Abstract
Objectives:
To provide a functional and phenotypic characterization of immune cells infiltrating small intestinal mucosa during non-IPEX autoimmune enteropathy (AIE), as to gain insights on the pathogenesis of this clinical condition.
Methods:
Duodenal biopsies from a patient with AIE at baseline and following drug-induced remission were analyzed by immunohistochemistry, immunofluorescence, and flow cytometry, and results were compared with those obtained from patients with active celiac disease, ileal Crohn’s disease and healthy controls. Lamina propria (LP) and intraepithelial (IELs) lymphocytes from AIE and controls were analyzed for mechanisms regulating cytokine production. Foxp3 expression and suppressive functions of LP regulatory T cells (Tregs) were analyzed.
Results:
The quantitative deficit of Foxp3 expression in Tregs in AIE associates with unrestrained IL-17 production by IELs. Interleukin (IL)-17-producing IELs were rare in the uninflamed duodenum and in the ileum of Crohn’s disease patients, and disappeared upon drug-induced AIE remission. IL-17 upregulation in CD4+IELs and CD4+LP T cells had different requirements for pro-inflammatory cytokines. Moreover, transforming growth factor-β (TGF-β) selectively enhanced IL-17 production by CD8+IELs. Intriguingly, although Foxp3lowTregs in AIE were poorly suppressive, they could upregulate GARP-LAP/TGF-β surface expression and enhanced IL-17 production selectively by CD8+IELs. Finally, phosphorylated Smad2/3 was detectable in duodenal CD8+ lymphocytes in active AIE in situ, indicating that they received signals from the TGF-β receptor in vivo.
Conclusions:
AIE is characterized by the appearance of unconventional IL-17-producing IELs, which could be generated locally by pro-inflammatory cytokines and TGF-β. These results suggest that Foxp3+Tregs and Treg-derived TGF-β regulate IL-17 production by IELs in the small intestine and in AIE.
Introduction
Autoimmune enteropathy (AIE) is a rare syndrome characterized by chronic diarrhea, malabsorption, and severe villous atrophy, generally associated with autoimmune comorbidities and circulating anti-enterocyte antibodies.1, 2 Most cases of AIE occur in the context of an X-linked genetic syndrome known as IPEX (immunodysregulation polyendocrinopathy enteropathy X-linked syndrome), a disease affecting male infants, and secondary to mutations in the coding region or in regulatory elements of Foxp3 gene.3, 4 Foxp3, also known as scurfin, is a forkhead transcription factor essential for the development and function of CD4+CD25+ regulatory T cells (Tregs), a subset of T lymphocytes that play a fundamental role in the regulation of T-cell activation and immune homeostasis.5 Consistently, IPEX patients display a profound defect in Tregs and a severe reduction of FoxP3 expression in most tissues.6 Nonetheless, in the last few years, non-IPEX adult-onset forms of AIE are being increasingly reported in both genders.7 These patients exhibit reduced expression of Foxp3 and reduced suppressive function of CD4+CD25+ Tregs,8 raising the possibility that defective autosomal genes could also lead to a deficit of Foxp3 expression and function. These findings have led to postulate that an unrestricted activation of gut-associated lymphoid tissue, due to an impairment in Tregs quantity and/or function, may be responsible for the intestinal inflammation and villous atrophy which dominate the pathological picture of the syndrome.1 Indeed, immunohistochemistry studies have documented a dense mixed T-cell infiltrate (CD4+ and CD8+) in the duodenal mucosa of patients with active AIE, which could be associated with a prominent intraepithelial lymphocytosis in a significant proportion of cases.9 This is consistent with the long-known role of activated T cells and T-cell-derived cytokines in the pathogenesis of enteropathy and villous atrophy in humans,10 and substantiates the clinical success of T-cell-directed therapies in both IPEX and non-IPEX AIEs. Indeed, even if clinical manifestations of AIE usually respond to corticosteroids, steroid-refractory cases are common, requiring rescue therapy with additional immunomodulators, such as azathioprine, cyclosporine, and tacrolimus, while bone marrow transplantation has been employed with some success in severe, drug-refractory cases.2 Despite these interventions, AIE is still affected by a high rate of complications and mortality, and novel therapeutic approaches to control the syndrome are urgently needed. In order to discover novel targets for selective and more effective drugs, it is thus highly relevant to obtain insights into characteristics of infiltrating intestinal inflammatory cells during active AIE. In this context, existing knowledge regarding AIE pathophysiology mostly derive from studies conducted on peripheral blood cells or histological sections,6, 9, 11 while very little is known regarding phenotypic and functional characteristics of inflammatory cells that infiltrate the intestinal mucosa.
Here, we report for the first time a detailed phenotypic and functional analysis of intraepithelial (IELs) and lamina propria (LP) T lymphocytes that infiltrate the small intestinal mucosa in a severe case of non-IPEX adult-onset AIE, following their variations during drug-induced disease remission. The appearance of unconventional IELs that co-produced IFN-γ and IL-17 was observed in the duodenal mucosa, which was largely absent in healthy controls and patients with celiac or Crohn’s disease, and which relieved upon pharmacological treatment. This was associated with a failure of LP CD4+CD25low Treg cells to control IL-17 induction in IELs. Intriguingly, activated Foxp3lowTregs in AIE upregulated membrane-bound transforming growth factor-β (TGF-β), and TGF-β strongly enhanced IL-17 induction in CD8+IELs. These results shed new light on the role of Foxp3+Tregs and TGF-β in the regulation of intestinal homeostasis in vivo.
Methods
AIE patient
A 26-year old woman was referred for a 6-month story of severe chronic watery diarrhea, hypokalemia, hypoalbuminemia, and severe malnutrition (body mass index 13.5). Additional findings included thrombocytopenia (60.000 platelets/mmc) and low-grade proteinuria. Upper endoscopy demonstrated scalloping of the duodenal mucosa. Celiac disease (CD) was ruled out on the basis of negative serological testing (anti-endomysial and anti-transglutaminase antibodies), normal immunoglobulin A levels, and negative genetic testing for HLA-DQ2 and DQ8. An elevated titer of anti-enterocyte IgG antibodies measured by indirect immunofluorescence confirmed the diagnosis of AIE. IPEX syndrome was excluded by the absence of mutations in the coding and/or in the poly-A region of Foxp3 gene by DNA sequencing (not shown). High-dose steroid treatment (prednisone 1 mg/kg) was initiated with progressive clinical and biochemical improvement. Ten weeks after initiation of steroid treatment, azathioprine therapy (2 mg/kg) was initiated to maintain disease remission, and steroid therapy was gradually weaned over 3 months until cessation. Follow-up endoscopies with duodenal biopsies were performed before treatments (active AIE, PRE), 8 weeks after initiation of steroid therapy, during clinical remission (T=1), and at 6 months, during azathioprine treatment (T=2).
