Abstract
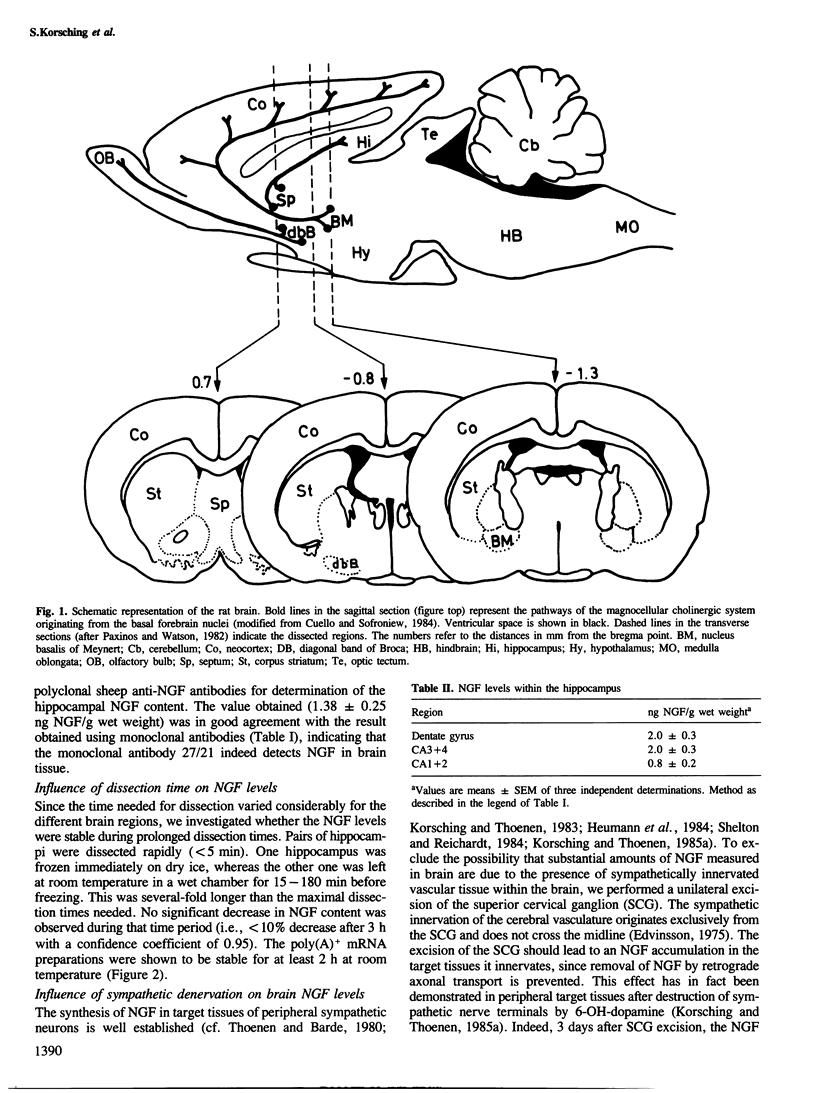
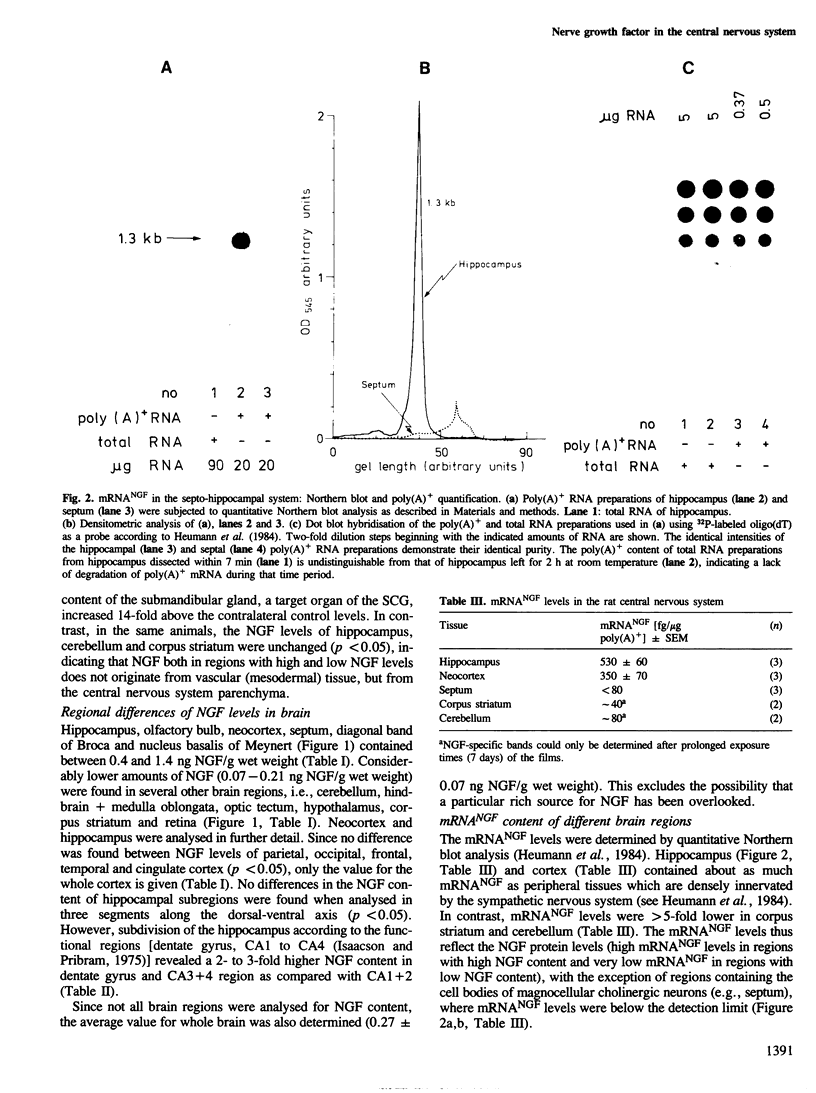
The levels of nerve growth factor (NGF) and its mRNA in the rat central nervous system were determined by two-site enzyme immunoassay and quantitative Northern blots, respectively. Relatively high NGF levels (0.4-1.4 ng NGF/g wet weight) were found both in the regions innervated by the magnocellular cholinergic neurons of the basal forebrain (hippocampus, olfactory bulb, neocortex) and in the regions containing the cell bodies of these neurons (septum, nucleus of the diagonal band of Broca, nucleus basalis of Meynert). Comparatively low, but significant NGF levels (0.07-0.21 ng NGF/g wet weight) were found in various other brain regions. mRNANGF was found in the hippocampus and cortex but not in the septum. This suggests that magnocellular cholinergic neurons of the basal forebrain are supplied with NGF via retrograde axonal transport from their fields of innervation. These results, taken together with those of previous studies showing that these neurons are responsive to NGF, support the concept that NGF acts as trophic factor for magnocellular cholinergic neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigl V., Woolf N. J., Butcher L. L. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982 Jun;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Crutcher K. A., Madison R., Davis J. N. A study of the rat septohippocampal pathway using anterograde transport of horseradish peroxidase. Neuroscience. 1981;6(10):1961–1973. doi: 10.1016/0306-4522(81)90036-1. [DOI] [PubMed] [Google Scholar]
- Dreyfus C. F., Peterson E. R., Crain S. M. Failure of nerve growth factor to affect fetal mouse brain stem catecholaminergic neurons in culture. Brain Res. 1980 Aug 4;194(2):540–547. doi: 10.1016/0006-8993(80)91239-1. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Sofroniew M. V. Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci. 1983 Nov;3(11):2286–2291. doi: 10.1523/JNEUROSCI.03-11-02286.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L. Neurogenic mechanisms in the cerebrovascular bed. Autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl. 1975;427:1–35. [PubMed] [Google Scholar]
- Fibiger H. C. The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Res. 1982 Nov;257(3):327–388. doi: 10.1016/0165-0173(82)90011-x. [DOI] [PubMed] [Google Scholar]
- Gnahn H., Hefti F., Heumann R., Schwab M. E., Thoenen H. NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a physiological role of NGF in the brain? Brain Res. 1983 Jul;285(1):45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- Godfrey D. A., Ross C. D., Herrmann A. D., Matschinsky F. M. Distribution and derivation of cholinergic elements in the rat olfactory bulb. Neuroscience. 1980;5(2):273–292. doi: 10.1016/0306-4522(80)90103-7. [DOI] [PubMed] [Google Scholar]
