Abstract
In the present study, nineteen new fluoro-benzimidazole derivatives, including nifuroxazide analogs, were synthesized by microwave-supported reactions and tested against a panel of pathogenic microorganisms consisting of resistant strains. The synthesized compounds were characterized and identified by FT-IR, 1H- and 13C-NMR, mass spectroscopy, and elemental analyses, respectively. In vitro antimicrobial and cytotoxic effects of the synthesized compounds were determined by microdilution and by [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) assay. The compound 4-[5(6)-fluoro-1H-benzimidazol-2-yl)-N'-(2-methylbenzylidene)]benzohydrazide (18) showed particularly high inhibitory activity against the gastro-intestinal pathogens, such as Escherichia coli O157:H7, Escherichiacoli ATCC 8739, Escherichia coli ATCC 35218 and Salmonella typhimurium ATCC 13311 standard strains, with minimum inhibitory concentrations (MIC90) ranging from 0.49–0.98 µg/mL. The microbial panel contained a total of ten pathogens including Klebsiella sp., Mycobacterium sp., MRSA, etc., for which the level of inhibitory activity measured was higher than that exhibited by the tested concentrations (MIC > 1000 µg/mL). In vitro cytotoxicity results revealed that the inhibitory concentration (IC50) value (210.23 µg/mL) of compound 18 against CCD 841 CoN cells (human intestinal epithelial cell line) is about 430 times higher than its MIC90 value against the tested Escherichia coli strains. Furthermore, the docking study of compound 18 suggested that its structure is very compatible with the active site pocket of the phosphofructokinase-2 enzyme.
Keywords: Benzimidazole, hydrazone, nifuroxazide, Escherichia coli, antimicrobial, mass spectroscopy
1. INTRODUCTION
Although there are currently several drugs to be used against microbial infections, much research is being conducted in this area on account of the unsatisfactory options available for treatment of illnesses caused by microorganisms, side effects, and toxic effects [1]. Besides, increased use and misuse of antimicrobial drugs have resulted in the development of resistant pathogens, and overcoming drug resistance has become an important issue in medicinal chemistry [2]. The discovery of novel and potent antibacterial and antifungal agents is an important strategy to solve the problem of microbial drug resistance [3]. However, the development of new drugs with completely new chemical structures is a very lengthy and costly process, and demands the effort of multi-disciplinary teams.
The optimization of available drugs is a financially accessible alternative that can provide promising antimicrobial agents [4–6]. Accordingly, in studies on novel drug development, research mostly concentrates on the chemical modification of the effective drugs or selected molecular structures to improve their bioavailability and potency, and consequently overcome antibiotic resistance mechanisms [7,8].
Hydrazones, which possess an azometine (-NHN=CH-) moiety have attracted a great deal of interest due to their increased importance in medicinal chemistry. Hydrazone derivatives isoniazid, nifuroxazide, furacilin, furazolidone, and ftivazide are the main drug examples, reported to display significant antimicrobial activity [9-11]. Many researchers have synthesized new target structures consisting of combinations of hydrazone and an active moiety in order to evaluate their biological activities [12–21]. Similarly, benzimidazole compounds have always been important pharmacophores in studies on antimicrobial agent development. The reason for this special interest can be explained by the structural similarity between purine and benzimidazole. It has been shown that during bacterial nucleic acid and protein synthesis, benzimidazoles compete with purines and inhibit the synthesis process [22,23]. Furthermore, the benzimidazole scaffold has an ability to form hydrogen bonds with biological enzymes and receptors and participates in π-π and hydrophobic interactions, which may be related to its mechanism of action [24,25]. Because of these features, several pharmacological and biochemical research studies have been performed, and many of them have supported the finding that benzimidazole derivatives are potent against various microorganism strains [26-39]. In the light of all these knowledge we previously synthesized antimicrobial potent benzimidazole derivatives in our laboratory [40-45]. Besides the antimicrobial activity, benzimidazoles have some other therapeutic affect on platelets and CB2 receptor [46,47].
Nifuroxazide, a clinically approved hydrazone-containing intestinal antiseptic and antibacterial drug, possesses a chemical structure that favours chemical modification by means of rational molecular design [48,49]. Hence, this drug can be thought of as an ideal lead molecule for the design of new analogues [4]. By evaluating the antimicrobial potency of benzimidazole and chemically modified nifuroxazide acting together, it may be determined whether the combination of benzimidazole and the hydrazone moiety exhibits increased antimicrobial activity.
2. EXPERIMENTAL
2.1. Chemistry
The chemicals used in the synthesis were purchased from Sigma-Aldrich Chemicals (Sigma-Aldrich Corp., St. Louis, MO, USA) or Merck Chemicals (Merck KGaA, Darmstadt, Germany). Melting points (M.p.) of the synthesized compounds were determined by MP90 digital melting point apparatus (Mettler Toledo, Ohio, USA) and were uncorrected. 1H NMR and 13C NMR spectra were recorded by a Bruker 500 MHz and 125 MHz digital FT-NMR spectrometer (Bruker Bioscience, Billerica, MA, USA) in DMSO-d6, respectively. The elemental compositions were recorded on a Leco CHNS-932 analyser (Leco, Michigan, USA). The IR spectra were obtained on a Shimadzu, IR Prestige-21 (Shimadzu, Tokyo, Japan). LC-MS-MS studies were performed on a Schimadzu, 8040 LC-MS-MS spectrophotometer (Shimadzu, Tokyo, Japan). The purities of compounds were checked by TLC on silica gel 60 F254 (Merck KGaA, Darmstadt, Germany).
2.1.1. Synthesis of methyl 4-(5(6)-fluoro-1H-benzimidazol-2-yl)benzoate (3)
Methyl-4-formylbenzoate (2) (4.8 g, 0.03 mol), sodium disulfite (5.7 g, 0.03 mol) and DMF (5 mL) were added into a vial (30 mL) of microwave synthesis reactor (Anton-Paar, Monowave 300, Austria). The reaction mixture, was heated under conditions of 240 oC and 10 bar for 5 min. The mixture was cooled down, 5-substituted-1,2-phenylenediamine (1) was added and then the final mixture was kept under the same reaction conditions in microwave reactor. After cooling, the mixture was poured into iced-water, precipitated product was washed with water, dried and recrystallized from ethanol.
Yield: 85%. M.p. 277.4 oC. 1H-NMR (300 MHz) (DMSO-d6) δ(ppm): 3.71 (3H, s, CH3), 7.06-7.11 (1H, m, C6H3-H), 7.44 (H, dd, J=9.40 Hz and J=2.20 Hz C6H3-H), 7.55-7.66 (H, m, C6H3-H), 7.97 (2H, d, J=8.50 Hz, C6H4-H), 8.26 (2H, d, J=8.50 Hz, C6H4-H), 12.84 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (75 MHz) (DMSO-d6) δ(ppm): 50.56, 101.84, 110.96, 111.31, 126.81, 128.21, 132.50, 134.64, 137.17, 152.28, 157.75, 160.88, 166.79. HRMS (M+H)+: calcd 271.0877, found 271.0881. Anal. calcd. For C15H11FN2O2, C, 66.66; H, 4.10; N, 10.37. Found: C, 66.72; H, 4.19; N, 10.46.
2.1.2. Synthesis of 4-(5(6)-fluoro-1H-benzimidazol-2-yl)benzoicacid hydrazide (4)
Methyl 4-(5(6)-fluoro-1H-benzimidazol-2-yl)benzoate (3) (0.02 mol) in ethanol (15 mL) and hydrazine hydrate (5 mL) were put into a vial (30 mL) of microwave synthesis reactor (MWI) (Anton-Paar Monowave 300, Austria). The reaction mixture was kept under the conditions of 150 oC and 10 bar for 10 min. After cooling, the mixture was poured into iced-water, precipitated product was washed with water, dried and recrystallized from ethanol.
Yield: 89%. M.p. 247 oC. 1H-NMR (300 MHz) (DMSO-d6) δ(ppm): 4.56 (2H, br.s, NH2), 7.05-7.12 (1H, m, C6H3-H), 7.41 (H, dd, J=9.40 Hz and J=2.20 Hz C6H3-H), 7.59-7.64 (H, m, C6H3-H), 7.98 (2H, d, J=8.50 Hz, C6H4-H), 8.21 (2H, d, J=8.50 Hz, C6H4-H), 9.92 (H, s, NH-CO), 12.79 (1H, s, Benzimidazole-NH, D2O exch). 13C-NMR (75 MHz) (DMSO-d6) δ(ppm): 101.94, 110.93, 111.27, 126.75, 128.11, 132.52, 134.74, 137.11, 152.22, 157.70, 160.82, 166.71. HRMS (M+H)+: calcd 271.0990, found 271.1002. Anal. calcd. For C14H11FN4O, C, 62.22; H, 4.10; N, 20.73. Found: C, 62.26; H, 4.13; N, 20.79.
2.1.3. Synthesis of 4-(5(6)-fluoro-1H-benzimidazol-2-yl)-N' (substitutedbenzylidene)benzohydrazide (6-24)
Appropriate quantity of 4-(5-substituted-1H-benzimidazol-2-yl)benzoic acid hydrazide (4) derivative (0.001 mol) was dissolved in ethanol. Benzaldehyde derivative (5) (0.001 mol) and a few drops of acetic acid were added into the solution. The reaction mixture was refluxed at 100 oC for 2 h. The residue was filtered, dried and recrystallized from butanol.
4-(5(6)-fluoro-1H-benzimidazol-2-yl)-N'-benzylidene-benzohydrazide (6)
Yield: 78%. M.p. 279 oC. IR νmax (ATR, cm-1): 3217 (N-H), 1664 (C=O), 1627-1614 (C=N), 1570-1420 (C=C), 854 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.27 (1H, dd, J=8.50 Hz and J=1.75 Hz, C6H5-H), 7.47 (3H, m, C6H5-H), 7.69-7.65 (2H, m, C6H5-H), 7.76 (2H, d, J=6.55 Hz, C6H4-H), 8.12 (2H, d, J=8.25 Hz, C6H4-H), 8.32 (2H, d, J=8.30 Hz, C6H4-H), 8.50 (H, s, N=CH), 11.98 (H, s, NH-CO), 13.29 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.80, 109.15, 116.10, 127.63, 128.83, 129.35, 130.65, 130.69, 132.76, 133.20, 137.52, 138.35, 141.43, 146.54, 151.82, 157.22, 162.93. EIS-MS (m/z): 359.05 [% 100, M+1], 360.10 [% 23.75, M+2]. Anal. calcd. For C21H15FN4O, C, 70.38; H, 4.22; N, 15.63. Found: C, 70.46; H, 4.21; N, 15.59.
