Abstract
Recent studies have indicated the clinical significance of tumor‐associated macrophages (TAM) in several malignant tumors including breast cancer. Although recent studies have focused on CD68‐positive or CD163‐positive TAM in breast cancer, no study has investigated the significance of CD204‐positive TAM in breast cancer. We found that CD204 expression on macrophages was evaluated following stimulation with the conditioned medium (CM) of breast cancer cell lines. Paraffin sections of 149 breast cancer samples which were diagnosed as invasive ductal carcinoma were immunohistochemically analyzed for CD68, CD163 and CD204 expression. The results of analyses indicated that a high number of CD204‐positive TAM was associated with worse clinical prognoses, including relapse‐free survival, distant relapse‐free survival and breast cancer‐specific survival; however, neither the numbers of CD68‐positive or CD163‐positive TAM were associated with clinical courses. Of the clinicopathological factors investigated, estrogen receptor, Ki‐67 index, hormone subtype, and histological grade were significantly related to the increased number of CD163‐positive and CD204‐positive TAM. These data indicate the clinical significance of CD204‐positive TAM in breast cancer progression and CD204 is a marker for predicting clinical prognosis in breast cancer.
Keywords: CD163, CD204, Ki‐67, macrophage, TAM
Invasive breast cancer is the most common cancer in women and accounts for 23% of all cancers in women.1, 2 Primary breast cancer exhibits many differences in morphology and in the expression of clinical biomarkers, such as estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth receptor 2 (HER2).3, 4 Such heterogeneity affects the complexity of pathological diagnosis and treatment protocols.
Tumor tissues, including breast cancer, consist not only of cancer cells but also of host‐derived normal cells, such as lymphocytes, fibroblasts and macrophages.5 Macrophages that infiltrate in tumor tissues are called tumor‐associated macrophages (TAM), and activated TAM are known to secrete several kinds of pro‐tumor molecules and EGFR ligands, including epidermal growth factor, heparin‐binding EGF‐like growth factor and oncostatin M.6, 7 A higher density of infiltrating CD68+ macrophages was shown to correlate well with an increased number of vessels and a poor clinical course in patients with breast cancer.8 Conversely, depletion of macrophages abrogated neovascularization in a murine model of breast cancer.9 Since the study of Pollard et al., which demonstrated that breast cancer metastasis was significantly abrogated in macrophage‐deficient Csf1op/Csf1op (op/op) mice,10 macrophage activation has been found to contribute to breast cancer progression and metastasis by means of many studies using gene engineered mice.11, 12
The receptor CD204 (scavenger receptor class A [SR‐A]) is specifically expressed on macrophages, and recent studies have demonstrated that high expression of CD204 is observed on M2‐like pro‐tumor macrophages.13, 14, 15 M2‐like macrophages have pro‐tumor characteristics that involve the production of angiogenic factors and immunosuppressive molecules.11, 12, 13 The progression and metastasis of ovarian cancer and pancreatic cancer were significantly inhibited in CD204‐deficient mice, and CD204‐deficient macrophages showed lower pro‐tumor activity than that of CD204‐positive macrophages.16, 17 Macrophages derived from CD204‐deficient mice showed anti‐tumor activity through the secretion of nitric oxide and interferon,17 and targeting of CD204‐positive macrophages abrogated ovarian cancer progression.18 These findings indicated that CD204 is involved in the pro‐tumor activation of macrophages.
In the present study, we found that CD204 expression on human monocyte‐derived macrophages was significantly increased by co‐culture with the conditioned medium (CM) of breast cancer cell lines, although other macrophage‐specific receptors, such as CD163 and CD206, were little changed by such co‐culture. This observation suggested the significance of CD204 expression on TAM in breast cancer. However, few studies have investigated CD204 in breast cancer. Therefore, we evaluated the relationship between CD204 expression on TAM and clinicopathological factors in patients with invasive breast cancer.
Materials and Methods
Breast cancer cell lines and conditioned medium
Three human breast cancer cell lines (MCF7, MDA‐MB‐453 and OCUB‐M) were obtained from the RIKEN Cell Bank (Tsukuba, Japan). All cells were cultured in DMEM/Ham's F‐12 (Wako, Japan) with 10% FBS. The CM of all three cell lines was collected as previously described.14
Macrophage culture
Peripheral blood mononuclear cells (PBMC) were obtained from three healthy voluntary donors in accordance with protocols approved by the Kumamoto University Hospital Review Board. CD14+ monocytes were isolated by using CD14‐microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). These monocytes were plated in 6‐well plates (2 × 105/well) and were cultured with 2% human serum, granulocyte macrophage‐colony stimulating factor (1 ng/mL, GM‐CSF, WAKO, Tokyo, Japan) and macrophage‐colony stimulating factor (100 ng/mL, M‐CSF, WAKO) for 7 days to induce differentiated macrophages.
