1. INTRODUCTION
Active immunotherapy of Alzheimer’s disease (AD) has been under study for its ability to elicit anti-amyloid-beta (Aβ) antibodies, induce Aβ clearance, and reduce Aβ accumulation in the brain [1]. Active immunotherapy also has the potential to be cost-effective for long-term therapy [2, 3]. Development of the first therapeutic vaccine for AD, AN1792, a full-length Aβ peptide (Aβ1-42), was discontinued because of meningoencephalitis, attributed to T-cell activation [4-6]. Clinical benefit was suggested, however, in anti-body responders to AN1792 [5]. ACC-001 (vanutide cridificar), a next-generation therapeutic vaccine consisting of N-terminal (Aβ1-7) peptides conjugated to a carrier protein, was designed to minimize potentially harmful T-cell responses [6]. This paper focuses on the safety of ACC-001 + QS-21 adjuvant in 3 long-term extensions of phase 2a studies of ACC-001 + QS-21 in subjects with mild to moderate AD conducted in the United States (parent study Clinicaltrials.gov ID: NCT00498602), the European Union (parent study NCT00479557), and Japan (parent Study 1, NCT-00752232; parent Study 2, NCT00959192) [7, 8]. Based on interim results of the parent studies, dosing in all extension studies was terminated in July 2013 due to lack of efficacy.
2. MATERIALS AND METHODS
2.1. Study Design and Analysis
The phase 2a, multicenter, long-term extension studies assessing safety, tolerability, and immunogenicity of ACC-001 + QS-21 adjuvant in subjects with mild to moderate AD were either third-party unblinded (EU/US studies) or open-label (Japan study). The studies were registered on Clinicaltrials.gov (EU ID: NCT00955409; US ID: NCT00960531; Japan ID: NCT01238991) and were conducted in compliance with ethical principles originating in or derived from the Declaration of Helsinki. Informed consent was obtained from all subjects or legal representatives and caregivers prior to enrollment. Results of the EU/US studies were analyzed together as planned in the study statistical analysis plans, and results from the Japan study were analyzed separately in a similar way. Descriptive summary statistics are provided for all endpoints in the analysis.
2.2. Subjects
Eligible subjects participated in 1 of 4 placebo-controlled, multiple-ascending-dose parent studies (EU, US, and 2 in Japan) and must have completed parent study week 78 (week 104 for Japan study cohort 1), received at least 3 doses of investigational product, and had a Mini-Mental State Examination (MMSE) score ≥10 at extension study screening. Subjects from the 2 Japan parent studies were enrolled in a single, long-term extension study. Screening evaluations were performed at parent study week 78 (week 104 for Japan study cohort 1). Subjects were excluded if they had significant neurological disease other than AD that could have affected cognition or function; a serious adverse event (SAE) in the parent study deemed related to ACC-001; brain magnetic resonance imaging (MRI) evidence of amyloid-related imaging abnormalities of edema or effusion (ARIA-E) during the parent study; history of or screening visit brain MRI scan indicative of any other significant abnormality, including, but not limited to, more than 4 microhemorrhages (<10 mm), evidence of a single prior hemorrhage >1 cm3, multiple lacunar infarcts (2 or more) or evidence of a single prior infarct >1 cm3, evidence of a cerebral contusion, encephalomalacia, aneurysms, vascular malformations, subdural hematoma, or space-occupying lesions (e.g., arachnoid cysts or brain tumors, such as meningioma); current presence of a clinically significant major psychiatric disorder (e.g., major depressive disorder) according to the criteria of the DSM-IV-TR, or symptoms (e.g., hallucinations, suicidal ideation, suicidal behavior) that could affect the subject’s ability to complete the study.
2.3. Treatment and Follow-Up
Subjects who received ACC-001 at a dose of 3, 10, or 30 μg with QS-21 adjuvant (50 μg) in the parent studies [7, 8] continued at the same dose level in the extension studies. Subjects who received ACC-001 alone in the parent studies continued at the same dose with QS-21 added in the extension studies. Subjects who received QS-21 alone or phosphate-buffered saline (PBS) control treatment in the parent studies received ACC-001 at the dose of their original ACC-001 cohort assignment plus QS-21. Four immunizations were planned during the extension studies at day 1, and month 6, 12, and 18, with 6 months of follow-up. Dosing was discontinued on July 12, 2013, due to lack of efficacy in the parent studies, and all patients were followed for safety for up to 6 months from the last dose they received.
2.4. Assessments
Safety/tolerability assessments included incidence and severity of treatment-emergent AEs (TEAEs), SAEs, and AEs of special circumstance defined as MRI evidence of ARIA-E, intracranial hemorrhage, vasculitis, and immune-mediated events following the injection (anaphylaxis, angioedema, urticaria, and clinical syndrome diagnostic of serum sickness). TEAEs were evaluated at each visit. Brain MRI was performed at screening and 2 weeks before each injection, and at week 104 or early termination. Fluid attenuated inversion recovery and T2*/gradient echo (GRE) sequences were used to assess ARIA-E and amyloid-related imaging abnormalities of microhemorrhage/characterized by hemosi-derin deposition (ARIA-H), respectively. Scanning protocols included T2*/GRE sequences to increase the sensitivity for and detection of microhemorrhages. MRI scans were read centrally at the screening visit in the EU/US studies and locally thereafter unless the local radiologist detected an abnormality, except in France, where all MRI scans were centrally read. All MRI scans were read locally in the Japan study unless the local radiologist detected an abnormality in a post-baseline scan, in which case it was centrally read. Exploratory assessments included immunogenicity of ACC-001 evaluated with anti-Aβ IgG titers measured by enzyme-linked immunosorbent assay (ELISA); blood samples for anti-Aβ IgG titers were drawn at EU/US extension study screening and weeks 4, 12, 24, 30, 36, 50, 56, 66, 76, 82, and 104 or early termination; and at Japan study screening and weeks 2, 4, 12, 24, 28, 30, 36, 50, 54, 56, 66, 76, 80, 82, 91, and 104 or early termination. Cognitive status was assessed by MMSE at extension study screening and weeks 12, 26, 36, 52, 66, 78, 91, and 104 or early termination in all studies.
