Summary
The pathogenesis of spondyloarthritis (SpA) involves activation of the innate immune system, inflammation and new bone formation. The two cytokines interleukin (IL)‐20 and IL‐24 have been shown to link innate immune activation and tissue homeostasis. We hypothesized that these two cytokines are secreted as part of activation of the innate immune system and affect bone homeostasis in SpA. IL‐20 and IL‐24 were measured in plasma from axial SpA patients (n = 83). Peripheral SpA patients (n = 16) were included for in‐vitro cell culture studies. The plasma IL‐20 and IL‐24 levels were increased in SpA patients compared with healthy controls (HCs) by 57 and 83%, respectively (both P < 0·0001). The Toll‐like receptor 4‐induced secretion of the two cytokines was greater in SpA peripheral blood mononuclear cells (PBMCs) compared with HC PBMCs. IL‐20 and IL‐24 increased the production of monocyte chemoattractant protein‐1 by activated SpA synovial fluid monocytes, decreased the production of Dickkopf‐1 by SpA fibroblast‐like synovial cells and induced mineralization in human osteoblasts. Taken together, our findings indicate disease‐aggravating functions of IL‐20 and IL‐24 in SpA.
Keywords: interleukin, monocyte, spondylitis, Toll‐like receptor
Introduction
Spondyloarthritis (SpA) is a group of immune‐mediated inflammatory diseases sharing a high frequency of the human leucocyte antigen (HLA)‐B27 allele and inflammation of joints, gut, skin and entheses 1, 2. The pathogenesis of SpA involves activation of the innate immune system, inflammation and new bone formation. The innate immune system is activated through pattern recognition receptors, including Toll‐like receptor 4 (TLR‐4). Inflammation is a result of increased production of proinflammatory cytokines and chemokines, including tumour necrosis factor (TNF)‐α and chemokine (C‐C motif) ligand 2 [CCL2, also known as monocyte chemoattractant protein 1 (MCP‐1)]. New bone formation by osteoblasts is determined by a balance between the inhibitory effect of Dickkopf‐1 (DKK1) and the stimulatory effect of bone morphogenetic proteins (BMPs). Treatment with drugs inhibiting tumour necrosis factor (TNF)‐α has shown great effect on inflammation while the effect on new bone formation is less pronounced 3, 4. This indicates that there are other pathways linking activation of the immune system and bone metabolism in SpA.
Interleukin (IL)‐20 and IL‐24 are members of the IL‐10 family of cytokines 5. The two cytokines are expressed by keratinocytes 6, 7, 8, but in early expression pattern studies were also found in TLR‐4‐stimulated monocytes 9, 10. They bind the two receptor complexes IL‐20R1/IL‐20R2 and IL‐22R/IL‐20R2 and signal through Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2) 11, 12, 13. The IL‐20R1 and IL‐22R subunits have been found primarily on epithelial cells, not leucocytes 9, 10, 12. In line with this, IL‐20 and IL‐24 have been suggested to be involved in tissue homeostasis and not immune activation 5. This has led to the hypothesis that therapeutic modulation of IL‐20 and IL‐24 might not result in the increased risk of infection seen with biological drugs such as the TNF‐α inhibitors 14, 15. The two cytokines could thus be interesting treatment targets in immune‐mediated inflammatory disease.
IL‐20 and IL‐24 have been described primarily to aggravate disease activity in immune‐mediated inflammatory conditions. This is mainly because transgenic mice over‐expressing IL‐20 16 and IL‐24 17 develop psoriasis‐like skin disease with hyperkeratosis, and the two cytokines have been associated with human psoriasis 6. In line with this, IL‐20 and IL‐24 have also been suggested to augment disease activity in arthritis 18, 19, 20, 21, 22, 23, inflammatory bowel disease 24, 25 and fibrotic disease 26. The effector mechanism of the two cytokines in SpA has not been studied previously.
Previously, we found elevated plasma and synovial fluid levels of IL‐20 and IL‐24 correlating with CCL2 plasma levels in a small group of SpA patients without any record of clinical disease activity 19. In this study, we aim to describe associations between IL‐20 and IL‐24 and disease activity and identify the sources and targets of the two cytokines in SpA.
Materials and methods
Study subjects
Plasma was included from a study population of SpA patients (n = 83) with symptoms restricted to the axial skeleton (aSpA) for measuring the concentration of IL‐20 and IL‐24. No cells were collected from these patients. The patients all met the European Spondyloarthropathy Study Group (ESSG) criteria (Table 1) 27. This study population was characterized with self‐assessment scores, clinical scores and test results. As described previously, these comprised patient global visual analogue scale (VAS) score, physician global assessment VAS score, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Metrology Index (BASMI), C‐reactive protein (CRP), HLA‐B27 status and radiography and magnetic resonance imaging (MRI) of the sacroiliac joint (SIJ) and entire spine 28, 29, 30, 31. The MRI activity scores were made using the Danish methods, differing primarily from the Spondyloarthritis Research Consortium of Canada (SPARCC) methods by using three‐dimensional assessment of the SIJ and the spine 28, 29, 32, 33. Further, treatment was registered for all patients at the time of cytokine measurement and during a 4‐year follow‐up period. The inclusion of patients was started in 2006, with only a small percentage of patients treated with a TNF‐α inhibitor. The patients not listed in Table 1 were treated with either physiotherapy alone or together with non‐steroidal anti‐inflammatory drug (NSAID) treatment.
Table 1.
Patient characteristics for patients with axial spondyloarthritis (SpA)
| Characteristics | Axial SpA (n = 83) |
|---|---|
| Age (years) | 38 (30–43) |
| Gender (percentage female) | 58 |
| HLA‐B27 (percentage positive) | 61 |
| Disease duration (years) | 7·5 (5·0–11·0) |
| Treatment (percentage of patients) | |
| Methotrexate | 11 |
| Salazopyrin | 12 |
| TNF‐α inhibitor | 8 |
| Self‐assessment scores | |
| BASDAI (0–100) | 29 (11–51) |
| BASFI (0‐100) | 15 (4–33) |
| Patient global (0–100) | 24 (7‐52) |
| Clinical scores | |
| BASMI (0–100) | 0 (0–0) |
| Physician global (0–100) | 10 (5–23) |
| Test results | |
| CRP (mg/l) | 2·1 (1·3–3·9) |
| SIJ MRI activity (0–40) | 5 (2–13) |
| Spine MRI activity (0–81) | 1 (0–4) |
| SIJ MRI chronicity (0–48) | 11 (2–27) |
| Spine MRI chronicity (0–207) | 0 (0–4) |
Numbers and parentheses express median and interquartile range (IQR). TNF‐α = tumour necrosis factor‐alpha; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BASFI = Bath Ankylosing Spondylitis Functional Index; BASMI = Bath Ankylosing Spondilitis Metrology Index; CRP = C‐reactive protein; MRI = magnetic resonance imaging; SIJ = sacroiliac joint; HLA = human leucocyte antigen.