4-(5(6)-Fluoro-1H-benzimidazol-2-yl)-N'-(2-chlorobenzy-lidene)benzohydrazide (7)
Yield: 86%. M.p. 206.4 oC. IR νmax (ATR, cm-1): 3215 (N-H), 1666 (C=O), 1629-1612 (C=N), 1558-1438 (C=C), 850 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.11 (1H, s, C6H3-H), 7.71-7.38 (5H, m, C6H4-H, C6H3-H), 8.06 (1H, d, J=6.05 Hz, C6H4-H), 8.14 (2H, d, J=7.90 Hz, C6H4-H), 8.32 (2H, d, J=7.90 Hz, C6H4-H), 8.91 (1H, s, N=CH), 12.20 (1H, s, NH-CO), 13.24 (1H, s, Benzimidazole-NH, D2O exch.).13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 103.04, 109.56, 116.77, 126.89, 127.41, 128.13, 128.89, 130.43, 132.04, 132.76, 133.74, 134.46, 137.43, 138.13, 138.49, 140.42, 151.98, 156.19, 162.98. EIS-MS (m/z): 393.05 [% 100, M+1], 394.10 [% 22.62, M+2], 395 [% 33.64, M+3], 396.05 [% 6.75, M+4]. Anal. calcd. For C21H14ClFN4O, C, 64.21; H, 3.59; N, 14.26. Found: C, 64.33; H, 3.58; N, 14.29.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(3-chlorobenzy-lidene)benzohydrazide (8)
Yield: 82%. M.p. 246.6 oC. IR νmax (ATR, cm-1): 3221 (N-H), 1664 (C=O), 1629-1614 (C=N), 1568-1429 (C=C), 844 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.10 (1H, s, C6H3-H), 7.51-7.71 (5H, m, C6H4-H, C6H3-H), 7.81(1H, s, C6H4-H), 8.11 (2H, d, J=7.90 Hz, C6H4-H), 8.31 (2H, d, J=7.90 Hz, C6H4-H), 8.47 (1H, s, N=CH), 12.11 (1H, s, NH-CO), 13.22 (1H, s, Benzimidazole-NH, D2O exch.).13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 103.12, 110.69, 116.40, 126.34, 126.81, 126.85, 128.88, 130.26, 131.24, 133.30, 134.16, 134.57, 137.69, 138.02, 140.51, 146.81, 151.88, 155.86, 163.09. EIS-MS (m/z): 393.05 [% 100, M+1], 394.10 [% 23.11, M+2], 395.05 [% 34.32, M+3], 396.10 [% 5.79, M+4]. Anal. calcd. For C21H14ClFN4O, C, 64.21; H, 3.59; N, 14.26. Found: C, 64.42; H, 3.60; N, 14.31.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(4-chlorobenzy-lidene)benzohydrazide (9)
Yield: 73%. M.p. 300.6 oC. IR νmax (ATR, cm-1): 3209 (N-H), 1662 (C=O), 1627-1614 (C=N), 1566-1427 (C=C), 842 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.11 (1H, t, J=9.0 Hz C6H3-H), 7.48 (1H, s, C6H3-H), 7.55 (1H, d, J=7.85 Hz, C6H4-H), 7.64 (1H, s, C6H3-H), 7.79 (1H, d, J=7.85 Hz, C6H4-H), 8.11 (2H, d, J=7.80 Hz, C6H4-H), 8.31 (2H, d, J=7.80 Hz, C6H4-H), 8.49 (1H, s, N=CH), 12.05 (1H, s, NH-CO), 13.24 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.65, 109.12, 116.38, 126.85, 128.85, 129.25, 130.45, 131.28, 133.72, 136.07, 137.98, 138.65, 140.86, 143.17, 152.27, 156.09, 162.97. EIS-MS (m/z): 393.05 [% 100, M+1], 394.05 [% 24.84, M+2 ], 395.05 [% 35.00, M+3], 396.10 [% 7.21, M+4]. Anal. calcd. For C21H14ClFN4O, C, 64.21; H, 3.59; N, 14.26. Found: C, 64.17; H, 3.58; N, 14.22.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(2,4-dichloro-benzylidene)benzohydrazide (10)
Yield: 76%. M.p. 257.3 oC. IR νmax (ATR, cm-1): 3219 (N-H), 1668 (C=O), 1622-1612 (C=N), 1589-1438 (C=C), 850 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.11 (1H, s, C6H3-H), 7.46-7.74 (3H, m, C6H3-H, C6H3-H), 8.05 (1H, d, J=8.45 Hz C6H3-H), 8.12 (2H, d, J=7.95 Hz, C6H4-H), 8.31 (2H, d, J=7.95 Hz, C6H4-H), 8.85 (1H, s, N=CH), 12.24 (1H, s, NH-CO), 13.23 (1H, s, Benzimidazole-NH, D2O exch.).13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.95, 109.80, 116.50, 126.88, 128.55, 127.83, 128.89, 129.28, 129.87, 131.15, 132.39, 133.40, 137.65, 138.63, 139.12, 140.33, 152.17, 154.37, 162.97. EIS-MS (m/z): 427.05 [% 100, M+1], 429.05 [% 21.02, M+3], 430.05 [% 16.10, M+4], 431.10 [% 11.83, M+5]. Anal. calcd. For C21H13Cl2FN4O, C, 59.03; H, 3.07; N, 13.11. Found: C, 58.83; H, 3.06; N, 13.14.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(2-nitrobenzyl-idene)benzohydrazide (11)
Yield: 72%. M.p. 285.7 oC. IR νmax (ATR, cm-1): 3257 (N-H), 1672 (C=O), 1629-1618 (C=N), 1556-1436 (C=C), 842 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.11 (1H, s, C6H3-H), 7.58-7.38 (2H, m, C6H4-H), 7.70 (1H, t, J=7.55 Hz, C6H4-H), 7.84 (1H, t, J=4.5 Hz, C6H4-H), 8.14 (4H, m, C6H4-H), 8.32 (2H, d, J=7.90 Hz, C6H4-H), 8.92 (1H, s, N=CH), 12.34 (1H, s, NH-CO), 13.25 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ (ppm): 102.75, 108.89, 116.56, 125.17, 126.93, 128.45, 128.96, 129.17, 131.22, 132.42, 134.23, 137.28, 138.74, 140.70, 143.77, 148.74, 151.30, 156.82, 163.13. EIS-MS (m/z): 404.05 [% 100, M+1], 405.05 [% 21.88, M+2]. Anal. calcd. For C21H14FN5O3, C, 62.53; H, 3.50; N, 17.36. Found: C, 62.37; H, 3.49; N, 17.39.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(3-nitrobenzyl-idene)benzohydrazide (12)
Yield: 65%. M.p. 323.4 oC. IR νmax (ATR, cm-1): 3219 (N-H), 1664 (C=O), 1631-1614 (C=N), 1562-1427 (C=C), 850 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.09 (1H, m, C6H3-H), 7.42-7.62 (2H, m, C6H4-H), 7.74 (1H, t, J=7.90 Hz, C6H4-H), 8.11 (2H, d, J=8.10 Hz, C6H4-H), 8.15 (1H, d, J=7.60 Hz, C6H4-H), 8.23 (1H, d, J=7.40 Hz, C6H4-H), 8.30 (2H, d, J=8.25 Hz, C6H4-H), 8.54 (1H, s, C6H4-H), 8.57 (1H, s, N=CH), 12.20 (1H, s, NH-CO), 13.21 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.86, 109.17, 116.48, 120.92, 126.43, 126.47, 128.56, 130.43, 132.89, 132.40, 133.93, 137.14, 138.23, 140.64, 145.55, 148.21, 152.72, 156.52, 162.70. EIS-MS (m/z): 404.05 [% 100, M+1], 405.10 [% 29.42, M+2]. Anal. calcd. For C21H14FN5O3, C, 62.53; H, 3.50; N, 17.36. Found: C, 62.63; H, 3.51; N, 17.41.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(4-nitrobenzyl-idene)benzohydrazide (13)
Yield: 67%. M.p. 304.4 oC. IR νmax (ATR, cm-1): 3211 (N-H), 1662 (C=O), 1629-1611 (C=N), 1564-1429 (C=C), 842 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.10 (1H, s, C6H3-H), 7.38-7.69 (2H, m, C6H3-H), 8.01 (2H, d, J=8.05 Hz, C6H4-H), 8.12 (2H, d, J=7.70 Hz, C6H4-H), 8.31 (4H, d, J=7.85 Hz, C6H4-H), 8.58 (1H, s, N=CH), 12.26 (1H, s, NH-CO), 13.22 (1H, s, Benzimidazole-NH, D2O exch.).13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.60, 109.88, 116.37, 124.41, 124.56, 126.89, 128.87, 132.10, 137.36, 138.43, 139.38, 141.06, 145.99, 150.34, 152.21, 156.07, 163.21. EIS-MS (m/z): 404 [% 100, M+1], 405.10 [% 22.88, M+2]. Anal. calcd. For C21H14FN5O3, C, 62.53; H, 3.50; N, 17.36. Found: C, 62.58; H, 3.51; N, 17.41.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(2-fluorobenzyl-idene)benzohydrazide (14)
Yield: 74%. M.p. 249.8 oC. IR νmax (ATR, cm-1): 3201 (N-H), 1662 (C=O), 1629-1614 (C=N), 1562-1440 (C=C), 848 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.11 (1H, s, C6H3-H), 7.31-7.71 (5H, m, C6H3-H), 7.98 (1H, t, J=7.20 Hz, C6H4-H), 8.12 (2H, d, J=7.90 Hz, C6H4-H), 8.32 (2H, d, J=7.90 Hz, C6H4-H), 8.74 (1H, s, N=CH), 12.10 (1H, s, NH-CO), 13.24 (1H, s, Benzimidazole-NH, D2O exch.).13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.51, 109.68, 116.44, 115.60, 118.25, 125.46, 127.04, 128.87, 130.13, 132.09, 132.61, 137.69, 138.71, 141.22, 142.98, 152.11, 156.20, 159. 69, 162.88. EIS-MS (m/z): 377.10 [% 100, M+1], 378.15 [% 30.23, M+2]. Anal. calcd. For C21H14F2N4O, C, 67.02; H, 3.75; N, 14.89. Found: C, 66.83; H, 3.74; N, 14.92.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(3-fluorobenzyl-idene)benzohydrazide (15)
