Abstract
Epstein–Barr virus (EBV) is closely associated with nasopharyngeal carcinoma (NPC). Serum IgA antibodies against early antigen (EA‐IgA) and viral capsid antigen (VCA‐IgA) are the most commonly used to screen for NPC in endemic areas. However, the prognostic value of serum EA‐IgA and VCA‐IgA in patients with NPC is less clear. We hypothesize that serum EA‐IgA and VCA‐IgA levels have prognostic impact for survival outcomes in NPC patients with undetectable pretreatment EBV (pEBV) DNA. In this series, 334 patients with non‐metastatic NPC and undetectable pEBV DNA were included. Serum EA‐IgA and VCA‐IgA were determined by ELISA. After analysis, serum EA‐IgA and VCA‐IgA loads correlated positively with T, N, and overall stage (all P < 0.05). Serum EA‐IgA was not associated with survival outcome in univariable analyses. But patients with serum VCA‐IgA >1:120 had significantly inferior 5‐year progression‐free survival (80.4% vs 89.6%, P = 0.025), distant metastasis‐free survival (88.4% vs 94.8%, P = 0.050), and locoregional relapse‐free survival (88.4% vs 95.6%, P = 0.023; log–rank test). Multivariable analyses revealed that N stage was the only independent prognostic factor (all P < 0.05), but the VCA‐IgA became insignificant. Further analyses revealed that serum VCA‐IgA was not an independent prognostic factor in early N (N0–1) or advanced N (N2–3) stage NPC. In summary, although both EA‐IgA and VCA‐IgA correlate strongly with TNM stage, our analyses do not suggest that these antibodies are prognostic biomarkers in patients with NPC and undetectable pEBV DNA.
Keywords: Early antigen, Epstein–Barr virus antibodies, nasopharyngeal carcinoma, prognostic value, viral capsid antigen
The highest incidence of NPC reportedly occurs in southern China, with the yearly incidence rate varying between 15 and 50 cases per 100 000 population.1 Despite improvements in the locoregional control rate due to the development of more precise imaging, radiotherapy technology, and eradication of potential metastasis by chemotherapy, the survival of patients with advanced NPC remains unsatisfactory.2 Therefore, the identification of new prognostic factors is of great importance to recognize patients at high risk.
Southern China has one of the highest incidences of EBV infection, and more than 95% of adults in southern China are infected with EBV3 Therefore, it is reasonable to speculate that EBV infection plays an important role in the etiology. In recent times, several studies4, 5, 6 have reported on the association of elevated pEBV DNA with adverse prognoses in patients with NPC. However, the prognostic value of IgA antibodies against early antigen (EA‐IgA) and viral capsid antigen (VCA‐IgA) are less clear. There have only been two studies on the prognostic impact of EBV antibodies in NPC, and they failed to detect an association between EBV antibodies and survival outcomes.7, 8 The relative risks seem highly heterogeneous, comprising patients harboring localized and metastatic disease in these studies. Therefore, the prognostic impact of serum EBV antibodies needs to be investigated in patients with non‐metastatic NPC and undetectable pEBV DNA.
On the basis of this premise, we undertook the current study to gain insight into the correlation between serum EBV antibodies and TNM stage from a prospectively created database, and evaluate the prognostic value of serum EBV antibodies for survival outcomes in patients with NPC and undetectable pEBV DNA.
Materials and Methods
Patient selection
This study was approved by the Institutional Review Board of Sun Yat‐sen University Cancer Center (Guangzhou, China). All NPC patients were identified from a prospectively created database from November 2009 and February 2012. The eligibility criteria were: (i) histologically confirmed NPC; (ii) no evidence of distant metastases; (iii) treated by radiotherapy with curative intent; (iv) undetectable (0 copies per mL) pEBV DNA; and (v) absence of secondary malignancy or pregnancy. Finally, a total of 334 patients were included in this study.
All patients underwent a pretreatment evaluation including a complete physical examination, MRI of the nasopharynx and neck, chest radiograph, abdominal sonography, electrocardiography, bone scan, and complete blood sampling (cell counts, biochemical profile, and EBV serology). Positron emission tomography/computed tomography (CT) was undertaken in 93 patients (27.8%). Patients were restaged by two radiation oncologists specializing in head and neck cancer according to the 2009 7th edition of the AJCC staging system,9 with disagreements resolved by consensus.
Radiotherapy
All patients received intensity‐modulated radiotherapy as a primary treatment, while immobilized in the supine position using a thermoplastic head and shoulder mask. Contrast‐enhanced planning CT (3‐mm slice thickness) images from the superior border of the frontal sinus to 2 cm below the sternoclavicular joint were obtained and transferred to the Monaco treatment planning system (version 3.02; Elekta Instrument AB, Stockholm, Sweden).
Target volumes and organs at risk were delineated on each slice of the CT images, as previously described,10 in agreement with International Commission on Radiation Units and Measurements Reports 6211 and 83.12 The GTV including GTVp and GTVnd was delineated on the basis of clinical, endoscopic, and MRI findings. Gross disease at primary site together with enlarged retropharyngeal lymph nodes was designated GTVp; clinically involved cervical lymph nodes was designated GTVnd. Two CTVs were delineated according to the GTV: CTV1, high‐risk regions encompassing GTVp plus 5–10 mm, including entire nasopharyngeal mucosa and 5 mm submucosal region; and CTV2, low‐risk regions containing CTV1 plus 5–10 mm, encompassing sites of microscopic extension and lymphatic regions. The PTVs, termed PTVp, PTV1, PTV2, and PTVnd, were constructed by expanding the GTVp, CTV1, CTV2, and CTVnd, respectively, by 3 mm; a 3‐mm margin was added to the brainstem and spinal cord to generate planning organ at risk volume.
