Abstract
OBJECTIVES:
Exercise has been scarcely studied in patients with cirrhosis, and prior evidence showed hepatic venous pressure gradient (HVPG) to be increased in response to exercise. The aim of this study was to investigate the effects of a supervised physical exercise program (PEP) in patients with cirrhosis.
METHODS:
In an open-label, pilot clinical trial, patients with cirrhosis were randomized to PEP (cycloergometry/kinesiotherapy plus nutritional therapy, n=14) or control (nutritional therapy, n=15); for 14 weeks. Primary outcomes were: the effect of PEP in HVPG, and quality of life (chronic liver disease questionnaire, CLDQ). As secondary outcomes we investigated changes in physical fitness (cardiopulmonary exercise testing), nutritional status (phase angle—bioelectrical impedance), ammonia levels, and safety.
RESULTS:
Twenty-two patients completed the study (11 each). HVPG decreased in subjects allocated to PEP (−2.5 mm Hg (interquartile range: −5.25 to 2); P=0.05), and increased in controls (4 mm Hg (0–5); P=0.039), with a significant between-groups difference (P=0.009). No major changes were noted in CLDQ in both groups. There was significant improvement in ventilatory efficiency (VE/VCO2) in PEP group (−1.9 (−3.12 to −0.1); P=0.033), but not in controls (−0.4 (−5.7 to 1.4); P=0.467). Phase angle improvement and a less-pronounced exercise-induced hyperammonemia were noted only in PEP group. No episodes of variceal bleeding or hepatic encephalopathy were observed.
CONCLUSIONS:
A supervised PEP in patients with cirrhosis decreases the HVPG and improves nutritional status with no changes in quality of life. Further studies evaluating physical training in cirrhosis are eagerly awaited in order to better define the benefits of sustained exercise. ClinicalTrials.gov:NCT00517738.
INTRODUCTION
There are many benefits related to physical exercise (PE) including increased bone mineral density, insulin sensitivity, quality of life (QoL); and decreased risk of cancer, cardiovascular, and metabolic diseases such as type 2 diabetes mellitus and dyslipidemia.1, 2, 3, 4 Although the recommended extent of PE needed to improve health status is ≥150 min per week in the general population, it is known that various PE durations and intensities are regarded as beneficial.2, 4 Moreover, there are different types of PE (aerobic, anaerobic—strength or resistance training—and flexibility) and thus the recommended regimen in each individual should take into account the presence of disease-specific states such as diabetes mellitus and cardiovascular or neurologic diseases, along with prevailing physical fitness.5
In liver diseases and cirrhosis there is no standardized recommendation regarding the type, intensity, and duration of exercise that is able to improve the health status. Moreover, the benefits PE could bring to patients with cirrhosis are not fully understood, and experience from clinical trials is particularly limited. Complications from portal hypertension and cirrhosis, such as malnutrition, fluid overload, ascites, and fatigue have greatly limited PE in this group of patients. Remarkably, malnutrition in cirrhosis have been linked to increased mortality, independently of disease severity,6, 7 both before and after liver transplantation. Limited evidence has suggested that QoL improves after PE in patients with cirrhosis, and this has been more recently shown in a randomized clinical trial. Improvement in sarcopenia in patients performing PE has been noted in the two published randomized clinical trials, along with amelioration of fatigue with enhanced physical fitness, and improvement in biochemical markers of hepatic dysfunction. Other potential benefits claimed for PE in cirrhotics, including the possibility of stimulating extra-hepatic ammonia metabolism and alleviating hepatic encephalopathy, remain to be proven.8, 9, 10, 11 The long-term effects of PE—particularly on mortality—are still unknown as well.
A major concern regarding PE is the increase in hepatic venous pressure gradient (HVPG), and the subsequent higher risk for variceal bleeding.12, 13 An initial study addressing acute changes in hepatic hemodynamics found a significant increase in HVPG in patients with cirrhosis and portal hypertension while exercising.12 However, in a subsequent study it was shown that non-selective beta-blockade was rather associated with a decrease in HVPG during exercise.13 The effect of a PE program (PEP), i.e., a sustained exercise regimen, on HVPG has not been evaluated in the setting of a randomized clinical trial to date.
The present study primarily aimed to evaluate the effect of aerobic exercise by means of a 14-week PEP on QoL and HVPG in patients with cirrhosis and portal hypertension. As secondary aims changes in physical fitness, nutritional status, ammonia levels, and safety were investigated.
METHODS
This was a randomized, open-label clinical trial conducted at two tertiary care centers, from March 2009 to March 2014. Local research and ethics committee approved this study protocol (Clinical Trials identifier: NCT00517738). It was designed and conducted according to the principles of the Declaration of Helsinki Principles. Every author had access to the study data, reviewed and approved the final manuscript.
Recruitment of participants
Patients (18–70 years old) were recruited from the Liver Unit at a tertiary care center, where clinical follow-up, biochemical, endoscopic, and nutritional evaluations were conducted. Cardiac evaluations and PEP were performed at the Cardiac Rehabilitation Unit from a cardiology-specialized center, where exercise was supervised by specially trained personnel.
Diagnosis of cirrhosis was based on liver biopsy, or the presence of portal hypertension (esophageal varices on endoscopy, ascites, and/or compatible features on ultrasonography) and markers of hepatocyte synthetic dysfunction. Subjects with any of the following conditions were excluded: renal failure (defined as a creatinine ≥1.5 mg/dl), prior surgical shunt for portal hypertension or transjugular intrahepatic portosystemic shunt, hepatic venous outflow obstruction, esophageal varices with high risk of bleeding (large or red signs), gastric or ectopic varices, ongoing treatment with peginterferon, cardiopulmonary disease, posttransplant status, neuropsychiatric disorders, personal history of stroke, musculoskeletal disease impairing exercise or other debilitating condition, insulin-requiring or poorly controlled diabetes, ongoing alcohol intake or smoking, rotating shift work, active infection or cancer, pregnancy, or an episode of decompensation necessitating hospital admission during the month prior to screening. All participants (both in PEP and control group) were receiving a non-selective beta-blocker (propranolol) by the time of recruitment. A complete history and physical examination were performed in all participating individuals during screening. Covert hepatic encephalopathy was defined clinically and with a PHES (Psychometric Hepatic Encephalopathy Score)<−4 s.d., using available Mexican-based norms.14
After the signing of informed consent, subjects were randomized in a 1:1 fashion to 14 weeks of a supervised PEP or to control. In order to accomplish allocation concealment an independent investigator generated sequentially numbered sealed envelopes containing the corresponding assignment, which were opened immediately after signing of informed consent. Patients in the control group were advised to continue with their regular daily activities, and not encouraged to engage in new activities demanding increased physical efforts during the 14 weeks of the study. In all cases, nutritional therapy was provided and treatment of cirrhosis complications optimized.
