Abstract
Background & Objective:
Thioredoxin-interacting protein (TXNIP) also known as thioredoxin binding protein-2 is a ubiquitously expressed protein that interacts and negatively regulates expression and function of Thioredoxin (TXN). Over the last few years, TXNIP has attracted considerable attention due to its wide-ranging functions impacting several aspects of energy metabolism. TXNIP acts as an important regulator of glucose and lipid metabolism through pleiotropic actions including regulation of β-cell function, hepatic glucose production, peripheral glucose uptake, adipogenesis, and substrate utilization. Overexpression of TXNIP in animal models has been shown to induce apoptosis of pancreatic β-cells, reduce insulin sensitivity in peripheral tissues like skeletal muscle and adipose, and decrease energy expenditure. On the contrary, TXNIP deficient animals are protected from diet induced insulin resistance and type 2 diabetes.
Summary:
Consequently, targeting TXNIP is thought to offer novel therapeutic opportunity and TXNIP inhibitors have the potential to become a powerful therapeutic tool for the treatment of diabetes mellitus. Here we summarize the current state of our understanding of TXNIP biology, highlight its role in metabolic regulation and raise critical questions that could help future research to exploit TXNIP as a therapeutic target.
Keywords: Diabetes mellitus, thioredoxin system, thioredoxin-interacting protein, oxidative stress, insulin resistance, pancreatic β-cell dysfunction, metabolic homoeostasis
1. INTRODUCTION
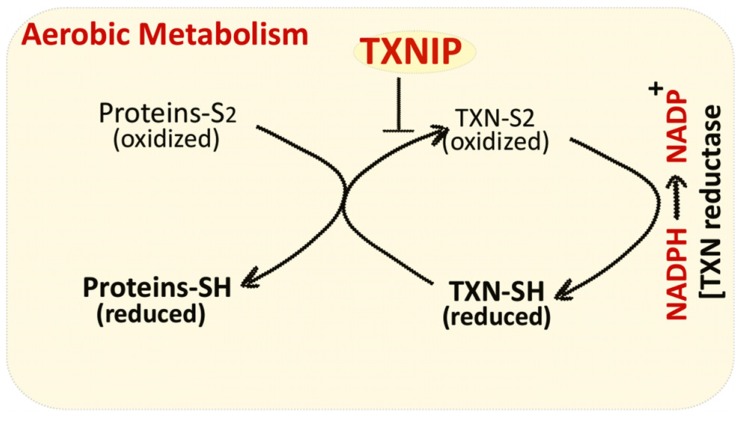
Cellular Redox balance (reduction/oxidation) is tightly controlled by the activity of several antioxidant systems including thioredoxin (TXN), a thiol oxidoreductase system that comprises of TXN, TXN reductase, and NADPH [1-3]. TXN is ubiquitously expressed in almost all species and plays a vital role in regulating cellular redox status. Oxidized proteins produced by reactive oxygen species (ROS) (generated as a result of aerobic metabolism) are reduced by the oxidoreductase activity of TXN. Oxidized TXN generated in the process is subsequently reduced by NADPH-dependent TXN reductase (Fig. 1). Thioredoxin interacting protein (TXNIP) binds and negatively modulates the activity of TXN, thereby influencing the cellular redox balance. TXNIP is a small 38 kDa protein that was originally named vitamin D upregulated protein-1 (VDUP-1), since it was identified in HELA cells that were stimulated with Vitamin D3 [4, 5]. But successive studies on TXNIP promoter analysis did not reveal a consensus vitamin D response element and vitamin-D induced transcription of TXNIP was not confirmed in other cell types [6]. TXNIP directly binds to two cysteine residues at the active catalytic site of TXN, thus impeding its reducing potential [7]. TXNIP can bind only to reduced form of TXN and requires two redox active cysteine residues on each protein [7]. This disulphide exchange reaction between TXNIP and reduced TXN is indispensable for the interaction between two proteins. TXNIP undergoes a structural reorganization upon binding to TXN, which involves formation of a de novo TXNIP Cys247-TXN Cys32 disulphide bond. TXNIP belongs to the family of α-arrestins but these cysteine residues are not preserved in the proteins of α-arrestins, thus, TXNIP is unique in its property to bind TXN and regulate its activity [7].
Fig. (1).
TXNIP reduces the activity of thioredoxin system by direct interaction with thioredoxin (TXN) protein.
In the last few years, TXNIP has emerged as a key regulator of glucose and lipid metabolism and it has been shown to influence metabolic regulation via multiple actions including insulin release from pancreatic β-cells, glucose production from liver and glucose uptake from peripheral tissues like muscle and adipose [8-13]. TXNIP also impacts whole body metabolism by acting as a nutrient sensor in discrete regions of brain and playing a vital role in the regulation of fuel utilization and energy expenditure [14, 15]. In addition, genetic and epigenetic variations in TXNIP are associated with chronic metabolic disorders such as diabetes and hypertension [16-21]. Interestingly, metformin and Glucagon like peptide-1 (GLP-1) agonists have been shown to downregulate the expression of TXNIP, which may contribute to their therapeutic efficacy in the treatment of diabetes mellitus [22-27]. As a result of these important findings, TXNIP has generated significant interest as a potential therapeutic target for the management of diabetes and other metabolic disorders.
2. TXNIP IS A KEY REGULATOR OF PANCREATIC Β–CELL BIOLOGY
Pancreatic β–cells play a vital role in metabolic regulation by sensing blood glucose levels and secreting hormone insulin, the main actor in charge of maintaining glucose homeostasis in the body. β–cell loss results in Type 1 diabetes mellitus (T1DM) and dysfunction of β–cells together with peripheral insulin resistance are essential components in the development of Type 2 diabetes mellitus (T2DM). Intense research efforts are ongoing to have a comprehensive understanding of β–cells gene expression in general and glucose-induced changes in particular to identify novel proteins that could be exploited to restore β–cell function and reestablish metabolic regulation in subjects with diabetes mellitus. In human islets, one of the strongly upregulated genes in response to glucose turned out to be TXNIP, signifying that it might play a key role in β–cell biology and possibly in metabolic disorders [28]. This discovery was particularly important since β–cells are susceptible to oxidative stress, and cell death due to apoptosis is a key factor in the pathogenesis of both type 1 and type 2 diabetes. Subsequent studies confirmed glucose induced stimulation of TXNIP expression in primary islets and we as well as in INS-1 β-cell line by quantity real-time PCR and immunoblotting [29]. TXNIP was shown to contain a well conserved E-box repeat that functions as a binding site for the carbohydrate response element binding protein (ChREBP) [29]. Predictably, TXNIP expression is markedly elevated in rodent models of diabetes mellitus and has a significant impact on the functioning of pancreatic β –cells [30-32].