3. RESULTS
3.1. Subjects
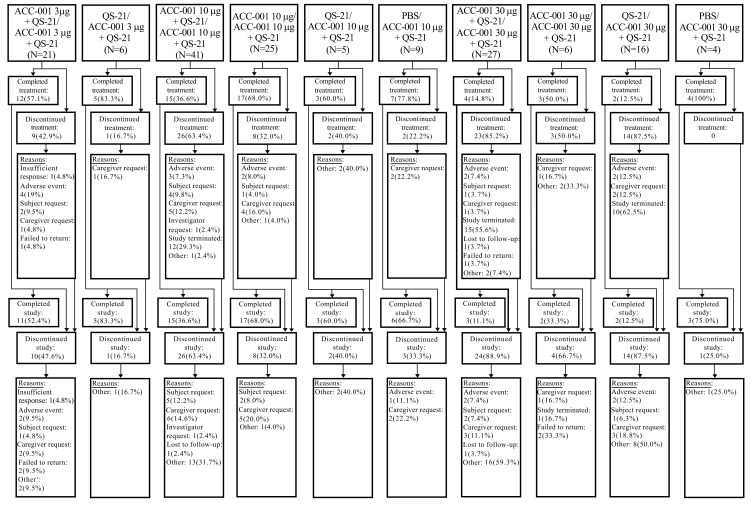
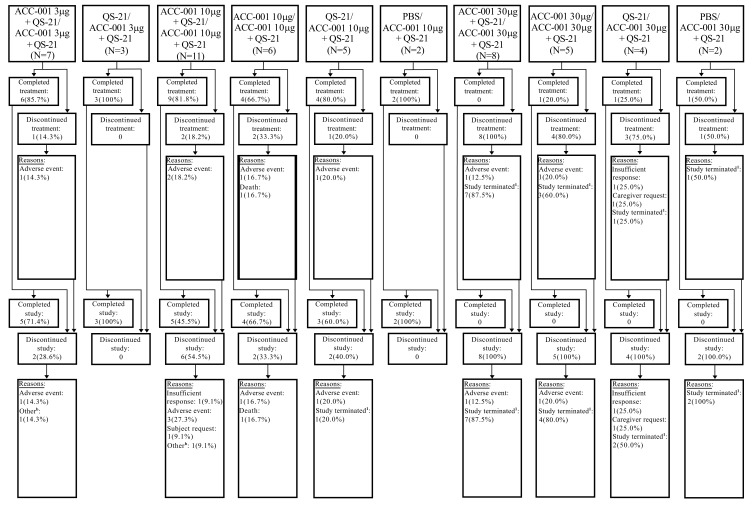
A total of 160 subjects were enrolled and 159 were treated in the EU/US studies, with first subject first visit (FSFV) on June 15, 2009, and last subject last visit (LSLV) on December 19, 2013. The Japan study enrolled and treated 53 subjects, with FSFV on December 20, 2010, and LSLV on December 13, 2013. In the EU/US studies, mean age was 70 years, with 59% female, mean duration of AD at parent study baseline was 5.25 years, mean baseline MMSE score was 18.8, and 93.8% of the subjects were receiving an acetylcholinesterase inhibitor or memantine at baseline. In the Japan study (N=53), the mean age was 69 years, with 68% female, mean duration of AD at extension study baseline was 5.6 years, mean baseline MMSE score was 21.1, and 84.9% of the subjects were receiving acetylcholinesterase inhibitor treatment for AD at baseline (Table 1). Treatment was completed by 45.0% and 58.5% of subjects in EU/US and Japan studies, respectively. Overall, the main reasons for study drug discontinuation in the EU/US and Japan studies, respectively, were study termination by the sponsor (23.1% and 22.6%), caregiver request (10.6% and 1.9%), subject request (5.0% and 0%), and AEs (8.1% and 13.2%). About 42% of subjects completed each study, including up to 6 months of post-treatment follow-up; subject disposition is provided in Figs. (1 and 2).
Fig. (1).
Subject disposition in EU/US studies (all enrolled subjects). N, number of subjects in each treatment group who were enrolled in the extension studies. The values in this row were used as the denominators for percentages; PBS, phosphate-buffered saline. a“Other” includes retrieval subjects and subjects who were discontinued from study drug due to early discontinuation of extension studies by sponsor who completed the 6 months of safety follow-up. Two subjects died during the studies. However, 1 subject was discontinued from the studies due to an adverse event, and died later due to a different adverse event. The second subject was discontinued due to an adverse event, with an outcome of death 2 months later. Treatment groups: treatment in the parent studies/treatment in the extension studies.
Fig. (2).
Subject disposition in Japan study (all enrolled subjects). aSix months’ follow-up after the last study drug injection for the subjects impacted by early discontinuation of the study is not collected in the same way as in the 1007/1008 studies. bAdmission to a nursing home. Treatment groups: Treatment in the parent studies/treatment in the extension studies. N, number of subjects in each treatment group who were enrolled in the extension study. The values in this row were used as the denominators for percentages; PBS, phosphate-buffered saline.
3.2. Safety and Tolerability
In the EU/US and Japan studies, respectively, TEAEs were reported in 91.2% (145/159) and 88.7% (47/53) of subjects; 39.6% and 67.9% of subjects had TEAEs that were assessed as treatment-related by EU/US and Japanese investigators, respectively; SAEs were reported in 22.0% and 18.9%, and were assessed as treatment-related in 3.1% (EU/US) and 11.3% (Japan) of subjects. AEs leading to withdrawal from treatment or the study occurred in 8.8% of subjects in the EU/US studies and 15.1% in the Japan study (Table 2). Asymptomatic ARIA-E was detected in 1 subject in the ACC-001 10 μg followed by ACC-001 10 μg + QS-21 group of the US study on the week 76 MRI scan, 169 days from the last injection and after the seventh injection (including 3 of 5 injections in the parent study and 2 injections in the extension study; the other 2 injections in the extension study were substituted with PBS per protocol because anti-Aβ IgG titers were >4000 U/mL). The ARIA-E resolved without treatment by week 104. Mild, asymptomatic hemorrhagic cerebral infarction was reported in 1 subject in the ACC-001 10 μg followed by ACC-001 10 μg + QS-21 group in the Japan study during follow-up on day 730, 182 days after the final injection of ACC-001 + QS-21 (9/9 including parent study injections), but the subject completed the study without treatment of the event. Four deaths occurred (2 during the EU/US studies and 1 after the 6-month follow-up; 1 in the Japan study); none were considered related to treatment by the investigators.
The most commonly reported TEAEs in EU/US and Japan studies are shown in Tables 3 and 4, respectively; most TEAEs were mild or moderate in severity. Overall, injection site reactions were the most common TEAEs, and were mild or moderate in severity. Most had a duration of <1 week and no dose relationship was evident. Urine protein-to-urine creatinine ratio (P/Cr) of ≥0.2 was observed in 15 subjects (9.4%) in the EU/US studies and 18 (34%) in the Japan study with no evidence of a dose relationship, and incidence was higher in subjects who received active treatment in the parent studies; serum creatinine was elevated in only 1 of these Japanese subjects.
Table 3.
Treatment-emergent adverse events in ≥5% of total subjects by preferred term in EU/US studies (safety population).*
| Preferred Term, n (%) |
Active/ACC-001
3 µg + QS-21 (N=21) |
Control/ACC-001 3 µg + QS-21
(N=6) |
Active/ACC-001 10 µg + QS-21
(N=65) |
Control/ACC-001 10 µg + QS-21
(N=14) |
Active/ACC-001
30 µg + QS-21 (N=33) |
Control/ACC-001 30 µg + QS-21
(N=20) |
Total
(N=159) |
|---|---|---|---|---|---|---|---|
| Injection site pain | 4 (19.0) | 0 | 11 (16.9) | 2 (14.3) | 5 (15.2) | 6 (30.0) | 28 (17.6) |
| Fall | 5 (23.8) | 1 (16.7) | 8 (12.3) | 2 (14.3) | 2 (6.1) | 3 (15.0) | 21 (13.2) |
| Injection site erythema | 4 (19.0) | 0 | 5 (7.7) | 1 (7.1) | 5 (15.2) | 4 (20.0) | 19 (11.9) |
| Urinary tract infection | 1 (4.8) | 4 (66.7) | 9 (13.8) | 1 (7.1) | 2 (6.1) | 1 (5.0) | 18 (11.3) |
| Agitation | 3 (14.3) | 4 (66.7) | 5 (7.7) | 2 (14.3) | 3 (9.1) | 1 (5.0) | 18 (11.3) |
| Injection site swelling | 3 (14.3) | 0 | 5 (7.7) | 0 | 4 (12.1) | 1 (5.0) | 13 (8.2) |
| Dizziness | 2 (9.5) | 0 | 5 (7.7) | 2 (14.3) | 2 (6.1) | 2 (10.0) | 13 (8.2) |
| Headache | 1 (4.8) | 0 | 6 (9.2) | 1 (7.1) | 3 (9.1) | 1 (5.0) | 12 (7.5) |
| Diarrhea | 5 (23.8) | 1 (16.7) | 3 (4.6) | 1 (7.1) | 2 (6.1) | 0 | 12 (7.5) |
| Contusion | 4 (19.0) | 1 (16.7) | 2 (3.1) | 1 (7.1) | 2 (6.1) | 1 (5.0) | 11 (6.9) |
| Depression | 3 (14.3) | 0 | 3 (4.6) | 3 (21.4) | 2 (6.1) | 0 | 11 (6.9) |
| Nasopharyngitis | 2 (9.5) | 1 (16.7) | 6 (9.2) | 0 | 1 (3.0) | 0 | 10 (6.3) |
| Syncope | 2 (9.5) | 0 | 3 (4.6) | 1 (7.1) | 2 (6.1) | 1 (5.0) | 9 (5.7) |
| Fatigue | 2 (9.5) | 1 (16.7) | 4 (6.2) | 0 | 1 (3.0) | 0 | 8 (5.0) |
| Anxiety | 2 (9.5) | 0 | 4 (6.2) | 1 (7.1) | 1 (3.0) | 0 | 8 (5.0) |
| Back pain | 0 | 0 | 4 (6.2) | 1 (7.1) | 1 (3.0) | 2 (10.0) | 8 (5.0) |
Table 4.
