Abstract
The gene for CF0 subunit I of ATP synthase has been located in wheat chloroplast DNA, between the genes for CF0 subunit III and α subunit of CF1. Nucleotide sequencing and analysis of RNA-DNA hybrids indicated that the gene is interrupted by an 823-bp intron which has boundaries similar to those previously described for the introns in protein-coding chloroplast genes of Euglena gracilis. The deduced amino acid sequence of CF0 subunit I indicates a polypeptide of 183 amino acid residues. However, N-terminal amino acid sequencing of the mature spinach CF0 subunit I suggests that the protein is synthesised with a N-terminal extension of 17 amino acid residues and is processed to give a protein of mol. wt. 19 001 of 166 amino acids residues. The mature CF0 subunit I shows similarities in primary and predicted secondary structure to F0 subunit b of Escherichia coli ATP synthase. A major transcript of 3.3 kb containing sequences from the genes for CF0 subunit III, subunit I and CF1 subunit α has been observed by RNA-DNA hybridisation.
Keywords: ATP synthase gene, chloroplast DNA, intron, transcripts, wheat
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aris J. P., Simoni R. D. Cross-linking and labeling of the Escherichia coli F1F0-ATP synthase reveal a compact hydrophilic portion of F0 close to an F1 catalytic subunit. J Biol Chem. 1983 Dec 10;258(23):14599–14609. [PubMed] [Google Scholar]
- Biggin M., Farrell P. J., Barrell B. G. Transcription and DNA sequence of the BamHI L fragment of B95-8 Epstein-Barr virus. EMBO J. 1984 May;3(5):1083–1090. doi: 10.1002/j.1460-2075.1984.tb01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Koller B. Sequence homologies between Escherichia coli and chloroplast ribosomal DNA as seen by heteroduplex analysis. J Mol Biol. 1980 Sep 15;142(2):247–261. doi: 10.1016/0022-2836(80)90048-0. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty A., Gray J. C. Synthesis of a dicyclohexylcarbodiimide-binding proteolipid by isolated pea chloroplasts. Eur J Biochem. 1980;108(1):131–136. doi: 10.1111/j.1432-1033.1980.tb04703.x. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Rochaix J. D. Chlamydomonas reinhardii gene for the 32 000 mol. wt. protein of photosystem II contains four large introns and is located entirely within the chloroplast inverted repeat. EMBO J. 1984 Dec 1;3(12):2753–2762. doi: 10.1002/j.1460-2075.1984.tb02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Deininger P. L., Bankier A., Barrell B. Homologous upstream sequences near Epstein-Barr virus promoters. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1565–1569. doi: 10.1073/pnas.80.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Sebald W. The proton conducting F0-part of bacterial ATP synthases. Biochim Biophys Acta. 1984 Apr 9;768(1):1–27. doi: 10.1016/0304-4173(84)90005-3. [DOI] [PubMed] [Google Scholar]
