Summary
Accumulating data show that the phenotypes and functions of distinctive mucosal dendritic cells (DCs) in the gut are regulated by retinoic acid (RA). Unfortunately, the exact role of butyrate in RA‐mediated mucosal DC differentiation has not been elucidated thoroughly to date. Mucosal‐like dendritic cell differentiation was completed in vitro by culturing bone marrow cells with growth factors [granulocyte–macrophage colony‐stimulating factor (GM‐CSF/interleukin (IL)‐4], RA and/or butyrate. The phenotypes, cytokine secretion, immune functions and levels of retinal dehydrogenase of different DCs were detected using quantitative polymerase chain reaction (qPCR), enzyme‐linked immunosorbent assay (ELISA) and flow cytometry, respectively. The results showed that RA‐induced DCs (RA‐DCs) showed mucosal DC properties, including expression of CD103 and gut homing receptor α4β7, low proinflammatory cytokine secretion and low priming capability to antigen‐specific CD4+ T cells. Butyrate‐treated RA‐DCs (Bu‐RA‐DCs) decreased CD11c, but increased CD103 and α4β7 expression. Moreover, the CD4+ T priming capability and the levels of retinal dehydrogenase of RA‐DCs were suppressed significantly by butyrate. Thus, butyrate and retinoic acid have different but synergistic regulatory functions on mucosal DC differentiation, indicating that immune homeostasis in the gut depends largely upon RA and butyrate to imprint different mucosal DC subsets, both individually and collectively.
Keywords: butyrate, dendritic cell, retinoic acid, short chain fatty acid, T cell
Introduction
It is well known that intestinal mucosa is the primary breeding place for flora and various foreign antigens. Thus, it is necessary for the mucosal immune system to create a protective immune response or to maintain tolerance 1. As with antigen‐presenting cells, dendritic cells are virtually omnipresent and play a pivotal role in instructing the initiation and activation and controlling the effects of the antigen‐induced immune response in peripheral immune organs, as well as immune tolerance in the gut 2. Dendritic cells (DCs) are remarkably plastic and have a notable ability to adapt to the resident microenvironment through modification of their phenotypes and functions 3. Since the discovery of DCs, various DC subsets, which show dramatically different phenotypes and immune functions, have been identified 4, 5. In mucosal tissue there are at least two different DC subsets, depending on the expression of CD103, and these DCs decide gut tolerance and intestinal inflammation 6, 7. Accumulating data have shown that metabolites or products derived from commensal bacteria have contributed greatly to the development, homeostasis and properties of DCs in the mammalian gastrointestinal tract 8, 9. If the composition or metabolism of the microflora is altered, inflammatory diseases such as inflammatory bowel disease (IBD), atherosclerosis and even colon cancer might be induced 10. Thus, these distinctive DC subsets and maintenance of a tolerant gut immune microenvironment depend largely upon the interaction between DCs and commensal bacterial populations.
In recent decades, studies have shown that vitamin A‐derived retinoic acid (RA) plays vital roles in maintaining gut immune homeostasis through the induction of gut homing in lymphocytes and the differentiation of regulatory T cells (Treg) 11. It also has been shown that RA has the capability to promote the development of mucosal DCs, including intestinal CD103+CD11b–(cDC1) and CD103+CD11b+ (cDC2) cells 9. In addition to RA, the fates of intestinal immune cells in the gut are also decided by many other factors, especially signals from commensal bacteria and their metabolites, such as short chain fatty acids (SCFAs) 12. As one of the richest SCFAs in the gut, butyrate is produced by intestinal bacteria through the fermentation of plant fibre, and functions in inducing the differentiation of Treg cells 13. Furthermore, studies have shown that lower levels of butyrate were detected in the lumen of patients with colitis, indicating a potential function of butyrate in regulating immune function in the gut 14. Unfortunately, at present, the exact role of butyrate in the differentiation of RA‐induced mucosal DCs is still not well demonstrated. In this study, by co‐culturing butyrate with RA to imprint bone marrow cell differentiation into DCs in vitro, we observed that butyrate co‐operates with RA to induce mucosal‐like DC differentiation. These results indicate a potential contribution of butyrate in maintaining gut immunity with tolerance properties.
