Abstract
OBJECTIVES:
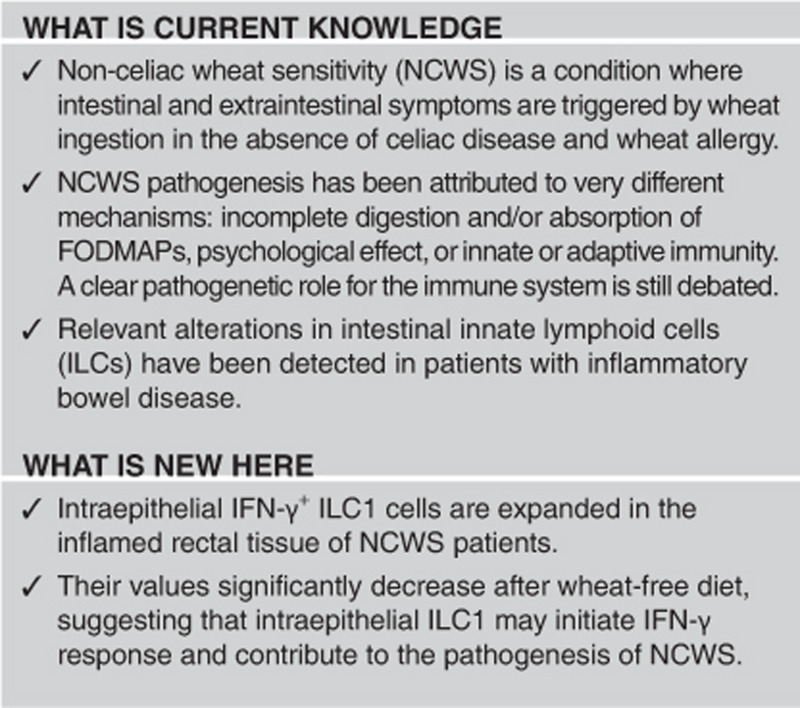
Non-celiac wheat sensitivity (NCWS) is defined as a reaction to ingested wheat after exclusion of celiac disease and wheat allergy. As its pathogenesis is incompletely understood, we evaluated the inflammatory response in the rectal mucosa of patients with well-defined NCWS.
METHODS:
The prospective study included 22 patients with irritable bowel syndrome (IBS)-like clinical presentation, diagnosed with NCWS by double-blind placebo-controlled challenge. Eight IBS patients not improving on wheat-free diet were used as controls. Two weeks after oral challenge was performed with 80 grams of wheat daily, cells were isolated from rectal biopsies and thoroughly characterized by fluorescence-activated cell sorting analysis for intracellular cytokines and surface markers.
RESULTS:
Rectal biopsies from wheat-challenged NCWS patients showed that a significant mucosal CD45+ infiltrate consisted of CD3+ and CD3− lymphocytes, with the latter spontaneously producing more interferon (IFN)-γ than IBS controls. About 30% of IFN-γ-producing CD45+ cells were T-bet+, CD56−, NKP44−, and CD117−, defining them as a type-1 innate lymphoid cells (ILC1). IFN-γ-producing ILC1 cells significantly decreased in 10 patients analyzed 2 weeks after they resumed a wheat-free diet.
CONCLUSIONS:
These data indicate that, in patients with active NCWS, IFN-γ-producing ILC1 cells infiltrate rectal mucosa and support a role for this innate lymphoid cell population in the pathogenesis of NCWS.
INTRODUCTION
Non-celiac gluten sensitivity (NCGS) has attracted increasing attention as a common cause of morbidity. NCGS is a condition where intestinal and extraintestinal symptoms are triggered by gluten ingestion in the absence of celiac disease and wheat allergy, as defined by discussions held at three different international consensus conferences.1, 2, 3
Despite the great interest in NCGS, much remains unknown about the pathogenesis. Some studies seem to suggest that wheat components other than gluten can cause the symptoms, and therefore the term “non-celiac wheat sensitivity” (NCWS) has been proposed instead of NCGS.4, 5 NCWS pathogenesis has been attributed to very different mechanisms: incomplete digestion and/or absorption of fermentable oligosacchararides and disacchararides, monosaccharides and polyols,6 psychological effect,7 or innate or adaptive immunity.8, 9, 10, 11
Innate lymphoid cells (ILCs) are a new, well-characterized population of innate immune cells, morphologically resembling T cells and derived from an Id2-dependent common lymphoid precursor.12, 13, 14, 15, 16, 17, 18 ILCs are divided into three major groups on the basis of a different transcriptional control and cytokine responsiveness and production. ILCs belonging to the group 1 include both natural killer (NK) and non-cytolytic ILC1. These non-cytolytic ILC1 are characterized by the expression of the transcription factor T-bet and production of interferon (IFN)-γ in response to interleukin (IL)-12, IL,-15 and IL-18 and thus resemble CD4+ T helper 1 cells innate counterparts.15, 19, 20, 21, 22, 23
Group 2 ILCs are dependent on GATA3 and retinoic acid–related orphan receptor α transcription factors for development. They produce type-2 cytokines such as IL-13, IL-5, IL-9, and amphiregulin in response to IL-25 and IL-33 produced upon infection by helminth parasites, airway responsiveness, and tissue repair.15, 24 Finally, group 3 ILCs, including lymphoid tissue cells and ILC3 cells, are under control of the retinoic acid–related orphan receptor γt transcription factor. ILC3 produce IL-17A and IL-22 in response to IL-23 and IL-1β produced upon infection by extracellular bacteria. They are functionally implicated in promoting inflammation or tissue repair, especially in the bowel.15, 25, 26, 27
Recently, relevant alterations in intestinal ILCs have been detected in patients with inflammatory bowel disease. Notably, it has been shown that patients affected by Crohn’s disease had an increase of IFN-γ producing ILC1 in the lamina propria and in the intraepithelial compartment, compared with normal controls without inflammation.26, 28, 29, 30 Hence, alterations of ILC populations could be associated with the pathogenesis and outcome of some human gastrointestinal diseases.