Several studies have shown TXNIP to be critical link between glucose toxicity and β-cell apoptosis [33-36]. Elevated glucose levels are known to have damaging effects on pancreatic β-cells and results in dysfunction of β-cells, reduction of insulin production and cell death by apoptosis. TXNIP plays a pivotal role in mediating the detrimental effects of elevated glucose on β-cells [34, 37]. TXNIP induces apoptosis of β-cells primarily by activating the mitochondrial death pathway through the stimulation of Apoptosis signal-regulating kinase 1 (ASK1) and involves cytochrome release and caspase -3 activation [38]. Saxena et al. demonstrated that TXNIP (localized primarily in cytosol and nucleus under normal conditions) translocates to the mitochondria in response to increased oxidative stress where it interacts with TXN-2 (mitochondrial TXN) [39]. TXN-2 is part of the mitochondrial antioxidant defense mechanism and it binds and inhibits the activity of ASK-1. TXNIP competes with ASK-1 to bind to TXN-2 resulting in the release of ASK-1 and initiation of the apoptotic signaling cascade in pancreatic β-cells [39]. Besides the activation of the mitochondrial death pathway and phosphorylation and activation of ASK1, TXNIP promotes β-cell apoptosis through the increased expression of pro-apoptotic miR-200 and inhibition of Zinc finger E-box-binding homeobox 1 (ZEB1) expression [40]. TXNIP has also been shown to mediate glucose dependent upregulation of islet amyloid polypeptide [41, 42]. Islet amyloid polypeptide (IAPP) is known to promote inflammation and beta-cell toxicity by aggregating into insoluble amyloid fibrils found in islets of most individuals with type 2 diabetes [43-45]. In addition, TXNIP controls the most significant β-cell function, i.e. expression and production of insulin [13, 46].
3. TXNIP CONTROLS PERIPHERAL INSULIN SENSITIVITY AND ADIPOGENESIS
TXNIP is expressed in metabolically important tissues like liver, adipose and skeletal muscle and is considered to be a key player in the regulation of glucose homeostasis [34]. The importance of TXNIP in metabolic regulation came to the fore when a mice strain (HcB-19) with a spontaneous nonsense mutation in TXNIP gene was found to have hyperlipidemia and features of dysregulated lipid metabolism [47]. The nonsense mutation resulted in a truncated TXNIP peptide lacking the region of the C terminus required for TXN binding. Several lines of evidence from subsequent studies have established the role of TXNIP in regulating multiple aspects of glucose and lipid metabolism in peripheral tissues of humans and rodents. Similar to β-cells, the expression of TXNIP is upregulated by glucose and repressed by insulin in human muscle and in cultured adipocytes [34]. Consistent with this, the expression of TXNIP is elevated in the skeletal muscle and adipose of T2DM subjects and is inversely correlated with whole body insulin stimulated glucose disposal [34]. Additionally, genetic manipulation of TXNIP expression affects both basal and insulin-stimulated glucose uptake in cultured adipocytes and in primary skeletal muscle myocytes. Overexpression of TXNIP diminished basal as well as insulin stimulated glucose uptake in human skeletal muscle myocytes and 3T3 L1 adipocytes [34, 48]. On the contrary, forced reduction of TXNIP expression by gene silencing significantly improved both basal and insulin-stimulated glucose uptake in these cells. Genetic ablation of TXNIP in mice enhances tissue insulin sensitivity and confers protection against diet-induced insulin resistance and development of T2DM [34]. TXNIP has also been shown to be a key component in the improvement of insulin sensitivity in response to caloric restriction (CR) in obese subjects. CR for 16 weeks resulted in marked reduction of TXNIP levels in the skeletal muscle of obese adults, which significantly correlated with the improvement in insulin sensitivity and enhanced non-oxidative disposal of glucose [49, 50]. Besides, TXNIP inhibits the process of adipogenesis in cultured cells and in animal models [51-53]. TXNIP null mice gain more weight and have higher fat mass, when fed on a high fat diet compared to the WT animals [52]. Interestingly, in spite of increased adiposity, TXNIP null mice have improved insulin sensitivity and do not develop features of metabolic syndrome. In 3T3 L1 adipocyte cells, TXNIP overexpression blunted adipogenesis whereas silencing of TXNIP enhanced differentiation of adipocytes. Similarly, embryonic fibroblasts (MEFs) derived from TXNIP knockout mice, show a marked increase in their potential to differentiate into mature adipocyte as compared to wild-type control MEFs [52].
4. TXNIP IS A KEY REGULATOR OF HEPATIC GLUCOSE PRODUCTION
Several studies have demonstrated that TXNIP plays a critical role in hepatic glucose production. HcB-19 mice and TXNIP knockout mice have inherent defect in maintaining blood glucose levels through glucose production [10, 54, 55]. Liver specific TXNIP knockout mice suffer from fasting hypoglycemia and elicit a diminished response to glucagon [52]. Hepatocytes isolated from TXNIP deficient animals produce significantly less glucose as compared to hepatocytes obtained from their wild type (wt) littermates. These hepatocytes are free from the influence of circulating hormones and substrates and recapitulate the observed in vivo metabolic abnormalities, supporting the evidence for an intrinsic defect in regulation of hepatocyte metabolism. No apparent flaw was noticed in glycogen metabolism and the activity of key gluconeogenic enzymes Glucose-6-phosphatase (G6-P) and Phosphoenolpyruvate carboxykinase (PEPCK) was unchanged in TXNIP deficient mice compared to their wt littermates [52]. TXNIP has been shown to influence cellular redox state and alter NADP/NADPH and NAD/NADH concentrations. This could have potentially explained the changes in glucose production from liver, since changes in the cellular redox state are known to control gluconeogenesis [56, 57]. However, in the fasted state, there was no significant difference in the ratios of NADP/NADPH and NAD/NADH and therefore, the altered redox state does not explain the changes seen in fasted TXNIP-deficient mice [10]. Interestingly, TXNIP deficiency only affected glucose production from lactate and not glycerol and TXNIP overexpression was associated with increased glucose production from lactate whereas glucose production from glycerol remained unchanged. In addition, the authors observed increased ketogenesis in TXNIP deficient animals. Together these results suggest that TXNIP deficiency affects mitochondrial function that leads to preferential routing of acetyl-CoA toward ketogenesis rather than gluconeogenesis. Hui et al. also showed that phosphoenolpyruvate a common precursor to glucose and lipids was selectively shunted to glyceroneogenesis rather than gluconeogenesis in HcB-19 mice [55]. Indeed, TXNIP deficient mice secrete more triglycerides from the liver, which can be attributed to increased fatty acid synthesis [10, 58].
5. NEURONAL TXNIP ACTS AS A NUTRIENT SENSOR AND REGULATOR OF ENERGY EXPENDITURE
TXNIP is highly expressed in discrete areas of hypothalamus and brainstem, which are critical centers of metabolic regulation suggesting the importance of neuronal TXNIP in the regulation of metabolic homeostasis and energy balance [59, 60]. Indeed, Blouet et al. demonstrated that hypothalamic TXNIP responds to hormonal and nutrient signals, and regulates adipose tissue metabolism and glucose homeostasis [15]. Hypothalamic expression of TXNIP is repressed by anorexigenic indicators (refeeding, insulin and leptin) and stimulated during fasting in healthy lean mice. TXNIP expression is elevated in various mouse models of obesity and diabetes and downregulation of hypothalamic TXNIP expression prevents diet-induced obesity and insulin resistance. Thus, hypothalamic TXNIP plays a critical role in nutrient sensing and the regulation of fuel utilization. Subsequent studies elucidated the role of TXNIP in Agouti-related protein (AGRP) neurons in mediating diet-induced obesity through the regulation of energy expenditure and adipose tissue metabolism [14]. Mice overexpressing TXNIP in AGRP neurons are vulnerable to diet- induced obesity because of increased adipogenesis and decreased energy expenditure. In contrast, AGRP neuron specific deletion of TXNIP protects the animals from diet-induced obesity by increasing energy expenditure and reducing adipose tissue storage. TXNIP is strongly upregulated during fasting induced and natural torpor (reduced body temperature and metabolic rate) and is believed to act as a key nutrient sensor to regulate energy homeostasis within the brain during prolonged periods of hypothermia and fasting [61]. The multiple functions of TXNIP in regulating metabolic homeostasis are summarized in Fig. (2).