Yield: 77%. M.p. 269.3 oC. IR νmax (ATR, cm-1): 3211 (N-H), 1664 (C=O), 1627-1614 (C=N), 1564-1427 (C=C), 854 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.11 (1H, m, C6H3-H), 7.28-7.71 (6H, m, C6H4-H,ve C6H3-H), 8.12 (2H, d, J=8.00 Hz, C6H4-H), 8.32 (2H, d, J=7.70 Hz, C6H4-H, 8.50 (1H, s, N=CH), 12.10 (1H, s, NH-CO), 13.25 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.67, 109.45, 114.67, 116.45, 117.63, 123.98, 126.90, 128.88, 131.38, 132.25, 134.62, 137.30, 138.36, 140.96, 147.13, 152.34, 155.93, 163.07, 163.87. EIS-MS (m/z): 377.10 [% 100, M+1], 378.15 [% 24.29, M+2]. Anal. calcd. For C21H14F2N4O, C, 67.02; H, 3.75; N, 14.89. Found: C, 66.94; H, 3.76; N, 14.86.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(4-fluorobenzyl-idene)benzohydrazide (16)
Yield: 82%. M.p. 288.7 oC. IR νmax (ATR, cm-1): 3209 (N-H), 1664 (C=O), 1627-1616 (C=N), 1568-1427 (C=C), 854 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.10 (1H, s, C6H3-H), 7.30-7.83 (6H, m, C6H4-H, C6H3-H), 8.10 (2H, d, J=8.30 Hz, C6H4-H), 8.30 (2H, d, J=8.30 Hz, C6H4-H), 8.48 (1H, s, N=CH), 11.99 (1H, s, NH-CO), 13.22 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.76, 109.43, 116.33, 116.51, 127.04, 128.83, 129.77, 131.40, 132.44, 137.76, 137.91, 140.85, 147.40, 152.48, 156.47, 164.65, 164.74. EIS-MS (m/z): 377.10 [% 100, M+1], 378.10 [% 28.74, M+2].Anal. calcd. For C21H14F2N4O, C, 67.02; H, 3.75; N, 14.89. Found: C, 66.87; H, 3.74; N, 14.93.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(2,4-difluoro-benzylidene)benzohydrazide (17)
Yield: 86%. M.p. 243 oC. IR νmax (ATR, cm-1): 3219 (N-H), 1660 (C=O), 1627-1618 (C=N), 1570-1427 (C=C), 856 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 7.10 (1H, s, C6H3-H), 7.20-7.70 (4H, m, C6H3-H), 7.99- 8.03 (1H, m, C6H3-H) 8.10 (2H, d, J=7.90 Hz, C6H4-H), 8.31 (2H, d, J=7.85 Hz, C6H4-H), 8.67 (1H, s, N=CH), 12.09 (1H, s, NH-CO), 13.23 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 102.75, 109.96, 111.16, 113.21, 113.31, 116.18, 126.88, 128.83, 132.31, 133.32, 137.49, 138.57, 140.53, 143.60, 152.76, 155.75, 161.39, 162.90, 164.84. EIS-MS (m/z): 395.10 [% 100, M+1], 396.10 [% 21.55, M+2]. Anal. calcd. For C21H13F3N4O, C, 63.96; H, 3.32; N, 14.21. Found: C, 64.12; H, 3.31; N, 14.18.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(2-methylbenzyl-idene)benzohydrazide (18)
Yield: 79%. M.p. 274.2 oC. IR νmax (ATR, cm-1): 3207 (N-H), 1635 (C=O), 1621-1612 (C=N), 1560-1435 (C=C), 850 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 2.49 (3H, s, -CH3), 7.09-7.13 (1H, m, C6H3-H), 7.28-7.53 (4H, m, C6H4-H,ve C6H3-H), 7.56 (1H, m, C6H3-H), 7.73 (1H, d, J=7.40 Hz, C6H4-H), 8.12 (2H, d, J=7.60 Hz, C6H4-H), 8.31 (2H, m, C6H4-H), 8.79 (1H, s, N=CH), 11.96 (1H, s, NH-CO), 13.25 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 19.48, 102.72, 109.41, 116.18, 124.91, 126.68, 127.01, 128.79, 129.33, 130.32, 131.36, 132.72, 135.99, 137.95, 138.41, 141.06, 143.18, 152.13, 156.18, 162.73. EIS-MS (m/z): 373.15 [% 100, M+1], 374.15 [% 27.92, M+2]. Anal. calcd. For C22H17FN4O, C, 70.96; H, 4.60; N, 15.05. Found: C, 70.76; H, 4.59; N, 15.08.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(3-methylbenzyl-idene)benzohydrazide (19)
Yield: 66%. M.p. 264.6 oC. IR νmax (ATR, cm-1): 3215 (N-H), 1662 (C=O), 1627-1616 (C=N), 1566-1429 (C=C), 854 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 2.36 (3H, s, -CH3), 7.08-7.12 (1H, m, C6H3-H), 7.25-7.71 (5H, m, C6H4-H, and C6H3-H), 8.11 (2H, d, J=7.85 Hz, C6H4-H), 8.31 (2H, d, J=7.50 Hz C6H4-H), 8.45 (1H, s, N=CH), 11.97 (1H, s, NH-CO), 13.23 (1H, s, Benzimidazole-NH, D2O exch.).13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 21.35, 102.89, 109.07, 116.76, 126.79, 127.86, 128.90, 129.22, 129.32, 131.36, 132.26, 133.16, 137.73, 137.85, 138.58, 140.98, 145.63, 152.47, 155.91, 162.96. EIS-MS (m/z): 373.15 [% 100, M+1], 374.15 [% 27.92, M+2]. Anal. calcd. For C22H17FN4O, C, 70.96; H, 4.60; N, 15.05. Found: C, 71.16; H, 4.61; N, 15.01.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(4-methylbenzyl-idene)benzohydrazide (20)
Yield: 64%. M.p. 319.2 oC. IR νmax (ATR, cm-1): 3213 (N-H), 1662 (C=O), 1627-1614 (C=N), 1571-1429 (C=C), 854 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 2.36 (3H, s, -CH3), 7.09-7.13 (1H, m, C6H3-H), 7.29 (2H, d, J=7.65 Hz, C6H4-H), 7.45-7.66 (4H, m, C6H4-H,and C6H3-H), 8.10 (2H, d, J=7.90, Hz, C6H4-H), 8.31 (2H, d, J=7.90 Hz, C6H4-H), 8.46 (1H, s, N=CH), 11.91 (1H, s, NH-CO), 13.23 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 21.52, 102.14, 109.23, 116.81, 126.83, 127.61, 128.80, 129.96, 130.34, 132.05, 137.86, 138.74, 139.72, 140.49, 146.57, 152.22, 155.84, 162.84. EIS-MS (m/z): 373.10 [% 100, M+1], 374.15 [% 22.62, M+2]. Anal. calcd. For C22H17FN4O, C, 70.96; H, 4.60; N, 15.05. Found: C, 70.83; H, 4.61; N, 15.02.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(2-methoxy-benzylidene)benzohydrazide (21)
Yield: 69%. M.p. 173.4 oC. IR νmax (ATR, cm-1): 3207 (N-H), 1631 (C=O), 1627-1612 (C=N), 1558-1436 (C=C), 852 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 3.89 (3H, s, -OCH3), 7.04-7.14 (3H, m, C6H3-H), 7.43-7.59 (3H, m, C6H4-H and C6H3-H), 7.90 (2H, d, J=7.60 Hz, C6H4-H), 8.12 (2H, d, J=7.90, Hz, C6H4-H), 8.30 (2H, d, J=7.90 Hz, C6H4-H), 8.85 (1H, s, N=CH), 11.97 (1H, s, NH-CO), 13.23 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 56.21, 101.98, 109.46, 112.40, 115.49, 116.48, 121.27, 126.86, 128.81, 130.89, 132.13, 132.25, 137.52, 138.16, 140.69, 144.00, 152.49, 156.31, 157.71, 167.96. EIS-MS (m/z): 389.10 [% 100, M+1], 390.10 [% 29.78, M+2]. Anal. calcd. For C22H17FN4O2, C, 68.03; H, 4.41; N, 14.43. Found: C, 68.12; H, 4.40; N, 14.38.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(3-methoxy-benzylidene)benzohydrazide (22)
Yield: 75%. M.p. 250.9 oC. IR νmax (ATR, cm-1): 3217 (N-H), 1662 (C=O), 1629-1614 (C=N), 1566-1429 (C=C), 850 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 3.82 (3H, s, -OCH3), 7.02 (1H, d, J=6.65 Hz, C6H4-H), 7.10 (1H, s, C6H3-H), 7.31 (2H, s, C6H4-H), 7.37-7.72 (3H, m, C6H4-H and C6H3-H), 8.10 (2H, d, J= 8.20 Hz, C6H4-H), 8.31 (2H, d, J=8.20 Hz, C6H4-H), 8.46 (1H, s, N=CH), 11.97 (1H, s, NH-CO), 13.21 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 55.67, 102.34, 109.79, 111.75, 116.34, 116.78, 120.60, 126.85, 128.83, 130.46, 132.21, 137.46, 138.78, 138.19, 140.85, 144.40, 152. 76, 156.29, 160.06, 162.98. EIS-MS (m/z): 389.10 [% 100, M+1], 390.10 [% 27.99, M+2]. Anal. calcd. For C22H17FN4O2, C, 68.03; H, 4.41; N, 14.43. Found: C, 68.21; H, 4.42; N, 14.46.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'-(4-methoxy-benzylidene)benzohydrazide (23)
Yield: 83%. M.p. 285.8 oC. IR νmax (ATR, cm-1): 3207 (N-H), 1662 (C=O), 1627-1614 (C=N), 1570-1446 (C=C), 850 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 3.82 (3H, s, -OCH3), 7.04 (2H, d, J=8.40 Hz, C6H4-H), 7.11 (1H, s, C6H3-H), 7.37-7.57 (2H, m, C6H3-H), 7.71 (2H, d, J=8.40 Hz, C6H4-H), 8.10 (2H, d, J= 8.10 Hz, C6H4-H), 8.31 (2H, d, J=8.10 Hz, C6H4-H), 8.44 (1H, s, N=CH), 11.86 (1H, s, NH-CO), 13.23 (1H, s, Benzimidazole-NH, D2O exch.).13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 55.78, 102.39, 109.58, 114.84, 116.58, 126.82, 127.30, 128.76, 129.24, 133.07, 137.95, 138.84, 140.56, 144.43, 152. 39, 155.74, 161.39, 162.7. EIS-MS (m/z): 389.10 [% 100, M+1], 390.15 [% 23.96, M+2]. Anal. calcd. For C22H17FN4O2, C, 68.03; H, 4.41; N, 14.43. Found: C, 67.86; H, 4.40; N, 14.41.