Western blot analysis
The macrophages were stimulated with IL‐4 (20 ng/mL, WAKO), IL‐10 (20 ng/mL, WAKO) or CM of breast cancer cell lines (concentration: 50%) for 48 h. Then the macrophages were collected, and cellular proteins were solubilized in Tris buffer containing 2% SDS and 10% glycerol. The amount of protein was quantified using the bicinchoninic acid assay. Equal amounts of protein were then separated on SDS‐PAGE, and were subsequently transferred to a polyvinylidene fluoride membrane. The following mouse antibodies were used for western blotting: anti‐CD204 (SRA‐E5; TransGenic, Kumamoto, Japan), anti‐CD163 (PM‐2K; TransGenic) and anti‐CD206 (5C11; Acris Antibodies, San Diego, CA, USA). For immunoblotting of CD204, cell lysates were first pretreated with N‐glycosidase (Roche, Basel, Switzerland). The membranes were re‐blotted with an anti‐β‐actin antibody as an internal control.
Tissue samples
Paraffin‐embedded tumor samples from 149 patients who were diagnosed with invasive ductal carcinoma from 2001 to 2012 in Kumamoto University Hospital were examined. Cases were classified as Luminal A/B‐like, Her 2‐positive and triple negative groups by means of immunostaining, as described previously,19 and cases of Luminal A‐like and Luminal B‐like were classified as Luminal‐like group. All samples were obtained with informed consent from patients in accordance with protocols approved by the Kumamoto University Hospital Review Board. Tissue samples were fixed in 10% neutral buffered formalin and were embedded in paraffin using routine methods, and tissue sections of the largest cross‐sectional area (one slide for each case) and no tissue microarray samples were used for this study.
Immunohistochemistry
Sections (3‐μm thick) obtained from paraffin‐embedded tumor samples were immunohistochemically stained. The following mouse monoclonal antibodies were used: anti‐CD204 (SRA‐E5; TransGenic), anti‐CD163 (10D6; Novocastra, Newcastle, UK), anti‐CD68 (PG‐M1; DAKO, Glostrup, Denmark) and anti‐Ki‐67(MIB‐1; DAKO, Glostrup, Denmark).20 After the samples were reacted with these first antibodies, the samples were incubated with HRP‐labeled secondary anti‐mouse antibody (Nichirei, Tokyo, Japan). The reaction was visualized using the Diaminobenzidine (DAB) system (Nichirei). Normal mouse immunoglobulin (DAKO) was used as a negative control and no signal was observed in these sections. CD68‐, CD163‐ and CD204‐positive cells were counted in four randomly selected areas of a high power field (×200) of a microscope by two pathologists (S.T. and M.Y.) who were blinded to information about the patients’ backgrounds or their prognosis. The data of the Ki‐67 labeling index was previously counted by our research group.21, 22 Double immunostaining of CD204, CD68, CD163 and Ki‐67 was performed as previously described.14 In brief, the sections were reacted with anti‐Ki‐67, CD163 or CD204 antibodies and visualized with DAB. After the sections were washed with citrate buffer (pH 2.2), they were reacted with anti‐CD68 or CD204 antibodies and visualized with HistoGreen (Linaris, Heiderberg, Germany).
Statistical analysis
Statistical analysis was carried out by using JMP10 (SAS Institute, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Conditioned medium of breast cancer cell lines upregulated CD204 expression on macrophages
We first tested whether incubation of macrophages with the CM of breast cancer cell lines could influence the expression of M2 macrophage markers such as CD163, CD204 and CD206. Western blot analysis indicated that CD204 expression was upregulated by the CM of all three breast cancer cell lines. The most significant change was induced by the CM of OCUB‐M cells (P = 0.014; Fig. 1a,b, n = 3). Neither CD163 nor CD206 expression was influenced by these CM, although IL‐4 and IL‐10 did induce CD206 and CD163, respectively (Fig. 1a).
Figure 1.
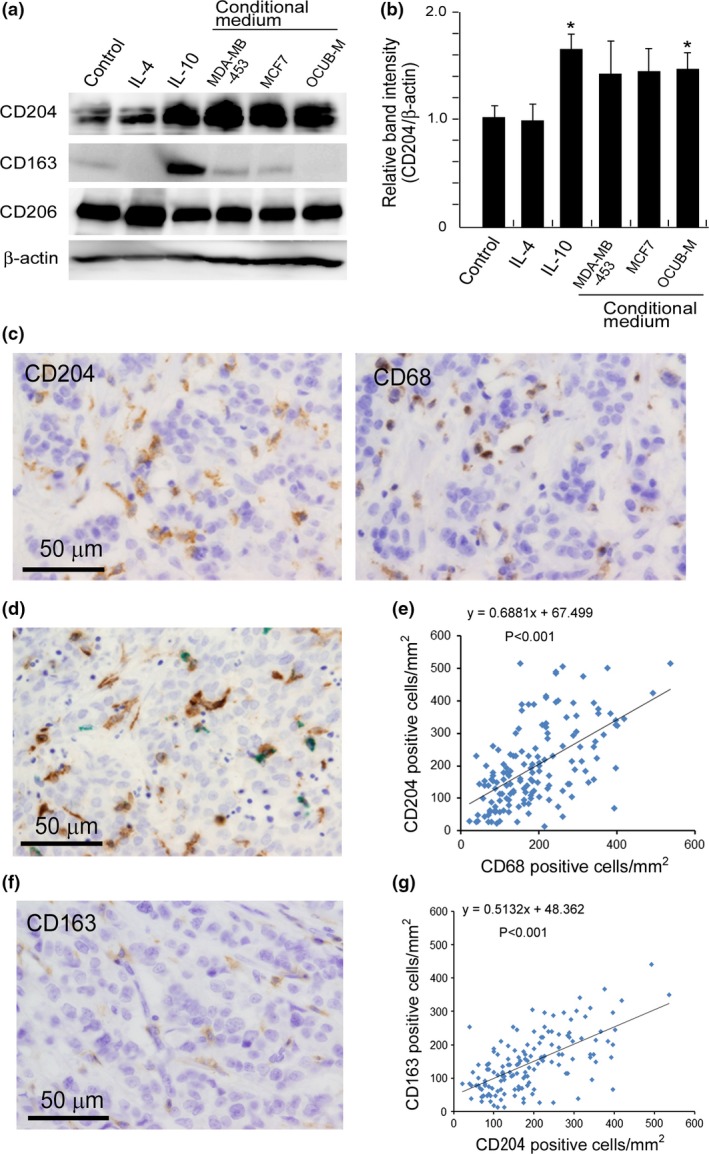
