Summary
The prevalence and clinical relevance of thyroid stimulating hormone (TSH) receptor (TSHR) blocking antibodies (TBAb) in patients with autoimmune thyroid disease (AITD) was investigated. Serum TBAb were measured with a reporter gene bioassay using Chinese hamster ovary cells. Blocking activity was defined as percentage inhibition of luciferase expression relative to induction with bovine TSH alone (cut‐off 40% inhibition). All samples were measured for TSHR stimulatory antibody (TSAb) and TSHR binding inhibiting immunoglobulins (TBII). A total of 1079 unselected, consecutive patients with AITD and 302 healthy controls were included. All unselected controls were negative for TBAb and TSAb. In contrast, the prevalence of TBAb‐positive patients with Hashimoto's thyroiditis and Graves' disease was 67 of 722 (9·3%) and 15 of 357 (4·2%). Of the 82 TBAb‐positive patients, thirty‐nine (48%), 33 (40%) and 10 (12%) were hypothyroid, euthyroid and hyperthyroid, respectively. Ten patients were both TBAb‐ and TSAb‐positive (four hypothyroid, two euthyroid and four hyperthyroid). Thyroid‐associated orbitopathy was present in four of 82 (4·9%) TBAb‐positive patients, with dual TSHR antibody positivity being observed in three. TBAb correlated positively with TBII (r = 0·67, P < 0·001) and negatively with TSAb (r = –0·86, P < 0·05). The percentage of TBII‐positive patients was higher the higher the level of inhibition in the TBAb assay. Of the TBAb‐positive samples with > 70% inhibition, 87% were TBII‐positive. Functional TSHR antibodies impact thyroid status. TBAb determination is helpful in the evaluation and management of patients with AITD. The TBAb assay is a relevant and important tool to identify potentially reversible hypothyroidism.
Keywords: bioassay, Hashimoto's thyroiditis, Graves' disease, TSH‐receptor blocking antibodies, TSH‐receptor stimulating antibodies
Introduction
Thyroid stimulating hormone (TSH)‐receptor antibodies (TSHR‐Ab) are involved directly in the pathogenesis of autoimmune thyroid diseases (AITD) 1. Certain TSHR‐Ab with stimulating function can mimic TSH activity and promote the autoimmune hyperthyroidism of Graves' disease (GD). Less commonly, however, TSHR‐Ab do not activate the TSHR, but rather block the physiological activity of TSH. This blocking function is associated often, but not uniformly, with hypothyroidism 2. The isolation of human monoclonal TSHR‐Ab has provided great insight into the molecular events associated with the interaction of autoantibodies with TSHR 3, 4. In addition, the assumption that patients have only one antibody type was negated by the demonstration that TSHR stimulating antibody (TSAb), TBAb and TSHR binding inhibiting immunoglobulins (TBII) can occur in the same patient 5. This dual positivity (TBAb and TSAb) may, in fact, explain the variable and sometimes unpredictable clinical presentation seen in AITD patients 6. In addition, TBII are understood to be a sum of TBAb and TSAb and, therefore, TBII levels mask the functional importance of TSHR‐Ab. A number of reports in the literature have highlighted the relevance of functional assessment of TSHR‐Ab in pregnancy in which neonatal hypothyroidism can be associated with maternal AITD 7, 8.
Although bioassays for measuring TSHR‐blocking and stimulating antibodies were described decades ago 9, the establishment of their clinical validity and utility were hampered by their complexity and unreliability. More recently, cell‐based bioassay technology has been improved significantly, which has enabled TSHR‐Ab bioassays to be brought into the clinical arena 10. While binding assays are able to detect the presence of the TSHR‐Ab, they are not able to distinguish their functionality 11, 12, 13. Recently, the highly variable sensitivity of various binding assays versus functional bioassays for TSHR‐Ab was noted 14. Towards the goal of establishing further the clinical relevance of assessing the functional activity of TSHR‐Ab, in the present work we investigated the prevalence and utility of TBAb in AITD.
