Abstract
Background:
Tanshinone IIA is a key active ingredient of danshen, which is derived from the dried root or rhizome of Salviae miltiorrhizae Bge. The tanshinone IIA has protective effects against the focal cerebral ischemic injury. However, the underlying mechanisms remain unclear.
Methods:
An in vitro model of cerebral ischemia was established by subjecting cultures of hippocampal neuronal cells to oxygen-glucose deprivation followed by reperfusion (OGD/R). The probes of 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) and 5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolylcarbocyanine,iodide (JC-1) were used to determine the mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) production. Western-blot was used to detect the expression of proteins in HT-22 cells.
Results:
The results of cell proliferative assays showed that the tanshinone IIA attenuated OGD/R-mediated neuronal cell death, with the evidence of increased cell viability. In addition, OGD/R exposure led to increase the levels of intracellular reactive oxygen species (ROS), which were significantly suppressed by tanshinone IIA treatment. Furthermore, tanshinone IIA treatment inhibited elevations in MMP and autophagy following exposure to OGD/R. Additionally, OGD/R promoted cell death with concomitant inhibiting phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/ mammalian target of Rapamycin (mTOR) pathway, which was reversed by tanshinone IIA.
Conclusion:
These results suggest that the tanshinone IIA protects against OGD/R-mediated cell death in HT-22 cells, in part, due to activating PI3K/Akt/mTOR pathway.
Keywords: Tanshinone IIA, cerebral ischemic, reactive oxygen species, mitochondrial membrane potential, neural cells
1. INTRODUCTION
Stroke is one of the most severe diseases which is a leading cause of death around the world. Cerebral ischemic stroke has been accounted for approximately 80% of all strokes [1, 2]. It suggests that the occlusion of a blood vessel by a thrombus, resulting in an immediate loss of the normal supply of oxygen and glucose to cerebral tissue, is the most common cause of ischemic stroke [3, 4]. Currently, several of therapeutic strategies are currently being considered as a means of minimizing the neuronal damage resulting from ischemia, leading to investigate the pathological mechanisms underlying stroke. The oxidative stress has been shown to severely impact the nervous system during the stroke through the generation of reactive oxygen species (ROS), which can result in mitochondrial dysfunction, the loss of DNA integrity, and the misfolding of proteins. The kinase cascade including phosphoinositide 3 kinase (PI3K), protein kinase B (Akt), and the mammalian target of rapamycin (mTOR) involved in oxidative stress in the nervous system, offers exciting prospects for the development of novel and safe clinical treatment avenues for nervous system disorders [5-7].
Danshen is a very important component of Chinese medicine derived from the dried root or rhizome of Salviae miltiorrhizae Bge. The danshen has been widely used in China for the treatment of cerebrovascular conditions in recent years, such as ischemic stroke [8]. Tanshinone IIA is a key active component of danshen [9]. Recent studies have demonstrated that tanshinone IIA has been shown to have protective effects against focal cerebral ischemia/reperfusion (I/R) injury in animal models [10-12]. Several studies reported that tanshinone IIA could protect against oxidative stress and cell death by significantly decreasing ROS levels in different system [13-16]. However, the mechanisms underlying tanshinone IIA-mediated protective effects on the cerebral I/R injury are still unclear. Therefore, the present study was intended to evaluate the potentially protective effects of tanshinone IIA in a stroke model in vitro and illuminate the involved mechanisms.
2. MATERIALS AND METHODS
2.1. Materials
Tanshinone IIA was bought from aladdin reagents (Shanghai, China).Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from GIBCO (Los Angeles, CA); Antibodies that including anti-phosphorylated Akt, and anti- phosphorylated p85 were obtained from Cell Signaling (Danvers, MA); anti-B cell lymphoma/lewkmia-2 Associated X protein (Bax), anti- Microtubule-associated protein 1A/1B-light chain 3(LC3) and anti- glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were bought from Abcam (Cambridge, UK); Lipofectamine RNAiMAX was purchased from Invitrogen (Carlsbad, CA); Fluorogenic probe 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) (Invitrogen, Eugene, OR); 5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolylcarbocyanine,iodide (JC-1) probe (Invitrogen, Carlsbad, CA); The small interfering RNAs (siRNAs) of mice protein kinase B (Akt),mammalian target of Rapamycin (mTOR) were synthesized by Gene Pharma (Shanghai, China).
