Abstract
Background and purpose:
Rheumatoid arthritis (RA) causes frequently cardiovascular complications, probably determined by early atherosclerosis in connection to chronic systemic inflammation. Purpose of our study was to assess subclinical cardiac and vascular dysfunction, and to evaluate the mechanisms of ventriculo-arterial interaction, in patients with correctly treated RA vs. normal subjects.
Methods:
We evaluated 46 subjects (55±10 years, 2 men): 29 patients with seropositive treated RA (mean duration of 11±9 years), without documented cardiovascular or pulmonary disease, and 17 control subjects, matched for age, sex, and distribution of conventional major risk factors. All RA patients were under long-term treatment (more than 6 months) with Methotrexat + Sulfasalasine (22 patients) or Methotrexat + Sulfasalasine + Infliximab (7 patients). We determined biomarkers of inflammation (P-selectin, interleukines 1, 6, 10, 18, seric amiloid A, á-TNF, ã-interferon, C-reactive protein, anti-oxidated LDL antibodies), myocardial fibrosis (â-crosslaps) and ventricular overload (BNP). We assessed the parameters of cardiac function by standard and tissue Doppler echocardiography, intima-media thickness and arterial stiffness by “e-tracking” and “wave intensity analysis” (at the level of the right carotid artery), endothelial function by flow mediated dilation (FMD), and carotid-femoral pulse wave velocity by the Complior method.
Results:
Biological parameters of inflammation, markers of myocardial fibrosis and of ventricular overload were not different between the 2 study groups. Also, parameters of subclinical cardiac and vascular function were similar between the two groups. RA patients had subclinical RV dysfunction, correlated to the duration of the disease. They also tended to have higher values of systolic pulmonary artery pressure than normals.
Conclusion:
Correctly treated patients with RA, with controlled systemic inflammation, have normal LV, endothelial and arterial function. However, in the absence of documented pulmonary disease, they do have subclinical RV dysfunction, correlated with the duration of disease. This suggests an intrinsic RV myocardial involvement but, since pulmonary artery pressure was also higher, a secondary mechanism might be also involved.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic disease of uncertain cause. Although it has many general symptoms, the characteristic feature of the disease is persistent inflammatory synovitis, which affects usually peripheral joints and evolves symmetrically, causing alterations of the joint cartilage and bone erosions. Despite its destructive potential, RA has variable outcomes. Some patients develop only a slight joint discomfort of short duration, while others have progressive disease, with significant functional impairment.
The prevalence of RA in the general population is of approximately 0.8% (0.3 -2.1%), women being affected 3 times more frequently than men. The prevalence grows with age, while frequency differences between sexes decrease with the increase of age. The first signs of the disease appear usually during the 4th and 5th decades of life and 80% of patients develop the disease between the age of 35 and 50 (1, 2).
RA associates a cardiovascular risk and mortality rate 3-5 times higher than the general population matched for sex and age. Cardiovascular disease is responsible for 35-50% of the mortality excess in patients with rheumatoid arthritis (RA), being followed by cerebrovascular disease as second cause of mortality in these patients (3-5). Traditional atherosclerosis risk factors don’t explain this excess of cardiovascular mortality and morbidity, indicating that other mechanisms may be involved, among which chronical inflammation is the most studied. Major cardiovascular events, such as myocardial infarction, unstable angina pectoris or sudden cardiac death occur approximatively 10 years earlier in patients with RA, suggesting that this disease, similarly to diabetes mellitus, represents a significant independent risk factor for early atherosclerosis (6). The immunological disorder and the systemic inflammation in RA play a major role in the development of early atherosclerosis and premature mortality. Factors determinating high cardiovascular mortality in patients with RA appear early in the natural history of the disease, because patients with newly diagnosed sero-positive RA have already elements of endothelial dysfunction (7) .
Consequently, the adequate control of chronic inflammation in RA might reduce cardiovascular risk. Many studies have demonstrated the fundamental role of inflammation in the pathogenesis of atherosclerosis. The presence of high grade systemic inflammation in RA might explain the development of cardiovascular disease (8). A series of similarities between atherogenic lesions and chronical synovitis have been underlined. Inflammation mediators from the synovial joint tissue reach systemic circulation in high concentrations and thus might affect the vascular endothelium and also the myocardium – generating proatherogenic lesions and myocardial fibrosis (9).
Despite the accumulated data regarding cardiovascular risk factors in RA, they mainly result from retrospective, not prospective studies.
The purpose of our study was to assess subclinical cardiac and vascular dysfunction, and to evaluate the mechanisms of ventriculo-arterial interaction, in patients with correctly treated RA versus normal subjects.
METHOD
Subjects
46 subjects (55±10 years, 2 men) were enrolled into the study: 29 patients with seropositive treated RA (mean duration of 11±9 years), without documented cardiovascular disease or pulmonary disease, and 17 control subjects, matched for age, sex, and distribution of conventional major risk factors. All RA patients were under long-term treatment (more than 6 months) with Methotrexat + Sulfasalasine (22 patients) or Methotrexat + Sulfasalasine + Infliximab (7 patients).