Materials and methods
A total of 2910 serum samples from 1079 unselected consecutive AITD patients [Hashimoto's thyroiditis (HT) and Graves' disease (GD)] and 302 unselected healthy controls (one sample each) were enrolled into the study. All controls were devoid of thyroid disorders and autoimmune diseases, had normal baseline serum thyroid‐related hormones and were negative for thyroperoxidase (TPO), thyroglobulin (Tg) and TSH receptor antibodies. HT was defined as a serum level of TPO‐Ab above the reference range with or without increased serum concentrations of Tg‐Ab, a hypoechoic appearance at thyroid ultrasound and eu‐ or hypothyroidism. GD was defined as positive TSHR‐Ab, suppressed baseline TSH, elevated free thyroid hormones [free triiodothyronine (fT3) and/or free thyroxine (fT4)], enhanced vascularization at thyroid ultrasound (‘thyroid inferno’), with or without clinical manifestations of orbital disease. Informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of the JGU Medical Center, and was carried out in accordance with the ethical guidelines of the Helsinki Declaration.
Bioassays for TSH‐receptor antibodies
Both bioassays use Chinese hamster ovary (CHO) cells expressing a chimeric TSHR (Mc4) and cyclic adenosine‐5'‐monophosphate (cAMP) response element (CRE)‐dependent luciferase. In the Mc4 construct, the amino acid (AA) residues 262–368 of human TSHR are replaced with AA residues 262–335 from rat luteinizing and chorionic gonadotrophin (LH‐CG) hormone receptor 15. In the plasmid construct used to make the stable CHO cell line, the TSHR cDNA is driven by the SV40 promoter/enhancer (SV40 pro) and a preceding beta globin intron; the firefly luciferase reporter gene is driven by a glycoprotein hormone alpha subunit promoter, which contains two CREB (cAMP response element‐binding protein) binding sites.
TSH‐receptor blocking antibody bioassay
Serum TBAb activity was measured as described previously 12, 13. Briefly, a frozen vial of CHO‐Mc4 cells (6·7 × 104 cells/well) was seeded and grown to a confluent cell monolayer in 96‐well plates for 15–18 h at 37°C, 5% CO2. The cells were plated in the 48 inner wells of a 96‐well plate with black‐walled, clear‐bottomed wells. For the sample preparation, 180 µl reaction buffer, 220 µl bovine (b)TSH (Sigma Aldrich, St Louis, MO, USA) and 40 µl of the sample were mixed. The final concentration of bTSH in the well was 100 mIU/l. The final sample dilution was 1 : 11 (40 µl/440 µl) and 100 µl of each sample was added to each well. Serum samples and three controls, consisting of a reference standard bTSH, normal serum and positive TBAb control, were tested in duplicate. After 3 h induction time, the luciferase expression levels of cell lysates were measured as relative light units (RLU) directly in the wells following the addition of substrate and lysis reagent using the luminometer (Tecan Infinite M200; Tecan GmbH, Crailsheim, Germany). Blocking activity was defined as percentage inhibition of luciferase expression relative to induction with bTSH alone [100 × (1 sample + bTSH/bTSH alone)]. Prior to calculating the percentage inhibition, the RLU of the background wells (normal serum diluted 1 : 11 in reaction buffer) were subtracted from the RLU of the test wells and the bTSH‐alone wells. The cut‐off for the TBAb assay is at 40% inhibition.
TSH‐receptor stimulating antibody bioassay
Serum TSAb activity was measured with a chimeric TSHR bioassay according to the manufacturer's instructions 15, 16. Briefly, a frozen vial of CHO‐Mc4 cells (6·7 × 104 cells/well) was seeded and grown to a confluent cell monolayer in 96‐well plates for 15–18 h at 37°C, 5% CO2. Serum samples, positive, reference and normal controls were diluted 1 : 11 in reaction buffer, added to the cell monolayers, and each plate was incubated for 3 h at 37°C, 5% CO2. Subsequently, the CHO‐Mc4 cells were lysed and the RLU were quantified in the luminometer (Tecan Infinite M200; Tecan). The cut‐off for the TSAb assay is specimen‐to‐reference ratio (SRR) at 140%.