Control biopsies
Duodenal biopsies from six age-matched consecutive patients presenting for gastroesophageal reflux-related symptoms were used as uninflamed controls. Standard histological examination of the duodenal mucosa ruled out organic diseases of the small intestine in all cases. In addition, ileal biopsies from five patients with endoscopically and histologically active Crohn’s disease not receiving steroids, immunosuppressants and/or anti-TNF therapy, were analyzed as inflamed controls. Crohn’s disease was diagnosed on the basis of a combination of clinical, laboratory, histologic, and radiologic findings.12 Duodenal biopsies from 10 patients with untreated active CD were used as additional controls. The histopathological diagnosis of CD was based on typical mucosal lesions with crypt hyperplasia and villous atrophy. All active CD patients were positive for anti-endomysial and anti-transglutaminase antibodies at the time of diagnosis. All patients gave their informed content to the study, which was performed in accordance with Helsinki declaration.
Immunohistochemistry and Immunofluorescence
Immunohistochemistry for CD3 (clone 2GV6), CD4 (clone SP35), and CD8 (SP57), was performed using an automated staining system (BenchMark ULTRA, Ventana, Roche, Tucson, AZ). Heat-induced antigen retrieval was automatically obtained using a 0.05 mol/l EDTA solution, pH 8.0 at 95 °C for 30 min. Reactions were revealed using ultraView Universal DAB Detection Kit (Ventana) in accordance with the manufacturer's instructions.
For immunofluorescence, paraffin sections from AIE biopsies (PRE and POST) and uninflamed duodenal biopsies as control were submitted to deparaffinization in xylene and hydration through a series of decreasing alcohol concentrations. Heat-induced antigen retrieval was obtained using Diva Decloaker solution (Biocare Medical, Concord, CA) at 120 °C for 3 min in the Decloaker chamber. After blocking and permeabilization with PBS-BSA 1%-NP40 1% for 30 min, tissues were incubated with rabbit polyclonal anti-CD8 antibody (1:100 ab4055, Abcam, Cambridge, UK) and goat polyclonal anti-phosphorylated SMAD2/3 antibody (1:50 sc-11769, Santa Cruz Biotechnology, Santa Cruz, CA) overnight. The sections were then washed and incubated with a donkey anti-rabbit Alexa 488 (1:1,000, Life Technologies, Carlsbad, CA) and a donkey anti-goat Alexa 568 (1:1,000, Life Technologies) for 45 min at room temperature. The negative control was performed omitting the primary antibodies and incubating tissues only with secondary antibodies for 45 min at room temperature. Tissues were then counterstained with DAPI and slides were mounted with Dako-Fluorescent mounting Media (Dako, Carpinteria, CA). Slides were observed using a Leica SP5 confocal microscope with a 63 × N.A. 1.4 PL APO objective.
Mucosal cell extraction
Fresh duodenal biopsies were collected in calcium- and magnesium-free Hanks’ balanced salt solution (HBSS, Euroclone, Italy) and rapidly processed. Biopsy specimens were initially incubated in a HBSS solution containing dithiothreitol (DTT, 0.145 mg/ml, Sigma-Aldrich, St. Louis, MO) for 15 min to remove mucus and adherent bacteria. After extensive washing in HBSS, biopsies were incubated for 50 min with a HBSS solution containing 0.37 mg/ml EDTA (Sigma-Aldrich) at 37 °C. At the end, supernatant was collected for intraepithelial lymphocytes (IELs) analysis. After extensive washing in HBSS, biopsies were then digested in RPMI 1640 medium (Euroclone, Italy), containing 400 U/ml collagenase D (Roche, Milan, Italy) in a 5% CO2 incubator at 37 °C for 1 h. After incubation, LP mononuclear cells (LPMCs) released from the tissue samples were passed through a 70 μm cell strainer and washed with complete 10% RPMI 1640 medium.
Flow cytometry
Eight-color flow cytometer analysis was performed using a FACS Canto II cytometer (Becton Dickinson, BD Biosciences, San Jose, CA) after staining with fluorochrome-conjugated antibodies. Unless indicated all antibodies were from eBioscence (San Diego, CA). For surface marker expression anti-CD4 (RPA-T4), anti-CD8 (SK1), anti-CD25 (BC96), anti-IL-7R (eBioRDR5) were used. For Foxp3 intranuclear staining, Foxp3 staining Buffer set (eBioscence) and anti-Foxp3 (PCH101) was used following manufacturer’s recommendations. For intracellular cytokine detection, LPMCs and IELs were incubated for 5 h in the presence or absence of PMA, ionomycin and brefeldin A (Sigma-Aldrich). Subsequently, cells were surface-labeled with conjugated antibodies. After fixation with 2% paraformaldehyde (Merck), cells were permeabilized with PBS-0.5% saponin (Sigma) and stained with anti-IL-10 (JES3-9D7), anti-IL-17 (eBio64DEC17), anti-IL-22 (22URTI), anti-IFN-γ (4S.B3) and anti-GM-CSF (BVD2-21C11) antibodies. FACS data were analyzed with FlowJo software (TreeStar, Ashland, OR). Phenotypic analysis of antigen-presenting cells (APC) cells in LP was performed by surface staining with anti-CD3 (SK7, BioLegend, San Diego, CA), CD16 (CD16, BD), -CD19 (HIB19, BD), -CD56 (B159, BD) as lineage markers, anti-CD11c (B-ly6, BD), anti-CD14 (61D3, BD), anti- HLA-DR (L243, BioLegend), anti-CD103 (Ber-ACT8, BD) and anti-CX3CR1 (2A9-1, Miltenyi Biotec, Auburn, CA). DC were gated as lineage−HLA-DR+CD11c+ and macrophages as HLA-DR+CD14+.