46 subjects (55±10 years, 2 men) were enrolled into the study: 29 patients with seropositive treated RA (mean duration of 11±9 years), without documented cardiovascular disease or pulmonary disease, and 17 control subjects, matched for age, sex, and distribution of conventional major risk factors. All RA patients were under long-term treatment (more than 6 months) with Methotrexat + Sulfasalasine (22 patients) or Methotrexat + Sulfasalasine + Infliximab (7 patients).
Biological assessment
We determined biomarkers of inflammation (P-selectin, interleukins 1, 6, 10, 18, seric amyloid A, á-TNF, and ã-interferon, C - reactive protein, and anti-oxidated LDL antibodies), myocardial fibrosis (â-crosslaps) and ventricular overload (BNP).
Echocardiography
Conventional echocardiography was performed on a commercially available ultrasound machine (General Electric VIVID 7), using a 1.5- 5 MHz transducer and consisted of M-mode, 2D, and Doppler blood flow measurements. Mmode tracings from the parasternal long axis view were used to measure aortic root, left atrium diameter, systolic and diastolic septal and posterior wall thickness, left ventricular diameters, fractional shortening, and left ventricular mass index (method of Devereaux with the application of Penn convention). Cross sectional images were recorded from the apex, and enddiastolic and end-systolic areas and left ventricular lengths were measured for the calculation of ejection fraction (modified biplane Simpson’s method). For the evaluation of right ventricular systolic function we assessed, using the apical 4 chamber vue, the right ventricular fractional area change (FAS), as: (RV end-diastolic area- RV end-systolic area)/RV end-diastolic area (12)
Diastolic function was assessed by pulsedwave Doppler of the transmitral flow; E/A ratio being calculated. Left ventricular inflow was recorded by color M-mode echocardiography, and flow propagation velocity was measured.
Tissue Doppler recordings were made in 4 incidences: parasternal long axis, apical four chambers, apical two chambers and apical three chambers, and were interpreted offline at the level of seven myocardial segments in order to assess longitudinal function from the mean velocities of six basal segments (from the apical four-chamber, two-chamber, and three-chamber view). Peak myocardial velocities in systole (STDI) and diastole (ETDI – early and ATDI – late diastolic waves) were measured.
On-line pulsed-wave tissue Doppler recordings were made at the level of the medial and lateral mitral annulus, as well as the lateral tricuspid annulus.
For the evaluation of the right ventricular function, we determined also, at the level of the lateral tricuspid annulus isovolumetric acceleration (IVA) and we calculated the RV myocardial performance index- RVMPI, according to current guidelines (12) as:
RVMPI = (IVCT+IVRT)/EjT
where IVCT- isovolumic contraction time, IVRT- isovolumic relaxation time, EjT-ejection time
2D - speckle tracking imaging was used in order to assess longitudinal myocardial deformation, according to the current recommendations (13). A frame rate of 70–80 frames/s was used during the whole examination, with an optimal sector width and image depth, without a dualfocusing option. Using off-line analysis, global longitudinal strain was obtained– a negative percent of deformation. We identified the frame in which LV endocardium was best defined and we traced manually the border of the endocardium, in order to identify the region of interest (ROI) between the endocardial and epicardial borders. All LV walls were divided into 3 sites: basal, medial, and apical. Cardiac cycle intervals were measured using pulsed wave tracing from the LV outflow tract (aortic valve opening – AVO, and aortic valve closure – AVC). Global longitudinal strain (GLS) was calculated from 18 ventricular sites from the apical chamber views. We also calculated RV GLS, from 6 right ventricular sites, using the 4 chamber apical view.
Arterial structure and function
After resting supine for 15 minutes, arterial assessment was performed at the right common carotid artery (RCCA) level, using another ultrasound machine (ALOKA SSD 5500, á 10), with a high resolution ultrasound transducer (7.5 MHz linear array probe). Intima-media thickness (IMT), was measured 1 cm bellow the bulb of the RCCA.
Echo-tracking and wave intensity analysis were used to assess arterial stiffness, forward and backward waves propagation, and ventriculoarterial coupling. Measurements were taken as mean of five beats. RCCA diameter waveforms change was obtained, and by calibration for blood pressure, augmentation index (AIx), an index of arterial stiffness was calculated according to the formula:
AIx = AP/PP x 100%
where AP is augmentation pressure (difference between the second and the first systolic peak on the arterial trace) and PP is pulse pressure.
Wave intensity (WI) was calculated according to the formula:
WI = (dP/dt) (dU/dt)
where P is blood pressure and U flow velocity, in respect to time (t). This is a validated method (14) that allows estimation of the forward and backward traveling arterial waves in the early and late systole (compression and expansion waves, respectively). As an index of ventriculoarterial coupling, we measured the amplitude of the forward, compression wave (CW).