Thyroid‐related hormones and antibodies
Serum levels of baseline TSH, fT3, fT4, Tg and TPO concentrations were measured using electrochemiluminescent immunoassays (CLIA; Abbott, Wiesbaden, Germany). TBII were measured with the Cobas e 411 (Roche Diagnostics, Mannheim, Germany). Both were utilized according to the manufacturer's instructions.
Statistical analysis
The baseline serum sample values of each patient were considered for statistical analysis. Data are presented as median and quartiles (25 and 75% percentiles) and analysed with spss version 23 (SPSS, Inc., Chicago, IL, USA) and sas version 9.3 (SAS Institute, Cary, NC, USA). Repeatability of the TBAb bioassay was analysed on five repeated measurements in 47 initially positive patients by fitting a linear random‐effects model and report the square root of the within‐patient variance as the precision parameter. This analysis was repeated with 45 patients with mean TBAb > 30% and 39 patients with mean TBAb > 40% to reduce a risk of bias due to accumulation of false‐positive findings near the cut‐off. A possible relationship between level and precision was investigated by Spearman's correlation coefficient between the means and standard deviations calculated for each patient. The correlation between assay findings with Spearman's correlation coefficients was quantified and tested for zero correlation with the corresponding test. All P‐values were two‐sided and a result was significant when P ≤ 0·05.
Results
Demographic data
A total of 1079 unselected, consecutive patients with AITD and 302 euthyroid healthy controls were included. The demographic and serological data are demonstrated in Table 1. The age of controls (median, 25 and 75% percentiles) was 25 (24–29) years, with 155 (51·3%) females. Compared with patients suffering from GD, the HT patients included in the study were younger (fivefold more children), included only 10% as many patients with thyroid‐associated orbitopathy (TAO), and had higher titres of TPO‐Ab and Tg‐Ab.
Table 1.
Demographic and laboratory data
| N = 1079 | GD | HT |
|---|---|---|
| n (%) | 357 (33) | 722 (67) |
| Female, n (%) | 276 (77) | 598 (83) |
| Age (years) | 47·9 (34·5–56) | 35·6 (17·4–49) |
| Children, n (< 18 years) | 14 (4) | 189 (26) |
| Adults, n (> 18 years) | 343 (96) | 533 (74) |
| Duration of AITD (years) | 2 (1–5) | 2 (0·1–8) |
| TAO, n (%) | 229 (64) | 44 (6) |
| TSH (0·4–4·9 mU/l) | 1·6 (0·01–4·7) | 2·0 (1–4) |
| fT3 (1·7–3·7 pg/ml) | 4·7 (3·7–5) | 3·0 (2·6–3·5) |
| fT4 (0·7–1·5 ng/dl) | 2·0 (1·2–16·2) | 1·2 (1–1·4) |
| Tg‐Ab (< 4·1 IU/ml) | 8·4 (1·8–58) | 58·4 (10–295) |
| TPO‐Ab (< 6 IU/ml) | 93 (7–519) | 182 (38–537) |
Values are represented in median and 25 and 75 quartiles [interquartile range (IQR)]. The normal range is shown for the thyroid‐related hormones and autoantibodies. GD = Graves' disease; HT = Hashimoto's thyroiditis; AITD = autoimmune thyroid disease; TAO = thyroid‐associated orbitopathy; TPO = thyroperoxidase; Ab = antibody; fT3 = free triiodothyronine; fT4 = free thyroxine.
