Abstract
The hedgehog signaling pathway regulates multiple morphogenetic processes during embryogenesis. Aberrant activation of the hedgehog pathway signal transduction in adult tissues is associated with the pathogenesis of hematologic malignancies and solid tumors. We report findings from an open‐label, multicenter phase I trial of the selective, small‐molecule hedgehog signaling inhibitor glasdegib (PF‐04449913) in Japanese patients with select advanced hematologic malignancies. Glasdegib was administered as once‐daily oral doses (25, 50 and 100 mg) in 28‐day cycles after a lead‐in dose on Day −5. The primary objectives were to determine first‐cycle dose‐limiting toxicities, safety, vital signs and laboratory test abnormalities. Secondary objectives included evaluation of pharmacokinetics, pharmacodynamics and preliminary evidence of clinical activity of glasdegib. No dose‐limiting toxicities were noted in the 13 patients in the present study. All patients experienced at least one treatment‐emergent, all‐causality adverse event. The most frequent treatment‐related adverse events (observed in ≥3 patients) were dysgeusia (n = 9), muscle spasms (n = 5), alopecia, decreased appetite (n = 4 each), and increased blood creatinine phosphokinase, constipation and diarrhea (n = 3 each). Two deaths occurred during the study and were deemed not to be treatment‐related due to disease progression. Glasdegib demonstrated dose‐proportional pharmacokinetics, marked downregulation of the glioma‐associated transcriptional regulator GLI1 expression in normal skin, and evidence of preliminary clinical activity, although data are limited. Glasdegib was safe and well tolerated across the dose levels tested. It is confirmed that the 100‐mg dose is safe and tolerable in Japanese patients, and this dose level will be examined in the future clinical trial.
Keywords: Glasdegib, hedgehog signaling pathway, hematologic malignancies, Japanese, smoothened
The Hedgehog (Hh) signaling pathway regulates multiple morphogenetic processes during embryogenesis, and is involved in the maintenance of neural and skin stem cells in adults. Binding of the Sonic, Indian or Desert Hh ligands to the transmembrane receptor patched (PTCH1) allows activation of the glioma‐associated transcriptional regulators GLI1 and GLI2, and modulation of target gene expression through smoothened (SMO)‐mediated signaling.1, 2 Aberrant activation of Hh pathway signal transduction in adult tissues may be implicated in the pathogenesis of chronic myeloid leukemia (CML), medulloblastoma and basal cell carcinoma.2, 3, 4, 5, 6
Glasdegib (PF‐04449913) is an oral, potent, selective, small‐molecule inhibitor of Hh signaling that functions through binding to the SMO receptor.7 In preclinical studies, glasdegib inhibited SMO in vitro and induced significant antitumor activity in vivo.8, 9, 10 Furthermore, administration of glasdegib resulted in a significant reduction in leukemic stem cell (LSC) burden in xenograft models, inhibition of Hh signaling, and a reduction in cell populations expressing LSC markers.9, 10 SMO inhibition comprises a new approach to the eradication of LSC, as demonstrated in preclinical models of CML, and this provides a rationale for investigating its clinical effects in patients with myeloid malignancies.
In a phase I dose‐escalation study conducted in patients with select advanced myeloid malignancies and solid tumors in the United States and Italy, glasdegib was well tolerated. Dysgeusia, decreased appetite and alopecia were the most common treatment‐related adverse events (AE) observed with glasdegib, and were consistent with the class effects observed with other Hh pathway inhibitors.11 Glasdegib demonstrated dose‐proportional pharmacokinetics (PK) over the 5–600 mg dose range. Furthermore, glasdegib showed preliminary clinical activity in approximately half of the patients in the study.12 The results from this study provided a rationale for phase II studies of glasdegib (as monotherapy or in combination with standard therapy) in a variety of hematologic malignancies, including acute myeloid leukemia (AML), myelodysplastic syndrome and myelofibrosis (MF).
This ongoing phase I study (data cut‐off on 1 March 2016) was conducted to evaluate the safety, PK, pharmacodynamics (PD) and preliminary clinical activity of glasdegib monotherapy in Japanese patients with select advanced hematologic malignancies.