Fig. (2).
TXNIP controls multiple aspects of energy metabolism by regulating key processes in adipose, brain, liver, muscle and pancreatic β-cells.
6. TXNIP REGULATES METABOLIC HOMEOSTASIS THROUGH MULTIPLE MECHANISMS
TXNIP functions primarily through the inhibition of TXN activity, which is a component of TXN system that plays a vital role in maintaining a reducing environment in the cell [62, 63]. Over-abundance of TXNIP impairs the protein reducing activity of TXN that leads to increased oxidative stress by generating excess reactive oxygen species (ROS) [64, 65]. Low levels of ROS are important for optimal cellular signaling but excessive generation of ROS impairs pancreatic cell function and reduces insulin sensitivity in skeletal muscle and adipose [66-69]. TXNIP is also involved in NOD-like receptor Protein-3 (NLRP3) inflammasome activation in a redox dependent manner [70, 71]. NLRs are present in cytoplasm where they act as receptors and detect danger signals emanating from cells undergoing stress, damage or abnormal death as well as by exogenous signals coming from pathogens. Once activated some NLRPs including NLRP3 form large protein complexes (inflammasome) that are responsible for the activation of caspase-1 and -5, which ultimately leads to the proteolytic activation of the pro-inflammatory cytokines [72]. TXNIP initiated NLRP3 inflammasome activation has been implicated in obesity-induced insulin resistance and beta cell failure [73-75]. Interestingly, the TXNIP KO mouse is similar to the Nlrp3 KO mouse in exhibiting improved glucose tolerance and insulin sensitivity [70].
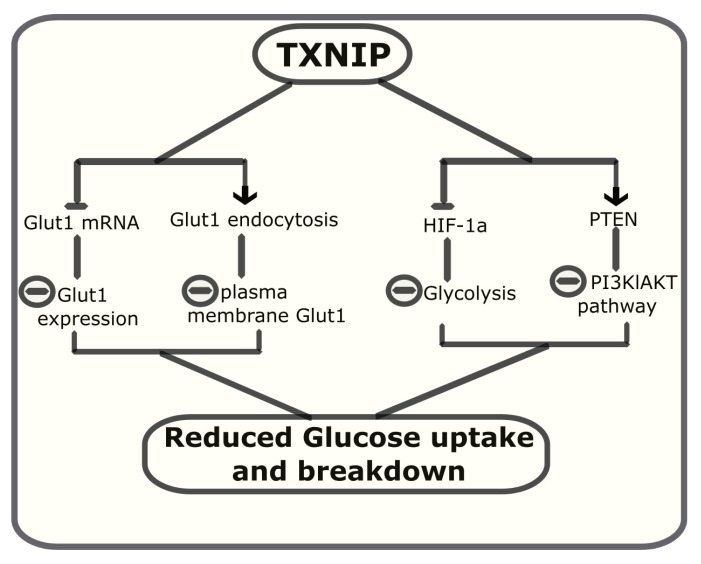
Although TXNIP was initially thought to exert most of its actions through the inhibition of TXN activity, evidence from several studies has indicated that many of the metabolic functions of TXNIP are independent of its ability to bind and inhibit TXN. Much of the information about TXN independent functions of TXNIP have come from the use of a missense variant of TXNIP (C247S) in which, substitution of cysteine by serine abolishes the ability of TXNIP to bind to TXN [7, 12]. Using this variant, Patwari et al. were the first to show that a major function of TXNIP, inhibition of glucose uptake does not require binding to TXN [7]. The authors observed that WT, C247S TXNIP or a related α-arrestin (Arrdc4 that does not bind TXN) impaired glucose uptake to a similar extent when overexpressed in 3t3 L1 adipocytes. Using TXNIP mutants and chimeric α-arrestins, the authors suggested that the metabolic functions of TXNIP and Arrdc4 are intrinsic to the arrestin domains and are essentially independent of TXN binding [7]. Subsequent studies revealed that TXNIP inhibits cellular glucose uptake by regulating the expression and modulating the localization of glucose transporter (Glut1) [76]. Loss of TXNIP in HEPG3 cells was accompanied by a dramatic increase in the expression of Glut1 mRNA suggesting a direct transcriptional control. The upregulation of Glut1 mRNA correlated with the increase in the glucose uptake by these cells. In addition, co-immunoprecipitation experiments revealed that TXNIP directly interacts with Glut1 and reducing its plasma membrane expression by facilitating its endocytosis [76].
TXNIP also inhibits glucose uptake by inhibiting glycolysis and increasing oxidative metabolism of pyruvate [77]. Deletion of TXNIP drives metabolic reprogramming toward aerobic glycolysis and cells lacking TXNIP show increased glucose uptake and lactate accumulation. TXNIP inhibits glycolysis by downregulating the expression of key glycolytic enzymes through the inhibition of hypoxia-induced transcription factor (HIF1α) expression [78]. HIF1α is responsible for increasing the glycolytic metabolism of glucose by upregulating the transcription of key glycolytic enzymes [79, 80]. TXNIP also regulates and activates Phosphatase and tensin homolog (PTEN) lipid phosphatase by a REDOX-sensitive mechanism [81]. PTEN is a natural inhibitor of PI3 kinase/AKT pathway and its activation negatively regulates glucose uptake and metabolism [82]. PTEN contains two cysteine residues in its active site that must remain in reduced state to maintain its phosphatase activity [83, 84]. PTEN activity is inhibited by insulin and growth factors by oxidation and formation of a disulphide bond between these cysteines. PTEN can be re-activated by reduction of cysteine residues by thioredoxin NADP (H) [85, 86]. Thioredoxin NADP(H)-mediated reactivation of PTEN is inhibited by NADH [87]. Loss of TXNIP results in the impairment of mitochondrial oxidation and increased NADH/NADP(H), which in turn blocks the reductive reactivation of PTEN, thus activation of AKT. Therefore, TXNIP deficiency is associated with inactivation of PTEN and increased PI3K/AKT signaling that leads to enhanced glucose transport and metabolism [81, 88]. TXNIP control of glucose uptake and breakdown is summarized in Fig. (3).
Fig. (3).
TXNIP controls glucose metabolism by inhibiting both its uptake and breakdown by cells.