Here we show that intraepithelial IFN-γ+ ILC1 cells are expanded in the inflamed rectal tissue of NCWS patients but values significantly decrease after gluten (wheat)-free diet, suggesting that intraepithelial ILC1 may initiate IFN-γ response and contribute to the pathogenesis of NCWS.
MATERIALS AND METHODS
Patients
We prospectively recruited adult patients with an irritable bowel syndrome (IBS)-like clinical presentation, according to the Rome II criteria, and a suspected diagnosis of NCWS. Most of the patients had been referred owing to intestinal symptoms whose onset, they reported, could be related to wheat ingestion and most of them also had extraintestinal symptoms. The patients were recruited between January 2014 and January 2015 at two centers: the Department of Internal Medicine at the University Hospital of Palermo and the Department of Internal Medicine of the Hospital of Sciacca.
During this period, >400 patients with suspected NCWS were examined, and after careful exclusion of Crohn’s disease, immunoglobulin E (IgE)-mediated wheat allergy (testing negative for serum-specific IgE and skin allergy tests), and inflammatory bowel disease diagnoses, 130 of them consented to enter one or more of our ongoing study protocols (see Supplementary File S1). These patients underwent an elimination diet and a subsequent double-blind placebo-controlled (DBPC) wheat challenge, using 80 g of wheat flour or rice flour for 2 weeks; the flour was given in packets and the patients consumed them after cooking (see Supplementary File S2).31 Sixty-one subjects tested positives to the challenge and received a definitive diagnosis of NCWS; of these, 22 patients (20 females, 2 males, age range 23–61 years, median 32 years) accepted to enter the present study. Table 1 shows the clinical characteristics of the patients enrolled in this study. These patients underwent rectal endoscopy with multiple mucosal biopsies at 5–15 cm from the anal verge at the end of the DBPC challenge, when they reported the reappearance of the symptoms. Ten patients who had reacted to the wheat challenge consented to rectoscopy and mucosal biopsies analysis 2 weeks after they resumed a wheat-free diet, when they again became asymptomatic. Of these, only 5 cases belonged to the already studied 22 patients on wheat challenge, while 5 cases were analyzed only 2 weeks after they resumed a wheat-free diet. Exclusion criteria were: (a) self-exclusion of wheat from the diet and refusal to reintroduce it, before entering the study; and (b) other “organic” gastrointestinal diseases.
Table 1. Individual clinical characteristics of the 22 patients with NCWS included in the study.
| Case | IBS kind | Extraintestinal symptoms | HLA DQ2/DQ8, yes/no | Duodenal histology | AGA IgG/IgA | Colon eos. infiltration | CD family history | Associated diseases | Other food intolerances |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IBS-A | None | DQ2 | Marsh 1 | Positives | Yes | No | Allergic rhinitis | Cow’s milk |
| 2 | IBS-D | Oligomenorrhea Headache Tiredness Foggy mind Numbness Oral aphtosis | DQ2 | Marsh 1 | Negatives | Yes | No | None | Cow’s milk |
| 3 | IBS-D | Oral aphtous Arthritis | DQ2 | Marsh 1 | Negatives | No | Yes | Ankylosing spondylitis Hashimoto disease | None |
| 4 | IBS-D | None | No | Marsh 1 | Negatives | No | No | Sarcoidosis Osteoporosis | Onion |
| 5 | IBS-C | None | No | Marsh 0 | Negatives | No | No | Allergic rhinitis | None |
| 6 | IBS-D | Headache Tiredness Foggy mind Numbness Anaemia | DQ2 | Marsh 1 | Negatives | No | Yes | None | None |
| 7 | IBS-A | Headache Tiredness Foggy mind Numbness Obesity Oligomenorrhea | No | Marsh 1 | Positives | No | No | None | None |
| 8 | IBS-D | None | DQ2 | Marsh 0 | Negatives | No | No | None | Cow’s Milk |