Finally, we assessed pulse wave velocity (PWV), using a validated non-invasive automated device (Complior, Artech Medical, Paris, France). After placing the transducers on the carotid and femoral arterial sites, carotid-femoral transit time (dt) has been measured. Distance (dD) traveled by the pulse wave was assessed with a zerolength measurement over the surface of the body, with a non-elastic tape. Pulse wave velocity was calculated as the distance divided by the transit time (dD/dt).
Simultaneously with arterial assessment, brachial arterial blood pressure was measured by an automated sphygmomanometer (Omron 705CP, Tokyo, Japan).
Endothelial function
Endothelial function was assessed with the same ALOKA 5500 á 10 machine. Internal diameter of the right brachial artery was monitored for 10 minutes: 1 min at rest, 5 min during a forearm ischemia induced by inflation up to 250 mmHg of a pneumatic forearm cuff, and 4 min after deflating the cuff. In order to minimize operator dependent error, a mechanical probe holder was used. Measurements were taken as a mean of five beats of every phase. Flow-mediated dilation (FMD) was calculated as the percent of maximal diameter change at the right brachial artery level, observed during reactive hyperaemia following deflation of the forearm occluding- cuff, according to the formula:
FMD = [(diameter after cuff deflation – resting diameter)/resting diameter] x 100% also measured.
Reproducibility
We have reported detailed studies of reproducibility of tissue Doppler data elsewhere (15- 17). Reproducibility of ultrasound assessment of arterial and endothelial function in our laboratory was measured in 20 subjects by two observers; intra-, and inter- variability are reported as standard deviation divided by its corresponding mean value, to give a coefficient of variation (CV in %) (18).
STATISTICAL ANALYSIS
Statistical analysis was performed with SPSS software (version 16.0) (SPSS Inc. Chicago, Illinois). Results are presented as mean value ±standard deviation. Differences between groups were tested for significance using the independent samples t-test. Linear regression was used to investigate the relation between two parametric variables in the total population. ANCOVA univariate analysis of variance was used with heart rate as a covariate, to assess influence of heart rate on the arterial stiffness parameters (19). A p<0.05 for a two-tailed test was considered significant.
RESULTS
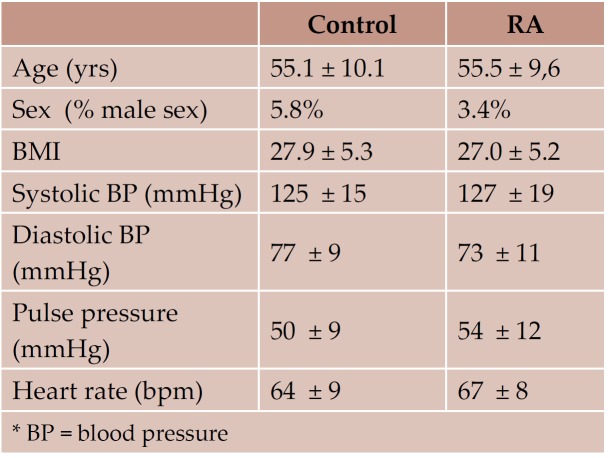
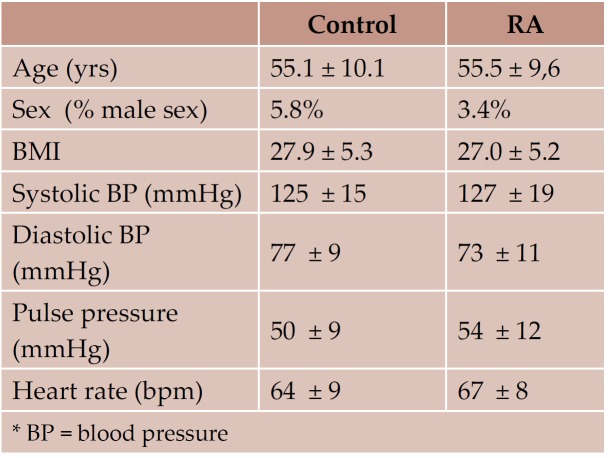
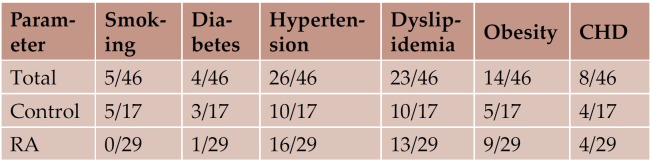
General characteristics of the study groups are shown in Table 1 and 2. There were no significant differences between the two groups for age, height, weight, body mass index, or distribution of classical cardiovascular risk factors. The incidence of already diagnosed chronic heart disease was similar in the 2 study groups. None of the RA patients had known family history of rheumatoid arthritis or other systemic disease.