Another study population, consisting of SpA patients (n = 16) with peripheral arthritis (pSpA), was included for obtaining synovial fluid for culturing paired synovial fluid mononuclear cells (SFMCs) and peripheral blood mononuclear cells (PBMCs) and for growing fibroblast‐like synovial cells (FLSs). These cells were used to study production and effects of IL‐20 and IL‐24 in vitro. Patients with peripheral arthritis contacted the clinic because of a knee joint effusion. No disease activity scores, prognosis scores or test results were obtained for this study population.
Plasma (n = 48) and PBMCs (n = 12) from healthy controls (HCs) were included from the Blood Bank at Aarhus University Hospital (Table 1). They were comparable regarding age and gender; median age 41 years [interquartile range (IQR) = 32–53] and 54% of females for the HCs included for measuring IL‐20 and IL‐24 plasma levels.
Sample handling
Blood samples from patients with axial SpA and HCs were collected in ethylenediamine tetraacetic acid (EDTA) tubes. Synovial fluid from patients with peripheral SpA was collected during therapeutic arthrocenthesis and transferred to tubes containing EDTA and blood samples were collected in EDTA tubes in continuation of the therapeutic arthrocenthesis. Cell‐free synovial fluid and plasma were pipetted after centrifugation at 300 g for 5 min and kept at −80°C until use. SFMCs and PBMCs from patients with peripheral SpA and PBMCs from HCs were isolated by conventional Ficoll‐Paque (GE Healthcare, Little Chalfont, UK) density‐gradient centrifugation and cryopreserved at −135°C until time of use. FLSs were grown from SFMCs as described previously 30, 34. Briefly, SFMCs were thawed and cultured in Dulbecco's modified Eagle's medium (DMEM; Lonza, Basel, Switzerland) supplemented with 10% fetal calf serum (FCS), penicillin, streptomycin and glutamine at 37°C and 5% CO2 at a density of 2 × 106 cells/ml in a humidified incubator, replacing the medium every 3–4 days. When the cell layer was 70% confluent, the FLSs were passaged by trypsin/EDTA treatment and used for analyses at passages 4–5.
IL‐20 and IL‐24 enzyme‐linked immunosorbent assays (ELISA)
The plasma concentrations of IL‐20 and IL‐24 were quantified with ELISA, validated as described previously 35. Antibodies, recombinant cytokines and streptavidin‐horseradish peroxidase (HRP) for the IL‐20 and IL‐24 ELISA were purchased from R&D Systems (Minneapolis, MN, USA; catalogue numbers DY1102 and DY1965). Nunc Maxisorp 96‐well microplates were coated with coat antibody at 2 μg/ml in phosphate‐buffered saline (PBS) and incubated overnight at room temperature (RT). Adding PBS with 5% skimmed milk to each well and incubating plates at RT for 2 h blocked non‐specific binding sites in the polystyrene wells. Then, samples, positive controls and standards prepared in assay diluent were added in duplicate and plates were incubated overnight at 4°C. The assay diluent contained protein‐free PBS blocking buffer (Thermo Scientific, Waltham, MA, USA) supplemented with 10 μg/ml mouse gamma globulin (Jackson ImmunoResearch, West Grove, PA, USA), 10 μg/ml human immunoglobulin (Ig) (Behring, King of Prussia, PA, USA) and 10 μg/ml goat gamma globulin (Jackson ImmunoResearch) to prevent potential unspecific binding of heterophilic antibodies in samples. Next, biotinylated detection antibody was added and plates were incubated for 1 h at RT. The anti‐IL‐20 detection antibody was used at 0·4 μg/ml, and the anti‐IL‐24 detection antibody was used at 0·8 μg/ml. Plates were then incubated with streptavidin‐HRP for 15 min at RT. Signals were amplified using biotinyl‐tyramide solution (PerkinElmer, Waltham, MA, USA) for 15 min at RT followed by streptavidin‐HRP for 30 min at RT. The plates were incubated finally with 3,3',5,5'‐tetramethylbenzidine (TMB) substrate solution at RT. Colour development was stopped with H2SO4. The optical density of each well was measured using a microplate reader set to 450 nm and wavelength correction set to 570 nm.
The concentration of IL‐20 and IL‐24 in supernatants was analysed as described for the plasma samples.
The detection limits of IL‐20 and IL‐24 ELISA systems were 31·3 and 15·6 pg/ml, respectively. All plasma samples were diluted 1 : 3 in assay diluent with the cut‐off for the plasma analyses at 93·9 pg/ml and 46·8 pg/ml.
Lipopolysaccharide (LPS) stimulation of PBMC and SFMC cultures
PBMCs from HCs and PBMCs and SFMCs from patients with peripheral SpA were incubated at a density of 2.0 × 106 cells/ml in I‐1640 (Lonza) supplemented with 10% FCS, penicillin, streptomycin and glutamine at 37°C and 5% CO2 for 48 h in a humidified incubator without changing medium. First, HC PBMCs were cultured with LPS (Sigma Aldrich, St Louis, MO, USA) at a concentration of 1, 10 or 100 ng/ml. Secondly, HC PBMCs and SpA PBMCs and SFMCs were cultured with or without LPS at a concentration of 100 ng/ml, as performed previously 31. Supernatants were harvested carefully after centrifugation of the culture plates at 300 g for 5 min and kept at −80°C until measuring concentrations of IL‐20 and IL‐24.