Materials and methods
Mice
C57BL/6 mice (aged 6–12 weeks) were purchased from the animal facility of the Medical Center at Yangzhou University, China. Ovalbumin (OVA)323–339 peptide‐specific T cell receptor (TCR) transgenic mice DO11.10 (BALB/c background) and forkhead box protein 3 green fluorescent protein (FoxP3GFP) mice (B6.Cg‐FoxP3tm1Mal/J) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). To prepare C57BL/6 × DO11.10 F1 mice, male C57BL/6 mice were crossed with female DO11.10 mice. Similarly, DO11.10 × FoxP3GFP F1 mice were generated by crossing female DO11.10 mice with male FoxP3GFP mice. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Jiangsu University in China.
Dendritic cells prepared with granulocyte–macrophage colony‐stimulating factor (GM‐CSF)/interleukin (IL)‐4 and RA or butyrate in vitro
Bone marrow‐derived dendritic cells were prepared by culturing bone marrow cells with GM‐CSF (10 ng/ml) and IL‐4 (1 ng/ml) (PeproTech, Rocky Hill, NJ, USA). To induce RA‐DC, Bu‐DC or RA‐Bu‐DC differentiation, RA (1 μM) (Sigma‐Aldrich, St Louis, MO, USA) and/or butyrate (0·5 mM) (Sigma‐Aldrich), respectively, were added into DC culture wells beginning at day 3. In some experiments, bone marrow cells were first labelled with carboxyfluorescein succinimidyl ester (CFSE) using the CellTraceTM CFSE Cell Proliferation Kit (Invitrogen, Carlsbad, CA, USA). DC cells were purified with anti‐CD11c magnetic beads (Stemcell Technologies Inc., Vancouver, Canada), according to the manufacturer's protocol.
Antibody and flow cytometer analysis
All fluorescein‐labelled antibodies [CD4, CD25, CD11c, CD11b, CD80, CD86, major histocompatibility complex (MHC)‐II, B7‐DC, CD103, Toll‐like receptor (TLR)‐4 and α4β7] and 7‐aminoactinomycin D (7‐AAD) were purchased from eBioscience (San Diego, CA, USA). Cells were collected and incubated with rat serum to block Fc receptors, then incubated with fluorescein‐labelled antibodies diluted to optimal concentrations for 20 min at 4°C. After being washed with phosphate‐buffered saline (PBS), cells were then analysed using a fluorescence activated cell sorter (FACS)Calibur flow cytometer or Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA, USA). All flow cytometer data were analysed using Accuri C6 analysis software or FlowJo software (Tree Star Inc., Ashland, OR, USA).
Quantitative real‐time polymerase chain reaction (qRT–PCR)
Total RNA was extracted from bone marrow dendritic cells (BMDCs) with TRIzol reagent, followed by cDNA synthesis with Superscript Reverse Transcriptase (ThermoScientific, Waltham, MA, USA USA). Quantitative polymerase chain reactions (PCR) were performed using fluorochrome SYBR Green Premix Ex TaqTM (Takara, Dalian, Liaoning, China) on a Bio‐Rad iCycler. Primers were synthesized by the Sangon Biotech Company (Shanghai, China). Total amounts of DNA were calculated using the 2–ΔΔCt method, and the relative mRNA levels of target genes were normalized against glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). The sequences of primers were as follows: GAPDH, forward 5′‐GGCATTGCTCTCAATGACAA‐3', reverse 5′‐TGTGAGGGAGATGCTCAGTG‐3′; IL‐6, forward 5′‐GAGGAGACTTCACAGAGGATAC‐3′, reverse 5′‐GACTCTGGCTTTGTCTTTCTTG‐3′; IL‐12, forward 5′‐GGTTTGCCATCGTTTTG‐3′, reverse 5′‐GGGTCTTTCCAGAGCCT‐3′; TNF‐α, forward 5′‐ GAACTGGCAGAAGAGGCACT‐3′, reverse 5′‐GGTCTGGGCCATAGAACTGA‐3′; Nos2, forward 5′‐CCAAGCCCTCACCTACTTCC‐3′, reverse 5′‐CTCTGAGGGCTGACACAAGG‐3′; ALDH1A2, forward 5′‐ACCGTGTTCTCCAACGTCACTGAT‐3′, reverse 5′‐TGCATTGCGGAGGATACCATGAGA‐3′.