The expression of CD163, CD204 and CD206 on macrophages. (a) Human monocyte‐derived macrophages were not stimulated (control) or were stimulated with IL‐4, IL‐10 or the conditioned media (CM) of three breast cancer cell lines, and the protein expression of CD204, CD163 and CD206 was evaluated using western blot analysis. β‐actin was assayed as a loading control. (b) The band intensity of CD204 and β‐actin in (a) was evaluated using Image J software (http://imagej.nih.gov/ij/). The calculated data were statistically analyzed using Student's t‐test. *P‐value <0.05. (c) Representative immunohistochemical staining of CD68 and CD204 in the same area of a breast cancer sample, and (d) double‐immunostaining of CD68 (green) and CD204 (brown) are shown. (e) The relationship between the density of CD68‐positive and CD204‐positive macrophages was evaluated using Spearman's correlation test. (f) Immunostaining of CD163 in the same area of the breast cancer sample shown in (c) is presented. (g) The relationship between the density of CD163‐positive and CD204‐positive macrophages was evaluated using Spearman's correlation test.
Density of CD204‐positive macrophages was higher than that of CD163‐positive macrophages in breast cancer tissues
CD68 is a well‐established pan‐macrophage marker, and some studies have shown that a high density of CD68‐positive macrophages correlates well with poor clinical prognosis.8 Therefore, we next investigated the relationship between CD68‐positive and CD204‐positive macrophages using invasive breast cancer cases. Immunostaining of CD68 and CD204 was performed on serial sections of breast cancer tissues and the density of CD68‐positive and CD204‐positive macrophages were counted in the same area (Fig. 1c). Double‐immunostaining showed that some of the CD204‐positive macrophages expressed CD68, whereas others express low levels of macrophages (Fig. 1d). The densities of CD68‐positive and CD204‐positive macrophages were well correlated; however, there seemed to be a higher density of CD204‐positive macrophages (mean: 205 cells/mm2) than of CD68‐positive macrophages (mean: 191 cells/mm2) (Fig. 1e). No increase in CD163 was observed on macrophages following incubation with breast cancer cell CM (Fig. 1a); however, CD163 expression on macrophages that have infiltrated breast cancer tissues has been reported.21 We next investigated the correlation between CD204 expression and CD163 expression in all cases. In immunostaining, the signal intensity of CD163 expression was weak compared with that of CD204 expression (Fig. 1f), and the density of CD163‐positive macrophages (mean: 146 cells/mm2) was lower than that of CD204‐positive macrophages, as shown in Figure 1g.
Higher density of CD204‐positive macrophages was associated with triple‐negative cancer cells and a higher Ki‐67 labeling index
We next investigated the significance of CD68‐, CD163‐ and CD204‐positive macrophages, as assessed by immunohistochemistry. After the density of macrophages was counted, the cases were divided into two groups (High and Low), as shown in Table 1. Statistical analysis showed that higher densities of CD68‐, CD163‐ and CD204‐positive macrophages were significantly associated with higher histological grade (P < 0.01; P = 0.01; P < 0.01, respectively) or ER‐negative status (CD204; P = 0.03; CD163; P = 0.04, respectively). Higher density of CD204‐positive macrophages was observed in triple negative patient groups than in the Luminal‐like group. The densities of CD68‐, CD163‐ and CD204‐positive macrophages were not correlated with age, menopausal status, tumor size or lymph node metastasis. Because a higher density of Ki‐67‐positive cancer cells was preferentially detected in the high CD204 group (Fig. 2a), we then tested the relationship of the densities of CD68‐, CD163‐ and CD204‐positive macrophages to the Ki‐67 labeling index. Double‐immunostaining of CD204 and Ki‐67 showed that no Ki‐67‐positive macrophages were observed in cancer tissues (Fig. 2b). Higher densities of CD163‐positive and CD204‐positive macrophages were interestingly associated with higher Ki‐67 labeling index (Fig. 2c,d and Table 1); however, there was no significant association between the number of CD68‐positive macrophages and Ki‐67 labeling index (Fig. 2e). The density of CD204‐positive macrophages was more closely related to the Ki‐67 labeling index than that of CD163‐positive macrophages.
Table 1.