4-(5(6)Fluoro-1H-benzimidazol-2-yl)-N'(2,4dimethoxy-benzylidene)benzohydrazide (24)
Yield: 77%. M.p. 171.6 oC. IR νmax (ATR, cm-1): 3211 (N-H), 1649 (C=O), 1624-1612 (C=N), 1554-1423 (C=C), 842 (1,4-disubstituted benzene). 1H-NMR (500 MHz) (DMSO-d6) δ(ppm): 3.83 (3H, s, -OCH3), 3.88 (3H, s, -OCH3), 6.65 (2H, s, C6H3-H), 7.10 (1H, s, C6H3-H), 7.38-7.68 (2H, m, C6H3-H), 7.83 (2H, d, J=8.75 Hz, C6H4-H), 8.10 (2H, d, J= 7.70 Hz, C6H4-H), 8.28 (2H, d, J=7.70 Hz, C6H4-H), 8.75 (1H, s, N=CH), 11.82 (1H, s, NH-CO), 13.21 (1H, s, Benzimidazole-NH, D2O exch.). 13C-NMR (125 MHz) (DMSO-d6) δ(ppm): 55.92, 56.26, 101.79, 102.25, 106.61, 109.66, 109.91, 116.58, 127.21, 128.74, 133.00, 134.94, 137.09, 137.94, 140.88, 144.08, 152.33, 155.70, 159.86, 162.50, 163.00. EIS-MS (m/z): 419.10 [% 100, M+1], 420.10 [% 65.13, M+2], 421.10 [% 10.06, M+3]. Anal. calcd. For C23H19FN4O3, C, 66.02; H, 4.58; N, 13.39. Found: C, 65.83; H, 4.56; N, 13.42.
2.2. Antimicrobial Activity
Microbiological studies were performed according to following guides: CLSI reference M24-A broth microdilution method [50] for Mycobacterium smegmatis (ATCC 14468), CLSI reference M07-A9 broth microdilution method [51] for bacterial strains and EUCAST definitive (EDef 7.1) method [52] for Candida albicans (ATCC 24433). Synthesized compounds were tested for their in vitro growth inhibitory activity against Staphylococcus aureus (SaMRSA) (ATCC 700699), Escherichia coli O157:H7, Escherichia coli (ATCC 8739), Escherichia coli (ATCC 35218), Escherichia coli (ATCC 25922), Mycobacterium smegmatis (ATCC 14468), Klebsiella pneumoniae (NCTC 9633), Salmonella typhimurium (ATCC 13311), Vibrio fischeri ChromaDex and Candida albicans (ATCC 24433). Chloramphenicol, ketoconazole, moxifloxacin, and nifuroxazide were used as control drugs.
2.2.1. Broth Microdilution Assay
The cultures were obtained from Mueller–Hinton broth (Difco) for the bacterial strains after overnight incubation at 37 °C. The yeasts were maintained in RPMI after overnight incubation at 37 °C. Mycobacterium smegmatis (ATCC 14468) in the Middle Brook medium were inoculated at 37 °C for 72 h. The inocula of test microorganisms adjusted to match the turbidity of a Mac Farland 0.5 standard tube as determined with a spectrophotometer and the final inoculum size was 0.5-2.5×105 cfu/mL for antibacterial and antifungal assays. Testing was carried out in Mueller–Hinton broth and RPMI at pH=7 and the two-fold serial dilutions technique was applied. The last well on the microplates containing only inoculated broth was kept as controls and the last well with no growth of microorganism was recorded to represent the MIC90 expressed in μg/mL. For both the antibacterial and antifungal assays the compounds were dissolved in DMSO. Further dilutions of the compounds and standard drugs in test medium were prepared at the required quantities of 1000, 500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9 and 1.95 μg/mL concentrations with Mueller–Hinton broth, RPMI and Middle Brook medium. The completed plates were incubated for 24h. At the end of the incubation, resazurin (20 µg/mL) was added into each well to control the growth in wells. Completed plates including Staphylococcus aureus (SaMRSA) (ATCC 700699), Escherichia coli O157:H7, Escherichia coli (ATCC 8739), Escherichia coli (ATCC 35218), Escherichia coli (ATCC 25922), Klebsiella pneumonia (NCTC 9633), Salmonella typhimurium (ATCC 13311), Vibrio fischeri ChromaDex and Candida albicans (ATCC 24433) were incubated for 2h. and Mycobacterium smegmatis (ATCC 14468) for 12h. MIC90 values were determined using a microplate reader at 590 nm excitation and 560 nm emission. Each experiment in the antimicrobial assays was replicated twice in order to define the MIC90 values, given in Table 1.
Table 1.
Antimicrobial activity (MIC90 µg/mL) of compounds 18 and reference drugs against pathogenic microorganisms.
| Compound | SaMRSA | E.coli 1 | E. coli 2 | E. coli 3 | E.coli 4 | Kp | St | Vf | Ms | Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| 18 | >1000 | 0.49 | 0.49 | 0.49 | 0.49 | >1000 | 0.98 | >1000 | >1000 | >1000 |
| Nifuroxazide | 15.62 | 31.25 | >1000 | 0.98 | 0.49 | 0.98 | 0.98 | 7.8 | >1000 | - |
| Chloram-phenicol | 62.5 | 15.62 | 3.9 | 0.98 | 0.98 | 3.9 | 0.98 | 3.9 | 125 | - |
| Moxifloxacin | 0.24 | 0.06 | 0.06 | 0.06 | 0.06 | 0.98 | 0.49 | 0.06 | 0.12 | - |
| Ketoconazole | - | - | - | - | - | - | - | - | - | 7.8 |
SaMRSA: Staphylococcus aureus (SaMRSA) (ATCC 700699), E. coli 1: Escherichia coli O157:H7, E. coli 2: Escherichia coli (ATCC 8739), E. coli 3: E. coli (ATCC 35218), E.coli 4: E. coli (ATCC 25922), Kp: Klebsiella pneumonia (NCTC 9633), St: Salmonella typhimurium (ATCC 13311), Vf: Vibrio fischeri, Ms: Mycobacterium smegmatis (ATCC 14468), Ca: Candida albicans (ATCC 24433).
2.3. Cytotoxic Activity
Healthy intestinal epithelial cell line CCD 841 CoN (ATCC® CRL-1790™) was used for cytotoxicity study. Cells were replicated into RPMI-1640 medium, containing 10% fetal bovine serum, 1% penicillin / streptomycin, 1% L-glutamine and amphotericin B at 37 °C with 95% relative humidity in 5% CO2 incubator. The proliferation of the human intestinal epithelial cells was assessed by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide) assay, reported in elsewhere [53]. Briefly, cells were inoculated into 96-well culture plates at densities of 5x103 cells per well. After 24 hours, they were treated with synthesized compounds (concentrations of 1.95-1000 µg/ml) for 24 h. After the incubations, MTT solution (5 mg/mL) was added to each well and incubated for 3 hours at 37ºC. At the end of the incubation, the purple MTT–formazan crystals were dissolved by adding 100 µg/mL of DMSO to each well. The plates were then read on a Microplate reader at 540 nm wavelength. The IC50 value was calculated from the plots of cell proliferations against concentrations by applying regression analyses on GraphPad Prism Version 5.
2.4. Theoretical Calculation of ADME Parameters
In order to predict pharmacokinetic profiles of the target compounds (6-24), some physicochemical parameters were calculated by using the Molinspiration property calculation program [54] and thus ADME properties of the compounds were evaluated.
2.5. Determination of Metabolic Stability
The stability of the selected compounds (7, 11, 14, 18, 19, 20 and 21) against metabolic activity of Escherichia coli O157:H7 was determined by following the same protocol explained in Broth Microdilution Assay. Single concentration (1.95 µg/mL for 18 and 1 mg/mL for the other derivatives) of the compounds were used in the test. After incubation period, DMSO, and the inocula with or without test compounds were filtered from 0.22 µm pore size membrane filter and then injected to LCMS-IT-TOF system (Shimadzu, Tokyo, Japan). The diluent (acetonitrile) application was also performed for determination of blank peaks. Mobile phase was used as solvent A; water (95%), solvent B; acetonitrile (5%), at a flow rate of 0.20 mL/min and a sample injection volume of 1 µl.
2.6. Docking Studies
Docking calculations were performed with the program AutoDock Vina [55]. The coordinates of PFK-2 is acquired from Escherichia coli (PDB ID: 3CQD) were obtained from the Protein Data Bank (PDB) (www.rcsb.org). AutoDock Tools (ADT, Version 1.5.6) [56], was used to add polar hydrogen atoms and partial charges for protein and ligand, which were saved in pdbqt format. For docking studies initial protein was prepared by removing ligands, all water molecules, any co-crystallized solvent. A grid box of 70 x 70 x 70, designed by ADT, was positioned in the middle of the protein (x=15.909, y=39.729, z=18.699). AutoDock Vina was used to dock the ligand into the active site of the protein. The poses of the docked ligands were analyzed, and the results were visualized by PyMOL 1.6.X [57].
