Summary
Persistent inflammatory response in cystic fibrosis (CF) airways is believed to play a central role in the progression of lung damage. Anti‐inflammatory treatment may slow lung disease progression, but adverse side effects have limited its use. Vitamin D has immunoregulatory properties. We randomized 16 CF patients to receive vitamin D2, vitamin D3 or to serve as controls, and investigated the effect of vitamin D supplementation on soluble immunological parameters, myeloid dendritic cells (mDCs) and T cell activation. Three months of vitamin D treatment were followed by two washout months. Vitamin D status at baseline was correlated negatively with haptoglobin, erythrocyte sedimentation rate and immunoglobulin A concentration. Total vitamin D dose per kg bodyweight correlated with the down‐modulation of the co‐stimulatory receptor CD86 on mDCs. Vitamin D treatment was associated with reduced CD279 (PD‐1) expression on CD4+ and CD8+ T cells, as well as decreased frequency of CD8+ T cells co‐expressing the activation markers CD38 and human leucocyte antigen D‐related (HLA‐DR) in a dose‐dependent manner. There was a trend towards decreased mucosal‐associated invariant T cells (MAIT) cell frequency in patients receiving vitamin D and free serum 25‐hydroxyvitamin D (free‐s25OHD) correlated positively with CD38 expression by these cells. At the end of intervention, the change in free‐s25OHD was correlated negatively with the change in CD279 (PD‐1) expression on MAIT cells. Collectively, these data indicate that vitamin D has robust pleiotropic immunomodulatory effects in CF. Larger studies are needed to explore the immunomodulatory treatment potential of vitamin D in CF in more detail.
Keywords: cystic fibrosis, immunity, immunoglobulins, T cells, vitamin D
Introduction
Cystic fibrosis (CF) is one of the most common life‐shortening autosomal recessive diseases in Caucasians. The major cause of morbidity and mortality is progressive lung disease, characterized by a vicious circle of infection and inflammation 1. The persistent inflammatory response in the airways is believed to play a central role in the progression of lung damage in CF 2. Invariant natural killer T cells (iNK T cells), T helper type 2 (Th2) and Th17 T cells are likely to be involved in the chronic inflammation affecting CF lungs 3, 4, 5. Anti‐inflammatory treatment may slow lung disease progression, but adverse side effects have limited its use 6, 7.
Vitamin D has a tolerogenic influence on dendritic cells (DCs) 8. Vitamin D inhibits proliferation, maturation, survival and differentiation of DCs in vitro which, in turn, leads to decreased capacity to induce T cell activation 9, 10. Simultaneously, it exerts a direct regulatory effect on T cells, inhibiting T cell proliferation and interleukin (IL)‐17 and interferon (IFN)‐γ production 11.
Vitamin D insufficiency occurs frequently in CF patients 12. Current vitamin D supplementation recommendations for CF were designed with a focus on bone health and recommend vitamin D3 (D3) over vitamin D2 (D2) 13, 14. However, a recent study has indicated that D2 may be as effective as D3 if given in a double dose 15. In vitro studies have suggested that vitamin D may have beneficial immunomodulatory effects in CF 16, 17. Adult CF patients admitted to hospital for pulmonary exacerbation and who received an oral bolus of 250 000 IU D3 showed reductions in serum IL‐6 and tumour necrosis factor (TNF) levels 18. Serum 25‐hydroxyvitamin D (s25OHD) levels in CF patients have been associated independently with total serum immunoglobulin (Ig)G levels 12, a marker of chronic inflammation 19. It is currently discussed whether vitamin D treatment should be used as adjunctive immunoregulatory therapy in CF 20.
Lipopolysaccharide (LPS) measured in plasma of CF patients is higher than in non‐CF individuals and is believed to be derived from Pseudomonas aeruginosa or other Gram‐negative bacteria 21. Serum LPS levels are higher in patients who were hospitalized previously for a CF exacerbation than in those who were not 22, and are associated positively with hospitalization rates in adult CF patients 23. At the same time, CF monocytes stimulated ex vivo with LPS had lost the ability to up‐regulate the triggering receptor expressed on myeloid cells‐1 (TREM‐1) 24. In line with this, expression of TREM‐1 on monocytes and the concentration of soluble TREM‐1 (sTREM‐1) in sera were shown to be pathologically low in CF. Interestingly, TREM‐1 was induced by active vitamin D in airway epithelial cells 25.
To our knowledge, the effect of vitamin D treatment on immunoglobulin concentrations, myeloid dendritic cells (mDCs) and T cell activation has not yet been evaluated in CF patients. The aim of this pilot trial was to investigate the effect of oral vitamin D supplementation on these parameters, using data collected in a previously published open‐label pilot trial 26.
Subjects and methods
Trial design
As described previously 26, sixteen CF patients of age ≥ 6 years and with baseline total s25OHD (tot‐s25OHD) < 75 nmol/l were randomized to receive D2, D3 or to serve as controls. Thirteen patients completed the study and were analysed. Clinical characteristics of patients completing the study were published previously 26, and completing relevant information is listed in Table 1. Three months of supplementation were followed by 2 months of washout. Patients below the age of 16 years randomized to the intervention arms received a starting weekly dose of 35 000 IU D2 or D3, whereas patients aged ≥ 16 years received a starting weekly dose of 50 000 IU D2 or D3. The weekly dose was given per os as seven once‐daily doses, and adjusted by tot‐s25OHD monitoring throughout the 3 months of intervention. The goal of the supplementation was to reach tot‐s25OHD > 100 nmol/l 26. There was no change in tot‐s25OHD levels in the control group 26. Patients receiving D2 had a tendency to increase tot‐s25OHD, while patients supplemented with D3 increased tot‐s25OHD levels significantly 26.
Table 1.
Clinical characteristics of patients completing the study
| All patients | Control group | Vit D2 group | Vit D3 group | |
|---|---|---|---|---|
| (n = 13) | (n = 4) | (n = 4) | (n = 5) | |
| Body mass index (kg/m2; mean ± s.d.)* | 19·5 ± 3·4 | 18·8 ± 2·7 | 19·0 ± 3·2 | 20.6 ± 4.5 |
| Pseudomonas aeruginosa chronic infection (n)† | 5 | 3 | 1 | 1 |
| Pseudomonas aeruginosa intermittent infection (n)‡ | 6 | 0 | 2 | 4 |
| Disease‐modifying drugs, prior or current use (n) | 0 | 0 | 0 | 0 |
| Short‐term oral corticosteroids, current use (n) | 2 | 1 | 0 | 1 |
| Inhaled corticosteroids, current use (n) | 3 | 1 | 1 | 1 |
*In patients < 20 years of age, body mass index (BMI) was assessed as isoBMI; s.d. = standard deviation. †Defined as positive serology assessed by exotoxin A antibodies, and/or ≥ 6 consecutive sputum cultures positive for Pseudomonas aeruginosa (with 1‐month interval between the samples). ‡Defined as ≥ 1 sputum culture positive for Pseudomonas aeruginosa, and < 6 consecutive sputum cultures positive for Pseudomonas aeruginosa within 6 months (with 1‐month interval between the samples) and negative exotoxin A serology.
The primary goal of the present study was to evaluate the effect of vitamin D treatment on soluble and cellular markers of immunological activation, and the secondary goal was to compare the effect of D2 with that of D3. Secondarily, we also aimed to investigate whether any effects of vitamin D were dose‐dependent.