T-cell stimulation
In vitro T cell receptor (TCR) stimulation of IELs and LPMCs isolated from the AIE patient or healthy controls was performed with plate-bound anti-CD3 and anti-CD28 Abs (2 μg/ml, BD) in the absence or presence of IL-17-promoting cytokines (TGF-β1, IL-1β, IL-6, and IL-23). All recombinant cytokines (R&D Systems, Minneapolis, MN) were added at 10 ng/ml, with the exception of TGF-β1 that were used at 1 ng/ml. After 4d on coated plates, cells were cultured for another 48 h in uncoated plates and then analyzed for intracellular cytokine production as previously described. To assess LAP (Latency-Associated Protein) and GARP (glycoprotein A repetitions predominant) expression, peripheral blood mononuclear cells were purified from the AIE patient and from 6 different healthy subjects using standard Ficoll-Paque gradient centrifugation. Different T-cell subsets were sorted on a FACSAriaII (BD) according to the expression of specific surface markers: Treg cells were sorted as CD4+IL7RlowCD25+ cells, whereas helper T cells were sorted as CD4+IL7RhighCD25− cells. After 3 days of TCR-dependent stimulation in plate-bound anti-CD3 and anti-CD28 Abs (2 μg/ml, BD Biosciences), Treg and helper T cells were stained with anti-GARP PeCy7 (G14D9, eBioscence) and anti-LAP (FNLAP, eBioscence) antibodies. Flow cytometry was performed using a FACS CANTO system (BD Biosciences) and FACS data were analyzed with FlowJo software (TreeStar).
Suppression assay
LPMCs and IELs from the AIE patient and from four untreated active CD as control were purified as previously described. CD4+ and CD8+ IELs were activated with anti-CD3/anti-CD28 Abs and IL-17 promoting cytokines for 4d in the presence or absence of autologous Treg cells (ratio 10:1) sorting isolated as CD4+IL7RlowCD25+ cells from LPMCs by a FACSAriaII (BD Biosciences). After 4d on coated plates, cells were cultured for another 48 h in uncoated plates and then analyzed for intracellular cytokine production as previously described.
Statistics
Statistical analysis was performed with Prism 5 software (GraphPad Software, La Jolla, CA). Student's t-tests for paired samples or one way anova (Tukey’s multiple comparison test) were used to evaluate differences between groups of variables. Statistical significance was set at *P<0.05, **P<0.005, and ***P<0.0005.
Results
Severe villous atrophy and massive duodenal T-cell infiltration in a case of severe AIE
Histological examination of the duodenum in AIE documented a severe villous atrophy and a massive infiltration of both the LP and the intraepithelial layer by mononuclear cells, including lymphocytes and eosinophilic granulocytes (Figure 1a). Steroid (T=1) and azathioprine treatment (T=2) determined a strong reduction of the inflammatory cell infiltrate, which was evident both in the LP and in the epithelial layer. Of note, a partial regrowth of intestinal villi was observed after the combined treatments at 6 months (T=2). Immunohistochemistry analysis further demonstrated that lymphocytes that infiltrated the LP and the epithelial layer contained both CD4+ and CD8+ T cells. TCR clonotyping on whole duodenal biopsies documented a polyclonal γ/δ T-cell repertoire (Figure 1b), thereby excluding the diagnosis of intestinal T-cell lymphoma.
Figure 1.
Massive duodenal lymphocytic infiltration in autoimmune enteropathy (AIE). Data from duodenal biopsies of the AIE patient at baseline (PRE), 8 weeks after initiation of steroid therapy (T=1) and at 6 months, under azathioprine therapy (T=2), are reported, together with those obtained from a cohort of subjects without signs of duodenal inflammation (CTR). (a) Histological sections (original magnification × 10) obtained from duodenal biopsies of the AIE patient and from a representative control. Hematoxylin and Eosin staining (H&E), and immunohistochemistry for CD3, CD4, and CD8 are reported. (b) TCR spectratyping of γ/δT cells revealed a polyclonal γ/δ T-cell pool and ruled out lymphoma.
Reduced expression of Foxp3, but not of IL-10, by duodenal CD4+ T cells in AIE
In order to characterize infiltrating T cells in AIE more in detail, we extracted mononuclear cells from the LP of duodenal biopsies before and after treatments, and compared them to non-inflamed duodenal control biopsies. Since non-IPEX AIE is characterized by reduced numbers of Foxp3-expressing Tregs in the CD4+ T-cell compartment, we first analyzed CD4+ regulatory and helper T-cell subsets. (Figure 2a). Of note, Foxp3+Tregs have a CD4+CD25hiIL-7Rlo phenotype in healthy individuals, while a second subset of Tregs in humans is also CD4+IL-7Rlo, but lacks CD25 and Foxp3 expression and produces large amounts of the anti-inflammatory cytokine IL-10.13 Data from mouse models indicates that Foxp3−IL-10+ “type 1 regulatory T cells (Tr1)” are also highly relevant for intestinal immune homeostasis.14 Both in AIE and controls the large majority of duodenal LP CD4+T cells displayed an IL-7RhiCD25−/lo helper phenotype (Figure 2a left panel). Only small fractions of regulatory CD25hiIL-7Rlo and CD25−IL-7R− subsets could be detected, and there were no major differences in the frequencies of these regulatory T-cell subsets between AIE and control subjects (Figure 2a right panel). We next analyzed LP CD4+ T cells for Foxp3 and IL-10 expression that are characteristic for Tregs and Tr1 cells, respectively. Consistent with previously described non-IPEX AIE cases,8 Foxp3 expression in intestinal CD4+CD25hiIL-7Rlo T cells was markedly reduced (23.3% vs. 64.1±1.6%, respectively, in AIE and controls), and only slightly increased upon treatments (Figure 2b). No mutations were identified by sequencing the Foxp3 gene (data not shown), ruling out that the patient was affected by IPEX. Conversely, IL-10 production was relatively high in active AIE and decreased upon treatments (Figure 2c). Thus, AIE is associated with defective expression of Foxp3, while IL-10 production by CD4+ T cells is not reduced.
Figure 2.