Griess reaction
The concentration of nitric oxide (NO) in samples was determined using the Griess reaction kit (Beyotime, Nanjing, China), according to the manufacturer's protocol. The NO concentrations in samples were determined quantitatively based on their correspondence to the absorbance from the standard plot.
Aldehyde dehydrogenase (ALDH) activity assay
ALDH activity in cells was determined by using the ALDEFLUOR staining kit (StemCell Technologies), according to the manufacturer's instructions. ALDEFLUOR‐stained cells were analysed using the FACSCalibur flow cytometer.
Antigen‐specific CD4+ T cell proliferation assay in vitro
DC cells were prepared as described previously and incubated with 1 mg/ml OVA323–339 peptide (Sigma‐Aldrich) at 37°C in a 5% CO2 incubator for more than 6 h to prepare OVA323–339‐loaded DCs. OVA323–339 peptide‐specific CD4+ T cells were collected from the spleens of C57BL/6 × DO11.10 F1 mice and purified using a CD4+ T cell selection kit (Stem Cell Technologies), according to the manufacturer's instructions. To induce CD4+ T cell proliferation, DCs (2 × 104) and CFSE‐labelled CD4+ T cells (2 × 105) were seeded into the wells of round‐bottomed 96‐well plates, and then co‐cultured in 5% CO2 at 37°C. On day 5, cells were collected and washed with PBS, and then incubated with phycoerythrin (PE)‐conjugated CD4 antibody and 7‐AAD (eBioscience, San Diego, CA, USA). The dilutions of CFSE in 7‐AAD‐negative CD4+ T cells were analysed using the FACSCalibur flow cytometer.
FoxP3+ regulatory cell differentiation in vitro
Naive CD4+ T cells in the splenocytes of C57BL/6 × FoxP3GFP F1 mice were purified using EasySep™ mouse naive CD4+ T cell isolation kit (Stem Cell Technologies), according to the manufacturer's protocol. To induce FoxP3+ Tregs differentiation in vitro, DC cells (2 × 104) and naive CD4+ T cells (2 × 105) were seeded into a round‐bottomed 96‐well plate in 200 μl medium, then co‐cultured further in 5% CO2 at 37°C for 4 days. On day 5, cells were harvested and incubated with fluorescein‐conjugated CD4 and CD25 antibodies and analysed by flow cytometry.
ELISA analysis for cytokines
The concentrations of IL‐2 or IL‐12p70 in the supernatant were determined, respectively, using an enzyme‐linked immunosorbent assay (ELISA) kit (eBioscience), according to the manufacturer's protocol.
Statistical analysis
The experimental data are presented as the mean ± standard error of the mean (s.e.m.). The statistical analysis was performed using one‐way analysis of variance (anova) in Graphpad Prism version 5; P < 0.05 was considered to be statistically significant.