Analytical methods
Blood was sampled and analysed for erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), tot‐s25OHD, albumin, vitamin D‐binding protein (DBP), cytokines, acute phase proteins and immunoglobulins at baseline and at 1, 4, 8, 12, 16 and 20 weeks. At the last study visit patients reported their sun exposure throughout the study, which was quantified as described previously 26. Serum albumin, calcium, ESR, CRP, anti‐trypsin, orosomucoid, haptoglobin, TNF‐α, IL‐6, IgG, IgM, IgA, leucocytes, monocytes, eosinophils, lymphocytes, basophils and tot‐s25OHD were analysed by standard methods at the Karolinska University Hospital Huddinge clinical laboratories. Plasma was collected and cryopreserved at −80°C. DBP was measured as described previously 26. Levels of IgE (Eagle Biosciences, Boston, MA, USA), TGF‐β (R&D Systems, Minneapolis, MN, USA), LL‐37 and sCD14 (Hycult Biotech, Uden, the Netherlands), as well as LPS and sTREM‐1 (My BioSource, San Diego, CA, USA), were measured in the plasma samples by enzyme‐linked immunosorbent assay (ELISA). Plasma IL‐10, IL‐17, IP‐10 and monocyte chemoattractant protein (MCP)‐1 were quantified by Luminex xMAP technology, using fluorescent‐coded beads (Merck Millipore, Darmstadt, Germany). Free s25OHD (free‐s25OHD) was calculated using tot‐s25OHD, albumin and DBP, as described previously 26.
Peripheral blood mononuclear cells (PBMCs) were isolated by density‐gradient sedimentation using Ficoll‐Paque (Lymphoprep, Axis‐Shield) at baseline, end of intervention and end of the study. PBMCs were stored in liquid nitrogen. Specimens were thawed and washed, and cell counts and viability were assessed using the Muse count and viability kit (Merck Millipore) before flow cytometry analysis. Cells were washed and stained at room temperature for 10 min in 96‐well V‐bottomed plates in the dark. Samples were then washed and fixed using fixation solution (BD Biosciences, San Jose, CA, USA) before data acquisition. The monoclonal antibodies used in flow cytometry were the following: anti‐CD3 AF700, anti‐CD4 allophycocyanin (APC)‐H7, anti‐CD11c phycoerythrin‐cyanin 5 (PE‐Cy5), anti‐CD38 PE‐Cy7, anti‐CD56 AF700, anti‐CD86 v450, anti‐CD161 APC, anti‐CD279 [programmed death 1 (PD‐1)] Brilliant Violet 421, anti‐human leucocyte antigen D‐related (HLA‐DR) APC (BD Biosciences), anti‐CD19 PE‐Texas Red (Abcam, Cambridge, UK), anti‐CD4 Qdot 705, anti‐CD8 Qdot 655, anti‐CD14 PE‐Texas Red and anti‐HLA‐DR PE‐Texas Red (Invitrogen, Carlsbad, CA, USA), anti‐Vα24 fluorescein isithiocyanate (FITC) and anti‐Vβ11 PE (Beckman Coulter, Fullerton, CA, USA) and anti‐Vα7.2 FITC (Biolegend, San Diego, CA, USA). Flow cytometry data were acquired using a BD LSR II instrument (BD Biosciences) and analysed using FlowJo version 9.6 (Tree Star, Ashland, OR, USA).
Statistical analysis
Statistical analyses were performed using statistica version 10 and Prism version 6, and figures were created in Prism version 6. For each variable, we plotted raw data to assess normality of distribution. For comparisons within groups between two time‐points, paired t‐test (normally distributed data) or Wilcoxon's matched‐pairs test (data without normal distribution) were used. For correlation analyses, Pearson's test or Spearman's test were used for normally or non‐normally distributed data, respectively. For some statistical analyses, values were log‐transformed (base e) if they were not distributed normally. Data were plotted as raw values. We considered P < 0·05 as statistically significant.
Results
25OHD levels increase during intervention and decrease during the washout period
There were no statistically significant differences in the baseline demographics of the study groups, as reported previously 26. The pooled group of patients receiving D2 or D3 increased their tot‐s25OHD and free‐s25OHD concentrations at the end of intervention. In this group, levels of both tot‐s25OHD and free‐s25OHD dropped significantly during the washout period (Table 2). There were no reported symptoms of vitamin D toxicity, but there was a transitory increase in albumin‐corrected plasma calcium in the D3 arm, compared with baseline (Supporting information, Fig. S1). The control group did not change their vitamin D status throughout the study (Table 2). There were no significant changes in any of the studied immunological parameters in the control group (Tables 2, 3, Supporting information, Tables S1 and S2).
Table 2.
Effect of vitamin D treatment on serum 25‐hydroxyvitamin D (s25OHD) levels, C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), acute phase proteins, soluble triggering receptor expressed on myeloid cells‐1 (sTREM‐1), lipopolysaccharide (LPS) and immunoglobulins
| Parameter | Group assignment | Baseline values | Change at week 12, compared with baseline | Change at week 20, compared with week 12 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n * | Median | Minimum | Maximum | n * | Delta | P‐value | n * | Delta | P‐value | ||
| Total‐s25OHD (nmol/l) | Vitamin D | 8 | 66 | 42 | 76 | 9 | 21 | 0·012 | 9 | −34 | 0·008 |
| Control | 3 | 38 | 17 | 92 | 4 | 29 | 0·285 | 4 | −23 | 0·144 | |
| Free‐S25OHD (pg/ml) | Vitamin D | 6 | 13·4 | 10·2 | 16·3 | 8 | 3·9 | 0·043 | 6 | −7·6 | 0·043 |
| Control | 3 | 6·7 | 3·4 | 22·6 | 3 | 5·3 | 1·000 | 3 | 0·1 | 1·000 | |
| CRP (mg/l) | Vitamin D | 9 | 0·0 | 0·0 | 16·0 | 9 | 0·0 | 1·000 | 9 | 0·0 | 1·000 |
| Control | 4 | 0·0 | 0·0 | 102·0 | 4 | 0·5 | 1·000 | 4 | 0·0 | 1·000 | |
| ESR (mm) | Vitamin D | 7 | 7·0 | 2·0 | 27·0 | 7 | 6·0 | 0·715 | 8 | −2·5 | 0·893 |
| Control | 2 | 20·5 | 17·0 | 24·0 | 4 | −8·0 | 1·000 | 2 | 2·0 | 1·000 | |
| Anti‐trypsin (g/l) | Vitamin D | 9 | 1·2 | 1·1 | 2·0 | 9 | 0·1 | 0·441 | 9 | −0·1 | 0·398 |
| Control | 4 | 1·5 | 1·3 | 2·6 | 4 | 0·1 | 0·285 | 4 | 0·0 | 0·584 | |
| Orosomucoid (g/l) | Vitamin D | 9 | 0·7 | 0·6 | 1·6 | 9 | 0·1 | 0·515 | 9 | 0·0 | 0·401 |
| Control | 4 | 0·9 | 0·8 | 3·0 | 4 | −0·1 | 0·273 | 4 | 0·1 | 0·465 | |
| Haptoglobin (g/l) | Vitamin D | 9 | 1·0 | 0·3 | 2·1 | 9 | 0·0 | 0·624 | 9 | 0·0 | 0·575 |
| Control | 4 | 1·6 | 1·0 | 4·0 | 4 | −0·3 | 0·715 | 4 | 0·0 | 0·715 | |
| sTREM‐1 (pg/ml) | Vitamin D | 6 | 145 | 83 | 935 | 8 | −9 | 0·080 | 7 | 38 | 0·249 |
| Control | 4 | 134 | 86 | 197 | 3 | −12 | 0·285 | 3 | 12 | 1·000 | |
| LPS (pg/l) | Vitamin D | 6 | 409 | 337 | 540 | 8 | −14 | 0·500 | 5 | 3 | 0·273 |
| Control | 4 | 472 | 359 | 1025 | 3 | −50 | 0·285 | 3 | 28 | 1·000 | |
| IgG (g/l) | Vitamin D | 9 | 10·4 | 4·2 | 15·8 | 9 | −0·2 | 0·834 | 9 | 0·1 | 0·484 |
| Control | 4 | 11·7 | 11·4 | 12·8 | 4 | 0·2 | 0·583 | 4 | −0·1 | 0·715 | |
| IgA (g/l) | Vitamin D | 9 | 1·6 | 0·5 | 4·4 | 9 | 0·1 | 0·499 | 9 | 0·0 | 0·249 |
| Control | 4 | 2·5 | 0·6 | 3·7 | 4 | −0·4 | 0·465 | 4 | 0·1 | 0·593 | |
| IgM (g/l) | Vitamin D | 9 | 1·2 | 0·4 | 1·6 | 9 | −0·2 | 0·753 | 9 | 0·0 | 0·722 |
| Control | 4 | 0·9 | 0·5 | 1·4 | 4 | 0·1 | 0·109 | 4 | 0·1 | 0·144 | |
| IgE (IU/l) | Vitamin D | 6 | 23251 | 11869 | 73347 | 8 | −262 | 0·686 | 7 | −4038 | 0·345 |
| Control | 4 | 67861 | 42463 | 117896 | 3 | 179 | 1·000 | 3 | 1297 | 1·000 | |
*Numbers might vary due to missing samples. Ig = immunoglobulin.