| 9 | IBS-A | Anemia Dyspepsia | No | Marsh 1 | Positives | Yes | No | Lichen planus | Cow’s milk |
| 10 | IBS-D | Hematochezia | DQ2 | Marsh 1 | Negatives | Yes | No | Asthma | None |
| 11 | IBS-C | Oligomenorrhea | No | Marsh 0 | Negatives | No | No | None | None |
| 12 | IBS-A | Artrhalgia Sicca syndrome | DQ2 | Marsh 1 | Negatives | Yes | No | Lichen | Cow’s Milk, egg, tomato, chocolate |
| 13 | IBS-D | Anemia | DQ2 | Marsh 0 | Negatives | Yes | Yes | Allergic rhinitis and asthma Lactose intolerance | Cow’s milk |
| 14 | IBS-C | Anemia Hypermenorrhea Headache Tiredness Foggy mind | DQ2 | Marsh 0 | Negatives | No | No | None | None |
| 15 | IBS-D | Headache Tiredness Foggy mind Numbness Artrhalgias | DQ8 | Marsh 1 | Negatives | No | No | None | None |
| 16 | IBS-D | Polymenorrhea Aphtosis | DQ2 | Marsh 0 | Negatives | No | Yes | None | Cow’s milk, egg |
| 17 | IBS-D | None | DQ2 | Marsh 1 | Negatives | Yes | No | None | Egg |
| 18 | IBS-A | Vomit Arthralgia Raynaud | DQ2 | Marsh 1 | Negatives | Yes | No | Orticaria | Cow’s milk |
| 19 | IBS-A | None | DQ2 | Marsh 1 | Positives | No | No | None | Cow’s milk |
| 20 | IBS-A | Anemia | No | Marsh 1 | Positives | No | No | None | None |
| 21 | IBS-D | Tiredness Foggy mind Numbness Aphtosis | DQ2 | Marsh 1 | Negatives | Yes | No | Recurrent vaginitis | Cow’s milk |
| 22 | IBS-A | None | No | Marsh 1 | Positives | No | No | None | Cow’s milk |
AGA, antigliadin antibodies; CD, celiac disease; eos., eosinophils; HLA, human leukocyte antigen; IBS, irritable bowel syndrome; IBS-A, IBS with alternating bowel movements; I IBS-C, IBS-constipation; BS-D, IBS-diarrhea; IgA/IgG, immunoglobulin A/G; NCWS, non-celiac wheat-sensitivity.
Intraepithelial colon eosinophil infiltration was defined according to our previous study (reference 31) as: a number of eosinophils >4 × high power fields.
As controls, we included eight subjects (seven females, one male) with IBS unrelated to NCWS or other types of food “intolerance”, who were consecutively recruited during the study period and sex- and age-matched with the NCWS patients. IBS diagnosis had been made in accordance with the Rome II criteria and none of these subjects improved on the same elimination diet as the NCWS patients. They underwent rectal mucosa biopsies when consuming a wheat containing diet—a minimum of 100 g—for at least 4 weeks (median 8 weeks, range 4–24 weeks).
This study was approved by the Ethics Committee of the University Hospital, Palermo, Italy where the patients were recruited and all of them gave written informed consent for the use and storage of tissue biopsies according to Declaration of Helsinki principles. The study was registered at clinicaltrials.gov (registration number: NCT01762579).
NCWS diagnosis
To diagnose NCWS, previously described criteria were adopted21, 22 (see Supplementary File S1).
Further inclusion criteria were: (a) age >18 years; (b) follow-up duration >6 months after the initial diagnosis; and (c) at least two outpatient visits during the follow-up period.
Isolation of rectal mucosa infiltrating cells and fluorescence-activated cell sorting analysis
Isolation of rectal mucosa–infiltrating cells was performed according to Carrasco et al.32 Briely, rectal biopsies were transferred in RPMI 1640 medium (Euroclone, Devon, UK) supplemented with 10% fetal calf serum (Euroclone), 2 mM L-glutamine (Euroclone), 20 mM HEPES (Euroclone), 100 U/ml penicillin (Euroclone), 100 μg/ml streptomycin (Euroclone), and 5 × 10−5 M 2-mercaptoethanol (Sigma, St Louis, MO) (complete medium). Tissue was first minced into small pieces followed by enzymatic digestion with 1.5 μg/ml type IV collagenase (Life Technologies, Carlsbad, CA), 20 μg/ml hyaluronidase (Sigma), and 50 μg/ml DNAase (Sigma) for 2 h at 37 °C.
