Abstract
Introduction:
Geraniin has many biological activities including anti-osteoporotic and anti-hyperglycemic efficacies.
Materials and Methods:
A rapid and simple method for the determination of geraniin in rat plasma using ultra performance liquid chromatography coupled to ultraviolet detector was developed. The plasma sample, spiked with epicatechin as an internal standard, was subjected to ethyl acetate extraction prior to analysis. Chromatographic separation was performed on the HSS T3 column and monitored at a wavelength of 280 nm. The limit of detection and lower limit of quantification was 0.07 μg/mL and 0.2 μg/mL in rat plasma, respectively.
Conclusion:
Good linearity was obtained in the range of 0.2 - 200 μg/mL, and the correlation coefficient was better than 0.997. The intra-day and inter-day precisions decreased 9.8%. The accuracy of QC samples ranged from 84.4% to 87.1%. The extraction recovery ranged from 88.4% to 90.3% and the matrix effect ranged from 84.4% to 87.2%. The analyte was stable in rat plasma when stored at room temperature for 12 hours, 4°C for 24 hours and -20°C for 15 days. t1/2 and t1/2 for i.v. was 0.21 ± 0.10 and 7.20 ± 2.20 h, respectively. Plasma clearance (CL) was 0.03 ± 0.02 L/h/kg and apparent volume of distribution (Vz) was 0.05 + 0.01 L/kg. The developed method was successfully applied to the pharmacokinetic study of geraniin in rats.
Keywords: Geraniin, UPLC, pharmacokinetics, epicatechin, rat, plasma
1. Introduction
Natural products are an important source in the discovery of leads for new drugs. Geraniin (GE) is the major active component in the rind of Nephelium lappaceum L. Phyllanthus muellerianus L. and Phyllanthus urinaria L. [1, 2]. GE exhibited a range of bioactive properties, such as being antiviral [3-5], anti-inflammatory [6, 7], antioxidant [8-12], anticarcinogenic [13], and antihypertensive [13-16]. In addition, GE possess in vitro hypoglycemic activity and has the good performances to prevent the formation of advanced glycation end-products. Recently, GE has also been used to prevent OVX-induced bone loss in bone mineral density and bone mineral level, to prove femur weight and bone calcium level [17, 18]. Some reviews agreed that geraniin is a potential functional food additive and antihyperglycemic agent [1, 2].
HPLC has been applied to detect the GE content in raw materials. Agyare et al. developed HPLC-UV to determine GE in raw materials to evaluate the quality control analysis of geraniin in dried leaves of Phyllanthus muellerianus [19]. Yin et al. established HPLC-UV to measure the dynamic GE concentration of Erodium stephanianum willd in different collection seasons. However, to our best knowledge, few work has been published on the pharmacokinetic of geraniin so far. In this study, a simple and rapid ultra-performance liquid chromatography (UPLC) was developed and validated to determine GE in rat plasma. The method has been successfully applied to the determination of GE in rat plasma after intravenous injection for pharmacokinetic study, which provided reference for further preclinical application of GE.
2. Experimental Section
2.1. Reagents and Materials
Geraniin (HPLC purity > 98%) and epicatechin (HPLC purity > 98%, IS) were purchased from Chengdu Must Bio-Technology Co. Ltd (Chengdou, China) (Fig. 1). Methanol and acetonitrile with HPLC grade were obtained from Merck (Merck, Darmstadt, Germany). Ethyl acetate was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ultrapure water (18.2 MΩ/cm) in the experiment was obtained from a Milli-Q purification system. All other reagents were of analytical grade.
Fig. (1).
Chemical structure of (A) geraniin and (B) epicatechin (IS).
2.2. Animals
6 Sprague-Dawley (SD) rats (220 ± 20 g) were provided from the Experimental Animal Center of Wenzhou Medical University (Wenzhou, China). The rats were fed in the condition of constant temperature (22 ± 2°C) and the humidity (55 ± 10%) with a 12 h light/dark cycle. All the experimental animals were housed under the above conditions for 7 days for acclimation, and were fasting overnight before experiments but maintained free access to water. The study was approved by the Animal Ethics Committee of Wenzhou Medical University (July 2, 2015; No: 2015–251).