Stimulation of SFMC and FLS cultures with IL‐20 and IL‐24
SFMCs from patients with peripheral SpA were cultured at a density of 1.0 × 106 cells/ml in RPMI‐1640 (Lonza) supplemented with 10% FCS, penicillin, streptomycin and glutamine at 37°C and 5% CO2 for 48 h in a humidified incubator without changing medium. To simulate IL‐20 and IL‐24 stimulation in vitro, cells were cultured with commercially available recombinant IL‐20 and IL‐24 proteins (R&D Systems) at 200 ng/ml. All supernatants were harvested after centrifugation of the culture plates at 300 g for 5 min and kept at −80°C until measuring CCL2 and DKK1 concentrations.
FLSs from patients with peripheral SpA were thawed and cultured in DMEM (Lonza) supplemented with 10% FCS, penicillin, streptomycin and glutamine at 37°C and 5% CO2 at a density of 5 × 104 cells/ml. FLSs were stimulated with IL‐20 or IL‐24 at 200 ng/ml for 48 h. A negative control culture without stimulation and a positive control culture with TNF‐α at 10 ng/ml were used in each experiment for comparison. Supernatants were harvested and kept at −80°C until measuring CCL2 and DKK1 concentrations.
Neutralizing IL‐20 and IL‐24 in SFMC cultures
To inhibit IL‐20 and IL‐24 in vitro, SFMCs from patients with peripheral SpA were incubated with neutralizing antibodies, as described previously 31. Polyclonal goat anti‐IL‐20 antibody (R&D Systems; AF1102) or polyclonal goat anti‐IL‐24 antibody (R&D Systems; AF1965) were used at a concentration of 5 µg/ml at 37°C for 48 h. A negative control culture with goat IgG (R&D Systems; AB‐108‐C) was used in each experiment for comparison.
CCL2 and DKK1 enzyme linked immunosorbent assays
Culture supernatants were analysed with commercially available CCL2 ELISA (Biolegend, San Diego, CA, USA) and DKK1 ELISA (R&D Systems) following the manufacturers’ instructions.
Flow cytometric detection of intracellular CCL2
SFMCs from patients with peripheral SpA were cultured at a density of 2.0 × 106 cells/ml in RPMI‐1640 (Lonza) supplemented with 10% FCS, penicillin, streptomycin and glutamine at 37°C and 5% CO2 in a humidified incubator for 24 h. Cells were stimulated with IL‐20 or IL‐24 at 200 ng/ml. A negative control culture without stimulation and a positive control culture with LPS at 10 ng/ml were used in each experiment for comparison. Golgi stop with brefeldin A (Sigma Aldrich) was used at a concentration of 10 μg/ml for the last 5 h of the stimulation. Cells were then harvested, transferred to polypropylene tubes (Nunc, Sigma Aldrich) and fixed using 4% formaldehyde (Sigma Aldrich) diluted in PBS. Cells were then permeabilized using 0·3% saponin (Sigma Aldrich) in PBS with 0·5% bovine serum albumin (BSA) (Calbiochem, San Diego, CA, USA) and 0·09% NaN3. Finally, the cells were stained for 30 min at 4°C with anti‐CD163 fluorescein isothiocyanate (FITC) (clone Mac2‐158; Trillium Diagnostics, Bangor, ME, USA), anti‐CD14 V500 (clone MφP9, BD Biosciences), anti‐CD16 PC7 (clone 3G8; Beckman Coulter) and anti‐CCL2 PE (clone 5D3‐F7; BD Biosciences) or a matching isotype control [IgG1 phycoerythrin (PE), clone MOPC‐21; BD Biosciences] in buffer supplemented with 10 μg/ml mouse gamma globulin (Jackson ImmunoResearch) to minimize non‐specific binding 36. Dead cells were excluded based on staining with Live/Dead fixable viability marker (near‐infra red; Life Technologies, Paisley, UK) added before fixation and permeabilization. The samples were analysed using an LSR Fortessa flow cytometer (BD Biosciences) and data were analysed using FlowJo software version 10 (Tree Star Inc., Ashland, OR, USA).
Osteoblast mineralization assay
Human osteoblasts (C‐12720; PromoCell, Heidelberg, Germany) were cultured and expanded in supplemented osteoblast growth medium (C‐27001; PromoCell). The cells were seeded in triplicate in 96‐well plates at a concentration of 100 000 cells/ml (20 000 cells/well) and cultured for 4 days. Cells were then cultured for 2 days in growth medium with or without either IL‐20 or IL‐24 at 50 ng/ml or bone morphogenic protein‐2 (BMP‐2) at 50 ng/ml, and subsequently in osteoblast mineralization medium (C‐27020; PromoCell). Osteoblast growth medium and mineralization medium with mediators were changed every 2–3 days for a total of 21 days. On day 21 the mineral formed was visualized using a commercial mineralization stain kit (OsteoImage, PA‐1503; Lonza) and a fluoroscan plate reader (Thermo Scientific; Fluoroscan Ascent FL). The staining was performed according to the manufacturer's protocol.
Statistics
All cytokine measurements, clinical scores and test results were expressed with the median and interquartile range (IQR). Comparisons of plasma IL‐20 and IL‐24 levels between groups were made using the Mann–Whitney U‐test. Correlation analyses between disease parameters and plasma cytokine levels were performed with Spearman's correlation. Comparisons of cell culture supernatant IL‐20 and IL‐24 concentrations were also analysed with non‐parametric statistics. The Mann–Whitney U‐test was used for unpaired comparisons and Wilcoxon's signed‐rank test was used for paired data. CCL2, DKK1 and mineralization ratios were log‐transformed and comparisons were made with the paired t‐test. A two‐tailed P‐value below 0·05 was considered statistically significant. Calculations and graphs were made with stata version 11 (StataCorp LP) and GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA).
Ethics
Samples from the SpA study groups were collected at the out‐patient clinic at Aarhus University Hospital. All samples were obtained after informed written consent, according to the Declaration of Helsinki, and approved by the Local Ethics Committee (project numbers 20121329 and 20058432) and the Danish Data Protection Agency.
Results
IL‐20 and IL‐24 plasma levels were increased in SpA patients compared with HCs
To test whether the peripheral blood levels of IL‐20 and IL‐24 were altered in SpA patients we measured the levels of the two cytokines in plasma from both patients with axial SpA and HCs. The plasma levels of IL‐20 and IL‐24 were increased in SpA patients compared with HCs by 57 and 83%, respectively (both P < 0·0001) (Fig. 1a,b). No associations were observed between gender or age and plasma levels of the two cytokines for either SpA patients or HCs.
Figure 1.
