Abstract
Background:
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, neu-ropathologically characterized by aggregates of β-amyloid peptides, which deposit as senile plaques, and of TAU protein, which forms neurofibrillary tangles. It is now widely accepted that neuroinflammation is implicated in AD pathogenesis.
Method:
Indeed, inflammatory mediators, such as cytokines and chemokines (chemotactic cytokines) can impact on the Alzheimer´s amyloid precursor protein by affecting its expression levels and amyloidogenic processing and/or β-amyloid aggregation. Additionally, cytokines and chemokines can influence kinases’ activities, leading to abnormal TAU phosphorylation. To date there is no cure for AD, but several thera-peutic strategies have been directed to prevent neuroinflammation. Anti-inflammatory, but also anti-amyloidogenic compounds, such as flavonoids were shown to favourably modulate some pathological events associated with neurodegeneration.
Conclusion:
This review focuses on the role of cytokines and chemokines in AD-associated pathologies, and summarizes the potential anti-inflammatory therapeutic approaches aimed at preventing or slowing down disease progression.
Keywords: Neuroinflammation, cytokines, chemokines, amyloid precursor protein, β-amyloid, TAU
1. BACKGROUND
Alzheimer’s disease (AD) is the most common chronic neurodegenerative disease and the leading cause of dementia, corresponding to around 60% of all cases [1]. AD prevalence increases with age and the majority of individuals with AD are aged 65 or older. The probability of developing the disease doubles every five years after the age of 65 and above 85, the risk reaches nearly 50% [2].
AD is clinically characterized by progressive cognitive decline leading to impaired memory function (including ability to form recent memories), later evolving to affect other intellectual functions. With the disease´s progression, and due to the widespread cortical dysfunction, patients become demented, aphasic, disorientated, immobile, and can, at later stages, become completely dependent on others.
A large number of factors have been associated with the increased risk of developing AD, namely genetic and non-genetic factors, where age is the single greatest etiological risk factor [3].
Two forms of AD exist: early-onset familial AD (EOFAD) associated with Mendelian inheritance that affects individuals less than 65 years old and represents around 5% of AD cases; and late-onset AD (LOAD), also known as sporadic AD, with no consistent mode of transmission that affects people more than 65 years old and represents the greater number of cases among older people (90-95% of AD cases). Early-onset FAD is mainly caused by rare, fully penetrant mutations in three different genes; the Alzheimer´s amyloid precursor protein (APP) and the presenilins (PSEN1 and PSEN2). Genetic polymorphisms of apolipoprotein E (APOE), and in particular APOE ε4 allele variant, are the major risk factors for sporadic cases, that, combined with life exposure factors, can strongly influence LOAD [4].
At the neuropathological level, AD is characterized by the presence of intracellular neurofibrillary tangles (NFTs) and extracellular amyloid neuritic or senile plaques (SPs), followed by alterations in synaptic signalling, synaptic loss and neuronal degeneration [5-7].
NFTs accumulate early in neuronal cytoplasm and arise due to abnormal TAU phosphorylation. Relevant kinases for TAU phosphorylation include cyclin-dependent kinase 5 (CDK5), glycogen synthase kinase-3β (GSK-3β) and p38 mitogen-activated protein kinases (p38-MAPK). In axons, the TAU protein, is one of the predominant microtubule-associated proteins, that normally binds to microtubules facilitating microtubular and cytoskeletal stability, and also promotes neurite outgrowth [8]. However, in its hyperphosphorylated form, TAU detaches from microtubules and aggregates, leading to microtubule instability and disrupting axonal transport [9-11]. Abnormal phosphorylation of TAU protein is neurotoxic and can cause neuronal death. NFTs are present in particular areas of AD brains, exhibiting a higher density in pyramidal neurons of the medial temporal lobe and moderate density in specific layers of the frontal, temporal and parietal lobes of the association cortex [12].
Senile plaques are associated with increased extracellular β-amyloid (Aβ) deposition distributed throughout the brain, but notably in the cerebral cortex and hippocampus of AD patients [13]. Aβ peptide overproduction tends to self-aggregate and form large insoluble β-sheet structures that lead to SPs formation and neurodegeneration. Aβ1-42 aggregates are the predominant form found deposited in AD SPs, due to the higher rate of fibrillization and insolubility comparatively to the Aβ1-40 peptide [6]. Aβ itself can contribute to TAU hyperphosphorylation by impacting either kinases or phosphatases activities [14-16].
This article reviews the role of the cytokines and chemokines on Alzheimer’s neuropathological hallmarks, focusing on their impact on APP processing, Aβ production and TAU phosphorylation.
2. APP PROCESSING AND Aβ PRODUCTION
The APP protein is a transmembrane protein, with a large extracellular portion, a hydrophobic transmembrane domain and a short C-terminus, designated the APP intracellular domain (AICD), which can suffer alternative splicing giving rise to at least eight APP isoforms [17]. It can be proteolytic processed by two distinct pathways: the non-amyloidogenic pathway and the amyloidogenic pathway, involving α-/β-secretases, respectively, and a γ-secretase complex that comprises PSEN, nicastrin, anterior pharynx defective-1 (APH-1) and presenilin enhancer-2 (Pen-2) [18]. The biochemical identities of the secretases have been unravelled. In particular three members of the desintegrin and metalloprotease (ADAM) family; the metalloproteinases ADAM9, ADAM10 and ADAM17/tumor necrosis factor-α converting enzyme (TACE) [19, 20], have been proposed to exert α-secretase activity, while the β-secretase activity has been mainly attributed to the β-site APP cleaving enzyme (BACE1) [21-24]. In the non-amyloidogenic pathway, subsequent cleavage by α-secretase and the γ-secretase complex precludes Aβ formation. The α-secretase cleavage originates the soluble APPα fragment (sAPPα) and a membrane-associated C-terminal fragment consisting of 83 amino acids (C83), which is then cleaved by γ-secretase complex, giving rise to P3 peptide and AICD [25]. In the amyloidogenic pathway, APP is processed by the β-secretase generating a soluble sAPPβ fragment and a membrane associated C-terminal fragment consisting of 99 amino acids (C99). The latter fragment is likewise a substrate for the γ-secretase complex, and cleavage leads to the release of AICD and Aβ peptide generation that can span from 1-38 to 1-43 residues. Under non-pathological conditions, Aβ1-40 is the peptide predominantly produced while Aβ1-42 is a minor species [26, 27]. APP processing and trafficking can be affected by several factors, including stress conditions [28, 29] and Aβ itself [30-32]. Excessive Aβ generation leads to several pathological events, including neurotoxicity, apoptosis, oxidative stress and neuroinflammation [33-35], as well as to TAU hyperphosphorylation [36] and NFTs formation. These anomalies culminate in synaptic damage and neuronal loss in the specific brain regions of AD affected patients, contributing to disease progression [37].
3. INFLAMMATION IN ALZHEIMER’S DISEASE
Brain inflammation is a neuropathological event implicated in AD. The cells involved in the neuroinflammatory reaction are microglia, astrocytes and neurons, that when stimulated can produce high levels of inflammatory mediators such as pro-inflammatory cytokines and chemokines (chemotactic cytokines) [33, 34]. Prostaglandins, leukotrienes, thromboxanes, coagulation factors, free radicals such as reactive oxygen species and nitric oxide, complement factors, proteases, protease inhibitors, and C-reactive protein can also be produced by these cells [33, 38, 39] and incite the inflammatory process.
During neuroinflammation acute short-lived insults (ranging from a few minutes to a few days) had no long-term effects in neuronal survival. In fact, moderate activation of microglia is thought to have beneficial effects in removing neurotoxins, cellular debris or dying cells, and also in promoting neuronal survival. However, problems may arise when the initial injury lasts for a long period that can even reach several years. This is known as chronic neuroinflammation, as is the case in AD that includes a persistent activation of microglia and release of inflammatory mediators. Hence, an inflammatory cycle is perpetuated since microglia and astrocytes are constantly activated, leading to further increases in the levels of cytokines and chemokines [40-43]. Disturbances in inflammatory and immune pathways in AD have been strongly associated with altered levels of some acute phase proteins and pro-inflammatory cytokines in blood, cerebrospinal fluid (CSF) and in AD brains [38, 44-46]. Signs of chronic inflammation occur in pathologically susceptible regions of AD brains as a response to Aβ peptide deposition and NFTs formation [33]. A raised hypothesis is that both SPs and tangles stimulate a chronic inflammatory reaction. In turn, inflammatory mediators can alter APP levels and amyloidogenic processing potentially increasing Aβ1-42 production (further discussed below). These circumstances can also inhibit the generation of sAPPα, a non-amyloidogenic fragment reported to have neuroprotective effects [47, 48]. Furthermore, Aβ itself can induce the expression of several pro-inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) by glial cells, leading to a vicious cycle [33, 43, 49-51]. Several chemokines such as CCL2 (also known as MCP-1, for monocyte chemoattractant protein-1), CXCL8 (IL-8), CXCL10 (IP-10) and CCL5 (also known as RANTES, for regulated on activation, normal T-cell expressed and secreted) are also produced in response to Aβ peptide deposition being responsible for the recruitment of peripheral immune cells [52]. Moreover, during the inflammatory state, non-resident central nervous system cells, as peripheral immune cells with chemokine receptors can cross the blood brain barrier and contribute to the inflammatory response in AD brains [53, 54]. The inflammatory events installed lead to both synaptic and neuronal damage thus contributing to neurodegeneration. Hence, the neuroinflammatory process, initially triggered by Aβ and perpetuated as a vicious cycle, can play an active role in AD progression.