3. RESULTS AND DISCUSSION
Prompted by previous research, we designed and synthesized nineteen new 4-(5(6)-fluoro-1H-benzimidazol-2-yl)benzoic acid substituted-benzylidene hydrazide derivatives (6–24). The synthetic route for the target compounds is outlined in Scheme 1. In the first and second steps, due to microwave irradiation, the products were obtained in good yields (85%–89%) with a short reaction time (10 min), while in previously reported work the products were obtained in lower yields with longer reaction times [58,59].
Scheme 1.
Synthesis of the compounds 6-24.
The chemical structures of the compounds (6–24) were confirmed by IR, 1H NMR, 13C NMR, mass spectral data and elemental analyses. Stretching absorptions of the N-H groups were observed at 3201–3257 cm-1, as expected. The carbonyl (C=O) group demonstrated a characteristic stretching absorption in the region 1631–1672 cm-1. Stretching absorptions at about 1423–1631 cm-1 were recorded for C=C and C=N double bonds. The stretching absorption belonging to 1,4-disubstituted benzene was determined at 842–856 cm-1. 1H NMR spectrum showed a broad singlet at 13.21–13.29 ppm due to the NH proton of the benzimidazole ring. The NH and CH protons of the hydrazone (-CONHN=CH-) moiety were recorded as singlets at 11.82–12.34 and 8.44–8.92 ppm, respectively. The aromatic protons belonging to 4-substituted phenyl groups gave peaks at 8.10–8.14 and 8.28–8.32 as two doublets. Aromatic protons of benzimidazole were observed at 6.65–7.83 ppm. The other aliphatic protons (-CH3 and -OCH3) belonging to variable groups in synthesized compounds (6–24) were recorded at 2.36–2.49 and 3.82–3.89 ppm as singlets. 13C NMR spectrum showed characteristic hydrazone (-CONHN=CH-) signals at 162.70–164.84 and 138.49–147.40 due to carbonyl (C=O) and azomethine (N=CH) carbons, respectively. The mass spectra (ES-MS) of the compounds showed [M+1] peaks, in agreement with their molecular formula. M+2, M+3, M+4, and M+5 peaks were also observed, especially for the chloro-substituted com-pounds (7–10) due to the high relative density of chlorine isotopes. Additionally, elemental analyses of all compounds gave satisfactory results.
3.1. Antimicrobial Activity
Synthesized compounds (6–24) were evaluated for antimicro-bial activity against various microorganisms such as Escherichia coli O157:H7 (enterohaemorrhagic serotype), Staphylococcus aureus (SaMRSA) (ATCC 700699), Escherichia coli (ATCC 8739), Escherichia coli (ATCC 35218), Escherichia coli (ATCC 25922), Mycobacterium smegmatis (ATCC 14468), Klebsiella pneumoniae (NCTC 9633), Salmonella typhimurium (ATCC 13311), Vibrio fischeri (ChromaDex, Boulder, USA) and Candida albicans (ATCC 24433). MIC90 values (Table 1) were revealed by fluorometric measurements using resazurin solution [60,61]. Chloramphenicol, ketoconazole, moxifloxacin and nifuroxazide were used as standard drugs in the activity test. Most of the compounds were found inactive against tested microbial strains, but compound 18 indicated very strong activity against four different Escherichia coli serotypes (MIC90 =0.49 µg/mL) and Salmonella typhimurium ATCC 13311 (MIC90 = 0.98 µg/mL). All of these strains are the members of intestinal flora, and thus the compound 18 may be suggested as an intestinal antiseptic. The antimicrobial spectrum of nifuroxazide, shown in Table 1, also supports this suggestion, as a clear similarity between the antimicrobial spectra of nifuroxazide and compound 18 can be seen. Furthermore, the MIC90 value (0.49 µg/mL) of compound 18 against Escherichia coli O157:H7 is 64 fold lower than that of nifuroxazide (31.25 µg/mL). It is known that the Escherichia coli O157:H7 serotype is highly pathogenic for humans and is responsible for tens of thousands of illnesses caused yearly. Children and the elderly are the most susceptible to severe complications. This serotype binds to the cells of the human intestinal tract and causes bloody diarrhea and a series of dangerous, often life-threatening complications [62]. Thus, the high potency of compound 18 in inhibiting Escherichia coli O157:H7 increases its antimicrobial importance.
3.2. Cytotoxic Activity
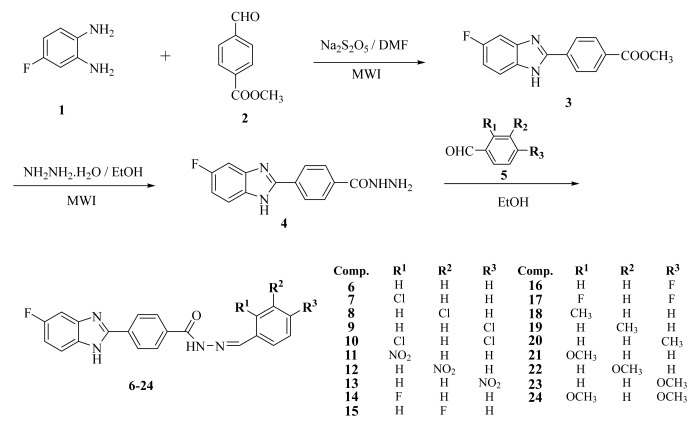
There are a number of criteria to be met for successful new drug development. The drug candidate should not only possess intrinsic activity, but should also be able to reach its target and not exhibit toxic effects. The toxicity of compound 18, which demonstrated significant antibacterial activity, was investigated by MTT assay. This assay is based upon the reduction of yellow MTT dye by metabolically active eukaryotic and prokaryotic cells to form the purple formazan product. The assay is generally used to examine cell viability and to estimate cell culture growth [63, 64]. MTT assay was performed using healthy intestinal epithelial cell line CCD 841 CoN (ATCC® CRL-1790™), since a significant proportion of antibacterial activity of compound 18 is against the intestinal flora. Nifuroxazide was also subjected to MTT assay in order to compare its cytotoxicity with that of compound 18. Fig. (1) presents the results, in which it is shown that the IC50 value (210.23 µg/mL) of compound 18 is about 430 fold higher than its MIC90 determined against intestinal bacteria. Furthermore, IC50 of compound 18 is about two fold higher than that of nifuroxazide (IC50 =121.90 µg/mL). These findings show that the antibacterial activity of the compound 18 is not due to general toxicity, but can be ascribed to its selective action against bacteria.
Fig. (1).
IC50 of the compound 18 and nifuroxazide onhealthy intestinal epithelial cell line CCD 841 CoN (ATCC® CRL-1790™).
3.3. Prediction of ADME Properties
In addition to essential biological activity, drug candidates should also have an ideal pharmacokinetic profile. Lipinski’s rule evaluates the absorption, distribution, metabolism and elimination (ADME) properties of drug like compounds and is important for the optimization of a biologically active compound. The rule requires that an orally active drug has no more than one violation [65]. To determine pharmacokinetic properties of the synthesized compounds 6–24, the theoretical calculations of ADME parameters (molecular weight, log P, topological polar surface are (tPSA), number of hydrogen donors and acceptors and volume) are presented in Table 2 along with violations of Lipinski’s rule. According to this data, all the compounds (6–24) follow Lipinski’s rule by causing no more than one violation. For compound 18, all calculated physicochemical parameters are compatible with Lipinski’s rule except for its log P value. Although the log P value of compound 18 (5.25) exceeds Lipinski’s limit, it shows that the compound has a lipophilic character, which is a key property that improves the ability of a drug to reach its target by trans membrane diffusion and to have a major influence on the final biological effect [66,67]. Furthermore, tPSA, described to be a predictive indicator of membrane penetration and calculated as 70.14, is found to be less than 140 Å2. This supports the idea that compound 18 may be an effective antibacterial agent [68].
Table 2.
Substituent pattern and some physicochemical parameters of the compounds 6-24 used in prediction of ADME profiles.
| Comp. | R1 | R2 | R3 | MW | logP | TPSA | HBA | HBD | Vol | Vio |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | H | H | H | 358.38 | 4.85 | 70.14 | 5 | 2 | 311.26 | 0 |
| 7 | Cl | H | H | 392.82 | 5.48 | 70.14 | 5 | 2 | 324.80 | 1 |
| 8 | H | Cl | H | 392.82 | 5.50 | 70.14 | 5 | 2 | 324.80 | 1 |
| 9 | H | H | Cl | 392.82 | 5.52 | 70.14 | 5 | 2 | 324.80 | 1 |
| 10 | Cl | H | Cl | 427.27 | 6.13 | 70.14 | 5 | 2 | 338.33 | 1 |
| 11 | NO2 | H | H | 403.37 | 4.76 | 115.97 | 8 | 2 | 334.59 | 0 |
| 12 | H | NO2 | H | 403.37 | 4.78 | 115.97 | 8 | 2 | 334.59 | 0 |
| 13 | H | H | NO2 | 403.37 | 4.80 | 115.97 | 8 | 2 | 334.59 | 0 |
| 14 | F | H | H | 376.37 | 4.96 | 70.14 | 5 | 2 | 316.19 | 0 |
| 15 | H | F | H | 376.37 | 4.99 | 70.14 | 5 | 2 | 316.19 | 0 |
| 16 | H | H | F | 376.37 | 5.01 | 70.14 | 5 | 2 | 316.19 | 1 |
| 17 | F | H | F | 394.36 | 5.10 | 70.14 | 5 | 2 | 321.12 | 1 |
| 18 | CH3 | H | H | 372.40 | 5.25 | 70.14 | 5 | 2 | 327.82 | 1 |
| 19 | H | CH3 | H | 372.40 | 5.27 | 70.14 | 5 | 2 | 327.82 | 1 |
| 20 | H | H | CH3 | 372.40 | 5.29 | 70.14 | 5 | 2 | 327.82 | 1 |
| 21 | OCH3 | H | H | 388.40 | 4.86 | 79.38 | 6 | 2 | 336.81 | 0 |
| 22 | H | OCH3 | H | 388.40 | 4.88 | 79.38 | 6 | 2 | 336.81 | 0 |
| 23 | H | H | OCH3 | 388.40 | 4.90 | 79.38 | 6 | 2 | 336.81 | 0 |
| 24 | OCH3 | H | OCH3 | 418.43 | 4.89 | 88.61 | 7 | 2 | 362.35 | 0 |
The data was determined with Molinspiration calculation software. MW: Molecular weight, logP: Octanol/water partition coefficient, tPSA: Topological polar surface area, HBA: Number of hydrogen acceptor, HBD: Number of hydrogen donor, Vol: Molecular volume, Vio: Number of violations.