Physical exercise program
A cardiac rehabilitation team composed of nurses, physical therapists, and cardiologists executed the PEP. Every patient performed 40 highly supervised sessions (online ECG, blood pressure, and continuous clinical monitoring) that included both aerobic training and kinesiotherapy. Aerobic training consisted of 40 min on a heart rate biofeedback workload cyclergometer (Ergoline,Bitz, Germany), with sessions scheduled three times per week (Mon-Wed-Fri) to an intensity of 12–14 of the Borg Rating of Perceived Exertion Scale (range of scale: 6–20) corresponding to 60–80% of the maximal theoretical age-adjusted heart rate. Every session had three phases: warm up, main phase, and cool down (10, 20, and 10 min, respectively). Kinesiotherapy included 30 min of rhythmic activities aimed to improve muscle strength and elasticity, coordination, and balance.
Nutritional therapy
Caloric intake was determined by the resting energy expenditure obtained with the Harris–Benedict equation plus 10% from the thermogenic effect of food. In the exercise group 30% of calories was added to account for the moderate physical activity from PEP, whereas only 10% from physical activity was added to the control group. The proportion of macronutrients in the diet was standardized in both groups and contained 65% of carbohydrates, 1.2 g/kg weight per day of protein (converted to the respective proportion), and the rest from lipids. Sodium intake was limited to 60–90 mEq/day or 1.5–2 g/day.
Follow up and standard of care
During the 14 weeks of the study and after completion of the study, all subjects were seen in clinic on a 4-weekly basis to address complications from liver disease, portal hypertension, and other comorbidities. Compliance with nutritional therapy, and medical management—including titration of beta-blockers and diuretics—were particularly emphasized. Routine laboratories (hematology, biochemistry, and coagulation) were mandatory before and at completion of study, and on an as-needed basis. No patient was started on any disease-specific therapy (apart from alcohol abstinence, where applicable) with the potential of decreasing portal hypertension during the study.
Physical fitness
Physical fitness was evaluated with a cardiopulmonary exercise test (CPET) at the beginning and the end of the study period. A Schiller CS-200 device (Baar, Switzerland) with a Trackmaster treadmill (Newton, KS) was used in all instances. A resting 12-lead ECG and spirometry were performed prior to the test. Electrocardiographic signal and blood pressure were recorded throughout the test, and during recovery phase. An automated medical gas analysis device (PowerCube, Niederlauer, Germany) was used to measure the volume, airflow and the fractional concentrations of oxygen and carbon dioxide in the exhaled air. CPET began with a 3-min resting period followed by a treadmill ramp protocol where one metabolic equivalents achieved in workload was added per minute.15 After maximal exercise was achieved, subjects continued to walk for 3 min at 2 km/h at 0% elevation, and then rested in the supine position for an additional 5 min (ACSM).15 The following variables were calculated from CPET and gas analysis: effort time; metabolic equivalents achieved; maximum heart rate (HRmax); chronotropic response; heart rate recovery at 1 min (HRR); blood pressure response during treadmill test (PR); respiratory quotient (RQ: CO2eliminated/O2consumed); maximal effort (VO2peak); VO2 at anaerobic threshold; minute ventilation to carbon dioxide production relationship or ventilatory efficiency (VE/VCO2); and myocardial oxygen consumption (MVO2). A cardiac defibrillator and a fully stocked resuscitation cart were present at all times.
HVPG measurement
In each participant, HVPG measurement was performed at baseline and at the end of the study (1–2 weeks before and after completion of the intervention). HVPG was measured by two interventional radiologists who were blinded to subject allocation, following standard recommendations.16 After internal jugular vein cannulation, a catheter was advanced into the hepatic vein. Free and wedged hepatic venous pressures were measured with a 7 Fr balloon catheter (Fogarty model 12TLW807F, Edwards Lifesciences, Irvine, CA). HVPG was calculated subtracting free pressure to wedged pressure. Each measurement was done in triplicate.
Quality of life
The chronic liver disease questionnaire (CLDQ) was applied to all participants before and at completion of the study.
Anthropometry and nutritional status
Weight and height were measured in each patient at the beginning of the study, and weight was rechecked at all follow up visits. Nutritional status was assessed by means of bioelectrical impedance (BIA)—RJL systems Quantum IV (Clinton Township, MI). This technique consists on applying alternating electric currents of 800 μA at 50 kHz with the aid of Ag/AgCl source and sensor electrodes to obtain R, Xc, and phase angle (i.e., the arc tangent of the ratio of reactance to resistance transformed to degrees). BIA was performed after an overnight fasting in supine position with arms and legs abducted from the body. Source and sensor electrodes were placed on the dorsum of both hand and foot on the right side of the body, respectively. Malnutrition was defined as PhA ≤4.9°, based on the corresponding cutoff reference.6
Circulating biomarkers
Blood samples were drawn from each individual on the day of the CPET and after a ≥8-h fasting period. At 0700 hours of the day of the CPET, a venous catheter was placed in the antecubital veins for serial blood drawings. Immediately after collection, each blood sample was placed on ice, centrifuged and analyzed for ammonia (before, at termination, 2, 4, and 6 h after CPET), lactic acid (before, at termination, 2 and 4 h after CPET), and creatine kinase (before, 2 and 4 h after CPET). All patients received a standard meal between 2 and 4 h after the CPET.
Statistical analyses
For sample size calculation it was assumed that PEP would positively impact QoL according to the CLDQ in at least one unit, compared with no change in controls. Estimation was based on the fact that changes in the magnitude of 1–2.5 points have been observed after liver transplantation.17 From a baseline CLDQ of 4.1±1.1 noted in Child-Turcotte-Pugh (CTP) A/B,18 and considering alpha and beta errors of 0.05 and 0.2, along with a 20% attrition rate, a total of 12 patients were needed per group.