Reduced Foxp3 expression, but enhanced interleukin (IL)-10 production in LP CD4+ T cells in AIE. (a) Left panel: surface expression of the IL-7R and CD25 on gated lamina propria (LP) CD4+ T cells from the AIE patient with active disease (PRE) or after cortisone (T=1) and azathioprine treatment (T=2) and a control patient as representative control. Right panel: frequencies of IL-7RhighCD25− helper T cells, IL-7RloCD25+ Tregs and IL-7R−CD25+ Tr1-like cells in autoimmune enteropathy (AIE) and six control donors. (b) Histogram showing intracellular reduced expression levels of FoxP3 on gated LP CD4+CD25+ Treg cells (red line) from the AIE patient and control, in comparison with expression in CD4+IL-7R+ helper T cells (blue line). Frequencies of IL-7RloCD25+ Tregs in AIE and six control donors are reported on the right. (c) IL-10 production by LP CD4+T cells after brief polyclonal stimulation in AIE and six control donors.
Uncontrolled IL-17 production by IELs in active AIE is reverted by immune-modulatory therapies
We next analyzed the frequencies and cytokine profiles of CD4+ and CD8+ T cells in the duodenal LP and in the intraepithelial compartments. We observed a selective and dramatic increase of CD8+ T cells but not of CD4+ T cells in the LP (Figure 3a). Of note, the intraepithelial compartment was largely unchanged displaying no major alterations in the frequencies of CD4+ and CD8+ T cells (Figure 3b).
Figure 3.
Autoimmune enteropathy (AIE) is associated with high interleukin (IL)-17 production by IELs. (a-b) Quantitative flow cytometric analysis of the relative percentages of CD4+ and CD8+ T cells among lymphocytes that were extracted from the duodenal lamina propria (LP mononuclear cells (LPMCs), (a) and from the intraepithelial compartment (IELs, (b) of the AIE patient before (PRE) and after treatments (POST) with steroids (8 weeks, T=1) or azathioprine (6 months, T=2) and from a cohort of six subjects without signs of duodenal inflammation (CTR). (c,d) Intracellular expression of IL-17 and interferon-γ (IFN-γ) in gated LP CD4+, CD8+ T cells as well as in CD4+ IELs and CD8+ IELs from the analyzed AIE patient at different time points (active disease: PRE; after 6 months: POST) and in control donors (CTR). Dot plots show intracellular IL-17 and IFN-γ production following brief in vitro polyclonal stimulation. (e,f) Percentages of CD4+ and CD8+ T cells that produce either IL-17 (left), IFN-γ (right) or that co-produce IFN-γ and IL-17 (middle) in the AIE patient at different time points as compared to the six controls in the LP (e) and intraepithelial compartments (f).
We next evaluated the effector cytokine profile of CD4+ and CD8+ T cells extracted from the LP (Figure 3c) and from the intraepithelial compartment (Figure 3d). We focused our attention on the expression of IFN-γ and IL-17, since T helper cells that co-produce these cytokines are associated with intestinal inflammation.15, 16, 17 We also assessed the production of IL-22 and GM-CSF, two additional key cytokines regulating intestinal homeostasis.18, 19 We observed increased levels of IL-17 production by LP CD4+lymphocytes in active AIE that was largely reverted upon therapy-induced resolution of inflammation (Figure 3e, upper panel). Conversely, no major differences were found between AIE and controls in the proportion of CD4+T cells producing IFN-γ (Figure 3e), GM-CSF or IL-22 (Supplementary Figure 1 online). LP CD8+ T cells expressed very high levels of IFN-γ in all cases, but IL-17-producing and IL-17/IFN-γ co-producing CD8+ T cells were rare (Figure 3e lower panel). Noteworthy, a dramatic increase of IL-17-producing lymphocytes was found in the intraepithelial compartment. (Figure 3f). As shown, IL-17-producing CD4+ T cells were more strongly increased in the intraepithelial compartment as compared to the LP, and IL-17/IFN-γ co-producing cells were also more frequent (Figure 3f). Moreover, in active AIE also CD8+ IELs produced very high levels of IL-17, the large majority of these IL-17+CD8+ IELs also co-producing IFN-γ (Figure 3f, lower panel). In marked contrast, in healthy controls IL-17/IFN-γ co-producing CD8+ IELs were nearly undetectable (Figure 3f). Furthermore, steroid and azathioprine treatments strongly reduced IL-17 production in particular in the intraepithelial compartment. To exclude that enhanced IL-17 expression was merely resulting from the ongoing mucosal inflammation, ileal biopsies from a group of patients with active Crohn’s disease were analyzed. As shown in Supplementary Figure 2 online, enhanced percentages of IL-17-producing CD4+ and CD8+ T cells were found both in the LP and in the intraepithelium compartment of active Crohn’s disease patients as compared to uninflamed controls as expected. However, while the fraction of IL-17-producing T cells in Crohn’s disease and AIE were similar in the LP, IL-17+ IELs were much more frequent in AIE, in particular in the CD8 compartment.
These data suggest that AIE is characterized by a selective increase of IL-17-producing LP CD4+ T cells, and by the massive appearance of unconventional IL-17 and IFN-γ co-producing CD4+ and CD8+ IELs. In particular IL-17-producing CD8+ IELs are largely absent in the healthy duodenum and rare in the ileum of Crohn’s disease patients, and disappear upon therapy-induced resolution of inflammation.