Material and Methods
Study design and patient selection
As part of an open‐label, multicenter phase I trial (NCT02038777), this study was performed in patients with select advanced hematologic malignancies in Japan. Glasdegib was administered as once‐daily (q.d.) oral doses in 28‐day cycles after a lead‐in dose on Day −5. Three doses (25, 50 and 100 mg) were evaluated using a 3 + 3 clinical study design. The primary objectives of the study were to determine first‐cycle dose‐limiting toxicities (DLT), safety, vital signs and laboratory test abnormalities. Secondary objectives included evaluation of PK, PD and preliminary evidence of clinical activity of glasdegib.
Patients aged ≥20 years with select advanced hematologic malignancies who were refractory, resistant or intolerant to prior therapies were included. Eligible patients were limited to myelodysplastic syndrome (MDS), MF, chronic myelomonocytic leukemia (CMML), CML (including T315I mutants) and AML. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and adequate renal, hepatic and cardiac functions. Patients were excluded from the study if they had active graft‐versus‐host disease, or life‐threatening or clinically significant uncontrolled infection. In addition, patients were not eligible if they were known to be refractory to platelet or packed red blood cell transfusions, had a history of serious cardiovascular disease in the previous 6 months, Fridericia‐corrected QTc (QTcF) >470 ms, or active central nervous system involvement by leukemia.
The study was conducted in compliance with the Declaration of Helsinki and followed the International Conference on Harmonisation Good Clinical Practices guidelines. The protocol was approved by the institutional review boards of the participating institutions and all patients provided signed informed consent. The study was supported by Pfizer and registered at ClinicalTrials.gov (NCT02038777).
Procedures
Glasdegib was administered as q.d. oral doses of 25, 50 and 100 mg using a 3 + 3 design. DLT were defined as treatment‐related AE observed from Day −5 to Day 28 of Cycle 1 that were possibly attributable to glasdegib. These included grade ≥3 nonhematologic toxicity, prolonged (≥42 days) myelosuppression (absolute neutrophil count <500/μL or platelet count <10 × 109/L with a normal bone marrow) and grade ≥ 3 QTc prolongation (QTc ≥501 ms after correction for reversible causes).
Assessments
Safety
Safety assessments included the collection of AE and serious AE, and were monitored for 28 days after the last study treatment or until all drug‐related toxicities were resolved. AE were graded for severity using NCI Common Terminology Criteria for Adverse Events, version 4.0. In addition, laboratory assessments were conducted.
Pharmacokinetics
Serial blood samples were collected for PK assessments on Day −5 following a single dose, and on Days 1, 8, 15 and 21 of Cycle 1, and on Day 1 of the following cycles. Samples were analyzed for plasma glasdegib concentrations at Covance Bioanalytical Services (Shanghai, China) using a validated analytical method (lower limit of quantification [LLOQ] of 3.00 ng/mL). PK parameters for glasdegib were evaluated, including the observed maximum plasma concentration (C max), time to maximum plasma concentration (T max) and area under the plasma concentration–time curve (AUC), and were estimated using noncompartmental analysis.
Pharmacodynamics
Normal skin biopsies were obtained at screening and on Cycle 1 Day 21 to analyze treatment‐related changes in the expression of Hh pathway‐regulated genes. When possible, PD skin sample collections were matched with PK sample collections for PK/PD evaluations.
Preliminary clinical activity
Clinical activity was assessed according to disease‐specific response criteria. The response criteria for CML was derived from Faderl et al. (1999) and Cohen et al. (2005).13, 14 Response criteria for CMML, MDS, MF and AML were derived and defined by the disease‐specific International Working Groups and World Health Organization guidelines.
Statistical analysis
No statistical sample size determination was performed. Safety, PK, PD and preliminary clinical activity data were summarized with descriptive statistics. Data from biomarker assays were analyzed using graphical methods and descriptive statistics.
Results
Patient characteristics and treatment
A total of 13 patients were enrolled in the study. Glasdegib was administered orally q.d. at 25 mg (N = 3), 50 mg (N = 4) and 100 mg (N = 6). Patient demographics and clinical characteristics are presented in Table 1 for all patients in the study. Seven patients had AML and four had MDS. The remaining patients had CMML (n = 1) and MF (n = 1).
Table 1.