The fact that, TXNIP is primarily localized in the nucleus and most of its target genes are downregulated prompted Xu et al. to assume that TXNIP might affect gene expression by regulating microRNA expression [46]. Indeed, they demonstrated that TXNIP upregulated expression of several miRNAs including miR-204 that mediated the TXNIP induced inhibition of insulin production. MiR-204 was shown to function through downregulation of MafA, which is a known transcriptional regulator of insulin gene [46]. TXNIP has also been shown to down-regulate miR-124a expression, a microRNA known to directly target FoxA2, a transcription factor that regulates expression of islet amyloid polypeptide [41, 42]. IAPP has been shown to aggregate into insoluble amyloid fibrils found in islets of most individuals with type 2 diabetes and promotes inflammation and beta-cell cytotoxicity [34-36]. TXNIP, therefore, enhances the expression of IAPP through miR-124a and FoxA2 [42]. In addition, TXNIP upregulates miR-200 expression in pancreatic β-cells, which targets a key transcription factor zinc finger E-box-binding homeobox 1 (Zeb1) [40]. Downregulation of Zeb1 by TXNIP/miR-200 results in β-cell apoptosis. MiR-200 also controls and increases expression of the epithelial marker E-cadherin, an important regulator of epithelial-mesenchymal transition (EMT), a process thought to be involved in β-cell expansion. MiRNA-200 increases E-cadherin expression by downregulating the expression of transcription factors ZEB1 and SIP1, which are known to negatively control E-cadherin expression [89]. Interestingly, the expression of miR-124a, miR200 and miR-204 are altered in the diabetic condition consistent with the increased expression of TXNIP [40]. TXNIP regulation of β-cell functioning through micro RNAs is illustrated in Fig. (4).
Fig. (4).
TXNIP regulates the expression of several micro RNAs to control multiple aspects of pancreatic β-cell biology.
7. TXNIP IS A POTENTIAL THERAPEUTIC TARGET
TXNIP has attracted considerable attention due to its multiple functions impacting several aspects of energy metabolism and numerous studies suggest that interventions designed to modulate the activity of TXNIP might be beneficial in the prevention/management/cure of diabetes mellitus. Several lines of evidence from animals and human studies strongly support the assumption that inhibition of TXNIP is a potential therapeutic approach to counter metabolic abnormalities associated with obesity and diabetes mellitus. First, TXNIP expression is strongly upregulated by glucose in a variety of cell types and elevated levels of TXNIP are found in diabetic human subjects and animal models of diabetes mellitus [34, 77, 90, 91]. Second, expression of TXNIP is also regulated by insulin, amino acids, nutritional signals like feed-fasting and anti-diabetic drug metformin, suggesting its importance in the regulation of metabolic homeostasis [10, 22, 24, 28, 29, 55, 92, 93]. Third, overexpression of TXNIP in animal models is associated with metabolic abnormalities including apoptosis of pancreatic β-cells; reduced insulin sensitivity and decreased energy expenditure [14, 35, 42, 94]. In contrast, deficiency of TXNIP is beneficial and animals lacking TXNIP have normal insulin sensitivity and do not develop diabetes or other metabolic abnormalities [34, 52, 94]. Anti-diabetic agents like Insulin, metformin, GLP-1 agonists and resveratrol have all been shown to inhibit TXNIP expression [24, 27, 95, 96]. Metformin and resveratrol are known activator of AMPK, which in turn inhibits expression of TXNIP mRNA and augments degradation of TXNIP protein [76, 92]. GLP-1 receptor agonists regulate expression of TXNIP by accelerating its proteosomal degradation in a cAMP/PKA dependent manner [27].
Prof Anath Shalev’s group in University of Alabama who were the first to discover TXNIP as the most dramatically up-regulated gene in response to glucose in pancreatic islets [28], identified verapamil (FDA approved drug for hypertension) as an inhibitor of TXNIP expression and demonstrated that oral verapamil restored β-cell health and ameliorated metabolic abnormalities in animal models of T1DM and T2DM [32, 97]. They are currently evaluating the efficacy of verapamil in a phase 2 randomized trial in patients who have developed T1DM within the previous three months. The idea is based on the assumption that newly diagnosed T1DM patients do not have comprehensive β-cell loss and the residual β-cells can be saved from further impairment by reducing the levels of TXNIP. This view was reinforced by a recent study in which diabetic subjects that received verapamil had fasting serum glucose levels significantly lower than their peers who were not receiving verapamil [98]. Remarkably, verapamil was very effective in subjects with T1DM or late-stage T2DM (both conditions characterized by β-cell death) but no effect was seen in subjects with early-stage T2DM (characterized by insulin resistance but no beta cell loss) [98]. This comes as a surprise because TXNIP is a significant player in regulating insulin sensitivity of peripheral tissues and reduced insulin sensitivity is hallmark of T2DM. One possible explanation is that verapamil may not downregulate TXNIP expression in adipose, liver and muscle and thus, has no effect on insulin sensitivity of these tissues. Apart from β-cells, verapamil had been shown to inhibit TXNIP expression in H9C2 cells and primary adult cardiomyocytes but verapamil effect on TXNIP expression in other cell types is not known [99]. Allopurinol, a drug used to treat gout and hyperuricemia also reduces TXNIP levels in animals and cultured cells. Interestingly, allopurinol has been shown to restore insulin sensitivity [100] and ameliorate cardiovascular [101, 102] and renal complications [103] associated with T2DM. Another drug, tranilast (n-[3,4-dimethoxycinnamoyl] anthranilic acid) used in Japan and South Korea for bronchial asthma, attenuates the up-regulation of TXNIP in streptozotocin induced diabetic animals [104] and several studies have highlighted the beneficial effects of tranilast in diabetic nephropathy and cardiomyopathy [105-107]. It remains to be seen whether the beneficial effects of these drugs on diabetic associated complications are a direct result of reduced TXNIP expression.
8. THERAPEUTIC TXNIP INHIBITION AND POSSIBLE RISKS
Intense efforts are on to develop small molecules that could inhibit TXNIP with high efficacy and selectivity and ameliorate metabolic abnormalities associated with diabetes mellitus. If successful, TXNIP based approach may achieve the requirement of a perfect anti-diabetic therapy by not only improving insulin secretion and sensitivity but also ameliorating global pathology of diabetes, including cardiovascular and microvascular complications. However, there are fears that loss of TXNIP might have serious consequences and one must proceed with caution with the use of TXNIP inhibitors. TXNIP expression is required for maintaining normal fasting glycaemia and TXNIP deficient animals suffer from hypoglycemia that is exacerbated by fasting and the liver of these animals is intrinsically defective in maintaining blood glucose levels through glucose production and release [10, 12]. Therefore, use of TXNIP inhibitors could potentially be associated with an elevated risk of hypoglycemic episodes. In addition, loss of TXNIP is associated with increased incidence of cancer and TXNIP expression is reduced in various human cancer cells [108-110]. TXNIP functions as a tumor suppressor and down-regulation of TXNIP contributes to cancer progression [62]. Consequently, one of the major concerns arising from pharmacological suppression of TXNIP is the possibility of increased risk of carcinogenesis.