Correlations between the density of tumor‐associated macrophages (TAM) and clinicopathological factors
| Clinicopathological feature | The density of CD204‐positive TAM (/mm2) | The density of CD163‐positive TAM (/mm2) | The density of CD68‐positive TAM (/mm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ≤200 | >200 | P‐value | ≤145 | >145 | P‐value | ≤190 | >190 | P‐value | ||
| Age | <50 | 36 | 21 | 15 | 0.49 | 17 | 19 | 0.16 | 21 | 15 | 0.47 |
| ≥50 | 113 | 66 | 47 | 64 | 49 | 65 | 48 | ||||
| Menopause | Pre‐ | 43 | 24 | 19 | 0.34 | 22 | 21 | 0.31 | 24 | 19 | 0.38 |
| Post‐ | 106 | 63 | 43 | 59 | 47 | 62 | 44 | ||||
| Tumor size | <2 cm | 62 | 35 | 27 | 0.33 | 35 | 27 | 0.39 | 33 | 29 | 0.13 |
| ≥2 cm | 85 | 51 | 34 | 46 | 39 | 53 | 32 | ||||
| LN metastasis | Negative | 95 | 59 | 36 | 0.13 | 54 | 41 | 0.25 | 55 | 40 | 0.47 |
| Positive | 53 | 28 | 25 | 27 | 26 | 31 | 22 | ||||
| Histological grade | 1 | 45 | 34 | 11 | <0.01† | 30 | 15 | 0.01† | 33 | 12 | <0.01† |
| 2 | 65 | 38 | 27 | 35 | 30 | 35 | 30 | ||||
| 3 | 34 | 10 | 24 | 11 | 23 | 13 | 21 | ||||
| ER | Negative | 34 | 15 | 19 | 0.03† | 14 | 20 | 0.04† | 17 | 17 | 0.15 |
| Positive | 115 | 72 | 43 | 67 | 48 | 69 | 46 | ||||
| PgR | Negative | 59 | 30 | 29 | 0.06 | 30 | 29 | 0.24 | 33 | 26 | 0.34 |
| Positive | 90 | 57 | 33 | 51 | 39 | 53 | 37 | ||||
| HER2 | Negative | 126 | 73 | 53 | 0.39 | 69 | 57 | 0.41 | 72 | 54 | 0.37 |
| Positive | 23 | 14 | 9 | 12 | 11 | 14 | 9 | ||||
| Ki67 | <20% | 47 | 36 | 11 | <0.01† | 32 | 15 | 0.01† | 29 | 18 | 0.23 |
| ≥20% | 98 | 48 | 50 | 46 | 52 | 54 | 44 | ||||
| Subtype | Luminal | 108 | 68 | 40 | <0.01† , ‡ | 63 | 45 | 0.14 | 64 | 44 | 0.49 |
| HER2 | 23 | 14 | 9 | 12 | 11 | 14 | 9 | ||||
| TN | 18 | 5 | 13 | 6 | 12 | 8 | 10 | ||||
TN, triple negative. †Statistically significant. Statistical analysis was performed by χ2‐test and Kruskal–Wallis H‐test. ‡Compared with triple negative group.
Figure 2.
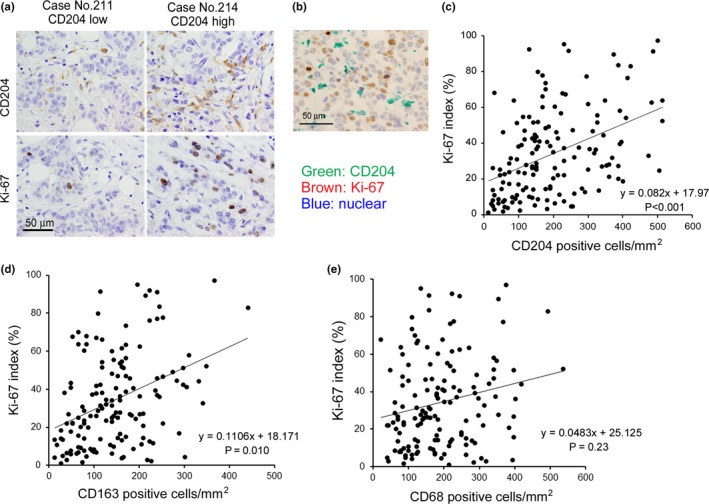
Correlation between the density of CD204‐positive macrophages and tumor cell proliferation. (a) The immunostaining data of two cases (a high CD204 case and a low CD204 case) are shown. Serial sections of cancer tissues were stained with the anti‐CD204 antibody and the anti‐Ki‐67 antibody, and pictures of the same area are shown. (b) Double immunostaining of CD204 and Ki‐67 in a representative breast cancer sample was performed in a high CD204 case. (c) The correlation between the density of CD204‐positive macrophages and the Ki‐67 index was evaluated using Spearman's correlation test. The correlations between the density of CD163‐positive macrophages and the Ki‐67 index (d) and between the density of CD68‐positive macrophages and the Ki‐67 index (e) were tested using Spearman's correlation test.