2.2. Cell Culture and Transfection
HT-22 cells (an immortalized mouse hippocampal cell line) were bought from Fu Xiang Biotech (Shanghai, China). HT-22 cells were cultured at 37 °C and 5% CO2 in DMEM with 10% heat-inactivated FBS. Transient transfection with siRNAs of Akt and mTOR was performed with using Lipofectamine RNAiMAX according to the manufacturer's procedures.
2.3. Oxygen and Glucose Deprivation/Reperfusion (OGD/R)
The model for oxygen and glucose deprivation/ reperfusion (OGD/R) was established using the anaeropack method as described previously [17]. Briefly, HT-22 cells were cultured in glucose-free DMEM and then placed inside a sealed air tight container which contains an anaeropack (Mitsubishi Gas Company, Tokyo, Japan), which resulted in a hypoxic atmosphere by absorbing oxygen and generating carbon dioxide. The cells were maintained in hypoxic conditions at 37°C for 6 hours. Thereafter, the medium was discarded, normal DMEM with glucose was added and culturing continued for 6-24 hours of reoxygenation under normoxic condition to produce OGD/R. HT-22 cells cultured in growth culture medium under normoxic condition served as a control.
2.4. Cell Viability Assay
The HT-22 cells (1×104 cells/well) were seeded in 96-well plates with 100 µl culture medium, and were incubated for overnight to allow the cells to attach to the bottom of the plate. The cell viability was analyzed with a microplate reader using cell counting kit-8 (CCK-8, Dojindo, Japan) following the treatments. The treatments included the tanshinone IIA (0.2, 1, 2 and 5 ug/ml) treatments, LY294002 (10 μM) adding and mTOR, Akt siRNAs transfection.
2.5. Measurement of Intracellular Reactive Oxygen Species (ROS) Production
Intracellular ROS levels used the CM-H2DCFDA staining. HT-22 cells were seeded onto 96-well plates similar to the cell viability test. After the indicated treatments, cultured medium were removed and incubated for 30 minutes with CM-H2DCFDA (10 µM) in DMEM. The cells were then washed twice with phosphate buffer saline (PBS) and ROS was visualized by fluorescence microscopy and quantified by a microplate reader (485 nm/528 nm).
2.6. Mitochondrial Membrane Potential (MMP) Analysis
Mitochondrial membrane potential was determined using JC-1 probe. HT-22 cells were seeded in 96-well plates similar to the cell viability test. After treatments, cultured medium were removed, and incubated for 30 min with 10 µg /ml of JC-1 dye (Invitrogen). The cells were washed, visualized and quantified by the fluorescence microscopy and microplate reader (485 nm/535 nm).
2.7. Immunofluorescence Staining
The immunofluorescence assays were performed with the methods we described previously [18]. Briefly, cells were incubated in 4% paraformaldehyde (PFA) for 10 minutes. The LC3 II antibody was then applied at an optimal concentration (1:100) overnight in a wet chamber. The cells were rinsed in PBS and incubated with the secondary antibodies. After washing three times with PBS, the secondary antibody conjugated to Alexa Fluor 488 was applied to these slides for 1 hour at room temperature. The nuclei were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI).