TGF-β and pro-inflammatory cytokines jointly induce IL-17 production in CD8+ IELs
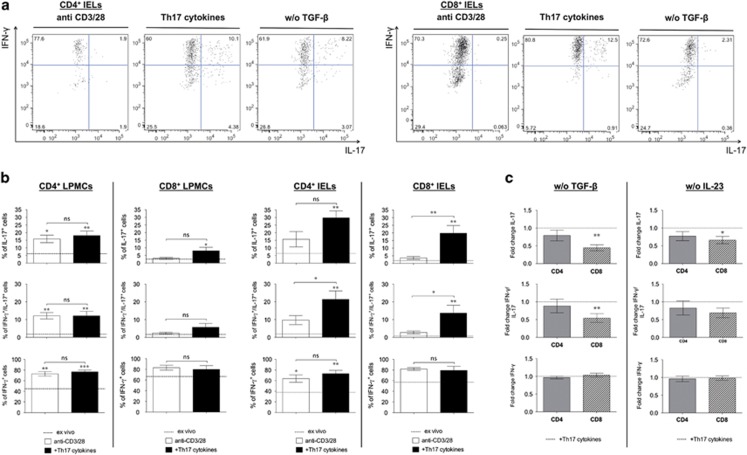
In order to understand if IL-17 and IFN-γ co-producing IELs could be generated locally upon inflammation, we activated IELs and LPMCs extracted from control biopsies with anti-CD3 and anti-CD28 antibodies in the absence and presence of an optimal combination of IL-17-promoting cytokines,17, 20, 21, 22, 23 namely high amounts of IL-1β, IL-6 and IL-23 and low amounts of TGF-β1 (Figure 4). CD4+ and CD8+ T cells from the intraepithelial compartment rapidly upregulated IL-17 production in response to TCR stimulation and Th17-promoting cytokines (Figure 4a). Interestingly, LP T cells had largely different requirements, since TCR stimulation alone was sufficient for the upregulation of IL-17 in LP CD4+ T cells, while IL-17 induction in LP CD8+ T cells was enhanced by cytokines, but inefficient when compared to other intestinal T-cell subsets (Figure 4b left panel). Of note, CD8+ IELs acquired very high IL-17-producing capacities in response to TCR stimulation and Th17-promoting cytokines, similar to the abundant levels observed in active AIE (Figure 4b right panel). IFN-γ production was highly expressed under all conditions, and consequently the majority of both CD4+ and CD8+ T cells that had upregulated IL-17 expression co-produced IFN-γ.
Figure 4.
Pro-inflammatory cytokines and transforming growth factor-β1 (TGF-β1) induce high levels of IL-17 in CD8+ IELs. Intracellular expression of interleukin (IL)-17, interferon-γ (IFN-γ) as well as co-expression interferon-γ (IFN-γ)/IL-17, in gated CD4+ and CD8+ (LP mononuclear cells (LPMCs) and intraepithelials (IELs)) extracted from a cohort of six subjects without signs of duodenal inflammation. T cells were activated with anti-CD3 and anti-CD28 in the absence (anti-CD3/28) or presence of IL-17 promoting cytokines (TGF-β1, IL-1β, IL-6 and IL-23; Th17 cytokines) and compared to ex vivo expression of IFN-γ and/or IL-17. (a) Representative stainings of stimulated CD4+ and CD8+ IELs. (b) Shown are the mean percentages and the s.e.m. * and ** at the top of the column indicates statistical significance as compared to ex vivo cytokine production (mean of ex vivo cytokine production is indicated by the black dotted line). (c) Mean variation of intracellular expression of IL-17 and/or IFN-γ induced with anti-CD3/CD28 antibodies and IL-17 promoting cytokines in CD4+ and CD8+ IELs in the selective absence of either TGF-β1 (w/o TGF-β) or IL-23 (w/o IL-23). Shown are the mean variations (fold change) and s.e.m. * and ** at the top of the column indicates statistical significance as compared with TCR stimulation in the presence of T helper 17 (Th17) cytokines indicated by the black dotted line.
The efficient cytokine-dependent induction of IL-17 in IELs prompted us to define the contribution of individual cytokines to this unexpected cellular plasticity. We therefore measured IFN-γ and IL-17 production in response to TCR stimulation and Th17-promoting cytokines in CD4+ and CD8+ IELs and analyzed their modulation in the selective absence of either IL-2317 or TGF-β123 (Figure 4c). The absence of TGF-β1 induced a strong and significant reduction of IL-17 production in CD8+ IELs, but had only a weak and not significant effect on IL-17 production by CD4+ IELs (Figure 4c, left panel). Furthermore, the effect of TGF-β1 was specific for IL-17, since IFN-γ production did not change significantly. Conversely, the absence of IL-23 had only a rather weak inhibitory effect on IL-17 induction in both CD4+ and CD8+ IELs (Figure 4c, right panel).
We conclude that TCR-activated IELs efficiently acquire IL-17-producing capacities without losing IFN-γ production in the presence of Th17-promoting cytokines. Intriguingly, TGF-β strongly enhanced the generation of IL-17 and IFN-γ co-producing CD8+ IELs, but had no relevant effect on CD4+ IELs.
Foxp3lowTregs upregulate TGF-β and enhance IL-17 induction in CD8+ IELs
Foxp3lowCD4+CD25+ Tregs from peripheral blood of AIE patients have reduced capacities to suppress the proliferation of circulating CD4+CD25− helper T cells,7 but efficiently suppress IL-2 and IFN-γ production.8 On the contrary, the capacity of LP Tregs to suppress intestinal T-cell responses in AIE has not been addressed yet, presumably due to the technical difficulties to isolate sufficient numbers of these rare Tregs (Figure 2a) from intestinal biopsies. In order to evaluate whether the increased expression of IL-17 in AIE by duodenal IELs was directly linked to a functional defect of Foxp3lowTregs, we set up a suppression assay where IL-17 production was induced in TCR-activated IELs with an optimal cytokine cocktail in the absence or presence of autologous Tregs isolated ex vivo from the LP. Duodenal Tregs from patients with CD, a clinical condition in which suppressive Tregs accumulate in the duodenum,24 were used as controls. Notably, ex vivo IL-17 and/or IFN-γ production by duodenal T-cell subsets were similar in CD patients and uninflamed control patients (Figure 5a), and in particular IL-17 production by CD8+ IELs were in both groups hardly detectable. Duodenal Tregs from CD patients strongly inhibited IL-17 upregulation of autologous CD8+ IELs, but had interestingly a weaker inhibitory effect on CD4+ IELs (Figure 5b). Moreover, IFN-γ production by CD8+ IELs was largely unaffected by the presence of Tregs, showing that mucosal Tregs control selectively the production of IL-17 by CD8+ IELs. In marked contrast, although duodenal Foxp3lowTregs in AIE also slightly reduced IL-17 expression in CD4+ IELs, they failed to inhibit IL-17 production by CD8+ IELs and even increased IL-17 secretion (Figure 5b).
Figure 5.