Higher density of CD204‐positive macrophages was associated with poor clinical prognosis
Next, the correlation between the densities of CD68‐, CD163‐ and CD204‐positive macrophages and clinical prognosis was statistically evaluated. A higher density of CD204‐positive macrophages was significantly associated with poor clinical prognosis, including relapse‐free survival (RFS), distant RFS (DRFS) and breast cancer‐specific survival (BCSS) (RFS, P = 0.001; DRFS, P < 0.001; BCSS, P < 0.001, respectively; Fig. 3a, Tables 2, 3, 4). The ratio of CD204‐positive macrophages (CD204+ cells/CD68+ cells) was also associated with DRFS and BCSS; however, more significant association was observed in the density of CD204‐positive macrophages and clinical course. In contrast, the densities of CD68‐positive and CD163‐positive macrophages had little association with the clinical course (Tables 2, 3, 4). Multivariate analysis showed that the density of CD204‐positive macrophages was an independent poor prognostic factor for RFS, DRFS and BCSS (RFS, P = 0.006; DRFS, P = 0.001; BCSS, P < 0.001, respectively; Tables 2, 3, 4). Because the cases shown in Figure 3a are comprised of three breast cancer subtypes (Luminal‐like, Her2‐positive and Triple negative), a similar analysis was performed for the 108 Luminal‐like cases. In these cases, the density of CD204‐positive macrophages was also significantly correlated with RFS and DRSF; however, there was no significant association between the density of CD204‐positive macrophages and BCSS (RFS, P = 0.045; DRFS, P = 0.013; BCSS, P = 0.10, respectively; Fig. 3b). Although similar associations with survival rate were found in triple negative cases, there was no statistically significant association between the density of CD204‐positive macrophages and clinical prognosis because of the low number of cases (data not shown).
Figure 3.
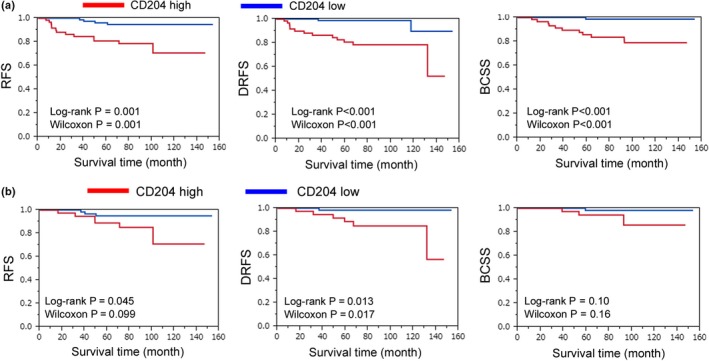
Correlation between the density of CD204‐positive macrophages and clinical prognosis. (a,b) Kaplan–Meyer analysis of relapse‐free survival (RFS), distant relapse‐free survival (DRFS) and breast cancer‐specific survival (BCSS) of the CD204 low density (CD204 low) and high density (CD204 high) subgroups of patients with CD204‐positive macrophages. A total of 149 patients with total invasive ductal carcinoma (a) and 108 patients with Luminal‐like type (b) were analyzed.
Table 2.
Univariate analysis and multivariate analysis in relapse free survival
| Variables | Reference | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P‐value | HR | 95% CI | P‐value | HR | 95% CI | |||||
| Lower | Upper | Lower | Upper | |||||||
| CD204 | Low vs high | Low | 0.001a | 5.35 | 1.89 | 19.01 | 0.006a | 4.61 | 1.51 | 17.29 |
| CD163 | Low vs high | Low | 0.892 | 1.07 | 0.40 | 2.80 | ||||
| CD68 | Low vs high | Low | 0.599 | 1.29 | 0.48 | 3.38 | ||||
| CD204/CD68 | Low vs high | Low | 0.054 | 2.56 | 0.98 | 7.05 | ||||
| CD163/CD68 | Low vs high | Low | 0.342 | 0.61 | 0.19 | 1.65 | ||||
| Ki67 | >20% vs ≦20% | ≦20% | 0.147 | 2.34 | 0.76 | 10.15 | ||||
| Age | <50, ≥50 | <50 | 0.629 | 0.62 | 0.23 | 1.83 | ||||
| Menopause | Pre vs post | Pre | 0.365 | 0.63 | 0.24 | 1.75 | ||||
| Tumor size | ≦2 cm vs >2 cm | ≦2 cm | 0.318 | 1.69 | 0.61 | 5.36 | ||||
| LN metastasis | Positive vs negative | Negative | 0.247 | 1.79 | 0.65 | 4.87 | ||||
| HG | 1,2 vs 3 | 1 + 2 | 0.035a | 2.89 | 1.08 | 7.58 | 0.978 | 1.02 | 0.29 | 3.36 |
| ER | Negative vs positive | Negative | 0.011a | 0.27 | 0.10 | 0.74 | 0.049a | 0.31 | 0.10 | 0.99 |
| PgR | Negative vs positive | Negative | 0.476 | 0.70 | 0.26 | 1.88 | ||||
| Her2 | Negative vs positive | Negative | 0.883 | 1.1 | 0.25 | 3.37 | ||||
Statistically significant.
Table 3.