2.8. Western-blot Assay
After treatments, the protein was extracted from HT-22 cells using the radio-immunoprecipitation assay (RIPA) buffer with protease inhibitor cocktail (Pierce). The soluble fraction of the cell lysates was isolated by centrifugation at 12, 000 g for 10 minutes in a microfuge. Bicinchoninic acid (BCA) reagent (Pierce, Rockford, IL, USA) was applied to determine protein concentration. The equal amounts of proteins (30ug/well) were separated by 4-12% dodecyl sulfate, sodium salt (SDS)-Polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes. The membranes were incubated overnight at 4°C with primary antibodies as following: anti-Akt (ser 473), anti-p85, anti-mTOR, anti-Bax, anti-LC3 and anti-GAPDH. After incubation, the membranes were washed with PBS (containing 0.1% Tween) and incubated with horseradish-peroxidase conjugated detected the antigen-antibody complexes using an electrochemiluminescence (ECL) Plus chemiluminescence reagent kit (Pierce, Rockford, IL, USA).
2.9. Statistical Analysis
Values are expressed as the mean±Standard Deviation (SD). The significance of differences between two groups was assessed by Student's t-test. Experiments have more than two groups and different doses, a dose response analysis was using the one way Analysis of Variance (ANOVA). P< 0.05 was considered significant.
3. RESULTS
3.1. Tanshinone IIA Inhibits OGD/R-mediated Cell Death in HT-22 Cells
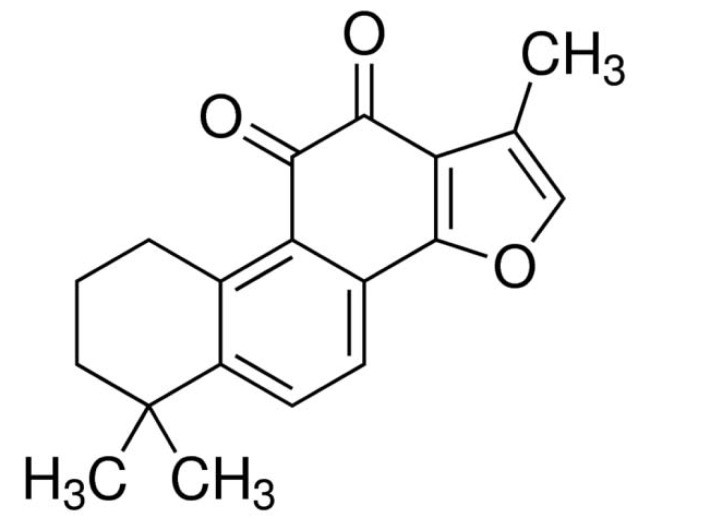
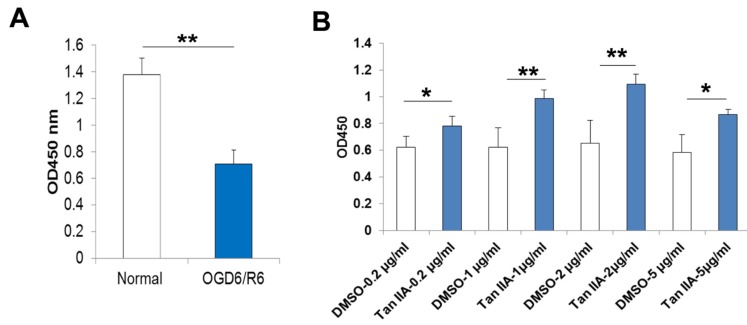
Tanshinone IIA is one of the key components from the Chinese herb Danshen (Radix Salviae Miltiorrhiza Bge) (Fig. 1). In this study, we firstly established an in vitro experimental model of OGD/R using the anaeropack as described previously [17]. HT-22 cells subjected to 6-hours of oxygen and glucose deprivation followed by 6-hours reoxygenation (OGD6/R6) showed a statistically significant 50% decrease in proliferation compared to the normoxia cells (Fig. 2). We further treated the HT-22 cells with different concentrations of tanshinone IIA (0.2-5 µg/ml) following 6-hours of OGD. It showed that treatment of the cells with tanshinone IIA significantly suppressed the OGD6/R6-mediated cell death (Fig. 2).
Fig. (1).
The structure of tanshinone IIA.
Fig. (2).