Treatment-emergent adverse events in ≥2 subjects in Japan study (safety population)*.
| Preferred Term, n (%) |
Active/ACC-001
3 µg + QS-21 (N=7) |
Control/ACC-001
3 µg + QS-21 (N=3) |
Active/ACC-001
10 µg + QS-21 (N=17) |
Control/ACC-001 10 µg + QS-21
(N=7) |
Active/ACC-001
30 µg + QS-21 (N=13) |
Control/ACC-001 30 µg
+ QS-21 (N=6) |
Total
(N=53) |
|---|---|---|---|---|---|---|---|
| Nasopharyngitis | 1 (14.3) | 1 (33.3) | 4 (23.5) | 2 (28.6) | 3 (23.1) | 1 (16.7) | 12 (22.6) |
| Injection site erythema | 2 (28.6) | 1 (33.3) | 2 (11.8) | 2 (28.6) | 2 (15.4) | 2 (33.3) | 11 (20.8) |
| Injection site pain | 1 (14.3) | 2 (66.7) | 1 (5.9) | 2 (28.6) | 2 (15.4) | 1 (16.7) | 9 (17.0) |
| Fall | 0 | 0 | 4 (23.5) | 0 | 0 | 1 (16.7) | 5 (9.4) |
| Back pain | 0 | 0 | 2 (11.8) | 1 (14.3) | 1 (7.7) | 1 (16.7) | 5 (9.4) |
| Injection site swelling | 1 (14.3) | 0 | 1 (5.9) | 1 (14.3) | 0 | 1 (16.7) | 4 (7.5) |
| Pyrexia | 1 (14.3) | 0 | 2 (11.8) | 1 (14.3) | 0 | 0 | 4 (7.5) |
| Tinea pedis | 0 | 0 | 3 (17.6) | 0 | 0 | 0 | 3 (5.7) |
| Hypertension | 0 | 0 | 1 (5.9) | 0 | 2 (15.4) | 0 | 3 (5.7) |
| Diarrhea | 0 | 0 | 1 (5.9) | 2 (28.6) | 0 | 0 | 3 (5.7) |
| Cognitive disorder | 0 | 0 | 1 (5.9) | 1 (14.3) | 0 | 1 (16.7) | 3 (5.7) |
| Cataract | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Conjunctival hemorrhage | 0 | 0 | 0 | 0 | 1 (7.7) | 1 (16.7) | 2 (3.8) |
| Constipation | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Dental caries | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Nausea | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Irritability | 0 | 0 | 1 (5.9) | 0 | 0 | 1 (16.7) | 2 (3.8) |
| Cystitis | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (16.7) | 2 (3.8) |
| Herpes zoster | 0 | 0 | 1 (5.9) | 0 | 0 | 1 (16.7) | 2 (3.8) |
| Blood pressure increased | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Protein urine present | 0 | 0 | 1 (5.9) | 0 | 1 (7.7) | 0 | 2 (3.8) |
| Spinal osteoarthritis | 0 | 0 | 0 | 0 | 2 (15.4) | 0 | 2 (3.8) |
| Lacunar infarction | 1 (14.3) | 0 | 0 | 0 | 1 (7.7) | 0 | 2 (3.8) |
| Anxiety | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Restlessness | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Urinary incontinence | 0 | 0 | 2 (11.8) | 0 | 0 | 0 | 2 (3.8) |
| Eczema | 0 | 0 | 1 (5.9) | 1 (14.3) | 0 | 0 | 2 (3.8) |
Active: Received ACC-001 + QS-21 or ACC-001 alone in the parent studies at the same dose as in the extension studies. Control: Received QS-21 or phosphate-buffered saline in the parent studies.
N, number of subjects in each treatment group who were enrolled in the extension study (the values in this row were used as the denominators for percentages); n, number of subjects in each category.
*The safety population includes all enrolled subjects who received at least 1 dose of study drug.
Classifications of adverse events are based on the Medical Dictionary for Regulatory Activities, v17.0.
3.3. Exploratory
There were no meaningful trends based on dose received in the present studies or treatment received in the parent studies in the MMSE change from baseline over time (Table 5).
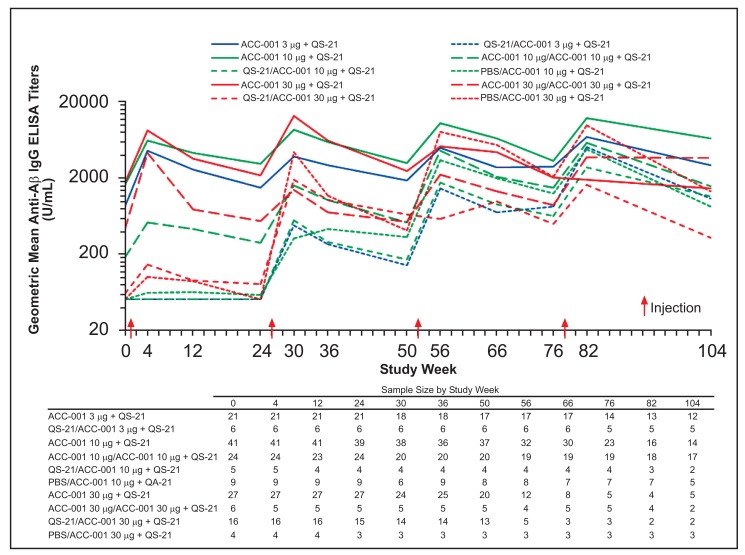
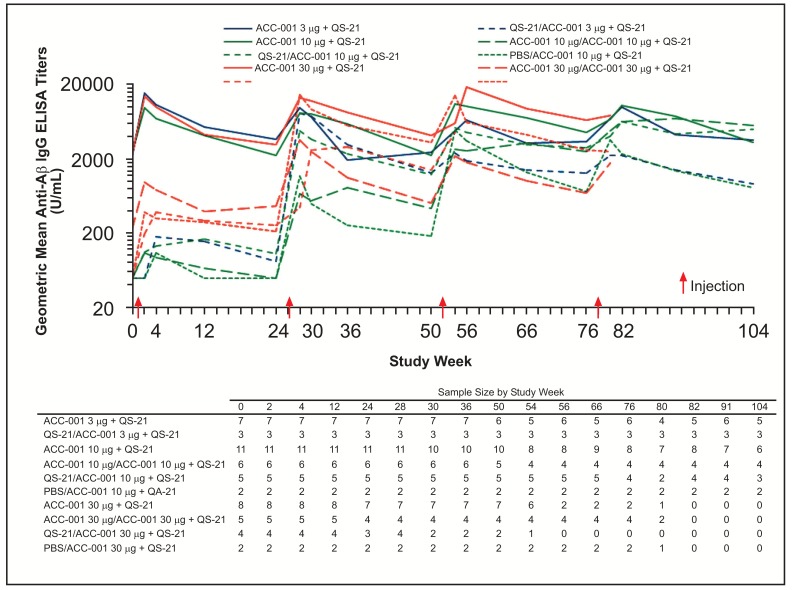
All groups that received placebo during the parent studies had anti-Aβ IgG geometric mean titers (GMTs) below the lower limit of quantitation at extension study baseline with the exception of 1 subject who also had a detectable titer at the parent study baseline, and showed sustained increases following the second injection (Figs. 3 and 4). Higher anti-Aβ IgG titers were observed in subjects who had received QS-21 + ACC-001 during the parent studies regardless of dose compared with those who had received ACC-001 alone or placebo during the parent studies.