Regulation of intraepithelial (IEL) interleukin (IL)-17 production and upregulation of latent TGF-β by CD25+Tregs. (a) Intracellular expression of IL-17 and/or IFN-γ in CD4+ and CD8+ IELs from the analyzed autoimmune enteropathy (AIE) patient (dashed line), in celiac disease (CD, black bars) and control donors (CTR, white bars). IFN-γ and/or IL-17 production was measured by intracellular staining following brief polyclonal re-stimulation. Shown are the mean percentages and s.e.m. (b) IELs and autologous duodenal LP CD25+IL-7Rlo Tregs from the AIE patient and from 4 celiac disease (CD) patients were co-cultured at a 10:1 ratio and stimulated with anti-CD3 and -CD28 antibodies and Th17-promoting cytokines for 6 days. IFN-γ and IL-17 production was measured by intracellular staining following brief polyclonal re-stimulation. Shown are the mean percentages and the statistical significances. (c) Peripheral blood CD25+IL-7Rlo Tregs and CD25−IL-7Rhigh helper T cells from the AIE patient or from healthy donors were stimulated with anti-CD3 and -CD28 antibodies for 3 days and surface LAP and GARP expression analyzed by flow cytometry. Histogram showing surface expression levels of GARP and LAP on LP CD4+CD25+ Treg cells from the AIE patient (blue line) and control (red line), in comparison with expression in CD4+IL-7R+ helper T cells (black dotted line). Mean of frequency (MFI) of GARP and LAP expression on IL-7RloCD25+ regulatory T cells (Tregs) in AIE (black dots) and control donors (white bars) are reported. (d) Duodenal biopsies from the AIE patient before (PRE) and after treatments with steroids (8 weeks, T=1) or azathioprine (6 months, T=2) and from a representative control were immune-stained for CD8 (green), p-SMAD2/3 (red), and nuclear stain (DAPI). Arrows indicates cells co-expressing CD8 and p-SMAD2/3. Scale bar: 50 um, original magnification 63x.
CD25+ Tregs are unable to produce pro-inflammatory cytokines, but unlike helper T cells they upregulate membrane-bound TGF-β in its latent form (LAP, Latency Associated Protein), which is presented by the Treg-specific surface receptor GARP (glycoprotein A repetitions predominant).25 Notably, TGF-β signaling in mouse effector T cells is required for the inhibition of experimental colitis by Foxp3+Tregs.26 We wondered if the dysfunctional Foxp3lowTregs in AIE could enhance IL-17 production of CD8+IELs via TGF-β, and to this end we compared the capacity of CD4+CD25+IL-7Rlow Tregs to upregulate GARP and LAP surface expression following TCR activation (Figure 5c). Foxp3lowTregs upregulated GARP and LAP similar to Tregs from healthy controls, indicating that the induction of membrane-bound TGF-β production was not inhibited in Foxp3lowTregs in AIE.
Finally, to address whether CD8+ IELs also received signals from TGF-β in AIE in vivo, we stained duodenal AIE sections for CD8 and phosphorylated Smad2/3, (p-SMAD2/3), since the latter are phosphorylated and thus activated upon TGF-β receptor (TGF-β R) engagement. As shown in Figure 5d, we observed a higher number of CD8+ cells (green cells) in active AIE (PRE) in situ compared to healthy controls that decreased upon therapy as expected. Moreover, p-Smad2/3 positive cells (red) were present both in the steady state and in AIE before and after treatment. Moreover, some CD8+ lymphocytes were positive for p-Smad2/3 in all cases, suggesting that they were exposed to and activated by TGF-β in vivo both in the healthy gut and in AIE.
In conclusion, while normal duodenal Tregs suppress IL-17 production by IELs, Foxp3lowTregs in AIE, not only failed to suppress, but even enhanced IL-17 production in CD8+ IELs. This paradoxic finding could be explained by the fact that Foxp3lowTregs efficiently upregulated membrane-bound TGF-β, which in turn could enhance IL-17 production by CD8+ IELs. Consistent with this scenario, TGF-β signaling in CD8+IELs could be detected in situ in active AIE, demonstrating that bioactive TGF-β was present under this inflammatory condition.
Enhanced CD103 expression on DC and macrophages in active AIE
Intestinal T-cell polarization towards effector or regulatory subclasses is largely dependent on cytokine production by mucosal antigen-presenting cells (APCs).27 To investigate whether alterations in mucosal APCs might be associated with the enhanced IL-17 production of T-cell subsets in AIE, we analyzed APC extracted from duodenal AIE LP at baseline and following drug-induced remission, and results were compared with healthy controls. Since intestinal APC subsets are distinguished by CD103 and CX3CR1 expression, we analyzed the expression of these surface markers among both DC and macrophages, identified as lin−HLA-DR+CD11c+ and HLA-DR+CD14+ cells, respectively.28 In comparison with healthy controls, a higher proportion of DCs and macrophages expressed CD103 in active AIE (Supplementary Figure 3 online), while a reduced proportion of CX3CR1+ cells were found. Steroid treatment reduced the expression of CD103, and increased the expression of CX3CR1 on both mucosal DC and on macrophages. Thus, the dysregulated T-cell homeostasis in AIE is associated with an increased frequency of CD103+DC and macrophages that is partially reverted by steroid therapy.
Discussion
AIE is a fascinating disease from an immunological perspective, as insights into its pathogenesis may lead to valuable information regarding mechanisms of tolerance and in vivo regulation of immune responses in the human gut. Specifically, the recognition that most AIE cases are secondary to reduced numbers and functions of Foxp3+ Tregs7 makes this rare disease a model to study a partial defect of Tregs in the modulation of intestinal immune homeostasis. However, most studies on AIE pathogenesis focused on the reduced numbers and functions of Tregs in peripheral blood rather than analyzing functional or phenotypic changes in regulatory and effector lymphocytes in the intestine. Nevertheless, such information would be of relevance in designing novel anti-inflammatory drugs and therapeutic approaches for this rare condition.