Protective effect of tanshinone IIA against cell death caused by OGD6/R6. As expected, the CCK-8 proliferative analysis indicated OGD6/R6 caused HT-22 cell death significantly (Normal versus OGD6/R6, 1.37±0.12 versus 0.70±0.10) (A), but this effect is effectively counteracted by tanshinone IIA treatment(0.2-5μg/ml) (B). *, p<0.05,**p<0.01.
3.2. Tanshinone IIA Suppresses OGD/R-Increased Intracellular ROS Production in HT-22 Cells
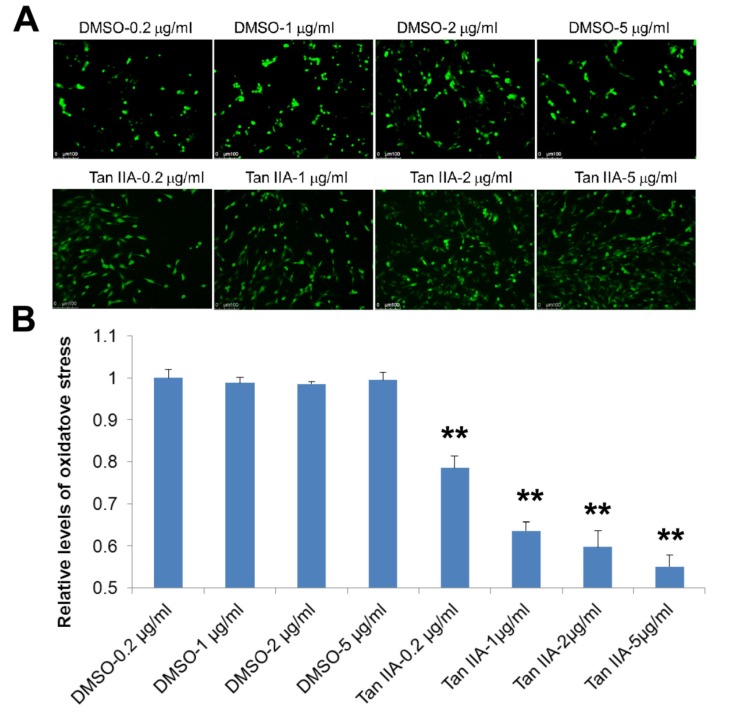
It has been demonstrated that the intracellular ROS production could be induced by OGD/R [19]. We thus evaluated whether tanshinone IIA inhibited OGD/R-mediated cell death through controlling oxidative stress response. To this end, we determine the intracellular ROS production using CM-H2DCFDA probe (CM-H2DCFDA can be oxidized from H2DCF to DCF, which is green fluorescence). As shown in Fig. (3), treatment with tanshinone IIA significantly suppressed the intracellular ROS production following reoxygenation (Fig. 3).
Fig. (3).
The tanshinone IIA treatment inhibits OGD/R-increased intracellular reactive oxygen species (ROS). Tanshinone IIA treatment significantly suppresses the OGD6/R6-increased the intracellular ROS that were detected with a CM-H2DCFDA probe. Representative photographs of fluorescence (A). The fluorescence is determined at 485/528 nm (B). Scale bar represents 50 µm. **p<0.01.