After digestion, harvested cells were washed twice in fresh complete medium and stained immediately or incubated (106/ml) overnight at 37 °C in 5% CO2 for the functional assay with medium alone, Ionomycin (1 μg/ml) and phorbol myristate acetate (150 ng/ml) (Sigma) or with IL-12 (10 ng/ml), IL-15 (50 ng/ml), and IL-18 (15 ng/ml) (Sigma). For intracellular analysis of cytokine expression, the Golgi blocker monensin (10 mg/ml) (Sigma) was added to each condition. For characterization of ILCs, cells freshly obtained from rectal tissue and incubated overnight with the appropriate stimuli were stained with the following fluorochrome-conjugated monoclonal antibodies (mAbs): anti-CD45 (2D1, BD Biosciences, San Diego, CA), anti-CD56 (MY31, BD Biosciences), anti-CD117 (YB5.B8, BD Biosciences), anti-NKp44 (P44-8, Biolegend, San Diego, CA), anti-CD3 (SK7, BD Biosciences), anti-CD19 (SJ25C1, BD Biosciences), anti-CD14 (M5E2, BD Biosciences), or with isotype-matched control mAbs (X40, BD Biosciences) in incubation buffer (phosphate-buffered saline (PBS) containing 1% fetal calf serum and 0.1% Na azide) for 30 min at 4 °C. The fixable viability dye 7-aminoactinomycin D (Sigma) was added to gate-only live cells. Cells were washed twice in PBS containing1% fetal calf serum, incubated for 30 min at 4 °C in a fixation/permeabilization solution (BD Biosciences), and thereafter intracellular staining was performed with anti-IFN-γ (B27, BD Biosciences), anti-TNF-α (anti-tumor necrosis factor-α 6401.1111, BD Biosciences), anti-IL-22 (142928 R&D Systems, Minneapolis, MN), anti-IL-17 (SCPL1362, BD Biosciences), anti-T-bet (H-210, Santa Cruz Biotechnology, Dallas, TX), and isotype-matched control mAbs (BD Biosciences). For T-bet detection, a purified primary antibody incubation was followed by fluorescein isothiocyanate–conjugated goat anti-rabbit antibody (BD Biosciences, San Diego, CA) staining. Samples were acquired on a FACSCanto II (BD Biosciences) and analyzed using the FlowJo software (Miltenyi Biotec, Cologne, Germany).
Immunofluorescence on paraffin-embedded tissue sections
Rectal biopsies from NCWS patients and IBS controls were fixed with formalin solution (Sigma) and embedded in paraffin. Immunofluorescence staining was performed on 5-μm-thick paraffin-embedded sections from tissue samples. Following rehydration, antigens were unmasked for 30 min at 95 °C using Dako Target retrieval solution (Glostrup, Denmark; pH 6.0), and the sections were incubated overnight at 4 °C with a purified mouse anti-CD3 (PC3/188A, Santa Cruz Biotechnology, 1:100 in PBS containing 3% bovine serum albumin and 0.05% Tween20). An isotype-matched irrelevant antibody was used as a negative control (ab27479, AbCam, Cambridge, UK).
Stained slides were then incubated with Cy5.5 goat anti-mouse IgG (A10524, Life Technologies, 1:200 in PBS containing 3% bovine serum albumin and 0.05% Tween20) for 2 h at room temperature.
For the evaluation of intracellular molecules, sections stained for surface molecules were then fixed with 2% paraformaldehyde for 30 min and permeabilized with Triton X -100 (BioRad, Hercules, CA) for 10 min. The purified rabbit anti-human IFN-γ (Q69, Novus Biologicals Europe, Abingdon Oxon, UK) was incubated 1:100 overnight at 4 °C and then goat anti-rabbit Alexa fluor 555 (A21428, Invitrogen, Carlsbad, CA) was added to the sections for 2 h. Stained slides were fixed with 2% paraformaldehyde for 30 min and then incubated for 3 h with purified rabbit anti-T-bet (H-210, Santa Cruz Biotechnology, 1:50). Positivity for T-bet was detected by incubation for 2 h with goat anti-rabbit Alexa fluor 488 (A11008, Life Technologies) in the presence of 200 ng/ml RNAse (Sigma). An isotype-matched polyclonal antibody (rabbit IgG ab27472, Abcam) was used as a negative control for intracellular staining. Finally, nuclei were counterstained with DAPI (4,6-diamidino-2-phenylindole; Sigma), and images were acquired using a confocal laser-scanning microscope (Leica Microsystems, Milan, Italy). Multiple cells were analyzed for each staining condition, with × 40 and × 63 magnifications.
Statistical analysis
Data were analyzed for statistical significance using Mann–Whitney test for two groups and Kruskal–Wallis test for more than two groups. Differences between groups with a probability of ≤0.05 were regarded as significant. All data were analyzed using GraphPad Prism version 6.0e (GraphPad, San Diego, CA).
RESULTS
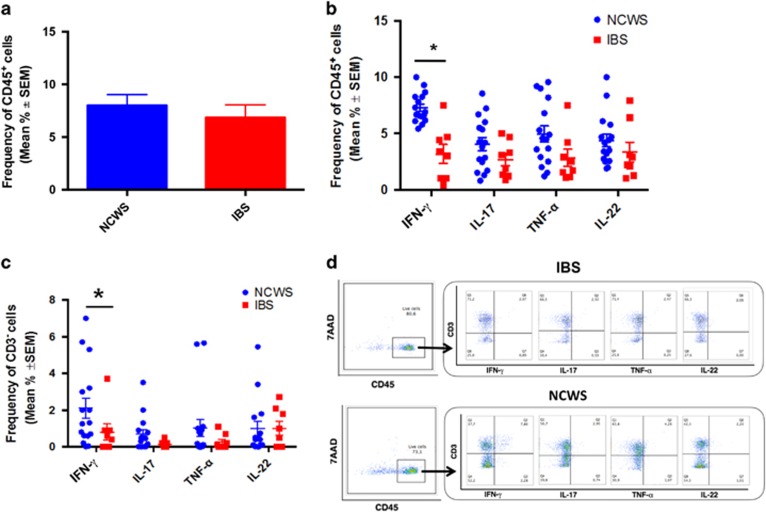
We challenged 22 NCWS patients, all on a gluten-free diet, as described under Methods section and collected rectal biopsy specimens at the end of challenge when they reported the reappearance of the symptoms. Single-cell suspensions were obtained from the specimens and were examined for cytokine expression by intracellular fluorescence-activated cell sorting analysis and compared with specimen samples from patients with IBS unrelated to NCWS or other types of food intolerance and who did not improve on wheat-free diet. As shown in Figure 1a, rectal biopsies from NCWS patients on wheat challenge had a relevant CD45+ leukocyte infiltrate (7.99±1.035%) comparable to that detected in IBS controls who did not improve on elimination diet (6.85±1.206%). The infiltrating CD45+ cells from NCWS patients spontaneously expressed IFN-γ and the percentages of CD45+IFN-γ+ cells were significantly higher than those detected in IBS patients. We also found that CD45+ cells from NCWS patients spontaneously expressed TNF-α, IL-17, and IL-22, but at lower levels than IFN-γ, even if percentages were not statistically significant compared with those detected in IBS patients (Figure 1b).