The prescribed doses to PTVp, PTVnd, PTV1, and PTV2 were 66–72, 64–70, 60–63, and 54–56 Gy, respectively, in 28–33 fractions (66–70 Gy to PTVp for T1 NPC, 68–72 Gy for T2–4 NPC; 68–70 Gy to nodes >1 cm, 64–68 Gy to clinically involved nodes ≤1 cm).13 The dose constraints for organs at risk and planning organ at risk volumes were as described for the RTOG‐0225 trial.14 All patients were treated following a routine schedule (one fraction daily, 5 days per week).
Chemotherapy
During the study period, we followed our institutional guidelines, which recommended radiotherapy alone for stage I, concurrent chemoradiotherapy for stage II, and concurrent chemoradiotherapy +/– neoadjuvant/adjuvant chemotherapy for stage III to IVB NPC, as defined by the 7th edition of the AJCC staging system. In patients with stage III to IVB disease, neoadjuvant chemotherapy was given when the waiting time for radiotherapy was considered to be longer than acceptable, or when it was considered advantageous to reduce bulky tumors. Neoadjuvant or adjuvant chemotherapy were cisplatin (80 mg/m2) with 5‐fluorouracil (800 mg/m2/day over 120 h), or cisplatin (80 mg/m2) with taxanes (80 mg/m2) given at 3‐week intervals for two or three cycles. Concurrent chemotherapy consisted of cisplatin (80 or 100 mg/m2) given in weeks 1, 4, and 7 of radiotherapy, or cisplatin (40 mg/m2) given weekly during radiotherapy, beginning on the first day of radiotherapy.
Quantification of plasma EBV DNA
Before treatment, peripheral venous blood (3 mL) was collected from each patient into EDTA‐containing tubes and centrifuged at 3000 g for 5 min. Total plasma DNA was extracted using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Fluorescence PCR was carried out using an EBV PCR quantitative diagnostic kit (Da‐An Genetic Diagnostic Center, Guangzhou, China) targeting the BamHI‐W region of the EBV genome. Data were analyzed using Applied Biosystems 7300 SDS software (Beijing, China). Undetectable EBV DNA was defined as 0 copies/mL.
Enzyme‐linked immunosorbent assay
The concentrations of serum EA‐IgA and VCA‐IgA in all samples were determined in duplicate using commercial ELISA kits (3400638; Berer Bioengineering, Beijing, China), as previously described,15 following the manufacturer's instructions. Briefly, negative control samples, positive control samples, and each serum sample diluted 1:10 were added to the plates. After washing, 100 μL HRP‐conjugated anti‐human IgA was added to each well. Color development and absorbance measurements were undertaken as described previously.16
Statistical analysis
The primary end‐point was PFS, and the secondary end‐points included OS, DMFS, and LRFS. Receiver operating characteristic curves were used to evaluate different cut‐off points for the EBV antibodies. Patients were stratified according to the cut‐off points. The AUC was used to assess the prognostic value of each serum EBV antibody. Patient demographic and disease data were compared using Pearson's χ2‐test (or Fisher's exact test, if indicated). Cumulative survival rates were estimated using the Kaplan–Meier method and the differences between survival curves were examined using the log–rank test. Multivariate analysis using a Cox proportional hazards model was used to test independent significance by backward elimination of insignificant explanatory variables. All statistical tests were two‐sided; P‐values <0.05 were considered significant. Statistical analysis was performed using R 3.1.2 (https://mirrors.tuna.tsinghua.edu.cn/CRAN/).
Results
Clinicopathological characteristics
The clinicopathological characteristics of the 334 patients, including 251 (75.1%) men and 83 (24.9%) women, are presented in Table 1. The median age at diagnosis was 45 years (range, 17–75 years). Based on the WHO criteria, 94% of patients had type III disease and 6% had type II disease. The TNM stage distribution based on the 7th AJCC staging system was stage I for 23 patients (6.9%), II for 69 (20.7%), III for 170 (50.9%), and IVA/B for 35 (21.6%). Eighty‐six percent of the patients (287/334) received chemotherapy. Of the patients receiving chemotherapy, 49% (142/287) received neoadjuvant chemotherapy, 13% (38/287) received adjuvant chemotherapy, and 100% (287/287) received concurrent chemotherapy.
Table 1.