4. CYTOKINES AND CHEMOKINES IN APP PROCESSING
As mentioned above, microglia and astrocytes are able to produce cytokines and chemokines that mediate inflammation and regulate the intensity and duration of the immune response [55]. Cytokines are a large and heterogeneous family of proteins that include the interleukins, TNF-α, IFN-γ and transforming growth factor-β (TGF-β). Since cytokines’ levels rapidly change in response to inflammation, some have been classified as pro-inflammatory, such as IL-1β/α, IL-6, IL-18, TNF-α, IFN-γ; while others, like IL-4, IL-10 and TGF-β1, present anti-inflammatory properties and counteract neuroinflammation. The effects of cytokines have been widely addressed in different cellular and animal models [56], and in this review, focus is given to their impact on AD-related pathologies.
Chemokines, an additional group of chemotactic cytokines, are likewise suspected to be major inflammatory mediators in AD. This family with over 50 different molecules, confers chemotaxis, tissue extravasation and functional modulation of leukocyte function during inflammation [57, 58]. Chemokines are classified into four families: CXC, CC, CX3C and C, based on the number and spacing of cysteine residues in the N-terminal, also known as α, β, γ and δ chemokines, respectively [59]. In the adult brain, microglia, astrocytes and neurons are believed to be the main source of chemokine and their receptors’ production [45, 60]. The central nervous system produces chemokines, like CCL2, CXCL8, CXCL10, CCL5 and CCL3 (also known as MIP-1α, for macrophage inflammatory protein 1-alpha) in response to several inflammatory and disease conditions. Chemokines may potently regulate microglial migration and recruitment of astrocytes to the area of neuroinflammation, favouring the extent of local inflammation.
In AD, the levels of several cytokines and chemokines have been found changed in vulnerable areas of patients’ brains and body fluids [38, 44-46] and cytokine polymorphisms associated in some cases with disease risk [56]. Dysregulated cytokines may prompt the inflammatory processes by enhancing Aβ production thus contributing to AD development. Consequences of these inflammatory proteins on APP processing and Aβ, as well as on TAU phosphorylation will be detailed (summarized in Table 1).
Table 1.
Effects of inflammatory mediators on APP, Aβ peptide and TAU in different cellular and animal models.
| APP | APP Processing | Aβ | TAU | Cellular/Animal Models | Refs. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYTOKINES | Pro-Inflammatory | IL-1β | ↑ APP mRNA ↑ or ↓ APP levels |
↑ sAPPα; ↓ sAPPβ ↑ γ-secretase & AICD ↑ α-secretase (ADAM17/TACE) ↓ β-secretase |
↑ or ↓ Aβ1-40 ↓ Aβ1-42 ↓ Aβ deposition ↑ Aβ ↓ Aβ1-40 ↓ Aβ1-42 ↓ Aβ deposition |
↑ TAU mRNA ↑ p-TAU ↓ p-TAU |
Cortical neurons; microglia; neuron-microglial co-cultures Endothelial, neuronal and glial cells IL-1β injected Sprague-Dawley rats IL-1β injected Long-Evans rats Neuroglioma U251 cells Retinal glial cells Neuroblastoma SK-N-SH cells; cortical neurons IL-1β overexpression in APP/PS1 tg mice IL-1β expression in APP/PS1 tg mice APP-based T20 cell line Rat anti-IL-1R blocking mAb injected 3xtg AD mice Cortical neurons; microglia; neuron-microglial co-cultures 3xtg AD/IL-1βXAT mice IL-1β pellet implanted Sprague-Dawley rats |
[64] [67] [68] [69] [70] [71] [72] [73] [74] [88] [65] [132] [135] [136] |
||||
| IL-1α | ↑ or ↓ APP levels | ↑ α-secretase (ADAM10; ADAM17/TACE) ↑ sAPPα |
↓ Aβ1-40
↓ Aβ1-42 |
--- | U373 MG astrocytoma cells | [76] | ||||||
| IL-6 | ↑ or = APP mRNA | --- | ↓ Aβ deposition |
↑ p-TAU | Cortical neurons; glial cells C57BL mice; Staggerer mutant mice Murine IL-6 overexpression in APP tg mice Hippocampal neurons |
[77] [78] [79] [133] |
||||||
| IL-18 | ↑ APP levels ↑ p-APP |
↑ sAPPβ ↑ β-secretase (BACE1) ↑ γ-secretase (PSEN1 & Pen-2) |
↑ Aβ1-40 | ↑ p-TAU | Differentiated SH-SY5Y neuroblastoma cells | [80] [134] |
||||||
| TNF-α | ↑ APP mRNA = APP levels ↓ APP levels |
↑ sAPPβ ↑ β-secretase (BACE1) ↑ γ-secretase & AICD |
↑ Aβ1-40
↑ Aβ1-42 ↑ or ↓ Aβ deposition ↓ Aβ1-40 ↓ Aβ1-42 ↓ or = Aβ deposition |
↑ p-TAU ↓ p-TAU |
APP expressing astrocytes; cortical neurons 3T3-L1 adipocytes Murine TNF-α expression in APP tg mice APP-based T20 cell line 3xtg AD mice/ TNF-α lowering agent (IDT) 3xtg AD mice/TNF-α lowering agent (3,6′-dithiothalidomide) APP/PS1 tg mice/ TNF-α decreasing agent (Infliximab) |
[51] [82] [85] [88] [83] [84] [139] |
||||||
| IFN-γ | = APP levels | ↑ β-secretase (BACE1) ↑ γ-secretase & AICD |
↑ Aβ1-40
↑ Aβ1-42 ↑ or ↓ Aβ deposition |
↓ p-TAU = or ↑ p-TAU |
APP expressing astrocytes; cortical neurons APP tg mice expressing IFN-γ IFN-γ injected B6/SJL mouse / U373 MG astrocytoma cells APP-based T20 cell line INF-γ expression in 3xtg AD mice Neuroglial cultures / JNPL3 mice; rTg4510 mice expressing INF-γ |
[51] [86] [87] [88] [137] [138] |
||||||
| Anti-Inflammatory | IL-4 | = APP levels | = CTFs | ↓ Aβ oligomerization ↑ or ↓ Aβ deposition ↑ Aβ1-40 ↑ Aβ1-42 |
↑ p-TAU | IL-4 expression in APP+PS1 tg mice IL-4 expression in APP/PS1 tg mice Murine IL-4 expression in APP tg mice |
[92] [93] [94] |
|||||
| CYTOKINES | IL-10 | --- | --- | ↑ Aβ deposition ↓ Aβ deposition ↓ Aβ1-40 ↓ Aβ1-42 |
--- | IL-10 expression in APP tg mice APP/PS1/IL10-/- tg mice |
[95] [96] |
|||||
| TGF-β1 | ↑ APP mRNA | --- | ↑ Aβ deposition |
--- | Astrocytes Microglial cell line BV-2 hAPP/TGF-β1 tg mice hAPP/TGF-β1 tg mice |
[98] [99] [100] [101] |
||||||
| Chemotactic |
CCL2 (MCP-1)/ CCR2* |
= APP levels | --- | ↑ Aβ1-40
↑ Aβ1-42 ↑ Aβ deposition ↑ Aβ oligomerization ↑ Aβ1-42 ↑ or = Aβ deposition |
--- | APP/CCL2 tg mice APP/CCL2 tg mice APP/PS1/CCL2-/- tg mice APP/PS1/CCR2-/- tg mice APP/CCR2-/- tg mice |
[105] [106] [107] [108] [109] |
|||||
|
CXCL8 (IL-8)/ CXCR2* |
--- |
↑ γ-secretase substrates (C99; C83) |
↓ Aβ1-40 ↓ Aβ1-42 |
--- |
CHO cell line expressing models; HEKsw cells/CXCR2 siRNA APP/PS1/CXCR2-/- tg mice |
[113] [114] |
||||||
| CXCL10 (IP-10)/ CXCR3* | = APP levels | --- |
↓ Aβ1-40 ↓ Aβ1-42 ↓ Aβ deposition |
--- | APP/PS1/CXCR3-/- tg mice | [117] | ||||||
|
CCL5 (RANTES)/ CCR5* |
--- |
↑ β-secretase (BACE1) ↑ γ-secretase substrates (C99) |
↑ Aβ1-42 ↑ Aβ deposition |
--- | CCR5-/- mice | [119] | ||||||
|
CCL3 (MIP-1α)/ CCR5* |
--- |
↑ β-secretase (BACE1) ↑ γ-secretase substrates (C99) |
↑ Aβ1-42 ↑ Aβ deposition |
--- | CCR5-/- mice | [119] | ||||||
|
CX3CL1 (fractalkine)/ CX3CR1* |
--- | --- |
↓ Aβ1-40 ↓ Aβ1-42 ↓ Aβ deposition |
↑ p-TAU |
APP/PS1/CX3CL1-/- tg mice hTAU-CX3CR1-/- tg mice APP/PS1/CX3CR1-/- tg mice / R1.40/CX3CR1-/- tg mice CRND8/CX3CR1-/- tg mice |
[140] [141] [142] [143] |
||||||
tg: transgenic; CHO: chinese hamster ovary; KO: knockout. References regarding TAU effects are in bold.
Data on cytokines modulators or KO/deficiency, as well as data not directly on chemokine but rather on receptor KO/deficiency (*) is in italics.