Patient baseline demographics and clinical characteristics
| Glasdegib dose | |||||||
|---|---|---|---|---|---|---|---|
| 25 mg n = 3 | 50 mg n = 4 | 100 mg n = 6 | Total | ||||
| Male | Female | Male | Female | Male | Female | ||
| Number of patients | 2 | 1 | 3 | 1 | 3 | 3 | 13 |
| Age, years | |||||||
| Median | 64.5 | 49.0 | 68.0 | 65.0 | 73.0 | 68.0 | 68.0 |
| Range | 63–66 | 49–49 | 64–72 | 65–65 | 71–81 | 55–72 | 49–81 |
| ECOG PS, n (%) | |||||||
| 0 | 1 (33) | 2 (50) | 4 (67) | 7 (54) | |||
| 1 | 2 (67) | 2 (50) | 1 (17) | 5 (38) | |||
| 2 | 0 | 0 | 1 (17) | 1 (8) | |||
| Primary diagnosis, n (%) | |||||||
| MDS | 2 (67) | 1 (25) | 1 (17) | 4 (31) | |||
| MF | 0 | 0 | 1 (17) | 1 (8) | |||
| CMML | 0 | 1 (25) | 0 | 1 (8) | |||
| AML | 1 (33) | 2 (50) | 4 (67) | 7 (54) | |||
| Prior regimens, n (%) | |||||||
| 1 | 1 (33) | 2 (50) | 3 (50) | 6 (46) | |||
| 2 | 1(33) | 0 | 1 (17) | 2 (15) | |||
| 3 | 0 | 1 (25) | 0 | 1 (8) | |||
| >3 | 1 (33) | 1 (25) | 2 (33) | 4 (31) | |||
AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; ECOG PS, Eastern Cooperative Oncology Group performance score; MDS, myelodysplastic syndrome; MF, myelofibrosis.
Safety and tolerability
No DLT were observed in the 13 patients in this study. All patients experienced at least one treatment‐emergent, all‐causality AE, the most frequent (observed in ≥3 patients) being dysgeusia, constipation, decreased appetite, muscle spasms, pyrexia, alopecia, and increased blood phosphokinase creatinine, diarrhea, fatigue, hypokalemia, thrombocytopenia, and decreased weight (Table 2). The most frequent grade 3 treatment‐emergent, all‐causality AE were anemia, hypokalemia, and thrombocytopenia (n = 2; 15% each). Grade 4 treatment‐emergent AE (leukopenia, neutropenia, thrombocytopenia and reduced platelet count) were observed (n = 1; 8% each).
Table 2.
Treatment‐emergent, all‐causality adverse events in ≥3 patients
| N = 13 | Treatment‐emergent, all‐causality adverse events | |
|---|---|---|
| All grades n (%) | ≥Grade 3 n (%) | |
| Any adverse event | 13 (100.0) | 7 (53.8) |
| Dysgeusia | 9 (69.2) | 0 |
| Constipation | 6 (46.2) | 0 |
| Decreased appetite | 6 (46.2) | 0 |
| Muscle spasms | 6 (46.2) | 0 |
| Pyrexia | 6 (46.2) | 1 (7.7) |
| Alopecia | 4 (30.8) | 0 |
| Blood creatinine phosphokinase increased | 3 (23.1) | 0 |
| Diarrhea | 3 (23.1) | 0 |
| Fatigue | 3 (23.1) | 0 |
| Hypokalemia | 3 (23.1) | 2 (15.4) |
| Thrombocytopenia | 3 (23.1) | 3 (23.1) |
| Weight decreased | 3 (23.1) | 0 |
Of the 13 patients in the study, 9 (69%) experienced treatment‐emergent, treatment‐related AE, which were all grade 1–3 in severity (Table 3). The most frequent treatment‐related AE (observed in ≥3 patients) were dysgeusia, muscle spasms, alopecia, decreased appetite, and increased blood creatinine phosphokinase, constipation and diarrhea.
Table 3.
Treatment‐emergent, treatment‐related adverse events in ≥3 patients
| N = 13 | Treatment‐emergent, treatment‐related adverse events | |
|---|---|---|
| All grades n (%) | Grade 3 n (%) | |
| Any adverse event | 9 (69.2) | 1 (7.7)a |
| Dysgeusia | 9 (69.2) | 0 |
| Muscle spasms | 5 (38.5) | 0 |
| Alopecia | 4 (30.8) | 0 |
| Decreased appetite | 4 (30.8) | 0 |
| Blood creatinine phosphokinase increased | 3 (23.1) | 0 |
| Constipation | 3 (23.1) | 0 |
| Diarrhea | 3 (23.1) | 0 |
No grade 4 or 5 treatment‐related adverse events were reported in this study.