Data are presented as percentages, mean±s.d. or median (interquartile range), as appropriate. The distribution of data was evaluated with Shapiro–Wilk test. Categorical variables were compared with χ2, Fischer’s exact test, or McNemar’s test, whereas continuous were compared with Wilcoxon, Friedman’s test, paired t-test, (within group changes), or Mann–Whitney U-test and t-test (between groups), as appropriate. Areas under the curve were constructed for repeated measurements of ammonia during each treadmill stress tests. Both per-protocol and intention to treat analysis were performed. A P-value ≤0.05 was considered statistically significant. Statistical analysis was carried out using the software package SPSS version 20 (SPSS, Armonk, NY).
RESULTS
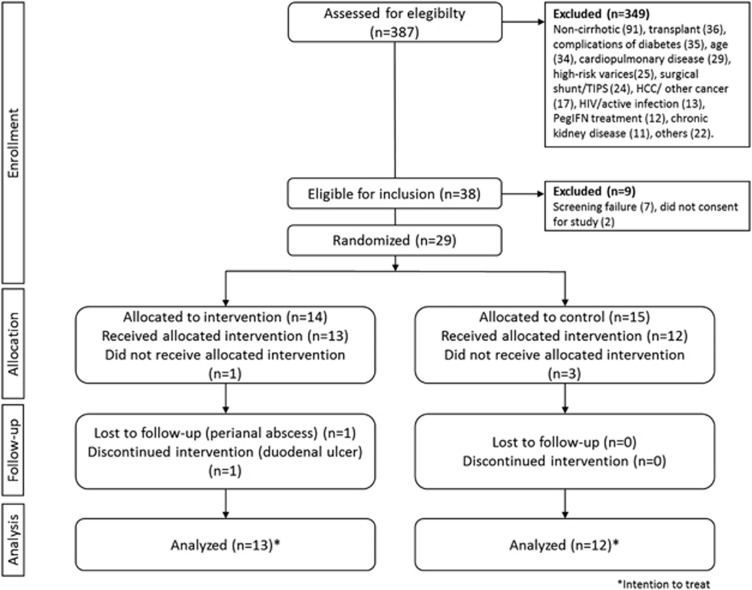
A total of 29 patients signed informed consent and were randomized (14 PEP, and 15 to control). Three subjects were not included after the baseline CPET disclosed silent cardiac ischemia (1 PEP, 2 control), and 1 was lost to follow up immediately after randomization (1 PEP); thus, none of them received any intervention. Two patients from the PEP group had to be terminated due to gastrointestinal complications, and one from the control group continued to drink alcohol during the study; thus, they were eliminated in the per-protocol analysis. Results on all endpoints are presented for patients receiving at least 1 day of study intervention (diet or exercise; modified intention to treat, n=25 patients; 13 PEP, 12 control). A per-protocol analysis was done for primary endpoints, including only patients that completed the protocol (n=22 patients; 11 PEP, 11 control; Figure 1). All patients (both PEP and control) received beta-blockers during the study.
Figure 1.
CONSORT diagram of the study protocol.
The etiology of cirrhosis was hepatitis C infection in 32% (n=8), non-alcoholic steatohepatitis in 28% (n=7), alcoholic and primary biliary cirrhosis in 12% each (n=3 each), autoimmune hepatitis, or other in 8% each (n=2 each). Table 1 shows demographic data, degree of liver impairment according to CTP and MELD scores, presence of cirrhosis-related complications, and other biochemical variables, according to the study group. Median age in total population was 52 (41.5—55.5) years, 76% (19/25) of the patients were male, and all patients had a CTP score A or B (16 and 9, respectively). There were no differences in biochemical or clinical parameters between PEP and control groups. All patients had a self-reported alcohol abstinence of at least 6 months by the time of study inclusion.
Table 1. Baseline characteristics of study population.
| Exercise group (n=13) | Control group (n=12) | P-value | |
|---|---|---|---|
| Gender, n (male/female) | 9/4 | 10/2 | 0.635 |
| Age, years | 53 (48–55) | 51 (38–57) | 0.478 |
| Weight, kg | 79.5 (59–82) | 71.2 (62–80) | 0.918 |
| Height, cm | 163 (152–175) | 166 (155–169) | 0.809 |
| BMI (kg/m2) | 27.5 (22.4–28.9) | 27.4 (25.3–30) | 0.705 |
| HR (bpm) | 59±6.2 | 60±6.5 | 0.527 |
| MAP (mm Hg) | 81.1±11.2 | 79.3±5.7 | 0.609 |
| Etiology of cirrhosis, n | 0.852 | ||
| HCV | 5 | 3 | 1.000 |
| NASH | 3 | 4 | |
| Alcohol | 1 | 2 | |
| Other | 4 | 3 | |
| Presence of HE (yes/no) | 3/8 | 3/8 | |
| Presence of ascites (yes/no) | 0/11 | 2/11 | 0.476 |
| History of variceal bleeding (yes/no) | 4/7 | 3/8 | 1.000 |
| Presence of small varices (yes/no) | 8/5 | 9/3 | 0.673 |
| Use of NSBB, % | 100 | 100 | |
| CTP score | 6 (5–7) | 6 (5–7) | 0.401 |
| MELD score | 9 (8–12) | 12 (7–14) | 0.606 |
| HVPG, mm Hg | 14.5 (12.3–18) | 11.5 (3.5–17.5) | 0.235 |
| Platelets, K/μl | 86 (64–130) | 67 (54–94) | 0.332 |
| Glucose, mg/dl | 100 (78–122) | 88 (81–107) | 0.847 |
| Creatinine, mg/dl | 0.8 (0.7–0.9) | 0.7 (0.6–0.8) | 0.217 |
| Sodium, mmol/l | 139 (137–140) | 139 (137–140) | 0.847 |
| Potassium, mmol/l | 4.3 (4.1–4.6) | 4.4 (4–4.6) | 0.847 |
| Carbon dioxide, mmol/l | 25 (24–25.5) | 24 (23–25.3) | 0.217 |
| Total bilirubin, mg/dl | 1.4 (0.7–2.3) | 2.0 (1.3–2.8) | 0.133 |
| ALT, U/l | 42 (32–77) | 40 (32–67) | 0.519 |
| AST, U/l | 68 (38–97) | 56 (40–78) | 0.606 |
| Albumin, g/dl | 3.6 (3.2–3.9) | 3.7 (2.8–3.7) | 0.797 |
| INR | 1.2 (1.1–1.3) | 1.3 (1.2–1.3) | 0.438 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CTP, Child-Turcotte-Pugh score; HCV, hepatitis C virus; HE, hepatic encephalopathy; HR, heart rate; HVPG, hepatic venous pressure gradient; INR, international normalized ratio; MAP, mean arterial pressure; MELD, model for end-stage liver disease; NASH, Non-alcoholic steatohepatitis; NSBB, non-selective beta-blockers.
