Abstract
The bacteriophage SP01 genome encodes a virus-specific type II DNA-binding protein, TF1. The bacterial proteins of this ubiquitous and evolutionarily conserved class are thought to bind non-specifically to DNA. In contrast, the experiments described here demonstrate that TF1 binds to specific sites in SP01 DNA. Several of these sites have been characterized by DNase I 'footprinting' and four of them have been shown to overlap strong phage promoters for Bacillus subtilis RNA polymerase holoenzyme. We speculate on the possible structural basis of site-selective DNA binding by a protein of this class.
Full text
PDF
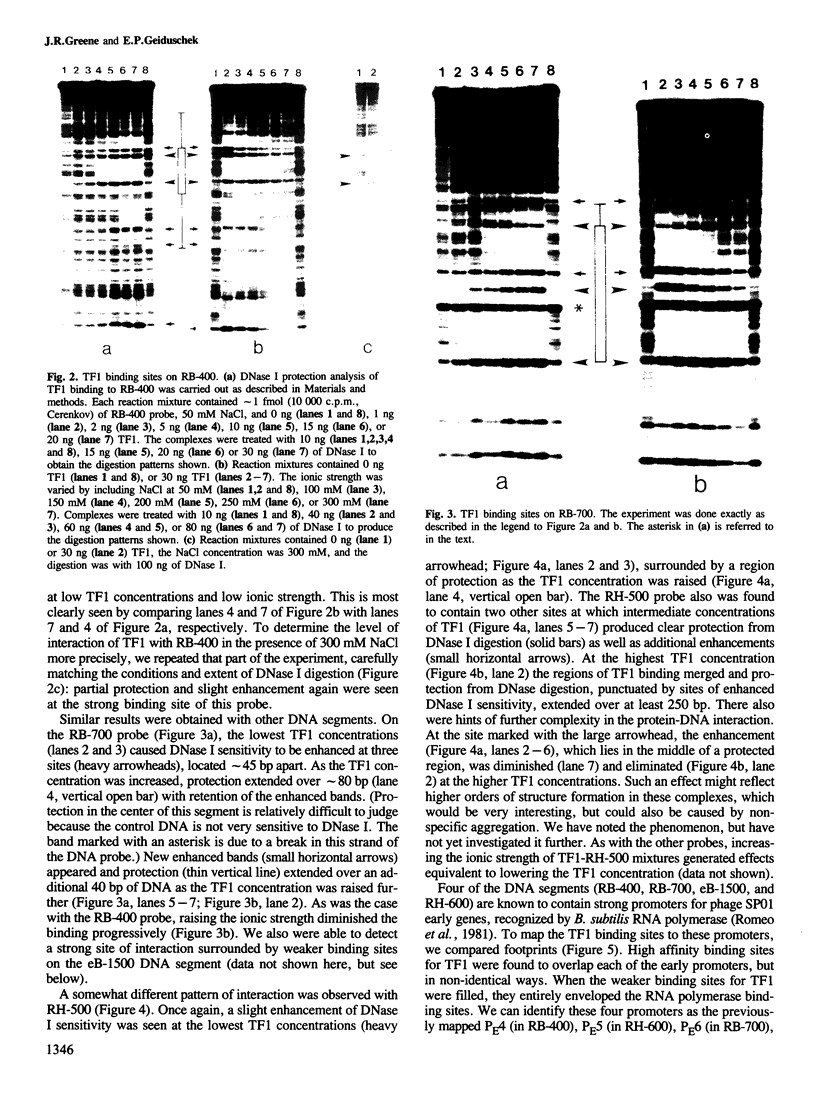

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthold V., Geider K. Interaction of DNA with DNA-binding proteins. The characterization of protein HD from Escherichia coli and its nucleic acid complexes. Eur J Biochem. 1976 Dec 11;71(2):443–449. doi: 10.1111/j.1432-1033.1976.tb11132.x. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Stewart C. R. EcoRI cleavage of DNA from Bacillus subtilis phage SPO1. Virology. 1978 Apr;85(2):601–605. doi: 10.1016/0042-6822(78)90464-6. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Leighton T., Rabinowitz J. C. Purification of Bacillus subtilis RNA polymerase with heparin-agarose. In vitro transcription of phi 29 DNA. J Biol Chem. 1979 Sep 25;254(18):9220–9226. [PubMed] [Google Scholar]
- Dickerson R. E. Base sequence and helix structure variation in B and A DNA. J Mol Biol. 1983 May 25;166(3):419–441. doi: 10.1016/s0022-2836(83)80093-x. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. J., Carver D., Gellert M. Synergistic effect of himA and gyrB mutations: evidence that him functions control expression of ilv and xyl genes. J Bacteriol. 1984 Feb;157(2):484–489. doi: 10.1128/jb.157.2.484-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Armelin M. C., Petrusek R., Bread C., Duffy J. J., Johnson G. Effects of the transciption inhibitory protein, TF1, on phage SP01 promoter complex formation and stability. J Mol Biol. 1977 Dec 25;117(4):825–842. doi: 10.1016/s0022-2836(77)80001-6. [DOI] [PubMed] [Google Scholar]
- Greene J. R., Brennan S. M., Andrew D. J., Thompson C. C., Richards S. H., Heinrikson R. L., Geiduschek E. P. Sequence of the bacteriophage SP01 gene coding for transcription factor 1, a viral homologue of the bacterial type II DNA-binding proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7031–7035. doi: 10.1073/pnas.81.22.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. G., Geiduschek E. P. Purification of the bacteriophage SP01 transcription factor 1. J Biol Chem. 1972 Jun 10;247(11):3571–3578. [PubMed] [Google Scholar]
- Johnson G. G., Geiduschek E. P. Specificity of the weak binding between the phage SPO1 transcription-inhibitory protein, TF1, and SPO1 DNA. Biochemistry. 1977 Apr 5;16(7):1473–1485. doi: 10.1021/bi00626a036. [DOI] [PubMed] [Google Scholar]
- Kimura M., Wilson K. S. On the DNA binding protein II from Bacillus stearothermophilus. II. The amino acid sequence and its relation to those of homologous proteins from other prokaryotes. J Biol Chem. 1983 Mar 25;258(6):4007–4011. [PubMed] [Google Scholar]
- Leong J. M., Nunes-Düby S., Lesser C. F., Youderian P., Susskind M. M., Landy A. The phi 80 and P22 attachment sites. Primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985 Apr 10;260(7):4468–4477. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Ohlendorf D. H., Anderson W. F., Matthews B. W. Many gene-regulatory proteins appear to have a similar alpha-helical fold that binds DNA and evolved from a common precursor. J Mol Evol. 1983;19(2):109–114. doi: 10.1007/BF02300748. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Pero J., Hannett N. M., Talkington C. Restriction cleavage map of SP01 DNA: general location of early, middle, and late genes. J Virol. 1979 Jul;31(1):156–171. doi: 10.1128/jvi.31.1.156-171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière-Yaniv J. Localization of the HU protein on the Escherichia coli nucleoid. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):439–447. doi: 10.1101/sqb.1978.042.01.047. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Tanaka I., Appelt K., Dijk J., White S. W., Wilson K. S. 3-A resolution structure of a protein with histone-like properties in prokaryotes. Nature. 1984 Aug 2;310(5976):376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Geiduschek E. P. A template-selective inhibitor of in vitro transcription. Proc Natl Acad Sci U S A. 1969 Feb;62(2):514–520. doi: 10.1073/pnas.62.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]