One patient experienced grade 3 treatment‐related pyrexia.
Treatment with glasdegib was permanently discontinued due to AE in 3 patients at 50 mg and 1 patient at 100 mg. Two deaths occurred during the study. One 68‐year‐old man developed grade 5 cerebral hemorrhage, which was due to disease progression, and a 65‐year‐old woman experienced grade 5 disease progression; both deaths were deemed not to be treatment‐related. A 72‐year‐old man developed grade 2 disseminated intravascular coagulation, which had not resolved by the end of the study. Temporary discontinuations occurred in 2 (15%) patients in the 25‐mg group, 1 patient in the 50‐mg group, and in 3 (23%) patients in the 100‐mg group for an all‐cause AE. Treatment was temporarily discontinued or the dose reduced in 3 (23%) patients due to a treatment‐related AE, including grade 1 muscle cramp, grade 1 dysgeusia and grade 1–3 pyrexia. All of these treatment‐related AE resolved following temporary treatment discontinuation. A 68‐year‐old woman in the 100‐mg group developed grade 1 alopecia and grade 1 dysgeusia that both resolved upon dose reduction of glasdegib. The median duration of treatment (from first dose to last dose) was 76 days (range, 36–332) for the 100‐mg group (Fig. 1).
Figure 1.
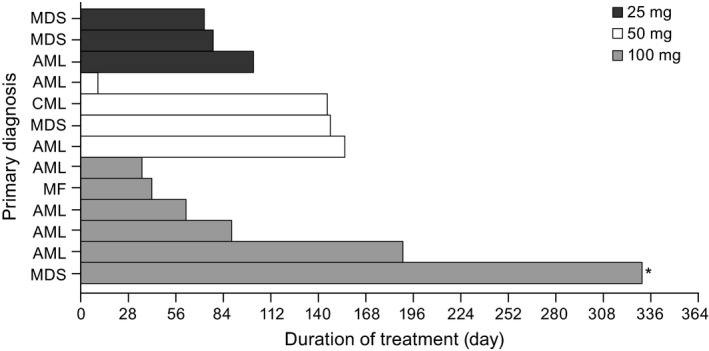
Duration of treatment with glasdegib across dose levels for individual patients. *Treatment is ongoing as of data cut. Duration of treatment: the duration from the date of first dose to one of the last dose (date of last dose – date of first dose + 1).
Pharmacokinetics
Maximum plasma concentration and exposure of glasdegib appeared to increase in a broadly dose‐proportional manner (25–100 mg). Median time to maximum plasma concentration was 2–4 h after single and multiple doses of glasdegib (Table4). The terminal elimination half‐life (t½) of glasdegib was 20.7 ± 7.7 h (mean ± SD) following a single dose of glasdegib (25–100 mg).
Table 4.
Summary of glasdegib pharmacokinetic parameters following single and multiple doses
| Parameter, units | Glasdegib dose | ||
|---|---|---|---|
| 25 mg | 50 mg | 100 mg | |
| Cycle 1 lead‐in day (single dose) | |||
| N | 3 | 4 | 6 |
| C max, ng/mL | 281 (96) | 321 (57) | 1019 (25) |
| T max, h | 2.0 (1.1–4.0) | 4.0 (2.0–7.3) | 2.0 (0.9–4.0) |
| AUCinf , ng•h/mL | 3346 (68) | 9587 (64) | 13102 (20) |
| CL/F, L/h | 7.47 (68) | 5.22 (64) | 7.63 (20) |
| Vz/F, L | 165 (66) | 225 (51) | 184 (21) |
| t ½, h | 15.3 (1.6) | 30.3 (5.4) | 17.1 (3.9) |
| Cycle 1/Day 21 (multiple dose) | |||
| n | 3 | 3 | 6 |
| C max, ng/mL | 357 (90) | 542 (9.9) | 1330 (12) |
| T max, h | 4.0 (3.9–4.0) | 4.0 (3.9–7.3) | 2.0 (0.9–3.9) |
| AUCtau, ng•h/mL | 4562 (99) | 9310 (14) | 15502 (26) |
| C avg, ng/mL | 190 (99) | 388 (14) | 646 (26) |
| C trough, ng/mL | 88 (139) | 240 (37) | 343 (35) |
Data are geometric mean (geometric %CV) for all parameters except: median (range) for T max; arithmetic mean (±SD) for t ½. AUCinf, area under the plasma concentration–time curve from time zero to infinity; AUCtau, area under the plasma concentration–time curve from time zero to 24 h; C avg, average plasma concentration at steady state; C max, maximum plasma concentration; C trough, lowest plasma concentration before the next dose; CV, coefficient of variation; t ½, terminal elimination half‐life; T max, time to maximum plasma concentration; Vz/F, volume of distribution.