Results
Butyrate modifies the phenotype of RA‐DCs
BM cells were cultured with GM‐CSF/IL‐4 with or without Bu and RA. As shown in Fig. 1a–c, there was no significant difference in the total cell numbers, cell size or cell divisions in different DCs. CD11c is one important biomarker of dendritic cells 15. Comparing BMDCs and RA‐DC cells, the expression of CD11c on Bu‐DC (GM‐CSF/IL‐4/Bu) and RA‐Bu‐DC (GM‐CSF/IL‐4/RA/Bu) was inhibited dramatically (Fig. 1d). Both co‐stimulatory molecules and MHC‐II molecules play important roles in DC‐mediated antigen presentation and T cell activation. The data in Fig. 2a show that the levels of CD80, CD86, MHC‐II and B7‐DC on DCs were obviously inhibited in RA‐DCs. CD103 is a biomarker of a proportion of mucosal DCs (CD103+DC) in mesenteric lymph nodes (MLNs) and colonic lamina propria (LP). The results in Fig. 2a show that RA treatment increased the level of CD103 in DCs (RA‐DC) significantly while butyrate had no effects, similar to DC treatment with RA. If DCs were treated with retinoic acid and butyrate simultaneously, higher CD103 were detected on DCs (RA‐Bu‐DC) (Fig. 2a). Interestingly, butyrate was found to have synergistic effects with RA in terms of the expression of gut homing receptor α4β7 in DCs (Fig. 2b).
Figure 1.

Butyrate suppresses CD11c expression in bone marrow differentiated dendritic cells (DCs). Bone marrow cells were labelled with carboxyfluorescein succinimidyl ester (CFSE) and then cultured with granulocyte–macrophage colony‐stimulating factor/interleukin 4 (GM‐CSF/IL‐4) with or without retinoic acid (RA) or butyrate (Bu) for 7 days to induced DC cell differentiation. On day 8, all cells were collected and total cell number (a), forward‐scatter (FSC) channel and CFSE dilution of cells in each well (b), total numbers of CD11b+CD11c+ DC cells (c) and CD11c expression (d) of DCs were analysed. FSC in the graph indicates cell size and CFSE dilution indicates cell proliferation or division. DC, cultured with GM‐CSF/IL‐4; RA‐DC, cultured with GM/IL‐4/RA; Bu‐DC, cultured with GM‐CSF/IL‐4/butyrate; RA‐Bu‐DC, cultured with GM‐CSF/IL‐4/RA/butyrate. Data are shown as the mean ± standard error of the mean. [Colour figure can be viewed at wileyonlinelibrary.com].
Figure 2.

Butyrate promotes retinoic acid (RA)‐induced dendritic cells (DCs) to express CD103 and α4β7 on the cell membrane. DC cells were prepared as in Fig. 1, and 1 μg/ml lipopolysaccharide (LPS) was added to purified cells on day 7 to induce DC cell maturation. The next day, cells were harvested to analyse the expression of CD80, CD86, major histocompatibility complex (MHC)‐II, B7‐DC, CD103 and Toll‐like receptor (TLR)‐4 (a) and α4β7 (b) using a flow cytometer. Numbers in the graphs are shown as the mean fluorescence intensities. [Colour figure can be viewed at wileyonlinelibrary.com].
Lower proinflammatory cytokines secreted by butyrate and RA‐induced DCs
Next, we investigated the effects of butyrate and retinoic acid on the cytokine profile of DCs. The data in Fig. 3a,b,d,e show that both butyrate and retinoic acid treatment suppressed the expression of IL‐6, tumour necrosis factor (TNF)‐α and IL‐12 in DCs (RA‐DC, Bu‐DC and RA‐Bu‐DC), which was detected using qPCR and ELISA. Interestingly, lipopolysaccharide (LPS)‐treated BMDCs up‐regulated nitric oxide synthase 2 (NOS2) levels and produced more NO, but these compounds were inhibited significantly in all RA and/or butyrate treated DCs (Fig. 3c,f). Thus, lower levels of proinflammatory cytokine secretion were detected in RA‐ and/or butyrate‐treated DCs.
Figure 3.

Effect of butyrate on the cytokine profiles of dendritic cells (DCs). DC cells (5 × 105/well) were re‐seeded into 24‐well plates and matured with 1 μg∕ml lipopolysaccharide (LPS) for 18 h. The relative mRNA levels of interleukin (I)L‐6 (a), tumour necrosis factor (TNF)‐α (b) and IL‐12 (d) in DCs were quantified by quantitative polymerase chain reaction (qPCR) analysis. The supernatants of cultured DC cells were used to detect IL‐12p70 protein using enzyme‐linked immunosorbent assay (ELISA) (e) (c, f). The mRNA levels of nitric oxide synthase 2 (NOS2) in DCs (c) and NO in the supernatants (f) were quantified with qPCR and the Griess reaction, respectively. Data are shown as the mean ± standard error of the mean. *P < 0·05; **P < 0·01; ***P < 0·001.