TBAb positivity
All healthy controls were negative for TBAb and TSAb (bioassay specificity 100%). In contrast, the prevalence of TBAb‐positive patients with HT and GD was 9·3 and 4·2%, respectively (Table 2). Approximately 82% of TBAb‐positive patients were diagnosed with HT and 18% GD. However, 3·1% (51 of 1621) samples of GD patients and 6·4% (82 of 1289) samples of HT patients were TBAb‐positive. The corresponding median (25 and 75% percentiles) TBAb percentage inhibition levels were −107 (–158·5; −60·5) for GD and −1 (–23·8; 16) for HT, respectively. The distribution of percentage inhibition of TBAb‐positive samples, according to thyroid function, reveals GD patients to be more hyperthyroid; HT patients, however, were more hypothyroid or euthyroid (Table 2). The prevalence and serum levels of TSAb and TBII have been investigated in all 1079 AITD patients (Table 3). TBAb percentage inhibition shows a clear trend to thyroid function (Table 4). The higher the percentage inhibition, the more hypothyroid was the patient. Of 82 TBAb‐positive patients, 39 were hypothyroid, 33 euthyroid and 10 hyperthyroid, with four (10·3%), two (6%) and four (40%) additionally testing TSAb‐positive. Four TBAb‐positive patients displayed TAO, two of whom had HT and two GD. Three of these four were additionally TSAb‐positive.
Table 2.
Prevalence and percentage inhibition in 82 thyroid stimulating hormone receptor‐blocking antibodies (TBAb)‐positive autoimmune thyroid disease (AITD) patients
| GD (n = 357) | HT (n = 722) | |
|---|---|---|
| TBAb‐pos. n (%) | 15 (4·2) | 67 (9·3) |
| TBAb (% inhibition) | 76 (51–86) | 55 (45–83) |
| Age (years) | ||
| Adults, n | 14 | 43 |
| Adults, % inhibition | 76·5 (54–86·3) | 56 (46–79) |
| Children, n | 1 | 24 |
| Children, % inhibition | 44 | 51·5 (42–92) |
| Gender | ||
| Female, n | 13 | 55 |
| Female, % inhibition | 77 (48–86·5) | 55 (44–78) |
| Male, n | 2 | 12 |
| Male, % inhibition | 70·5 (65–70·5) | 60 (47–99) |
| Thyroid function, n (%) | ||
| Hypothyroid | 5 (33) | 36 (54) |
| Euthyroid | 4 (27) | 27 (40) |
| Hyperthyroid | 6 (40) | 4 (6) |
| Percentage inhibition | ||
| Hypothyroid | 77 (76–77) | 60 (45–92) |
| Euthyroid | 83·5 (61·5–93) | 54 (43–78) |
| Hyperthyroid | 48 (43–66) | 66·5 (47–82) |
| TAO | ||
| n (%) | 2 (13·33) | 2 (3) |
| % inhibition | 42·5 (41–43) | 74·5 (43–82) |
| Specific treatment | ||
| • L‐T4, n | 3 | 23 |
| • L‐T4, % inhibition | 86 (84–86) | 47 (41–84) |
| • Methimazole, n | 3 | 0 |
| • Methimazole, % inhibition | 76 (44–76) | – |
| TBAb% inhibition (10 TSAb‐positive patients) | 44 (41–44) | 60 (50·5–68) |
Values are represented in median and 25 and 75 quartiles (interquartile range). GD = Graves' disease; HT = Hashimoto's thyroiditis; TAO = thyroid‐associated orbitopathy; TBAb = thyroid stimulating hormone receptor‐blocking antibodies; TSAb = thyroid stimulating hormone receptor‐stimulating antibodies.
Table 3.
Prevalence and serum levels of TSAb and TBII in all 1079 AITD patients
| GD (n = 357) | HT (n = 722) | |
|---|---|---|
| TSAb‐positive, n (%) | 309 (87) | 68 (9) |
| TSAb (SRR %) | 422 (280–516) | 47 (36–71) |
| TBII positive, n (%) | 269 (75) | 68 (9) |
| TBII (< 1·75 IU/l) | 6·65 (2.53–19·8) | 0.5 (0·5–0·5) |
Values are represented in median and 25 and 75 quartiles (IQR). TSAb = thyroid stimulating hormone receptor (TSHR) stimulating antibody; TBII = TSHR binding inhibiting immunoglobulins; AITD = autoimmune thyroid disease; SRR = specimen‐to‐reference ratio.