Clinicopathological features of 334 patients
| Characteristic | No. of patients | % |
|---|---|---|
| Age, years | ||
| <50 | 237 | 71.0 |
| ≥50 | 97 | 29.0 |
| Sex | ||
| Male | 251 | 75.1 |
| Female | 83 | 24.9 |
| Histology | ||
| WHO II | 20 | 6.0 |
| WHO III | 314 | 94.0 |
| History of smoking | ||
| No | 224 | 67.1 |
| Yes | 110 | 32.9 |
| T stage† | ||
| T1 | 62 | 18.6 |
| T2 | 49 | 14.7 |
| T3 | 167 | 50.0 |
| T4 | 56 | 16.8 |
| N stage† | ||
| N0 | 66 | 19.8 |
| N1 | 193 | 57.8 |
| N2 | 56 | 16.8 |
| N3 | 19 | 5.7 |
| Clinical stage† | ||
| I | 23 | 6.9 |
| II | 69 | 20.7 |
| III | 170 | 50.9 |
| IVA/B | 72 | 21.6 |
| Chemotherapy | ||
| CCRT alone | 107 | 32.0 |
| NACT + CCRT | 142 | 42.5 |
| CCRT + AC | 38 | 11.4 |
| No chemotherapy | 47 | 14.1 |
†According to the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system. AC, adjuvant chemotherapy; CCRT, concurrent chemoradiotherapy; NACT, neoadjuvant chemotherapy.
Correlation between EBV antibodies and clinicopathological characteristics
In the study, ROC curve was used to evaluate different cut‐off points for the EBV antibodies. The cut‐off of EA‐IgA for PFS was 1:15 (sensitivity 0.528, specificity 0.580; AUC = 0.545), and 1:120 of VCA‐IgA for PFS (sensitivity 0.736, specificity 0.431; AUC = 0.604). The correlations between the serum levels of these antibodies and various clinicopathological features were examined. Patients with age ≥50 years, advanced T stage (T3/4), advanced N stage (N2/3), and advanced clinical stage (stage III–IV) were more likely to present high EA‐IgA (>1:15) and high VCA‐IgA (>1:120) (all P < 0.05; Tables 2, 3). But there was no significant difference in sex, histology, smoking, or chemotherapy between patients with high and low EA‐IgA or high and low VCA‐IgA (all P > 0.05).
Table 2.
Baseline characteristics of the patients with nasopharyngeal carcinoma stratified by low IgA antibodies against early antigen (EA‐IgA) versus high EA‐IgA
| Characteristic | Low EA‐IgA | High EA‐IgA | P‐value* |
|---|---|---|---|
| Age, years | 0.028 | ||
| <50 | 124 (66.0) | 113 (77.4) | |
| ≥50 | 64 (34.0) | 33 (22.6) | |
| Sex | 0.799 | ||
| Male | 140 (74.5) | 111 (76.0) | |
| Female | 48 (25.5) | 35 (24.0) | |
| Histology | 0.819 | ||
| WHO II | 12 (6.4) | 8 (5.5) | |
| WHO III | 176 (93.6) | 138 (94.5) | |
| History of smoking | 0.197 | ||
| No | 132 (70.2) | 92 (63.0) | |
| Yes | 56 (29.8) | 54 (37.0) | |
| T stage† | <0.001 | ||
| T1 | 51 (27.1) | 11 (7.5) | |
| T2 | 27 (14.4) | 22 (15.1) | |
| T3 | 86 (45.7) | 81 (55.5) | |
| T4 | 24 (12.8) | 32 (21.9) | |
| N stage† | <0.001 | ||
| N0 | 49 (26.1) | 17 (11.6) | |
| N1 | 109 (58.0) | 84 (57.5) | |
| N2 | 21 (11.2) | 35 (24.0) | |
| N3 | 9 (4.8) | 10 (6.9) | |
| Clinical stage† | <0.001 | ||
| I | 21 (11.2) | 2 (1.4) | |
| II | 46 (24.5) | 23 (15.8) | |
| III | 88 (46.8) | 82 (56.2) | |
| IVA‐B | 33 (17.6) | 39 (26.7) | |
| Chemotherapy | 0.434 | ||
| No | 29 (15.4) | 18 (12.3) | |
| Yes | 159 (84.6) | 128 (87.7) |
*P‐values were calculated using the χ2‐test. †According to the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system. High EA‐IgA, >1:15; Low EA‐IgA, ≤1:15.
Table 3.
Baseline characteristics of the patients with nasopharyngeal carcinoma stratified by low versus high IgA antibodies against viral capsid antigen (VCA‐IgA)
| Characteristic | Low VCA‐IgA | High VCA‐IgA | P‐value* |
|---|---|---|---|
| Age, years | 0.002 | ||
| <50 | 83 (61.5) | 154 (77.4) | |
| ≥50 | 52 (38.5) | 45 (22.6) | |
| Sex | 0.606 | ||
| Male | 99 (73.3) | 152 (76.4) | |
| Female | 36 (26.7) | 47 (23.6) | |
| Histology | 0.815 | ||
| WHO II | 9 (6.7) | 11 (5.5) | |
| WHO III | 126 (93.3) | 188 (94.5) | |
| History of smoking | 0.154 | ||
| No | 97 (71.9) | 127 (63.8) | |
| Yes | 38 (28.1) | 72 (36.2) | |
| T stage† | 0.002 | ||
| T1 | 38 (28.1) | 24 (12.1) | |
| T2 | 21 (15.6) | 28 (14.1) | |
| T3 | 57 (42.2) | 110 (55.3) | |
| T4 | 19 (14.1) | 37 (18.6) | |
| N stage† | <0.001 | ||
| N0 | 38 (28.1) | 28 (14.1) | |
| N1 | 85 (63.0) | 108 (54.3) | |
| N2 | 7 (5.2) | 49 (24.6) | |
| N3 | 5 (3.7) | 14 (7.0) | |
| Clinical stage† | <0.001 | ||
| I | 17 (12.6) | 6 (3.0) | |
| II | 35 (25.9) | 34 (17.1) | |
| III | 59 (43.7) | 111 (55.8) | |
| IVA‐B | 24 (17.8) | 48 (24.1) | |
| Chemotherapy | 0.112 | ||
| No | 24 (17.8) | 23 (11.6) | |
| Yes | 111 (82.2) | 176 (88.4) |
*P‐values were calculated using the χ2‐test. †According to the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system. High VCA‐IgA, >1:120; Low VCA‐IgA, ≤1:120.