3.4. Metabolic Stability and Structure Activity Relationship Studies
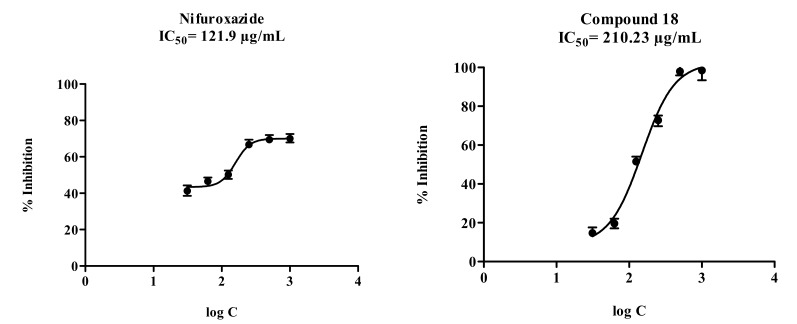
The synthesized compounds were designed to bear various patterns of substituents. For this purpose, -NO2, -Cl, -F, -CH3 and -OCH3 substituents, possessing different electronic characters were preferred. We evaluated the effects of various substituents and osition effects on antimicrobial activity. Only compound 18 showed significant antimicrobial activity while the other com-pounds remained inactive. This unexpected finding created a very difficult situation from which to carry out clear evaluations of structure-activity relationships. Nevertheless, we attempted to explain this interesting result by hypothesizing that the antimicrobial activity of compound 18 is related to its cytotoxic action. However, cytotoxicity studies revealed that the antibacterial activity of compound 18 is not due to general toxicity but can be ascribed to the selectivity against bacteria. Then we focused on the probable stability distance of the compounds 6–24. It is well known that hydrazones are essential compounds for prodrug designs owing to their poor metabolic stability, and it has been reported that the hydrazone moiety is usually not stable in vivo and in vitro. The hydrolytic stability of hydrazones, therefore, is related to substi-tuents in their chemical structure [69-71]. The active compound 18, which carries a 2-methyl substituent on the benzylidene sub-structure, as well as the inactive compounds 7, 11, 14, 19, 20 and 21, which contain 2-chloro, 2-nitro, 2-fluoro, 3-methyl, 4-methyl, and 2-methoxy, respectively, were again subjected to an antimicro-bial assay using Escherichia coli O157:H7 strain. After the incubation period, the inocula containing the synthesized compounds were analyzed in a liquid chromatography/mass spectrometry-ion trap-time of flight (LCMS-IT-TOF) system. The aim was to determine whether there is a structural change in any of the compounds as a consequence of bacterial metabolic activity. No change between the spectra of compound 18 before and after incubation with bacteria was observed and there was no new peak assigned for a probable metabolite of compound 18 as seen in Fig. 2. Similar findings were obtained for the other tested compounds 7, 11, 14, 19, 20 and 21, respectively. These results suggest that the hydrazones reported in the present study have in vitro stability against bacterial metabolic activity.
Fig. (2).
LCMS-IT-TOF chromatogram and spectra of the compound 18.
A: The LC-MS chromatogram of compound 18 before incubation with E. coli O157:H7.
B: The positive ionisation HR-MS spectra of compound 18 before incubation with E. coli O157:H7.
C:The LC-MS chromatogram of Escherichia coli O157:H7inocula with compound 18 after incubation.
D: The positive ionisation HR-MS spectra of E. coli O157:H7 inocula with compound 18 after incubation.
Another possible way to explain the significant antibacterial activity of compound 18 is through electronic parameters, which may have an influence on biological activity. The electronic constants of substituents reported by Hansch et al., [72] were used in this study as reported in Table 3, where only the methyl group has a negative field effect (−0.04). This suggests that only the methyl group has an inductive electron donating ability among the tested substituents. Moreover, it is known that Hammett substituent constants cannot be measured for ortho- substituents since these substituents have steric effects [73]. Therefore, it may be suggested that due to steric hindrance property of the 2-methyl substituent in compound 18, this compound is more active than the 3- and 4-methyl substituted compounds 19 and 20 against Escherichia coli.
Table 3.
Some electronic constants of the substituents in the compounds 6-24.
| Compound | Substituent | σm | σp | F | R |
|---|---|---|---|---|---|
| 6 | H | - | - | - | - |
| 7 | o-Cl | - | - | 0.41 | -0.15 |
| 8 | m-Cl | 0.37 | - | 0.41 | -0.15 |
| 9 | p-Cl | - | 0.23 | 0.41 | -0.15 |
| 10 | o,p-DiCl | - | 0.23 | 0.41 | -0.15 |
| 11 | o-NO2 | - | - | 0.67 | 0.16 |
| 12 | m-NO2 | 0.71 | - | 0.67 | 0.16 |
| 13 | p-NO2 | - | 0.78 | 0.67 | 0.16 |
| 14 | o-F | - | - | 0.43 | -0.34 |
| 15 | m-F | 0.34 | - | 0.43 | -0.34 |
| 16 | p-F | - | 0.06 | 0.43 | -0.34 |
| 17 | o,p-DiF | - | 0.06 | 0.43 | -0.34 |
| 18 | o-CH3 | - | - | -0.04 | -0.13 |
| 19 | m-CH3 | -0.07 | - | -0.04 | -0.13 |
| 20 | p-CH3 | - | -0.17 | -0.04 | -0.13 |
| 21 | o-OCH3 | - | - | 0.26 | -0.51 |
| 22 | m-OCH3 | 0.12 | - | 0.26 | -0.51 |
| 23 | p-OCH3 | - | -0.27 | 0.26 | -0.51 |
| 24 | o,p-DiOCH3 | - | -0.27 | 0.26 | -0.51 |
σm: Hammett substituent constant for meta position; σp: Hammett substituent constant for para position; F: Field effect constant; R: Resonance effect constant
Molecular Modelling Studies
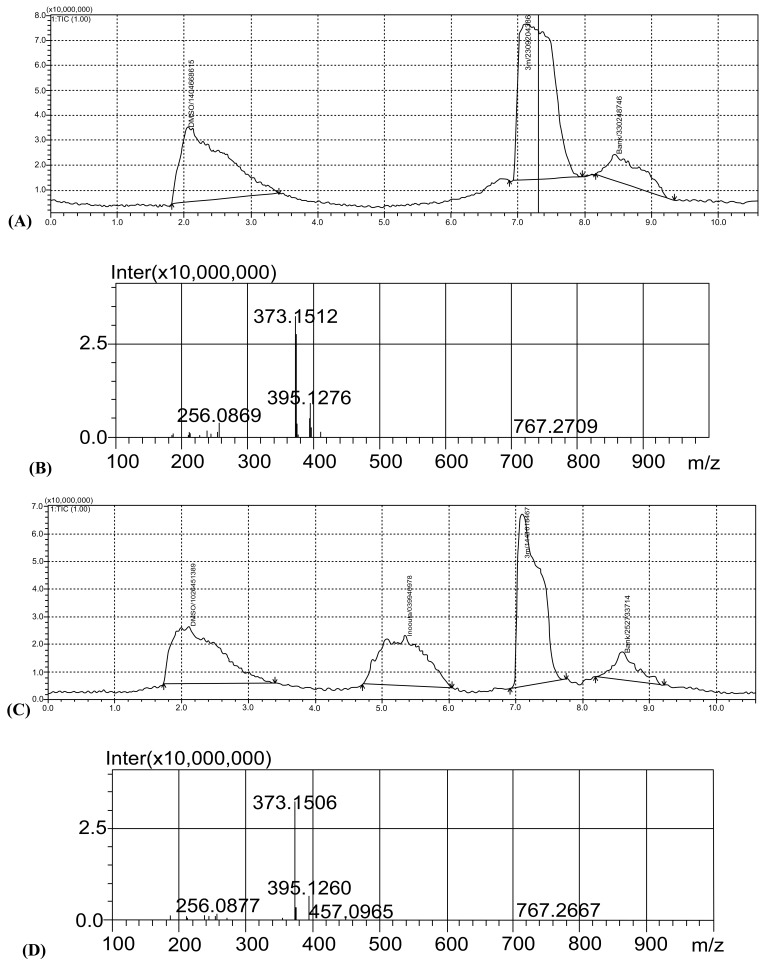
Substrate inhibition by adenosine triphosphate (ATP) is a regulatory feature of the phosphofructokinase isoenzymes (PFK-1 and PFK-2) of Escherichia coli. Under gluconeogenic conditions, the loss of this regulation of PFK-2 causes substrate cycling of fructose-6-phosphate (fructose-6-P) and futile consumption of ATP, delaying growth [74]. Thus, docking studies were carried out to find a possible binding mode for compound 18 with PFK-2. The structure of the enzyme was gained from the Protein Data Bank (PDB ID: 3CQD) [75,76]. Compound 18 was docked into the active site of the enzyme, and low energy docked coordinates were selected to determine the most possible interactions with the enzyme. The best docking pose, showing residues in the active site, is presented in Fig. (3). The docking study suggests that compound 18 is very compatible with the active site pocket of PFK-2. It interacts with the amino acids Lys27, Arg29, Gly38, Gly40, Val107, Ser139, Ser168, Glu170, Lys189, Glu190, Ala193, Val252 and Leu294. The nitrogen atom of the benzimidazole ring system results in the formation of a hydrogen bond with the carbonyl group of Gly40. The phenyl group, in the middle of the structure, settles down in π-π stacking with Lys27. There is another hydrogen bond between the oxygen atom of the carbonyl group and the hydroxyl group of Ser168. It is also thought that a van der Waals interaction between the 2-methyl group of compound 18, and the active region of the enzyme provides a steadier binding, hence enabling compound 18 to bind to the pocket. The compounds 19, 20 and 21, which bear 3-methyl, 4-methyl and 2-methoxy substituents respectively were also docked with PFK-2. However, none of the compounds settled in the active site pocket.