Table 4.
Thyroid function in 82 TBAb‐positive patients with AITD according to TBAb percentage inhibition
| % Inhibition | 40–50 | 51–60 | 61–70 | 71–100 | Σ (%) |
|---|---|---|---|---|---|
| Hypothyroid | 14 | 7 | 1 | 17 | 39 (48) |
| Euthyroid | 12 | 7 | 2 | 12 | 33 (40) |
| Hyperthyroid | 4 | 2 | 2 | 2 | 10 (12) |
| Patients (n) | 30 | 16 | 5 | 31 | 82 |
| TBII | 5 (16·6%) | 6 (37·5%) | 3 (60%) | 27 (87·1%) | 41 (50%) |
TBII = thyroid stimulating hormone receptor (TSHR)‐binding inhibitory immunoglobulins; AITD = autoimmune thyroid disease; TBAb = TSHR‐blocking antibodies.
Both TSAb (Fig. 1a) and TBAb (Fig. 1b) bioassays were utilized simultaneously along with the binding (TBII) test. Ten patients (six with HT and four with GD) were positive in all assays (Fig. 1a, red circle). The thyroid function of these patients was as follows: four hypothyroid, two euthyroid and four hyperthyroid. Among all 133 TBAb‐positive samples, 59% were TBII‐positive and TSAb‐negative, 7·5% were positive for both TBII and TSAb and 33·5% were negative for both TSAb and TBII (Table 5). Not surprisingly, TBAb correlated positively with TBII (r =0·67, P < 0·001) and negatively with TSAb (r = –0·86, P < 0·05). Samples with high TBAb positivity (> 70% inhibition) were more likely to be positive in the binding assay. When comparing the TBAb percentage inhibition to TBII positivity (Table 4) an ascending relation was apparent, such that of the samples with 40–50% inhibition only 17% were TBII‐positive, whereas of those samples with > 70% inhibition, 87% were TBII‐positive.
Figure 1.
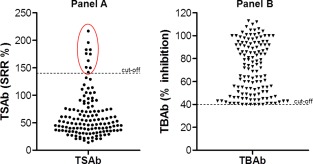
Distribution of thyroid stimulating hormone receptor‐stimulating antibodies (TSAb) (a) and TSHR‐blocking antibodies (TBAb) (b) in 133 TBAb‐positive serum samples with autoimmune thyroid disease (AITD). (a) The dots demonstrate TSAb measured in the TSAb bioassay (cut‐off at 140 specimen‐to‐reference ratio (SRR %) as a dotted line. Included in the red circle are the 10 TBAb‐ and TSAb‐positive samples. (b) The triangles TBAb measured in the TBAb bioassay (cut‐off at 40% inhibition as a dotted line). [Colour figure can be viewed at wileyonlinelibrary.com].
Table 5.
Classification of TSHR‐antibody positivity in bioassays and a binding assay in 133 TBAb‐positive samples
| TBAb | TSAb | TBII | n (%) |
|---|---|---|---|
| + | – | + | 78 (59) |
| + | – | – | 45 (33·5) |
| + | + | + | 10 (7·5) |
TBAb = thyroid stimulating hormone receptor (TSHR)‐blocking antibodies; TSAb = TSHR stimulating antibodies; TBII = TSHR‐binding inhibitory immunoglobulins.
Repeatability
For the TBAb assay, reproducibility data were generated from 622 sera of 47 AITD patients, initially TBAb‐positive. The root mean squared error was 9·1% for patients with mean TBAb from 40 to 50% inhibition and of 8·8% when restricting data to patients with mean TBAb > 30 or > 40%. A significant trend towards increasing precision with higher TBAb levels was observed (Spearman's r = –0·33, P = 0·02, between individual means and standard deviations).