Results are expressed as mean (s.d.), median (IQR) or relative frequencies. Statistical test Mann–Whitney U-test or χ2 or Fisher’s test as appropriate.
Baseline upper endoscopy revealed signs of portal hypertension in all patients (small esophageal varices in 64% (n=16), portal hypertensive gastropathy in 76% (n=19)), and prior variceal bleeding had occurred in 32% (n=8). Covert hepatic encephalopathy was present in 28% (n=7). Only three patients had ascites at the time of the recruitment (grade I). The proportion of patients with esophageal varices, encephalopathy, or ascites, did not differ between groups.
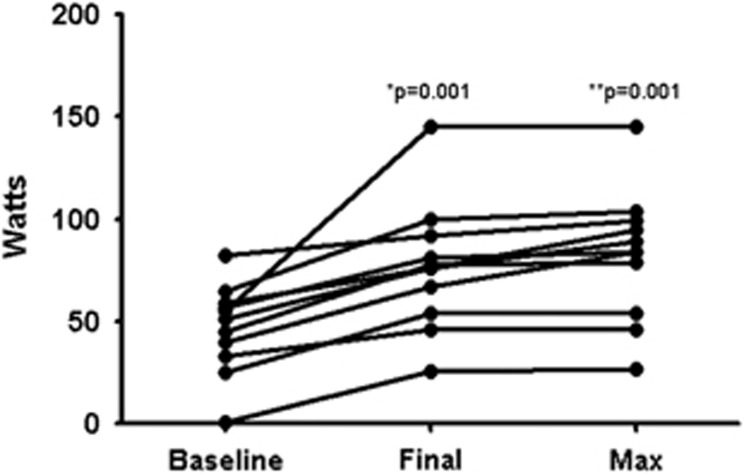
Adherence to study protocol was excellent with no dropouts in either group, although final CPET and HVPG could not be performed in one patient each (control and PEP groups, respectively). Attendance rate to exercise visits was 97% among patients randomized to PEP. Regarding cycloergometry, the workload increased from 46.6±21.7 watts to 76.5±31 watts (P=0.001) after completion of the program, and the maximum achieved workload during the training period was 82.3±31.7 watts (P<0.001, compared with baseline). An increase of the workload was seen in all patients by the end of the study (Figure 2). In the control group only one patient did not follow the recommendation of not engaging in new activities demanding physical effort during the study period; this patient joined a gym and decided to exercise on his own.
Figure 2.
Changes in workload in the exercise group. * change between baseline and final workload, ** change between baseline and maximum workload.
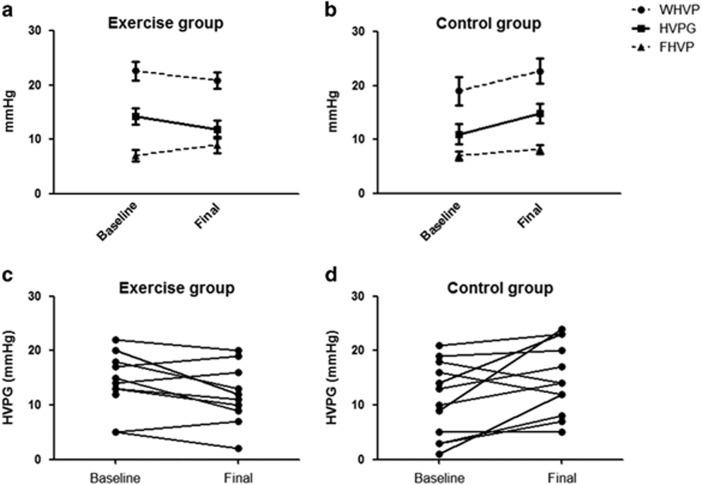
A drop in HVPG was noted in the PEP group (14.5 (12.3–18) and 11.5 (8.5–16.7) mmHg, for baseline and final measurements, respectively, P=0.05) contrasting an increase in HVPG in controls (11.5 (3.5–17.5) and 14 (9–22.2) mmHg, baseline and final measurements, P=0.039) by the end of study (Figure 3). The difference between groups (final—baseline measurement) was statistically significant (−2.5 (−5.2 to 2) and 4 (0.25 to 8) mmHg, P=0.009). When analyzed per patient, the HVPG decreased in 7/10 in the PEP group and increased in 3/10; whereas it decreased in 2/12, remained unchanged in 1/12, and increased in 9/12 in the control group.
Figure 3.
Changes in hepatic hemodynamics at baseline and final evaluations in exercise (a) and control groups (b). Individual changes on HVPG in exercise (c) and control groups (d). HVPG, hepatic venous pressure gradient; FHVP, free hepatic venous pressure; WHVP, wedged hepatic venous pressure.
In the per protocol analysis (n=22, 11 each), a drop in the HVPG was noted in the PEP group (14.5 (11–18.5) and 11.5 (8.5–16.7) mmHg, for baseline and final measurements, respectively), compared with an increase in the control group (13 (5–18) and 14 (8–23) mmHg, baseline and final measurements). This disclosed a median change of −2.5 (−5.2 to 2) in HVPG for PEP group, and 4 (0–5) mm Hg for control (P=0.016 for between-groups difference). When analyzed per patient, the HVPG decreased in 7/10 in the PEP group and increased in 3/10; whereas it decreased in 2/11, remained unchanged in 1/11, and increased in 8/11 in the control group (Supplementary Figure 1 online). Elimination of 3 patients in the control group showing an unexplained increase in HVPG (in 15, 11, and 9 units) abolished the trend toward increased HVPG in controls (baseline 13 (4–18.5) and final 14 (7.5–18.5); P=0.25), but did not change the significant difference noted between groups (P=0.05).