Univariate analysis and multivariate analysis in distant relapse free survival
| Variables | Reference | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P‐value | HR | 95% CI | P‐value | HR | 95% CI | |||||
| Lower | Upper | Lower | Upper | |||||||
| CD204 | Low vs high | Low | <0.001a | 10.24 | 2.82 | 65.54 | 0.001a | 8.57 | 2.12 | 59.13 |
| CD163 | Low vs high | Low | 0.667 | 1.25 | 0.44 | 3.59 | ||||
| CD68 | Low vs high | Low | 0.728 | 1.20 | 0.42 | 3.34 | ||||
| CD204/CD68 | Low vs high | Low | 0.004a | 4.65 | 1.58 | 16.87 | ||||
| CD163/CD68 | Low vs high | Low | 0.504 | 0.69 | 0.21 | 1.97 | ||||
| Ki67 | >20% vs ≦20% | ≦20% | 0.021a | 6.31 | 1.25 | 114.65 | 0.567 | 1.83 | 0.28 | 35.69 |
| Age | <50, ≥50 | <50 | 0.944 | 1.04 | 0.35 | 3.80 | ||||
| Menopause | Pre vs post | Pre | 0.907 | 0.93 | 0.33 | 3.03 | ||||
| Tumor size | ≦2 cm vs >2 cm | ≦2 cm | 0.158 | 2.40 | 0.72 | 10.76 | ||||
| LN metastasis | Positive vs negative | Negative | 0.141 | 2.22 | 0.76 | 6.80 | ||||
| HG | 1,2 vs 3 | 1 + 2 | 0.007a | 4.25 | 1.50 | 12.8 | 0.946 | 0.96 | 0.27 | 3.58 |
| ER | Negative vs positive | Negative | 0.001a | 0.18 | 0.06 | 0.52 | 0.014a | 0.21 | 0.05 | 0.73 |
| PgR | Negative vs positive | Negative | 0.051 | 0.35 | 0.11 | 1.01 | ||||
| Her2 | Negative vs positive | Negative | 0.898 | 1.09 | 0.24 | 3.49 | ||||
Statistically significant.
Table 4.
Univariate analysis and multivariate analysis in breast cancer specific survival
| Variables | Reference | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P‐value | HR | 95% CI | P‐value | HR | 95% CI | |||||
| Lower | Upper | Lower | Upper | |||||||
| CD204 | Low vs high | Low | <0.001a | 16.00 | 3.06 | 293.66 | <0.001a | 15.87 | 2.74 | 302.04 |
| CD163 | Low vs high | Low | 0.989 | 1.01 | 0.29 | 3.35 | ||||
| CD68 | Low vs high | Low | 0.750 | 0.82 | 0.21 | 2.72 | ||||
| CD204/CD68 | Low vs high | Low | 0.002a | 8.04 | 2.07 | 52.76 | ||||
| CD163/CD68 | Low vs high | Low | 0.366 | 0.55 | 0.12 | 1.92 | ||||
| Ki67 | >20% vs ≦20% | ≦20% | 0.060 | 4.94 | 0.94 | 90.56 | ||||
| Age | <50, ≥50 | <50 | 0.551 | 1.56 | 0.40 | 10.24 | ||||
| Menopause | Pre vs post | Pre | 0.777 | 1.21 | 0.34 | 5.52 | ||||
| Tumor size | ≦2 cm vs >2 cm | ≦2 cm | 0.085 | 3.33 | 0.85 | 21.88 | ||||
| LN metastasis | Positive vs negative | Negative | 0.261 | 1.97 | 0.54 | 6.85 | ||||
| HG | 1,2 vs 3 | 1 + 2 | 0.031a | 3.75 | 1.13 | 13.04 | 0.449 | 0.57 | 0.14 | 2.53 |
| ER | Negative vs positive | Negative | <0.001a | 0.09 | 0.02 | 0.31 | 0.001a | 0.09 | 0.02 | 0.39 |
| PgR | Negative vs positive | Negative | 0.105 | 0.37 | 0.09 | 1.23 | ||||
| Her2 | Negative vs positive | Negative | 0.964 | 1.04 | 0.15 | 4.03 | ||||
Statistically significant.
Discussion
In the present study, we report a significant association between CD204‐positive macrophages and clinicopathological factors in patients with invasive breast cancer. Since the 1990s there have been several studies that have described a significant correlation between CD68‐positive macrophages and the clinical course of patients with invasive breast cancer.8 Although CD204 has been reported to be a marker for pro‐tumor macrophages in esophageal cancer, pancreatic cancer, glioma and lung cancers,14, 15, 23, 24, 25 no study of the relationship between CD204 and breast cancer samples has been published. Therefore, this study is the first report to describe the significance of CD204 in breast cancer.
In the present study, we first showed that CM of breast cancer cell lines significantly increased CD204 expression on macrophages; however, we have never identified the cancer‐cell derived molecules associated with upregulation of CD204. Breast cancer cells are known to secrete colony stimulating factor 1/2 and monocyte chemoattractant protein‐1, which is closely associated with macrophage activation.26 Cyr61 is known to be secreted from breast cancer cells,27 and cancer cell‐derived Cyr61 increased CD204 expression on macrophages via activation of MEK/ERK pathway.28 These molecules are suggested to be involved in CD204 overexpression in TAM.
CD68 is a widely‐used marker for pan‐macrophages, as described above. Our study demonstrated that the densities of CD68‐positive and CD204‐positive macrophages were very similar but the density of CD204‐postitive macrophages was slightly higher than that of CD68‐postitive macrophages. A similar observation was seen in our previous research related to human glioma.14, 29 Because CD68 is a lysosomal‐associated membrane protein, and not a cell membrane protein, this molecule seemed to be downregulated in some macrophages, which were possibly with less phagocytic features. It is also interesting to note that the Ki‐67 index of tumor cells was significantly associated with the density of CD204‐positive macrophages, but not with the density of CD68‐positive macrophages.