Butyrate regulates the priming functions of RA‐DCs on CD4+ T cells
A hallmark of DCs is their ability to stimulate antigen‐restricted T cell activation and proliferation. Comparing the priming capabilities of different DCs in Fig. 4a, we found that RA‐treated DCs (RA‐DCs) showed a decreased ability to prime CD4+ T cell proliferation than DCs/LPS. Remarkably, the proliferations of CD4+ T cells were suppressed more significantly in both Bu‐DCs and RA‐Bu‐DCs compared to RA‐DCs (Fig. 4a). This was confirmed further by detecting the levels of IL‐2 in the supernatant of DC/CD4+ T cells culture wells (Fig. 4b). We next assessed the function of RA and/or butyrate on regulatory T cell differentiation in vitro. The data in Fig. 4c show that RA treatment obviously promoted CD4+ Treg cell differentiation. Unfortunately, fewer FoxP3+ T cells were found in butyrate‐treated groups, indicating that butyrate itself displays inefficient regulation of the differentiation of CD4+ regulatory T cells. Furthermore, the data in Fig. 4d show lower levels of IL‐2 in RA‐DCs and RA‐Bu‐DCs, indicating that Treg cells consumed more IL‐2 proteins in these groups.
Figure 4.
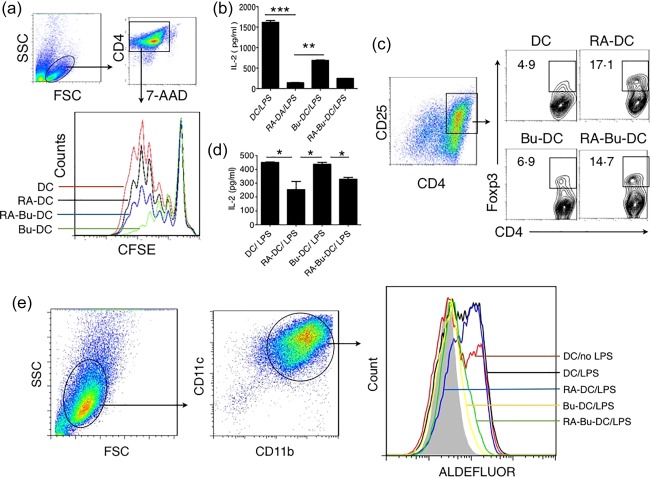
Butyrate regulates the functions of retinoic acid (RA)‐induced dendritic cells (DCs). (a,b) Butyrate regulated the capability of RA‐DCs negatively in priming the proliferation of CD4+ T cells. DC cells were cultured with carboxyfluorescein succinimidyl ester (CFSE)‐labelled ovalbumin (OVA)323–339‐specific CD4+ T cells at a ratio of 1 : 10 in U‐bottomed 96‐well plates. Cells were cultured further for 4 days. The divisions of 7‐aminoactinomycin D (7‐AAD)–CD4+ T cells were analysed with a flow cytometer (a), and the levels of interleukin (IL)‐2 protein in the supernatants were detected using enzyme‐linked immunosorbent assay (ELISA) (b). (c,d) Butyrate had little effect on regulatory cell differentiation. Naive CD4+ T cells were purified from the spleens of forkhead box protein 3 green fluorescent protein (FoxP3–GFP) × DO11.10 F1 mice and cultured with OVA323–339 peptide‐loaded DCs at a ratio of 1 : 10. After being cultured for 4 days, cells were collected. CD4+CD25+FoxP3+ regulatory T cells were identified with a flow cytometer (c), and the levels of IL‐2 in the supernatants were detected using ELISA (d). The numbers in the graphs indicate the percentage of FoxP3+ cells in the CD4+CD25+cells. (e) Butyrate inhibits aldehyde dehydrogenase in RA‐DCs. The activity of aldehyde dehydrogenase (ALDH) in DCs was detected using ALDEFLUOR staining with a flow cytometer (e). Data are shown as the mean ± standard error of the mean. *P < 0·05; **P < 0·01; ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com].