Discussion
This work entailed the largest study of TSHR‐blocking antibodies from consecutive and unselected patients with AITD from a single institution. It shows clearly the high prevalence and clinical relevance of TBAb such that, overall, approximately 10% of patients with AITD are TBAb‐positive. Most TBAb‐positive patients have HT, but patients with GD may also be TBAb‐positive, particularly during or after anti‐thyroid drug treatment. Consistent with the functional relevance of TBAb, the majority of TBAb‐positive patients were hypothyroid. TBAb‐positivity, however, was not predominant in patients with TAO. TBAb was correlated highly with TBII. Gender, age and treatment had no impact upon either TBAb prevalence or degree of inhibition.
The high number of unselected subjects emphasizes the importance of this field of research. As well as the high number of patients, the novelty of this paper encompasses diligent testing involving not only the functional blocking and stimulating cell‐based bioassays, but also the conventional binding assay. This allowed us to detect the seldom prevalence of dual TSH receptor antibody positivity. In the meantime, we were able to detect a further ‘double‐positive’ patient (with two samples) who was not included in the following data. In addition, our serological data enabled us to identify the thyroid state responsible for symptoms and relevant for clinical presentation. Thus far, the investigation of the results obtained with thyroid function is unique to bioassay testing.
Subsequent to development 12, analytical performance and validation of this TBAb bioassay was performed recently 13. The goal of the present study was to illuminate the prevalence and clinical importance of TBAb, an under‐studied biomarker despite its increasing relevance in AITD. Due possibly to its ephemeral nature, measurement of TBAb presents far more difficulty than measurement of TSAb. A mass screening of more than 1000 AITD patients detected 82 TBAb‐positive patients, thus allowing closer examination of this uncommon biomarker. Results that compare the binding assay and bioassay emphasize the sensitivity of the cell‐based bioassay and highlights that the binding assay is unable to distinguish the functionality of TSHR antibody.
This is the only study, to our knowledge, where an unselected, consecutively followed collection of AITD patients were compared with a large unselected control group devoid of thyroid or autoimmune disease. In contrast, several previous publications have encompassed smaller numbers of patients and did not compare the results of various testing methods, including established commercial assays 2, 17, 18. To the best of our knowledge, only one study looked at a relatively large paediatric collective in which TBAb percentage inhibition was analysed after neonatal screening of potentially hypothyroid newborns. A prevalence of TBAb‐positivity of 1·4% (11 of 790) was shown to be associated mainly with mothers with AITD 7.
The large number of patients is the relevant pool, and the novel functional bioassays are important tools. TBAb determination primarily provides an important tool to identify AITD, mainly in the form of reversible hypothyroidism. Independently from its detection, the progress, development or remission of the disease can be measured by the percentage of antibody inhibition. The blood withdrawals took part at different stages of the respective AITD patients: some were undergoing therapy, others were at the beginning of disease progress and some had previously unknown antibody titres. The TBAb testing, however, showed a tendency of according thyroid functions. The higher the percentage of TBAb inhibition, the more clinically apparent (hypothyroidism). Therefore, antibody monitoring corresponds to the severity of the disease.
TBAb percentage inhibition shows a clear trend towards thyroid function. The higher the percentage inhibition, the more hypothyroid was the patient. Of those patients with TBAb positivity in GD, a higher percentage inhibition was detectable in patients during or post‐anti‐thyroid drug treatment. However, 33 TBAb‐positive patients were euthyroid at the time of sampling. The presence of concomitant TSAb positivity in the serum may explain biochemical euthyroidism. Another possibility is the magnitude of TBAb level in the serum, with hypothyroidism occurring above a certain antibody concentration.