The pro-inflammatory cytokine IL-1 was described to regulate APP processing and Aβ production. IL-1 was previously reported to affect the synthesis and processing of APP, increasing the release of sAPP and potentially augmenting Aβ production, which may contribute to plaque formation and progression, dystrophic neurite proliferation and to neuronal loss [61-64]. More recently, Kitazawa et al. [65] described that blocking IL-1 signalling in 3xtg AD mice with an IL-1R blocking antibody was beneficial, since it leads to a decrease in certain Aβ fibrillar forms and plaques. Among the IL-1 family members are, IL1-β and IL-1α, both increased in AD brain tissue [46, 66]. Controversial data have been reported for IL-1β. While some authors showed that this pro-inflammatory cytokine can render in increased APP mRNA or APP levels [64, 67-69] others mentioned a decrease [70, 71]; it is feasible that the different effects may depend on the cell line an incubation period used. Furthermore, many studies reported an increase in sAPPα secretion in response to IL-1β [64, 70, 72], an effect proposed to be dependent on MEK1/2 and JNK-activated α-secretase cleavage [70] or ADAM17/TACE up-regulation [72]. Moreover, it was likewise described that IL-1β can reduce sAPPβ, Aβ1-40 and Aβ1-42 levels as a result of decreased β-secretase cleavage [72], suggesting in this case that IL-1β can act as an anti-amyloidogenic factor. In agreement, Shaftel et al. [73] showed that IL-1β overexpression in APP/PS1 tg mice promotes microglia activation, reduction of amyloid pathology associated with increased plaque phagocytosis and decreased insoluble Aβ1-40 and Aβ1-42 concentrations. It was also suggested that increased Aβ clearance by microglia in models of sustained IL-1β neuroinflammation could involve Th2 cytokines, such as IL-4 [74]. A feedback signalling loop between Aβ and IL-1β was proposed in which Aβ can induce the production of IL-1β [39]. In turn, the presence of IL-1β greatly increases the secretion of the cytokine IL-6 as well as of the chemokine CXCL8 by human astrocytoma cells (U-373 MG) [75], which can also have an impact on APP processing.
IL-1α was shown to activate the non-amyloidogenic pathway in astrocytes, via stimulation of the α-secretase pathway (ADAM10 and even more so ADAM17/TACE) and consequent sAPPα secretion. Different time periods lead to dual effects on APP. While a short treatment with IL-1α stimulated cell-associated APP, a longer treatment lead to a decrease in both APP and Aβ1-40 and Aβ1-42 levels [76].
As mentioned, other inflammatory cytokines, such as IL-6, IL-18, IL-10, TNF-α and TGF-β1 can also affect APP metabolism, for example by augmenting APP expression and impacting on Aβ production/deposition (see summary Table 1 and Fig. 1).
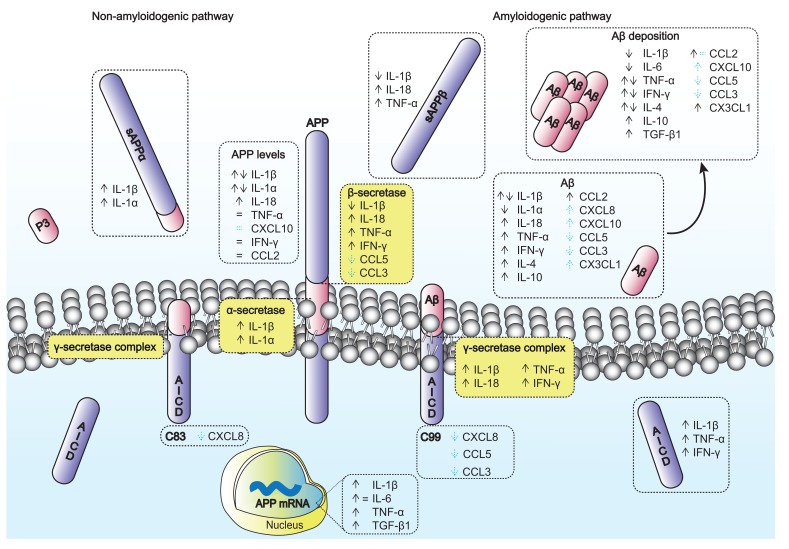
Fig. (1).
Impact of cytokines and chemokines on APP and Aβ peptide. APP is proteolytically processed by two main pathways: the non-amyloidogenic and the amyloidogenic pathways, which leads to Aβ production. Aβ can aggregate and deposit as SPs. The arrows and equality symbol represent the effects prompted by cytokines and chemokines on APP, APP resulting fragments (sAPPα, sAPPβ and AICD), APP secretases (α-, β- and γ-secretases) and on Aβ and its deposition. Dashed arrows and equality symbol in blue represent the putative effects based on chemokines receptor KO/deficiency data.
The pro-inflammatory cytokine, IL-6 has been described as having pleiotropic effects and increased levels in AD brains [38, 66]. Previous studies demonstrated differential effects for IL-6 on APP expression, depending on the cellular model. In particular, IL-6 lead to induction of APP expression, in primary rat cortical neurons while no differences were observed in glial cells [77]. Brugg et al. [78] showed that increases in both IL-6 and IL-1β mRNA correlate with changes in the expression pattern of APP isoforms (decreases in APP695 and increases in APP KPI levels) in specific brain regions. More recently, Chakrabarty et al. [79] showed that IL-6 overexpression induces extensive gliosis and suppresses Aβ deposition in vivo in an APP tg mice model, with no significant alterations on APP processing. IL-18 was mentioned to be elevated in brain specimens from AD patients, and to be able to increase the levels of proteins involved in APP processing, namely BACE1 and members of the γ-secretase complex (PSEN1 and Pen-2), as well as the APP levels and phosphorylation in differentiated SH-SY5Y cells [80]. These events were also accompanied by enhanced Aβ1-40 production and sAPPβ secretion.
TNF-α is another mediator, actively produced by microglia during inflammation, although its levels are low in healthy brains making it difficult to determine its physiological function. It has been demonstrated to play a key role in neuroinflammation-mediated cell death in different neuropathologies, including AD, and hence its inhibition may constitute a therapeutic strategy for different neurodegenerative disorders [81]. Yamamoto and colleagues [51], showed that TNF-α stimulates BACE1 expression and enhances amyloidogenic processing from APP expressing astrocytes and cortical neurons. Further, TNF-α was described to induce APP mRNA expression in a dose-dependent manner via nuclear factor k B activation [82]. Consistently, TNF-α modulation decreased fibrillar amyloid accumulation [83] and chronic administration of TNF-α lowering agents decreased APP levels, soluble Aβ1-42 and Aβ deposition in old 3xtg AD mice [84]. However, expression of murine TNF-α in APP tg mice at early stage rendered in attenuated Aβ1-42 and Aβ1-40 plaque burden and deposition, without difference in APP levels [85].
IFN-γ is another cytokine up-regulated in AD brains with pleiotropic effects, exhibiting both deleterious and protective functions [66]. In a 3xtg AD mice model (APP tgCRND8) that expressed murine IFN-γ, microglia and astrocytes activation was exacerbated and correlated with a decrease in Aβ deposition, possibly due to synergistic effects of activated glia and innate immune system components that trigger Aβ phagocytosis [86]. No differences were observed in APP C-terminal fragments (CTFs) production or on APP levels. Nonetheless, as reported for TNF-α, IFN-γ could enhance Aβ production and deposition in APP expressing astrocytes and cortical neurons, possible via BACE1 expression and suppression of Aβ clearance [51, 87]. Additionally, using a cell based reporter gene assay IFN-γ, TNF-α and IL-1β were all able to stimulate γ-secretase activity leading to both increased AICD and Aβ production [88]. Moreover IFN-γ was also shown to induce the expression of IL-18 [89], which in turn is capable of promoting the production of toxic inflammatory molecules, such as IL-1β [90] and IFN-γ itself [91], pointing to cytokines interplay.
A cytokine with controversial effects on AD is IL-4. IL-4 overexpression rendered in attenuated Aβ pathology [92, 93], while short-term expression had no effect on APP but exacerbated amyloid deposition [94], which may relate with the glial clearance activity in the different models.
Chakrabarty et al. [95] recently showed that the anti-inflammatory cytokine IL-10, increased Aβ deposition and impaired cognition in APP transgenic mouse models expressing this interleukin. Interestingly, increased APOE expression (which, depending on the allele, may enhance Aβ deposition) and decreased Aβ phagocytosis by microglia was also reported. In agreement, Guillot-Sestier et al. [96] reported that IL-10 deficient APP/PS1 tg mice exhibited reduced Aβ abundance in brains and enhanced microglial phagocytosis of the peptide. Further, IL-10 deficiency preserves synaptic integrity and attenuates cognitive disturbance driven by APP/PS1 tg mice, supporting the notion that IL-10 can at some point contribute to AD pathology.
TGF-β1 plays a central role in the brain response to injury, and elevated TGF-β1 levels have been found in CSF and serum of AD patients [38, 46, 97]. However different data were obtained regarding its impact on APP and Aβ. TGF-β1 was previously shown to increase APP isoform differential expression in cultured astrocytes [98] and microglia cells [99]. Later on, this cytokine was shown to induce Aβ deposition in an APP/TGF-β1 transgenic mice [100] and Aβ accumulation, preferentially in cerebral blood vessels and not in parenchymal plaques [101]. This latter event was associated with robust microglia activation and enhanced inflammatory mediators, which resulted in Aβ clearance and reduction of plaque burden.