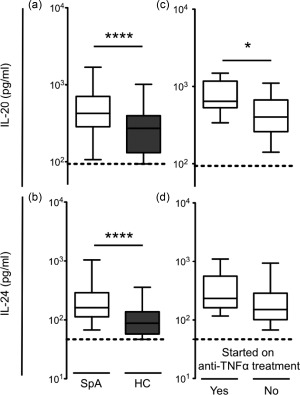
Plasma concentrations of interleukin (IL)‐20 and IL‐24 in patients with axial spondyloarthritis (SpA) (n = 83) and healthy controls (HC) (n = 48) and associations between plasma concentrations of IL‐20 and IL‐24 and clinical disease in SpA. (a) The median value of IL‐20 was 423 pg/ml [interquartile range (IQR) = 284–706 pg/ml] in plasma from SpA patients and 270 (IQR = 131–395 pg/ml) in plasma from HC. (b) The median value of IL‐24 was 161 pg/ml (IQR = 113–289 pg/ml) in plasma from SpA patients and 88 (IQR = 58–138 pg/ml) in plasma from HC. (c,d) Plasma concentrations of IL‐20 and IL‐24 in patients with SpA who were (n = 7) or were not switched to anti‐tumour necrosis factor (TNF)‐α treatment during the time from inclusion to the follow‐up 4 years later. (c) The median value of IL‐20 was 644 pg/ml (IQR = 528–1172 pg/ml) in plasma from patients switched to anti‐TNF‐α treatment and 400 (IQR = 260–671 pg/ml) in plasma from patients not switched to anti‐TNF‐α treatment. (d) The median value of IL‐24 was 234 pg/ml (IQR = 162–559 pg/ml) in plasma from patients switched to anti‐TNF‐α treatment and 151 (IQR = 101–287 pg/ml) in plasma from patients not switched to anti‐TNF‐α treatment. Data were analysed using the Mann–Whitney U‐test. Boxes indicate median and IQR and bars indicate 10th and 90th percentiles. Truncated lines indicate assay detection limit. *P < 0·05; ****P < 0·0001.
A possible association between IL‐20 and IL‐24 and disease activity in SpA was studied with correlations between the levels of the two cytokines and registered disease parameters. IL‐20 plasma levels associated with the physician global VAS score (rho = 0·24, P = 0·042). IL‐20 and IL‐24 did not correlate with any other scores of disease activity (Table 2). However, there was a trend towards associations between plasma levels of the two cytokines and future switch to biological therapy (P = 0·031 and P = 0·11, respectively) (Fig. 1c,d) and spine MRI chronicity score (P = 0·10 and P = 0·15, respectively) (Table 2).
Table 2.
Correlations between plasma concentrations of IL‐20 and IL‐24 and disease activity scores in patients with axial spondyloarthritis (SpA)
| Cytokine conc. | ||
|---|---|---|
| IL‐20 | IL‐24 | |
| Patient self‐assessment scores | ||
| BASDAI | 0·021 (0·87) | −0·18 (0·13) |
| BASFI | 0·10 (0·41) | −0·19 (0·15) |
| Patient global | 0·017 (0·89) | −0·14 (0·26) |
| Clinical scores | ||
| BASMI | 0·11 (0·34) | 0·13 (0·26) |
| Physician global | 0·24 (0·042) | 0·094 (0·42) |
| Test results | ||
| CRP | −0·059 (0·61) | −0·035 (0·76) |
| SIJ MRI activity | 0·025 (0·83) | 0·015 (0·90) |
| Spine MRI activity | 0·069 (0·55) | 0·039 (0·73) |
| SIJ MRI chronicity | 0·070 (0·54) | 0·070 (0·54) |
| Spine MRI chronicity | 0·19 (0·10) | 0·16 (0·15) |
BASDAI = Bath Ankylosing Spondylitis Disease Activity Index; BASFI = Bath Ankylosing Spondylitis Functional Index; BASMI = Bath Ankylosing Spondilitis Metrology Index; CRP = C‐reactive protein; IL = interleukin; MRI = magnetic resonance imaging; MTX = methotrexate; SIJ = sacroiliac joint; SpA = spondyloarthritis. Data were analysed using Spearman's correlation. Numbers indicate Spearman's rho with P‐value in parenthesis. Bold numbers indicate P < 0·05.
TLR‐4‐induced IL‐20 and IL‐24 production by PBMCs was increased in SpA patients compared with HCs
We first studied the production of IL‐20 and IL‐24. We stimulated SFMCs and PBMCs from patients with peripheral SpA and PBMCs from HCs with the TLR‐4 ligand LPS and measured the production of IL‐20 and IL‐24 by ELISA. The secretion of both IL‐20 and IL‐24 from HC PBMCs showed a dose‐dependent increase after stimulation with LPS at a concentration of 1, 10 and 100 ng/ml (Fig. 2a,b). LPS at 100 ng/ml increased the secretion of IL‐20 and IL‐24 in all cultures with detectable cytokine concentrations of SpA SFMCs (one of six and five of six, respectively), SpA PBMCs (four of six and six of six, respectively) and HC PBMCs (nine of 12 and 12 of 12, respectively) (Fig. 2c,d). There was a greater increase in secretion of IL‐24 by SpA PBMCs compared with HC PBMCs (P = 0·031) (Fig. 2c,d).
Figure 2.
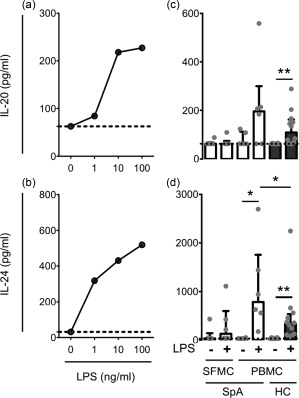
Secretion of interleukin (IL)‐20 and IL‐24 by synovial fluid mononuclear cells (SFMCs) and peripheral blood mononuclear cells (PBMCs) from patients with peripheral spondyloarthritis (SpA) and PBMCs from healthy controls (HC) stimulated with lipopolysaccharide (LPS). (a,b) Stimulation of HC PBMCs with a 10‐fold titration of the LPS concentration (representative, n = 3). (c,d) Stimulation of SpA SFMCs (n = 6) and PBMCs (n = 6) and HC PBMCs (n = 12) with LPS at a concentration of 100 ng/ml. Data were analysed using the Mann–Whitney U‐test comparing unpaired samples and the Wilcoxon signed‐rank test comparing paired samples. Boxes and bars indicate median and interquartile range (IQR). Truncated lines indicate assay detection limit. *P < 0·05; **P < 0·01.