Fig. (3).
Geometric mean anti-Aβ IgG titers by study week in EU/US studies. When an assay result was below the lower limit of quantification of 100 U/mL, 50 U/mL was assigned for IgG titers.
Fig. (4).
Geometric mean anti-Aβ IgG titers by study week in Japan study. When an assay result was below the lower limit of quantification of 100 U/mL, 50 U/mL was assigned for IgG titers.
4. DISCUSSION
4.1. Safety and Tolerability
The present studies provide the most long-term clinical information on active immunotherapy for AD to date, showing that ACC-001 + QS-21 adjuvant was well tolerated with an acceptable safety profile. The most common TEAEs were injection site reactions of mild to moderate severity, similar to the ACC-001 parent studies and a phase 2a study with long-term extension of the active immunotherapy CAD106 (Aβ1-6) [9]. CAD106 treatment in the parent study was associated with high rates of local reactions when administered subcutaneously (ecchymosis, erythema, induration, swelling, or pain was reported by ~97% of subjects after at least 1 of the 3 injections), while incidence with intramuscular injection (local reactions reported by ~44%) was similar to that of the ACC-001 studies. Data on injection reactions were not reported for the CAD106 extension study [9]. Intramuscular injection may prove a favorable route of administration for active immunotherapy for AD. The spectrum of TEAEs observed in the active immunotherapy studies with ACC-001 and CAD106 was similar. However, cardiac disorders were reported in 12.8% of CAD106-treated patients in the parent study (but no patients in the placebo arm) and 15.6% in the extension study; these events were less common in the ACC-001 studies, suggesting different effects of the different Aβ peptides tested or random differences in events occurring in the small elderly study populations. Both studies confirm that this active approach to immunotherapy was not associated with the meningoencephalitis observed in subjects treated with AN-1792, even with long-term treatment [9].
The rates of ARIA-E were similar to those in the parent studies [7, 8], during which 2 cases were reported in the EU/US studies and none in the Japan study. The rates of ARIA-E were lower than in studies of passive immunotherapy with bapineuzumab (4.2%-16.7% depending on ApoE ε4 carrier status and bapineuzumab dose) [10-12]. Interestingly, ARIA related to hemorrhage was observed in 2 (4.4%) of the subjects treated with CAD106 and 1 placebo-treated subject, but ARIA-E was not observed. The low incidence of ARIA events across the active immunotherapy studies relative to passive immunotherapy with bapineuzumab suggests that active vaccination is less prone to induce these events.
Overall rates of TEAEs and SAEs were similar between the EU/US and Japan extension studies. These studies were not designed to be compared with each other; however, there were several notable differences between the EU/US and Japan studies related to AEs. The percentage of TEAEs reported as treatment-related by Japanese investigators was higher than their Western counterparts. Discontinuation from treatment because of TEAEs was also higher in the Japan study. The reasons for these differences are unknown, and there was no placebo comparison in the extension studies that would help determine whether there were real differences in patient reactions to ACC-001 + QS-21 in these studies. In the Japan parent studies, at least 1 TEAE was reported for nearly all subjects, and assignment of treatment-relatedness was somewhat higher in the ACC-001 groups (33.3% to 50%) compared with control groups (16.7% for QS-21 and 25% for PBS) in Study 1, but not in Study 2 (44.4% to 83.3% in ACC-001 groups vs 50% in the QS-21 group) [7]. Treatment-relatedness of TEAEs was not reported in the publication of the EU/US parent studies.
Some differences in the rates of specific AEs were also noted between the EU/US studies and the Japan study. Urine P/Cr ≥0.2 occurred in a higher percentage of Japanese subjects in these extension studies. These urine P/Cr elevations were not associated with other renal laboratory abnormalities or investigator-reported AEs, and were not considered clinically important. Hypertension and diabetes are well-established risk factors for kidney disease [13], and if the study populations differed in the prevalence of these comorbidities, this could have contributed to the differences observed in P/Cr elevations. However, the rate of current type 2 diabetes mellitus reported in the medical history of study subjects was very similar between the EU/US studies and the Japan study, and the rate of current hypertension in the medical history was actually higher in the EU/US studies (data not shown). The rate of blood pressure control may be lower in Japan than in the US and some areas of Europe based on population survey data reports [14-17], which could be a contributing factor; however, uncontrolled hypertension was an exclusion criterion for the parent studies [7, 8]. The laboratories used for analysis of samples differed between the EU/US and Japan studies, which may also have played a role. Interestingly, reporting of injection site pain was identical in both study populations, while injection site erythema was reported in more subjects in Japan; it is possible that Japanese subjects are more likely to have this reaction or physicians more sensitive to this TEAE in their population. However, given the small number of subjects, heterogeneity between individual study sites might be higher than the between-study heterogeneity.
4.2. Exploratory
No meaningful differences in cognitive decline as measured by the MMSE were observed between groups treated with ACC-001 in the parent and extension studies and groups that initiated ACC-001 in the long-term extension. MMSE assessment was included in these safety extension studies to monitor for cognitive worsening with treatment, as was observed in the phase 3 trial of semagacestat [18]. No efficacy trends were detected in the extension studies despite longer-term therapy. Similar lack of efficacy was also reported for CAD106 [9]. Numerical decreases in cerebrospinal fluid (CSF) p-tau were observed in the CAD106 extension study regardless of parent study treatment, which the authors attributed to either long-term CAD106 treatment or natural progression of disease. Significant reductions in CSF p-tau were seen in the initial 2 of 4 phase 3 bapineuzumab studies, which, although favorable trends were observed, was not replicated in the second 2 studies, most likely because of early termination and resultant small sample size in the CSF p-tau sub-study. Immunotherapeutic approaches specifically targeting the reduction of neurofibrillary tangles composed of tau in the central nervous system, another hallmark of AD, have shown benefit in animal models [19]. Given the inter-relationship between Aβ and tau pathologies in AD (summarized in Rosenmann [19]), potential benefit from a combined immunotherapeutic approach should be evaluated in future research.
In all groups who received active treatment (ACC-001 + QS-21 or ACC-001 alone) during the parent studies, anti-Aβ IgG GMTs were sustained, with peak levels above those in the parent studies [7, 8]. Anti-Aβ IgG GMTs were lower for groups that did not receive active immunization in the parent studies, although this trend was less evident in the Japan study. These findings suggest that repeated injections of ACC-001 + QS-21 with an interval of 6 months or longer elicited the most robust and sustained immune responses. Unfortunately, methodologic differences in the reporting of antibody titers between the ACC-001 studies and the CAD106 study preclude comparison of the immunogenicity of these 2 vaccines, and adequate antibody titers for benefit have not been established.