Several chemokines and their receptors can likewise be found altered in AD brains or in AD models [45], suggesting a disease pathogenic role for these inflammatory mediators. Exposure of microglial cells to Aβ itself causes their activation and leads to the production not only of cytokines but also of chemokines [102]. As mentioned, astrocytes, the most common cells in the brain, can be activated by Aβ peptides to synthetize various pro-inflammatory molecules, similar to those produced by microglia. In particular, CCL2 is produced by microglial cells and astrocytes [103] and its levels are likewise increased in AD patients’ brains [104]. Yamamoto et al. [105] showed that overexpression of APP and CCL2 did not render in alterations in APP processing (APP levels and CTFs formation) but it enhanced Aβ levels, aggregation and deposition in APP/CCL2 mice. Further, CCL2 expression renders in increased APOE levels, which may relate to enhanced Aβ deposition due to reduced Aβ clearance. Consistently, CCL2 enhanced Aβ oligomerization, microgliosis and accelerated cognitive dysfunction [106]. Not only CCL2 overexpression affected AD-related processes, but also CCL2 [107] and CC-chemokine receptor 2 (CCR2) deficiencies [108,109] contributed to amyloid pathology and disease progression. CCR2 deficiencies can either result in no significant differences at the amyloid deposition level [108] or lead to Aβ accumulation particular around blood vessels. Taken together data suggest a relevant role for CCL2-CCR2 signalling in AD pathogenesis.
CXCL8, a chemokine produced by macrophage and other cell types, important for the recruitment of activated microglia into sites of damaged brain, was found significantly increased in serum, CSF and AD brains when compared to control individuals [110, 111]. In fact, not only are chemokines altered but also their receptors are increased in AD brains. CXCR2, an CXCL8 receptor is such an example, which is highly up-regulated in dystrophic neurites of SPs [112]. Bakshi and collaborators showed that knockdown or depletion of CXCR2, and treatment with a CXCR2 antagonist, resulted in decreased Aβ1-42 and Aβ1-40 production and accumulation of γ-secretase substrates C99 and C83, while treatment with agonists enhanced Aβ1-40 production. The inhibitory effect of the antagonist is mediated via γ-secretase, in particular via reduction of presenilin expression (on component of γ-secretase complex) [113, 114]. This data suggested that CXCR2 up-regulation can render in increased γ-secretase activity and enhanced Aβ production.
In AD brains and AD animal models, the chemokine CXCL10, also known as interferon γ-inducible protein 10 (IP-10), was also found in higher levels [115, 116], and Aβ positive plaques were co-localized with high IP-10 expression in APP transgenic mice [116]. A pathogenic role for this chemokine and its receptor CXCR3 in AD may thus be proposed. In agreement, CXCR3 deficiency in APP/PS1 tg mice leads to a reduction in plaque burden and Aβ levels, accompanied by an increase in microglial Aβ uptake [117]. In this model no differences for APP or CTFs were observed, suggesting that CXCR3 might not exert a role on APP processing but rather impact at Aβ clearance level by modulation of microglia. By contrast, the CCL5 and CCL3 chemokine receptor CCR5, similarly found elevated in post-mortem AD brains [104, 118], appear to have a suppressive effect on the development and progression of AD pathology. CCR5 knockout (KO) (CCR5-/- mice model) resulted in higher levels of Aβ1-42, Aβ deposition, and expression of BACE1 and C99 when compared with CCR5+/+ mice, as shown by Lee et al. [119].
In summary, cytokines and chemokines appear to be critical players in AD, either as promoters or suppressors of disease related pathogenic events. These inflammatory mediators can act at different levels, including at the APP proteolytic processing by affecting the secretases’ activities, Aβ production and deposition into SPs (see summary Fig. 1).
Additional inflammatory players, with consequences for APP processing and metabolism, may be considered during the neuroinflammatory process observed in AD [38,120]. Besides cytokines’ and chemokines’ release, complement proteins, acute phase proteins and oxidative mediators can also be expressed. Complement proteins are essential for the elimination of cell debris and potentially toxic protein aggregates. For instance complement factor C3 is a fundamental component of the complement system and a crucial inflammatory protein activated in AD. It has been suggested that this complement factor could have a beneficial impact in neurons and plaque clearance by reducing Aβ deposition, since its deficiency leads to accelerated Aβ deposition and neurodegeneration. Furthermore, it can modulate microglia phenotype, as demonstrated when using complement C3-deficient APP transgenic AD mouse models [121]. Both α1-antichymotrypsin and α2-macroglobulin are examples of acute phase proteins, increased during inflammation, found in association with SPs in AD. α1-antichymotrypsin, is overexpressed in the AD patients’ brains, serum and CSF [122], it promotes Aβ polymerization and deposition both in vitro and in vivo [123, 124]. In contrast, α2-macroglobulin is capable of maintaining Aβ in a soluble state, preventing its aggregation and fibril formation [125, 126]. The cyclooxygenase-2 and cytosolic phospholipase A2 are inflammatory mediators associated with oxidative stress and reactive oxygen species generation [127]. In particular, cytosolic phospholipase A2α has been involved in neurodegenerative processes, by increasing APP expression [128]. Further, according to Sagy-Bross et al. [129] Aβ is capable of inducing APP expression via cytosolic phospholipase A2α, prostaglandin E2 release and activation of the cyclic adenosine monophosphate response element binding protein.
Other types of inflammatory mediators can be involved in AD pathogenesis. Peroxisome proliferator activated receptor γ (PPARγ), is such an example, since it is a nuclear receptor capable of inhibiting the inflammatory response, and whose levels are altered in AD brains. It has been reported that PPARγ supresses BACE1 gene promoter activity, and hence BACE1 mRNA; consequently PPARγ depletion will result in increased BACE1 expression and Aβ generation [130, 131]. These data support the hypothesis that PPARγ activation may act as a protective mechanism in AD pathophysiology.
5. CYTOKINES AND TAU PHOSPHORYLATION
Inflammation can also contribute to abnormal TAU phosphorylation, although literature is scarce, several cytokines can promote TAU phosphorylation, potentially impacting its function and accelerating NFTs formation. The cytokines IL-1, IL-1β, IL-6, IL-18, TNF-α and IFN-γ, are examples of inflammatory proteins that can modify TAU phosphorylation (see Table 1) and have implications for AD pathogenesis. Most of these cytokines act on several TAU kinases in the brain, including CDK5, GSK-3β, and p38-MAPK, by increasing their activity and leading to TAU hyperphosphorylation in several residues, including Ser199, Ser202 and Thr205 [64, 65, 132-135]. It has been suggested that besides its increases in Tau phosphorylation, IL-1β may increase the levels of TAU mRNA [136] and that blocking IL-1 signalling lead to decreased Tau phosphorylation [65]. Further, IFN-γ can be either associated with reduced phosphorylated TAU (p-TAU) levels [137] or increased soluble TAU phosphorylation [138]. Different TNF-α modulators were also shown to decrease TAU phosphorylation in APP/PS1 tg mice [139], APP 3xtg AD mice [84], and to reduce paired helical filaments TAU in the same APP 3xtg model [83], supporting a role for this cytokine in TAU pathology. Additionally, unpublished data by our group suggests that both CCL2 and CXCL8 chemokines can also impact on p-TAU, in a neuroblastoma cell model.
Of note, it is important to refer that some cytokines and chemokines can have opposite effects on both TAU and Aβ pathologies. As an example, Ghosh et al. [135] showed that sustained IL-1β overexpression leads to robust increases in TAU phosphorylation (most probably via p38-MAPK and GSK-3β) despite a clear reduction (about 70-80%) in the amyloid load. A similar opposite effect on TAU and amyloid pathologies was observed for IFN-γ [137]. Further, deficiency of membrane-anchored chemokine CX3CL1 (fractalkine) in APP/PS1 tg mice also lead to enhanced TAU phosphorylation, via the p38-MAPK pathway, and reduced Aβ deposition [140]. Consistently, CX3CR1 receptor deficiency was previously shown to increase TAU phosphorylation [141] and to ameliorate Aβ levels and deposition [142, 143].
Inflammatory mediators, such as cytosolic phospholipase A2 and PPARs have been likewise associated with alterations in TAU phosphorylation levels at Ser214 and Ser199 and in TAU kinases, namely CDK5 and phosphorylated extracellular signal-regulated kinases 1/2 [144, 145]. α1-antichy-motrypsin is capable of inducing TAU phosphorylation at specific residues (Ser202, Thr231, Ser262) and hence tangle formation, through the activation of c-Jun N-terminal kinases, extracellular signal-regulated kinases and GSK-3α/β [122, 146].
In essence, neuroinflammation is a key process that can contribute to the formation of both SPs and NFTs, the major AD neuropathological hallmarks, and thus to disease pathogenesis. Identification of the inflammatory mediators most relevant to disease pathogenesis may aid in the design of novel therapeutic strategies or in the selection of an inflammatory biomarker panel that may potentially aid in AD diagnosis.
6. ANTI-INFLAMMATORY DRUGS AND AD HALLMARKS
Anti-inflammatory drugs like non-steroidal anti-inflammatory drugs (NSAIDs) have been tested in the last decades as an attempt to prevent the onset or to slow down AD progression [38, 147, 35]. As the mode of action NSAIDs can act by targeting AD neuroinflammation and neuropathological hallmarks. In particular, NSAIDS have been proposed to inhibit cyclooxygenase (enzyme responsible for the formation of several eicosanoids involved in inflammation) or to target nuclear factor-κB, Rho-GTPases, PPARγ and APP secretases, impacting on APP processing during the inflammatory state [148]. For example, NSAID ibuprofen treatment reduced the expression of cyclooxygenase-2 and Aβ1-42 levels [130], and long in vivo treatment of APP transgenic mice, with this drug substantially decreased Aβ deposition [149]. Additionally, NSAIDs can bind to PPARγ activating its transcriptional regulatory activities and inhibiting β-secretase [130, 150], thus rendering in decreased Aβ production. Weggen et al. [151] described that a subset of NSAIDs can subtly alter the γ-secretase complex, leading to decreased Aβ1-42 levels, independently of cyclooxygenase activity. In addition, NSAIDs were shown to decrease TAU phosphorylation. As reported by Tortosa and colleagues [152], phosphorylated TAU levels at Ser422 decreased, after cell treatment with the acetylsalicylic acid NSAID. Later, a study carried out by McKee et al. [153] showed that ibuprofen treatment decreased TAU phosphorylation in 3xtg AD mice. Despite the promising effects on APP and TAU, the actual therapeutic potential of these drugs in AD is still controversial. The failure of NSAIDs in clinical trials have been attributed to various factors including: short duration and inappropriate timing of the trials related to late drug delivery to patients (patients too old or too ill); class of drugs used; NSAID’s dose and concentration that can effectively reach the brain; and finally the genetic variability of the patients [154, 155].