IL‐20 and IL‐24 induced the production of CCL2 by synovial fluid monocytes from patients with SpA
We then studied the cellular targets of IL‐20 and IL‐24. To study the role of the two cytokines in inflammation in SpA we first stimulated SFMCs and FLSs from patients with peripheral SpA with IL‐20 and IL‐24 and measured the production of the proinflammatory chemokine CCL2 by ELISA. Stimulation with the two cytokines increased the secretion of CCL2 by all SpA SFMC cultures but not by SpA FLS cultures (Fig. 3a,c). To study the effect of endogenous IL‐20 and IL‐24 on CCL2 secretion, we then neutralized IL‐20 and IL‐24 in SpA SFMC cultures. Inhibition of IL‐20 or IL‐24 reduced the secretion of CCL2 only modestly in four of six and five of six cultures, respectively (Fig. 3b).
Figure 3.
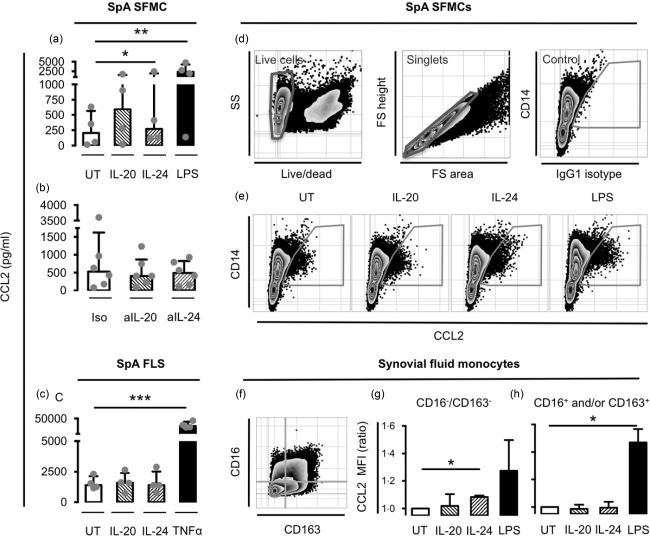
Effect of interleukin (IL)‐20 and IL‐24 on chemokine (C‐C motif) ligand 2 (CCL2) production by synovial fluid mononuclear cells (SFMCs), fibroblast‐like synovial cells (FLSs) and synovial monocyte subsets from patients with peripheral spondyloarthritis (SpA). (a) CCL2 concentration in supernatants after stimulation of SpA SFMCs with IL‐20 or IL‐24 at 200 ng/ml (n = 4). (b) CCL2 concentration in supernatants after treating SpA SFMCs with anti‐IL‐20 (aIL‐20) or anti‐IL‐24 (aIL‐24) neutralizing antibodies at 5 μg/ml (n = 4). (c) CCL2 concentration in supernatants after stimulation of SpA FLSs with IL‐20 or IL‐24 at 200 ng/ml (n = 4). For statistical analysis, data were normalized to untreated cultures, expressed as a ratio, log‐transformed and analysed with the paired t‐test. (d–h) Intracellular staining of CCL2 in SpA SFMCs after stimulation with IL‐20 or IL‐24 at 200 ng/ml (n = 4) or lipopolysaccharide (LPS) at 10 ng/ml. (d) Gating strategy to identify CCL2‐producing cells gating on live cells, single cells and using immunoglobulin (Ig)G1 isotype control‐stained sample as a negative control. (e) Representative plots showing intracellular staining of CCL2 in monocytes from untreated and stimulated cultures. Cells were plotted using CD14 to visually discriminate monocytes and lymphocytes. (f) Defining monocyte subsets based on the markers CD16 and CD163. (g) Median fluorescence intensity (MFI) of CCL2 in CCL2+/CD16–/CD163– monocytes. (h) Median fluorescence intensity (MFI) of CCL2 in CCL2+/CD16+and/or CD163+ monocytes. This subset was also analysed as three separate subsets (CD16+, CD163+ and CD16+/CD163+) with the same result. For statistical analysis, data were normalized to untreated cultures, expressed as a ratio, log‐transformed and analysed using the paired t‐test. Boxes and bars indicate median and interquartile range (IQR). *P < 0·05; **P < 0·01; ***P < 0·001.
We then wanted to identify the specific cellular targets of IL‐20 and IL‐24 among the cells in the SpA SFMC cultures. Therefore, cellular expression of intracellular CCL2 was studied by flow cytometry. Constitutive CCL2 production was observed in CD14+ monocytes in all untreated SpA SFMC cultures. The percentage of CCL2‐producing cells was not increased after IL‐20 or IL‐24 stimulation compared with untreated cultures. However, the production of CCL2 [as measured by median fluorescence intensity (MFI)] in CCL2+ monocytes was increased in all stimulated cultures, reflecting potentiated CCL2 production in cells already producing some CCL2 (Fig. 3e). This increase in CCL2 MFI after stimulation with the two cytokines was found specifically in the CD16–/CD163– monocyte subset (Fig. 3g). In contrast, there was no increase in CCL2 MFI in CD16+ monocytes, CD163+ monocytes or when pooling these in a subset of CD16+ and/or CD163+ monocytes (Fig. 3h).
IL‐20 and IL‐24 induced DKK1 production by SpA FLSs and mineralization by human osteoblasts
To study the possible role of IL‐20 and IL‐24 in new bone formation in SpA we first examined the effect of the two cytokines on production of the osteoblast inhibitory factor DKK1 by SFMCs and FLSs from patients with peripheral SpA. Stimulation with the two cytokines decreased the secretion of DKK1 by all SpA FLS cultures. The production of DKK1 was very low in the SFMC cultures, and was not altered by IL‐20 and IL‐24 stimulation (Fig. 4a,b).
Figure 4.