The here analyzed AIE patient had severe duodenal inflammation, an elevated titer of anti-enterocyte antibodies and reduced Foxp3 expression in CD25+ Tregs; moreover γ/δ lymphoma, IPEX as well as CD were ruled out, indicating that she was actually affected by AIE.6, 8 Intestinal homeostasis requires not only Foxp3+Tregs but also IL-10,29 that can be provided by both Foxp3+ and Foxp3− CD4+ Tregs in mice.14, 30, 31, 32 Consistently, defects in the IL-10/IL-10R pathway induces severe early-onset colitis in humans.33 We detected relatively high IL-10 production by CD4+ T cells in active AIE (PRE) that diminished upon treatment-induced remission, indicating that defective Foxp3 expression in Tregs but not impaired IL-10 production is the underlying defect in AIE. Consistent with previous reports,34 we found that the small intestinal mucosa was massively infiltrated with T cells during active AIE, with a preponderance of CD8+ over CD4+ T cells in the LP. In addition, we observed a very selective upregulation of IL-17 in LP CD4+T cells. These alterations were largely reverted upon treatment-induced mucosal healing. IL-17 is important for epithelial barrier function and has anti-microbial as well as pro-inflammatory activities, and the upregulation of IL-17 might be an unsuccessful attempt by the intestinal immune system to re-establish gut homeostasis in AIE. Indeed, while anti-IFN-γ antibodies had a beneficial effect in IBD patients, anti-IL-17A was unexpectedly detrimental.35 Nevertheless, mucosal Th17 cells and in particular those co-producing IL-17 and IFN-γ or GM-CSF could also drive chronic intestinal inflammation, and might thus also play a pathogenic role.15, 19, 36
A second major change in active AIE that was reverted upon mucosal healing was the abundant IL-17 production by IELs, in particular IL-17 and IFN-γ-co-producing CD8+IELs. Importantly, IL-17-producing CD8+ IELs were rare in other inflammatory diseases of the small intestine, such as CD and ileal Crohn’s disease. CD8+IELs have been implicated in multiple pathological processes of the small intestine, including the progression of inflammatory bowel diseases,37 as well as in the pathogenesis of CD by directly targeting intestinal epithelial cells and causing villous atrophy.38, 39 In addition, IFN-γ/IL-17 co-producing CD8+ T cells have been described in other immunological disorders of the intestine, including active CD40 and microscopic colitis.41 Interestingly, recent evidence in mice suggests that IELs may be under the direct control of intestinal Tregs, as an experimental reduction in numbers and functionality of Tregs leads to a severe form of intestinal inflammation characterized by infiltration of the intraepithelial compartment by IL-17-secreting CD8+ T cells.42 Consistently, we found that the induction of IL-17-producing CD8+IELs in humans was inhibited by Tregs in control patients, but not in AIE. IL-17 and IFN-γ co-producing T cells can be generated from IL-17-producing CD4+ T cells with cytokines that induce IFN-γ, such as IL-1β and IL-23 or IL-12.20, 43 In contrast, we showed here that IFN-γ-producing CD8+IELs could acquire IL-17 production without losing IFN-γ production in response to a cocktail of IL-17-promoting cytokines, i.e., IL-1β, IL-6, IL-23 and TGF-β1. IELs are thus not terminally differentiated cells but are highly plastic,43 and could change their cytokine profile in response to locally uncontrolled cytokine production. Pro-inflammatory cytokines in the LP are probably derived from myeloid cells, and interestingly we observed increased frequencies of CD103+ myeloid cells in active AIE. Indeed, intestinal CD103+DC produce pro-inflammatory cytokines and induce Th17 differentiation in mice.44
An important and unexpected finding of this study was that IL-17 production by IELs and LP T cells had largely different requirements.45 Thus, while LP CD4+ T cells rapidly upregulated IL-17 upon TCR stimulation in the absence of exogenous cytokines, LP CD8+ T cells poorly upregulated IL-17 production even under optimal Th17 conditions, consistent with their low IL-17 production in active AIE. Intriguingly, the anti-inflammatory cytokine TGF-β, which strongly inhibits the induction of IFN-γ production in naïve CD4+ T cells19, 44 and restrains colitis,26 enhanced IL-17 production in CD8+IELs, but had no inhibitory effect on IFN-γ. This enhancing effect of TGF-β was specific for CD8+ IELs, because TGF-β had no enhancing effect on CD4+IELs. Thus, pro-inflammatory cytokines were sufficient to induce IL-17 in TCR-activated CD4+IELs, possibly because they contain pre-committed Th17 cell precursors46, 47 which do not require TGF-β to upregulate IL-17.46, 48 Finally, our data suggests that a relevant source of intestinal TGF-β, which promotes IL-17 induction in CD8+ IELs in AIE, might be the defective Foxp3lowTregs themselves. Indeed, latent TGF-β was efficiently and selectively upregulated on Foxp3lowTregs in AIE, and the latter further enhanced IL-17 production selectively by CD8+IELs. Thus, the defective Foxp3lowTregs might enhance IL-17 production by CD8+IELs via TGF-β Moreover, some duodenal CD8+T cells contained phosphorylated Smad2/3, demonstrating that they received signals from the TGF-βR also under inflammatory conditions in vivo and suggesting that bioactive TGF-β was available. Indeed, it was previously shown in mice that the presence of apoptotic cells and pathogen-associated molecular patterns in the inflamed intestine is a physiological situation that leads to the induction of both TGF-β and pro-inflammatory cytokines.49 Overall these findings indicate that TGF-β plays an important role not only in the steady state, but also under inflammatory conditions.
In conclusion, our data shows that in AIE the quantitative deficit of Foxp3 expression in intestinal CD4+CD25+Tregs is associated with a selectively dysregulated IL-17 production in particular by CD8+ lymphocytes in the epithelium, which is reverted upon successful pharmacological treatment. The enhanced IL-17 production by both CD4+ and CD8+IELs in AIE suggests that human Foxp3+Tregs have a non-redundant role to control IL-17 production by IELs. Moreover, TGF-β, possibly derived from defective Tregs in AIE, could be selectively important for IL-17 production by CD8+IELs. This data broadens our understanding on the role of Foxp3+Tregs and TGF-β in intestinal immune homeostasis, and raises the question if mongersen, an efficient treatment of Crohn’s disease50 that enhances TGF-β responsiveness, is also an appropriate treatment for AIE.
Study Highlights

Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Flavio Caprioli, MD, PhD.
Specific author contributions: Designed, performed and analyzed experiments and wrote the paper: M.P.; designed experiments and wrote the paper: J.G.; designed and supervised the study and experiments and wrote the paper: F.C.; collected and analyzed histological data: U.G.; critically reviewed the manuscript for important intellectual content: A.M., S.T., G.N., P.L., G.E., M.P., L.E., S.A., D.C.; All Authors approved the final draft submitted.