Prognostic value of EBV antibodies in patients with NPC
The results of univariable analyses are shown in Table 4. In the log–rank test, high EA‐IgA was not associated with inferior PFS (HR, 1.47; 95% CI, 0.86–2.53; P = 0.157), OS (HR, 1.15; 95% CI, 0.54–2.45; P = 0.714), DMFS (HR, 1.14; 95% CI, 0.56–2.34; P = 0.716), and LRFS (HR, 1.89; 95% CI, 0.90–3.95; P = 0.087; Fig. 1). Likewise, we did not observe any difference in OS between patients with high VCA‐IgA and low VCA‐IgA (90.5% vs 92.1%, P = 0.282; Fig. 2). However, patients with high VCA‐IgA had a significantly inferior prognosis in terms of PFS (80.4% vs 89.6%, P = 0.025; Fig. 2a), DMFS (88.4% vs 94.8%, P = 0.050; Fig. 2c), and LRFS (88.4% vs 95.6%, P = 0.023; Fig. 2d) than patients with low VCA‐IgA. Thus, VCA‐IgA may be a better predictor compared to VCA‐IgA for patients with undetectable pEBV DNA. After adjusting for the TNM classification and EBV antibodies, the N stage was the only significant prognostic factor for PFS (HR, 2.18; 95% CI, 1.22–3.89; P = 0.008), OS (HR, 2.90; 95% CI, 1.36–6.20; P = 0.006) and DMFS (HR, 2.16; 95% CI, 1.31–4.67; P = 0.031), but VCA‐IgA became insignificant for predicting PFS (HR, 1.52; 95% CI, 0.80–2.89; P = 0.205), DMFS (HR, 1.77; 95% CI, 0.73–4.31; P = 0.208), and LRFS (HR, 2.45; 95% CI, 0.78–7.68; P = 0.125) (Table 5).
Table 4.
Univariable analyses of prognostic factors for the whole cohort of patients with nasopharyngeal carcinoma (n = 334)
| Variable | Progression‐free survival | Overall survival | Distant metastasis‐free survival | Locoregional relapse‐free survival | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value* | HR (95% CI) | P‐value* | HR (95% CI) | P‐value* | HR (95% CI) | P‐value* | |
| Age, years | 0.88 (0.47–1.65) | 0.694 | 1.37 (0.62–3.05) | 0.440 | 0.56 (0.21–1.45) | 0.230 | 0.87 (0.37–2.04) | 0.755 |
| <50 | ||||||||
| ≥50 | ||||||||
| Sex | 1.11 (0.60–2.04) | 0.742 | 0.52 (0.18–1.50) | 0.224 | 0.91 (0.39–2.13) | 0.836 | 1.42 (0.65–3.12) | 0.383 |
| Male | ||||||||
| Female | ||||||||
| Histology | 0.78 (0.28–2.17) | 0.639 | 1.72 (0.23–12.70) | 0.596 | 1.89 (0.26–13.90) | 0.531 | 0.39 (0.13–1.11) | 0.078 |
| WHO II | ||||||||
| WHO III | ||||||||
| Smoking | 1.80 (1.05–3.09) | 0.033 | 2.65 (1.24–5.66) | 0.012 | 1.87 (0.91–3.83) | 0.087 | 1.51 (0.72–3.15) | 0.278 |
| No | ||||||||
| Yes | ||||||||
| T stage | 1.40 (0.76–2.58) | 0.278 | 1.35 (0.57–3.21) | 0.492 | 1.40 (0.62–3.15) | 0.413 | 1.34 (0.59–3.02) | 0.482 |
| T1–2 | ||||||||
| T3–4 | ||||||||
| N stage | 2.51 (1.45–4.35) | 0.001 | 2.93 (1.37–6.26) | 0.006 | 2.50 (1.20–5.19) | 0.014 | 2.02 (0.94–4.34) | 0.072 |
| N0–1 | ||||||||
| N2–3 | ||||||||
| Clinical stage | 1.47 (0.76–2.86) | 0.254 | 1.61 (0.61–4.26) | 0.337 | 1.58 (0.64–3.85) | 0.319 | 1.23 (0.52–2.87) | 0.636 |
| I–II | ||||||||
| III–IVA/B | ||||||||
| VCA‐IgA | 1.98 (1.08–3.65) | 0.028 | 1.57 (0.69–3.59) | 0.370 | 2.28 (0.98–5.31) | 0.050 | 2.73 (1.11–6.69) | 0.029 |
| <1:120 | ||||||||
| ≥1:120 | ||||||||
| EA‐IgA | 1.47 (0.86–2.53) | 0.159 | 1.15 (0.54–2.45) | 0.715 | 1.14 (0.56–2.34) | 0.716 | 1.89 (0.90–3.95) | 0.092 |
| <1:15 | ||||||||
| ≥1:15 | ||||||||
| Chemotherapy | 2.88 (0.87–9.61) | 0.084 | 1.04 (0.89–8.97) | 0.997 | 5.30 (0.71–39.80) | 0.105 | 2.15 (0.49–9.45) | 0.312 |
| CCRT alone | ||||||||
| NACT + CCRT | ||||||||
| CCRT + AC | ||||||||
| No chemotherapy | ||||||||
Values in bold are significant (P < 0.05). *P‐values were calculated using the unadjusted log–rank test. AC, adjuvant chemotherapy; CI, confidence interval; CCRT, concurrent chemoradiotherapy; EA‐IgA, IgA antibodies against early antigen; HR, hazard ratio; NACT, neoadjuvant chemotherapy; VCA‐IgA, IgA antibodies against viral capsid antigen.