- Howe C. J., Auffret A. D., Doherty A., Bowman C. M., Dyer T. A., Gray J. C. Location and nucleotide sequence of the gene for the proton-translocating subunit of wheat chloroplast ATP synthase. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6903–6907. doi: 10.1073/pnas.79.22.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabin G. D., Farley M., Hallick R. B. Chloroplast gene for Mr 32000 polypeptide of photosystem II in Euglena gracilis is interrupted by four introns with conserved boundary sequences. Nucleic Acids Res. 1984 Jul 25;12(14):5801–5812. doi: 10.1093/nar/12.14.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
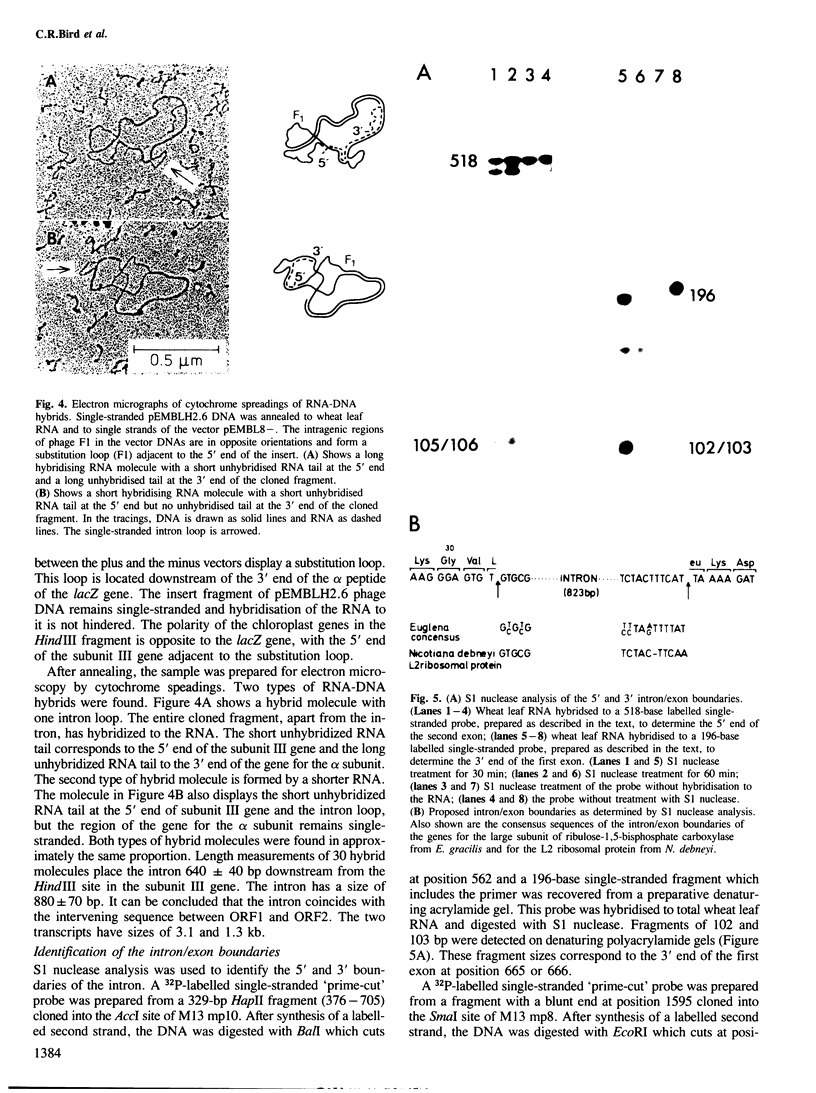
- Koller B., Delius H., Dyer T. A. The organization of the chloroplast DNA in wheat and maize in the region containing the LS gene. Eur J Biochem. 1982 Feb;122(1):17–23. doi: 10.1111/j.1432-1033.1982.tb05842.x. [DOI] [PubMed] [Google Scholar]
- Koller B., Delius H. Intervening sequences in chloroplast genomes. Cell. 1984 Mar;36(3):613–622. doi: 10.1016/0092-8674(84)90341-6. [DOI] [PubMed] [Google Scholar]
- Koller B., Gingrich J. C., Stiegler G. L., Farley M. A., Delius H., Hallick R. B. Nine introns with conserved boundary sequences in the Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. Cell. 1984 Feb;36(2):545–553. doi: 10.1016/0092-8674(84)90247-2. [DOI] [PubMed] [Google Scholar]
- Krebbers E. T., Larrinua I. M., McIntosh L., Bogorad L. The maize chloroplast genes for the beta and epsilon subunits of the photosynthetic coupling factor CF1 are fused. Nucleic Acids Res. 1982 Aug 25;10(16):4985–5002. doi: 10.1093/nar/10.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola-Morgenthaler L. R., Morgenthaler J. J., Price C. A. Synthesis of coupling factor CF1 protein by isolated spinach chloroplasts. FEBS Lett. 1976 Feb 1;62(1):96–100. doi: 10.1016/0014-5793(76)80025-7. [DOI] [PubMed] [Google Scholar]
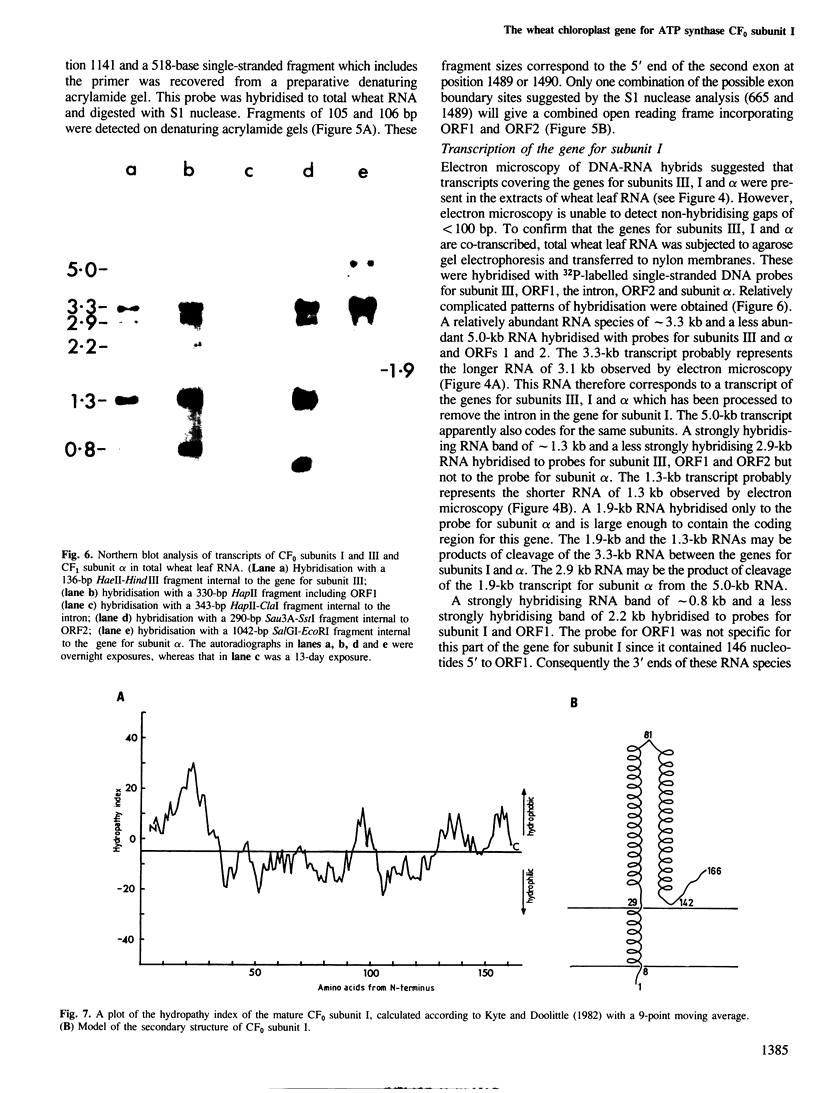
- Michel F., Dujon B. Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast- and nuclear-encoded members. EMBO J. 1983;2(1):33–38. doi: 10.1002/j.1460-2075.1983.tb01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon P. E., Stutz E. Nucleotide sequence of a Euglena gracilis chloroplast genome region coding for the elongation factor Tu; evidence for a spliced mRNA. Nucleic Acids Res. 1983 Sep 10;11(17):5877–5892. doi: 10.1093/nar/11.17.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Eytan E., Notsani B. E., Sigrist H., Sigrist-Nelson K., Gitler C. Isolation of a chloroplast N,N'-dicyclohexylcarbodiimide-binding proteolipid, active in proton translocation. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2375–2378. doi: 10.1073/pnas.74.