In an anti-inflammatory perspective, is an emerging group of natural compounds known as polyphenols, and more specifically flavonoids that appear to reduce AD severity. Flavonoids are natural compounds present in fruits, vegetables, plants and beverages. Besides their anti-inflammatory potential, flavonoids were also described to have anti-oxidant and anti-amyloidogenic properties [156, 157]. In AD these compounds are capable of modulating the production of pro-inflammatory cytokines, for instance by decreasing Aβ aggregation and toxicity, TNF-α and IL-1β generation in microglia [158] or by reducing Aβ-induced cytokine production, possibly via PPARs activation [159]. The beneficial effects of these natural compounds have been likewise attributed to their capacity to inhibit neuronal apoptosis and certain TAU kinases like CDK5 [160] and GSK-3β [161], that disrupt Aβ aggregates and modulate APP processing by acting on α- and β-secretases leading to decreased Aβ production [162]. The mechanisms underlying flavonoid based neuroprotection are currently the focus of research, and evidence supports that supplementation of these natural compounds may constitute a new therapeutic approach for AD. Future studies should address the potential of these anti-inflammatory strategies in preventing or slowing down disease progression.
CONCLUSION
Neuroinflammation is a key event linked to AD pathogenesis in which inflammatory molecules such as cytokines and chemokines are released in response to Aβ peptide. These mediators can act at multiple levels and trigger many neurodegenerative events associated with pathology development, including altered APP processing that may in turn render in increased Aβ production, aggregation, and abnormal TAU phosphorylation, thus perpetuating a vicious cycle. Hence, supporting the notion that AD-related inflammatory mediators could represent suitable targets or useful biomarker candidates in AD therapeutics or diagnosis. Based on this and on the fact that anti-inflammatory drugs could revert at least in part the effects prompted by these inflammatory mediators, many studies were directed at NSAIDs, although thus so far, clinical trials have not been completely satisfactory. Flavonoids show great potential as a novel strategy for AD therapy. Nonetheless, collective characterization of the patients, including individual heterogeneity and disease stages; drug effective dosage; standardization of methods and protocols; correlation of cytokines levels with imagiology information and with gold standard biomarkers, as the case of CSF Aβ and TAU, are some of the aspects that should be considered in future cytokines and therapeutic evaluation studies in AD.
ACKNOWLEDGEMENTS
This work was financed by PIDC/DTP-PIC/5587/2014, Fundação para a Ciência e Tecnologia of the Ministério da Educação e Ciência, Feder funds through compete 2020. It was also supported by iBiMED (UID/BIM/04501/2013).
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- ADAM
A desintegrin and metalloprotease
- APH-1
Anterior pharynx defective-1
- APOE
Apolipoprotein E
- APP
Amyloid precursor protein
- AICD
APP intracellular domain
- Aβ
β-amyloid
- BACE
β-site APP cleaving enzyme
- C83
C-terminal fragment consisting of 83 amino acids
- C99
C-terminal fragment consisting of 99 amino acids
- CDK5
Cyclin-dependent kinase 5
- CHO
Chinese hamster ovary
- CSF
Cerebrospinal fluid
- FAD
Early-onset familial Alzheimer’s Disease
- HEK
Human embryonic kidney
- IP-10
Interferon γ-inducible protein 10
- IFN-γ
Interferon-γ
- IL
Interleukin
- KO
Knockout
- LOAD
Late-onset Alzheimer’s Disease
- MCP-1
Monocyte chemoattractant protein-1
- MIP-1α
Macrophage inflammatory protein 1-alpha
- NFTs
Neurofibrillary tangles
- NSAIDs
Non-steroidal anti-inflammatory drugs
- Pen-2
Presenilin enhancer-2
- PPARγ
Peroxisome proliferator activated receptor γ
- p-tau
Phosphorylated tau
- PSEN
Presenilin
- RANTES
Regulated on activation normal T-cell expressed and secreted
- SPs
Senile plaques
- sAPP
Soluble APP fragment
- TACE
Tumor necrosis factor-α converting enzyme
- TGF-β
Transforming growth factor-β
- tg
Transgenic
- TNF-α
Tumor necrosis factor-α
CONFLICT OF INTEREST
The authors declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kalaria R.N., Maestre G.E., Arizaga R., Friedland R.P., Galasko D., Hall K., et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookmeyer R., Gray S., Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harman D. Alzheimer’s disease pathogenesis: role of aging. Ann. N. Y. Acad. Sci. 2006;1067(1):454–460. doi: 10.1196/annals.1354.065. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi R.E. The genetics of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:1–10. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Cruz e Silva O.A., Henriques A.G., Domingues S.C. da Cruz e Silva EF. Wnt signalling is a relevant pathway contributing to amyloid beta-peptide-mediated neuropathology in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 2010;9:720–726. doi: 10.2174/187152710793237458. [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1(1):1–23. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriques A.G., Oliveira J.M., Carvalho L.P. da Cruz e Silva OAB. Aβ Influences cytoskeletal signaling cascades with consequences to Alzheimer’s disease. Mol. Neurobiol. 2015;52:1391–1407. doi: 10.1007/s12035-014-8913-4. [DOI] [PubMed] [Google Scholar]
- 8.Mandelkow E.M., Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998;8:425–427. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- 9.Gustke N., Steiner B., Mandelkow E.M., Biernat J., Meyer H.E., Goedert M., et al. The Alzheimer-like phosphorylation of tau protein reduces microtubule binding and involves Ser-Pro and Thr-Pro motifs. FEBS Lett. 1992;307:199–205. doi: 10.1016/0014-5793(92)80767-b. [DOI] [PubMed] [Google Scholar]
- 10.Alonso A.C., Zaidi T., Grundke-Iqbal I., Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging. 2003;24(8):1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Cvetkovi D. Neuropathological hallmarks of Alzheimer’s disease. Arch. Oncol. 2001;9(3):195–199. [Google Scholar]
- 13.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 14.Chung S-H. Aberrant phosphorylation in the pathogenesis of Alzheimer’s disease. BMB Rep. 2009;42:467–474. doi: 10.5483/bmbrep.2009.42.8.467. [DOI] [PubMed] [Google Scholar]
- 15.Vintém A.P., Henriques A.G. da Cruz e Silva OAB, da Cruz e Silva EF. PP1 inhibition by Aβ peptide as a potential pathological mechanism in Alzheimer’s disease. Neurotoxicol. Teratol. 2009;31:85–88. doi: 10.1016/j.ntt.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Blurton-Jones M., LaFerla F.M. Pathways by which Abeta facilitates tau pathology. Curr. Alzheimer Res. 2006;3(5):437–448. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- 17.Sandbrink R., Masters C.L., Beyreuther K. Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J. Biol. Chem. 1994;269(2):1510–1517. [PubMed] [Google Scholar]
- 18.Wolfe M.S., Haass C. The role of presenilins in gama-secretase activity. J. Biol. Chem. 2001;276:5413–5416. doi: 10.1074/jbc.R000026200. [DOI] [PubMed] [Google Scholar]
- 19.Buxbaum J.D., Liu K.N., Luo Y., Slack J.L., Stocking K.L., Peschon J.J., et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 20.Allinson T.M., Parkin E.T., Turner A.J., Hooper N.M. ADAMs family members as amyloid precursor protein alpha-secretases. J. Neurosci. Res. 2003;74:342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 21.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Rogers G, et al. Beta-Secretase cleavage of Alzheimer’s Amyloid Precursor Protein by the transmembrane aspartic protease BACE Sci. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 22.Bennett B.D., Babu-Khan S., Loeloff R., Louis J.C., Curran E., Citron M., et al. Expression analysis of BACE2 in brain and peripheral tissues. J. Biol. Chem. 2000;275:20647–20651. doi: 10.1074/jbc.M002688200. [DOI] [PubMed] [Google Scholar]
- 23.Cai H., Wang Y., McCarthy D., Wen H., Borchelt D.R., Price D.L., et al. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 24.Cole S.L., Vassar R. The Alzheimer’s disease Beta-secretase enzyme, BACE1. Mol. Neurodegener. 2007;2(1):1–25. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Ma Q., Zhang Y., Xu H. Proteolytic processing of Alzheimer’s β‐amyloid precursor protein. J. Neurochem. 2012;120(Suppl. 1):9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haass C., Schlossmacher M.G., Hung A.Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B.L., et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 27.Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 28.Henriques A.G., Domingues S.C., Fardilha M. da Cruz e Silva EF, da Cruz e Silva OA. Sodium azide and 2-deoxy-D-glucose-induced cellular stress affects phosphorylation-dependent AβPP processing. J. Alzheimers Dis. 2005;7:201–212. doi: 10.3233/jad-2005-7302. [DOI] [PubMed] [Google Scholar]
- 29.Domingues S.C., Henriques A.G., Wu W. da Cruz e Silva EF, da Cruz e Silva OAB. Altered subcellular distribution of the Alzheimer’s amyloid precursor protein under stress conditions. Ann. N. Y. Acad. Sci. 2007;1096:184–195. doi: 10.1196/annals.1397.085. [DOI] [PubMed] [Google Scholar]
- 30.Henriques A.G., Vieira S.I., Crespo-López M.E., de Oliveira M.A. da Cruz e Silva EF, da Cruz e Silva OAB. Intracellular sAPP retention in response to Aβ is mapped to cytoskeleton-associated structures. J. Neurosci. Res. 2009;87:1449–1461. doi: 10.1002/jnr.21959. [DOI] [PubMed] [Google Scholar]
- 31.Henriques A.G., Vieira S.I. da Cruz e Silva EF, da Cruz e Silva OAB. Abeta hinders nuclear targeting of AICD and Fe65 in primary neuronal cultures. J. Mol. Neurosci. 2009;39:248–255. doi: 10.1007/s12031-009-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriques A.G., Vieira S.I. da Cruz e Silva EF, da Cruz e Silva OAB. Abeta promotes Alzheimer’s disease-like cytoskeleton abnormalities with consequences to APP processing in neurons. J. Neurochem. 2010;113(3):761–771. doi: 10.1111/j.1471-4159.2010.06643.x. [DOI] [PubMed] [Google Scholar]