Table 3.
Effect of vitamin D treatment on dendritic cells (DC) and T cells
| Parameter | Group assignment | Baseline values | Change at week 12, compared with baseline | Change at week 20, compared with week 12 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n * | Median | Minimum | Maximum | n * | Delta | P‐value | n * | Delta | P‐value | ||
| DC (% of PBMC) | Vitamin D | 9 | 2·0 | 1·2 | 3·8 | 9 | 0·0 | 0·314 | 8 | −0·1 | 0·263 |
| Control | 4 | 2·0 | 0·8 | 2·3 | 4 | −0·2 | 0·465 | 4 | −0·6 | 0·273 | |
| CD40 (DC MFI†) | Vitamin D | 9 | 92 | 85 | 133 | 9 | −7 | 0·110 | 8 | −2 | 0·674 |
| Control | 4 | 122 | 106 | 140 | 4 | −7 | 0·465 | 4 | −12 | 0·465 | |
| CD86 (DC MFI†) | Vitamin D | 9 | 774 | 602 | 961 | 9 | 10 | 0·515 | 8 | −83 | 0·575 |
| Control | 4 | 730 | 638 | 814 | 4 | 101 | 0·068 | 4 | 42 | 1·000 | |
| CD80 (DC MFI†) | Vitamin D | 9 | 27 | 9 | 42 | 9 | −2 | 0·953 | 8 | −4 | 0·208 |
| Control | 4 | 33 | 24 | 41 | 4 | −1 | 0·465 | 4 | −3 | 1·000 | |
| CD8+ (% of T cells) | Vitamin D | 9 | 33 | 21 | 45 | 8 | 3 | 0·600 | 8 | −3 | 0·310 |
| Control | 4 | 36 | 25 | 45 | 4 | −1 | 1·000 | 4 | 0 | 0·715 | |
| CD38+ (% of CD8 T cells) | Vitamin D | 9 | 83 | 76 | 96 | 8 | 5 | 0·069 | 8 | −5 | 0·237 |
| Control | 4 | 90 | 82 | 92 | 4 | −1 | 1·000 | 4 | 1 | 0·465 | |
| CTLA‐4+ (% of CD8 T cells) | Vitamin D | 9 | 0·9 | 0·8 | 1·7 | 8 | 0·1 | 0·779 | 8 | −0·1 | 0·866 |
| Control | 4 | 1·2 | 0·8 | 4·9 | 4 | 0·0 | 0·465 | 4 | 0·1 | 0·465 | |
| HLA‐DR+ (% of CD8 T cells) | Vitamin D | 9 | 16 | 5 | 32 | 8 | −8 | 0·012 | 8 | 7 | 0·043 |
| Control | 4 | 15 | 12 | 19 | 4 | −4 | 0·715 | 4 | 2 | 0·273 | |
| PD‐1+ (% of CD8 T cells) | Vitamin D | 9 | 27 | 9 | 49 | 8 | −2 | 0·036 | 8 | −1 | 1·000 |
| Control | 4 | 17 | 12 | 33 | 4 | 1 | 0·465 | 4 | −2 | 0·715 | |
| CD38+HLA‐DR+ (% of CD8 T cells) | Vitamin D | 9 | 24 | 11 | 32 | 8 | −11 | 0·036 | 8 | 6 | 0·028 |
| Control | 4 | 26 | 21 | 29 | 4 | −5 | 0·715 | 4 | −1 | 0·273 | |
| CD4+ (% of T cells) | Vitamin D | 9 | 53 | 45 | 66 | 8 | −3 | 0·484 | 8 | 2 | 0·310 |
| Control | 4 | 51 | 37 | 61 | 4 | −2 | 0·715 | 4 | 2 | 0·273 | |
| CD4/CD8 T cell ratio | Vitamin D | 9 | 1·56 | 0·99 | 3·02 | 8 | −0·08 | 0·889 | 8 | 0·04 | 0·499 |
| Control | 4 | 1·50 | 0·88 | 2·32 | 4 | −0·08 | 0·715 | 4 | 0·12 | 0·715 | |
| CD38+ (% of CD4 T cells) | Vitamin D | 9 | 67 | 45 | 87 | 8 | −2 | 0·208 | 8 | −1 | 0·176 |
| Control | 4 | 70 | 59 | 85 | 4 | 1 | 0·273 | 4 | −1 | 0·144 | |
| CTLA4+ (% of CD4 T cells) | Vitamin D | 9 | 2·0 | 1·1 | 3·7 | 8 | 0·0 | 0·484 | 8 | −0·5 | 0·499 |
| Control | 4 | 1·5 | 1·2 | 2·1 | 4 | 0·4 | 0·465 | 4 | 0·0 | 1·000 | |
| HLA‐DR+ (% of CD4 T cells) | Vitamin D | 9 | 8 | 5 | 23 | 8 | −1 | 0·208 | 8 | 0 | 0·866 |
| Control | 4 | 16 | 10 | 27 | 4 | 0 | 0·465 | 4 | 2 | 0·465 | |
| PD‐1+ (% of CD4 T cells) | Vitamin D | 9 | 24 | 16 | 40 | 8 | −3 | 0·123 | 8 | 0 | 0·398 |
| Control | 4 | 26 | 13 | 37 | 4 | −3 | 0·068 | 4 | 1 | 0·273 | |
| CD38+HLA‐DR+ (% of CD4 T cells) | Vitamin D | 9 | 6 | 4 | 21 | 8 | 0 | 0·263 | 8 | 1 | 0·735 |
| Control | 4 | 14 | 11 | 20 | 4 | 0 | 0·465 | 4 | 2 | 0·715 | |
| iNK T cell frequency (% of T cells) | Vitamin D | 9 | 0·03 | 0·01 | 0·84 | 9 | 0·00 | 0·441 | 8 | −0·01 | 0·889 |
| Control | 4 | 0·04 | 0·02 | 0·14 | 4 | −0·02 | 0·144 | 4 | 0·01 | 0·715 | |
| MAIT cell frequency (% of T cells) | Vitamin D | 9 | 0·40 | 0·19 | 2·21 | 9 | −0·03 | 0·0506 | 8 | −0·05 | 0·401 |
| Control | 3 | 0·85 | 0·43 | 2·69 | 3 | 0·49 | 1·000 | 3 | −0·51 | 1·000 | |
| CD38+ (% of MAIT) | Vitamin D | 9 | 9 | 4 | 31 | 9 | 3 | 0·953 | 8 | −5 | 0·779 |
| Control | 4 | 11 | 0 | 30 | 4 | −2 | 0·285 | 4 | 1 | 0·273 | |
| PD–1+ (% of MAIT) | Vitamin D | 9 | 25 | 15 | 47 | 9 | −5 | 0·374 | 8 | −2 | 0·575 |
| Control | 4 | 19 | 10 | 70 | 4 | 1 | 0·465 | 4 | 2 | 0·068 | |
| HLA‐DR+ (% of MAIT) | Vitamin D | 9 | 11 | 2 | 16 | 9 | −3 | 0·515 | 8 | 0 | 0·484 |
| Control | 4 | 9 | 0 | 14 | 4 | 0 | 0·715 | 4 | 0 | 0·465 | |
*Numbers might vary due to missing samples. †MFI, mean fluorescence intensity; PBMC = peripheral blood mononuclear cells; CTLA‐4 = cytotoxic T lymphocyte 4; HLA‐DR = human leucocyte antigen D‐related; PD‐1 = programmed death 1; iNK T = invariant natural killer T cells; MAIT = mucosal‐associated invariant T cells.