Financial support: This work was supported by the Cariplo Foundation and the Italian Ministry of health (Giovani Ricercatori GR-2011- 02352001).
Potential competing interests: None.
Supplementary Material
References
- Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep 2012; 14: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram S, Murray JA, Pardi DS et al. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol 2007; 5: 1282–1290 quiz 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol 2012; 3: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res 2007; 38: 112–121. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061. [DOI] [PubMed] [Google Scholar]
- Moes N, Rieux-Laucat F, Begue B et al. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology 2010; 139: 770–778. [DOI] [PubMed] [Google Scholar]
- Ruemmele FM, Brousse N, Goulet O. Autoimmune enteropathy: molecular concepts. Curr Opin Gastroenterol 2004; 20: 587–591. [DOI] [PubMed] [Google Scholar]
- Zuber J, Viguier M, Lemaitre F et al. Severe FOXP3+ and naive T lymphopenia in a non-IPEX form of autoimmune enteropathy combined with an immunodeficiency. Gastroenterology 2007; 132: 1694–1704. [DOI] [PubMed] [Google Scholar]
- Bishu S, Arsenescu V, Lee EY et al. Autoimmune enteropathy with a CD8+ CD7- T-cell small bowel intraepithelial lymphocytosis: case report and literature review. BMC Gastroenterol 2011; 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med 1988; 167: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khalidi H, Kandel G, Streutker CJ. Enteropathy with loss of enteroendocrine and paneth cells in a patient with immune dysregulation: a case of adult autoimmune enteropathy. Hum Pathol 2006; 37: 373–376. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Dignass A, Panes J et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27. [DOI] [PubMed] [Google Scholar]
- Haringer B, Lozza L, Steckel B et al. Identification and characterization of IL-10/IFN-gamma-producing effector-like T cells with regulatory function in human blood. J Exp Med 2009; 206: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011; 34: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007; 204: 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh R, Kozhaya L, McKevitt K et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 2014; 211: 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern PP, Izcue A, Maloy KJ et al. The interleukin-23 axis in intestinal inflammation. Immunol Rev 2008; 226: 147–159. [DOI] [PubMed] [Google Scholar]
- Seiderer J, Brand S. IL-22: a two-headed cytokine in IBD? Inflamm Bowel Dis 2009; 15: 473–474. [DOI] [PubMed] [Google Scholar]
- Griseri T, McKenzie BS, Schiering C et al. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity 2012; 37: 1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastirr I, Maglie S, Paroni M et al. IL-21 is a central memory T cell-associated cytokine that inhibits the generation of pathogenic Th1/17 effector cells. J Immunol 2014; 193: 3322–3331. [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008; 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M, Harrison OJ, Schiering C et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med 2012; 209: 1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006; 24: 179–189. [DOI] [PubMed] [Google Scholar]
- Zanzi D, Stefanile R, Santagata S et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am J Gastroenterol 2011; 106: 1308–1317. [DOI] [PubMed] [Google Scholar]
- Tran DQ, Andersson J, Wang R et al. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci USA 2009; 106: 13445–13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlen L, Read S, Gorelik L et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med 2005; 201: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 2008; 8: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzoli G, Krietsch J, Weick A et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013; 122: 932–942. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW et al. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999; 190: 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Harrington LE, Janowski KM et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol 2007; 8: 931–941. [DOI] [PubMed] [Google Scholar]
- Uhlig HH, Coombes J, Mottet C et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol 2006; 177: 5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997; 389: 737–742. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Klein C et al. IL-10 and IL-10 receptor defects in humans. Ann NY Acad Sci 2011; 1246: 102–107. [DOI] [PubMed] [Google Scholar]
- Murch SH, Fertleman CR, Rodrigues C et al. Autoimmune enteropathy with distinct mucosal features in T-cell activation deficiency: the contribution of T cells to the mucosal lesion. J Pediatr Gastroenterol Nutr 1999; 28: 393–399. [DOI] [PubMed] [Google Scholar]
- Hueber W, Sands BE, Lewitzky S et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Flavell RA. Life, death, and miracles: Th17 cells in the intestine. Eur J Immunol 2012; 42: 2238–2245. [DOI] [PubMed] [Google Scholar]
- Cheroutre H. In IBD eight can come before four. Gastroenterology 2006; 131: 667–670. [DOI] [PubMed] [Google Scholar]
- Meresse B, Chen Z, Ciszewski C et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 2004; 21: 357–366. [DOI] [PubMed] [Google Scholar]
- Hue S, Mention JJ, Monteiro RC et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004; 21: 367–377. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Sarra M, Del Vecchio Blanco G et al. Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol 2010; 184: 2211–2218. [DOI] [PubMed] [Google Scholar]
- Kumawat AK, Strid H, Tysk C et al. Microscopic colitis patients demonstrate a mixed Th17/Tc17 and Th1/Tc1 mucosal cytokine profile. Mol Immunol 2013; 55: 355–364. [DOI] [PubMed] [Google Scholar]
- Park SG, Mathur R, Long M et al. T regulatory cells maintain intestinal homeostasis by suppressing gammadelta T cells. Immunity 2010; 33: 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geginat J, Paroni M, Maglie S et al. Plasticity of human CD4 T cell subsets. Front Immunol 2014; 5: 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson EK, Uronen-Hansson H, Semmrich M et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 2013; 38: 958–969. [DOI] [PubMed] [Google Scholar]
- Santarlasci V, Maggi L, Capone M et al. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol 2009; 39: 207–215. [DOI] [PubMed] [Google Scholar]
- Cosmi L, De Palma R, Santarlasci V et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med 2008; 205: 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer F, Khaitan A, Kozhaya L et al. Differentiation of IL-17-producing effector and regulatory human T cells from lineage-committed naive precursors. J Immunol 2014; 193: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastirr I, Crosti M, Maglie S et al. Signal Strength and Metabolic Requirements Control Cytokine-Induced Th17 Differentiation of Uncommitted Human T Cells. J Immunol 2015; 195: 3617–3627. [DOI] [PubMed] [Google Scholar]
- Torchinsky MB, Garaude J, Martin AP et al. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature 2009; 458: 78–82. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Neurath MF, Ardizzone S et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn's disease. N Engl J Med 2015; 372: 1104–1113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.