- 33.Meraz-Ríos M.A., Toral-Rios D., Franco-Bocanegra D., Villeda-Hernández J., Campos-Peña V. Inflammatory process in Alzheimer’s disease. Front. Integr. Nuerosci. 2013;7(59):1–15. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales I., Guzmán-Martínez L., Cerda-Troncoso C. Farías G a, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8(112):1–9. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walters A., Phillips E., Zheng R., Biju M., Kuruvilla T. Evidence for neuroinflammation in Alzheimer’s disease. Prog. Neurol. Psychiatry. 2016;20(5):25–31. [Google Scholar]
- 36.Oliveira J.M., Henriques A.G., Martins F., Rebelo S. da Cruz e Silva OAB. Amyloid-β modulates both AβPP and Tau phosphorylation. J. Alzheimers Dis. 2015;45(2):495–507. doi: 10.3233/JAD-142664. [DOI] [PubMed] [Google Scholar]
- 37.Shankar G.M., Walsh D.M. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol. Neurodegener. 2009;4:1–13. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubio-Perez J.M., Morillas-Ruiz J.M. A review: inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012;2012:1–15. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mrak R.E., Griffin W.S. Glia and their cytokines in progression of neurodegeneration. Neurobiol. Aging. 2005;26(3):349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Rogers J., Strohmeyer R., Kovelowski C.J., Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40(2):260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- 41.Rivest S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009;9(6):429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 42.Dzamba D., Harantova L., Butenko O., Anderova M. Glial Cells - The Key Elements of Alzheimer’s Disease. Curr. Alzheimer Res. 2016;13(8):894–911. doi: 10.2174/1567205013666160129095924. [DOI] [PubMed] [Google Scholar]
- 43.Calsolaro V., Edison P. Neuroinflammation in Alzheimer’s disease : current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Swardfager W., Lanctôt K., Rothenburg L., Wong A., Cappell J., Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Liu C., Cui G., Zhu M., Kang X., Guo H. Neuroinflammation in Alzheimer’s disease: chemokines produced by astrocytes and chemokine receptors. Int. J. Clin. Exp. Pathol. 2014;7:8342–8355. [PMC free article] [PubMed] [Google Scholar]
- 46.Brosseron F., Krauthausen M., Kummer M., Heneka M.T. Body fluid cytokine levels in Mild Cognitive Impairment and Alzheimer’s disease: a comparative overview. Mol. Neurobiol. 2014;50:534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattson M.P., Cheng B., Culwell A.R., Esch F.S., Lieberburg I., Rydel R.E. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10(2):243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 48.Ma T., Zhao Y., Kwak Y-D., Yang Z., Thompson R., Luo Z., et al. Statin’s excitoprotection is mediated by sAPP and the subsequent attenuation of calpain-induced truncation events, likely via rho-ROCK signaling. J. Neurosci. 2009;29(36):11226–11236. doi: 10.1523/JNEUROSCI.6150-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Combs C.K., Karlo J.C., Kao S.C., Landreth G.E. Beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001;21(4):1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindberg C., Hjorth E., Post C., Winblad B., Schultzberg M. Cytokine production by a human microglial cell line: effects of beta-amyloid and alpha-melanocyte-stimulating hormone. Neurotox. Res. 2005;8:267–276. doi: 10.1007/BF03033980. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto M., Kiyota T., Horiba M., Buescher J.L., Walsh S.M., Gendelman H.E., et al. Interferon-γ and Tumor Necrosis Factor-α regulate Amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. Am. J. Pathol. 2007;170(2):680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiala M., Zhang L., Gan X., Sherry B., Taub D., Graves M.C., et al. Amyloid-beta induces chemokine secretion and monocyte migration across a human blood-brain barrier model. Mol. Med. 1998;4:480–489. [PMC free article] [PubMed] [Google Scholar]
- 53.Britschgi M., Wyss-Coray T. Systemic and acquired immune responses in Alzheimer’s disease. Int. Rev. Neurobiol. 2007;82:205–233. doi: 10.1016/S0074-7742(07)82011-3. [DOI] [PubMed] [Google Scholar]
- 54.Bonotis K., Krikki E., Holeva V., Aggouridaki C., Costa V., Baloyannis S. Systemic immune aberrations in Alzheimer’s disease patients. J. Neuroimmunol. 2008;193(1-2):183–187. doi: 10.1016/j.jneuroim.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Tuppo E.E., Arias H.R. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Zheng C., Zhou X-W., Wang J-Z. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 2016;5:1–15. doi: 10.1186/s40035-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luster A. Chemokines-chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;338(7):436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 58.Owens T., Babcock A.A., Millward J.M., Toft-Hansen H. Cytokine and chemokine inter-regulation in the inflamed or injured CNS. Brain Res. Brain Res. Rev. 2005;48:178–184. doi: 10.1016/j.brainresrev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Murphy P.M. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 2002;54:227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- 60.Le Thuc O., Blondeau N., Nahon J.L., Rovère C. The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects. Ann. N. Y. Acad. Sci. 2015;1351:127–140. doi: 10.1111/nyas.12855. [DOI] [PubMed] [Google Scholar]
- 61.Goldgaber D., Harris H.W., Hla T., Maciag T., Donnelly R.J., Jacobsen J.S., et al. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. USA. 1989;86(19):7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buxbaum J.D., Oishi M., Chen H.I., Pinkas-Kramarski R., Jaffe E.A., Gandy S.E., et al. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc. Natl. Acad. Sci. USA. 1992;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogers J.T., Leiter L.M., McPhee J., Cahill C.M., Zhan S.S., Potter H., et al. Translation of the alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J. Biol. Chem. 1999;274(10):6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- 64.Griffin W.S., Liu L., Li Y., Mrak R.E., Barger S.W. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J. Neuroinflammation. 2006;3:1–9. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitazawa M., Cheng D., Tsukamoto M.R., Koike M.A., Wes P.D., Vasilevko V., et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 2011;187(12):6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGeer E.G., McGeer P.L. Inflammatory Cytokines in the CNS. Possible role in the pathogenesis of neurodegenerative disorders and therapeutic implications. CNS Drugs. 1997;7:214–228. [Google Scholar]
- 67.Forloni G., Demicheli F., Giorgi S., Bendotti C., Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells : modulation by interleukin-I. Brain Res. Mol. Brain Res. 1992;16:128–134. doi: 10.1016/0169-328x(92)90202-m. [DOI] [PubMed] [Google Scholar]
- 68.Sheng J.G., Ito K., Skinner R.D., Mrak R.E., Rovnaghi C.R., van Eldik L.J., et al. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol. Aging. 1996;17(5):761–766. doi: 10.1016/0197-4580(96)00104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song C., Zhang Y., Dong Y. Acute and subacute IL-1 β administrations differentially modulate neuroimmune and neurotrophic systems: possible implications for neuroprotection and neurodegeneration. J. Neuroinflammation. 2013;10(59):1–15. doi: 10.1186/1742-2094-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma G., Chen S., Wang X., Ba M., Yang H., Lu G. Short-term interleukin-1(beta) increases the release of secreted APP(alpha) via MEK1/2-dependent and JNK-dependent alpha-secretase cleavage in neuroglioma U251 cells. J. Neurosci. Res. 2005;80(5):683–692. doi: 10.1002/jnr.20515. [DOI] [PubMed] [Google Scholar]
- 71.Anderson P., Watts H., Jen S., Gentleman S.M., Moncaster J.A., Walsh D.T., et al. Differential effects of interleukin-1β and S100B on amyloid precursor protein in rat retinal neurons. Clin. Ophthalmol. 2009;3:235–242. doi: 10.2147/opth.s2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tachida Y., Nakagawa K., Saito T., Saido T.C., Honda T., Saito Y., et al. Interleukin-1 beta up-regulates TACE to enhance alpha-cleavage of APP in neurons: resulting decrease in Abeta production. J. Neurochem. 2008;104(5):1387–1393. doi: 10.1111/j.1471-4159.2007.05127.x. [DOI] [PubMed] [Google Scholar]
- 73.Shaftel S.S., Kyrkanides S., Olschowka J.A., Miller J.H., Johnson R.E., O’Banion M.K. Sustained hippocampal IL-1 β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 2007;117(6):1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cherry J.D., Olschowka J.A., O’Banion M.K. Arginase 1+ microglia reduce Aβ plaque deposition during IL-1β -dependent neuroinflammation. J. Neuroinflammation. 2015;12(203):1–13. doi: 10.1186/s12974-015-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gitter B.D., Cox L.M., Rydel R.E., May P.C. Amyloid beta peptide potentiates cytokine secretion by interleukin-1 beta-activated human astrocytoma cells. Proc. Natl. Acad. Sci. USA. 1995;92(23):10738–10741. doi: 10.1073/pnas.92.23.10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bandyopadhyay S.M., Hartley D., M.Cahill C, Debomay K. Chattopadhyay N Rogers J. IL-1alpha stimulates non-amyloidogenic pathway by alpha-secretase (ADAM-10 and ADAM-17) cleavage of APP in human astrocytic cells involving p38 MAP Kinase. J. Neurosci. Res. 2006;84:106–118. doi: 10.1002/jnr.20864. [DOI] [PubMed] [Google Scholar]