2.3. Instrument and Chromatographic Conditions
UPLC analyses were performed using a Waters Acquity - UPLC (Waters, Milford, MA, USA) equipped with an autosampler. The chromatographic column was ACQUITY UPLC HSS T3 (100 mm × 2.1 mm, 1.8 μm, Waters, USA) with the mobile phase of 0.1% trifluoroacetic acid (A) and acetonitrile (B). The optimal isogradient elution was as follows: 83% A and 17% B. The column temperature and injection volume were 30°C and 5 μL, respectively. The flow rate was 0.3 mL/min and the detection wavelength was 280 nm.
2.4. Preparation of Standard and Quality Control Solutions
1 mg/mL of GE and IS stock solutions (pH=5.0, adjustment with acetic acid) were prepared by 10.0 mg corresponding solutes dissolved in methanol using 10 mL of brown volumetric flask, and were all kept in 4°C, respectively. The stock solutions were serially diluted with methanol to prepare standard solutions at desired concentrations before analysis. Calibration samples for GE was prepared by spiking 10 μL standard solutions and 10 μL IS solution in blank plasma to obtain final plasma concentrations of 0.2, 0.5, 1.0, 2.0, 5.0, 10.0, 20.0, 50, 100 and 200 μg/mL for GE and 10 μg/mL for IS. All standard solutions were kept in 4°C until analysis. Quality Control (QC) samples were prepared in blank plasma at low, medium and high concentrations of 0.5, 10, 150 µg/mL for GE.
2.5. Sample Preparation
Plasma (100 μL) was mixed with 10 μL of 10% of ascorbic acid solution (pH=5.0, adjustment with acetic acid) and 10 μL of IS solution. Then they thoroughly mixed by vortexing for 30 s and left at room temperature for 2 min. Ethyl acetate (400 μL) was added and mixed by vortexing vigorously for 3 min to extract GE and IS. The sample was then centrifuged briefly (4000 rpm) at 4°C for 10 min and 350 μL of ethyl acetate layer was transferred to a clean Eppendorf tube containing 10 μL of 20% ascorbic acid solution. A second extraction was performed by adding 400 μL of ethyl acetate into the plasma-containing tube, and vortexing for another 3 min. The sample was further centrifuged at 5000 rpm for 10 min. The supernatant of 350 μL was collected and combined with the first fraction. The mixed supernatant of 700 μL was dried under nitrogen stream at 50°C for 10 min. The residue was reconstituted in 100 μL of 50% methanol/water/0.01% ascorbic acid and vortexed for 2 min. The solution was centrifuged at 15,000 × g for 10 min and the supernatant (∼80 μL) was transferred to an autosampler vial for injection.
2.6. Method Validation
The established method for selectivity, linearity, accuracy, precision, recovery and stability was validated according to the FDA guideline of bioanalytical methods [20]. The selectivity was assessed by analyzing six blank plasma samples with corresponding plasma samples spiked with GE and IS, and with plasma samples after iv. The standard calibration curve was determined by plotting the peak area ratio (y) of GE vs IS with the nominal concentrations (x) of GE in rat plasma. The regression equation was calculated by using the weighted (1/x2) linear least-squares regression. The limit of detection (LOD) and quantification (LLOQ) was defined as a signal to noise ratio (S/N) of 3:1 and 10:1, respectively. The precision and accuracy were determined by analysis of six replicates at LLOQ and each QC level (low, mid and high) on three consecutive days. The accuracy was calculated by the percentage of the measured concentration to the nominal concentration. The extraction recovery of GE was calculated by comparing the measured concentration of QC samples with corresponding standard solutions of GE added into the post-extracted supernatant from blank plasma matrix. The extraction recovery of IS (10 μg/mL) was obtained according to the same procedure. This procedure was repeated for five replicates at three QC concentration levels. For evaluating the stability of the method, the concentrations of QCs were determined in the condition of 4°C for 12 h, room temperature for 24 h, -20°C for 15 days and three freeze-thaw cycles on three consecutive days, respectively.
2.7. Pharmacokinetic Study
GE was dissolved in water/:propanediol (8:2, v/v) and finally diluted to an appropriate concentration prior to animal experiment. Six SD rats were anesthetized with intramuscular 10% Chloral Hydrate (5mg/kg). They were then received sublingual intravenous administrations of GE (10 mg/kg) and blood samples (about 0.3 mL) were collected in heparinized tubes at 0.083, 0.167, 0.333, 0.667, 1, 2, 4, 8, 12 and 24 h post-dosing through the caudal veins. Blood samples were centrifuged at 5000 rpm for 10 min immediately and the supernatant was separated and preserved at -20°C until analysis. The pharmacokinetics parameters were calculated by DAS software (version 3.0, Shanghai, China).