4.3. Study limitations
The most important study limitation was the early termination of dosing by the sponsor. Approximately half of the subjects completed the extension studies overall, which could have affected both the safety and exploratory immunogenicity and efficacy assessments. Nonetheless, the findings suggest that continued vaccination with ACC-001 + QS-21 provides a more robust anti-Aβ immune response with a safety profile consistent with shorter-term treatment.
CONCLUSION
ACC-001 was well tolerated in these long-term extension studies and no new safety signals were observed. ARIA-E rates were consistent with prior experience with ACC-001 + QS-21 and asymptomatic. Anti-Aβ antibody responses to ACC-001 + QS-21 were most robust in the groups who continued ACC-001 + QS-21 from the parent studies. The low safety risks and ease of administration suggest that active immunization against Aβ may be a feasible option in patients with amyloid deposits and a high risk for dementia. Larger trials of adequate duration, with optimized dosing, may be needed to demonstrate efficacy with active (therapeutic) immunization.
Table 1.
Subject demographics and baseline characteristics (all enrolled subjects).
A. EU/US studies
| ACC-001 3 µg + QS-21/ACC-001 3 µg + QS-21 | QS-21/ACC-001 3 µg + QS-21 | ACC-001 10 µg + QS-21/ACC-001 10 µg + QS-21 | ACC-001 10 µg/ACC-001 10 µg + QS-21 | QS-21/ACC-001 10 µg + QS-21 | PBS/ACC-001 10 µg + QS-21 | ACC-001 30 µg + QS-21/ACC-001 30 µg + QS-21 | ACC-001 30 µg/ACC-001 30 µg + QS-21 | QS-21/ACC-001 30 µg + QS-21 | PBS/ACC-001 30 µg + QS-21 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 21 | 6 | 41 | 25 | 5 | 9 | 27 | 6 | 16 | 4 | 160 |
| Age, y | |||||||||||
| Mean | 68.7 | 67.7 | 69.7 | 72.4 | 75.2 | 69.6 | 71.3 | 66.3 | 69.5 | 75.8 | 70.4 |
| SD | 6.94 | 9.52 | 9.29 | 9.12 | 8.23 | 7.94 | 9.20 | 9.42 | 6.95 | 7.89 | 8.61 |
| Sex, n (%) | |||||||||||
| Female | 11 (52.4) | 4 (66.7) | 21 (51.2) | 12 (48.0) | 3 (60.0) |
8 (88.9) | 16 (59.3) | 3 (50.0) | 14 (87.5) | 2 (50.0) | 94 (58.8) |
| Male | 10 (47.6) | 2 (33.3) | 20 (48.8) | 13 (52.0) | 2 (40.0) | 1 (11.1) | 11 (40.7) | 3 (50.0) | 2 (12.5) | 2 (50.0) | 66 (41.3) |
| Country, n (%) | |||||||||||
| France | 1 (4.8) | 0 | 2 (4.9) | 3 (12.0) | 1 (20.0) | 1 (11.1) | 2 (7.4) | 1 (16.7) | 3 (18.8) | 1 (25.0) | 15 (9.4) |
| Germany | 6 (28.6) | 1 (16.7) | 8 (19.5) | 5 (20.0) | 0 | 0 | 6 (22.2) | 0 | 0 | 0 | 26 (16.3) |
| Spain | 0 | 0 | 3 (7.3) | 1 (4.0) | 0 | 1 (11.1) | 2 (7.4) | 0 | 2 (12.5) | 0 | 9 (5.6) |
| United States | 14 (66.7) | 5 (83.3) | 28 (68.3) | 16 (64.0) | 4 (80.0) | 7 (77.8) | 17 (63.0) | 5 (83.3) | 11 (68.8) | 3 (75.0) | 110 (68.8) |
| Race, n (%) | |||||||||||
| White | 20 (95.2) | 6 (100.0) | 40 (97.7) | 25 (100.0) | 4 (80.0) | 9 (100.0) | 26 (96.3) | 6 (100.0) | 16 (100.0) | 4 (100.0) | 156 (97.5) |
| Black/African American | 0 | 0 | 1 (2.4) | 1 | 1 (20.0) | 0 | 1 (3.7) | 0 | 0 | 0 | 3 (1.9) |
| Asian | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 1 (4.8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.6) |
| AD duration, ya | |||||||||||
| Mean | 6.62 | 6.12 | 4.78 | 5.10 | 3.85 | 4.36 | 5.77 | 4.07 | 4.35 | 7.98 | 5.25 |
| Baseline MMSE scoreb | |||||||||||
| Mean | 16.7 | 18.3 | 18.4 | 19.6 | 21.8 | 20.0 | 18.7 | 20.5 | 19.1 | 20.0 | 18.8 |
| SD | 4.64 | 5.61 | 5.92 | 5.45 | 4.21 | 6.14 | 4.98 | 4.76 | 4.84 | 4.69 | 5.29 |
| MMSE stratum, n (%)b | |||||||||||
| <10c | 1 (4.8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.6) |
| 10-15 | 10 (47.6) | 2 (33.3) | 15 (36.6) | 5 (20.0) | 0 | 2 (22.2) | 7 (25.9) | 1 (16.7) | 3 (18.8) | 1 (25.0) | 46 (28.8) |
| 16-20 | 5 (23.8) | 2 (33.3) | 12 (29.3) | 10 (40.0) | 2 (40.0) | 3 (33.3) | 11 (40.7) | 2 (33.3) | 6 (37.5) | 0 | 53 (33.1) |
| 21-26 | 5 (23.8) | 2 (33.3) | 9 (22.0) | 7 (28.0) | 2 (40.0) | 3 (33.3) | 8 (29.6) | 3 (50.0) | 6 (37.5) | 3 (75.0) | 48 (30.0) |
| >26 | 0 | 0 | 5 (12.2) | 3 (12.0) | 1 (20.0) | 1 (11.1) | 1 (3.7) | 0 | 1 (6.3) | 0 | 12 (7.5) |
| Drug treatment for AD, n (%)d | |||||||||||
| Yes | 21 (100.0) | 6 (100.0) | 38 (92.7) | 23 (92.0) | 4 (80.0) | 9 (100.0) | 25 (92.6) | 5 (83.3) | 15 (93.8) | 4 (100.0) | 150 (93.8) |
| No | 0 | 0 | 3 (7.3) | 2 (8.0) | 1 (20.0) | 0 | 2 (7.4) | 1 (16.7) | 1 (6.3) | 0 | 10 (6.3) |
AD, Alzheimer’s disease; MMSE, mini-mental state exam; N, number of subjects in each treatment group who were enrolled in the extension study, the values in this row were used as the denominators for percentages; n, number of subjects in each category; PBS, phosphate buffered saline; SD, standard deviation.
Treatment groups: treatment in the parent studies/treatment in the extension studies.
aStart date for calculating AD duration is obtained at Screening from the parent studies.
bBaseline is the Screening for the extension/week 78 from the parent studies.
cThis subject's MMSE score was artificially low due to language predominant features and the subject actually presented with moderate AD. The subject was therefore approved for enrollment in the study by the Sponsor. Subsequent to this decision, the subject continued to decline and was discontinued from the study.
dAcetylcholinesterase inhibitor and/or memantine.