Fig. (3).
The binding of compound 18 at the active site of Pfk-2enzyme. The amino acids were determined as follows: Lys27, Arg29, Gly38, Gly40, Val107, Ser139, Ser168, Glu170, Lys189, Glu190, Ala193, Val252, Leu294.
CONCLUSION
In conclusion, antimicrobial activity screening of the new fluoro-benzimidazole derivatives displayed the potency of compound 18 as an intestinal antiseptic. Cytotoxicity, ADME prediction and molecular docking studies also supported this suggestion. In addition, findings of the present study may have an impact on medicinal chemists, stimulating them to synthesize more effective compounds bearing chemical structures similar to that of compound 18.
ACKNOWLEDGEMENTS
This study was financially supported by Anadolu University Scientific Projects Fund, Project No: 1502S060 and 1605S316. J. Bueno was supported by the Tubitak 2221- Fellowship Program for Visiting Scientists.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Javetz E., Melnick J.L., Adelberg E.A. Review of Medical Microbiology. Lange, California: 1984. p. 122. [Google Scholar]
- 2.Mitscher L.A., Pillai S.P., Gentry E.J., Shankel D.M. Multiple drug resistance. Med. Res. Rev. 1999;19(6):477–496. doi: 10.1002/(sici)1098-1128(199911)19:6<477::aid-med2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Grare M., Mourer M., Fontanay S., Regnouf-de-Vains J.B., Finance C., Duval R.E. In vitro activity of para-guanidinoethylcalix [4]arene against susceptible and antibiotic-resistant Gram-negative and Gram-positive bacteria. J. Antimicrob. Chemother. 2007;60(3):575–581. doi: 10.1093/jac/dkm244. [DOI] [PubMed] [Google Scholar]
- 4.Jorge S.D., Masunari A., Rangel-Yagui C.O., Pasqualoto K.F., Tavares L.C. Design, synthesis, antimicrobial activity and molecular modeling studies of novel benzofuroxan derivatives against Staphylococcus aureus. Bioorg. Med. Chem. 2009;17(8):3028–3036. doi: 10.1016/j.bmc.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 5.DiMasi J.A., Hansen R.W., Grabowski H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. 2003;22(2):151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 6.Gök Y, Akkoç S, Erdoğan H, Albayrak S. In vitro antimicrobial studies of new benzimidazolium salts and silver N-heterocyclic carbene complexes. J Enzyme Inhib Med Chem. 2016:1–6. doi: 10.3109/14756366.2015.1132210. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes M.B., Goncalves J.E., Scotti M.T., De Oliveira A.A., Tavares L.C., Storpirtis S. Caco-2 cells cytotoxicity of nifuroxazide derivatives with potential activity against methicillin-resistant Staphylococcus aureus. Toxicol. In Vitro. 2012;26(3):535–540. doi: 10.1016/j.tiv.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Alasmary F.A., Snelling A.M., Zain M.E., Alafeefy A.M., Awaad S., Karodia N. Synthesis and evaluation of selected benzimidazole derivatives as potential. Antimicrob Agents Mol. 2015;20(8):15206–15223. doi: 10.3390/molecules200815206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chornous V.A., Bratenko M.K., Vovk M.V., Sidorchuk I.I. Synthesis and antimicrobial activity of pyrazole-4-carboxylic acid hydrazides and N-(4-pyrazoyl) hydrazones of aromatic and heteroaromatic aldehyde. Pharm. Chem. J. 2001;35(4):26–28. [Google Scholar]
- 10.Alsaeedi H.S., Aljaber N.A., Ara I. Synthesis and investigation of antimicrobial activity of some nifuroxazide analogues. Asian J. Chem. 2015;27(10):3639. [Google Scholar]
- 11.Velezheva V., Brennan P., Ivanov P., et al. Synthesis and antituberculosis activity of indole–pyridine derived hydrazides, hydrazide–hydrazones, and thiosemicarbazones. Bioorg. Med. Chem. Lett. 2016;26(3):978–985. doi: 10.1016/j.bmcl.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Altıntop M.D., Özdemir A., Turan-Zitouni G., et al. Synthesis and in vitro evaluation of new nitro-substituted thiazolyl hydrazone derivatives as anticandidal and anticancer agents. Molecules. 2014;19(9):14809–14820. doi: 10.3390/molecules190914809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rollas S., Küçükgüzel S.G. Biological activities of hydrazone derivatives. Molecules. 2007;12(8):1910–1939. doi: 10.3390/12081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim H.S., Abou-seri S.M., Ismail N.S., Elaasser M.M., Aly M.H., Abdel-Aziz H.A. Bis-isatin hydrazones with novel linkers: Synthesis and biological evaluation as cytotoxic agents. Eur. J. Med. Chem. 2016;108:415–422. doi: 10.1016/j.ejmech.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Şenkardeş S., Kaushik-Basu N., Durmaz İ., et al. Synthesis of novel diflunisal hydrazide–hydrazones as anti-hepatitis C virus agents and hepatocellular carcinoma inhibitors. Eur. J. Med. Chem. 2016;108:301–308. doi: 10.1016/j.ejmech.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Souza L.G., Almeida M.C., Lemos T.L., et al. Synthesis, antibacterial and cytotoxic activities of new biflorin-based hydrazones and oximes. Bioorg. Med. Chem. 2016;26(2):435–439. doi: 10.1016/j.bmcl.2015.11.095. [DOI] [PubMed] [Google Scholar]
- 17.De Azambuja Carvalho P.H., Duval A.R., Manzolli Leite F.R., Nedel F., Cunico W., Lund R.G. (7-Chloroquinolin-4-yl) arylhydrazones: Candida albicans enzymatic repression and cytotoxicity evaluation, Part 2. J. Enzyme Inhib. Med. Chem. 2016;31(1):126–131. doi: 10.3109/14756366.2015.1010527. [DOI] [PubMed] [Google Scholar]
- 18.Puskullu M.O., Shirinzadeh H., Nenni M., Gurer-Orhan H., Suzen S. Synthesis and evaluation of antioxidant activity of new quinoline-2-carbaldehyde hydrazone derivatives: bioisosteric melatonin analogues. J. Enzyme Inhib. Med. Chem. 2016;31(1):121–125. doi: 10.3109/14756366.2015.1005012. [DOI] [PubMed] [Google Scholar]
- 19.Imran S., Taha M., Ismail N.H., et al. Synthesis of novel flavone hydrazones: in-vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. Eur. J. Med. Chem. 2015;105:156–170. doi: 10.1016/j.ejmech.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Velezheva V., Brennan P., Ivanov P., et al. Synthesis and antituberculosis activity of indole–pyridine derived hydrazides, hydrazide–hydrazones, and thiosemicarbazones. Bioorg. Med. Chem. 2016;26(3):978–985. doi: 10.1016/j.bmcl.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 21.Prasanna V.L., Narender R. Synthesis and antimicrobial activity of new imidazole-hydrazone derivatives. Asian J. Chem. 2015;27(10):3605. [Google Scholar]
- 22.Spasov A.A., Yozhitsa I.N., Bugaeva L.I., Anisimova V.A. Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharm. Chem. J. 1999;33(5):232–243. [Google Scholar]
- 23.Arjmand F., Mohani B., Ahmad S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu (II) complex. Eur. J. Med. Chem. 2005;40(11):1103–1110. doi: 10.1016/j.ejmech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Shaker Y.M., Omar M.A., Mahmoud K., et al. Synthesis, in vitro and in vivo antitumor and antiviral activity of novel 1-substituted benzimidazole derivatives. J. Enzyme Inhib. Med. Chem. 2015;30(5):826–845. doi: 10.3109/14756366.2014.979344. [DOI] [PubMed] [Google Scholar]
- 25.Tonelli M., Boido V., La Colla P., et al. Pharmacophore modeling, resistant mutant isolation, docking, and MM-PBSA analysis: combined experimental/computer-assisted approaches to identify new inhibitors of the bovine viral diarrhea virus (BVDV). Bioorg. Med. Chem. 2010;18(6):2304–2316. doi: 10.1016/j.bmc.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 26.Desai N.C., Kotadiya G.M. Microwave-assisted synthesis of benzimidazole bearing 1,3,4-oxadiazole derivatives: Screening for their in vitro antimicrobial activity. Med. Chem. Res. 2014;23(9):4021–4033. [Google Scholar]
- 27.Desai N.C., Shihory N., Bhatt M., Patel B., Karkar T. Studies on antimicrobial evaluation of some 1-((1-(1H-benzo [d]imidazol-2-yl) ethylidene) amino)-6-((arylidene) amino)-2-oxo-4-phenyl-1, 2-dihydropyridine-3, 5-dicarbonitriles. Synth. Commun. 2015;45(23):2701–2711. [Google Scholar]
- 28.Desai N.C., Kotadiya G.M. Synthesis, antimicrobial, and cytotoxic activities of novel benzimidazole derivatives bearing cyanopyridine and 4-thiazolidinone motifs. Med. Chem. Res. 2014;23(8):3823–3835. [Google Scholar]
- 29.Subhashini N.J., Boddu L., Amanaganti J. Synthesis, characterization, and antimicrobial activity of new bis-1, 2, 3-triazol-H-yl-substituted 2-arylbenzimidazoles. Russ. J. Gen. Chem. 2014;84(7):1442–1449. [Google Scholar]
- 30.Montalvão S., Leino T.O., Kiuru P.S., Lillsunde K.E., Yli‐Kauhaluoma J., Tammela P. Synthesis and biological evaluation of 2‐amino-benzothiazole and benzimidazole analogs based on the clathrodin structure. Arch Pharm Chem Life Sci. 2016;349:137–149. doi: 10.1002/ardp.201500365. [DOI] [PubMed] [Google Scholar]
- 31.Joshi D., Parikh K. Synthesis and evaluation of novel benzimidazole derivatives as antimicrobial agents. Med. Chem. Res. 2014;23(3):1290–1299. [Google Scholar]
- 32.Jiang L., Wang M.Y., Wan F.X., Qu Z.Q. Synthesis and biological activity of tri-substituted 1,2,4-triazoles bearing benzimidazole moiety. Phosphorus Sulfur. 2015;190(10):1599–1605. [Google Scholar]