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Schatz G. Biosynthesis and assembly of the proton-translocating adenosine triphosphatase complex from chloroplasts. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1361–1364. doi: 10.1073/pnas.77.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Cox D. N., Senior A. E. Integration of F1 and the membrane sector of the proton-ATPase of Escherichia coli. Role of subunit "b" (uncF protein). J Biol Chem. 1983 Aug 25;258(16):9793–9800. [PubMed] [Google Scholar]
- Pick U., Racker E. Purification and reconstitution of the N,N'-dicyclohexylcarbodiimide-sensitive ATPase complex from spinach chloroplasts. J Biol Chem. 1979 Apr 25;254(8):2793–2799. [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E. Secondary and tertiary structure of membrane proteins involved in proton translocation. Biochim Biophys Acta. 1983 Jul 15;726(2):81–95. doi: 10.1016/0304-4173(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Deno H., Kato A., Sugiura M. Overlap and cotranscription of the genes for the beta and epsilon subunits of tobacco chloroplast ATPase. Gene. 1983 Oct;24(2-3):147–155. doi: 10.1016/0378-1119(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G. L., Matthews H. M., Bingham S. E., Hallick R. B. The gene for the large subunit of ribulose-1,5-bisphosphate carboxylase in Euglena gracilis chloroplast DNA: location, polarity, cloning, and evidence for an intervening sequence. Nucleic Acids Res. 1982 Jun 11;10(11):3427–3444. doi: 10.1093/nar/10.11.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohdoh N., Sugiura M. The complete nucleotide sequence of 16S ribosomal RNA gene from tobacco chloroplasts. Gene. 1982 Feb;17(2):213–218. doi: 10.1016/0378-1119(82)90074-9. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Auffret A. D., Carne A., Gurnett A., Hanisch P., Hill D., Saraste M. Solid-phase sequence analysis of polypeptides eluted from polyacrylamide gels. An aid to interpretation of DNA sequences exemplified by the Escherichia coli unc operon and bacteriophage lambda. Eur J Biochem. 1982 Apr 1;123(2):253–260. doi: 10.1111/j.1432-1033.1982.tb19761.x. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. E. coli F1-ATPase interacts with a membrane protein component of a proton channel. Nature. 1982 Aug 26;298(5877):867–869. doi: 10.1038/298867a0. [DOI] [PubMed] [Google Scholar]
- Willey D. L., Auffret A. D., Gray J. C. Structure and topology of cytochrome f in pea chloroplast membranes. Cell. 1984 Feb;36(2):555–562. doi: 10.1016/0092-8674(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Junctions of the large single copy region and the inverted repeats in Spinacia oleracea and Nicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res. 1984 Aug 24;12(16):6547–6558. doi: 10.1093/nar/12.16.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]