Biological assessment
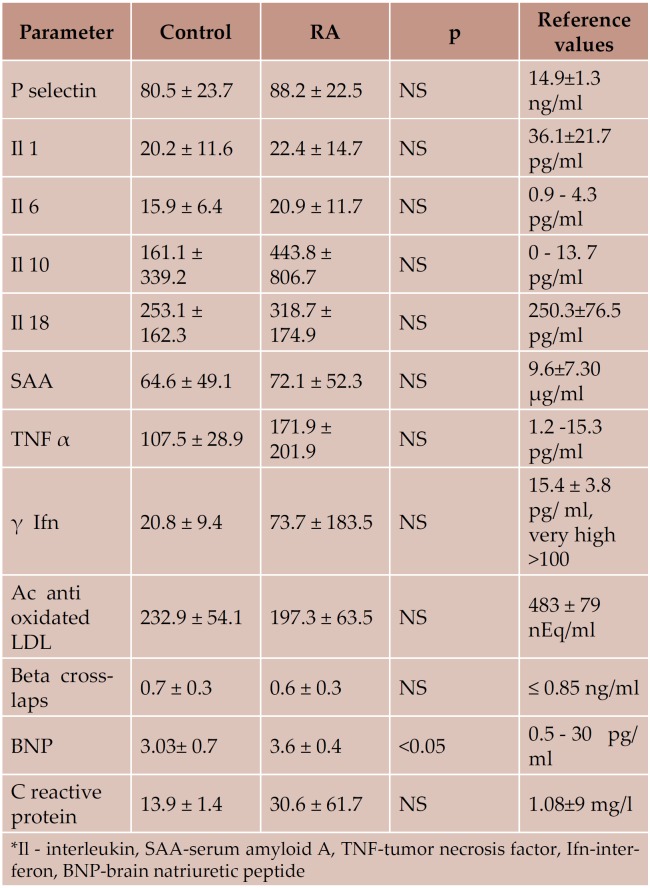
There was no significant difference between biological markers of inflammation, myocardial fibrosis and ventricular overload between controls and RA (Table 3). We noticed in both groups elevated values of some markers of inflammation (P selectin, interleukin 6 and 10, serum amyloid A, TNF β, δ interferon and C reactive protein). Markers of myocardial fibrosis were in normal limits in both study groups. BNP had significantly higher values in the RA group, but both values were in normal range. There were no statistical significant correlations between biological markers and the echocardiographic parameters (standard, Tissue Doppler) or those of vascular function.
Echocardiography
Conventional echo data are listed in Table 4. There were no statistical significant differences between the 2 groups and the values obtained were in the normal range. Myocardial systolic and diastolic velocities, as well as GLS, assessed by tissue Doppler and speckle tracking, are also shown in Table 4.
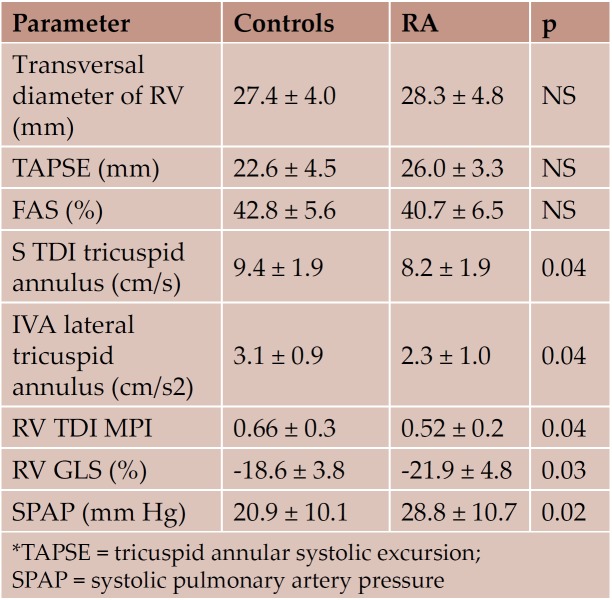
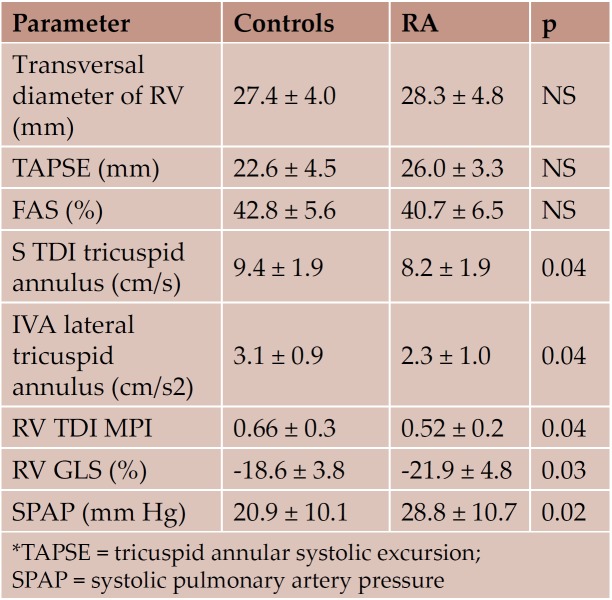
Regarding the evaluation of the right heart, standard echocardiography parameters were not different between the 2 study groups. TDI tricuspid annular velocities, IVA, MPI and RV GLS were significantly lower in the RA group. RVGLS inversely correlated with the duration of the disease (r=-0.45, p=0.04) (Figure 1). Although SPAP was normal in both study groups, SPAP values were significantly higher in the RA group (Table 5).