- 33.Mavrova A.T., Yancheva D., Anastassova N., et al. Synthesis, electronic properties, antioxidant and antibacterial activity of some new benzimidazoles. Bioorg. Med. Chem. 2015;23(19):6317–6326. doi: 10.1016/j.bmc.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Keller P., Müller C., Engelhardt I., et al. An antifungal benzimidazole derivative inhibits ergosterol biosynthesis and reveals novel sterols. Antimicrob. Agents Chemother. 2015;59(10):6296–6307. doi: 10.1128/AAC.00640-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soodamani V., Patel D., Nayakanti D., Josyula R. An efficient synthesis of novel 2-(5-indolyl)-1H-benzimidazole derivatives and evaluation of their antimicrobial activities. J. Heterocycl. Chem. 2015;52(5):1457–1466. [Google Scholar]
- 36.Jiang Y., Han Q., Shen R., Zang X., Wang B. Synthesis and antimicrobial activity of some new 4H-pyrrolo [1, 2-a] benzimidazoles. Chem. Res. Chin. Univ. 2014;30(5):755–758. [Google Scholar]
- 37.Desai N.C., Pandya D.D., Kotadiya M.G., Desai P. Synthesis of pyridyl benzimidazoles encompassing 4-thiazolidinone derivatives as potential antimicrobial agents. Lett. Drug Des. Discov. 2013;10(10):942–950. [Google Scholar]
- 38.Vashist N., Sambi S.S., Kumar P., Narasimhan B. Development of QSAR for antimicrobial activity of substituted benzimidazoles. Drug Res. 2015;65(5):225–230. doi: 10.1055/s-0034-1371828. [DOI] [PubMed] [Google Scholar]
- 39.Tonelli M., Novelli F., Tasso B., et al. Antiviral activity of benzimidazole derivatives. III. Novel anti-CVB-5, anti-RSV and anti-Sb-1 agents. Bioorg. Med. Chem. 2014;22(17):4893–4909. doi: 10.1016/j.bmc.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 40.Altintop MD, Mohsen UA, Ozkay Y, Demirel R, Kaplancikli ZA. Synthesis and antimicrobial activity of benzimidazole-based acetamide derivatives. Turk J Pharm Sci. 2015;12(1) [Google Scholar]
- 41.Yurttas L., Ozkay Y., Karaca H., Tunali Y., Kaplancıkli Z.A. Synthesis and antimicrobial evaluation of some 2,5-disubstituted benzimidazole derivatives. Lett. Drug Des. Discov. 2013;10(6):486–491. [Google Scholar]
- 42.Ozkay Y., Tunalı Y., Karaca H., Işıkdağ İ. Antimicrobial activity of a new series of benzimidazole derivatives. Arch. Pharm. Res. 2011;34(9):1427–1435. doi: 10.1007/s12272-011-0903-8. [DOI] [PubMed] [Google Scholar]
- 43.Ozkay Y., Tunalı Y., Karaca H., Işıkdağ İ. Antimicrobial activity of a new combination system of benzimidazole and various azoles. Arch. Pharm. (Weinheim) 2011;344(4):264–271. doi: 10.1002/ardp.201000172. [DOI] [PubMed] [Google Scholar]
- 44.Ozkay Y., Tunalı Y., Karaca H., Işıkdağ İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur. J. Med. Chem. 2010;45(8):3293–3298. doi: 10.1016/j.ejmech.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Ozkay Y., Tunali Y., Karaca H., Isikdag I. Synthesis and antimicrobial activity of some novel benzimidazole hydrazides. Asian J. Chem. 2011;23(4):1503. [Google Scholar]
- 46.Cichero E., Ligresti A., Allarà M., et al. Homology modeling in tandem with 3D-QSAR analyses: a computational approach to depict the agonist binding site of the human CB2 receptor. Eur. J. Med. Chem. 2011;46(9):4489–4505. doi: 10.1016/j.ejmech.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Di Braccio M., Grossi G., Signorello M.G., et al. Synthesis, in vitro antiplatelet activity and molecular modelling studies of 10-substituted 2-(1-piperazinyl) pyrimido [1,2-a]benzimidazol-4(10H)-ones. Eur. J. Med. Chem. 2013;62:564–578. doi: 10.1016/j.ejmech.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 48.Zorzi R.R., Jorge S., Palace-Berl D.F., et al. Exploring 5-nitrofuran derivatives against nosocomial pathogens: synthesis, antimicrobial activity and chemometric analysis. Bioorg. Med. Chem. 2014;22(10):2844–2854. doi: 10.1016/j.bmc.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Abdel-Aziz H.A., Eldehna W.M., Fares M., et al. Synthesis, biological evaluation and 2D-QSAR study of halophenyl bis-hydrazones as antimicrobial and antitubercular agents. Int. J. Mol. Sci. 2015;16(4):8719–8743. doi: 10.3390/ijms16048719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clinical and Laboratory Standards Institute Susceptibility testing of mycobacteria, nocardiae, and other aerobic - actinomycetes; Approved Standard-Second Edition. . CLSI document M24-A. . [PubMed] [Google Scholar]
- 51.Clinical and Laboratory Standards Institute Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard-Ninth Edition. . CLSI document M07-A9. [Google Scholar]
- 52.EUCAST Definitive Document EDef 7.1: Method for the determination of broth dilution mics of antifungal agents for fermentative yeasts. . doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 53.Zıtounı G.T., Altıntop M.D., Özdemir A., Kaplancıklı Z.A., Dikmen M. Synthesis of some hydrazone derivatives bearing purine moiety as anticancer agents. Turk J Pharma Sci. 2014;11(1):55–66. [Google Scholar]
- 54.Bratislava C.M. Cheminformatics M. Bratislava, Slovak Republic, http://www.molinspiration.com/services/properties.html. (accessed Feb 2016). [Google Scholar]
- 55.Trott O., Olson A.J. AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimi-zation and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ADT/PMV/ViewerFramework Michel F. Sanner. Python: a programming language for software integration and development. J. Mol. Graph. Model. 1999;17:57–61. [PubMed] [Google Scholar]
- 57. The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC. [Google Scholar]
- 58.Navarrete‐Vázquez G., Moreno‐Diaz H., Estrada‐Soto S., et al. Microwave‐assisted one‐pot synthesis of 2‐(substituted phenyl)‐1H‐benzimidazole derivatives. Synth. Commun. 2007;37(17):2815–2825. [Google Scholar]
- 59.Navarrete-Vázquez G., Moreno-Diaz H., Aguirre-Crespo F., et al. Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbene bioisosteres. Bioorg. Med. Chem. 2006;16(16):4169–4173. doi: 10.1016/j.bmcl.2006.05.082. [DOI] [PubMed] [Google Scholar]
- 60.Borra R.C., Lotufo M.A., Gagioti S.M., Barros F.D., Andrade P.M. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009;23(3):255–262. doi: 10.1590/s1806-83242009000300006. [DOI] [PubMed] [Google Scholar]
- 61.Palomino J.C., Martin A., Camacho M., Guerra H., Swings J., Portaels F. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002;46(8):2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rangel J.M., Sparling P.H., Crowe C., Griffin P.M., Swerdlow D.L. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg. Infect. Dis. 2005;11:604–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pozzolini M., Scarfì S., Benatti U., Giovine M. Interference in MTT cell viability assay in activated macrophage cell line. Anal. Biochem. 2003;313(2):338–341. doi: 10.1016/s0003-2697(02)00631-0. [DOI] [PubMed] [Google Scholar]
- 64.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 65.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;19:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 66.Testa B., Crivori P., Reist M., Carrupt P.A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: concepts and examples. Perspect. Drug Discov. Des. 2000;19(1):179–11. [Google Scholar]
- 67.Patil M., Hunoor R., Gudasi K. Transition metal complexes of a new hexadentate macroacyclic N2O4-donor Schiff base: inhibitory activity against bacteria and fungi. Eur. J. Med. Chem. 2010;45(7):2981–2986. doi: 10.1016/j.ejmech.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 68.Veber D.F., Johnson S.R., Cheng H.Y., Smith B.R., Ward K.W., Kopple K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45(12):2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 69.Kitaev Y.P., Buzykin B.I. The Reactions of Hydrazones. Russ. Chem. Rev. 1972;41(6):495–515. [Google Scholar]
- 70.Gülerman N.N., Oruç E.E., Kartal F., Rollas S. In vivo metabolism of 4-fluorobenzoic acid [(5-nitro-2-furanyl) methylene]hydrazide in rats. Eur. J. Drug Metab. Pharmacokinet. 2000;25(2):103–108. doi: 10.1007/BF03190075. [DOI] [PubMed] [Google Scholar]
- 71.Kömürcü Ş.G., Rollas S., Ülgen M., Gorrod J.W., Çevikbaş A. Evaluation of some arylhydrazones of p-aminobenzoic acid hydrazide as antimicrobial agents and their in vitro hepatic microsomal metabolism. Boll Chim Farmaceutico-Anno. 1995;134(7):281–285. [PubMed] [Google Scholar]
- 72.Imramovsky´ A., Polanc S., Vinšová J., et al. A new modification of anti-tubercular active molecules. Bioorg. Med. Chem. 2007;15(7):2551–2559. doi: 10.1016/j.bmc.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 73.Hansch C., Leo A., Unger S.H., Kim K.H., Xikaitani D., Lien E.J. Aromatic substituent constants for structure-activity correlations. J. Med. Chem. 1973;16(11):1207–1216. doi: 10.1021/jm00269a003. [DOI] [PubMed] [Google Scholar]
- 74.Khan M.T. Recent Trends on QSAR in the pharmaeutical perceptions. Bentham Science Publishers; 2012. [Google Scholar]
- 75.Mushtaq K., Sheikh J.A., Amir M., Khan N., Singh B., Agrewala J.N. Rv2031c of Mycobacterium tuberculosis: a master regulator of Rv2028–Rv2031 (HspX) operon. Fron Microbiol; 2015. p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cabrera R., Baez M., Pereira H.M., Caniuguir A., Garratt R.C., Babul J. The Crystal Complex of Phosphofructokinase-2 of Escherichia coli with Fructose-6-phosphate kinetic and structural analysis of the allosteric atp inhibition. J. Biol. Chem. 2011;286(7):5774–5783. doi: 10.1074/jbc.M110.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]