B. Japan study (all enrolled subjects)
| ACC-001 3 µg + QS-21/ACC-001 3 µg + QS-21 | QS-21/ACC-001 3 µg + QS-21 | ACC-001 10 µg + QS-21/ACC-001 10 µg + QS-21 | ACC-001 10 µg/ACC-001 10 µg + QS-21 | QS-21/ACC-001 10 µg + QS-21 | PBS/ACC-001 10 µg + QS-21 | ACC-001 30 µg + QS-21/ACC-001 30 µg + QS-21 | ACC-001 30 µg/ACC-001 30 µg + QS-21 | QS-21/ACC-001 30 µg + QS-21 | PBS/ACC-001 30 µg + QS-21 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 7 | 3 | 11 | 6 | 5 | 2 | 8 | 5 | 4 | 2 | 53 | ||||||||
| Age, y | |||||||||||||||||||
| Mean | 69.0 | 73.3 | 67.5 | 69.5 | 65.2 | 70.5 | 73.9 | 71.2 | 64.0 | 68.5 | 69.2 | ||||||||
| SD | 9.8 | 9.2 | 9.8 | 6.9 | 6.7 | 7.8 | 5.4 | 2.0 | 7.1 | 2.1 | 7.6 | ||||||||
| Sex, n (%) | |||||||||||||||||||
| Male | 3 (42.9) | 0 | 3 (27.3) | 2 (33.3) | 2 (40.0) | 0 | 4 (50.0) | 2 (40.0) | 0 | 1 (50.0) | 17 (32.1) | ||||||||
| Female | 4 (57.1) | 3 (100.0) | 8 (72.7) | 4 (66.7) | 3 (60.0) | 2 (100.0) | 4 (50.0) | 3 (60.0) | 4 (100.0) | 1 (50.0) | 36 (67.9) | ||||||||
| Race, n (%) | |||||||||||||||||||
| Asian | 7 (100.0) | 3 (100.0) | 11 (100.0) | 6 (100.0) | 5 (100.0) | 2 (100.0) | 8 (100.0) | 5 (100.0) | 4 (100.0) | 2 (100.0) | 53 (100.0) | ||||||||
| AD duration, ya | |||||||||||||||||||
| Mean | 4.1 | 5.1 | 5.6 | 8.2 | 4.5 | 5.8 | 4.9 | 5.6 | 6.4 | 6.7 | 5.6 | ||||||||
| Baseline MMSE scoreb | |||||||||||||||||||
| Mean | 23.1 | 21.0 | 20.7 | 19.3 | 19.6 | 22.0 | 21.4 | 22.4 | 20.5 | 21.5 | 21.1 | ||||||||
| SD | 3.2 | 6.6 | 4.4 | 6.1 | 7.6 | 1.4 | 2.4 | 2.1 | 2.6 | 2.1 | 4.2 | ||||||||
| MMSE stratum, n (%)b | |||||||||||||||||||
| <16 | 0 | 1 (33.3) | 2 (18.2) | 1 (16.7) | 2 (40.0) | 0 | 0 | 0 | 0 | 0 | 6 (11.3) | ||||||||
| 16-20 | 1 (14.3) | 0 | 3 (27.3) | 2 (33.3) | 0 | 0 | 2 (25.0) | 1 (20.0) | 2 (50.0) | 1 (50.0) | 12 (22.6) | ||||||||
| 21-26 | 4 (57.1) | 1 (33.3) | 4 (36.4) | 2 (33.3) | 2 (40.0) | 2 (100.0) | 6 (75.0) | 4 (80.0) | 2 (50.0) | 1 (50.0) | 28 (52.8) | ||||||||
| >26 | 2 (28.6) | 1 (33.3) | 2 (18.2) | 1 (16.7) | 1 (20.0) | 0 | 0 | 0 | 0 | 0 | 7 (13.2) | ||||||||
|
Active/ACC-001 3 µg N=7 |
Control/ACC-001 3 µg N=3 |
Active/ACC-001 10 µg N=17 |
Control/ACC-001 10 µg N=7 |
Active/ACC-001 30 µg N=13 |
Control/ACC-001 30 µg N=6 |
Total N=53 |
|||||||||||||
| Acetylcholinesterase treatment for AD | |||||||||||||||||||
| n (%) | 6 (85.7) | 3 (100.0) | 16 (94.1) | 7 (100.0) | 8 (61.5) | 5 (83.3) | 45 (84.9) | ||||||||||||
AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination; N, number of subjects in each treatment group who were enrolled in the extension study, the values in this row were used as the denominators for percentages; n, number of subjects in each category; PBS, phosphate-buffered saline; SD, standard deviation.
Treatment groups: treatment in the parent studies/treatment in the extension studies. Active: received ACC-001 + QS-21 or ACC-001 alone in the parent studies at the same dose as in the extension studies. Control: received QS-21 or phosphate-buffered saline in the parent studies.
aStart date for calculating AD duration is obtained at Screening from the extension studies.
bBaseline is the Screening for the extension/week 78 from the parent studies (week 104 of cohort 1 in Japanese study 1).
Table 2.
Overview of adverse events (safety population)*
| n (%) |
Active/ACC-001
3 µg + QS-21 (N=21) |
Control/ACC-001
3 µg + QS-21 (N=6) |
Active/ACC-001
10 µg + QS-21 (N=65) |
Control/ACC-001 10 µg + QS-21
(N=14) |
Active/ACC-001
30 µg + QS-21 (N=33) |
Control/ACC-001 30 µg
+ QS-21 (N=20) |
Total
(N=159) |
|---|---|---|---|---|---|---|---|
| AEs | 21 (100.0) | 6 (100.0) | 64 (98.5) | 14 (100.0) | 29 (87.9) | 19 (95.0) | 153 (96.2) |
| TEAEs | 21 (100.0) | 6 (100.0) | 61 (93.8) | 13 (92.9) | 26 (78.8) | 18 (90.0) | 145 (91.2) |
| Life-threatening TEAEs | 2 (9.5) | 0 | 2 (3.1) | 0 | 0 | 0 | 4 (2.5) |
| AEs of special circumstance |
0 | 0 | 1 (1.5) | 0 | 0 | 0 | 1 (0.6) |
| Injection site reactions > 0 |
9 (42.9) | 0 | 21 (32.3) | 4 (28.6) | 10 (30.3) | 8 (40.0) | 52 (32.7) |
| SAEs | 6 (28.6) | 1 (16.7) | 18 (27.7) | 2 (14.3) | 4 (12.1) | 4 (20.0) | 35 (22.0) |
| AEs causing discontinuation of study drug or withdrawal from study | 4 (19.0) | 0 | 5 (7.7) | 1 (7.1) | 2 (6.1) | 2 (10.0) | 14 (8.8) |
| AEs causing death | 2 (9.5) | 0 | 0 | 0 | 0 | 0 | 2 (1.3) |
B. Japan study
| n (%) |
ACC-001 3 µg + QS-21/ACC-001
3 µg + QS-21 (N=7) |
QS-21/ACC-001
3 µg + QS-21 (N=3) |
Active/ACC-001
10 µg + QS-21 (N=17) |
Control/ACC-001 10 µg + QS-21
(N=7) |
Active/ACC-001
30 µg + QS-21 (N=13) |
Control/ACC-001 30 µg
+ QS-21 (N=6) |
Total
(N=53) |
|---|---|---|---|---|---|---|---|
| Subjects with AEs | |||||||
| All causality | 7 (100.0) | 3 (100.0) | 16 (94.1) | 5 (71.4) | 10 (76.9) | 6 (100.0) | 47 (88.7) |
| AEs of special circumstance | 0 | 0 | 1 (5.9) | 0 | 0 | 0 | 1 (1.9) |
| Injection site reaction >0 | 3 (42.9) | 2 (66.7) | 3 (17.6) | 3 (42.9) | 3 (23.1) | 3 (50.0) | 17 (32.1) |
| Subjects with severe AEs | |||||||
| All causality | 0 | 0 | 3 (17.6) | 0 | 1 (7.7) | 0 | 4 (7.5) |
| Subjects with SAEs | |||||||
| All causality | 0 | 0 | 7 (41.2) | 1 (14.3) | 2 (15.4) | 0 | 10 (18.9) |
| Subjects discontinued the study drug due to AEs | |||||||
| All causality | 1 (14.3) | 1 (33.3) | 4 (23.5) | 1 (14.3) | 2 (15.4) | 0 | 9 (17.0) |
Treatment groups: treatment in the parent studies/treatment in the extension studies. Active: received ACC-001 + QS-21 or ACC-001 alone in the parent studies at the same dose as in the extension studies. Control: received QS-21 or phosphate-buffered saline in the parent studies.