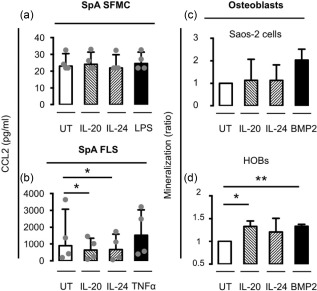
Effect of interleukin (IL)‐20 and IL‐24 on Dickkopf‐1 (DKK1) production by synovial fluid mononuclear cells (SFMCs) and fibroblast‐like synovial cells (FLSs) from patients with peripheral spondyloarthritis (SpA) and on osteoblast mineralization. (a,b) DKK1 concentration in supernatants after stimulation of SpA SFMCs and FLSs with IL‐20 or IL‐24 at 200 ng/ml (n = 4). (c,d) Mineralization measured as hydroxyapatite deposition in osteoblast cultures after stimulation with IL‐20 or IL‐24 at 200 ng/ml (n = 3). Data were normalized to untreated cultures, expressed as a ratio, log‐transformed and analysed using the paired t‐test. Boxes and bars indicate median and interquartile range (IQR). *P < 0·05; **P < 0·01.
We then tested the direct effect of IL‐20 and IL‐24 on osteoblast activity using a commercial mineralization assay. Stimulation with both cytokines increased mineralization in all primary human osteoblast cultures but not in cultures of the sarcoma osteogenic (Saos‐2) cell line (Fig. 4c,d).
Discussion
IL‐20 and IL‐24 are believed to be important for tissue homeostasis at epithelial surfaces, but not for activation of the immune system 5. This means that modulation of the two cytokines might not result in the increased risk of infection seen with other disease‐modifying drugs such as TNF‐α inhibitors 14, 15. This makes them interesting treatment target candidates in immune‐mediated inflammatory diseases. IL‐20 and IL‐24 have already been associated with inflammatory arthritis 18, 19, 20, 21, 22, 23, 37, and anti‐IL‐20 antibody treatment has shown efficacy in rheumatoid arthritis (RA) 38. However, little is known about IL‐20 and IL‐24 in SpA.
In psoriasis and arthritis, IL‐20 and IL‐24 have been characterized primarily as proinflammatory, inducing the production of other cytokines and chemokines 19, 20, 39. However, in a recent comprehensive study of skin infection, IL‐20 and IL‐24 were found to decrease host immune defence mechanisms 40. In this study, increased plasma levels of IL‐20 and IL‐24 were not associated with disease activity scores. However, the concentrations of the two cytokines were increased in the small group of patients who needed to switch to anti‐TNF‐α therapy later. These findings point to disease‐aggravating but not necessarily proinflammatory functions of IL‐20 and IL‐24 in SpA. This is because the patients started on anti‐TNF‐α therapy have been selected based on many factors, including inflammatory activity, chronic structural changes and disease progression.
This study points to TLR‐4 stimulated monocytes as a source of IL‐20 and IL‐24 in peripheral blood. LPS induced the production of the two cytokines from both SpA and HC PBMCs. In fact, the LPS‐induced increase in IL‐24 production was significantly greater in SpA PBMCs compared with HC PBMCs. The possible link between TLR‐4 and IL‐20 and IL‐24 is interesting in SpA. First, TLR‐4 is up‐regulated in SpA 41, 42. This could explain the increased responsiveness to LPS seen in this study. Secondly, several studies indicate that both pathogen‐associated molecular pattern (PAMPs) and danger‐associated molecular pattern (DAMPs) are involved in the inflammatory process in SpA; for example, HLA‐B27 transgenic rats do not develop autoinflammatory disease in an environment without presence of germs and PAMPs 43. Also, host interactions with pathogens and the microbiota are involved critically in reactive arthritis 44, 45, 46. Finally, TLR‐4 agonistic DAMPs such as high mobility group box protein 1 (HMGB1) and hyaluronic acid fragments are increased in plasma from SpA patients compared with HCs 47, 48.
The role of IL‐20 and IL‐24 in inflammation and new bone formation in SpA was studied by examining the effects of IL‐20 and IL‐24 on SFMCs, FLSs and osteoblasts in vitro.
Inflammation in SpA involves cytokines and chemokines. CCL2 is essential for the attraction of monocytes to the joints and was suggested recently as a novel biomarker in SpA 49. This study indicates that IL‐20 and IL‐24 potentiate CCL2 secretion by already activated synovial monocytes in SpA. Thus, IL‐20 and IL‐24 increased the production of CCL2 only in monocytes already activated in vivo in the inflamed joint. This was a small subset of CD16–/CD163– monocytes already producing CCL2. The constitutive CCL2 production seen in our untreated cultures has been reported previously in macrophages isolated from the synovial membrane 50. Here, we show that IL‐20 and IL‐24 may contribute to maintenance of inflammation by potentiating this CCL2 production in CD16–/CD163– monocytes. Our findings are in line with recent studies showing higher numbers of CD16– monocytes in ankylosing spondylitis with the capability to produce IL‐6 51, 52. In contrast to previous studies using RA FLSs, we did not see any effect of the two cytokines on SpA FLSs 20.
Bone homeostasis is a combination of osteoclast and osteoblast activity. IL‐20 and IL‐24 decrease the secretion of DKK1 by SpA FLSs and stimulate mineralization by human osteoblasts. DKK1 is an important negative regulator of osteoblastogenesis. Therefore, the two cytokines could lead to increased new bone formation by both indirect and direct mechanisms. Supporting this hypothesis, increased plasma concentrations of IL‐20 and IL‐24 seem to be associated with more chronic changes on MRI in the axial SpA patients. However, we and others have also found stimulatory effects of the two cytokines on osteoclasts 23, 37. The net effect of these mechanisms needs further clarification.
In conclusion, plasma levels of IL‐20 and IL‐24 were increased in SpA compared with HCs. In vitro, IL‐20 and IL‐24 were produced by TLR‐4‐stimulated PBMCs and the two cytokines targeted a subset of activated SpA synovial fluid monocytes, SpA FLSs and osteoblasts. Taken together, our findings indicate that IL‐20 and IL‐24 could be novel links between activation of the innate immune system and new bone formation in SpA. This indicates a possible role of anti‐IL‐20 or anti‐IL‐24 antibodies in the treatment of SpA. It is not known whether inhibition of these cytokines will affect inflammation and/or new bone formation in SpA in vivo. The relatively modest stimulatory capacity of IL‐20 and IL‐24 on CCL2 production by SFMCs found in this study indicates that neutralizing these cytokines might not result in a substantial anti‐inflammatory effect. However, inhibition of CCL2 production by subsets of synovial monocytes might prove beneficial, and the effect on osteoblast mineralization holds promise for further study. Future studies including inhibition of these cytokines, their common receptors and JAK1 or TYK2 will show if IL‐20 and IL‐24 could be new targets in SpA treatment.