3. Results and Discussion
3.1. Optimization of Methods
The stability of analyte in solvent is important for the development and validation of assay method. GE was easily oxidized under alkaline or neutral environments for its structure with several phenolic hydroxyl groups. In preliminary stability experiments, 1 μg/mL of GE in methanol was put in the condition of 4°C for 12 h, 4°C for one week (pH=5.0, adjustment with acetic acid) and room temperature for 4 h to investigate its stability. The results indicated that the degradation rate of GE was about 90%, 98% and 75%, respectively, suggesting that the stability in the condition of room temperature and 4°C for 12 h were easily oxidized and unacceptable for biological assay. In the procedure of optimization of extraction methods, ascorbic acid (antioxidant) and acetic acid (acidity adjustment) were thus added into plasma sample to prevent the oxidation of GE. In addition, several variables, including extraction solution, temperature and time of nitrogen stream, were selected for the optimization procedure. We finalized the optimal sample extraction protocol in section 'Sample Preparation'. Epicatechin had similar molecular structure with GE and was used as IS.
The sensitivity of detection was very important for the analysis of biological samples. In this work, four types of detectors including mass spectrometry (Waters Quattro Micro mass spectrometer), fluorescence (RF-5301PC, SHIMADZU), ultraviolet (ACQUITY UPLC PDA) and electrochemical detector (CHI650b Electrochemical Analyzer, USA) were investigated to select the optimal detector. 1 μg/mL of GE in methanol was injected into four detectors to investigate the respective value of response signal. In contrast, after the full optimization of four detector parameters, the detection sensitivity of ultraviolet detector was higher than that of other detectors. Ultraviolet absorption of GE had two maximum absorption bands at wavelength of 220 nm and 280 nm. Because the wavelength of 280 nm had no significant interference within the retention time, it was set as the detection wavelength to determine the content of GE and IS.
Analysis was performed on several different types of UPLC columns, including a BEH Shield RP18 column (100 mm × 2.1 mm, 1.7 μm), BEH C18 column (100 mm × 2.1 mm, 1.7 μm) and HSS T3 column (100 mm × 2.1 mm, 1.8 μm). HSS T3 column provided symmetrical peak shapes, better separation and suitable retention time for GE and IS in contrast with the other chromatographic columns. The percentage of acetonitrile/water, methanol/water and acidic modifiers (trifluoroacetic acid, acetic acid and phosphoric acid) in the mobile phase were optimized to obtain better separation and peak shapes. The presence of acetonitrile and water with 0.1% trifluoroacetic acid in the mobile phase obviously improved the response of GE and IS.
In general, the more simple and fast extraction method is and the more popular it is. Protein precipitation is one of the most simple and fast extraction method and was thus firstly applied to extract GE in rat plasma. Our results showed that extraction recovery of protein precipitation is about 60%, while the interference peaks of samples increased in the chromatograms, resulting in the worse selectivity of the method. Similarly, the addition of acetonitrile into rat plasma caused the diluted plasma concentration of GE and made the detection more difficult. According to the chemical polarity of GE, ethyl acetate was selected as the extraction solvent and the results showed that extraction rate reached more than 88% and the extraction method had less interference peaks and better selectivity.