9. MOVING FORWARD
TXNIP has emerged as a leading player in the regulation of metabolism and a prospective therapeutic candidate. Great progress has been made in the last few years and our understanding of TXNIP biology and its role in metabolic regulation has become fairly clear. However, more work is needed to comprehensively understand the signaling mechanisms involved in the physiological functions of TXNIP. In particular, little is known about the specific mechanism(s) by which TXNIP regulates insulin sensitivity in peripheral tissues although the role of AMPK and PTEN phosphatase has been pointed out. It is also not clear whether increased TXNIP expression is just a consequence or actually a cause of diabetes. The most likely possibility is that up-regulation of TXNIP is a short term response to high calories diet but repeated bouts of over-nutrition lead to a permanent increase in TXNIP expression and accompanying adverse effects. One of the critical questions regarding the molecular function of TXNIP and other α-arrestins is whether they are capable of regulating the trafficking of receptors as β-arrestins do. Recent evidence suggests that α-arrestins like ARRDC3 and ARRDC4 are involved in the regulation of endosomal sorting and intracellular signaling of β2-Adrenergic receptor [111-113]. G-protein coupled receptors (GPCRs) are involved in almost all the aspects of metabolic homeostasis [114-119] and it is highly likely that TXNIP, which has apparent structural similarity to ARRDC3 and ARRDC4 may be involved in the trafficking and signaling of GPCRs. Furthermore, TXNIP has been shown to bind glucose transporter (Glut1) and regulate its endocytosis in TRVb-1 cells [76] and it remains to be seen whether TXNIP directly interacts with glucose transporters in insulin sensitive tissues like muscle and adipose. TXNIP does inhibit glucose transport in these tissues [52, 120]; however, it is not clear if this is facilitated by direct binding of TXNIP to the glucose transporters.
CONCLUSION
Notwithstanding the gaps in the knowledge, data from studies on animals, cell lines and human subjects have provided considerable and convincing evidence that TXNIP impacts almost all the aspects of energy metabolism. In animal models, there is definite proof indicating the contribution of TXNIP in the development of retinopathy, nephropathy and cardiovascular complications associated with diabetes mellitus, even though it remains to be demonstrated in humans [23, 121-123]. Therefore, targeting TXNIP is believed to offer a unique therapeutic opportunity to not only improve insulin secretion and sensitivity but also ameliorating global pathology of diabetes, including cardiovascular and microvascular complications. Efforts are on to find novel TXNIP inhibitors for anti-diabetic therapy and attention is likely to focus on the identification of inhibitors that could only reduce the expression and/or activity of TXNIP to non-diabetic levels so as to avoid damaging consequences of complete loss of this protein.
ACKNOWLEDGEMENTS
This study was supported by King Abdullah International Medical Research Center (KAIMRC) through research grant RC13/268/R awarded to SM.
LIST OF ABBREVIATIONS
- TXN
Thioredoxin
- TXNIP
Thioredoxin interacting protein
- HIF1α
Hypoxia induced factor
- ROS
Reactive oxygen species
- MafA
Musculoaponeurotic Fibrosarcoma Oncogene Homolog A
- CHREBP
Carbohydrate Response Element Binding Protein
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
AUTHOR CONTRIBUTION
Sameer Mohammad, Saeed Al Mahri, Naif Mohammad Alhawiti, Shuja Shafi Malik and Mohammad Azhar Aziz searched and scrutinized the literature. Sameer Mohammad wrote the paper. All authors read and approved the final manuscript.
REFERENCES
- 1.Yodoi J., Masutani H., Nakamura H. Redox regulation by the human thioredoxin system. Biofactors. 2001;15(2-4):107–111. doi: 10.1002/biof.5520150212. [DOI] [PubMed] [Google Scholar]
- 2.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 3.Nordberg J., Arner E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama A., Matsui M., Iwata S., et al. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999;274(31):21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 5.Chen K.S., DeLuca H.F. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim. Biophys. Acta. 1994;1219(1):26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig D.L., Kotanides H., Le T., Chavkin D., Bohlen P., Witte L. Cloning, genetic characterization, and chromosomal mapping of the mouse VDUP1 gene. Gene. 2001;269(1-2):103–112. doi: 10.1016/s0378-1119(01)00455-3. [DOI] [PubMed] [Google Scholar]
- 7.Patwari P., Higgins L.J., Chutkow W.A., Yoshioka J., Lee R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006;281(31):21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muoio D.M. TXNIP links redox circuitry to glucose control. Cell Metab. 2007;5(6):412–414. doi: 10.1016/j.cmet.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Shalev A. Minireview: Thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Mol. Endocrinol. 2014;28(8):1211–1220. doi: 10.1210/me.2014-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheth S.S., Castellani L.W., Chari S., et al. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J. Lipid Res. 2005;46(1):123–134. doi: 10.1194/jlr.M400341-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Schulze P.C., Yoshioka J., Takahashi T., He Z., King G.L., Lee R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004;279(29):30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 12.Chutkow W.A., Patwari P., Yoshioka J., Lee R.T. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J. Biol. Chem. 2008;283(4):2397–2406. doi: 10.1074/jbc.M708169200. [DOI] [PubMed] [Google Scholar]
- 13.Oka S., Yoshihara E., Bizen-Abe A., et al. Thioredoxin binding protein-2/thioredoxin-interacting protein is a critical regulator of insulin secretion and peroxisome proliferator-activated receptor function. Endocrinology. 2009;150(3):1225–1234. doi: 10.1210/en.2008-0646. [DOI] [PubMed] [Google Scholar]
- 14.Blouet C., Liu S.M., Jo Y.H., Chua S., Schwartz G.J. TXNIP in Agrp neurons regulates adiposity, energy expenditure, and central leptin sensitivity. J. Neurosci. 2012;32(29):9870–9877. doi: 10.1523/JNEUROSCI.0353-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blouet C., Schwartz G.J. Nutrient-sensing hypothalamic TXNIP links nutrient excess to energy imbalance in mice. J. Neurosci. 2011;31(16):6019–6027. doi: 10.1523/JNEUROSCI.6498-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowluru R.A., Mishra M. Contribution of epigenetics in diabetic retinopathy. Sci. China Life Sci. 2015;58(6):556–563. doi: 10.1007/s11427-015-4853-0. [DOI] [PubMed] [Google Scholar]