Figure 1.

Kaplan–Meier curves for 334 patients with nasopharyngeal carcinoma, stratified by IgA antibodies against early antigen (EA‐IgA) (<1:15 vs ≥1:15). (a) Progression‐free survival, (b) overall survival, (c) distant metastasis‐free survival, and (d) locoregional relapse‐free survival. CI, confidence interval; HR, hazard ratio.
Figure 2.
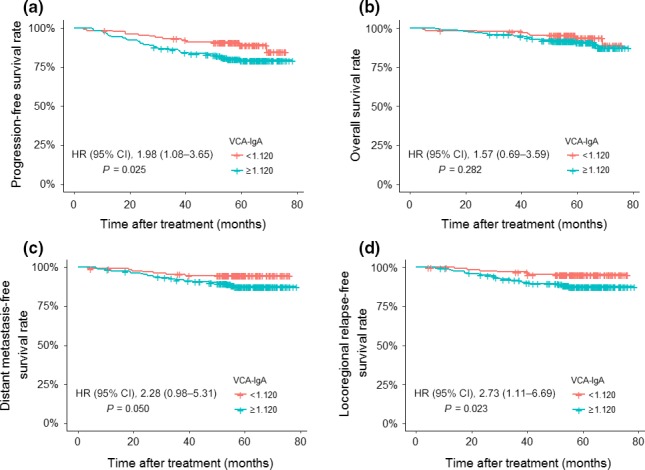
Kaplan–Meier curves for 334 patients with nasopharyngeal carcinoma stratified by IgA antibodies against viral capsid antigen (VCA‐IgA) (<1:120 vs ≥1:120). (a) Progression‐free survival. (b) Overall survival. (c) Distant metastasis‐free survival. (d) Locoregional relapse‐free survival. CI, confidence interval; HR, hazard ratio.
Table 5.
Multivariate analysis of prognostic factors for the whole cohort of patients with nasopharyngeal carcinoma (n = 334)
| HR (95% CI) | P‐value* | |
|---|---|---|
| Progression‐free survival | ||
| Smoking, no versus yes | 1.71 (1.00–2.95) | 0.052 |
| N stage, N0–1 vs N2–3 | 2.18 (1.22–3.89) | 0.008 |
| VCA‐IgA, <1:120 vs ≥1:120 | 1.52 (0.80–2.89) | 0.205 |
| Overall survival | ||
| Smoking, no versus yes | 2.62 (1.23–5.61) | 0.013 |
| N stage, N0–1 vs N2–3 | 2.90 (1.36–6.20) | 0.006 |
| Distant metastasis‐free survival | ||
| Smoking, no versus yes | 1.77 (0.86–3.64) | 0.119 |
| N stage, N0–1 vs N2–3 | 2.16 (1.31–4.67) | 0.031 |
| VCA‐IgA, <1:120 vs ≥1:120 | 1.77 (0.73–4.31) | 0.208 |
| Locoregional relapse‐free survival | ||
| Histology, WHO II vs III | 0.37 (0.13–1.08) | 0.070 |
| N stage, N0–1 vs N2–3 | 1.51 (0.68–3.34) | 0.309 |
| VCA‐IgA, <1:120 vs ≥1:120 | 2.45 (0.78–7.68) | 0.125 |
| EA‐IgA, <1:15 vs ≥1:15 | 1.05 (0.42–2.63) | 0.920 |
*P‐values were calculated with an adjusted Cox proportional hazards model. CI, confidence interval; EA‐IgA, IgA antibodies against early antigen; HR, hazard ratio; VCA‐IgA, IgA antibodies against viral capsid antigen.
Prognostic value of VCA‐IgA in NPC patients with early or advanced N stage
Finally, we further analyzed the prognostic value of VCA‐IgA in the subgroups of NPC patients with early and advanced N stage. In the early N stage (N0–1) subgroup, there was no significant difference survival between patients with high VCA‐IgA and low VCA‐IgA in terms of PFS (85.3% vs 90.2%; P = 0.217), OS (93.4% vs 95.1%; P = 0.627), DMFS (91.2% vs 95.1%; P = 0.220), or LRFS (90.4% vs 95.1%; P = 0.146; Fig. S1). Likewise, VCA‐IgA was not associated with PFS (HR, 1.72; 95% CI, 0.40–7.40; P = 0.459), OS (HR, 0.84; 95% CI, 0.18–3.84; P = 0.822), DMFS (HR, 1.94; 95% CI, 0.25–15.07; P = 0.517), or LRFS (P = 0.146; Fig. S2) in the advanced N stage (N2–3) subgroup.