3.2. Method Validated
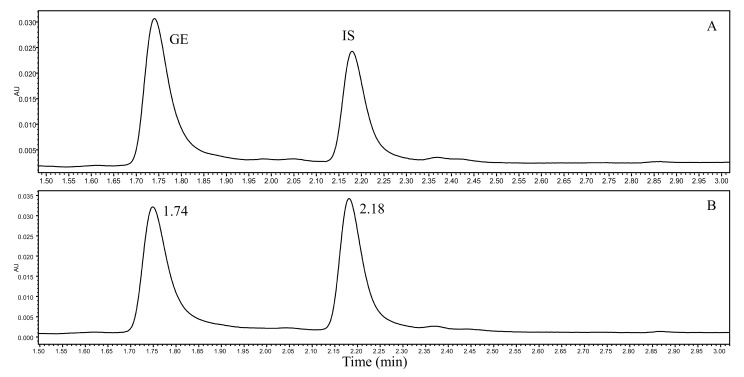
The typical chromatograms of blank plasma, blank plasma spiked with standard solution of GE and IS, and rat plasma samples were shown in Fig. (2). The retention time of GE and IS were 1.74 and 2.18 min, respectively. There was no obvious interference within the retention time. The linearity of this method was in the range of 0.2 -200 μg/mL with LLOQ (0.2 μg/mL) and LOD (0.07 μg/mL). The regression equation of GE was Y = 0.01270*x - 0.008268 (R2=0.9974) and the accuracy of each point of calibration curve was in the range of 85% - 106%. As shown in Table 1, the intra-day precision (RSD) of this method was 4.6% - 8.7%, and inter-day precision was 5.1% - 9.8%. Accuracy ranged between 84.4% and 88.8%. The extraction recovery of six reduplicates of QCs and IS was at the range of 88.7% - 90.3% and 82.2%, respectively. It was indicated that method met the requirements for drug analysis in vivo. The detailed result was listed in Table 2. Under the condition of 4°C, room temperature (20°C), three freeze-thaw cycles and Long-term stability (at -20°C for 15 days), the RSD of GE was all below 12.2%, 14.5%, 13.0% and 14.5%, respectively, which showed excellent stability.
Fig. (2).
UPLC chromatograms of (A) blank rat plasma spiked with GE (10 μg/mL) and IS (10 μg/mL) and, (B) rat plasma sample collected 30 min after sublingual intravenous injection of 10 mg/kg.
Table 1.
Intra- and inter-day accuracy and precision of QCs and LLOQ.
|
Spiked Concentration
(µg/ mL) |
Intra-Day Concentration (Mean + SD) |
Precision
(% RSD) |
Accuracy
(%) |
Inter-Day Concentration (Mean+SD) |
Precision
(% RSD) |
Accuracy
(%) |
|---|---|---|---|---|---|---|
| 150 | 130.7 + 6.1 | 4.6 | 87.1 | 133.2 + 6.8 | 5. 1 | 88.8 |
| 10 | 8.5 + 0.6 | 6.7 | 85.2 | 8.5 + 0.5 | 6.2 | 85.3 |
| 0.5 | 0.4 + 0.04 | 8.7 | 84.4 | 0.4 + 0.04 | 9.8 | 85.8 |
| 0.2 | 0.2 + 0.03 | 12.2 | 82.5 | 0.2 + 0.03 | 13.4 | 82.1 |
Table 2.
Extraction recovery of QCs (n =5).
|
Spiked plasma concentration
(µg/ mL) |
Extraction recovery
(mean + SD) |
|---|---|
| 150 | 90.1 + 8.2 |
| 10 | 88.7 + 8.3 |
| 0.5 | 90.3 + 12.5 |
| 10 (IS) | 82.2 ± 7.9 |
3.3. Pharmacokinetic Study
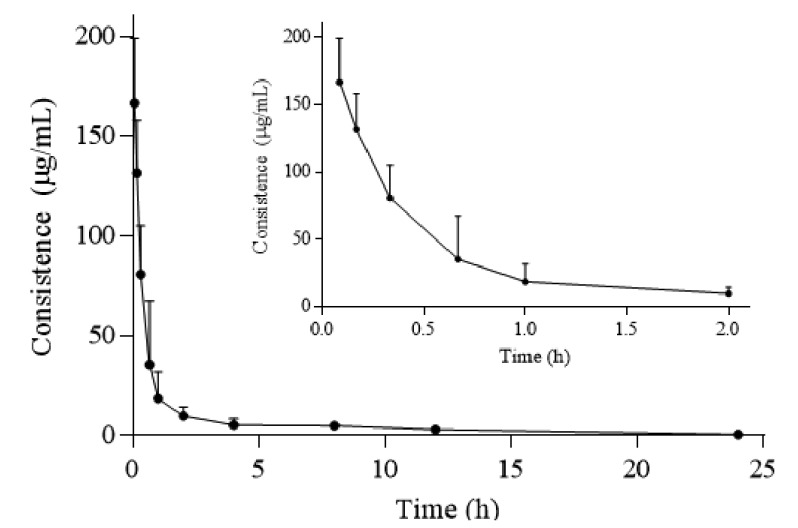
After sublingual intravenous administrations of GE in rats, the means of plasma concentration-time curve (n=6) showed in Fig. (3). The data was calculated by DAS 3.0 program and was listed in Table 3. According to Akaike Information Criterion (AIC) minimum principle and the biological fitness of concentration-time curve, Sublingual intravenous administration was fitted as two-compartmental model (weight coefficient = 1/c2). As showed in Table 3 and Fig. (3), t1/2α for i.v. was 0.21 + 0.10 h, it suggested that GE was distributed in vivo rapidly, while t1/2β was 7.20 ± 2.20 h, suggesting that the elimination of GE was slow. CL for i.v. was 0.03 ± 0.02 L/h/kg suggested that GE experienced a slow elimination in vivo after intravenous administration.Vz for i.v. was 0.05 ± 0.01 L/kg demonstrated that GE was mainly distributed in plasma. In preliminary experiments, another two rats were received oral administrations of GE (100 mg/kg) and blood samples (about 0.3 mL) were collected in heparinized tubes at 0.167, 0.333, 0.667, 1, 2, 4, 8, 12 and 24 h post-dosing through the caudal veins. However, the content of GE was not detected for all samples of rat plasma, suggesting that GE had a low bioavailability.
