C, Fan X, Dikiv S, Van der Veeken J, De Roos P et al Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature 2013; 504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504:446–50. [DOI] [PubMed] [Google Scholar]
- 72. Murri M, Leiva I, Gomez‐Zumaquero JM, Tinahones FJ, Cardona F, Soriquer F et al Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case–control study. BMC Med 2013; 21:11–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Davis‐Richardson AG, Ardissone AN, Dias R, Simell V, Leonard MT, Kemppainen KM et al Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol 2014; 5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kostic AD, Gevers D, Siljander H, Vatanen T, Hyötyläinen T, Hämäläinen AM et al The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015; 17:260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alkanani AK, Hara N, Gottlieb PA, Ir D, Robertson CE, Wagner BD et al Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 2015; 64:3510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meiía‐León ME, Petrosino JF, Aiami NJ, Domínguez‐Bello MG, De La Barca AM. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci Rep 2014; 4:3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Paun A, Yau C, Danska JS. The influence of the microbiome on type 1 diabetes. J Immunol 2017; 198:590–5. [DOI] [PubMed] [Google Scholar]
- 78. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol 2015; 15:545–58. [DOI] [PubMed] [Google Scholar]
- 79. Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV et al Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014; 83:1022–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C et al Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011; 479:538–41. [DOI] [PubMed] [Google Scholar]
- 81. Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T‐cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2011; 108:4615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ochoa‐Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque‐Begum S et al Role of gut commensal microbiota in the development of experimental autoimmune encephalomyelitis. J Immunol 2009; 183:6041–50. [DOI] [PubMed] [Google Scholar]
- 83. Forsythe P, Kunze WA. Voices from within: gut microbes and the CNS. Cell Mol Life Sci 2013; 70:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ochoa‐Repáraz J, Mielcarz DW, Haque‐Begum S, Kasper LH. Induction of regulatory B population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes 2010; 1:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol 2015; 17:344. [DOI] [PubMed] [Google Scholar]
- 86. Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G et al A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL‐10 producing regulatory T cells. PLoS One 2010; 5:e9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bhargava P, Mowey EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep 2014; 14:492. [DOI] [PubMed] [Google Scholar]
- 88. Jhangi S, Gandhi R, Glanz B, Cook S, Nejad P, Ward D et al Increased Archaea species and changes with therapy in gut microbiome of multiple sclerosis subjects. Neurology 2014; 82:S24.001. [Google Scholar]
- 89. Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T et al Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One 2015; 10:e0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J et al Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Invest Med 2015; 63:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bang C, Weidenbach K, Gutsmann T, Heine H, Schmitz RA. The intestinal archaea Methanosphaera stadtmanae and Methanobrevibacter smithii activate human dendritic cells. PLoS One 2014; 9:e99411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, de Vos WM. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin‐degrader Akkermansia muciniphila . Front Microbiol 2011; 2:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ganesh BP, Klopfleisch R, Loh G, Blaut M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium‐infected gnotobiotic mice. PLoS One 2013; 8:e74963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jangi S, Gandhi R, Cox LM, Li N, Von Glehn F, Yan R et al Alterations of the human microbiome in multiple sclerosis. Nat Commun 2016; 7:12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA et al Linking long‐term dietary patterns with gut microbial enterotypes. Science 2011; 334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MM et al Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016; 6:28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Colpitts SL, Kasper LH. Influence of the gut microbiome on autoimmunity in the central nervous system. J Immunol 2017; 198:596–604. [DOI] [PubMed] [Google Scholar]
- 98. Malmström V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nat Rev Immunol 2017; 17:60–75. [DOI] [PubMed] [Google Scholar]
- 99. Klareskog L, Padyukov L, Lorentzen J, Alfredsson L. Mechanisms of disease: genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat Clin Pract Rheumatol 2006; 2:425–33. [DOI] [PubMed] [Google Scholar]
- 100. Brusca SB, Abramson SB, Scher JU. Microbiome and mucosal inflammation as extra‐articular triggers for rheumatoid arthritis and autoimmunity. Curr Opin Rheumatol 2014; 26:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dorożyńska I, Majewska‐Szczepanik M, Marcińska K, Szczepanik M. Partial depletion of natural gut flora by antibiotic aggravates collagen induced arthritis (CIA) in mice. Pharmacol Rep 2014; 66:250–5. [DOI] [PubMed] [Google Scholar]
- 102. Liu X, Zeng B, Zhang J, Li W, Mou F, Wang H et al Role of the gut microbiome in modulating arthritis progression in mice. Sci Rep 2016; 6:30594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A et al Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist‐deficient mice. J Exp Med 2000; 191:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abdollahi‐Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR et al Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest 2008; 118:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rogier R, Koenders MI, Abdollahi‐Roodsaz S. Toll‐like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res 2015; 2015:527696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gomez A, Luckey D, Yeoman CJ, Marietta EV, Berg Miller ME, Murray JA et al Loss of sex and age driven differences in the gut microbiome characterize arthritis‐susceptible 0401 mice but not arthritis‐resistant 0402 mice. PLoS One 2012; 7:e36095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y et al Gut‐residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010; 32:815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C et al Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013; 2:e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K et al Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol 2016; 68:2646–61. [DOI] [PubMed] [Google Scholar]