Pharmacodynamics
A marked (>80%) downregulation of GLI1 expression from skin biopsies of glasdegib‐treated patients was observed at steady state in the 50 and 100 mg groups. However, marked downregulation of GLI1 expression was not observed at steady state in the 25‐mg group (Figs 2 and 3). For the 100‐mg group, the mean average plasma concentration (C avg) was 646 ng/mL, with low variability (coefficient of variation of 26%).
Figure 2.
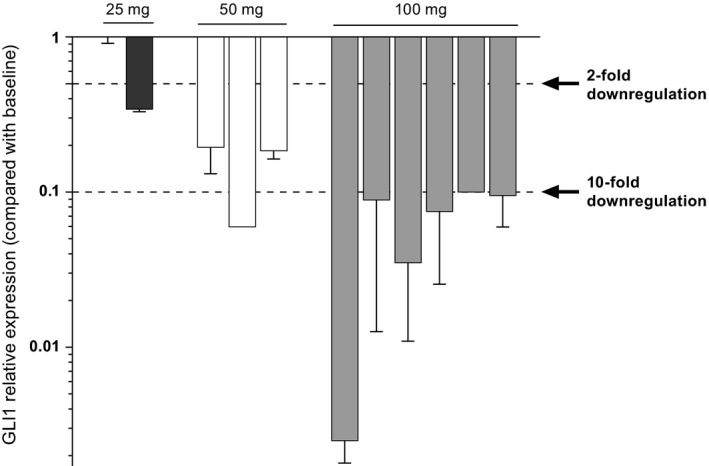
GLI1 relative expression (versus baseline) from skin biopsies following treatment with glasdegib across dose levels.
Figure 3.
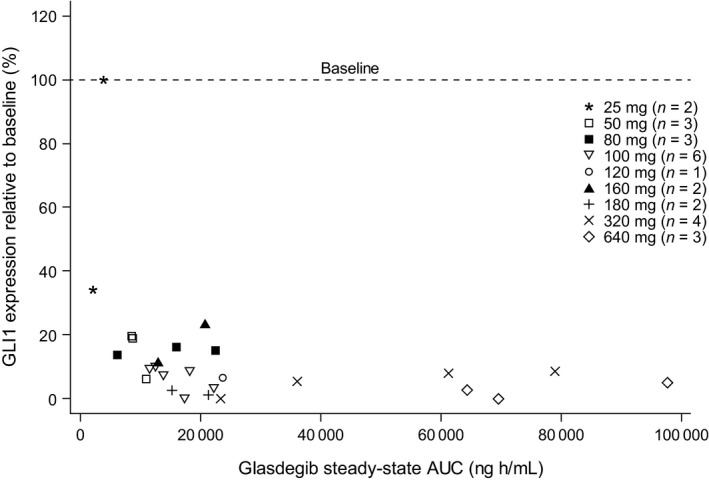
GLI1 inhibition versus AUC.
Preliminary clinical activity
Preliminary clinical activity data showed that 1 (8%) patient with AML in the 100‐mg group achieved morphological complete remission as the best response. Four patients (31%) with AML (1 each at 25 and 50 mg, and 2 in the 100‐mg group) achieved stable disease. One patient with MDS in the 100‐mg group achieved marrow complete remission. Two (16%) with MDS (1 each at 25 and 100 mg) achieved stable disease.
Discussion
In this ongoing study, glasdegib appeared safe and well tolerated with dose‐proportional PK and evidence of clinical activity in Japanese patients with select hematologic malignancies across the dose levels tested (25–100 mg). Similar treatment‐related AE were observed in a study of glasdegib in a phase I study in European and American patients with hematologic malignancies,12 and were considered to be consistent with the toxicities observed with other Hh pathway inhibitors.11, 12, 15 None of the deaths observed in this study were considered to be treatment‐related.