Figure 1.
Leukocyte infiltration and cytokine expression in rectal biopsies of non-celiac wheat-sensitive (NCWS) patients on wheat challenge. (a) Mean percentages of leukocytes (live CD45+ cells) infiltrating the rectal tissue of NCWS patients on wheat challenge and irritable bowel syndrome (IBS) controls. (b) Percentage of CD45+ cells and (c) percentage of CD3− cells expressing different cytokines in the rectal mucosa of NCWS patients on wheat challenge and IBS controls. Data are expressed as mean±s.e.m. *P<0.05. (d) Representative dot plots showing the expression of several cytokines by CD3+ and CD3− subsets of CD45+ cells infiltrating the rectal mucosa of IBS controls (upper panel) and NCWS patients on wheat challenge (upper panel). IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; 7AAD, 7-aminoactinomycin D.
The finding that IFN-γ expression was upregulated in human NCWS tissues after gluten challenge prompted us to investigate the source of this cytokine. We found that the major IFN-γ-producing cells were CD3+ and CD14+ cells, which were also the major source of TNF-α, IL-17, and IL-22 (data not shown). Interestingly, additional analyses of CD45+ infiltrating the rectal mucosa of wheat-challenged patients highlighted a significantly higher frequency of CD3−CD14− cells (Figure 1c) producing IFN-γ (2.6±0.9% of CD45+ cells), compared with IBS controls (0.8±0.4% of CD45+ cells) (P<0.05; Figure 1c). Of note, this CD3−CD14− fraction accounted for about one-third (32%) of all CD45+IFN-γ+ cells. Figure 1d shows representative results from one NCWS patient and one IBS control.
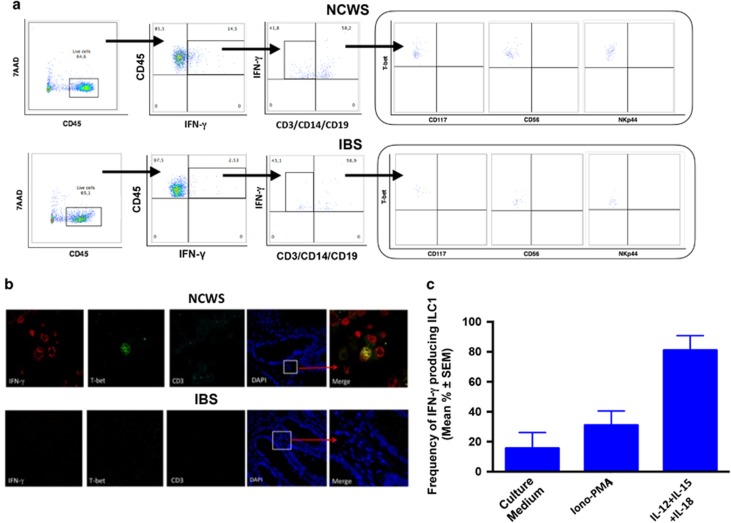
We next examined the characteristics of the CD45+CD3−CD14− population expressing IFN-γ+ in tissues from NCWS patients. As shown in Figure 2a, the whole infiltrating IFN-γ+ CD45+CD3−CD14− cells had a T-bet+, CD56−, NKP44−, and CD117− phenotype, indicating that these cells are ILC1 and are not classical NK cells. ILC1 were similarly present in the rectal mucosa of IBS controls, but overall frequencies were significantly lower (only about 3% of these cells were T-bet+ in IBS controls) than in NCWS patients upon challenge (about 15% of these cells were T-bet+).
Figure 2.