- Hefti F., Hartikka J., Eckenstein F., Gnahn H., Heumann R., Schwab M. Nerve growth factor increases choline acetyltransferase but not survival or fiber outgrowth of cultured fetal septal cholinergic neurons. Neuroscience. 1985 Jan;14(1):55–68. doi: 10.1016/0306-4522(85)90163-0. [DOI] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Scott J., Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J. 1984 Dec 20;3(13):3183–3189. doi: 10.1002/j.1460-2075.1984.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger P., Lenoir D. Nerve growth factor (NGF) stimulation of cholinergic telencephalic neurons in aggregating cell cultures. Brain Res. 1982 Feb;255(2):229–238. doi: 10.1016/0165-3806(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Tsukahara S., Tsuji T., Sugita K., Nagata T. Histochemical studies on the regeneration of aminergic nerves in rat cerebral artery after superior cervical ganglionectomy. Histochemistry. 1983;77(1):57–62. doi: 10.1007/BF00496636. [DOI] [PubMed] [Google Scholar]
- Konkol R. J., Mailman R. B., Bendeich E. G., Garrison A. M., Mueller R. A., Breese G. R. Evaluation of the effects of nerve growth factor and anti-nerve growth factor on the development of central catecholamine-containing neurons. Brain Res. 1978 Apr 14;144(2):277–285. doi: 10.1016/0006-8993(78)90154-3. [DOI] [PubMed] [Google Scholar]
- Korsching S., Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3513–3516. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S., Thoenen H. Nerve growth factor supply for sensory neurons: site of origin and competition with the sympathetic nervous system. Neurosci Lett. 1985 Mar 15;54(2-3):201–205. doi: 10.1016/s0304-3940(85)80079-3. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Nagy J. I., Atmadia S., Fibiger H. C. The nucleus basalis magnocellularis: the origin of a cholinergic projection to the neocortex of the rat. Neuroscience. 1980;5(7):1161–1174. doi: 10.1016/0306-4522(80)90195-5. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Aloe L., Mugnaini E., Oesch F., Thoenen H. Nerve growth factor induces volume increase and enhances tyrosine hydroxylase synthesis in chemically axotomized sympathetic ganglia of newborn rats. Proc Natl Acad Sci U S A. 1975 Feb;72(2):595–599. doi: 10.1073/pnas.72.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Loy R., Moore R. Y. Anomalous innervation of the hippocampal formation by peripheral sympathetic axons following mechanical injury. Exp Neurol. 1977 Nov;57(2):645–650. doi: 10.1016/0014-4886(77)90096-6. [DOI] [PubMed] [Google Scholar]