3.3. Tanshinone IIA Suppresses OGD/R-Reduced the Mitochondrial Membrane Potential in HT-22 Cells
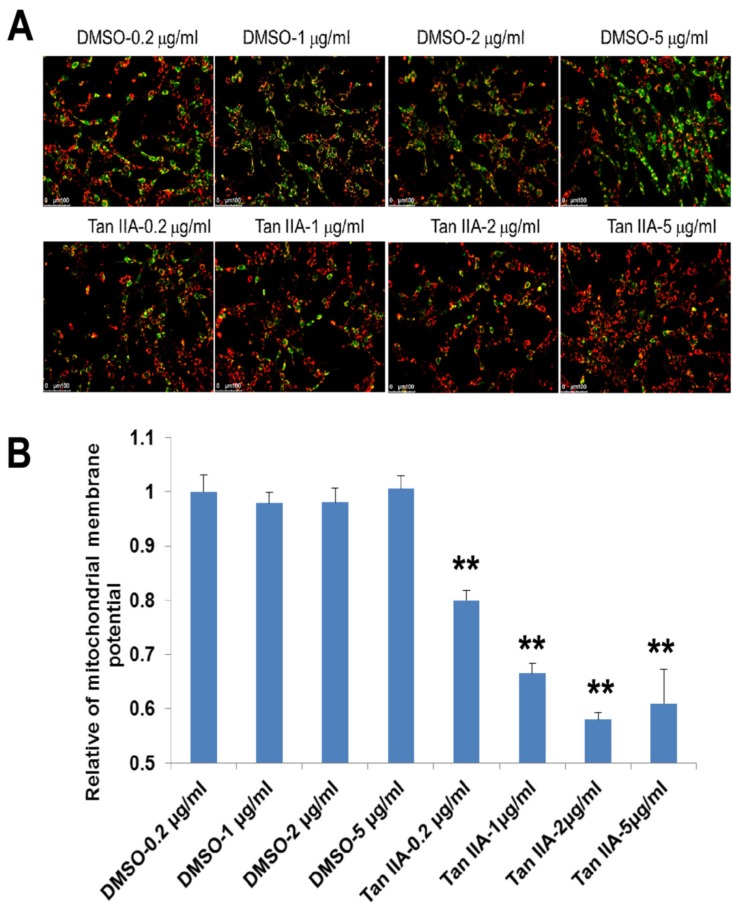
ROS production is known a reason that can cause mitochondrial injury and to disrupt mitochondrial membrane potential (MMP). We here measured MMP using JC-1 staining. The red fluorescence indicates JC-1 existed in the aggregated form in mitochondrial membranes at resting potential. The green fluorescence indicates the existence of free JC-1 at the depolarized MMP. As shown in Fig. (4), the green fluorescence associated with reduction of MMP was markedly suppressed by tanshinone IIA (Fig. 4).
Fig. (4).
Effect of tanshinone IIA treatment on mitochondrial membrane potential (MMP). Tanshinone IIA inhibited decreasing of OGD6/R6-increased MMP that was detected by a JC-1 assay. Representative photographs of fluorescence (A). Quantification of the fluorescence (B) Scale bar represents 50 µm. **p<0.01.
3.4. Tanshinone IIA Suppresses OGD/R-Caused Autophagy in HT-22 Cells
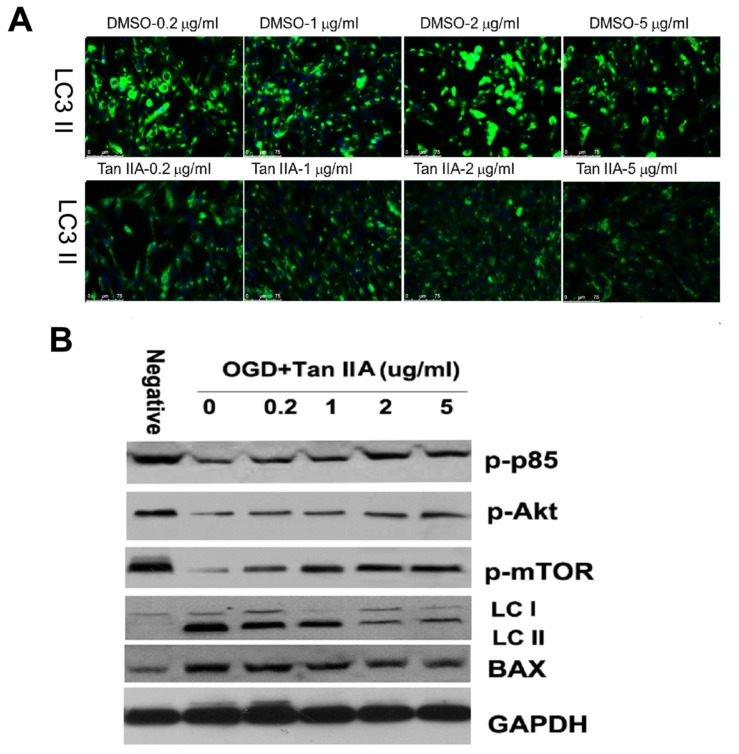
Oxidative stress can activate neural cells autophagy, which helps to break down macromolecules and recycle their components to preserve cellular energy and clear damaged proteins and mitochondria. We here further investigated whether tanshinone IIA exerted its neuroprotection by inhibiting autophagic cell death. As shown in Fig. 5, tanshinone IIA treatment decreased the OGD/R-mediated LC3 II expression in HT-22 cells (Fig. 5). The PI3K/Akt/ mTOR axis plays important roles in autophagy inhibition. It showed that tanshinone IIA activated the PI3K/Akt/mTOR signal pathway by increased key components of this pathway, including the p85, Akt and mTOR (Fig. 5).