- 17.De Marinis Y., Cai M., Bompada P., et al. Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney Int. 2016;89(2):342–353. doi: 10.1016/j.kint.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Ramus S.M., Cilensek I., Petrovic M.G., Soucek M., Kruzliak P., Petrovic D. Single nucleotide polymorphisms in the Trx2/TXNIP and TrxR2 genes of the mitochondrial thioredoxin antioxidant system and the risk of diabetic retinopathy in patients with Type 2 diabetes mellitus. J. Diabetes Complications. 2016;30(2):192–198. doi: 10.1016/j.jdiacomp.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 19.van Greevenbroek M.M., Vermeulen V.M., Feskens E.J., et al. Genetic variation in thioredoxin interacting protein (TXNIP) is associated with hypertriglyceridaemia and blood pressure in diabetes mellitus. Diabet. Med. 2007;24(5):498–504. doi: 10.1111/j.1464-5491.2007.02109.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira N.E., Omae S., Pereira A., et al. Thioredoxin interacting protein genetic variation is associated with diabetes and hypertension in the Brazilian general population. Atherosclerosis. 2012;221(1):131–136. doi: 10.1016/j.atherosclerosis.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Chambers J.C., Loh M., Lehne B., et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 2015;3(7):526–534. doi: 10.1016/S2213-8587(15)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Kover K.L., Heruth D.P., et al. New insight into metformin action: regulation of chrebp and foxo1 activities in endothelial cells. Mol. Endocrinol. 2015;29(8):1184–1194. doi: 10.1210/ME.2015-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong C.R., Chan W.P., Nguyen T.H., et al. Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc. Drugs Ther. 2014;28(4):347–360. doi: 10.1007/s10557-014-6538-5. [DOI] [PubMed] [Google Scholar]
- 24.Chai T.F., Hong S.Y., He H., et al. A potential mechanism of metformin-mediated regulation of glucose homeostasis: inhibition of Thioredoxin-interacting protein (Txnip) gene expression. Cell. Signal. 2012;24(8):1700–1705. doi: 10.1016/j.cellsig.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Einbinder Y., Ohana M., Benchetrit S., et al. Glucagon-Like-Peptide-1 and Vitamin D: anti-inflammatory response in diabetic kidney disease in db/db mice and in cultured endothelial cells. Diabetes Metab. Res. Rev. 2016;32(8):805–815. doi: 10.1002/dmrr.2801. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y.S., Jun H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19. doi: 10.1016/j.metabol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Shao W., Yu Z., Fantus I.G., Jin T. Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell. Signal. 2010;22(8):1240–1246. doi: 10.1016/j.cellsig.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Shalev A., Pise-Masison C.A., Radonovich M., et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology. 2002;143(9):3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- 29.Minn A.H., Hafele C., Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146(5):2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 30.Price S.A., Gardiner N.J., Duran-Jimenez B., Zeef L.A., Obrosova I.G., Tomlinson D.R. Thioredoxin interacting protein is increased in sensory neurons in experimental diabetes. Brain Res. 2006;1116(1):206–214. doi: 10.1016/j.brainres.2006.07.109. [DOI] [PubMed] [Google Scholar]
- 31.Chen J., Hui S.T., Couto F.M., et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008;22(10):3581–3594. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Cha-Molstad H., Szabo A., Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am. J. Physiol. Endocrinol. Metab. 2009;296(5):E1133–E1139. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto M., Yamaoka M., Takei M., et al. Endogenous hydrogen sulfide protects pancreatic beta-cells from a high-fat diet-induced glucotoxicity and prevents the development of type 2 diabetes. Biochem. Biophys. Res. Commun. 2013;442(3-4):227–233. doi: 10.1016/j.bbrc.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Parikh H., Carlsson E., Chutkow W.A., et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4(5):e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Saxena G., Mungrue I.N., Lusis A.J., Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57(4):938–944. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbett J.A. Thioredoxin-interacting protein is killing my beta-cells! Diabetes. 2008;57(4):797–798. doi: 10.2337/db08-0055. [DOI] [PubMed] [Google Scholar]
- 37.Shalev A. Lack of TXNIP protects beta-cells against glucotoxicity. Biochem. Soc. Trans. 2008;36(Pt 5):963–965. doi: 10.1042/BST0360963. [DOI] [PubMed] [Google Scholar]
- 38.Lu J., Holmgren A. Thioredoxin system in cell death progression. Antioxid. Redox Signal. 2012;17(12):1738–1747. doi: 10.1089/ars.2012.4650. [DOI] [PubMed] [Google Scholar]
- 39.Saxena G., Chen J., Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010;285(6):3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filios S.R., Xu G., Chen J., Hong K., Jing G., Shalev A. MicroRNA-200 is induced by thioredoxin-interacting protein and regulates Zeb1 protein signaling and beta cell apoptosis. J. Biol. Chem. 2014;289(52):36275–36283. doi: 10.1074/jbc.M114.592360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pu J., Zhao B., Wang E.J., et al. Racial/Ethnic Differences in Gestational Diabetes Prevalence and Contribution of Common Risk Factors. Paediatr. Perinat. Epidemiol. 2015;29(5):436–443. doi: 10.1111/ppe.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing G., Westwell-Roper C., Chen J., Xu G., Verchere C.B., Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J. Biol. Chem. 2014;289(17):11807–11815. doi: 10.1074/jbc.M113.525022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayden M.R., Tyagi S.C., Kerklo M.M., Nicolls M.R. Type 2 diabetes mellitus as a conformational disease. JOP. 2005;6(4):287–302. [PubMed] [Google Scholar]
- 44.Marzban L., Tomas A., Becker T.C., et al. Small interfering RNA-mediated suppression of proislet amyloid polypeptide expression inhibits islet amyloid formation and enhances survival of human islets in culture. Diabetes. 2008;57(11):3045–3055. doi: 10.2337/db08-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zraika S., Hull R.L., Verchere C.B., et al. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia. 2010;53(6):1046–1056. doi: 10.1007/s00125-010-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu G., Chen J., Jing G., Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 2013;19(9):1141–1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bodnar J.S., Chatterjee A., Castellani L.W., et al. Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat. Genet. 2002;30(1):110–116. doi: 10.1038/ng811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patwari P., Chutkow W.A., Cummings K., et al. Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J. Biol. Chem. 2009;284(37):24996–25003. doi: 10.1074/jbc.M109.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson E.J. Cutting Calories and TXNIP From the Skeletal Muscle to Restore Insulin Sensitivity. Diabetes. 2016;65(1):16–18. doi: 10.2337/dbi15-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson M.L., Distelmaier K., Lanza I.R., et al. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes. 2016;65(1):74–84. doi: 10.2337/db15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahsan M.K., Okuyama H., Hoshino Y., et al. Thioredoxin-binding protein-2 deficiency enhances methionine-choline deficient diet-induced hepatic steatosis but inhibits steatohepatitis in mice. Antioxid. Redox Signal. 2009;11(10):2573–2584. doi: 10.1089/ars.2009.2385. [DOI] [PubMed] [Google Scholar]
- 52.Chutkow W.A., Birkenfeld A.L., Brown J.D., et al. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59(6):1424–1434. doi: 10.2337/db09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chutkow W.A., Lee R.T. Thioredoxin regulates adipogenesis through thioredoxin-interacting protein (Txnip) protein stability. J. Biol. Chem. 2011;286(33):29139–29145. doi: 10.1074/jbc.M111.267666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshioka J., Imahashi K., Gabel S.A., et al. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ. Res. 2007;101(12):1328–1338. doi: 10.1161/CIRCRESAHA.106.160515. [DOI] [PubMed] [Google Scholar]