The clinically recommended dose of glasdegib that was identified in previously reported studies in European and American patients was 100 mg.16, 17 The recommended dose was selected based on a comprehensive review of the available clinical data (safety, efficacy and PK outcomes) from the phase I evaluation of glasdegib. Therefore, the primary objective of this study was also to determine first‐cycle DLT up to 100 mg. However, this study was initiated from the lower dose (50 mg) because this study was the first in Japanese patients with select hematologic malignancies. In addition, it was decided to add the 25‐mg dose cohort to understand the glioma‐associated transcriptional regulator GLI1 expression of glasdegib globally in the lower dose level, as the 25‐mg dose level had not been tested globally. It was confirmed that the 100‐mg dose was safe and tolerable in Japanese patients, and the maximum tolerated dose was not reached in this study. Glasdegib demonstrated dose‐proportional PK across the dose range in this study (25–100 mg). Although data are limited, this ongoing study showed that glasdegib demonstrated similar PK parameters to those reported in European and American patients with hematologic malignancies following single and multiple dosing.12 Furthermore, GLI1 was suppressed at 100 mg. The 100‐mg dose level will be used for the further assessment of the clinical activity of glasdegib in Japanese patients.
Downregulation of GLI1 is associated with inhibition of tumor growth.18 As seen with other SMO inhibitors,19 substantial and sustained Hh pathway inhibition (indicated by reduced GLI1 expression) was seen in patients with disease control, which is consistent with the role of Hh pathway signaling in the pathogenesis of basal cell carcinoma,20, 21, 22 and indicates an association between GLI1 inhibition and tumor response. Although there is no clear direct evidence that GLI1 suppression in skin correlates with suppression in hematologic malignancy, the mechanism of action of glasdegib was confirmed, taking into account preclinical data such as tissue distribution and xenograft models.20, 21, 22 A marked (>80%) downregulation of GLI1 expression was observed in the skin tissue in this Japanese population following treatment with glasdegib at doses ≥50 mg. This was consistent with findings from a PK/PD analysis of glasdegib at steady state in European and American patients with hematologic malignancies, which showed similar downregulation of GLI1 expression at daily doses ranging from 120 to 270 mg.12 Our findings were also consistent with those from a study of glasdegib in patients with advanced solid tumors (80–640 mg), which reported a > 80% downregulation of GLI1 expression in the surrogate tissue of glasdegib‐treated patients.16 Although marked downregulation of GLI1 expression was not observed at the 25‐mg dose level, it was observed at 50 mg and above. This observation indicates that at the clinically recommended dose of 100 mg, glasdegib adequately modulates the Hh signaling pathway.
Although data are limited, preliminary evidence of clinical activity was noted in this study following treatment with glasdegib. In a phase I study in European and American patients with hematologic malignancies, some suggestion of clinical activity was noted in 23 of 47 patients.12 Another phase I study in patients with solid tumors reported evidence of stable disease in 35% of patients and prolonged (≥6 months) stabilization of disease in 13% of patients following glasdegib monotherapy.16 Preliminary evidence of clinical activity in Japanese patients was considered to be consistent with the preliminary efficacy in European and American patients.
In conclusion, glasdegib was safe and well tolerated across the dose levels tested (25–100 mg). The safety, tolerability and PK profiles of glasdegib in Japanese patients with select advanced hematologic malignancies were consistent with the results previously reported in European and American patients. Although data are limited, preliminary clinical activity was observed across dose levels tested, warranting further investigation in this patient population. The present study is currently enrolling separate cohorts of Japanese patients with previously untreated AML or high‐risk MDS to assess the safety, tolerability and PK of glasdegib administered in combination with low‐dose cytarabine or cytarabine plus daunorubicin.
Disclosure Statement
Dr Y Minami received research funding from Bristol‐Myers Squibb. Dr H Minami received research funding from Novartis, Chugai, Eisai, Boehringer Ingelheim, Taiho, Pfizer, Ono, AstraZeneca, Bristol‐Myers Squibb, Amgen Astellas BioPharma, Bayer, Kyowa Hakko Kirin, Eli Lilly, Daiichi Sankyo, Astellas and Takeda. Drs T Miyamoto, G Yoshimoto, W Munakata, Y Onishi, M Kobayashi and T Naoe have no conflicts of interest to declare. Dr Y Kobayashi received research funding from Pfizer, Ohtsuka and Astellas, and other payments (e.g. trips, travel or gifts, which were not related to research) from Pfizer. Drs G Chan, A Woolfson, C Ono, M Naveed Shaik, Y Fujii, and X Zheng were full‐time employees of Pfizer during the conduct of this study. Dr M Ikuta is a former employee of Pfizer.