As understanding of the pathogenesis of GD has evolved, it has been suspected that certain patients might produce not only TSAb but also TBAb, and that this might account for their alternating clinical presentation 19, 20. The isolation of separate monoclonal antibodies (MAbs) with stimulatory and blocking activity from the same patient's lymphocytes confirmed this suspicion 5. Structure–function analyses using these MAbs have provided insight into the mechanism of their functional activity 3.The co‐presence of TBAb and TSAb in the serum of a patient has been speculated to have a counteracting effect, similar to a tug‐of‐war 6. In TSAb bioassays, the co‐presence of TBAb and TSAb created a net sum, possibly evening out and remaining non‐detectable. TSAb detects net TSAb, but cannot detect TBAb. TBAb detects either net TBAb (positive inhibition) or net TSAb (negative inhibition). Neither assay detects them ‘independently’ 12.
Despite the large collection of patients in the present study, because of the low prevalence of TBAb, the number of TBAb‐positive patients was relatively small. The cross‐sectional nature of this study limits the ability to obtain information about TBAb development. New trials are planned to utilize prospective longitudinal studies as well as long‐term follow‐up. The depicted patients were determined via cross‐section and do not display the fluctuation of TSHR‐Ab in a singular patient's lifetime – in particular, in extreme situations such as puberty and pregnancy 21. Antibody‐switching between TBAb and TSAb has been reported in recent literature 22, 23. These switches have not only become apparent during the therapy of HT, with L‐T4 resulting in hyperthyroidism, but also during anti‐thyroid drug therapy in GD leading to hypothyroidism 6. Therefore, in AITD ongoing monitoring of both is essential, independently of what could be a misleading clinical appearance. Integrating the measurement of functional TSHR‐Ab into the management of AITD can assist physicians with difficult cases, and simultaneous measurement of TBAb and TSAb can help in the interpretation of thyroid dysfunction.
Disclosure
T. D., J. K., J. Kö. and M. K. have nothing to disclose. P. D. O. and G. J. K. consult for Quidel, USA. This paper entails parts of the PhD thesis of J.K.
References
- 1. Kahaly GJ, Diana T. TSH receptor antibody functionality and nomenclature. Front Endocrinol (Lausanne) 2017; 8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiovato L, Vitti P, Santini F et al Incidence of antibodies blocking thyrotropin effect in vitro in patients with euthyroid or hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab 1990; 71:40–5. [DOI] [PubMed] [Google Scholar]
- 3. Sanders P, Young S, Sanders J et al Crystal structure of the TSH receptor (TSHR) bound to a blocking‐type TSHR autoantibody. J Mol Endocrinol 2011; 46:81–99. [DOI] [PubMed] [Google Scholar]
- 4. Smith BR, Furmaniak J, Sanders J. TSH receptor blocking antibodies. Thyroid 2008; 18:1239. [DOI] [PubMed] [Google Scholar]
- 5. Evans M, Sanders J, Tagami T et al Monoclonal autoantibodies to the TSH receptor, one with stimulating activity and one with blocking activity, obtained from the same blood sample. Clin Endocrinol (Oxf) 2010; 73:404–12. [DOI] [PubMed] [Google Scholar]
- 6. McLachlan SM, Rapoport B. Thyrotropin‐blocking autoantibodies and thyroid‐stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid 2013; 23:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown RS, Bellisario RL, Botero D et al Incidence of transient congenital hypothyroidism due to maternal thyrotropin receptor‐blocking antibodies in over one million babies. J Clin Endocrinol Metab 1996; 81:1147–51. [DOI] [PubMed] [Google Scholar]