- 55.Hui T.Y., Sheth S.S., Diffley J.M., et al. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J. Biol. Chem. 2004;279(23):24387–24393. doi: 10.1074/jbc.M401280200. [DOI] [PubMed] [Google Scholar]
- 56.Ferraz M., Brunaldi K., Oliveira C.E., Bazotte R.B. Hepatic glucose production from L-alanine is absent in perfused liver of diabetic rats. Res. Commun. Mol. Pathol. Pharmacol. 1997;95(2):147–155. [PubMed] [Google Scholar]
- 57.Fulgencio J.P., Kohl C., Girard J., Pegorier J.P. Troglitazone inhibits fatty acid oxidation and esterification, and gluconeogenesis in isolated hepatocytes from starved rats. Diabetes. 1996;45(11):1556–1562. doi: 10.2337/diab.45.11.1556. [DOI] [PubMed] [Google Scholar]
- 58.Donnelly K.L., Margosian M.R., Sheth S.S., Lusis A.J., Parks E.J. Increased lipogenesis and fatty acid reesterification contribute to hepatic triacylglycerol stores in hyperlipidemic Txnip-/- mice. J. Nutr. 2004;134(6):1475–1480. doi: 10.1093/jn/134.6.1475. [DOI] [PubMed] [Google Scholar]
- 59.Levendusky M.C., Basle J., Chang S., Mandalaywala N.V., Voigt J.M., Dearborn R.E., Jr Expression and regulation of vitamin D3 upregulated protein 1 (VDUP1) is conserved in mammalian and insect brain. J. Comp. Neurol. 2009;517(5):581–600. doi: 10.1002/cne.22195. [DOI] [PubMed] [Google Scholar]
- 60.Aon-Bertolino M.L., Romero J.I., Galeano P., et al. Thioredoxin and glutaredoxin system proteins-immunolocalization in the rat central nervous system. Biochim. Biophys. Acta. 2011;1810(1):93–110. doi: 10.1016/j.bbagen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Hand L.E., Saer B.R., Hui S.T., et al. Induction of the metabolic regulator Txnip in fasting-induced and natural torpor. Endocrinology. 2013;154(6):2081–2091. doi: 10.1210/en.2012-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou J., Chng W.J. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion. 2013;13(3):163–169. doi: 10.1016/j.mito.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Yamawaki H., Berk B.C. Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels. Curr. Opin. Nephrol. Hypertens. 2005;14(2):149–153. doi: 10.1097/00041552-200503000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Kaimul A.M., Nakamura H., Masutani H., Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free Radic. Biol. Med. 2007;43(6):861–868. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 65.Shah A., Xia L., Goldberg H., Lee K.W., Quaggin S.E., Fantus I.G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013;288(10):6835–6848. doi: 10.1074/jbc.M112.419101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016;40(4):272–279. doi: 10.4093/dmj.2016.40.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892.. doi: 10.1155/2010/453892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishikawa T., Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007;9(3):343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 70.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 71.Schroder K., Zhou R., Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 72.Abderrazak A., Syrovets T., Couchie D., et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mori M.A., Bezy O., Kahn C.R. Metabolic syndrome: is Nlrp3 inflammasome a trigger or a target of insulin resistance? Circ. Res. 2011;108(10):1160–1162. doi: 10.1161/RES.0b013e318220b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen H., Ting J.P., O’Neill L.A. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat. Immunol. 2012;13(4):352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandanmagsar B., Youm Y.H., Ravussin A., et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu N., Zheng B., Shaywitz A., et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol. Cell. 2013;49(6):1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu F.X., Chai T.F., He H., Hagen T., Luo Y. Thioredoxin-interacting protein (Txnip) gene expression: sensing oxidative phosphorylation status and glycolytic rate. J. Biol. Chem. 2010;285(33):25822–25830. doi: 10.1074/jbc.M110.108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farrell M.R., Rogers L.K., Liu Y., Welty S.E., Tipple T.E. Thioredoxin-interacting protein inhibits hypoxia-inducible factor transcriptional activity. Free Radic. Biol. Med. 2010;49(9):1361–1367. doi: 10.1016/j.freeradbiomed.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marin-Hernandez A., Gallardo-Perez J.C., Ralph S.J., Rodriguez-Enriquez S., Moreno-Sanchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev. Med. Chem. 2009;9(9):1084–1101. doi: 10.2174/138955709788922610. [DOI] [PubMed] [Google Scholar]
- 80.Cantley J., Grey S.T., Maxwell P.H., Withers D.J. The hypoxia response pathway and beta-cell function. Diabetes Obes. Metab. 2010;12(Suppl. 2):159–167. doi: 10.1111/j.1463-1326.2010.01276.x. [DOI] [PubMed] [Google Scholar]
- 81.Hui S.T., Andres A.M., Miller A.K., et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc. Natl. Acad. Sci. USA. 2008;105(10):3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson J.E., Thompson C.B. Putting the rap on Akt. J. Clin. Oncol. 2004;22(20):4217–4226. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 83.Meuillet E.J., Mahadevan D., Berggren M., Coon A., Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN’s tumor suppressor activity. Arch. Biochem. Biophys. 2004;429(2):123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 84.Lee S.R., Yang K.S., Kwon J., Lee C., Jeong W., Rhee S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277(23):20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 85.Leslie N.R., Bennett D., Lindsay Y.E., Stewart H., Gray A., Downes C.P. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22(20):5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahadev K., Zilbering A., Zhu L., Goldstein B.J. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J. Biol. Chem. 2001;276(24):21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 87.Pelicano H., Xu R.H., Du M., et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J. Cell Biol. 2006;175(6):913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ren X., Ma H., Qiu Y., et al. The downregulation of thioredoxin accelerated Neuro2a cell apoptosis induced by advanced glycation end product via activating several pathways. Neurochem. Int. 2015;87:128–135. doi: 10.1016/j.neuint.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Park S.M., Gaur A.B., Lengyel E., Peter M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang S., Jin Y., Zheng H., et al. High glucose condition upregulated Txnip expression level in rat mesangial cells through ROS/MEK/MAPK pathway. Mol. Cell. Biochem. 2011;347(1-2):175–182. doi: 10.1007/s11010-010-0626-z. [DOI] [PubMed] [Google Scholar]
- 91.Cha-Molstad H., Saxena G., Chen J., Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J. Biol. Chem. 2009;284(25):16898–16905. doi: 10.1074/jbc.M109.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaked M., Ketzinel-Gilad M., Cerasi E., Kaiser N., Leibowitz G. AMP-activated protein kinase (AMPK) mediates nutrient regulation of thioredoxin-interacting protein (TXNIP) in pancreatic beta-cells. PLoS One. 2011;6(12):e28804. doi: 10.1371/journal.pone.0028804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gondo Y., Satsu H., Ishimoto Y., Iwamoto T., Shimizu M. Effect of taurine on mRNA expression of thioredoxin interacting protein in Caco-2 cells. Biochem. Biophys. Res. Commun. 2012;426(3):433–437. doi: 10.1016/j.bbrc.2012.08.116. [DOI] [PubMed] [Google Scholar]