Discussion
According to previous studies, the prognosis of patients with NPC is far from clearly defined.17 To our knowledge, this is the first large‐scale study to show the impact of serum EBV antibodies on the prognosis of patients with non‐metastatic NPC from a population with a high prevalence of both EBV infection and NPC. In endemic areas, previous studies18, 19 have mainly focused on the impact of EBV DNA in NPC. However, to the best of our knowledge, few studies have investigated the influence of serum EBV antibodies on the prognosis of NPC patients, especially those with undetectable pEBV DNA. The current study did not indicate any association between serum EA‐IgA, VCA‐IgA, and treatment outcomes in patients with undetectable pEBV DNA.
Necessity of serum EBV antibodies research
It is well recognized that EBV infection is a major risk factor for NPC progress.20, 21 Previous studies have shown that EBV can latently infect human nasopharyngeal epithelial cells, and induce lytic infection by triggering capillary expansion, altered protein localization, gene activation, DNA damage responses, and mutations during viral replication.22 Progression of NPC may induce EBV to enter the replication phase and express EA and VCA, stimulating production of transcript‐related EBV antibodies. With recent advances, several high‐quality, repeatable, and economical ELISA kits have been commercialized for testing serum EBV antibodies, including VCA‐IgA and EA‐IgA. Thus, the quantification of serum EBV antibodies in patients with NPC may reflect the tumor burden and provide reliable indexes for clinical TNM staging.8, 23 However, to date, only two studies have evaluated the prognostic value of EBV antibodies in NPC.7, 8 Given the lack of studies, the prognostic value of serum EBV antibodies remains unclear.
Predictive validity of serum EBV antibodies
Compared with EA‐IgA, VCA‐IgA may be better at determining survival outcomes. Although high VCA‐IgA was significantly associated with inferior PFS, DMFS, and LRFS in the log–rank test, it became insignificant in multivariable analyses. Moreover, subgroup analyses revealed that VCA‐IgA had no significant prognostic value in patients with early or advanced N stage NPC. Similar findings were obtained in previous studies. In a critical analysis of the value of EBV antibodies as prognostic biomarkers, Cai et al.7 failed to detect an association between serum EBV antibodies and survival outcomes. Recently, Sun et al.8 analyzed the prognostic value of serum EA‐IgA, VCA‐IgA, and EBV DNA, and found EBV DNA was a more sensitive and valuable molecular biomarker to enhance the traditional TNM system in comparison with serum EBV antibodies. While these study cohorts seem highly heterogeneous comprising of patients harboring localized and metastatic disease in these studies. One caveat of comparing our findings with the studies presented above is the fact that only patients with non‐metastatic NPC and undetectable pEBV DNA were eligible for the current study, which may reduce interference by EBV DNA on the prognostic value of EBV antibodies. With respect to this issue, we have confidence that serum EA‐IgA and VCA‐IgA have limited value for adding prognostic value to conventional clinical indices in identifying patients with NPC.
Correlation between EBV antibodies and clinicopathological characteristics
The associations between serum EA‐IgA, VCA‐IgA, and various clinicopathological features have been reported before. Sun et al.8 reported that the pretreatment serum VCA‐IgA and EA‐IgA titers increased with disease stage (2009 AJCC staging system) in 779 NPC patients treated at a single institution. Additionally, the same authors identified a positive association between EBV antibodies and histological subtype. Similarly, a strong, positive association was observed between EBV antibodies and disease stage in this study; however, we failed to detect an association between EBV antibodies and histological subtype, which may be due to the relatively small sample size (334 patients). Consistent with Baizig et al.,24 we found that patients with age >50 years at initial diagnosis were more likely to show high EBV antibody titers. The potentially longer exposure time to EBV in patients with age >50 years may partly explain this phenomenon; however, further investigation is required to fully explain the mechanism between age and increased EBV antibodies in patients with NPC.
High levels of pEBV DNA predict inferior survival outcomes in endemic areas
In the current study, our results suggest that high titers of EBV antibodies were likely to be present in advanced stage patients. Furthermore, high levels of EBV DNA are predictive of poor survival outcomes for NPC patients.4, 19, 25, 26 However, some articles27 indicate that EBV positivity was associated with extended OS, PFS, and LRFS. The following factors may explain the discrepancies. Pathologically, NPC can be classified according to the WHO system into keratinizing (WHO type I) and non‐keratinizing (WHO types II and III) subtypes,28 and EBV infection is closely associated with the development of nearly all undifferentiated NPC (WHO types II and III). Because more than 95% of NPC patients are infected with EBV and presented with WHO types II and III in endemic areas,3 in situ hybridization to determine the status of EBV infection was usually omitted. In daily clinical practice, serologic testing for EBV DNA load using PCR analysis has become common, as it is a non‐invasive and convenient method that complements imaging examinations. Approximately 25% of NPC patients were pathologically classified as WHO type I in North America,29 which is associated with human papillomavirus,30, 31, 32 and EBV status determined by in situ hybridization was negative for this group of patients. It is well recognized that the WHO histologic type has been shown to be an independent prognostic factor, and survival advantage is chiefly seen for WHO II/III over WHO I.33 Therefore, patients infected with EBV tended to have better survival outcomes relative to those uninfected, which was consistent with the finding suggested by Jiang et al.'s study.27
Limitations and future directions of study
A major limitation of our study is that a single measurement of EBV antibodies and the data were obtained exclusively at one center, and the measurement of serum EBV antibodies still needs to be globally standardized. Another limitation is that we failed to include data regarding other EBV antibodies including EBV nuclear antigen 1‐IgA and ‐IgG, Zta‐IgA, and Rta‐IgG. However, prior studies have indicated that serum EA‐IgA and VCA‐IgA were the most important screening markers of EBV antibodies.3, 34 The third concern was that we failed to include data regarding post‐treatment EBV antibodies. Future studies need to continue to evaluate the prognostic value of post‐treatment EBV antibodies in NPC patients.