- 77.Del Del-Bo R., Angeretti N., Lucca E., De Simoni M.G., Forloni G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and fl-amyloid production in cultures. Neurosci. Lett. 1995;188:70–74. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- 78.Brugg B., Dubreuil Y.L., Huber G., Wollman E.E., Delhaye-Bouchaud N., Mariani J. Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc. Natl. Acad. Sci. USA. 1995;92(7):3032–3035. doi: 10.1073/pnas.92.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chakrabarty P., Jansen-West K., Beccard A., Ceballos-Diaz C., Levites Y., Verbeeck C., et al. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24(2):548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sutinen E.M., Pirttilä T., Anderson G., Salminen A., Ojala J.O. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J. Neuroinflammation. 2012;9(1):1–14. doi: 10.1186/1742-2094-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tweedie D., Sambamurti K., Greig N.H. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr. Alzheimer Res. 2007;4(4):378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 82.Sommer G., Kralisch S., Lipfert J., Weise S., Krause K., Jessnitzer B., et al. Amyloid precursor protein expression is induced by tumor necrosis factor alpha in 3T3-L1 adipocytes. J. Cell. Biochem. 2009;108(6):1418–1422. doi: 10.1002/jcb.22382. [DOI] [PubMed] [Google Scholar]
- 83.Gabbita S.P., Johnson M.F., Kobritz N., Eslami P., Poteshkina A., Varadarajan S., et al. Oral TNFα modulation alters neutrophil infiltration, improves cognition and diminishes tau and amyloid pathology in the 3xTgAD mouse model. PLoS One. 2015;10:1–28. doi: 10.1371/journal.pone.0137305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tweedie D., Ferguson R.A., Fishman K., Frankola K.A., Van Praag H., Holloway H.W., et al. Tumor necrosis factor-α synthesis inhibitor 3,6 ′ - dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer’s disease. J. Neuroinflammation. 2012;9(106):1–16. doi: 10.1186/1742-2094-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chakrabarty P., Herring A., Ceballos-diaz C., Das P., Golde T.E. Hippocampal expression of murine TNFα results in attenuation of amyloid deposition in vivo. Mol. Neurodegener. 2011;6(16):1–10. doi: 10.1186/1750-1326-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chakrabarty P., Ceballos-Diaz C., Beccard A., Janus C., Dickson D., Golde T., et al. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J. Immunol. 2010;184(9):5333–5343. doi: 10.4049/jimmunol.0903382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho H.J., Su Kyoung K., Min Jin S., Eun Mi H., Sik Kim Y., Kyoon H., et al. IFN-gama-induced BACE1 expression is mediated by activation of JAK2 and ERK1/2 signaling pathways and direct binding of STAT1 to BACE1 promoter in astrocytes. Glia. 2007;55(3):253–262. doi: 10.1002/glia.20451. [DOI] [PubMed] [Google Scholar]
- 88.Liao Y-F., Wang B-J., Cheng H-T., Kuo L-H., Wolfe M.S. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gama stimulate gama-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J. Biol. Chem. 2004;279(47):49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 89.Kim Y-M., Im J.Y., Han S.H., Kang H.S. Choi I. IFN-gama up-regulates IL-18 gene expression via IFN consensus sequence-binding protein and activator protein-1 elements in macrophages. J. Immunol. 2000;165(6):3198–3205. doi: 10.4049/jimmunol.165.6.3198. [DOI] [PubMed] [Google Scholar]
- 90.Joosten L.A., Radstake T.R., Lubberts E., van den Bersselaar L.A., van Riel P.L., van Lent P.L., et al. Association of interleukin-18 expression with enhanced levels of both interleukin-1beta and tumor necrosis factor alpha in knee synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(2):339–347. doi: 10.1002/art.10814. [DOI] [PubMed] [Google Scholar]
- 91.Okamura H, Tsutsi H, Komatsu T, Yutsudo M. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 92.Kiyota T., Okuyama S., Swan R.J., Jacobsen M.T., Gendelman H.E., Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24(8):3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Latta C.H., Sudduth T.L., Weekman E.M., Brothers H.M., Abner E.L., Popa G.J., et al. Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-β using BV2 microglial cells and APP/PS1 transgenic mice. J. Neuroinflammation. 2015;12(41):1–13. doi: 10.1186/s12974-015-0243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chakrabarty P., Tianbai L., Herring A., Ceballos-diaz C., Das P., Golde T.E. Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition. Mol. Neurodegener. 2012;7(36):1–12. doi: 10.1186/1750-1326-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chakrabarty P., Andrew L., Ceballos-Diaz C., Eddy J., Funk C., Moore B., et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85(3):519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guillot-Sestier M-V., Doty K.R., Gate D., Jr J.R., Leung B.P., Rezai-Zadeh K., et al. IL10 deficiency re-balances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85(3):534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chao C.C., Hu S., Frey W.H., Ala T.A., Tourtellotte W.W., Peterson P.K. Transforming growth factor beta in Alzheimer’s disease. Clin. Diagn. Lab. Immunol. 1994;1:109–110. doi: 10.1128/cdli.1.1.109-110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gray C.W., Patel A.J. Regulation of beta-amyloid precursor protein isoform mRNAs by transforming growth factor-beta1 and interleukin-1beta in astrocytes. Brain Res. Mol. Brain Res. 1993;19:251–256. doi: 10.1016/0169-328x(93)90037-p. [DOI] [PubMed] [Google Scholar]
- 99.Mönning U., Sandbrink R., Banati R.B., Masters C.L., Beyreuther K. Transforming growth factor beta mediates increase of mature transmembrane amyloid precursor protein in microglial cells. FEBS Lett. 1994;342(3):267–272. doi: 10.1016/0014-5793(94)80514-8. [DOI] [PubMed] [Google Scholar]
- 100.Wyss-Coray T., Masliah E., Mallory M., McConlogue L., Johnson-Wood K., Lin C., et al. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer’s disease. Nature. 1997;389(6651):603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- 101.Wyss-Coray T., Lin C., Yan F., Yu G.Q., Rohde M., McConlogue L., et al. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7(5):612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 102.Rogers J., Lue L.F. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem. Int. 2001;39:333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 103.Lee K.S., Chung J.H., Choi T.K., Suh S.Y., Oh B.H., Hong C.H. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2009;28(4):281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- 104.Liao Y., Guan Z., Ravid R. Changes of nuclear factor and inflammatory chemotactic factors in brain of patients with Alzheimer’s disease. Zhonghua Bing Li Xue Za Zhi. 2011;40:585–589. [PubMed] [Google Scholar]
- 105.Yamamoto M., Horiba M., Buescher J.L., Huang D., Gendelman H.E., Ransohoff R.M., et al. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am. J. Pathol. 2005;166(5):1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiyota T., Yamamoto M., Xiong H., Lambert M.P., Klein W.L., Gendelman H.E., et al. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One. 2009;4(7):1–12. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kiyota T., Gendelman H.E., Weir R.A., Higgins E.E., Zhang G., Jain M. CCL2 affects beta-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2013;34:1060–1068. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naert G., Rivest S. CC Chemokine Receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011;31(16):6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C., et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 110.Li K., Liu S., Yao S., Wang B., Dai D., Yao L. Interaction between interleukin-8 and methylenetetrahydrofolate reductase genes modulates Alzheimer’s disease risk. Dement. Geriatr. Cogn. Disord. 2009;27:286–291. doi: 10.1159/000204766. [DOI] [PubMed] [Google Scholar]
- 111.Alsadany M.A., Shehata H.H., Mohamad M.I. R GM. Histone deacetylases enzyme, copper, and IL-8 levels in patients with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2012;28:54–61. doi: 10.1177/1533317512467680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xia M., Qin S., McNamara M., Mackay C., Hyman B.T. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimer’s disease. Am. J. Pathol. 1997;150(4):1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 113.Bakshi P., Margenthaler E., Laporte V., Crawford F., Mullan M. Novel role of CXCR2 in regulation of gamma-secretase activity. ACS Chem. Biol. 2008;3(12):777–789. doi: 10.1021/cb800167a. [DOI] [PubMed] [Google Scholar]
- 114.Bakshi P., Margenthaler E., Reed J., Crawford F., Mullan M. Depletion of CXCR2 inhibits γ-secretase activity and amyloid-β production in a murine model of Alzheimer’s disease. Cytokine. 2011;53(2):163–169. doi: 10.1016/j.cyto.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 115.Xia M.Q., Bacskai B.J., Knowles R.B., Qin S.X., Hyman B.T. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer’s disease. J. Neuroimmunol. 2000;108(1-2):227–235. doi: 10.1016/s0165-5728(00)00285-x. [DOI] [PubMed] [Google Scholar]
- 116.Duan R-S., Yang X., Chen Z-G., Lu M-O., Morris C., Winblad B., et al. Decreased fractalkine and increased IP-10 expression in aged brain of APP(swe) transgenic mice. Neurochem. Res. 2008;33(6):1085–1089. doi: 10.1007/s11064-007-9554-z. [DOI] [PubMed] [Google Scholar]