- 94.Jo S.H., Kim M.Y., Park J.M., Kim T.H., Ahn Y.H. Txnip contributes to impaired glucose tolerance by upregulating the expression of genes involved in hepatic gluconeogenesis in mice. Diabetologia. 2013;56(12):2723–2732. doi: 10.1007/s00125-013-3050-6. [DOI] [PubMed] [Google Scholar]
- 95.Bedarida T., Baron S., Vibert F., et al. Resveratrol Decreases TXNIP mRNA and Protein Nuclear Expressions With an Arterial Function Improvement in Old Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(6):720–729. doi: 10.1093/gerona/glv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nivet-Antoine V., Cottart C.H., Lemarechal H., et al. trans-Resveratrol downregulates Txnip overexpression occurring during liver ischemia-reperfusion. Biochimie. 2010;92(12):1766–1771. doi: 10.1016/j.biochi.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 97.Xu G., Chen J., Jing G., Shalev A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61(4):848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khodneva Y., Shalev A., Frank S.J., Carson A.P., Safford M.M. Calcium channel blocker use is associated with lower fasting serum glucose among adults with diabetes from the REGARDS study. Diabetes Res. Clin. Pract. 2016;115:115–121. doi: 10.1016/j.diabres.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cha-Molstad H., Xu G., Chen J., et al. Calcium channel blockers act through nuclear factor Y to control transcription of key cardiac genes. Mol. Pharmacol. 2012;82(3):541–549. doi: 10.1124/mol.112.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takir M., Kostek O., Ozkok A., et al. Lowering Uric Acid With Allopurinol Improves Insulin Resistance and Systemic Inflammation in Asymptomatic Hyperuricemia. J. Investig. Med. 2015;63(8):924–929. doi: 10.1097/JIM.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 101.Rajesh M., Mukhopadhyay P., Batkai S., et al. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J. Cell. Mol. Med. 2009;13(8B):2330–2341. doi: 10.1111/j.1582-4934.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roncal C.A., Reungjui S., Sanchez-Lozada L.G., et al. Combination of captopril and allopurinol retards fructose-induced metabolic syndrome. Am. J. Nephrol. 2009;30(5):399–404. doi: 10.1159/000235731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu P., Chen Y., Wang B., Zhang F., Wang D., Wang Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin. Endocrinol. (Oxf.) 2015;83(4):475–482. doi: 10.1111/cen.12673. [DOI] [PubMed] [Google Scholar]
- 104.Tan S.M., Zhang Y., Cox A.J., Kelly D.J., Qi W. Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol. Dial. Transplant. 2011;26(1):100–110. doi: 10.1093/ndt/gfq355. [DOI] [PubMed] [Google Scholar]
- 105.Mifsud S., Kelly D.J., Qi W., et al. Intervention with tranilast attenuates renal pathology and albuminuria in advanced experimental diabetic nephropathy. Nephron, Physiol. 2003;95(4):83–91. doi: 10.1159/000074845. [DOI] [PubMed] [Google Scholar]
- 106.Akahori H., Ota T., Torita M., Ando H., Kaneko S., Takamura T. Tranilast prevents the progression of experimental diabetic nephropathy through suppression of enhanced extracellular matrix gene expression. J. Pharmacol. Exp. Ther. 2005;314(2):514–521. doi: 10.1124/jpet.105.084772. [DOI] [PubMed] [Google Scholar]
- 107.Kelly D.J., Zhang Y., Connelly K., et al. Tranilast attenuates diastolic dysfunction and structural injury in experimental diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2007;293(5):H2860–H2869. doi: 10.1152/ajpheart.01167.2006. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe R., Nakamura H., Masutani H., Yodoi J. Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2. Pharmacol. Ther. 2010;127(3):261–270. doi: 10.1016/j.pharmthera.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Zhou J., Yu Q., Chng W.J. TXNIP (VDUP-1, TBP-2): a major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2011;43(12):1668–1673. doi: 10.1016/j.biocel.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 110.Sheth S.S., Bodnar J.S., Ghazalpour A., et al. Hepatocellular carcinoma in Txnip-deficient mice. Oncogene. 2006;25(25):3528–3536. doi: 10.1038/sj.onc.1209394. [DOI] [PubMed] [Google Scholar]
- 111.Tian X., Irannejad R., Bowman S.L., et al. The alpha-Arrestin ARRDC3 Regulates the Endosomal Residence Time and Intracellular Signaling of the beta2-Adrenergic Receptor. J. Biol. Chem. 2016;291(28):14510–14525. doi: 10.1074/jbc.M116.716589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dores M.R., Lin H.N JG, Mendez F, Trejo J. The alpha-arrestin ARRDC3 mediates ALIX ubiquitination and G protein-coupled receptor lysosomal sorting. Mol. Biol. Cell. 2015;26(25):4660–4673. doi: 10.1091/mbc.E15-05-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shea F.F., Rowell J.L., Li Y., Chang T.H., Alvarez C.E. Mammalian alpha arrestins link activated seven transmembrane receptors to Nedd4 family e3 ubiquitin ligases and interact with beta arrestins. PLoS One. 2012;7(12):e50557. doi: 10.1371/journal.pone.0050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Puca L., Brou C. Alpha-arrestins - new players in Notch and GPCR signaling pathways in mammals. J. Cell Sci. 2014;127(Pt 7):1359–1367. doi: 10.1242/jcs.142539. [DOI] [PubMed] [Google Scholar]
- 115.Kang D.S., Tian X., Benovic J.L. Role of beta-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr. Opin. Cell Biol. 2014;27:63–71. doi: 10.1016/j.ceb.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohammad S. Role of Free Fatty Acid Receptor 2 (FFAR2) in the Regulation of Metabolic Homeostasis. Curr. Drug Targets. 2015;16(7):771–775. doi: 10.2174/1389450116666150408103557. [DOI] [PubMed] [Google Scholar]
- 117.Mohammad S. GPR40 Agonists for the Treatment of Type 2 Diabetes Mellitus: Benefits and Challenges. Curr. Drug Targets. 2016;17(11):1292–1300. doi: 10.2174/1389450117666151209122702. [DOI] [PubMed] [Google Scholar]
- 118.Mohammad S., Ramos L.S., Buck J., Levin L.R., Rubino F., McGraw T.E. Gastric inhibitory peptide controls adipose insulin sensitivity via activation of cAMP-response element-binding protein and p110beta isoform of phosphatidylinositol 3-kinase. J. Biol. Chem. 2011;286(50):43062–43070. doi: 10.1074/jbc.M111.289009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohammad S., Patel R.T., Bruno J., Panhwar M.S., Wen J., McGraw T.E. A naturally occurring GIP receptor variant undergoes enhanced agonist-induced desensitization, which impairs GIP control of adipose insulin sensitivity. Mol. Cell. Biol. 2014;34(19):3618–3629. doi: 10.1128/MCB.00256-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peterson C.W., Stoltzman C.A., Sighinolfi M.P., Han K.S., Ayer D.E. Glucose controls nuclear accumulation, promoter binding, and transcriptional activity of the MondoA-Mlx heterodimer. Mol. Cell. Biol. 2010;30(12):2887–2895. doi: 10.1128/MCB.01613-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shah A., Xia L., Masson E.A., et al. Thioredoxin-Interacting Protein Deficiency Protects against Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015;26(12):2963–2977. doi: 10.1681/ASN.2014050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh L.P. Thioredoxin Interacting Protein (TXNIP) and Pathogenesis of Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013:4. doi: 10.4172/2155-9570.1000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Y., Li X., Tang S. Retrospective analysis of the relationship between elevated plasma levels of TXNIP and carotid intima-media thickness in subjects with impaired glucose tolerance and early Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2015;109(2):372–377. doi: 10.1016/j.diabres.2015.05.028. [DOI] [PubMed] [Google Scholar]