Haptoglobin correlates inversely with vitamin D status at baseline and change in vitamin D levels during treatment
Haptoglobin, orosomucoid and anti‐trypsin are systemic markers of inflammation that are released in the acute phase of infection, and are increased in CF 27. Reductions in haptoglobin are associated with improvements in lung function 28 and with the overall clinical improvement with antibiotic therapy 29. The effect of vitamin D on acute phase proteins in CF has not yet been studied. In this pilot study, haptoglobin was correlated negatively with free‐25OHD at baseline (Fig. 1a). Changes in haptoglobin concentration were correlated negatively with changes in free‐s25OHD at the end of intervention (Fig. 1b) and with changes in tot‐s25OHD at the end of the study (Fig. 1c). These observations suggest that s25OHD and haptoglobin levels are correlated inversely in CF.
Figure 1.
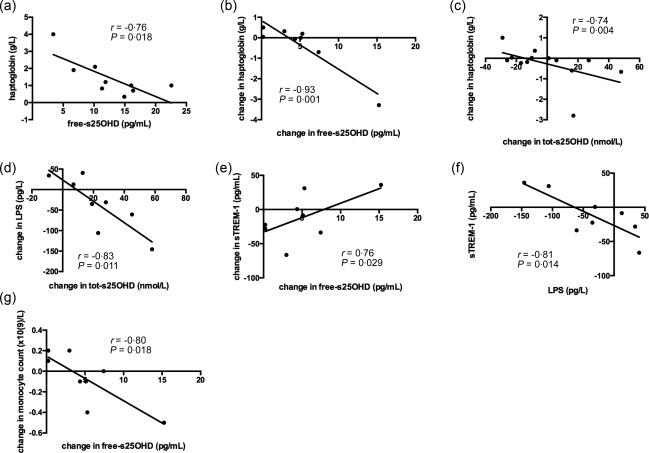
Haptoglobin correlates inversely with vitamin D status at baseline and change in vitamin D during treatment. Lipopolysaccharide (LPS) and soluble triggering receptor expressed on myeloid cells‐1 (sTREM‐1) correlate with change in vitamin D levels during treatment. Free serum 25‐hydroxyvitamin D (free‐s25OHD) was calculated and haptoglobin, LPS and sTREM‐1 analysed as described in Subjects and methods. Haptoglobin was correlated negatively with free‐s25OHD at baseline (a). Changes in haptoglobin concentration were correlated negatively with changes in free‐s25OHD at the end of intervention (b), and with changes in tot‐s25OHD at the end of the study (c). Change from baseline in tot‐s25OHD versus change from baseline in plasma LPS levels at the end of supplementation in all patients (d). Change from baseline in free‐s25OHD versus change from baseline in plasma sTREM‐1 levels at the end of supplementation in all patients (e). Change from baseline in LPS versus change from baseline in sTREM‐1 at the end of supplementation in all patients (f). Change from baseline in free‐s25OHD versus change from baseline in circulating monocyte count at the end of supplementation in all patients (g). P < 0·05 in all. Results are plotted as raw data from all subjects participating in the study irrespective of treatment arm (a–g). The number of patients presented in the individual graphs varies due to a few missing samples (see Table 2).
LPS and sTREM‐1 correlate with change in vitamin D levels during treatment
It was shown previously that CF monocytes stimulated ex vivo with LPS had lost the ability to up‐regulate TREM‐1 24, and sTREM‐1 levels in sera were shown to be pathologically low in CF. Another study indicated that active vitamin D can induce TREM‐1 in airway epithelial cells 25. Therefore we studied effect of vitamin D treatment on sTREM and LPS levels. At the end of supplementation, change from baseline in tot‐s25OHD was correlated negatively with change in LPS (Fig. 1d), change in free‐s25OHD correlated positively with change in plasma sTREM‐1 and changes in sTREM‐1 correlated with changes in LPS (Fig. 1e and f). At the same time‐point, the change from baseline in free‐s25OHD was correlated inversely with changes in monocyte count (Fig. 1g). This suggests that vitamin D may influence the LPS–sTREM‐1 axis in CF.
Vitamin D levels correlate with erythrocyte sedimentation rate and immunoglobulin concentrations
Erythrocyte sedimentation rate (ESR) is a marker of inflammation, which is determined largely by plasma fibrinogen and globulins, including immunoglobulins. IgG levels increase with age and are in CF correlated negatively with lung function 30, as well as with exercise capacity and oxygen uptake 31. In bronchial epithelial lining fluid, IgG, IgA and IgM concentrations are 2·5–6 times greater in stable patients with mild CF compared with control subjects 32. It is believed that the persistent inflammatory response in the airways plays a central role in the progression of lung damage in CF 2. 1,25‐dihydroxyvitamin D3 decreases B cell proliferation, plasma cell differentiation and immunoglobulin secretion 33, 34. In light of this background, we investigated the effect of vitamin D treatment on ESR and immunoglobulin concentrations. At baseline, tot‐s25OHD and free‐s25OHD were correlated negatively with ESR (Fig. 2a,b). At the end of supplementation, the change in ESR was correlated inversely with the change in tot‐s25OHD from baseline (Fig. 2c). At baseline, tot‐s25OHD and free‐s25OHD were correlated negatively with serum total IgA levels (Fig. 3a,b). At 1 week of supplementation, changes in free‐s25OHD were correlated negatively with changes in serum total IgM (Fig. 3c). D2, but not D3 (data not shown), supplementation tended to decrease serum total IgG concentration at 8 weeks of supplementation (P = 0·068; Supporting information, Fig. S2). Changes in free‐s25OHD correlated with changes in plasma total IgE at 4 weeks of supplementation (Fig. 3d). At the end of supplementation, there was an inverse correlation between both total vitamin D dose ingested and sun exposure and plasma total IgE (Fig. 3e,f). Collectively, these findings indicate that higher levels of vitamin D are associated with lower immunoglobulin production in CF in a dose‐dependent manner.
Figure 2.
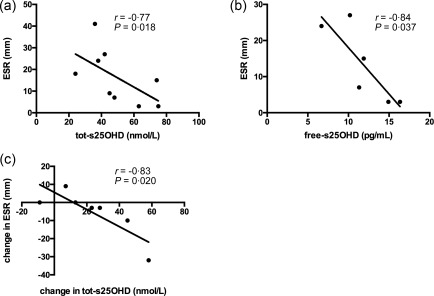
Vitamin D levels correlate with erythrocyte sedimentation rate (ESR). Free serum 25‐hydroxyvitamin D (free‐s25OHD) was calculated and ESR was analysed as described in Subjects and methods. At baseline, tot‐s25OHD (a) and free‐s25OHD (b) were correlated negatively with ESR. At the end of supplementation, the change in ESR was correlated inversely with the change in tot‐s25OHD from baseline (c). All Ps < 0·05. Results are plotted as raw data from all subjects participating in the study irrespective of treatment arm (a–c). The number of patients presented in the individual graphs varies due to a few missing samples (see Table 2).
Figure 3.