Butyrate inhibits the activity of retinal dehydrogenase in RA‐DCs
The metabolism of retinoic acid depends largely upon the function of members of the retinal dehydrogenase family, especially ALDH1a2. The data in Fig. 4e show that ALDH1a2 could be detected in the BMDCs and increased further in response to LPS stimulation. Similar to previously reported data, RA treatment increased the ALDH1a2 activity in RA‐DCs. It is worth noting that the activities of ALDH1a2 were suppressed dramatically in both groups of butyrate treated DCs (Bu‐DC and RA‐Bu‐DC).
Discussion
The exact effect of butyrate on the differentiation of RA‐induced mucosal DCs has not been elucidated thoroughly to date. In this study, we observed that butyrate treatment suppressed the expression of co‐stimulatory MHC‐II molecules but increased the levels of CD103 and α4β7 in RA‐induced DCs (RA‐Bu‐DCs). Moreover, the decreased capability of RA‐DCs to prime OVA323–339 peptide antigen‐specific CD4+ T cell proliferation was inhibited by butyrate. Thus, this study shows that butyrate had synergistic functions with RA to induce mucosal‐like DC differentiation from bone marrow cells in vitro.
DCs work as the conductor in deciding the results of an immune response. As one of the DC subsets, CD103+ DCs represent the DC population in the intestinal LP and the MLN, and play an important role in mediating immunological tolerance in the gut 16, 17. This is largely dependent upon the relationship between the flexibility of CD103+ DCs and gut microenvironment. Evidence shows that retinoic acid induces DC cells to express α4β7, and confers mucosal DC properties on them 9, 18, 19. These data indicate the direct effects of retinoic acid on the differentiation of gut‐resident DC cells. Here, we found that RA treatment promoted BMDC differentiation with mucosal‐like DC properties. RA‐treated cells express higher CD103 and α4β7 expression, but decreased the ability to induce antigen CD4+T cell proliferation (Fig. 4). Indeed, the positive expression of CD103 and α4β7 indicate that these RA‐DCs were endowed with the correct set of homing receptors for subsequent entry into mucosal effector tissues. Moreover, these RA‐DCs have a reduction in the production of proinflammatory cytokines (TNF‐α, IL‐6) and IL‐12 (Fig. 3) and exhibit promotion of Treg cell differentiation (Fig. 4).
Studies have shown that bacterial members of the clostridium, bacillus and butyrivibrio genera can produce and maintain high concentrations of butyrate in the small intestine and colon. Published works show that butyrate regulates the differentiation and maturation of DCs, and changes the cells' capability to capture and prime antigen‐specific T cell activation 20, 21. These characteristics were confirmed in both butyrate‐treated DC groups (Bu‐DCs and RA‐Bu‐DCs) in this study, indicating the potential roles of butyrate in the gut. Outside the intestine, dietary fermentable fibre can also shape the immunological environment in the lung and influence the severity of allergic inflammation through SCFAs 22. Interestingly, we found that CD103 and α4β7 expression were only increased in RA‐treated DCs (RA‐DCs and RA‐Bu‐DCs), suggesting that CD103 induced transcription is related closely to vitamin (retinol) metabolism, not to butyrate.
Certain mucosal DC subsets have reduced ability to prime CD4+ T proliferation than DC cohorts in peripheral tissue. Indeed, the priming ability of CD4+ T cell activation and proliferation was impaired seriously in all RA‐DCs, Bu‐DCs and RA‐Bu‐DCs (Fig. 4), indicating the regulatory functions of RA and butyrate on DCs. Studies have also shown that CD103+DCs can induce the generation of FoxP3+ regulatory cells from naive T cells in the presence of retinoic acid 16. Other studies also revealed that RA stifles autoimmunity through up‐regulating Treg cell development, thus inhibiting the ongoing autoimmune disease 23. We noticed that retinoic acid‐treated DCs (RA‐DCs and RA‐Bu‐DCs) had a stronger capability to induce Treg cell differentiation, which was not inhibited significantly with butyrate treatment (Fig. 4). A series of contradictory statements exists in published data regarding the effects of butyrate on Treg cells 24, 25. The exact role of SCFAs on T cell differentiation still needs more exploration.