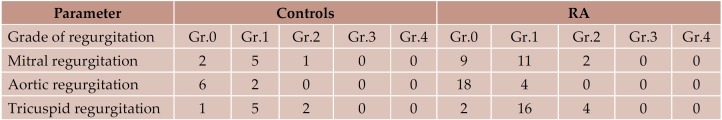
None of the examined patients presented hemodynamic significant valvular heart disease. We detected only mild or medium regurgitation, with no valvular stenosis (Table 6).
Arterial and endothelial function
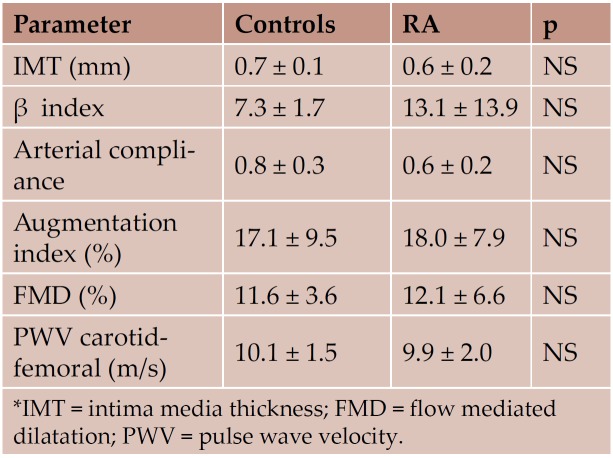
The results of the assessment of arterial and endothelial functions are shown in Table 7. The vascular function parameters were in normal ranges for the age of the patients, with no statistical significant values between the 2 groups.
Reproducibility
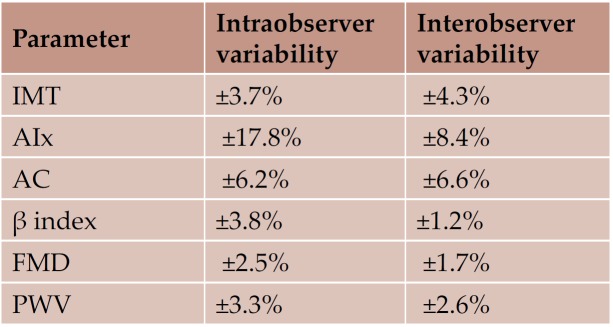
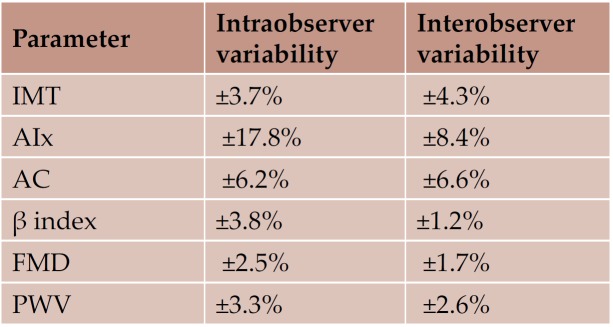
Reproducibility of tissue Doppler data was previously reported (14-16); interobserver variability was ± 6.8% for the radial velocities, and between ± 2.0% and ± 6.1% for the longitudinal velocities, while intraobserver variability was ± 2.7% for the radial velocities, and between ±1.8% and ± 2.5% for the longitudinal velocities. Reproducibility of the assessment of arterial and endothelial functions is shown in Table 8 (18).
Subgroups
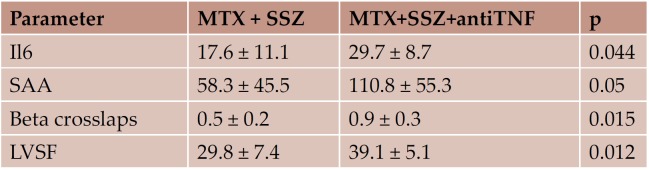
Regarding the subgroups analysis of the RA patients, there were no statistical relevant differences between most of the biological parameters, excepting Il6, SAA and beta-crosslaps. Concerning echocardiographic parameters, the LVEF was significantly higher in the subgroup treated with anti TNF agents. Differences between subgroups are shown in Table 9.
Table 1.
Table 1. Demographical characteristics of the study groups
Table 2.
Table 2. Distribution of classical risk factors and known chronic heart disease (number of patients)
Table 3.
Table 3. Biological assessment- RA vs. control group.
Table 4.