In summary, our study findings did not confirm the role of EA‐IgA and VCA‐IgA as prognostic biomarkers among patients with NPC and undetectable pEBV DNA. However, it is plausible that EA‐IgA and VCA‐IgA might enhance patient stratification by providing an additional layer of information on disease burden. In support of this suggestion, EA‐IgA and VCA‐IgA were positively associated with T stage, N stage, and clinical stage. Further prospective studies of large cohorts of patients with NPC that include an analysis of post‐treatment EBV antibodies are warranted.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AJCC
American Joint Committee on Cancer
- AUC
area under the ROC curve
- CI
confidence interval
- CT
computed tomography
- CTV
clinical target volume
- DMFS
distant metastasis‐free survival
- EA‐IgA IgA
antibodies against early antigen
- EBV
Epstein–Barr virus
- GTV
gross tumor volume
- GTVnd
GTV with clinically involved cervical lymph nodes
- GTVp
GTV of primary nasopharyngeal tumor with enlarged retropharyngeal lymph nodes
- HR
hazard ratio
- LRFS
locoregional relapse‐free survival
- NPC
nasopharyngeal carcinoma
- OS
overall survival
- pEBV
pretreatment EBV
- PFS
progression‐free survival
- PTV
planning target volume
- ROC
receiver operating characteristic
- VCA‐IgA
IgA antibodies against viral capsid antigen
Supporting information
Fig. S1. Kaplan–Meier curves for patients with early N category (N0–1) nasopharyngeal carcinoma stratified by IgA antibodies against viral capsid antigen (VCA‐IgA) (<1:120 vs ≥1:120).
Fig. S2. Kaplan–Meier curves for patients with advanced N category (N2–3) nasopharyngeal carcinoma stratified by IgA antibodies against viral capsid antigen (VCA‐IgA) (<1:120 vs ≥1:120).
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81372409), the Sun Yat‐sen University Clinical Research 5010 Program (No. 2012011), and the National Natural Science Foundation of China (No. 81402532).
Cancer Sci 108 (2017) 1640–1647
Funding Information
National Natural Science Foundation of China; Sun Yat‐sen University
Contributor Information
Zhen‐Yu Qi, Email: qizhy@sysucc.org.cn.
Ying Sun, Email: sunying@sysucc.org.cn.
References
- 1. Jemal A, Bray F, Center MM et al Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Yi JL, Gao L, Huang XD et al Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten year experience of a single institution. Int J Radiat Oncol Biol Phys 2006; 65: 161–8. [DOI] [PubMed] [Google Scholar]
- 3. Du JL, Chen SH, Huang QH et al Subtype distribution and long‐term titer fluctuation patterns of serum anti‐Epstein‐Barr virus antibodies in a non‐nasopharyngeal carcinoma population from an endemic area in South China: a cohort study. Chin J Cancer 2016; 35(1): 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin JC, Wang WY, Chen KY et al Quantification of plasma Epstein‐Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 2004; 350: 2461–70. [DOI] [PubMed] [Google Scholar]
- 5. Tang LQ, Chen QY, Fan W et al Prospective study of tailoring whole‐body dual‐modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein‐Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013; 31: 2861–9. [DOI] [PubMed] [Google Scholar]
- 6. Leung SF, Chan KC, Ma BB et al Plasma Epstein‐Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced‐stage nasopharyngeal carcinoma. Ann Oncol 2014; 25: 1204–8. [DOI] [PubMed] [Google Scholar]
- 7. Cai YL, Li J, Lu AY et al Diagnostic significance of combined detection of Epstein‐Barr virus antibodies, VCA/IgA, EA/IgA, Rta/IgG and EBNA1/IgA for nasopharyngeal carcinoma. Asian Pac J Cancer Prev 2014; 15: 2001–6. [DOI] [PubMed] [Google Scholar]
- 8. Sun P, Chen C, Cheng YK et al Serologic biomarkers of Epstein‐Barr virus correlate with TNM classification according to the seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol 2014; 271: 2545–54. [DOI] [PubMed] [Google Scholar]
- 9. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–4. [DOI] [PubMed] [Google Scholar]
- 10. Lai SZ, Li WF, Chen L et al How does intensity‐modulated radiotherapy versus conventional two‐dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011; 80: 661–8. [DOI] [PubMed] [Google Scholar]
- 11. ICRU report . Prescribing, Recording, and Reporting Photon Beam Therapy, Vol. 62 Maryland: International Commission on Radiation Units and Measurements, 1999. [Google Scholar]
- 12. ICRU Report . Prescribing, Recording, and Reporting Photon‐Beam Intensity‐Modulated Radiation Therapy (IMRT), Vol. 83 Maryland: International Commission on Radiation Units and Measurements, 2010. [Google Scholar]
- 13. Su SF, Han F, Zhao C et al Long‐term outcomes of early‐stage nasopharyngeal carcinoma patients treated with intensity‐modulated radiotherapy alone. Int J Radiat Oncol Biol Phys 2012; 82: 327–33. [DOI] [PubMed] [Google Scholar]