CD163 has also recently been found to be a marker for pro‐tumor macrophages in several kinds of malignant tumors,13, 30 and some studies have demonstrated the significance of CD163‐positive macrophages in invasive breast cancer.31, 32, 33 Macrophage expression of CD163 was previously shown to be induced by the CM of MDA‐MB231 cells, but not by the CM of MCF‐7 or T47D cells.31 MDA‐MB231 cells have been shown to have more stem‐cell like properties or mesenchymal differentiation than MCF‐7 cells.34 Cancer cells that display high stem‐cell properties were shown to significantly induce immunosuppressive M2 macrophages via soluble factors, including M‐CSF, TGF‐β and MIC‐1.35 The fact that no CD163 upregulation was observed in our study of macrophage culture with the CM of MCF7, MDA‐MB‐453 and OCUB‐M cells may indicate the low stem‐cell properties of these cell lines. In the present study, we showed that the number of CD163‐positive macrophages in breast cancer samples was lower than that of CD204‐positive macrophages in the data shown in Figure 1. These observations are similar to those of our previous study.36 These findings might indicate that CD163 is expressed on more specialized macrophages than CD204 in breast cancer.
The Ki‐67 index is a well‐known marker for the prediction of clinical prognosis in patients with a malignant tumor, including breast cancer.37, 38 In the present study, we found a positive correlation between the density of CD163‐positive and CD204‐positive macrophages and the Ki‐67 index. A similar correlation was previously seen in glioma in which Stat3 activation was significantly involved in the cell–cell interaction between glioma cells and macrophages.39 Cancer cell‐derived soluble molecules, including annexin A1, heat shock proteins, fibronectin and galectin‐1, were suggested to bind and stimulate CD204.16 Therefore, we tried to investigate the detailed mechanisms of the cell–cell interaction between breast cancer cells and macrophages using cultured cell lines and human macrophages. However, no significant cell–cell interactions between macrophages and breast cancer cell lines were observed (unpublished data). Further studies are necessary to uncover the detailed functions of CD204‐positive macrophages in the breast cancer microenvironment.
In this study, we demonstrated that a high density of CD204‐positive macrophages was associated with worse clinical course, including DRFS, RFS and BCSS, in patients with invasive breast cancer. A high Ki‐67 index was also correlated with worse DRFS, but not with RFS and BCSS in this study. Notably, in multivariate analysis a high density of CD204‐positive macrophages was the only independent factor associated with clinical course, including DRFS, RFS and BCSS. This result indicated that the density of CD204‐positive macrophages is a useful marker for prediction of clinical prognosis and might be a better marker than the Ki‐67 index. In preliminary data, we tested whether macrophage‐derived factors influenced the cancer cell proliferation and invasion; however, no significant involvement was observed (unpublished data).
The present study revealed that higher expression of CD204 is related to worse clinical prognosis in breast cancer. Although the detailed mechanism of CD204 upregulation has never been clarified, it is reported that hyaluronan (HA) is one of the breast cancer‐derived factors that upregulates M2 marker expression, such as CD204, CD206, IL‐10 and TGF‐β, via STAT3 activation.40 In a murine ovarian cancer model, immunotherapy with anti‐CD204 immunotoxin inhibited tumor progression.7 In murine lymphoma, CD204−/− macrophages inhibited tumor growth by means of producing nitric oxide and interferon‐γ, which are M1 markers.17 Thus, CD204 might be a target for anti‐breast cancer therapy.
In conclusion, we have shown that the density of CD204‐positive macrophages is significantly related to the Ki‐67 index and to worse clinical prognosis in patients with breast cancer. Thus, in addition to Ki‐67, CD204 is also considered to be useful for the prediction of clinical prognosis. The fact that CD204 expression was upregulated by the conditioned medium of breast cancer cell lines further indicated the significance of CD204 in macrophage activation. However, further studies are necessary to uncover the detailed mechanisms of CD204‐related macrophage activation.
Disclosure statement
The authors have no conflicts of interest to declare.
Acknowledgments
We thank Ms Emi Kiyota, Mr Osamu Nakamura, Ms Yui Hayashida and Mr Takenobu Nakagawa for their technical assistance. This work was supported by JSPS KAKENHI (No. 16H05162).
Cancer Sci 108 (2017) 1693–1700
Funding Information
This work was supported by JSPS KAKENHI (No. 16H05162).