Disclosure
The authors declare no financial conflicts of interest.
Author contributions
T. W. K. helped to design the study, helped to collect the SFMC and PBMC samples, helped to carry out the experiments, analysed and interpreted the data and drafted the manuscript. M. N. A. helped to plan and carry out the experiments. B. S. C. and A. G. J. helped to collect the patient samples and information. M. H. and B. D. helped to design the study and supervised the project. All authors helped to analyse and interpret the data, were involved in revising the manuscript and read and approved the final manuscript.
Acknowledgements
We thank Karin Skovgaard Sørensen (Department of Biomedicine, Aarhus University) for excellent technical assistance concerning the ELISA systems and the mineralization assay. All flow cytometric analyses were performed using the LSR Fortessa at the FACS core facility at Aarhus University. The Danish Rheumatism Association, Aage Bangs Foundation and the Faculty of Health at Aarhus University funded this work.
References
- 1. Dougados M, Baeten D. Spondyloarthritis. Lancet 2011; 377:2127–37. [DOI] [PubMed] [Google Scholar]
- 2. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016; 374:2563–74. [DOI] [PubMed] [Google Scholar]
- 3. Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long‐term anti‐TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis 2014; 73:710–5. [DOI] [PubMed] [Google Scholar]
- 4. Lories RJU, Luyten FP, de Vlam K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther 2009; 11:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutz S, Wang X, Ouyang W. The IL‐20 subfamily of cytokines – from host defence to tissue homeostasis. Nat Rev Immunol 2014; 14:783–95. [DOI] [PubMed] [Google Scholar]
- 6. Kunz S, Wolk K, Witte E et al Interleukin (IL)‐19, IL‐20 and IL‐24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol 2006; 15:991–1004. [DOI] [PubMed] [Google Scholar]
- 7. Otkjaer K, Kragballe K, Johansen C et al IL‐20 gene expression is induced by IL‐1beta through mitogen‐activated protein kinase and NF‐kappaB‐dependent mechanisms. J Invest Dermatol 2007; 127:1326–36. [DOI] [PubMed] [Google Scholar]
- 8. Otkjaer K, Holtmann H, Kragstrup TW et al The p38 MAPK regulates IL‐24 expression by stabilization of the 3' UTR of IL‐24 mRNA. PLOS ONE 2010; 5:e8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL‐10 family members? J Immunol 2002; 168:5397–402.] [DOI] [PubMed] [Google Scholar]
- 10. Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R. Expression patterns of IL‐10 ligand and receptor gene families provide leads for biological characterization. Int Immunopharmacol 2004; 4:577–92. [DOI] [PubMed] [Google Scholar]
- 11. Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL‐19, IL‐20 and MDA‐7 through IL‐20 receptor complexes of two types. J Immunol 2001; 167:3545–9. [DOI] [PubMed] [Google Scholar]
- 12. Parrish‐Novak J, Xu W, Brender T et al Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor–ligand interactions mediate unique biological functions. J Biol Chem 2002; 277:47517–23. [DOI] [PubMed] [Google Scholar]
- 13. Logsdon NJ, Deshpande A, Harris BD, Rajashankar KR, Walter MR. Structural basis for receptor sharing and activation by interleukin‐20 receptor‐2 (IL‐20R2) binding cytokines. Proc Natl Acad Sci USA 2012; 109:12704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leng R‐X, Pan H‐F, Tao J‐H, Ye D‐Q. IL‐19, IL‐20 and IL‐24: potential therapeutic targets for autoimmune diseases. Expert Opin Ther Targets 2011; 15:119–26. [DOI] [PubMed] [Google Scholar]
- 15. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL‐22‐IL‐22R1 system. Nat Rev Drug Discov 2014; 13:21–38. [DOI] [PubMed] [Google Scholar]
- 16. Blumberg H, Conklin D, Xu WF et al Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell 2001; 104:9–19. [DOI] [PubMed] [Google Scholar]
- 17. He M, Liang P. IL‐24 transgenic mice: in vivo evidence of overlapping functions for IL‐20, IL‐22, and IL‐24 in the epidermis. J Immunol 2010; 184:1793–8. [DOI] [PubMed] [Google Scholar]
- 18. Alanärä T, Karstila K, Moilanen T, Silvennoinen O, Isomäki P. Expression of IL‐10 family cytokines in rheumatoid arthritis: elevated levels of IL‐19 in the joints. Scand J Rheumatol 2010; 39:118–26. [DOI] [PubMed] [Google Scholar]
- 19. Kragstrup TW, Otkjaer K, Holm C et al The expression of IL‐20 and IL‐24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine 2008; 41:16–23. [DOI] [PubMed] [Google Scholar]
- 20. Hsu Y‐H, Li H‐H, Hsieh M‐Y et al Function of interleukin‐20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum 2006; 54:2722–33. [DOI] [PubMed] [Google Scholar]
- 21. Lebre MC, Jonckheere CL, Kraan MC et al Expression of IL‐20 in synovium and lesional skin of patients with psoriatic arthritis: differential response to alefacept treatment. Arthritis Res Ther 2012; 14:R200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scrivo R, Conigliaro P, Riccieri V et al Distribution of interleukin‐10 family cytokines in serum and synovial fluid of patients with inflammatory arthritis reveals different contribution to systemic and joint inflammation. Clin Exp Immunol 2015; 179:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kragstrup TW, Greisen SR, Nielsen MA et al The interleukin‐20 receptor axis in early rheumatoid arthritis: novel links between disease‐associated autoantibodies and radiographic progression. Arthritis Res Ther 2015; 18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fonseca‐Camarillo G, Furuzawa‐Carballeda J, Granados J, Yamamoto‐Furusho JK. Expression of interleukin (IL)‐19 and IL‐24 in inflammatory bowel disease patients: a cross‐sectional study. Clin Exp Immunol 2014; 177:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fonseca‐Camarillo G, Furuzawa‐Carballeda J, Llorente L, Yamamoto‐Furusho JK. IL‐10‐ and IL‐20‐expressing epithelial and inflammatory cells are increased in patients with ulcerative colitis. J Clin Immunol 2013; 33:640–8. [DOI] [PubMed] [Google Scholar]