In mammals, vitamin A is taken from food in the form of retinol, retinoic ester or beta‐carotene. In the cell, retinol can be oxidized into retinal by retinol dehydrogenase (RDH) and then turn into retinoic acid by retinaldehyde dehydrogenase (ALDH) 26. Accumulating data have shown that intestinal CD103+ DCs express retinal dehydrogenase (ALDH) 2 highly, which works as an enzyme to convert retinal to RA and then induce gut‐homing T and B cells 27. Furthermore, DCs can acquire high levels of retinoic acid‐producing capacities in response to vitamin D3 28. Here, our data also confirmed that the levels of RALDH were increased in RA‐treated DCs (RA‐DCs) (Fig. 4), indicating the importance of RA in the differentiation of immune cells in the gut. Unfortunately, the exact role of butyrate on ALDH is waiting to be demonstrated. Data from high‐fibre feeding models showed that fibre‐derived SCFAs, particularly acetate and butyrate, enhanced oral tolerance and protected against food allergy by enhancing ALDH activity in CD103+ DCs in vivo 28. In contrast, our data showed that butyrate inhibited the levels of ALDH in DCs significantly. The research model used in the experiments may have caused this difference. Previous studies also showed that butyrate significantly induced IL‐23 expression and promoted IL‐17 secretion, indicating its contribution to increasing the severity of dextran sulphate sodium (DSS) colitis 29. Interestingly, one colonic DC subset (CD103+CD11b+ALDH–) was identified recently, which displays a unique capacity to polarize T helper type 17 (Th17) cells 30. Similar to the CD103+CD11b+ALDH– DC subset, our data show that butyrate and RA treatment suppress the expression of CD11c and ALDH synergistically, but increase CD103 in RA‐Bu‐DCs. These data indicate the potential roles of butyrate and RA in the differentiation and function of this special colonic CD103+CD11b+ALDH–DC subset.
Collectively, the data in this study suggest that CD103+ mucosal DC subsets, which control T cell homeostasis in the gut, are dependent upon the regulation of butyrate and RA, both individually and collectively.
Disclosure
No conflicts of interest.
Acknowledgement
This study was funded by grants from the National Natural Science Foundation of China (31570879 and 31428006).