Some discrete improvement in QoL was noted only in the PEP group (Table 2). According to the CLDQ questionnaire, the PEP group showed an improvement in the worry domain. Although the CLDQ overall score improved among patients allocated to the PEP group, the difference was not statistically significant. In the per-protocol analysis, an improvement in the emotional role domain in the control group (5.5 (4.5–6.5) and 6.3 (5.1–6.8) for the baseline and final measurements, P=0.022) was noted as well, with no additional differences from modified intention to treat analysis.
Table 2. Changes on quality of life during the study (CLDQ).
|
Exercise (n=13) |
Control (n=12) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Final | P-value | Baseline | Final | P-value | |
| Abdominal symptoms | 4.3 (2.8–6.3) | 6.0 (4.6–6.7) | 0.123 | 5.5 (4.7–6.6) | 5.6 (4.7–6.5) | 0.475 |
| Fatigue | 4.0 (2.4–6.0) | 5.8 (3.4–6.2) | 0.721 | 5.6 (4.0–6.4) | 4.9 (4.5–5.3) | 0.262 |
| Systemic symptoms | 5.2 (3.3–6.2) | 5.6 (4.4–6.8) | 0.341 | 6.2 (5.4–6.7) | 6.0 (5.2–6.6) | 0.858 |
| Activity | 5.3 (3.6–6.3) | 5.6 (4.7–6.7) | 0.635 | 5.3 (4.0–6.1) | 6.0 (4.5–6.0) | 0.254 |
| Emotional function | 4.5 (3.4–5.7) | 5.6 (4.1–6.1) | 0.169 | 5.5 (4.4–6.4) | 6.1 (5.1–6.6) | 0.091 |
| Worry | 3.4 (1.8–5.9) | 5.8 (4.0–6.6) | 0.008 | 5.1 (4.1–6.3) | 6.2 (5.5–6.6) | 0.289 |
| CLDQ overall score | 4.0 (3.2–5.9) | 5.8 (4.5–6.1) | 0.182 | 5.5 (4.4–6.1) | 5.7 (4.9–6.2) | 0.480 |
CLDQ, chronic liver disease questionnaire.
Data are expressed as median (interquartile range). Wilcoxon test.
Changes in CPET parameters before and after completion of the study protocol are presented in Table 3. Patients in the PEP showed an improvement in the blood pressure response during exercise, and in the VE/VCO2. Regarding ventilatory efficiency the improvement noted in the PEP group was −1.9 (−3.12 to −0.1), P=0.033, compared to −0.4 (−5.7 to 1.4) in controls, P=0.6. In the control group, only the chronotropic response showed improvement by the end of the study. No other changes were observed in the CPET parameters evaluated. Results on CPET did not change after excluding the subject that deviated from protocol in the control group.
Table 3. Changes between baseline and final CPET.
|
Exercise (n=13) |
Control (n=12) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Final | P-value | Baseline | Final | P-value | |
| ET | 10.3 (8.6–12.1) | 9.9 (9.0–11.1) | 0.689 | 8.8 (7.1–11) | 9.1 (6.8–11.2) | 0.333 |
| MET | 7.8±1.8 | 8.0±1.6 | 0.807 | 7.5±2.6 | 7.9±3.1 | 0.312 |
| HRmax (%) | 75.5±10.4 | 73.2±11.6 | 0.373 | 71.7±8.0 | 71.2±11.5 | 0.648 |
| CR (b.p.m./MET) | 7.6±1.9 | 7.2±2.5 | 0.468 | 8.8±3.1 | 7.4±2.5 | 0.019 |
| HRR at 1 min (beats) | 18.0±7.9 | 18.7±5.8 | 0.766 | 20.6±12.4 | 20.9±9.9 | 0.907 |
| PR (mm Hg/MET) | 6.1±2.2 | 4.1±2.5 | 0.023 | 3.3±2.0 | 3.3±2.0 | 1.000 |
| RQ | 1.19±0.14 | 1.21±0.14 | 0.138 | 1.20±0.10 | 1.21±0.12 | 0.741 |
| Peak VO2 (mlO2/kg/min) | 28.5±5.5 | 27.6±4.9 | 0.605 | 24.4±9.1 | 26.1±8.5 | 0.116 |
| AT (mlO2/kg/min) | 19.8±5.7 | 16.7±6.8 | 0.313 | 14.5±6.0 | 17.0±3.6 | 0.202 |
| VE/VCO2 | 27.6±3.6 | 25.6±2.9 | 0.033 | 30.1±6.2 | 28.2±6.2 | 0.467 |
| VO2/RER | 19.8±5.7 | 18.6±3.5 | 0.508 | 15.4±4.1 | 16.8±3.2 | 0.332 |
| MVO2 | 1.3±0.9 | 1.5±0.8 | 0.625 | 1.4±0.8 | 1.8±0.8 | 0.221 |
AT, anaerobic threshold; b.p.m., beats per minute; CR, chronotropic response; EF, Effort time; HRmax, maximum heart rate; MET, metabolic equivalent; MVO2, myocardial oxygen consumption; PR, blood pressure response; RER, respiratory exchange ratio; RQ, respiratory quotient; VCO2, carbon dioxide production; VE, minute ventilation; VE/VCO2, minute ventilation/CO2 production; VO2, oxygen consumption.
There were no changes in anthropometry in the PEP or control group (Table 4). Differences in phase angle were observed only in the PEP group, as well as a statistically significant improvement when between-groups difference was taken into account. Although no statistical difference could be noted, 2/13 and 1/12 presented malnutrition at the beginning of the study, and this changed to 1/11 and 4/11 by the end of the study, for the PEP and control groups, respectively.
Table 4. Changes in nutritional parameters.
|
Exercise group (n=13) |
Control group (n=12) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Final | P-value | Baseline | Final | P-value | |
| Weight (kg) | 81.5 (62.7–89.5) | 80 (58.2–82) | 0.447 | 71.4 (62.5–85) | 73 (64.5–77.5) | 0.959 |
| BMI (kg/m2) | 27.9 (23–29.4) | 28.2 (22.0–29.8) | 0.889 | 27.5 (25.3–30.1) | 27.1 (25.7–28.6) | 0.695 |
| Phase angle (°) | 5.8 (5.2–6.6) | 6.1 (5.5–7.0) | 0.029 | 5.6 (5.2–6.8) | 5.6 (4.5–6.3) | 0.622 |
| Delta for phase angle | 0.4 (0 to 0.8) | 0 (−0.7 to 0.2) | 0.023 | |||
BMI, body mass index.