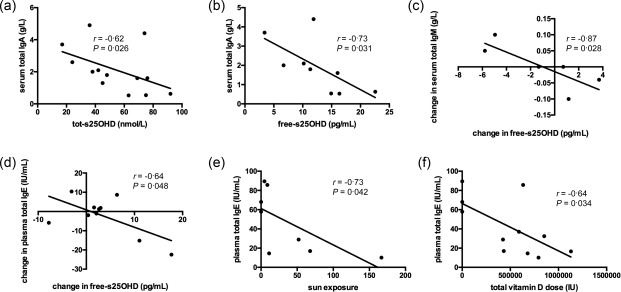
Vitamin D levels correlate with immunoglobulin concentrations. Free serum 25‐hydroxyvitamin D (free‐s25OHD) was calculated and immunoglobulin (Ig)A, IgM, IgG and IgE were analysed as described in Subjects and methods. Sun exposure was quantified as described previously 26. Total‐s25OHD (a) and free‐s25OHD (b) were correlated negatively with serum total IgA levels at baseline. Changes in free‐s25OHD were correlated negatively with changes in serum total IgM at 1 week of supplementation (c). Changes in free‐s25OHD correlated with changes in plasma total IgE at 4 weeks of supplementation (d). At the end of supplementation, there was an inverse correlation between both sun exposure (e) and total vitamin D dose ingested (f) and plasma total IgE. P < 0·05 in all, unless stated otherwise. Results are plotted as raw data from all subjects participating in the study irrespective of treatment arm (a–f). The number of patients presented in the individual graphs varies due to a few missing samples (see Table 2).
Total vitamin D dose per kg bodyweight correlates with the down‐modulation of the co‐stimulatory molecule CD86 on myeloid dendritic cells
1,25‐dihydroxyvitamin D3 decreases differentiation, maturation and immunostimulatory capacity of DCs by inhibiting the expression of CD40, CD80, CD86 and major histocompatibility complex (MHC) class II molecules 35, 36, 37, 38. Therefore, we hypothesized that vitamin D treatment would also decrease the expression of co‐stimulatory molecules on mDCs in CF patients. mDCs were identified as described previously 39. In line with this hypothesis, the total vitamin D dose per kg bodyweight correlated with the down‐modulation of the co‐stimulatory molecule CD86 on mDCs at the end of the study (Fig. 4). This suggests that vitamin D is likely to inhibit the immunostimulatory capacity of DCs in CF in a dose‐dependent manner.
Figure 4.
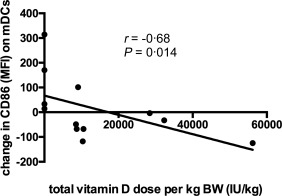
Total vitamin D dose per kg bodyweight correlates with the down‐modulation of the co‐stimulatory molecule CD86 on myeloid dendritic cells (mDCs). The total vitamin D dose per kg bodyweight correlated with the down‐modulation of CD86 on mDCs at the end of the study. Results are plotted as raw data from all subjects participating in the study irrespective of treatment arm. The number of observations presented in the graph is smaller than the total number of patients due to a few missing samples (see Table 3).
Vitamin D treatment reduces CD279 (PD‐1) expression on CD8+ T cells as well as frequency of CD8+ T cells co‐expressing CD38 and HLA‐DR
In the scenario of chronic T cell activation, vitamin D is believed to suppress T cell proliferation and cytokine production (as reviewed in 40). To test whether vitamin D could down‐modulate T cell activation in CF, we evaluated the effect of vitamin D treatment on CD8+ and CD4+ T cells. The combined group of patients receiving D2 or D3 reduced their HLA‐DR and CD279 (PD‐1) expression on CD8+ T cells at the end of supplementation (Fig. 5a,b). The frequency of CD8+ T cells co‐expressing CD38 and HLA‐DR was also decreased at the end of supplementation (Fig. 5c). The frequency of CD8+ T cells expressing HLA‐DR and co‐expressing CD38 and HLA‐DR increased significantly during the washout period, returning to baseline levels, while expression of CD279 (PD‐1) by CD8+ T cells remained down‐regulated until the end of the study (Fig. 5a–c). HLA‐DR expression on CD8+ T cells correlated with the total vitamin D dose per kg bodyweight at the end of intervention (Fig. 5d), and the down‐regulation of HLA‐DR expression on CD8+ T cells correlated with the change in tot‐s25OHD at the end of the study (Fig. 5e). Patients receiving D3, but not controls and patients receiving D2 (data not shown), displayed decreased CD279 (PD‐1) expression on CD8+ T cells at the end of supplementation (P = 0·027, Fig. 5f). Collectively, these observations indicate a modulatory effect of vitamin D treatment on CD8+ T cell activation and exhaustion in CF.
Figure 5.
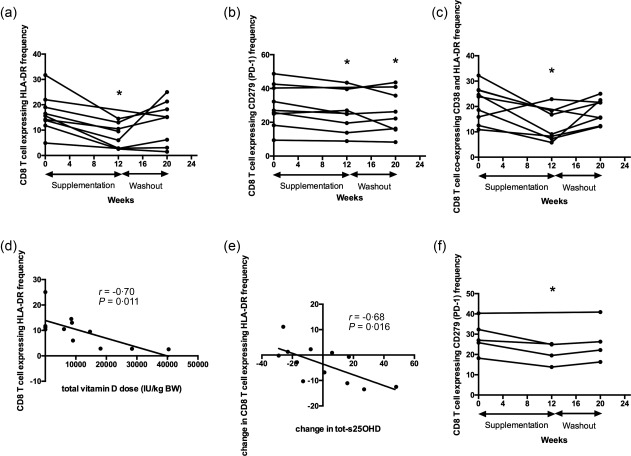
Vitamin D treatment reduces CD279 [programmed death 1 (PD‐1)] expression on CD8+ T cells as well as frequency of CD8+ T cells co‐expressing CD38 and human leucocyte antigen D‐related (HLA‐DR). The combined group of patients receiving D2 or D3 reduced their HLA‐DR (a) and CD279 (PD‐1) (b) expression on CD8+ T cells at the end of supplementation. The frequency of CD8+ T cells co‐expressing CD38 and HLA‐DR was also decreased at the end of supplementation (c). HLA‐DR expression on CD8+ T cells correlated with the total vitamin D dose per kg bodyweight at the end of intervention (d), and the down‐regulation of the HLA‐DR expression on CD8+ T cells correlated with change in total‐serum 25‐hydroxyvitamin D (s25OHD) at the end of the study (e). Patients receiving D3 displayed decreased CD279 (PD‐1) expression on CD8+ T cells at the end of supplementation (f). P < 0·05 in all. Results are plotted as raw data from the subjects in the pooled vitamin D2 and vitamin D3 treatment group (a–c), raw data from all subjects participating in the study irrespective of treatment arm (d,e) and as raw data from subjects in the vitamin D3 treatment group (f). The number of patients presented in the individual graphs varies due to a few missing samples (see Table 3).
Vitamin D supplementation decreases the frequency of CD4+ T cells expressing CD279 (PD‐1) in a dose‐dependent manner
At baseline, free‐s25OHD was correlated negatively with the expression of HLA‐DR by CD4+ T cells and with the frequency of CD4+ T cells co‐expressing CD38 and HLA‐DR (Fig. 6a,b). The frequency of CD4+ T cells co‐expressing CD38 and HLA‐DR was not affected by vitamin D supplementation (Supporting information, Fig. S3). At the end of the study, the combined group of patients receiving vitamin D2 or D3 experienced a decreased frequency of CD4+ T cells expressing CD279 (PD‐1) (Fig. 6c). At this time‐point, total vitamin D dose received was correlated negatively with change in CD279 (PD‐1) expression by CD4+ T cells (Fig. 6d) and correlated positively with change in CD4/CD8 ratio (Fig. 6e). This suggests that vitamin D treatment may ameliorate exhaustion of CD4+ T cells in CF.
Figure 6.