*The safety population includes all enrolled subjects who received at least 1 dose of study drug.
AE, adverse event; N, number of subjects in each treatment group who were enrolled in the extension study, the values in this row were used as the denominators for percentages; n, number of subjects in each category; PBS, phosphate-buffered saline; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Table 5.
Descriptive summary statistics for Mini-Mental State Examination over time (safety population).
A. EU/US studies
| Study Week |
Active/ACC-001
3 µg (N=21) |
Control/ACC-001
3 µg (N=6) |
Active/ACC-001
10 µg (N=65) |
Control/ACC-001
10 µg (N=14) |
Active/ACC-001
30 µg (N=33) |
Control/ACC-001 30 µg
(N=20) |
|---|---|---|---|---|---|---|
| Screening: n Mean (SD) |
21 16.7 (4.6) |
6 18.3 (5.6) |
65 18.9 (5.8) |
14 20.6 (5.4) |
33 19.0 (4.9) |
20 19.3 (4.7) |
| Week 12: n Mean (SD) |
21 16.2 (5.5) |
6 19.3 (6.1) |
65 18.4 (6.1) |
13 18.1 (6.6) |
32 18.4 (5.2) |
20 19.0 (4.1) |
| Week 26: n Mean (SD) |
21 14.2 (5.5) |
6 16.2 (6.2) |
62 17.0 (7.2) |
12 18.7 (7.6) |
31 17.3 (4.7) |
18 17.6 (4.1) |
| Week 36: n Mean (SD) |
18 15.6 (3.8) |
6 16.5 (7.0) |
55 17.5 (6.4) |
10 19.5 (8.5) |
31 16.7 (5.2) |
17 18.5 (4.8) |
| Week 52: n Mean (SD) |
19 14.1 (5.7) |
6 16.0 (7.0) |
53 15.9 (7.6) |
11 15.4 (7.4) |
24 16.4 (6.6) |
16 17.6 (5.3) |
| Week 66: n Mean (SD) |
17 13.5 (5.4) |
6 13.8 (6.7) |
48 16.2 (7.8) |
11 18.2 (7.3) |
14 17.6 (7.1) |
5 14.2 (4.3) |
| Week 78: n Mean (SD) |
14 13.1 (5.9) |
5 13.4 (5.5) |
41 16.1 (8.1) |
11 17.3 (6.9) |
10 18.2 (6.1) |
6 15.0 (4.5) |
| Week 91: n Mean (SD) |
15 12.6 (6.3) |
5 15.0 (3.6) |
33 14.0 (8.3) |
9 18.6 (6.1) |
7 18.9 (6.7) |
5 13.4 (4.7) |
| Week 104: n Mean (SD) |
11 12.0 (6.9) |
5 12.4 (4.3) |
30 15.5 (8.4) |
9 17.4 (7.2) |
6 16.8 (6.3) |
5 12.0 (6.7) |
B. Japan study
| Study Week |
Active/ACC-001
3 µg (N=7) |
Control/ACC-001
3 µg (N=3) |
Active/ACC-001
10 µg (N=17) |
Control/ACC-001
10 µg (N=7) |
Active/ACC-001
30 µg (N=13) |
Control/ACC-001 30 µg
(N=6) |
|---|---|---|---|---|---|---|
| Screening: n Mean (SD) |
7 23.1 (3.2) |
3 21.0 (6.6) |
17 20.2 (4.9) |
7 20.3 (6.4) |
13 21.8 (2.2) |
6 20.8 (2.3) |
| Week 12: n Mean (SD) |
7 23.1 (4.5) |
3 21.7 (5.9) |
17 19.6 (4.3) |
7 20.1 (7.3) |
13 22.0 (3.9) |
6 20.0 (3.1) |
| Week 26: n Mean (SD) |
7 21.9 (4.8) |
3 20.0 (5.0) |
17 19.5 (4.6) |
7 19.6 (7.3) |
11 22.2 (3.5) |
6 18.7 (3.9) |
| Week 36: n Mean (SD) |
7 23.0 (4.4) |
3 21.3 (7.2) |
13 19.1 (5.7) |
7 18.6 (7.7) |
11 21.9 (3.2) |
4 18.3 (2.4) |
| Week 52: n Mean (SD) |
6 22.8 (3.7) |
3 22.0 (4.6) |
13 17.9 (7.6) |
7 17.0 (8.8) |
11 20.7 (3.2) |
3 18.0 (2.6) |
| Week 66: n Mean (SD) |
5 22.6 (5.5) |
3 21.7 (4.9) |
12 17.8 (6.7) |
7 18.3 (8.9) |
6 21.7 (2.0) |
2 15.5 (3.5) |
| Week 78: n Mean (SD) |
6 19.5 (5.4) |
3 20.0 (4.6) |
12 17.5 (6.3) |
6 19.7 (6.5) |
6 21.2 (2.9) |
2 15.0 (7.1) |
| Week 91: n Mean (SD) |
5 21.2 (5.3) |
3 21.7 (4.2) |
11 16.6 (6.7) |
6 20.0 (6.7) |
NR | NR |
| Week 104: n Mean (SD) |
5 19.4 (6.2) |
3 20.3 (5.0) |
9 15.4 (7.0) |
5 19.0 (8.5) |
NR | NR |
N, number of subjects in each treatment group who were enrolled in the extension study; n, number of subjects in each category; SD, standard deviation; NR, not reported.
Active/ACC-001: Received ACC-001 + QS-21 or ACC-001 alone at the designated dose in the parent study and ACC-001 + QS-21 at the designated dose in the extension study.
Control/ACC-001: Received QS-21 or phosphate-buffered saline in the parent study and ACC-001 + QS-21 at the designated dose in the extension study.
ACKNOWLEDGEMENTS
M. Hüll was PI (European study branch); he collected and interpreted data, drafted and revised the manuscript, and read and approved the final manuscript.
C. Sadowsky performed research/study, collected and analyzed data, and drafted and revised the manuscript.
H. Arai designed and performed the study, and drafted and revised the manuscript.
G. Le Prince Leterme designed the protocol, interpreted data, generated final study reports, drafted and revised the manuscript, and approved the final manuscript.
A. Holstein designed the protocol, interpreted data, generated final study reports, drafted and revised the manuscript, and approved the final manuscript.
K. Booth participated in study coordination and conduct, served as medical lead for the 1007/8 studies, interpreted data, drafted and revised the manuscript, and read and approved the final manuscript.
Y. Peng supported data collection, analysis, and interpretation, and drafted and revised the manuscript.
T. Yoshiyama designed and performed the study, analyzed data, and drafted and revised the manuscript.
H. Suzuki designed and performed the study, collected data, and drafted and revised the manuscript.
N. Ketter reviewed the data outputs, drafted and revised sections of the manuscript, and reviewed the manuscript.
E. Liu contributed to the study design, participated in data review and interpretation, drafted and revised the manuscript, and read and approved the final manuscript.
J.M. Ryan contributed to the study design and conduct, interpreted data, and revised and approved the final manuscript.