- 8. Brown RS, Keating P, Mitchell E. Maternal thyroid‐blocking immunoglobulins in congenital hypothyroidism. J Clin Endocrinol Metab 1990; 70:1341–6. [DOI] [PubMed] [Google Scholar]
- 9. Vitti P, Chiovato L, Fiore E, Mammoli C, Rocchi R, Pinchera A. Use of cells expressing the human thyrotropin (TSH) receptor for the measurement of thyroid stimulating and TSH‐blocking antibodies. Acta Med Austriaca 1996; 23:52–6. [PubMed] [Google Scholar]
- 10. Kamijo K, Murayama H, Uzu T, Togashi K, Olivo PD, Kahaly GJ. Similar clinical performance of a novel chimeric thyroid‐stimulating hormone receptor bioassay and an automated thyroid‐stimulating hormone receptor binding assay in Graves' disease. Thyroid 2011; 21:1295–9. [DOI] [PubMed] [Google Scholar]
- 11. Jordan NJ, Rinderle C, Ashfield J et al A luminescent bioassay for thyroid blocking antibodies. Clin Endocrinol (Oxf) 2001; 54:355–64. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Kim J, Diana T, Klasen R, Olivo PD, Kahaly GJ. A novel bioassay for anti‐thyrotrophin receptor autoantibodies detects both thyroid‐blocking and stimulating activity. Clin Exp Immunol 2013; 173:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diana T, Li Y, Olivo PD et al Analytical performance and validation of a bioassay for thyroid‐blocking antibodies. Thyroid 2016; 26:734–40. [DOI] [PubMed] [Google Scholar]
- 14. Diana T, Wuster C, Kanitz M, Kahaly GJ. Highly variable sensitivity of five binding and two bio‐assays for TSH‐receptor antibodies. J Endocrinol Invest 2016; 39:1159–65. [DOI] [PubMed] [Google Scholar]
- 15. Lytton SD, Li Y, Olivo PD, Kohn LD, Kahaly GJ. Novel chimeric thyroid‐stimulating hormone‐receptor bioassay for thyroid‐stimulating immunoglobulins. Clin Exp Immunol 2010; 162:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diana T, Kanitz M, Lehmann M, Li Y, Olivo PD, Kahaly GJ. Standardization of a bioassay for thyrotropin receptor stimulating autoantibodies. Thyroid 2015; 25:169–75. [DOI] [PubMed] [Google Scholar]
- 17. Arikawa K, Ichikawa Y, Yoshida T et al Blocking type antithyrotropin receptor antibody in patients with nongoitrous hypothyroidism: its incidence and characteristics of action. J Clin Endocrinol Metab 1985; 60:953–9. [DOI] [PubMed] [Google Scholar]
- 18. Yoshikawa N, Nishikawa M, Horimoto M, Uno C, Taniguchi N, Inada M. Activity of thyroid stimulating antibody and thyroid stimulation blocking antibody determined by radioiodine uptake into FRTL‐5 cells. Endocrinol Jpn 1989; 36:55–63. [DOI] [PubMed] [Google Scholar]
- 19. Lenzner C, Morgenthaler NG. The effect of thyrotropin‐receptor blocking antibodies on stimulating autoantibodies from patients with Graves' disease. Thyroid 2003; 13:1153–61. [DOI] [PubMed] [Google Scholar]
- 20. Miyauchi A, Amino N, Tamaki H, Kuma K. Coexistence of thyroid‐stimulating and thyroid‐blocking antibodies in a patient with Graves' disease who had transient hypothyroidism. Am J Med 1988; 85:418–20. [DOI] [PubMed] [Google Scholar]
- 21. Kiefer FW, Klebermass‐Schrehof K, Steiner M et al Fetal/neonatal thyrotoxicosis in a newborn from a hypothyroid woman with Hashimoto's thyroiditis. J Clin Endocrinol Metab 2016; 102:6–9. [DOI] [PubMed] [Google Scholar]
- 22. Inoue A, Koizumi S, Matsuda A et al Graves' hyperthyroidism showing transient hypothyroidism during interferon therapy for chronic hepatitis type C. Endocr J 2005; 52:293–8. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida K, Aizawa Y, Kaise N et al Role of thyroid‐stimulating blocking antibody in patients who developed hypothyroidism within one year after 131I treatment for Graves' disease. Clin Endocrinol (Oxf) 1998; 48:17–22. [DOI] [PubMed] [Google Scholar]