Data are expressed as median (interquartile range).
Serial ammonia determinations in relation to the final CPET are shown in Table 5. As can be noted, the levels of blood ammonia immediately after completion of the CPET significantly rose above baseline in the control group, but not in the PEP group. Between-groups comparisons were not significantly different except for the ammonia determination immediately after completion of CPET, corresponding to a less-pronounced exercise-induced hyperammonemia for the PEP group. Ammonia blood concentration did not change when analyzed as areas under the curve for the whole duration of sampling (6 h) or up until 2 h after completion of the CPET (Table 6).
Table 5. Ammonia levels during final cardiopulmonary exercise test.
| Pre | Post | 2 h | 4 h | 6 h | |
|---|---|---|---|---|---|
| Exercise group | 116±69 | 149±89 | 116±59 | 123±50 | 113±57 |
| Control group | 103±48 | 197±93* | 122±61** | 129±52 | 124±51 |
CPET, cardiopulmonary exercise test.
Ammonia is expressed in mcg/dl. Mean±s.d.; *P=0.005 and **P=0.015 for the change between pre–post and post 2 h the CPET.
Table 6. Ammonia levels in baseline and final CPET.
|
AUC 6 h |
AUC 2 h |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Δ (Final—Baseline) | aP-value | Baseline | Final | Δ (Final—Baseline) | aP-Value | |
| Exercise group | 430±180 | 432±206 | 3±91 | 0.878 | 317±135 | 315±161 | −2±83 | 0.878 |
| Control group | 438±203 | 487±196 | 49±264 | 0.530 | 320±152 | 361±157 | 41±222 | 0.530 |
AUC, areas under the curve; CPET, cardiopulmonary exercise test; PEP, physical exercise program.
Paired t-test. n=25, 13 PEP and 12 control.
Mean±s.d.. Final values were imputed as no change for the 2 patients in the PEP group lost to follow up.
Safety
Baseline CPET was able to identify silent ischemia in three patients, and as mentioned above, these patients were excluded from the study. During the study two patients in the PEP group developed complications thought not to be directly associated with the intervention. One subject had an episode of upper GI bleeding secondary to a duodenal ulcer induced by self-prescribed NSAIDs, and second one developed a perianal abscess (terminated from study). The frequency of complications was not different among groups (2/12 in the PEP and 0/12 in the control group, P=0.480). Another patient in the PEP group had an episode of severe hypertension during the final CPET causing premature termination of the test, but no ischemic complication was noted. However, no events of variceal bleeding or hepatic encephalopathy were seen during the study. Although lactate levels significantly increased after the CPET, there was a return to baseline at 2 h post-test with no clinical consequences noted (Table 7). No significant changes were noted in serial creatine kinase levels in relation to CPET (data not shown).
Table 7. Changes on lactate levels during baseline and final CPET.
| Baseline | Final | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | 2 h | 4 h | Pre | Post | 2 h | 4 h | |
| Exercise group | 1.4 (1.2–1.9) | 5.6 (2.5–7.3)a | 1.6 (1.4–2.0)b | 2.0 (1.7–2.6) | 2 (1.3–2.7) | 5.3 (1.8–8.8) | 2.5 (1.5–3.5) | 1.8 (1.5–5.1) |
| Control group | 1.2 (1.2–1.5) | 4.3 (2.5–5.6)a | 1.9 (1.5–2.2)b | 1.7 (1.3–2.3) | 1.7 (1.5–2.0) | 7.3 (3.2–11.7)a | 1.9 (1.6–2.1)b | 1.8 (1.6–2.3) |
CPET, cardiopulmonary exercise test.
Lactate levels are expressed as mmol/l. Data are expressed as Median (Interquartile range)
Difference between pre and post lactate levels, and between
Post and 2 h after in the corresponding CPET, P<0.05. No final values for 2 patients in the exercise group.
DISCUSSION
Despite the multiple benefits conveyed by physical exercise to the general population, its specific value has been scarcely addressed in cirrhosis. Our study expands the limited knowledge on the benefits a sustained exercise regimen can yield to patients with cirrhosis. The most important finding was the decrease in HVPG noted in patients allocated to the PEP. HVPG is an accurate surrogate of sinusoidal or portal hypertension, and as portal hypertension represents the underlying cause for most of the cirrhosis-related complications and mortality,19 any measure capable of lowering the HVPG should translate into positive clinical outcomes in cirrhosis. Apart from timely treatment of underlying liver disease and liver transplantation, pharmacological therapies aiming to reduce HVPG are not exempt of side effects, limiting its use in some patients.20, 21, 22 Thus, this finding renders physical exercise an attractive therapeutic approach for portal hypertension in patients with cirrhosis.
Although the mechanisms explaining the change in HVPG after physical training remain to be elucidated, it can be hypothesized that improved endothelial function, by attenuating the inflammatory cascade, and a decreased activation of the renin–angiotensin system might have played a major role.23, 24 The changes in HVPG after physical training seen in our study are explained by lowering of wedged hepatic pressure and an increase in free hepatic pressure. Decreased wedged hepatic pressure reflects a lower intrasinusoidal resistance and could be related to modulation in many pathophysiological pathways induced by exercise, including nitric oxide metabolism, oxidative stress, and insulin resistance, as well as changes in portal inflow and in splanchnic vasodilation.25, 26, 27, 28 On the other hand, increased free hepatic pressure after exercise might be secondary to increased cardiac preload, which in fact has been associated to increased survival in cirrhosis.29 Noteworthy, there were no weight shifts within each group or differences in nutritional therapy and between groups that might have accounted for the changes in HVPG, as was recently suggested by another study,30 and all patients received beta-blockers before inclusion in the study, with no differences in titration protocol between groups.