Type-1 innate lymphoid cells (ILC1) infiltrate the inflamed rectal mucosa of non-celiac wheat-sensitive (NCWS) patients upon wheat challenge. (a) Representative dot plots showing the gating strategy for the ex vivo identification and characterization of ILC1 in the rectal mucosa of NCWS patients. (b) Representative images of confocal analysis of T-bet (green), interferon (IFN)-γ (red), and negative CD3 (cyan) co-localization in the rectal mucosa of a wheat-challenged NCWS patient (upper panel) and of an irritable bowel syndrome (IBS) control (lower panel). The merge panels show triple staining of the analyzed ILC1. Nuclei were counterstained with DAPI (4,6-diamidino-2-phenylindole). (c) IFN-γ production by ILC1 infiltrating the rectal mucosa of a NCWS patient and cultured for 24 h either alone (medium) or with Ionomycin/phorbol myristate acetate (PMA) or with a combination of interleukin (IL)-12, IL-15, and IL-18. 7AAD, 7-aminoactinomycin D.
Figure 2b shows confocal microscopic analysis of the IFN-γ+ cells that infiltrated the rectal mucosa of a representative NCWS patient on wheat challenge and an IBS control: cells were detected as simultaneously positive for nuclear expression of T-bet and cytosolic expression of IFN-γ but negative for surface expression of CD3.
Functional experiments confirmed that the IFN-γ+ CD45+CD3−CD14− cell population infiltrating tissues from NCWS patients was in fact ILC1, as culture of these cells with IL-12, IL-15, and IL-18 triggered sixfold more IFN-γ production compared with culture with medium alone (81% vs. 15.6%), and even threefold more IFN-γ production compared with stimulation with Ionomycin–phorbol myristate acetate (81% vs. 31%) (Figure 2c).
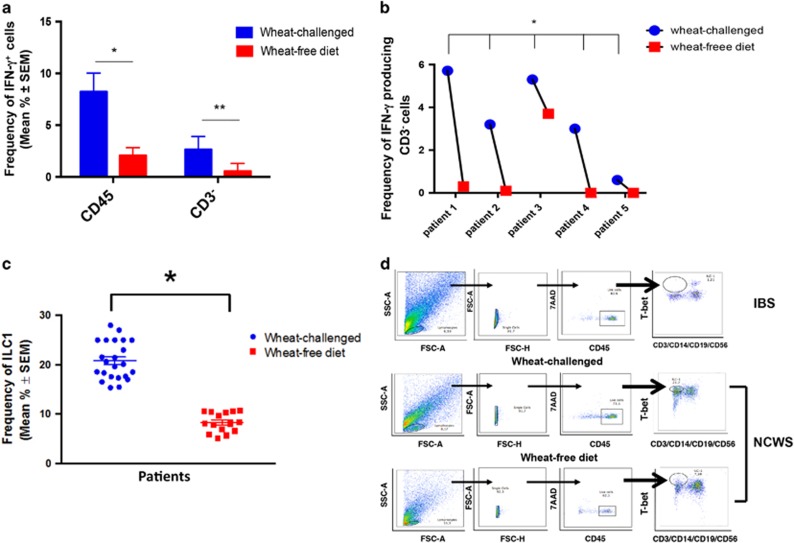
To determine whether infiltrating ILC1 cells were correlated with the pathogenesis of NCWS, we retested 10 NCWS patients 2 weeks after they resumed a wheat-free diet, when they again became asymptomatic. Of these, 5 cases belonged to the 22 patients already studied on wheat challenge and who agreed to repeat the mucosal study and 5 cases were not previously studied.
As shown in Figure 3, NCWS patients on wheat-free diet (10 cases) showed a significantly lower frequency of infiltrating IFN-γ-producing CD45+ and their CD3−CD14− fraction (P<0.01; Figure 3a), which was evident in all the 5 NCWS patients already studied on wheat challenge (Figure 3b). Accordingly, the frequency of ILC1 significantly decreased after wheat-free diet, when compared with wheat-challenged patients, as shown by cumulative data reported in Figure 3c and data from one individual patient (Figure 3d).
Figure 3.
Effect of wheat-free diet on type-1 innate lymphoid cells (ILC1) infiltrating the rectal mucosa. (a) Percentages of cytokine-producing CD45+ cells and CD3− cells infiltrating the rectal mucosa of non-celiac wheat-sensitive (NCWS) patients on wheat challenge and after wheat-free diet. Data are expressed as mean±s.e.m. *P<0.05; **P<0.01 (Mann–Whitney test). (b) Percentages of cytokine-producing CD3− cells infiltrating the rectal mucosa of five individual NCWS patients on wheat challenge and after wheat-free diet. (c) ILC1 before (or on wheat challenge) and after wheat-free diet in NCWS patients. Data are shown as mean percentage±s.e.m. *P<0.05. (d) Representative dot plots showing the percentage of ILC1 in irritable bowel syndrome (IBS) control (upper panel) and NCWS patients on wheat challenge (middle panel) and after wheat-free diet (lower panel). FSC, forward scatter; IFN, interferon; SSC, side scatter; 7AAD, 7-aminoactinomycin D.