Acknowledgments
Medical writing support was provided by Neel Misra of Engage Scientific Solutions and was funded by Pfizer.
Cancer Sci 108 (2017) 1628–1633
Funding Information
This study was supported by Pfizer
Clinical trial registration
ClinicalTrials.gov identifier NCT02038777.
References
- 1. Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001; 15: 3059–87. [DOI] [PubMed] [Google Scholar]
- 2. Irvine DA, Copland M. Targeting hedgehog in hematologic malignancy. Blood 2012; 119: 2196–204. [DOI] [PubMed] [Google Scholar]
- 3. McMillan R, Matsui W. Molecular pathways: the hedgehog signaling pathway in cancer. Clin Cancer Res 2012; 18: 4883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med 2009; 9: 873–86. [DOI] [PubMed] [Google Scholar]
- 5. Dierks C, Grbic J, Zirlik K et al Essential role of stromally induced hedgehog signaling in B‐cell malignancies. Nat Med 2007; 13: 944–51. [DOI] [PubMed] [Google Scholar]
- 6. Peacock CD, Wang Q, Gesell GS et al Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA 2007; 104: 4048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Munchhof MJ, Li Q, Shavnya A et al Discovery of PF‐04449913, a potent and orally bioavailable inhibitor of smoothened. ACS Med Chem Lett 2012; 3: 106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sadarangani A, Pineda G, Lennon KM et al GLI2 inhibition abrogates human leukemia stem cell dormancy. J Transl Med 2015; 13: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukushima N, Minami Y, Kakiuchi S et al Small‐molecule Hedgehog inhibitor attenuates the leukemia‐initiation potential of acute myeloid leukemia cells. Cancer Sci 2016; 107: 1422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson‐Fisher A, McMahon MJ, Lam J et al PF‐04449913, a small molecule inhibitor of the hedgehog signaling pathway, is effective in inhibiting tumor growth in preclinical models. Clin Cancer Res 2011; 71: 4504. [Google Scholar]
- 11. Rudin CM, Hann CL, Laterra J et al Treatment of medulloblastoma with hedgehog pathway inhibitor GDC‐0449. N Engl J Med 2009; 361: 1173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinelli G, Oehler VG, Papayannidis C et al Treatment with PF‐04449913, an oral smoothened antagonist, in patients with myeloid malignancies: a phase 1 safety and pharmacokinetics study. Lancet Haematol 2015; 2: e339–46. [DOI] [PubMed] [Google Scholar]
- 13. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med 1999; 131: 207–19. [DOI] [PubMed] [Google Scholar]
- 14. Cohen MH, Johnson JR, Pazdur R. U.S. Food and Drug Administration Drug Approval Summary: conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin Cancer Res 2005; 11: 12–9. [PubMed] [Google Scholar]
- 15. Axelson M, Liu K, Jiang X et al U.S. Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res 2013; 19: 2289–93. [DOI] [PubMed] [Google Scholar]
- 16. Wagner AJ, Messersmith WA, Shaik MN et al A phase I study of PF‐04449913, an oral hedgehog inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2015; 21: 1044–51. [DOI] [PubMed] [Google Scholar]
- 17. Shaik MN, LaBadie RR, Rudin D, Levin WJ. Evaluation of the effect of food and ketoconazole on the pharmacokinetics of the smoothened inhibitor PF‐04449913 in healthy volunteers. Cancer Chemother Pharmacol 2014; 74: 411–8. [DOI] [PubMed] [Google Scholar]
- 18. Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 2010; 1805: 181–208. [DOI] [PubMed] [Google Scholar]
- 19. Migden MR, Guminski A, Gutzmer R et al Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double‐blind phase 2 trial. Lancet Oncol 2015; 16: 716–28. [DOI] [PubMed] [Google Scholar]
- 20. Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer 2008; 8: 743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gailani MR, Stahle‐Backdahl M, Leffell DJ et al The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet 1996; 14: 78–81. [DOI] [PubMed] [Google Scholar]
- 22. Reifenberger J, Wolter M, Knobbe CB et al Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol 2005; 152: 43–51. [DOI] [PubMed] [Google Scholar]