Prior evidence on the effect of exercise on HVPG in portal hypertension had been limited to HVPG determination while patients were performing cycloergometry, showing an increase in HVPG in patients without proper beta-blockade, followed by normalization of the gradient.12 Accumulated evidence from more recent years has failed to prove an increased risk for adverse events in patients with cirrhosis and portal hypertension undergoing physical training,31, 32, 33 and our results are in agreement with these findings. Conceivably, repeated aerobic exercise of moderate intensity can trigger adaptation mechanisms in portal hypertension resulting in improved organ homeostasis, just as reported for cardiovascular diseases.
Another interesting finding in our study was the numerical improvement in nutritional status (according to phase angle) observed in patients with cirrhosis allocated to the PEP, which became statistically significant when compared with the numerical deterioration in the control group. Although the clinical relevance of this finding is unclear, it proves the concept that deterioration in muscle mass can be prevented with physical training. Phase angle from bioelectrical impedance is a novel marker of malnutrition in cirrhosis, and we have recently validated its predictive role for mortality in our population of cirrhotics.6 Two randomized trials evaluating the role of exercise in muscle mass were also able to show improved malnutrition by quantifying muscle mass according to ultrasound-measured thickness and/or thigh circumference.31, 32 However, evidence from more accurate methods such as cross-sectional imaging is lacking. Despite the independent association between malnutrition and mortality in cirrhosis, few studies proposing feasible strategies to improve nutritional status have been conducted. Attempts with protein or amino acid supplementation have shown that exercise is essential in order to favor protein synthesis and hypertrophy of skeletal muscle leading to increased muscle mass.34, 35 Indeed, Roman et al.,31 and our study did not find any benefit from protein/amino acid supplementation in control groups not exposed to physical training.
Exercise, particularly under anaerobic conditions, is known for causing elevated levels of ammonia in blood. On the CPET done by the end of the study, only patients in the PEP group did not exhibit a significant elevation in blood ammonia levels immediately after the test, what was noted at the baseline CPET in both groups. As skeletal muscle functions as an ammonia scavenger in patients with hyperammonemia, this finding is not surprising and a similar phenomenon has been observed in non-cirrhotic subjects.36 It remains to be tested whether physical training could help abating hyperammonemia and even become a therapeutic strategy for hepatic encephalopathy.8
Surprisingly, no significant changes were noted between PEP and control groups in self-reported QoL, and only moderate changes on CPET parameters. Regarding QoL the expected increase of at least one unit in the CLDQ overall score was accomplished in the PEP group but data were widely dispersed thus precluding statistical significance. The isolated improvement noted in the worry item in the PEP group in the modified intention to treat analysis has, therefore, dubious clinical relevance. Our results contrast findings by similar studies on physical training in cirrhosis where more consistent improvements across several items of self-reported measures of physical and mental health have been informed.31, 32, 37 As for the physical fitness parameters evaluated in CPET, the significantly increased workload noted in the PEP group did not reflect into an increased VO2peak, but it did reflect improved ventilator efficiency or VE/VCO2. Our patients, particularly those included in the PEP group, had a rather good aerobic fitness at baseline as reflected in their VO2peak thus not leaving too much room for improvement, what might explain differences reported with another study including patients with cirrhosis.32 However, the improved ventilatory efficiency (drop in VE/VCO2) is a very positive finding as this marker is known to provide additional prognostic information in patients with cardiopulmonary disease beyond that provided by VO2peak.38, 39, 40 Although no major safety issues derived from the intervention, there were two complications likely associated with PEP (NSAIDs-related duodenal ulcer and perianal abscess), and CPET disclosed three cases of silent ischemia and one hypertensive crisis in relation to pre-existing cardiac disease.
The strengths of this study were its randomized design and inclusion of a control group, the measurement of HVPG both baseline and post-intervention, and the direct supervision of the PEP in a cardiac rehabilitation center vastly experienced in the management of high-risk patients. This study has some limitations as well. First, we were not able to show positive findings for QoL, for what our sample size was calculated; second, per design it is unclear whether our findings on HVPG are underpowered; third only CTP A or B patients were included, so these results are not applicable to patients with more advanced disease; fourth, our PEP was a very specific supervised program and would be difficult to implement in many health care systems; fifth, we did not use a physical activity tracker or performed random alcohol testing to validate adherence to study recommendations. We systematically assessed daily physical activity and alcohol drinking on each follow-up visit and were able to identify two deviations from protocol (one patient restarted drinking alcohol, and another joined an exercise program); however, we cannot rule out that other protocol deviations might have occurred. Finally, although there is a chance for bias in HPVG determination, we believe this is small as all measurements were performed by widely experienced providers adhering to international recommendations.
In conclusion, this pilot randomized trial evaluated the effect of a supervised physical exercise program in patients with cirrhosis and portal hypertension, undergoing treatment with non-selective beta-blockers, showing a decrease in HVPG as well as improved nutritional status. Further studies evaluating physical training in cirrhosis are eagerly awaited in order to better define the risks and benefits of sustained exercise. The final goal for hepatologists should be to fine-tune an exercise regimen prescription that can positively impact our patients with cirrhosis and portal hypertension, as it has been described for other chronic diseases.
Study Highlights
Acknowledgments
We are indebted to our interventional radiologists, Manuel Guerrero-Martínez and Adrián González-Aguirre, for their excellent support and technical assistance.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Andrés Duarte-Rojo, MD, DSc.
Specific author contributions: Study concept and design: R.U.M.-R., H.I.-L., S.P.-d.-L.R., F.V.-V., A.T., and A.D.-R.; acquisition of the data: R.U.M.-R., H.I.-L., A.R.-M., O.G.-F., and A.D.-R.; analysis, interpretation of data and drafting of the manuscript: R.U.M.-R., H.I.-L., A.R.-M., and A.D.-R.; critical revision of the manuscript: S.P.-d.-L.R.; obtained the study funding: R.U.M.-R., A.T., and A.D.-R. All authors approved the final version of the manuscript.
Financial support: This study was fully supported by National Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología, CONACYT) Mexico. Andres Duarte-Rojo was also supported by the "Angeles Espinosa Yglesias" Award 2009 from FUNSALUD/FUNDHEPA, México.
Potential competing interests: None.