Table 4. Standard echocardiographic data and myocardial velocities (cm/s) assessed by tissue Doppler imaging in the study groups (mean ± SD).
Table 5.
Table 5. RV structural and functional parameters (standard echocardiography and TDI, mean ± SD)
Table 6.
Table 6. The presence and grading of valvular regurgitation in the 2 study groups
Table 7.
Table 7. Vascular function parameters (mean ± SD)
Figure 1.
Figure 1.
Table 8.
Table 8. Reproducibility of vascular function parameters (Bland Altman coeffi cient) (17)
Table 9.
Table 9. Subgroup analysis of the RA patients (statistically signifi cant diff erences, mean ± SD).
Table 10.
Table 10. Similarities between atherosclerosis and RA (9)
Table 11.
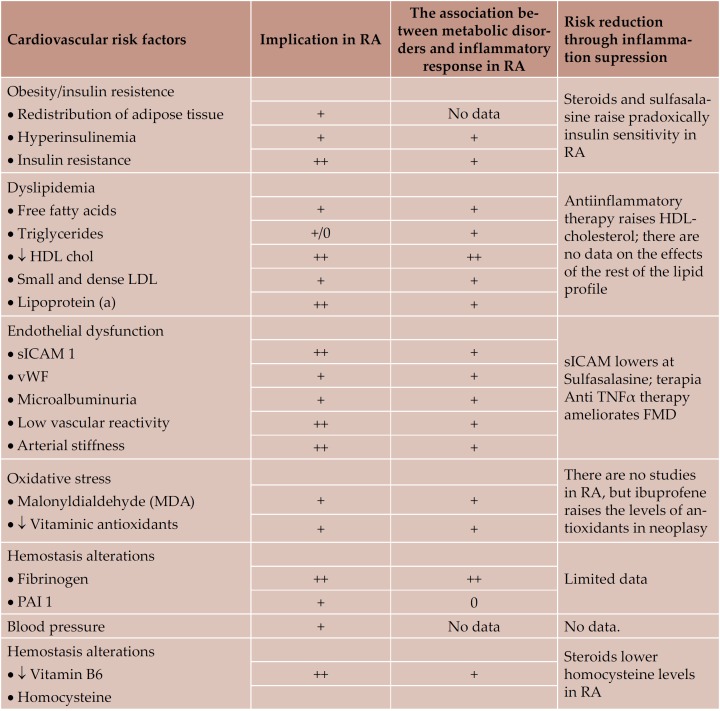
Table 11. Cardiovascular risk factors in RA, their association with infl ammatory response and the eff ects of infl ammation supression on them (26)
Table 12.
Table 12. Therapeutic strategies in RA (1, 29)
DISCUSSSION
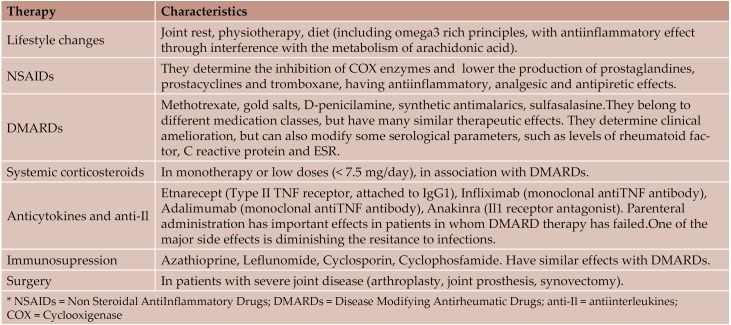
RA is a chronic inflammatory disease, with headquarters at the joint level, but which can affect most of the main inner organs. Having similar mechanisms with those of atherosclerosis (Table 10) and being frequently associated with classical atherosclerosis risk factors (Table 11), RA comes with high cardiovascular morbidity and mortality (4-6, 9). Recent studies have suggested a much higher cardiovascular affectation in RA than in normals matched in sex and age, even similar to that in diabetes mellitus (6, 20). There are multiple therapeutic alternatives in RA, which act through antiinflammatory and immunosupressive mechanisms and often lead to a limitation of joint disease and augmentation of the quality of life (Table 12) (1, 2, 21-25). Their effects on subclinical cardiovascular dysfunction has been little studied until now (21-25, 26).
Our study has aimed to perform an extensive cardiovascular evaluation in patients with long term (over 6 months) treated RA, through biological methods, quantifying inflammation, myocardial fibrosis and cardiac dysfunction, as well as through modern ultrasonographic methods, compared to normal subjects, matched in sex, age, and distribution of classical cardiovascular risk factors. The purpose was to verify the efficacy of antirheumatoid therapies on subclinical atherosclerotic disease.