References
- 1. Fitzmaurice C, Dicker D, Pain A et al The global burden of cancer 2013. JAMA Oncol 2015; 1: 505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mallory MA, Losk K, Lin NU et al The influence of radiology image consultation in the surgical management of breast cancer patients. The influence of radiology image consultation in the surgical management of breast cancer patients. Ann Surg Oncol 2015; 22: 3383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ‘omics. Breast Cancer Res 2015; 17: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 2014; 144: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jinushi M, Komohara Y. Tumor‐associated macrophages as an emerging target against tumors: creating a new path from bench to bedside. Biochim Biophys Acta 2015; 1855: 123–30. [DOI] [PubMed] [Google Scholar]
- 6. O'Sullivan C, Lewis CE, Harris AL, McGee JO. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet 1993; 342: 148–9. [DOI] [PubMed] [Google Scholar]
- 7. Vlaicu P, Mertins P, Mayr T et al Monocytes/macrophages support mammary tumor invasivity by co‐secreting lineage‐specific EGFR ligands and a STAT3 activator. BMC Cancer 2013; 13: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 1996; 56: 4625–9. [PubMed] [Google Scholar]
- 9. Lin EY, Li JF, Gnatovskiy L et al Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 2006; 66: 11238–46. [DOI] [PubMed] [Google Scholar]
- 10. Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony‐stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001; 193: 727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noy R, Pollard JW. Tumor‐associated macrophages: from mechanisms to therapy. Immunity 2014; 41: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015; 15: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 2014; 105: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti‐inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008; 216: 15–24. [DOI] [PubMed] [Google Scholar]
- 15. Kurahara H, Shinchi H, Mataki Y et al Significance of M2‐polarized tumor‐associated macrophage in pancreatic cancer. J Surg Res 2011; 167: e211–19. [DOI] [PubMed] [Google Scholar]
- 16. Neyen C, Plüddemann A, Mukhopadhyay S et al Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol 2013; 190: 3798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Komohara Y, Takemura K, Lei XF et al Delayed growth of EL4 lymphoma in SR‐A‐deficient mice is due to upregulation of nitric oxide and interferon‐gamma production by tumor‐associated macrophages. Cancer Sci 2009; 100: 2160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bak SP, Walters JJ, Takeya M, Conejo‐Garcia JR, Berwin BL. Scavenger receptor‐A‐targeted leukocyte depletion inhibits peritoneal ovarian tumor progression. Cancer Res 2007; 67: 4783–9. [DOI] [PubMed] [Google Scholar]
- 19. Goldhirsch A, Wood WC, Coates AS et al Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22: 1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakagawa T, Ohnishi K, Kosaki Y et al Optimum immunohistochemical procedures for analysis of macrophages in human and mouse formalin fixed paraffin‐embedded tissue samples. J Clin Exp Hematop 2017; accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamamoto‐Ibusuki M, Yamamoto Y, Yamamoto S et al Comparison of prognostic values between combined immunohistochemical score of estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, Ki‐67 and the corresponding gene expression score in breast cancer. Mod Pathol 2013; 26: 79–86. [DOI] [PubMed] [Google Scholar]
- 22. Xu C, Yamamoto‐Ibusuki M, Yamamoto Y et al High survivin mRNA expression is a predictor of poor prognosis in breast cancer: a comparative study at the mRNA and protein level. Breast Cancer 2014; 21: 482–90. [DOI] [PubMed] [Google Scholar]
- 23. Shigeoka M, Urakawa N, Nakamura T et al Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Sci 2013; 104: 1112–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshikawa K, Mitsunaga S, Kinoshita T et al Impact of tumor‐associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci 2012; 103: 2012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ito M, Ishii G, Nagai K, Maeda R, Nakano Y, Ochiai A. Prognostic impact of cancer‐associated stromal cells in patients with stage I lung adenocarcinoma. Chest 2012; 142: 151–8. [DOI] [PubMed] [Google Scholar]
- 26. Yoshimura T, Imamichi T, Weiss JM et al Induction of monocyte chemoattractant proteins in macrophages via the production of granulocyte/macrophage colony‐stimulating factor by breast cancer cells. Front Immunol 2016; 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP‐1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res 2001; 61: 8917–23. [PubMed] [Google Scholar]
- 28. Shigeoka M, Urakawa N, Nishio M et al Cyr61 promotes CD204 expression and the migration of macrophages via MEK/ERK pathway in esophageal squamous cell carcinoma. Cancer Med 2015; 4: 437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeya M, Komohara Y. Role of tumor‐associated macrophages in human malignancies: friend or foe? Pathol Int 2016; 66: 491–505. [DOI] [PubMed] [Google Scholar]
- 30. Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011; 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sousa S, Brion R, Lintunen M et al Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res 2015; 17: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tiainen S, Tumelius R, Rilla K et al High numbers of macrophages, especially M2‐like (CD163‐positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 2015; 66: 873–83. [DOI] [PubMed] [Google Scholar]
- 33. Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012; 12: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasquier J, Thawadi HA, Ghiabi P et al Microparticles mediated cross‐talk between tumoral and endothelial cells promote the constitution of a pro‐metastatic vascular niche through Arf6 up regulation. Cancer Microenviron 2014; 7: 41–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu A, Wei J, Kong LY et al Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol 2010; 12: 1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Komohara Y, Hasita H, Ohnishi K et al Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci 2011; 102: 1424–31. [DOI] [PubMed] [Google Scholar]
- 37. Yamashita H, Ogiya A, Shien T et al Clinicopathological factors predicting early and late distant recurrence in estrogen receptor‐positive, HER2‐negative breast cancer. Breast Cancer 2015; 23: 830–43. [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto S, Ibusuki M, Yamamoto Y et al Clinical relevance of Ki67 gene expression analysis using formalin‐fixed paraffin‐embedded breast cancer specimens. Breast Cancer 2013; 20: 262–70. [DOI] [PubMed] [Google Scholar]
- 39. Komohara Y, Horlad H, Ohnishi K et al Importance of direct macrophage‐tumor cell interaction on progression of human glioma. Cancer Sci 2012; 103: 2165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang G, Guo L, Yang C et al A novel role of breast cancer‐derived hyaluronan on inducement of M2‐like tumor‐associated macrophages formation. Oncoimmunology 2016; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]