DISCUSSION
Despite some residual skepticism, NCWS is emerging as a new clinical entity, although it is not yet well defined. The hypothesis that it is a heterogeneous condition and that people suffering from NCWS can be symptomatic due to different pathogenic mechanisms—not exclusively dependent on gluten ingestion—should be acknowledged, as suggested by many authors.5, 31, 33, 34, 35, 36, 37, 38
Recently we contributed data supporting an immunological effect of wheat ingestion,33 showing that NCWS patients have a strong tendency to autoimmunity, characterized by both associated autoimmune diseases and the presence of serum antinuclear antibodies positivity.34
However, very little is known about the immunological mechanism that starts in the intestinal mucosa as a consequence of wheat ingestion. Both innate8, 9 and adaptive immunity10, 11, 39 have been suggested as being involved in the immunological response of NCWS patients. Furthermore, experimental data showed that a component of wheat, amylase trypsin inhibitors, acts as potent stimulators of innate immune reactions by the stimulation of Toll-like receptor 4 in monocytes, macrophages, and dendritic cells.40
Our data strongly support the role of innate immunity in the pathogenesis of NCWS. We demonstrated a significant infiltration of CD45+/CD3−CD14− cells in the rectal mucosa of NCWS patients upon wheat challenge. Analysis of cytokine production demonstrated dominant spontaneous IFN-γ production by these cells, which were further identified as an ILC1 population expressing T-bet and producing IFN-γ.
A possible role for IFN-γ production in NCWS was also demonstrated in a Norwegian study,10 but authors also reported an increased infiltration of CD3+ T cells in the patient duodenal mucosa. In our study, we also detected CD3+ cells in the rectal mucosa of NCWS patients (data not shown), and studies are ongoing in our laboratories to identify their phenotypic and functional characteristics. However, the original feature of our study was the finding of ILC1 cells among infiltrating leukocytes, which decreased on wheat-free diet, indicating that they are involved in the pathogenesis of NCWS.
Innate lymphocytes are actually divided into three major groups on the basis of different transcriptional control and cytokine responsiveness and production: ILC1 are under control of the T-bet transcription factor and produce IFN-γ in response to IL-12, IL-15, or IL-18 stimulation.23, 41 Their functional response was also demonstrated in the experiments that we performed on the ILC1 cell population of the rectal mucosa of NCWS patients included in the present study.
We focused on the colon and not on the duodenal mucosa of NCWS patients as the patients included in the present study—as most of those included in the previous studies published recently—suffered from IBS-like symptoms and it is well known that IBS is very often characterized by colon mucosa inflammation.42, 43, 44 Consequently and in agreement with our unpublished data, we hypothesized that the immunological response could be more prominent in the colon rather than in the duodenal mucosa of NCWS patients.
However, the unique previous study that looked at the colon mucosa of NCWS patients did not show any difference in CD4+, CD68+, CD79+ lymphocyte infiltration during gluten-free and gluten-containing diet, but only significant decreases in the expression of zonula occludens 1, claudin-1, and occludin in rectosigmoid mucosa was demonstrated on gluten-containing diet.11
The limits of our study must be noted. First, we studied patients referred to tertiary centers with experience in Crohn’s disease and NCWS and this factor clearly led to a selection bias: therefore, our results cannot be extended to the broad population of self-treated or self-diagnosed NCWS patients. Moreover, a small patient sample was studied and we agree that a percentage of self-reported NCWS patients did not have an immunological mechanism as the basis of their symptoms.
Second, we cannot actually exclude a role for adaptive immunity in NCWS pathogenesis, although not demonstrated in the present study. Our current working hypothesis is that adaptive immunity could have a very relevant role in a “second pathogenic phase”. The exact role for the mucosal eosinophil infiltrate that we had shown in both the duodenal and colon mucosa of NCWS patients31, 34 or mast cells remains to be clarified.
Finally, our study did not look at the duodenal mucosa and we are not able to give data on this site.
On the other hand, we show a clear-cut innate immunological activation in patients suffering from NCWS and diagnosed by DBPC wheat challenge. The significant reduction in ILC1 after wheat-free diet in all the patients who underwent rectal biopsies after wheat was withdrawn from the diet indicates a close correlation between administration of foods containing wheat, expansion of ILC1, and NCWS and suggests a therapeutic role for a wheat-free diet.
In conclusion, our data indicate that, in people with active NCWS, ILC1 infiltrate their rectal mucosa and express transcripts encoding the pro-inflammatory cytokine IFN-γ, supporting a role for this lymphoid cell subset in the pathogenesis of NCWS.
Study Highlights
Acknowledgments
We wish to thank Mr Frank Adamo for revising the text and Alessandro Gorgone for his technical support.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Antonio Carroccio, MD.
Specific author contributions: A. Carroccio and F. Dieli had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: A. Carroccio, F. Dieli, D. Di Liberto, P. Mansueto, A. D’Alcamo. Acquisition of data: A. Carroccio, A. D’Alcamo, G. Iacono, P. Mansueto. Endoscopy study: G. Geraci. Cell and cytokine analyses: D. Di Liberto, M. Lo Pizzo, E. Lo Presti, F. Fayer, G. Guggino. Analysis and interpretation of data: A. Carroccio, F. Dieli, D. Di Liberto, M. Lo Pizzo, E. Lo Presti, F. Fayer, G. Guggino, A. D’Alcamo, P. Mansueto. Drafting of the manuscript: A. Carroccio, F. Dieli, D. Di Liberto. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: D. Di Liberto, E. Lo Presti.
Financial support: The study was supported by the Italian Foundation for Celiac Disease (FC) Grant for Project 013/2014.
Potential competing interests: None.