Fig. (3).
Mean plasma concentration-time of GE after sublingual intravenous administration of 10 mg/kg (n=6).
Table 3.
The PK parameters of geraniin after sublingual administration of 10 mg/kg (n = 6).
| Parameter | Units | i.v. (10 mg/kg) |
|---|---|---|
| t1/2α | h | 0.21 ± 0.10 |
| t1/2β | h | 7.20 ± 2.20 |
| Vz | L/kg | 0.05 ± 0.01 |
| CL | L/h/kg | 0.03 ± 0.02 |
| AUC(0-t) | mg/L*h | 482.1 ± 171.1 |
| AUC(0-∞) | mg/L*h | 503.3 ± 169.2 |
| K10 | 1/h | 0.68 ± 0.51 |
| K12 | 1/h | 3.06 ± 1.15 |
| K21 | 1/h | 0.24 ± 0.11 |
CONCLUSION
A rapid and simple UPLC method was firstly developed and validated to determine GE in rat plasma. The proposed method was successfully applied to the pharmacokinetic study of GE after intravenous administration. Although GE is unstable in rat plasma at room temperature, it was found that its stability was obviously increased by adding ascorbic acid and acetic acid immediately to the collected plasma samples. The extraction method and UPLC elution condition described provided a practical means to analyze GE in biological matrix. Compared with electrochemical, fluorescence or mass spectrometric detectors, UV offered a higher sensitivity for GE.
ACKNOWLEDGEMENTS
The authors acknowledge financial support from the Nature Foundation Committee of Wenzhou in China (Y20140002), Special Program for Guangdong High-tech Zone (2012B011000050) and Guangdong social development Program (2013B021800232).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Perera A., Ton S.H., Palanisamy U.D. Perspectives on geraniin, a multifunctional natural bioactive compound. Trends Food Sci. Technol. 2015;44(2):243–257. [Google Scholar]
- 2.Elendran S., Wang L.W., Prankerd R., Palanisamy U.D. The physicochemical properties of geraniin, a potential antihyperglycemic agent. Pharm. Biol. 2015;53(12):1719–1726. doi: 10.3109/13880209.2014.1003356. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y.J., Xiu J.H., Zhang L.F., Qin C., Liu J.N. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine. 2012;20(1):67–70. doi: 10.1016/j.phymed.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y., Zhang L., Fan X., Qin C., Liu J. Bioorg. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Med. Chem. Lett. 2012;22(6):2209–2211. doi: 10.1016/j.bmcl.2012.01.102. [DOI] [PubMed] [Google Scholar]
- 5.Yeo S.G., Song J.H., Hong E.H., Lee B.R., Kwon Y.S., Chang S.Y., Kim S.H., Lee S.W., Park J.H., Ko H.J. Antiviral effects of Phyllanthus urinaria containing corilagin against human enterovirus 71 and Coxsackievirus A16 in vitro. Arch. Pharm. Res. 2015;38(2):193–202. doi: 10.1007/s12272-014-0390-9. [DOI] [PubMed] [Google Scholar]
- 6.Velazquez-Gonzalez C., Carino-Cortes R., Gayosso de Lucio J.A., Ortiz M.I., De la O.A.M., Altamirano-Baez D.A., Angeles L. J., Bautista-Avila M. Antinociceptive and anti-inflammatory activities of Geranium bellum and its isolated compounds. BMC Complement. Altern. Med. 2014;14:506. doi: 10.1186/1472-6882-14-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boakye Y.D., Agyare C., Abotsi W.K. Study and analysis of energy efficiency in the main energy systems of the Hospital Homero Castanier Crespo: Thermal system. Planta Med. 2013;79(13):1142–1142. [Google Scholar]