The biological parameters used were those mentioned in literature to be associated with inflammation in RA as well as in early atherosclerosis (8, 9, 27). We also used markers of myocardial fibrosis and ventricular overload (BNP). Concerning the echocardiographic examination, we have extended beyond standard parameters, using new methods, such as Tissue Doppler Imaging or Speckle Tracking, which allow the detection of early systolic or diastolic dysfunction. We also focused on the ultrasonographic evaluation of ventriculo-arterial coupling, because arterial stiffness induced by atherosclerotic determinations may lead over time to cardiac dysfunction. (28). Endothelial function was determined through vascular ultrasound using the method of flow mediated dilation. Also, we have included in our examination the measurement of pulse wave velocity (COMPLIOR method), as supplementary marker of arterial rigidity, mainly of great arteries.
The right heart was also evaluated, because of the frequent occurence of pulmonary hypertension and consequent right ventricular dysfunction in autoimmune disease with associated vasculitis (29, 30).
The results of our study have shown that in treated RA there are no significant differences compared to normals. Biological parameters of inflammation, myocardial fibrosis and ventricular overload, as well as ultrasonographic determination of systolic and diastolic function, were similar in the 2 study groups and were situated in normal range. Both subjects with RA and normals, had an endothelial function (assessed through FMD) and an arterial stiffness, according to their age.
An important finding of our study concerns the right heart: RA patients, although correctly treated, have subclinical RV dysfunction, correlated to the duration of the disease. Also, RA patients tend to have higher values of systolic pulmonary artery pressure than normals. This should be taken into consideration for the follow up of patients with RA and also when implementing prevention therapies.
Regarding the 2 subgroups of RA patients (Methotrexate + Sulfasalasine vs. Methotrexate +Sulfasalasine +AntiTNF-Infliximab) the studied biological and ultrasound parameters were in their majority similar, with some exceptions. In the infliximab group, we have noticed significantly higher values of interleukine 6 and SAA (suggesting less control of inflammation) as well as of γ crosslaps (as markers of subclinical myocardial fibrosis). On the other hand, the infliximab group had a higher LV shortening fraction, probably acting as an early compensatory mechanism for myocardial fibrosis.
The results of our study indicate that current therapeutic methods in RA are efficient not only regarding the control of joint disease, but also of systemic inflammation and cardiovascular morbidity.
CONCLUSSION
Patients with correctly treated RA and controlled systemic inflammation, regardless of the disease duration, do not have subclinical cardiac and vascular dysfunction. Being considered a disease with significant cardiovascular risk, similar to diabetes, RA needs quick implementa- TABLE 12. Therapeutic strategies in RA (1, 29) tion of cardiovascular prevention and an early start of treatment.
Conflict of interests: none declared.
Financial support: This work was supported by a grant of the Romanian Ministry of National Education and Scientific Research [grant CEEX I 14/2006] and by the Sectorial Operational Programme Human Resources Development (SOPHRD) financed by the European Social Fund and the Romanian Governement under the contract number POSDRU 141531 [POSDRU/159/1.5/S/141531 to SLM].
Acknowledgements statement: The authors of the article entitled “The assessment of subclinical cardiovascular dysfimction in treated rheumatoid arthritis”,would like to thank dr. Aurora Arghir, for her review of this article, as tutor of the first author, according to POSDRU 141531.
Contributor Information
Stefania L MAGDA, Department of Cardiology, University and Emergency Hospital, Bucharest, Romania.
Raluca I MINCU, Department of Cardiology, University and Emergency Hospital, Bucharest, Romania.
Maria FLORESCU, Department of Cardiology, University and Emergency Hospital, Bucharest, Romania.
Andrea O CIOBANU, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania.
Gabriela F UDREA, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania.
Mircea CINTEZA, Department of Cardiology, University and Emergency Hospital, Bucharest, Romania; University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania.
Dragos VINEREANU, Department of Cardiology, University and Emergency Hospital, Bucharest, Romania; University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania.