- 26. Sziksz E, Pap D, Lippai R et al Fibrosis related inflammatory mediators: role of the IL‐10 cytokine family. Mediat Inflamm 2015; 2015:764641–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dougados M, van der Linden S, Juhlin R et al The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991; 34:1218–27. [DOI] [PubMed] [Google Scholar]
- 28. Madsen KB, Jurik AG. Magnetic resonance imaging grading system for active and chronic spondylarthritis changes in the sacroiliac joint. Arthritis Care Res (Hoboken) 2010; 62:11–8. [DOI] [PubMed] [Google Scholar]
- 29. Madsen KB, Jurik AG. MRI grading method for active and chronic spinal changes in spondyloarthritis. Clin Radiol 2010; 65:6–14. [DOI] [PubMed] [Google Scholar]
- 30. Kragstrup TW, Jalilian B, Hvid M et al Decreased plasma levels of soluble CD18 link leukocyte infiltration with disease activity in spondyloarthritis. Arthritis Res Ther 2014; 16:R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kragstrup TW, Andersen T, Holm C et al Toll‐like receptor 2 and 4 induced interleukin‐19 dampens immune reactions and associates inversely with spondyloarthritis disease activity. Clin Exp Immunol 2015; 180:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maksymowych WP, Inman RD, Salonen D et al Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 2005; 53:703–9. [DOI] [PubMed] [Google Scholar]
- 33. Maksymowych WP, Inman RD, Salonen D et al Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005; 53:502–9. [DOI] [PubMed] [Google Scholar]
- 34. Stebulis JA, Rossetti RG, Atez FJ, Zurier RB. Fibroblast‐like synovial cells derived from synovial fluid. J Rheumatol 2005; 32:301–6. [PubMed] [Google Scholar]
- 35. Kragstrup TW, Vorup‐Jensen T, Deleuran B, Hvid M. A simple set of validation steps identifies and removes false results in a sandwich enzyme‐linked immunosorbent assay caused by anti‐animal IgG antibodies in plasma from arthritis patients. Springerplus 2013; 2:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andersen MN, Al‐Karradi SNH, Kragstrup TW, Hokland M. Elimination of erroneous results in flow cytometry caused by antibody binding to Fc receptors on human monocytes and macrophages. Cytometry A 2016; 89:1001–9. [DOI] [PubMed] [Google Scholar]
- 37. Kragstrup TW. The IL‐20 receptor axis in immune‐mediated inflammatory arthritis: novel links between innate immune recognition and bone homeostasis. Scand J Rheumatol 2016; 45:53–7. [DOI] [PubMed] [Google Scholar]
- 38. Šenolt L, Leszczynski P, Dokoupilová E et al Efficacy and safety of anti‐interleukin‐20 monoclonal antibody in patients with rheumatoid arthritis: a randomized phase IIa trial. Arthritis Rheum 2015; 67:1438–48. [DOI] [PubMed] [Google Scholar]
- 39. Sa SM, Valdez PA, Wu J et al The effects of IL‐20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 2007; 178:2229–40. [DOI] [PubMed] [Google Scholar]
- 40. Myles IA, Fontecilla NM, Valdez PA et al Signaling via the IL‐20 receptor inhibits cutaneous production of IL‐1β and IL‐17A to promote infection with methicillin‐resistant Staphylococcus aureus . Nat Immunol 2013; 14:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Rycke L, Vandooren B, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor alpha blockade treatment down‐modulates the increased systemic and local expression of Toll‐like receptor 2 and Toll‐like receptor 4 in spondylarthropathy. Arthritis Rheum 2005; 52:2146–58. [DOI] [PubMed] [Google Scholar]
- 42. Assassi S, Reveille JD, Arnett FC et al Whole‐blood gene expression profiling in ankylosing spondylitis shows upregulation of Toll‐like receptor 4 and 5. J Rheumatol 2011; 38:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taurog JD, Richardson JA, Croft JT et al The germfree state prevents development of gut and joint inflammatory disease in HLA‐B27 transgenic rats. J Exp Med 1994; 180:2359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomson GT, DeRubeis DA, Hodge MA, Rajanayagam C, Inman RD. Post‐Salmonella reactive arthritis: late clinical sequelae in a point source cohort. Am J Med 1995; 98:13–21. [DOI] [PubMed] [Google Scholar]
- 45. Zhang X, Glogauer M, Zhu F, Kim T‐H, Chiu B, Inman RD. Innate immunity and arthritis: neutrophil Rac and toll‐like receptor 4 expression define outcomes in infection‐triggered arthritis. Arthritis Rheum 2005; 52:1297–304. [DOI] [PubMed] [Google Scholar]
- 46. Inman R. Innate immunity of spondyloarthritis: the role of Toll‐like receptors. New York: Springer, 2009. [DOI] [PubMed] [Google Scholar]
- 47. Oktayoglu P, Em S, Tahtasiz M et al Elevated serum levels of high mobility group box protein 1 (HMGB1) in patients with ankylosing spondylitis and its association with disease activity and quality of life. Rheumatol Int 2013; 33:1327–31. [DOI] [PubMed] [Google Scholar]
- 48. Duruöz MT, Turan Y, Cerrahoglu L, Isbilen B. Serum hyaluronic acid levels in patients with ankylosing spondylitis. Clin Rheumatol 2008; 27:621–6. [DOI] [PubMed] [Google Scholar]
- 49. Romero‐Sanchez C, Tsou H‐K, Jan M‐S et al Serum monocyte chemotactic protein‐1 concentrations distinguish patients with ankylosing spondylitis from patients with mechanical low back pain. J Spinal Disord Tech 2011; 24:202–7. [DOI] [PubMed] [Google Scholar]
- 50. Koch AE, Kunkel SL, Harlow LA et al Enhanced production of monocyte chemoattractant protein‐1 in rheumatoid arthritis. J Clin Invest 1992; 90:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Surdacki A, Sulicka J, Korkosz M et al Blood monocyte heterogeneity and markers of endothelial activation in ankylosing spondylitis. J Rheumatol 2014; 41:481–9. [DOI] [PubMed] [Google Scholar]
- 52. Conrad K, Wu P, Sieper J, Syrbe U. In vivo pre‐activation of monocytes in patients with axial spondyloarthritis. Arthritis Res Ther 2015; 17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