- 8.Anokwuru C.P., Sinisi A., Samie A., Taglialatela-Scafati O. Antibacterial and antioxidant constituents of Acalypha wilkesiana. Nat. Prod. Res. 2015;29(12):1180–1183. doi: 10.1080/14786419.2014.983105. [DOI] [PubMed] [Google Scholar]
- 9.Bing S.J., Ha D., Kim M.J., Park E., Ahn G., Kim D.S., Ko R.K., Park J.W., Lee N.H., Jee Y. Geraniin down regulates gamma radiation-induced apoptosis by suppressing DNA damage. Food Chem. Toxicol. 2013;57:147–153. doi: 10.1016/j.fct.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Priya O.S., Viswanathan M.B., Balakrishna K., Venkatesan M. Chemical constituents and in vitro antioxidant activity of Phyllanthus wightianus. Nat. Prod. Res. 2011;25(10):949–958. doi: 10.1080/14786419.2010.517203. [DOI] [PubMed] [Google Scholar]
- 11.Kang K.A., Lee I.K., Zhang R., Piao M.J., Kim K.C., Kim S.Y., Shin T., Kim B.J., Lee N.H., Hyun J.W. Radioprotective effect of geraniin via the inhibition of apoptosis triggered by γ-radiation-induced oxidative stress. Cell Biol. Toxicol. 2011;2(2):83–94. doi: 10.1007/s10565-010-9172-4. [DOI] [PubMed] [Google Scholar]
- 12.Ito H. Metabolites of the Ellagitannin geraniin and their antioxidant activities. Planta Med. 2011;77(11):1110–1115. doi: 10.1055/s-0030-1270749. [DOI] [PubMed] [Google Scholar]
- 13.Ko H. Bioorg. Geraniin inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration, invasion and anoikis resistance. Med. Chem. Lett. 2015;25(17):3529–3534. doi: 10.1016/j.bmcl.2015.06.093. [DOI] [PubMed] [Google Scholar]
- 14.Lin S.Y., Wang C.C., Lu Y.L., Wu W.C., Hou W.C. Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem. Toxicol. 2008;46(7):2485–2492. doi: 10.1016/j.fct.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Hsu F.L., Lu F.H., Cheng J.T. Influence of Acetonylgeraniin, a Hydrolyzable Tannin from Euphoria longana, on Orthostatic Hypotension in a Rat Model. Planta Med. 1994;60(4):297–300. doi: 10.1055/s-2006-959487. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J.T., Chang S.S., Hsu F.L. Antihypertensive action of geraniin in rats. J. Pharm. Pharmacol. 1994;46(1):46–49. doi: 10.1111/j.2042-7158.1994.tb03718.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y., He B., Zhang X., Yang R., Li S., Song B., Zhang Y., Yun Y., Yan H., Chen P., Shen Z. Osteoprotective effect of geraniin against ovariectomy-induced bone loss in rats. Bioorg. Med. Chem. Lett. 2015;25(3):673–679. doi: 10.1016/j.bmcl.2014.11.081. [DOI] [PubMed] [Google Scholar]
- 18.He B., Hu M., Li S.D., Yang X.T., Lu Y.Q., Liu J.X., Chen P., Shen Z.Q. Effects of geraniin on osteoclastic bone resorption and matrix metalloproteinase-9 expression. Bioorg. Med. Chem. Lett. 2013;23(3):630–634. doi: 10.1016/j.bmcl.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Agyare C., Rogge S., Baumann A., Lechtenberg M., Hensel A. Planta Med. 2010;76(12):1323–1323. [Google Scholar]
- 20.US Food and Drug Administration . Guidance for industry bioanalytical method validation. Rockville, MD: US Department of Health and Human Services, CDER and CVM; 2001. [Google Scholar]