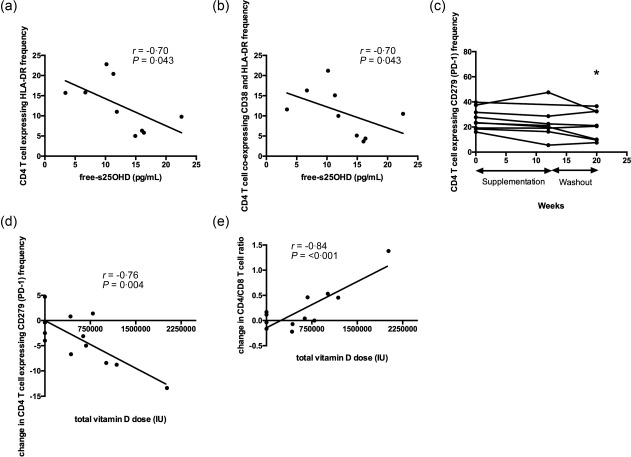
Vitamin D supplementation decreases the frequency of CD4+ T cells expressing CD279 [programmed death 1 (PD‐1)] in a dose‐dependent manner. At baseline, free‐serum 25‐hydroxyvitamin D (s25OHD) was correlated negatively with the expression of human leucocyte antigen D‐related (HLA‐DR) by CD4+ T cells (a) and with the frequency of CD4+ T cells co‐expressing CD38 and HLA‐DR (b). At the end of the study, the combined group of patients receiving vitamin D2 or D3 experienced a decreased frequency of CD4+ T cells expressing CD279 (PD‐1) (c). At this time‐point, total vitamin D dose received was correlated negatively with change in CD279 (PD‐1) expression by CD4+ T cells (d) and correlated positively with change in CD4/CD8 ratio (e). P < 0·05 in all. Results are plotted as raw data from all subjects participating in the study irrespective of treatment arm (a,b,d,e) and as raw data from the subjects in the pooled vitamin D2 and vitamin D3 treatment group (c). The number of patients presented in the individual graphs varies due to a few missing samples (see Table 3).
Trend towards decreased mucosal‐associated invariant T cells (MAIT) cell frequency in patients receiving vitamin D and free‐s25OHD correlates positively with CD38 expression by these cells
MAIT cells are believed to contribute to protection from pulmonary infections 41, 42, and reduced MAIT cell frequency in peripheral blood is associated with more severe lung disease in CF 43. In asthma patients, MAIT cell frequencies correlate with vitamin D levels 44. Therefore we asked whether there is any association between vitamin D and MAIT cells in CF patients. At baseline, free‐s25OHD was correlated positively with CD38 expression and tended to correlate with HLA‐DR expression by MAIT cells (Fig. 7a,b). MAIT cells were identified by the co‐expression of CD161 and Vα7·2 in T cells (Fig. 7c). There was a trend towards decreased MAIT cell frequency at the end of intervention compared with baseline in the combined group of patients receiving vitamin D2 or D3 (P = 0·0506; Fig. 7c,d). At the end of intervention, the change in free‐s25OHD was correlated negatively with the change in CD279 (PD‐1) expression on MAIT cells (Fig. 7e). Jointly, these observations indicate that vitamin D may influence MAIT cells’ activation state as well as their frequency in peripheral blood in CF.
Figure 7.
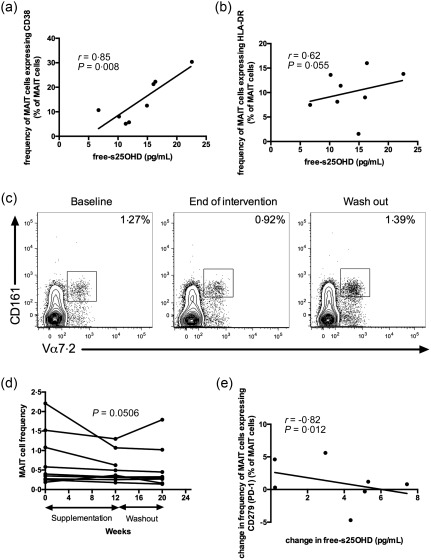
There is a trend towards decreased mucosal‐associated invariant T cell (MAIT) frequency in patients receiving vitamin D and free‐serum 25‐hydroxyvitamin D (s25OHD) correlates positively with CD38 expression by these cells. At baseline, free‐s25OHD was correlated positively with CD38 expression, P = 0·008, (a) and tended to correlate with human leucocyte antigen D‐related (HLA‐DR) expression, P = 0·055, (b) by MAIT cells. MAIT cells were identified by the combined expression of CD161 and Vα7.2 by T cells (c). There was a trend towards decreased MAIT cell frequency at the end of intervention compared with baseline in the combined group of patients receiving vitamin D2 or D3, P = 0·0506 (d). At the end of intervention, the change in free‐s25OHD was correlated negatively with the change in CD279 (programmed death 1) expression on MAIT cells (e). P < 0·05 in all, unless stated otherwise. Results are plotted as raw data from all subjects participating in the study irrespective of treatment arm (a,b,e) and as raw data from the subjects in the pooled vitamin D2 and vitamin D3 treatment group (d). The number of patients presented in the individual graphs varies due to a few missing samples (see Table 3).
By influencing pulmonary macrophages, iNK T cells can improve the course of P. aeruginosa or Mycobacterium tuberculosis pneumonia infection 45, 46. The vitamin D receptor is required for iNK T cell development in mice 47, but low iNK T cell numbers could not be corrected by later intervention with vitamin D in a mouse model 48. In line with this, the frequency of iNK T cells (Vα24+ Vβ11+) did not change upon vitamin D supplementation in the present study (Supporting information, Fig. S4a,b).
Discussion
This pilot study supports the notion that high‐dose once‐daily vitamin D supplementation may decrease immune activation in CF in a dose‐dependent manner. For several of the parameters that were affected by vitamin D supplementation there was also an association with the change in the level of tot‐ or free‐s25OHD, reinforcing the biological relevance of our findings.
In this trial, vitamin D treatment had a much more profound effect on CD8+ T cells compared with CD4+ T cells (Figs 5 and 6). Similarly, the vitamin D analogue calcipotriol was reported previously to reduce the frequency of IL17+CD8+ T cells in psoriasis, while leaving the CD4+ T cells unaffected 49. In experimental inflammatory bowel disease, VDR expression was required to prevent replication of quiescent CD8+ T cells 50. One may speculate that vitamin D targets CD8+ T cells directly in CF, reducing their activation, while its effect on CD4+ T cells may be more indirect or mediated through different pathways.
We have described previously a negative association between serum total IgG levels and vitamin D status in a large cohort of Scandinavian CF patients 12. In this cohort, D2 supplementation was associated more closely with alterations in IgG levels compared with D3 supplementation (unpublished observations). Those observations are in line with the results of the present pilot trial, where D2 but not D3 supplementation tended to decrease IgG (Supporting information, Fig. S2). Conversely, the vast majority of significant immunological changes in this trial were observed in the combined group of patients receiving D2 or D3. We speculate that D2 and D3 may both have some immunomodulatory effects, possibly affecting different pathways with different magnitude, which would speak in favour of using a combination supplement. In this pilot trial the average D2 daily dose was almost double the D3 dose, due to the lower efficacy of D2 at increasing s25OHD concentration 26. Local immunomodulatory effects of vitamin D in the gut are well documented 51, and may partially explain the difference between the effect of D2 and D3 on IgG levels. Taken together, our results generate a hypothesis that D2 exerts some immunomodulatory effect when given in high doses despite being less efficient at improving vitamin D status. Larger studies are needed to test this hypothesis. Conversely, only patients receiving D3 and not patients receiving D2 displayed decreased PD‐1 expression on CD8 T cells (Fig. 5f). We speculate that this might be explained by the fact that D2 supplementation was less efficient at increasing s25OHD, as only patients supplemented with D3 increased tot‐s25OHD levels significantly 26.
In a recent trial by Simoneau 15 there was a trend towards a reduction in IgE in CF patients receiving a weekly dose 50 000 IU D3, and low s25OHD has been shown to be associated with high IgE concentrations 52. This is in line with the results of this pilot trial, where we observed a negative correlation between changes in IgE and changes in free‐s25OHD (Fig. 3d), as well as between vitamin D doses and IgE (Fig. 3f). These findings suggest that vitamin D treatment may have a dose‐dependent negative effect on plasma total IgE levels. Future studies are needed to confirm this in a larger CF population, as well as to determine the clinical relevance of these findings.