- 14. Lee N, Harris J, Garden AS et al Intensity‐modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009; 27: 3684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peng YH, Xu YW, Qiu SQ et al Combination of autoantibodies against NY‐ESO‐1 and viral capsid antigen immunoglobulin A for improved detection of nasopharyngeal carcinoma. Oncol Lett 2014; 8: 1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ai P, Wang T, Zhang H et al Determination of antibodies directed at EBV proteins expressed in both latent and lytic cycles in nasopharyngeal carcinoma. Oral Oncol 2013; 49: 326–31. [DOI] [PubMed] [Google Scholar]
- 17. Lee AW, Ng WT, Chan LK et al The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol 2012; 48: 1007–13. [DOI] [PubMed] [Google Scholar]
- 18. Lo YM, Chan LY, Lo KW et al Quantitative analysis of cell‐free Epstein‐Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 1999; 59: 1188–91. [PubMed] [Google Scholar]
- 19. Tang LQ, Chen QY, Guo SS et al The impact of plasma Epstein– Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Br J Cancer 2014; 111: 1102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu FH, Xiong D, Xu YF et al An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein‐Barr virus activation. J Natl Cancer Inst 2012; 104: 1396–410. [DOI] [PubMed] [Google Scholar]
- 21. Chien YC, Chen JY, Liu MY et al Serologic markers of Epstein‐Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 2001; 345: 1877–82. [DOI] [PubMed] [Google Scholar]
- 22. Hau PM, Deng W, Jia L et al Role of ATM in the formation of the replication compartment during lytic replication of Epstein‐Barr virus in nasopharyngeal epithelial cells. J Virol 2015; 89: 652–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun R, Wang X, Li X. Correlation Analysis of Nasopharyngeal Carcinoma TNM Staging with Serum EA IgA and VCA IgA in EBV and VEGF‐C and ‐D. Med Sci Monit 2015; 21: 2105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baizig NM, Morand P, Seigneurin JM et al Complementary determination of Epstein‐Barr virus DNA load and serum markers for nasopharyngeal carcinoma screening and early detection in individuals at risk in Tunisia. Eur Arch Otorhinolaryngol 2012; 269: 1005–11. [DOI] [PubMed] [Google Scholar]
- 25. Leung SF, Zee B, Ma BB et al Plasma Epstein‐Barr viral deoxyribonucleic acid quantitation complements tumornode‐ metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24: 5414–8. [DOI] [PubMed] [Google Scholar]
- 26. Bortolin MT, Pratesi C, Dolcetti R et al Clinical value of Epstein‐ Barr virus DNA levels in peripheral blood samples of Italian patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Lett 2006; 233: 247–54. [DOI] [PubMed] [Google Scholar]
- 27. Jiang W, Chamberlain PD, Garden AS et al Prognostic value of p16 expression in Epstein‐Barr virus‐positive nasopharyngeal carcinomas. Head Neck 2016; 38(Suppl 1): E1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet 2005; 365: 2041–54. [DOI] [PubMed] [Google Scholar]
- 29. Lo EJ, Bell D, Woo JS et al Human papillomavirus and WHO type I nasopharyngeal carcinoma. Laryngoscope 2010; 120: 1990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi W, Pataki I, MacMillan C et al Molecular pathology parameters in human nasopharyngeal carcinoma. Cancer 2002; 94: 1997–2006. [DOI] [PubMed] [Google Scholar]
- 31. Punwaney R, Brandwein MS, Zhang DY et al Human papillomavirus may be common within nasopharyngeal carcinoma of Caucasian Americans: investigation of Epstein‐Barr virus and human papillomavirus in eastern and western nasopharyngeal carcinoma using ligation‐dependent polymerase chain reaction. Head Neck 1999; 21(1): 21–9. [DOI] [PubMed] [Google Scholar]
- 32. Giannoudis A, Ergazaki M, Segas J et al Detection of Epstein‐Barr virus and human papillomavirus in nasopharyngeal carcinoma by the polymerase chain reaction technique. Cancer Lett 1995; 89: 177–81. [DOI] [PubMed] [Google Scholar]
- 33. Sun LM, Li CI, Huang EY, Vaughan TL. Survival differences by race in nasopharyngeal carcinoma. Am J Epidemiol 2007; 165: 271–8. [DOI] [PubMed] [Google Scholar]
- 34. Leung SF, Tam JS, Chan AT et al Improved accuracy of detection of nasopharyngeal carcinoma by combined application of circulating Epstein‐Barr virus DNA and anti‐Epstein‐Barr viral capsid antigen IgA antibody. Clin Chem 2004; 50: 339–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Kaplan–Meier curves for patients with early N category (N0–1) nasopharyngeal carcinoma stratified by IgA antibodies against viral capsid antigen (VCA‐IgA) (<1:120 vs ≥1:120).
Fig. S2. Kaplan–Meier curves for patients with advanced N category (N2–3) nasopharyngeal carcinoma stratified by IgA antibodies against viral capsid antigen (VCA‐IgA) (<1:120 vs ≥1:120).