- 117.Krauthausen M., Kummer M.P., Zimmermann J., Reyes-irisarri E., Terwel D., Bulic B., et al. CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer’s disease model. J. Clin. Invest. 2015;125(1):365–378. doi: 10.1172/JCI66771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tripathy D., Thirumangalakudi L., Grammas P. RANTES upregulation in the Alzheimer’s disease brain: a possible neuroprotective role. Neurobiol. Aging. 2010;31:8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee Y.K., Kwak D.H., Oh K.W., Nam S-Y., Lee B.J., Yun Y.W., et al. CCR5 deficiency induces astrocyte activation, Abeta deposit and impaired memory function. Neurobiol. Learn. Mem. 2009;92(3):356–363. doi: 10.1016/j.nlm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maier M., Peng Y., Jiang L., Seabrook T.J., Carroll M.C., Lemere C.A. Complement C3 deficiency leads to accelerated amyloid plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J. Neurosci. 2008;28(25):6333–6341. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tyagi E., Fiorelli T., Norden M., Padmanabhan J. Alpha 1-antichymotrypsin, an inflammatory protein overexpressed in the brains of patients with Alzheimer’s disease, induces Tau hyperphosphorylation through c-Jun N-terminal kinase activation. Int. J. Alzheimers Dis. 2013;2013:1–12. doi: 10.1155/2013/606083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eriksson S., Janciauskiene S., Lannfelt L. Alpha1-antichymotrypsin regulates Alzheimer beta-amyloid peptide fibril formation. Proc. Natl. Acad. Sci. USA. 1995;92:2313–2317. doi: 10.1073/pnas.92.6.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nilsson L.N., Bales K.R., DiCarlo G., Gordon M.N., Morgan D., Paul S.M., et al. Alpha-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2001;21(5):1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du Y., Bales K.R., Dodel R.C., Liu X., Glinn M.A., Horn J.W., et al. Alpha2-macroglobulin attenuates beta-amyloid peptide 1-40 fibril formation and associated neurotoxicity of cultured fetal rat cortical neurons. J. Neurochem. 1998;70(3):1182–1188. doi: 10.1046/j.1471-4159.1998.70031182.x. [DOI] [PubMed] [Google Scholar]
- 126.Hughes S.R., Khorkova O., Goyal S., Knaeblein J., Heroux J., Riedel N.G., et al. Alpha2-macroglobulin associates with beta-amyloid peptide and prevents fibril formation. Proc. Natl. Acad. Sci. USA. 1998;95(6):3275–3280. doi: 10.1073/pnas.95.6.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hsieh H-L., Yang C-M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res. Int. 2013;2013:1–18. doi: 10.1155/2013/484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Desbene C, Malaplate-Armand C, Youssef I, Garcia P, Stenger C, Sauvee M, et al. Critical role of cPLA2 in Abeta oligomer-induced neurodegeneration and memory deficit. Neurobiol Aging. 2012;33(6) doi: 10.1016/j.neurobiolaging.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 129.Sagy-Bross C., Kasianov K., Solomonov Y., Braiman A., Friedman A., Hadad N., et al. The role of cytosolic phospholipase A2 α in amyloid precursor protein induction by amyloid beta1-42 : implication for neurodegeneration. J. Neurochem. 2015;132:559–571. doi: 10.1111/jnc.13012. [DOI] [PubMed] [Google Scholar]
- 130.Heneka M.T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C., et al. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1-42 levels in APPV717I transgenic mice. Brain. 2005;128(6):1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 131.Sastre M., Dewachter I., Rossner S., Bogdanovic N., Rosen E., Borghgraef P., et al. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc. Natl. Acad. Sci. USA. 2006;103(2):443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y., Liu L., Barger S.W., Griffin W.S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci. 2003;23(5):1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Quintanilla R.A., Orellana D.I., González-Billault C., Maccioni R.B. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp. Cell Res. 2004;295(1):245–257. doi: 10.1016/j.yexcr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 134.Ojala J.O., Sutinen E.M., Salminen A., Pirttilä T. Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells. J. Neuroimmunol. 2008;205(1-2):86–93. doi: 10.1016/j.jneuroim.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 135.Ghosh S., Wu M.D., Shaftel S.S., Kyrkanides S., LaFerla F.M., Olschowka J.A., et al. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 2013;33(11):5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sheng J.G., Zhu S.G., Jones R.A., Griffin W.S., Mrak R.E. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp. Neurol. 2000;163(2):388–391. doi: 10.1006/exnr.2000.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mastrangelo M.A., Sudol K.L., Narrow W.C., Bowers W.J. Interferon-γ differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am. J. Pathol. 2009;175(5):2076–2088. doi: 10.2353/ajpath.2009.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li A., Ceballos-diaz C., DiNunno N., Levites Y., Cruz P.E., Lewis J., et al. IFN-gama promotes tau phosphorylation without affecting mature tangles. FASEB J. 2015;29(10):4384–4398. doi: 10.1096/fj.15-275834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shi J-Q., Shen W., Chen J., Wang B-R., Zhong L-L., Zhu Y-W., et al. Anti-TNF-α reduces amyloid plaques and tau phosphorylation and induces CD11c-positive dendritic-like cell in the APP/PS1 transgenic mouse brains. Brain Res. 2011;1368:239–247. doi: 10.1016/j.brainres.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 140.Lee S., Xu G., Jay T.R., Bhatta S., Kim K., Jung S., et al. Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway. J. Neurosci. 2014;34(37):12538–12546. doi: 10.1523/JNEUROSCI.0853-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68(1):19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee S., Varvel N.H., Konerth M.E., Xu G., Cardona A.E., Ransohoff R.M., et al. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am. J. Pathol. 2010;177(5):2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Z., Condello C., Schain A., Harb R., Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar Aβ phagocytosis. J. Neurosci. 2010;30(50):17091–17101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De-Paula V.J., Schaeffer E.L., Talib L.L., Gattaz W.F., Forlenza O.V. Inhibition of phospholipase A2 increases tau phosphorylation at Ser214 in embryonic rat hippocampal neurons. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82(1):57–60. doi: 10.1016/j.plefa.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 145.Barroso E., del Valle J., Porquet D., Santos A.M., Salvadó L., Rodríguez-Rodríguez R., et al. Tau hyperphosphorylation and increased BACE1 and RAGE levels in the cortex of PPARβ/δ-null mice. Biochim. Biophys Acta Mol Basis Dis. 2013;1832(8):1241–1248. doi: 10.1016/j.bbadis.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 146.Padmanabhan J., Levy M., Dickson D.W., Potter H. Alpha1-antichymotrypsin, an inflammatory protein overexpressed in Alzheimer’s disease brain, induces tau phosphorylation in neurons. Brain. 2006;129(11):3020–3034. doi: 10.1093/brain/awl255. [DOI] [PubMed] [Google Scholar]
- 147.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sastre M., Walter J., Gentleman S.M. Interactions between APP secretases and inflammatory mediators. J. Neuroinflammation. 2008;5(25):1–11. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lim G.P., Yang F., Chu T., Chen P., Beech W., Teter B., et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000;20(15):5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sastre M., Dewachter I., Landreth G.E., Willson T.M., Klockgether T., van Leuven F., et al. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J. Neurosci. 2003;23(30):9796–9804. doi: 10.1523/JNEUROSCI.23-30-09796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weggen S., Eriksen J.L., Das P., Sagi S.A., Wang R., Pietrzik C.U., et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 152.Tortosa E., Avila J., Pérez M. Acetylsalicylic acid decreases tau phosphorylation at serine 422. Neurosci. Lett. 2006;396:77–80. doi: 10.1016/j.neulet.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 153.McKee A.C., Carreras I., Hossain L., Ryu H., Klein W.L., Oddo S., et al. Ibuprofen reduces Aβ, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–236. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sastre M., Gentleman S.M. NSAIDs: how they work and their prospects as therapeutics in Alzheimer’s disease. Front. Aging Neurosci. 2010;2:1–6. doi: 10.3389/fnagi.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imbimbo B.P., Solfrizzi V., Panza F. Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Front. Aging Neurosci. 2010;2:1–14. doi: 10.3389/fnagi.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tuñón M.J., García-Mediavilla M.V., Sánchez-Campos S., González-Gallego J. Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr. Drug Metab. 2009;10(3):256–271. doi: 10.2174/138920009787846369. [DOI] [PubMed] [Google Scholar]
- 157.Williams R.J., Spencer J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012;52(1):35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 158.Wang S., Wang Y-J., Su Y., Zhou W., Yang S., Zhang R., et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology. 2012;33(3):482–490. doi: 10.1016/j.neuro.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 159.Valles SL, Dolz-Gaiton P, Gambini J, Borras C. Estradiol or genistein prevent Alzheimer’s disease-associated inflammation correlating with an increase PPARγ expression in cultured astrocytes. Brain Res. 2010;1312:138–144. doi: 10.1016/j.brainres.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 160.Leclerc S., Garnier M., Hoessel R., Marko D., Bibb J.A., Snyder G.L., et al. Indirubins inhibit glycogen synthase kinase-3beta and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 161.Gong E.J., Park H.R., Kim M.E., Piao S., Lee E., Jo D., et al. Morin attenuates tau hyperphosphorylation by inhibiting GSK3β. Neurobiol. Dis. 2011;44(2):223–230. doi: 10.1016/j.nbd.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Baptista F.I., Henriques A.G., Silva A.M., Wiltfang J. da Cruz e Silva OAB. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chem. Neurosci. 2014;5(2):83–92. doi: 10.1021/cn400213r. [DOI] [PMC free article] [PubMed] [Google Scholar]