- McKinney M., Coyle J. T., Hedreen J. C. Topographic analysis of the innervation of the rat neocortex and hippocampus by the basal forebrain cholinergic system. J Comp Neurol. 1983 Jun 10;217(1):103–121. doi: 10.1002/cne.902170109. [DOI] [PubMed] [Google Scholar]
- Melera P. W., Rusch H. P. A characterization of ribonucleic acid in the myxomycete Physarum polycephalum. Exp Cell Res. 1973 Nov;82(1):197–209. doi: 10.1016/0014-4827(73)90262-0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosko S., Lynch G., Cotman C. W. The distribution of septal projections to the hippocampus of the rat. J Comp Neurol. 1973 Nov 15;152(2):163–174. doi: 10.1002/cne.901520204. [DOI] [PubMed] [Google Scholar]
- Olson L., Ebendal T., Seiger A. NGF and anti-NGF: evidence against effects on fiber growth in locus coeruleus from cultures of perinatal CNS tissues. Dev Neurosci. 1979;2(4):160–176. doi: 10.1159/000112451. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Sofroniew M. V., Cuello A. C., Powell T. P., Eckenstein F., Esiri M. M., Wilcock G. K. Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the Alzheimer's type demonstrated by immunohistochemical staining for choline acetyltransferase. Brain Res. 1983 Dec 19;289(1-2):375–379. doi: 10.1016/0006-8993(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Rennels M. L., Gregory T. F., Blaumanis O. R., Fujimoto K., Grady P. A. Evidence for a 'paravascular' fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985 Feb 4;326(1):47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Otten U., Agid Y., Thoenen H. Nerve growth factor (NGF) in the rat CNS: absence of specific retrograde axonal transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979 Jun 8;168(3):473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Scott J., Selby M., Urdea M., Quiroga M., Bell G. I., Rutter W. J. Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature. 1983 Apr 7;302(5908):538–540. doi: 10.1038/302538a0. [DOI] [PubMed] [Google Scholar]
- Seiler M., Schwab M. E. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984 May 21;300(1):33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7951–7955. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., Gagnon C., Guroff G., Thoenen H. Purification of nerve growth factor antibodies by affinity chromatography. J Neurochem. 1976 Jun;26(6):1207–1211. doi: 10.1111/j.1471-4159.1976.tb07008.x. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis R. H., Rubin H. Calcium protects DNase I from proteinase K: a new method for the removal of contaminating RNase from DNase I. Anal Biochem. 1980 Sep 1;107(1):260–264. doi: 10.1016/0003-2697(80)90519-9. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]