Contributor Information
Q. Shao, Email: shao_qx@ujs.edu.cn
S. Xia, Email: xiasheng1519@163.com.
References
- 1. Persson EK, Jaensson E, Agace WW. The diverse ontogeny and function of murine small intestinal dendritic cell/macrophage subsets. Immunobiology 2010; 215:692–7. [DOI] [PubMed] [Google Scholar]
- 2. Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest 2009; 119:2441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Novak N, Allam JP, Betten H, Haberstok J, Bieber T. The role of antigen presenting cells at distinct anatomic sites: they accelerate and they slow down allergies. Allergy 2004; 59:5–14. [DOI] [PubMed] [Google Scholar]
- 4. Chiang MC, Tullett KM, Lee YS et al Differential uptake and cross‐presentation of soluble and necrotic cell antigen by human DC subsets. Eur J Immunol 2016; 46:329–39. [DOI] [PubMed] [Google Scholar]
- 5. Haniffa M, Collin M, Ginhoux F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv Immunol 2013; 120:1–49. [DOI] [PubMed] [Google Scholar]
- 6. Muzaki AR, Tetlak P, Sheng J et al Intestinal CD103(+)CD11b(‐) dendritic cells restrain colitis via IFN‐gamma‐induced anti‐inflammatory response in epithelial cells. Mucosal Immunol 2016; 9:336–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koyama M, Cheong M, Markey KA et al Donor colonic CD103+ dendritic cells determine the severity of acute graft‐versus‐host disease. J Exp Med 2015; 212:1303–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mann ER, Bernardo D, Al‐Hassi HO et al Human gut‐specific homeostatic dendritic cells are generated from blood precursors by the gut microenvironment. Inflamm Bowel Dis 2012; 18:1275–86. [DOI] [PubMed] [Google Scholar]
- 9. Zeng R, Bscheider M, Lahl K, Lee M, Butcher EC. Generation and transcriptional programming of intestinal dendritic cells: essential role of retinoic acid. Mucosal Immunol 2016; 9:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune‐mediated inflammatory diseases. Front Microbiol 2016; 7:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villablanca EJ, Wang S, de Calisto J et al MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology 2011; 141:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu W, Sun M, Chen F et al Microbiota metabolite short‐chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 2016, doi:10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arpaia N, Campbell C, Fan X et al Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature 2013; 504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan J, McKenzie C, Potamitis M et al The role of short‐chain fatty acids in health and disease. Adv Immunol 2014; 121:91–119. [DOI] [PubMed] [Google Scholar]
- 15. Sivakumaran S, Henderson S, Ward S et al Depletion of CD11c(+) cells in the CD11c.DTR model drives expansion of unique CD64(+) Ly6C(+) monocytes that are poised to release TNF‐alpha. Eur J Immunol 2016; 46:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coombes JL, Siddiqui KR, Arancibia‐Carcamo CV et al A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF‐beta and retinoic acid‐dependent mechanism. J Exp Med 2007; 204:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Worbs T, Bode U, Yan S et al Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med 2006; 203:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guerra‐Perez N, Frank I, Veglia F et al Retinoic acid imprints a mucosal‐like phenotype on dendritic cells with an increased ability to fuel HIV‐1 infection. J Immunol 2015; 194:2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klebanoff CA, Spencer SP, Torabi‐Parizi P et al Retinoic acid controls the homeostasis of pre‐cDC‐derived splenic and intestinal dendritic cells. J Exp Med 2013; 210:1961–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nastasi C, Candela M, Bonefeld CM et al The effect of short‐chain fatty acids on human monocyte‐derived dendritic cells. Sci Rep 2015; 5:16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte‐derived dendritic cells and macrophages. Clin Exp Immunol 2002; 130:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trompette A, Gollwitzer ES, Yadava K et al Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20:159–66. [DOI] [PubMed] [Google Scholar]
- 23. Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All‐trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)‐dependent suppression of interferon‐gamma‐producing T‐cells without affecting Th17 cells. Diabetes 2009; 58:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park J, Goergen CJ, HogenEsch H, Kim CH. Chronically elevated levels of short‐chain fatty acids induce T cell‐mediated ureteritis and hydronephrosis. J Immunol 2016; 196:2388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan J, McKenzie C, Vuillermin PJ et al Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 2016; 15:2809–24. [DOI] [PubMed] [Google Scholar]
- 26. Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem 2000; 267:4315–24. [DOI] [PubMed] [Google Scholar]
- 27. Agace WW, Persson EK. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol 2012; 33:42–8. [DOI] [PubMed] [Google Scholar]
- 28. Sato T, Kitawaki T, Fujita H et al Human CD1c(+) myeloid dendritic cells acquire a high level of retinoic acid‐producing capacity in response to vitamin D(3). J Immunol 2013; 191:3152–60. [DOI] [PubMed] [Google Scholar]
- 29. Berndt BE, Zhang M, Owyang SY et al Butyrate increases IL‐23 production by stimulated dendritic cells. Am J Physiol Gastrointest Liver Physiol 2012; 303:G1384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Janelsins BM, Lu M, Datta SK. Altered inactivation of commensal LPS due to acyloxyacyl hydrolase deficiency in colonic dendritic cells impairs mucosal Th17 immunity. Proc Natl Acad Sci USA 2014; 111:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