- 110. Liu X, Zou Q, Zeng B, Fang Y, Wei H. Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Curr Microbiol 2013; 67:170–6. [DOI] [PubMed] [Google Scholar]
- 111. Chen J, Wright K, Davis JM, Jeraldo O, Marietta EV, Murray J et al An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genoma Med 2016; 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ciccia F, Ferrante A, Guggino G, Triolo G. The role of the gastrointestinal tract in the pathogenesis of rheumatic diseases. Best Pract Res Clin Rheumatol 2016; 30:889–900. [DOI] [PubMed] [Google Scholar]
- 113. Di Paola M, Cavalieri D, Albanese D, Sordo M, Pindo M, Donati C et al Alteration of fecal microbiota profiles in juvenile idiopathic arthritis. Associations with HLA‐B27 allele and disease status. Front Microbiol 2016; 7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011; 365:2110–21. [DOI] [PubMed] [Google Scholar]
- 115. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol 2016; 12:605–20. [DOI] [PubMed] [Google Scholar]
- 116. Tsokos GC, Lo MS, Reis PC, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol 2016; 12:716–30. [DOI] [PubMed] [Google Scholar]
- 117. Van de Wiele TV, Van Praet JT, Marzorati M, Drennan MB, Elewaut D. How the microbiota shapes rheumatic diseases. Nat Rev Rheumatol 2016; 12:398–411. [DOI] [PubMed] [Google Scholar]
- 118. Zhang H, Liao X, Sparks JB, Luo XM. Dynamics of gut microbiota in autoimmune lupus. Appl Environ Microbiol 2014; 80:7551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hsieh CC, Lin BF. Dietary factors regulate cytokines in murine models of systemic lupus erythematosus. Autoimmun Rev 2011; 11:22–7. [DOI] [PubMed] [Google Scholar]
- 120. Johnson BM, Gaudreau MC, Al‐Gadban MM, Gudi R, Vasu C. Impact of dietary deviation on disease progression and gut microbiome composition in lupus‐prone SNF1 mice. Clin Exp Immunol 2015; 181:323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Van Praet JT, Donovan E, Vanassche I, Drennan MB, Windels F, Dendooven A et al Commensal microbiota influence systemic autoimmune responses. EMBO J 2015; 34:466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hevia A, Milani C, López P, Cuervo A, Arboleva S, Duranti S et al Intestinal dysbiosis associated with systemic lupus erythematosus. MBio 2014; 5:e01548–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. He Z, Shao T, Li H, Xie Z, Wen C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog 2016; 8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. López P, de Paz B, Rodrígues‐Carrio J, Hevia A, Sánchez B, Margolles A et al Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci Rep 2016; 6:24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 2010; 7:503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT et al Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 2017; 117:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dolpady J, Sorini C, Di Pietro C, Cosorich I, Ferrarese R, Saita D et al Oral probiotic VSL#3 prevents autoimmune diabetes by modulating microbiota and promoting indoleamine 2,3‐dioxygenase‐enriched tolerogenic intestinal environment. J Diabetes Res 2016; 2016:7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Uusitalo U, Liu X, Yang J, Aronsson CA, Hummel S, Butterworth M et al Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr 2016; 170:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ezendam J, De Klerk A, Gremmer ER, Van Loveren H. Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents. Clin Exp Immunol 2008; 154:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A et al Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol 2013; 146:217–27. [DOI] [PubMed] [Google Scholar]
- 131. Takata K, Kinoshita M, Okuno T, Moriya M, Kohda T, Honorat JA et al The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL‐10‐producing regulatory T cells. PLoS One 2011; 6:e27644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ochoa‐Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum‐Haque S, Dasgupta S et al Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol 2010; 185:4101–8. [DOI] [PubMed] [Google Scholar]
- 133. Ochoa‐Repáraz J, Mielcarz DW, Wang Y, Begum‐Haque S, Dasgupta S, Kasper DL et al A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol 2010; 3:487–95. [DOI] [PubMed] [Google Scholar]
- 134. Kouchaki E, Tamtaii OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E et al Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double‐blind, placebo‐controlled trial. Clin Nutr 2016; 16:30214‐X. [DOI] [PubMed] [Google Scholar]
- 135. Abhari K, Shekarforoush SS, Hosseinzadeh S, Nazifi S, Sajedianfard J, Eskandari MH. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr Res 2016; 60:30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pineda MA, Thompson SF, Summers K, De Leon F, Pope J, Reid G. A randomized, double‐blinded, placebo‐controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit 2011; 17:CR347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Vaghef‐Mehrabany E, Alipour B, Homayouni‐Rad A, Sharif SK, Asghari‐Jafarabadi M, Zavvari ZS. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014; 30:430–5. [DOI] [PubMed] [Google Scholar]
- 138. Zamani B, Golkar HR, Farshbaf S, Emadi‐Baygi M, Tajabadi‐Ebrahimi M, Jafari P et al Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double‐blind, placebo‐controlled trial. Int J Rheum Dis 2016; 19:869–79. [DOI] [PubMed] [Google Scholar]
- 139. Vong L, Lorentz RJ, Assa A, Glogauer M, Sherman PM. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J Immunol 2014; 192:1870–7. [DOI] [PubMed] [Google Scholar]
- 140. Mu Q, Zhang H, Luo XM. SLE: another autoimmune disorder influenced by microbes and diet? Front Immunol 2015; 6:608. [DOI] [PMC free article] [PubMed] [Google Scholar]