REFERENCES
- Harrison’s Principles of Internal Medicine - Harrison’s Principles of Internal Medicine. Sixteenth Edition, McGraw- Hill. 2005:1968–1977. [Google Scholar]
- Alehata D, Tuhina N, Silman AJ and al. - 2010 rheumatoid arthritis classifi cation criteria: an American College of Cardiology/ European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;6:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- Kaplan JM. - Cardiovascular disease in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18:289–297. doi: 10.1097/01.bor.0000218951.65601.bf. [DOI] [PubMed] [Google Scholar]
- Prior P, Symmons DP, Scott DL, et al. - Cause of death in rheumatoid arthritis. Br J Rheumatol. 1984;23:92–99. doi: 10.1093/rheumatology/23.2.92. [DOI] [PubMed] [Google Scholar]
- Jacobsson LT, Knowler WC, Pillemer S, et al. - Rheumatoid arthritis and mortality. A longitudinal study in Pima Indians. Arthritis Rheum. 1993;36:1045–1053. doi: 10.1002/art.1780360804. [DOI] [PubMed] [Google Scholar]
- Del Rincon ID, Williams K, Stern MP, et al. - High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- Bergholm R, Leirisalo-Repo M, Vehkavaara S, et al. - Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol. 2002;22:1637–1641. doi: 10.1161/01.atv.0000033516.73864.4e. [DOI] [PubMed] [Google Scholar]
- Danesh J, Whincup P, Walker M, et al. - Low grade infl ammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasceri V, Yeh ET. - A tale of two diseases : atherosclerosis and rheumatoid arthritis. Circulation. 1999;100:2124–2126. doi: 10.1161/01.cir.100.21.2124. [DOI] [PubMed] [Google Scholar]
- Sugawara J, Maeda S, Otsuki T, Tanabe T, Ajisaka R, Matsuda M. - Effects on nitric oxide synthase inhibitor on decrease in peripheral arterial stiff ness with acute low-intensity aerobic exercise. Am J Physiol Heart Circ Physiol. 2004;287:2666–69. doi: 10.1152/ajpheart.00077.2004. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, et al. - International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelialdependent fl ow-mediated vasodilation of the brachial artery: a report of the International brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Rudski LG, Lai WW, Afi lalo J, et al. - Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Amundsen BH, Helle-Valle T, Edvardsen T, et al. - Noninvasive myocardial strain measurment by speckle tracking echocardiography: validation against sonomicrometry and tagged resonance imaging. J Am Coll Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Niki K, Sugawara M, Chang D, et al. - A new noninvasive measurement system for wave intensity: evaluation of carotid arterial wave intensity and reproducibility. Heart Vessels. 2002;17:12–21. doi: 10.1007/s003800200037. [DOI] [PubMed] [Google Scholar]
- Florescu M, Stoicescu C, Magda S, et al. - Supranormal cardiac function in athletes related to beK er arterial and endothelial function; Ventriculo-arterial coupling in athletes. Echocardiography. 2010;27:659–67. doi: 10.1111/j.1540-8175.2009.01121.x. [DOI] [PubMed] [Google Scholar]
- Vinereanu D, Florescu N, Sculthorpe N, et al. – Diff erentiation between pathologic and physiologic left ventricular hypertrophy by tissue Doppler assessment of long-axis function in patients with hypertrophic cardiomyopathy or systemic hypertension and in athletes. Am J Cardiol. 2001;88:53–58. doi: 10.1016/s0002-9149(01)01585-5. [DOI] [PubMed] [Google Scholar]
- Vinereanu, Khokhar A, Fraser AG. - Reproducibility of pulsed wave tissue Doppler echocardiography. Echocardiogr. 1999;12:492–99. doi: 10.1016/s0894-7317(99)70086-6. [DOI] [PubMed] [Google Scholar]
- Magda SL, Ciobanu A, Florescu M, Vinereanu D - Comparative reproducibility of the noninvasive ultrasound methods for the assessment of vascular function. Heart and Vessels. 2013;28:143–50. doi: 10.1007/s00380-011-0225-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, MacCallum H, Flint L, et al. - The infl uence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Halm VP, Peters MJ, Voskuyl AE, et al. - Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE Investigation, Ann Rheum Dis. 2009;68:1395–400. doi: 10.1136/ard.2008.094151. [DOI] [PubMed] [Google Scholar]
- Hurlimann D, Forster A, Noll G, et al. - Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- Popa C, Netea MG, Radstake T, et al. - Infl uence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson LT, Turesson C, Gulfe A, et al. - Treatment with tumor necrosis factor blockers is associated with a lower incidence of fi rst cardiovascular events in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1213–1218. [PubMed] [Google Scholar]
- Fischer LM, Schlienger RG, Ma! er CM, et al. - Discontinuation of nonsteroidal anti-infl ammatory drug therapy and risk of acute myocardial infarction. Arch Intern Med. 2004;164:2472–2476. doi: 10.1001/archinte.164.22.2472. [DOI] [PubMed] [Google Scholar]
- Del Rincon I, O’Leary DH, Haas RW, Escalante A - Eff ect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis Rheum. 2004;50:3813–3822. doi: 10.1002/art.20661. [DOI] [PubMed] [Google Scholar]
- McCarey DW, McInnes IB, Madhok R, et al. – Trial of Atorvastatin in Rheumatoid Arthritis (TARA): a double-blind randomised placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- Sattar N, McCarey D, Capell H, McInnes IB. - Explaining how « high grade » systemic infl ammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108:2957–2963. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- Prabhu S. - Altered left ventricular-arterial coupling precedes pum dysfunction in early heart failure. Heart Vessels. 2007;22:170–177. doi: 10.1007/s00380-006-0954-9. [DOI] [PubMed] [Google Scholar]
- Carreira PE. - Pulmonary hypertension in autoimmune rheumatic diseases. Autoimmunity rewievs. 2004;3:313–320. doi: 10.1016/j.autrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Magda S, Vinereanu D. - Poliartrita reumatoida si disfunctia cardiovasculara subclinica. Progrese in Cardiologie vol III, Media Med Publicis. 2008:151–68. [Google Scholar]