These studies were sponsored by Pfizer Inc and Janssen Alzheimer Immunotherapy, R&D, LLC. Editorial writing support was provided by Marsha Scott, PhD, of Phase Five Communications, and was funded by Pfizer Inc. Hideo Suzuki and J. Michael Ryan were employees of Pfizer Inc at the time the study was completed. The authors would like to extend their appreciation to Ronald Black, MD, for his extensive expertise in Alzheimer's disease and seminal scientific contribution to the strategy and development of the ACC Program, and to Nick Jackson, PhD, for his deep expertise in vaccines and his invaluable contributions to the strategy and development of the ACC Program. The authors would like to thank the following principal investigators: France: Prof. Anne-Sophie Rigaud; Dr. Bruno Dubois; Dr. Pierre-Jean Ousset; Prof. Bruno Vellas; Dr. Sophie Auriacombe; Prof. Jean-François Dartigues; Dr. Bernard-François Michel; Dr. Jacques Touchon; Prof. Florence Pasquier; Germany: Dr. Oliver Peters; Prof. Dr. Lutz Frölich; Prof. Dr. Alexander Friedrich Kurz; Dr. Michael Heneka; Dr. Anja Schneider; Japan: Dr. Nobuto Shibata; Dr. Yosuke Ichimiya; Dr. Megumi Takahashi; Dr. Satoshi Orimo; Dr. Norifumi Tsuno; Dr. Hiroshi Yoneda; Dr. Eiichi Oguni; Dr. Hayato Tabu; Dr. Kazuma Kaneko; Dr. Shinichi Miyao; Dr. Shizuo Hatashita; Spain: Dr. Pedro Gil Gregorio; Dr. José M. Ribera-Casado; Dr. José Luis Molinuevo Guix; Dr. Jordi Peña-Casanova; Dr. Rafael Blesa Gonzalez; United States: Dr. Raymond Scott Turner; Dr. Joel Steven Ross; Dr. Paul Robert Solomon; Dr. Scott M. McGinnis; Dr. Stephen P. Salloway; Dr. Christopher H. Van Dyck; Dr. Karen Lynette Bell; Dr. Marwan Noel Sabbagh; Dr. John Carl Morris; Dr. Pierre N. Tariot; Dr. Kerri Louise Wilks; Dr. Adam Louis Boxer. The authors would also like to extend their thanks to all of the subjects and caregivers for their contributions throughout the course of these studies.
CONFLICT OF INTEREST
M. Hüll has received payment for participation in advisory boards from Roche and Lilly. He has received payment for preclinical studies and lectures from Schwabe. He has been an investigator in clinical trials for AbbVie and Pfizer.
C. Sadowsky has received payment for participation in advisory boards and speaker bureaus for Novartis, Lilly, Forest, and Accera. He has received payment for participation in an advisory board for Cognoptix, has received payment for participation in a speaker bureau for Pamlab, and has received payment for participation in clinical trials for Abbott, Avanir, Avid, En Vivo, GE, Pfizer, Neuronetrix, and Neuronix.
H. Arai was a coordinating investigator for the Japan study.
G. Le Prince Leterme is an employee of Pfizer Inc and was during the conduct of the studies.
A. Holstein is an employee of Pfizer Inc and was during the conduct of the studies.
K. Booth is an employee of Pfizer Inc, has stock options, and owns company stock.
Y. Peng is an employee of Pfizer Inc and was during the conduct of the studies.
T. Yoshiyama is an employee of Pfizer Japan Inc.
H. Suzuki was an employee of Pfizer Japan Inc. during the conduct of the studies.
N. Ketter is an employee of Janssen Research and Development and was during the conduct of the studies.
E. Liu was an employee of Janssen Research and Development during the conduct of the studies and while the manuscript was developed.
J.M. Ryan was an employee of Pfizer Inc during the conduct of the studies and currently owns company stock.
REFERENCES
- 1.Panza F., Solfrizzi V., Imbimbo B.P., Tortelli R., Santamato A., Logroscino G. Amyloid-based immunotherapy for Alzheimer’s disease in the time of prevention trials: The way forward. Expert Rev. Clin. Immunol. 2014;10:405–419. doi: 10.1586/1744666X.2014.883921. [DOI] [PubMed] [Google Scholar]
- 2.Yang K.C., Chen H.H. Probabilistic cost-effectiveness analysis of vaccination for mild or moderate Alzheimer’s disease. Curr. Alzheimer Res. 2016;13:809–816. doi: 10.2174/1567205013666160129095012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agadjanyan M.G., Petrovsky N., Ghochikyan A. A fresh perspective from immunologists and vaccine researchers: Active vaccination strategies to prevent and reverse Alzheimer’s disease. Alzheimers Dement. 2015;11:1246–1259. doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orgogozo J.M., Gilman S., Dartigues J.F., et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 5.Gilman S., Koller M., Black R.S., et al. AN1792(QS-21)-201 Study Team. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 6.Pride M., Seubert P., Grundman M., Hagen M., Eldridge J., Black R.S. Progress in the active immunotherapeutic approach to Alzheimer’s disease: Clinical investigations into AN1792-associated meningoencephalitis. Neurodegener. Dis. 2008;5:194–196. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- 7.Arai H., Suzuki H., Yoshiyama T. Vanutide cridificar and the QS-21 adjuvant in Japanese subjects with mild to moderate Alzheimer’s disease: results from two phase 2 studies. Curr. Alzheimer Res. 2015;12:242–254. doi: 10.2174/1567205012666150302154121. [DOI] [PubMed] [Google Scholar]
- 8.Pasquier F., Sadowsky C., Holstein A., et al. Two phase 2 multiple ascending-dose studies of vanutide cridificar (ACC-001) and QS-21 adjuvant in mild-to-moderate Alzheimer’s disease. J. Alzheimers Dis. 2016;51:1131–1143. doi: 10.3233/JAD-150376. [DOI] [PubMed] [Google Scholar]
- 9.Farlow M.R., Andreasen N., Riviere M.E., et al. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res. Ther. 2015;7:1–13. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salloway S., Sperling R., Gilman S., et al. Bapineuzumab 201 clinical trial investigators. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salloway S., Sperling R., Fox N.C., et al. Bapineuzumab 301 and 302 Clinical Trial Investigators Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenberghe R., Rinne J.O., Boada M., et al. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res. Ther. 2016;8:1–13. doi: 10.1186/s13195-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saran R., Li Y., Robinson B., Agodoa L.Y., Ayanian J., Bragg-Gresham J., et al. US renal data system 2015 annual data report: Epidemiology of kidney disease in the united states. Am. J. Kidney Dis. 2016;67(1):S1–S434. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda N., Sapienza D., Guerrero R., et al. Control of hypertension with medication: a comparative analysis of national surveys in 20 countries. Bull. World Health Organ. 2014;92:10–19C. doi: 10.2471/BLT.13.121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura K., Nagai M., Ohkubo T. Epidemiology of hypertension in Japan: Where are we now? Circ. J. 2013;77:2226–2231. doi: 10.1253/circj.cj-13-0847. [DOI] [PubMed] [Google Scholar]
- 16.Joffres M., Falaschetti E., Gillespie C., et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open. 2013;3:e003423. doi: 10.1136/bmjopen-2013-003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf-Maier K., Cooper R.S., Banegas J.R., et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 18.Doody R.S., Raman R., Farlow M., et al. A phase 3 trial of semagacestat for the treatment of Alzheimer’s disease. N. Engl. J. Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 19.Rosenmann H. Immunotherapy for targeting tau pathology in Alzheimer’s disease and tauopathies. Curr. Alzheimer Res. 2013;10:217–228. doi: 10.2174/1567205011310030001. [DOI] [PubMed] [Google Scholar]