Supplementary Material
References
- Sapone A, Bai JC, Ciacci C et al. Spectrum of gluten related disorders: consensus on new nomenclature and classification. BMC Med 2012; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catassi C, Bai JC, Bonaz B et al. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients 2013; 5: 3839–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catassi C, Elli L, Bonaz B et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients 2015; 7: 4966–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroccio A, Rini G, Mansueto P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology 2014; 146: 320–321. [DOI] [PubMed] [Google Scholar]
- Fasano A, Sapone A, Zevallos V et al. Non-celiac gluten-sensitivity. Gastroenterology 2015; 148: 1195–1204. [DOI] [PubMed] [Google Scholar]
- Biesiekierski JR, Peters SL, Newnham ED et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013; 145: 320–328. [DOI] [PubMed] [Google Scholar]
- Peters SL, Biesiekierski JR, Yelland GW et al. Randomised clinical trial: gluten may cause depression in subjects with non-coeliac gluten sensitivity: an exploratory randomised clinical study. Aliment Pharmacol Ther 2014; 39: 1104–1112. [DOI] [PubMed] [Google Scholar]
- Sapone A, Lammers KM, Mazzarella G et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 2010; 152: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapone A, Lammers KM, Casolaro V et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 2011; 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brottveit M, Beitnes AC, Tollefsen S et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol 2013; 108: 842–850. [DOI] [PubMed] [Google Scholar]
- Vazquez-Roque MI, Camilleri M, Smyrk T et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013; 144: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier M, Sawa S, Eberl G. Notch, Id2 and RORγt, sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med 2012; 209: 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyler T, Klose CSN, Souabni A et al. Gata3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 2012; 37: 634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol 2012; 30: 647–675. [DOI] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 2011; 12: 21–27. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Withers DR. Immunology. Innate lymphoid cell relations. Science 2010; 330: 594–595. [DOI] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC et al. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 1999; 17: 189–220. [DOI] [PubMed] [Google Scholar]
- Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol 2008; 9: 473–475. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Pross H et al. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol 1975; 5: 117–121. [DOI] [PubMed] [Google Scholar]
- Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol 2006; 118: 1–10. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol 2004; 22: 405–429. [DOI] [PubMed] [Google Scholar]
- Ganal SC, Sanos SL, Kallfass C et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 2012; 37: 171–186. [DOI] [PubMed] [Google Scholar]
- Schulthess J, Meresse B, Ramiro-Puig E et al. Interleukin-15-dependent NKp46(+) innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity 2012; 37: 108–121. [DOI] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol 2012; 13: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 2011. a; 12: 383–390. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM et al. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 2011. b; 34: 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Cárcamo CV, Fleming MP et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 2011; 208: 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama T, Kamada N, Chinen H et al. Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology 2010; 139: 882. [DOI] [PubMed] [Google Scholar]
- Bernink JH, Peters CP, Munneke M et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013; 14: 221. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 2013; 38: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroccio A, Mansueto P, Iacono G et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol 2012; 107: 1898–1906. [DOI] [PubMed] [Google Scholar]
- Carrasco A, Mañe J, Santaolalla R et al. Comparison of lymphocyte isolation methods for endoscopic biopsy specimens from the colonic mucosa. J Immunol Methods 2013; 389 (1-2): 29–37. [DOI] [PubMed] [Google Scholar]
- Carroccio A, D'Alcamo A, Cavataio F et al. High proportions of people with non-celiac wheat sensitivity have autoimmune disease or anti-nuclear antibodies. Gastroenterology 2015; 149: 596–603. [DOI] [PubMed] [Google Scholar]
- Carroccio A, Mansueto P, D'Alcamo A et al. Non-celiac wheat sensitivity as an allergic condition: personal experience and narrative review. Am J Gastroenterol 2013; 108: 1845–1852. [DOI] [PubMed] [Google Scholar]
- Nijeboer P, Bontkes HJ, Mulder CJ et al. Non-celiac gluten sensitivity. Is it in the gluten or the grain? J Gastrointestin Liver Dis 2013; 22: 435–440. [PubMed] [Google Scholar]
- Mooney PD, Aziz I, Sanders DS. Non-celiac gluten sensitivity: clinical relevance and recommendations for future research. Neurogastroenterol Motil 2013; 25: 864–871. [DOI] [PubMed] [Google Scholar]
- Guandalini S, Polanco I. Non-celiac gluten sensitivity or wheat intolerance syndrome? J Pediatr 2015; 166: 805–811. [DOI] [PubMed] [Google Scholar]
- Volta U, Caio G, Tovoli F et al. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol 2013; 10: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon J, Puppa EL, Greenwald B et al. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015; 7: 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker Y, Zeissig S, Kim S et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of Toll-like receptor 4. J Exp Med 2012; 209: 2395–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010; 464: 1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 2010; 7: 163–173. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012; 303: G775–G785. [DOI] [PubMed] [Google Scholar]
- Mansueto P, D’Alcamo A, Seidita A et al. Food allergy in irritable bowel syndrome: the case of non-celiac wheat sensitivity. World J Gastroenterol 2015; 21: 7089–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.