In this pilot study, changes in s25OHD were associated negatively with changes in plasma LPS and positively with changes in sTREM‐1. Several in‐vitro and mouse studies have shown that vitamin D receptor signalling is crucial for mucosal barrier homeostasis 53, 54, 55. Moreover, active vitamin D abrogated the destructive effect of LPS on monolayer permeability by restoring tight junction proteins 56 and prevented airway epithelial barrier disruption 57. In light of this background, we speculate that vitamin D treatment in this study may have strengthened the airway epithelial barrier, thus decreasing LPS translocation from the lungs, which enabled TREM‐1 up‐regulation.
There are indications in the literature that there might be an association between vitamin D and MAIT cells 44, but this has not been investigated in more detail. In this trial, patients randomized to receive D2 or D3 vitamin tended to display decreased MAIT cell frequency at the end of intervention (Fig. 7c,d), and the change in free‐s25OHD was correlated negatively with the change in CD279 (PD‐1) expression on MAIT cells (Fig. 7e). We speculate that vitamin D treatment might enhance migration of activated MAIT cells to the lungs, decreasing their numbers as well as their exhaustion markers in the circulation.
Some of the immunological changes that were observed following vitamin D treatment were short‐lived, returning to baseline levels during the washout period. This was evident for the frequency of CD8+ T cells co‐expressing CD38 and HLA‐DR (Fig. 5c), as well as the total frequency of HLA‐DR+ CD8 T cells (Fig. 5a). Conversely, other parameters, such as CD279 (PD‐1) expression on CD8+ T cells (Fig. 5b) or IL‐8 26, remained down‐regulated throughout the washout period, despite the fact that vitamin D levels dropped during washout. This underscores that vitamin D has pleiotropic effects affecting numerous arms of the immune system and that its impact on these arms may be short or sustained for longer time‐periods.
In summary, this pilot trial indicates that vitamin D supplementation may modulate immune activation in CF in a complex manner. It also suggests that vitamin D2 may be a non‐inferior substitute for the currently recommended vitamin D3, if given in higher doses. This study has generated novel observations that need to be follow‐ up in larger long‐term placebo‐controlled studies.
Disclosure
The authors have nothing to disclose.
Clinical trial registration
The study was registered at ClinicalTrials.gov as NCT01321905.
Author contributions
T. P. and L. H. designed the study and delineated hypotheses. T. P. collected the data, analysed and interpreted them, with contributions from L. H., D. P. P., M. F. T. and J. S. J. S. and D. P. P. had primary responsibility for design of the flow cytometric panels. T. P. and D. P. P. performed the experiments. T. P. wrote the paper; T. P. and L. H. had primary responsibility for final content. All authors read and approved the final manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Albumin‐corrected plasma calcium concentration increased at 1 week of supplementation and at the end of the study in the vitamin D3 group (P < 0·05).
Fig. S2. Vitamin D2 supplementation tended to decrease serum total immunoglobulin (Ig)G concentration at 8 weeks of supplementation (P = 0·068). Results are plotted as raw data from subjects in the vitamin D2 treatment group.
Fig. S3. The frequency of CD4+ T cells co‐expressing CD38 and human leucocyte antigen D‐related (HLA‐DR) was not affected by vitamin D supplementation (not significant). Results are plotted as raw data from the subjects in the pooled vitamin D2 and vitamin D3 treatment group.
Fig. S4. Vitamin D does not influence invariant natural killer T cell frequency. Gating strategy for invariant natural killer T cells (iNK T) cells (Vα24+ Vβ11+) is shown in (a). The frequency of iNK T cells did not change upon vitamin D supplementation (not significant) in the present study (b). Results are plotted as raw data from the subjects in the pooled vitamin D2 and vitamin D3 treatment group (b).
Table S1. Effect of vitamin D treatment on soluble immunological parameters.
Table S2. Effect of vitamin D treatment on peripheral blood leucocyte counts.
Acknowledgements
This study was supported by the Karolinska Institutet, the Stockholm County Council, Stiftelsen Samariten, Erica Lederhausens Minnesstiftelse, the Swedish Cystic Fibrosis Association, Stiftelsen Frimurare Barnhuset i Stockholm, the Swedish Research Council, the Heart and Lung Foundation and the Swedish Cancer Society. D. P.‐P. is the recipient of a postdoctoral fellowship from the Canadian Institutes of Health Research.
Data from this paper were presented at a meeting: 36th European Cystic Fibrosis Conference, Lisbon, Portugal, 14 June 2013, Abstract WS16.3.
References
- 1. Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med 2012; 18:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Rose V. Mechanisms and markers of airway inflammation in cystic fibrosis. Eur Respir J 2002; 19:333–40.] [DOI] [PubMed] [Google Scholar]
- 3. Siegmann N, Worbs D, Effinger F et al Invariant natural killer T (iNKT) cells prevent autoimmunity, but induce pulmonary inflammation in cystic fibrosis. Cell Physiol Biochem 2014; 34:56–70. [DOI] [PubMed] [Google Scholar]
- 4. Tiringer K, Treis A, Fucik P et al A Th17‐ and Th2‐skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2013; 187:621–9. [DOI] [PubMed] [Google Scholar]
- 5. Kushwah R, Gagnon S, Sweezey NB1. Intrinsic predisposition of naïve cystic fibrosis T cells to differentiate towards a Th17 phenotype. Respir Res 2013; 14:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torphy TJ, Allen J, Cantin AM et al Considerations for the conduct of clinical trials with antiinflammatory agents in cystic fibrosis. A cystic fibrosis foundation workshop report. Ann Am Thorac Soc 2015; 12:1398–406. [DOI] [PubMed] [Google Scholar]
- 7. Cheng K, Ashby D, Smyth RL. Oral steroids for long‐term use in cystic fibrosis. Cochrane Database Syst Rev 2015; CD000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bscheider M, Butcher EC. Vitamin D immunoregulation through dendritic cells. Immunology 2016; 148:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griffin MD, Kumar R. Effects of 1alpha,25(OH)2D3 and its analogs on dendritic cell function. J Cell Biochem 2003; 88:323–6. [DOI] [PubMed] [Google Scholar]
- 10. van Etten E, Mathieu C. Immunoregulation by 1,25‐dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 2005; 97:93–101. [DOI] [PubMed] [Google Scholar]
- 11. Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015; 7:3011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pincikova T, Nilsson K, Moen IE et al Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr 2011; 65:102–9. [DOI] [PubMed] [Google Scholar]
- 13. Tangpricha V, Kelly A, Stephenson A et al An update on the screening, diagnosis, management, and treatment of vitamin D deficiency in individuals with cystic fibrosis: evidence‐based recommendations from the Cystic Fibrosis Foundation. J Clin Endocrinol Metab 2012; 97:1082–93. [DOI] [PubMed] [Google Scholar]
- 14. Sermet‐Gaudelus I, Bianchi ML, Garabédian M et al European Cystic Fibrosis Bone Mineralisation guidelines. J Cyst Fibros 2011; 10: S16–23. [DOI] [PubMed] [Google Scholar]
- 15. Simoneau T, Sawicki GS, Milliren CE, Feldman HA, Gordon CM. A randomized controlled trial of vitamin D replacement strategies in pediatric CF patients. J Cyst Fibros 2016; 15:234–41. [DOI] [PubMed] [Google Scholar]
- 16. Dauletbaev N, Herscovitch K, Das M et al Down‐regulation of IL‐8 by high‐dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up‐regulation of DUSP1. Br J Pharmacol 2015; 172:4757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schögler A, Muster RJ, Kieninger E et al Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL‐37. Eur Respir J 2016; 47:520–30. [DOI] [PubMed] [Google Scholar]