Supplementary Material
References
- Orozco LJ, Buchleitner AM, Gimenez-Perez G et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev 2008: CD003054. [DOI] [PubMed]
- Wen CP, Wai JP, Tsai MK et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011; 378: 1244–1253. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Chen H, Wagenknecht LE et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308: 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Jakicic JM, Ard JD et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2960–2984. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- Ruiz-Margain A, Macias-Rodriguez RU, Duarte-Rojo A et al. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: A prospective cohort study. Dig Liver Dis 2015; 47: 309–314. [DOI] [PubMed] [Google Scholar]
- Montano-Loza AJ, Meza-Junco J, Prado CM et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012; 10: 166–173 173 e1. [DOI] [PubMed] [Google Scholar]
- Duarte-Rojo A, Torres-Vega MA, Villamil-Ramirez H et al. Changes in peripheral blood mononuclear cells glutamine synthetase mRNA after exercise in healthy volunteers: exploring an alternative proposal for non hepatic ammonia metabolism. Rev Invest Clin 2012; 64: 164–172. [PubMed] [Google Scholar]
- Di Francescomarino S, Sciartilli A, Di Valerio V et al. The effect of physical exercise on endothelial function. Sports Med 2009; 39: 797–812. [DOI] [PubMed] [Google Scholar]
- Ji LL, Zhang Y. Antioxidant and anti-inflammatory effects of exercise: role of redox signaling. Free Radic Res 2014; 48: 3–11. [DOI] [PubMed] [Google Scholar]
- You T, Arsenis NC, Disanzo BL et al. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med 2013; 43: 243–256. [DOI] [PubMed] [Google Scholar]
- Garcia-Pagan JC, Santos C, Barbera JA et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology 1996; 111: 1300–1306. [DOI] [PubMed] [Google Scholar]
- Bandi JC, Garcia-Pagan JC, Escorsell A et al. Effects of propranolol on the hepatic hemodynamic response to physical exercise in patients with cirrhosis. Hepatology 1998; 28: 677–682. [DOI] [PubMed] [Google Scholar]
- Duarte-Rojo A, Estradas J, Hernández-Ramos R et al. Validation of the psychometric hepatic encephalopathy score (PHES) for identifying patients with minimal hepatic encephalopathy. Dig Dis Sci 2011; 56: 3014–3023. [DOI] [PubMed] [Google Scholar]
- Balady GJ, Arena R, Sietsema K et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010; 122: 191–225. [DOI] [PubMed] [Google Scholar]
- Bosch J, Abraldes JG, Berzigotti A et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroentero lHepatol 2009; 6: 573–582. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Cordoba J, Garin O et al. Validity of the Spanish version of the Chronic Liver Disease Questionnaire (CLDQ) as a standard outcome for quality of life assessment. Liver Transpl 2006; 12: 95–104. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Guyatt G, Kiwi M et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999; 45: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014; 383: 1749–1761. [DOI] [PubMed] [Google Scholar]
- Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010; 362: 823–832. [DOI] [PubMed] [Google Scholar]
- Tandon P, Abraldes JG, Berzigotti A et al. Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis. J Hepatol 2010; 53: 273–282. [DOI] [PubMed] [Google Scholar]
- Trebicka J, Hennenberg M, Laleman W et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology 2007; 46: 242–253. [DOI] [PubMed] [Google Scholar]
- Braith RW, Welsch MA, Feigenbaum MS et al. Neuroendocrine activation in heart failure is modified by endurance exercise training. J Am Coll Cardiol 1999; 34: 1170–1175. [DOI] [PubMed] [Google Scholar]
- Adamopoulos S, Parissis J, Kroupis C et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J 2001; 22: 791–797. [DOI] [PubMed] [Google Scholar]
- Francque S, Verrijken A, Mertens I et al. Visceral adiposity and insulin resistance are independent predictors of the presence of non-cirrhotic NAFLD-related portal hypertension. Int J Obes (Lond) 2011; 35: 270–278. [DOI] [PubMed] [Google Scholar]
- Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis—current status and future directions. J Hepatol 2014; 61: 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005; 115: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Assar M, Angulo J, Rodriguez-Manas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 2013; 65: 380–401. [DOI] [PubMed] [Google Scholar]
- Moller S, Hobolth L, Winkler C et al. Determinants of the hyperdynamic circulation and central hypovolaemia in cirrhosis. Gut 2011; 60: 1254–1259. [DOI] [PubMed] [Google Scholar]
- Berzigotti AG, Albillos A, Villanueva JC et al. Lifestyle intervention by a 16-week programme of supervised diet and physical exercise ameliorates portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology 2014; 60: 253A. [DOI] [PubMed] [Google Scholar]
- Roman E, Torrades MT, Nadal MJ et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci 2014; 59: 1966–1975. [DOI] [PubMed] [Google Scholar]
- Zenith L, Meena N, Ramadi A et al. Eight Weeks of Exercise Training Increases Aerobic Capacity and Muscle Mass and Reduces Fatigue in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2014; 12: 1920–1926. [DOI] [PubMed] [Google Scholar]
- Pattullo V, Duarte-Rojo A, Soliman W et al. A 24-week dietary and physical activity lifestyle intervention reduces hepatic insulin resistance in the obese with chronic hepatitis C. Liver Int 2013; 33: 410–419. [DOI] [PubMed] [Google Scholar]
- Cermak NM, Res PT, de Groot LC et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012; 96: 1454–1464. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 1994; 330: 1769–1775. [DOI] [PubMed] [Google Scholar]
- Lo PY, Dudley GA. Endurance training reduces the magnitude of exercise-induced hyperammonemia in humans. J Appl Physiol (1985) 1987; 62: 1227–1230. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Pattullo V, Garcia-Saenz-de-Sicilia M et al. Can a lifestyle intervention improve risk factors for a negative response to peginterferon treatment in patients with chronic hepatitis C, cirrhosis, and obesity? J Hepatol 2014; 60: S478. [Google Scholar]
- Thirapatarapong W, Armstrong HF, Bartels MN. Comparison of cardiopulmonary exercise testing variables in COPD patients with and without coronary artery disease. Heart Lung 2014; 43: 146–151. [DOI] [PubMed] [Google Scholar]
- Gitt AK, Wasserman K, Kilkowski C et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002; 106: 3079–3084. [DOI] [PubMed] [Google Scholar]
- MacGowan GA, Panzak G, Murali S. Exercise-related ventilatory abnormalities are more specific for functional impairment in chronic heart failure than reduction in peak exercise oxygen consumption. J Heart Lung Transplant 2001; 20: 1167–1173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.