- 18. Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr 2012; 66:1072–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levy H, Kalish LA, Huntington I et al Inflammatory markers of lung disease in adult patients with cystic fibrosis. Pediatr Pulmonol 2007; 42:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chesdachai S, Tangpricha V. Treatment of vitamin D deficiency in cystic fibrosis. J Steroid Biochem Mol Biol 2016; 164:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. del Campo R, Martínez E, del Fresno C et al Translocated LPS might cause endotoxin tolerance in circulating monocytes of cystic fibrosis patients. PLOS ONE 2011; 6:e29577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ngan DA, Wilcox PG, Aldaabil M et al The relationship of systemic inflammation to prior hospitalization in adult patients with cystic fibrosis. BMC Pulm Med 2012; 12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilcox PG, Aldaabil M, Ngan DA et al Elevated plasma lipopolysaccharide levels are associated with more frequent hospitalizations in adult cystic fibrosis patients. Am J Respir Crit Care Med 2011; 183:A1117. [Google Scholar]
- 24. del Fresno C, Gomez‐Pina V, Lores V et al Monocytes from cystic fibrosis patients are locked in an LPS tolerance state: down‐regulation of TREM‐1 as putative underlying mechanism. PLOS ONE 2008; 3:e2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rigo I, McMahon L, Dhawan P et al Induction of triggering receptor expressed on myeloid cells (TREM‐1) in airway epithelial cells by 1,25(OH)(2) vitamin D. Innate Immun 2012; 18:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pincikova T, Paquin‐Proulx D, Sandberg JK, Flodström‐Tullberg M, Hjelte L. Clinical impact of vitamin D treatment in cystic fibrosis: a pilot randomized, controlled trial. Eur J Clin Nutr 2017; 71:203–205. [DOI] [PubMed] [Google Scholar]
- 27. Emmett M, Miller JL, Crowle AJ. Protein abnormalities in adult respiratory distress syndrome, tuberculosis, and cystic fibrosis sera. Proc Soc Exp Biol Med 1987; 184:74–82. [DOI] [PubMed] [Google Scholar]
- 28. Sagel SD, Thompson V, Chmiel JF et al Effect of treatment of cystic fibrosis pulmonary exacerbations on systemic inflammation. Ann Am Thorac Soc 2015; 12:708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valletta EA, Rigo A, Bonazzi L, Zanolla L, Mastella G. Modification of some markers of inflammation during treatment for acute respiratory exacerbation in cystic fibrosis. Acta Paediatr 1992; 81:227–30. [DOI] [PubMed] [Google Scholar]
- 30. Proesmans M, Els C, Vermeulen F, De Boeck K. Change in IgG and evolution of lung function in children with cystic fibrosis. J Cyst Fibros 2011; 10:128–31. [DOI] [PubMed] [Google Scholar]
- 31. van de Weert‐van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CL, van der Ent CK, Arets HG. Chronic infection and inflammation affect exercise capacity in cystic fibrosis. Eur Respir J 2012; 39:893–8. [DOI] [PubMed] [Google Scholar]
- 32. Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med 1994; 150:448–54. [DOI] [PubMed] [Google Scholar]
- 33. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25‐dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007; 179:1634–47. [DOI] [PubMed] [Google Scholar]
- 34. Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha,25‐dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest 1984; 74:657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferreira GB, Overbergh L, Verstuyf A, Mathieu C. 1α,25‐Dihydroxyvitamin D3 and its analogs as modulators of human dendritic cells: a comparison dose‐titration study. J Steroid Biochem Mol Biol 2013; 136:160–5. [DOI] [PubMed] [Google Scholar]
- 36. Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25‐hydroxyvitamin D3–1 alpha‐hydroxylase and production of 1 alpha,25‐dihydroxyvitamin D3 by human dendritic cells. Blood 2003; 102:3314–6. [DOI] [PubMed] [Google Scholar]
- 37. Penna G, Adorini L. 1 Alpha,25‐dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 2000; 164:2405–11. [DOI] [PubMed] [Google Scholar]
- 38. Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor‐dependent pathway that promotes a persistent state of immaturity in vitro and in vivo . Proc Natl Acad Sci USA 2001; 98:6800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paquin‐Proulx D, Santos BA, Carvalho KI et al Dysregulated CD1 profile in myeloid dendritic cells in CVID is normalized by IVIg treatment. Blood 2013; 121:4963–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci 2014; 1317:70–5. [DOI] [PubMed] [Google Scholar]
- 41. Napier RJ, Adams EJ, Gold MC, Lewinsohn DM. The role of mucosal associated invariant T cells in antimicrobial immunity. Front Immunol 2015; 6:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci USA 2013; 110:E3119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith DJ, Hill GR, Bell SC, Reid DW. Reduced mucosal associated invariant T‐cells are associated with increased disease severity and Pseudomonas aeruginosa infection in cystic fibrosis. PLoS One 2014; 9:e109891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hinks TS, Zhou X, Staples KJ et al Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J Allergy Clin Immunol 2015; 136:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nieuwenhuis EE, Matsumoto T, Exley M et al CD1d‐dependent macrophage‐mediated clearance of Pseudomonas aeruginosa from lung. Nat Med 2002; 8:588–93. [DOI] [PubMed] [Google Scholar]
- 46. Sada‐Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis‐infected macrophages, produce interferon‐gamma, and kill intracellular bacteria. PLOS Pathog 2008; 4:e1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci USA 2008; 105:5207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu S, Cantorna MT. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol 2011; 186:1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dyring‐Andersen B, Bonefeld CM, Bzorek M et al The vitamin D analogue calcipotriol reduces the frequency of CD8+ IL‐17+ T cells in psoriasis lesions. Scand J Immunol 2015; 82:84–91. [DOI] [PubMed] [Google Scholar]
- 50. Chen J, Bruce D, Cantorna MT. Vitamin D receptor expression controls proliferation of naïve CD8+ T cells and development of CD8 mediated gastrointestinal inflammation. BMC Immunol 2014; 15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li YC, Chen Y, Du J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J Steroid Biochem Mol Biol 2015; 148:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hyppönen E, Berry DJ, Wjst M, Power C. Serum 25‐hydroxyvitamin D and IgE – a significant but nonlinear relationship. Allergy 2009; 64:613–20. [DOI] [PubMed] [Google Scholar]
- 53. Kong J, Zhang Z, Musch MW et al Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008; 294:G208–16. [DOI] [PubMed] [Google Scholar]
- 54. Firrincieli D, Zúñiga S, Rey C et al Vitamin D nuclear receptor deficiency promotes cholestatic liver injury by disruption of biliary epithelial cell junctions in mice. Hepatology 2013; 58:1401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu W, Chen Y, Golan MA et al Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 2013; 123:3983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen SW, Wang PY, Zhu J et al Protective effect of 1,25‐dihydroxyvitamin d3 on lipopolysaccharide‐induced intestinal epithelial tight junction injury in caco‐2 cell monolayers. Inflammation 2015; 38:375–83. [DOI] [PubMed] [Google Scholar]
- 57. Li W, Dong H, Zhao H et al 1,25‐Dihydroxyvitamin D3 prevents toluene diisocyanate‐induced airway epithelial barrier disruption. Int J Mol Med 2015; 36:263–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Albumin‐corrected plasma calcium concentration increased at 1 week of supplementation and at the end of the study in the vitamin D3 group (P < 0·05).
Fig. S2. Vitamin D2 supplementation tended to decrease serum total immunoglobulin (Ig)G concentration at 8 weeks of supplementation (P = 0·068). Results are plotted as raw data from subjects in the vitamin D2 treatment group.
Fig. S3. The frequency of CD4+ T cells co‐expressing CD38 and human leucocyte antigen D‐related (HLA‐DR) was not affected by vitamin D supplementation (not significant). Results are plotted as raw data from the subjects in the pooled vitamin D2 and vitamin D3 treatment group.
Fig. S4. Vitamin D does not influence invariant natural killer T cell frequency. Gating strategy for invariant natural killer T cells (iNK T) cells (Vα24+ Vβ11+) is shown in (a). The frequency of iNK T cells did not change upon vitamin D supplementation (not significant) in the present study (b). Results are plotted as raw data from the subjects in the pooled vitamin D2 and vitamin D3 treatment group (b).
Table S1. Effect of vitamin D treatment on soluble immunological parameters.
Table S2. Effect of vitamin D treatment on peripheral blood leucocyte counts.