Fig. (5).
Effect of tanshinone IIA on OGD/R-caused autophagy in HT-22 cells. Tanshinone IIA inhibited significantly inhibited the expression of Bax and LC3 II but promoted expression of p85, mTOR and Akt (The main components of PI3K/Akt/mTOR pathway). Representative photographs of fluorescence for the autophagy marker LC 3II (A). Western-blots analysis (B) Scale bar represents 50 µm.
3.5. PI3K/Akt/mTOR Signals are Involved in Tanshinone IIA-mediated Protective Effects in OGD/R-Mediated HT-22 Cell Death
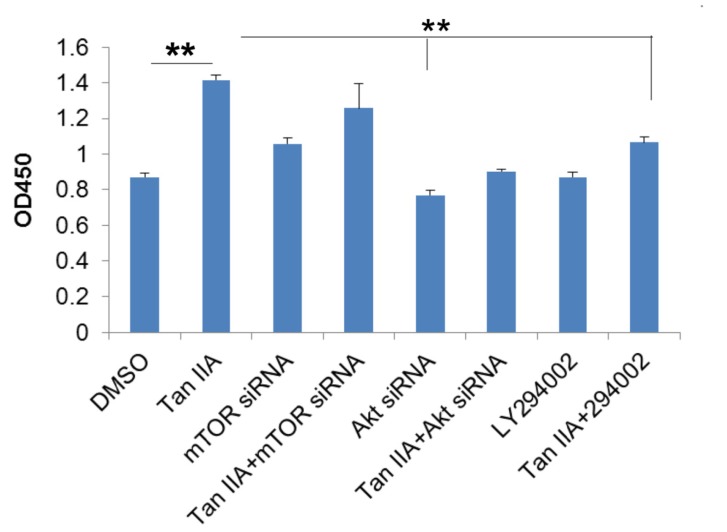
Based on our hypothesis that PI3K/Akt/mTOR are involved in tanshinone IIA-mediated protective role in OGD/R-mediated neural injury, we thus transfected siRNAs that were specific to mTOR or Akt into the HT-22 cells, or treated HT-22 cells with LY294002, and then exposure to OGD6/R6. As shown in Fig. (6), the viability of cells with tanshinone IIA treatment exceeded that of ODG6/R6 treated cells, but cells transfected with Akt siRNA, mTOR siRNA or LY294002 counteracted the effects of tanshinone IIA (Fig. 6).
Fig. (6).
PI3K/Akt/mTOR pathway is essential to tanshinone IIA-mediated protective effects in OGD/R-mediated HT-22 cell death. The CCK-8 assay showed that tanshinone IIA treatment increased the viability of HT-22 cells in OGD/R model, but this effect was abrogated by mTOR siRNA, Akt siRNA transfection or LY294002 treatment. **p<0.01.
3.6. Tanshinone IIA Counteracts OGD/R-Increased Intracellular ROS Levels and Reduced MMP via PI3K/Akt/mTOR Pathway
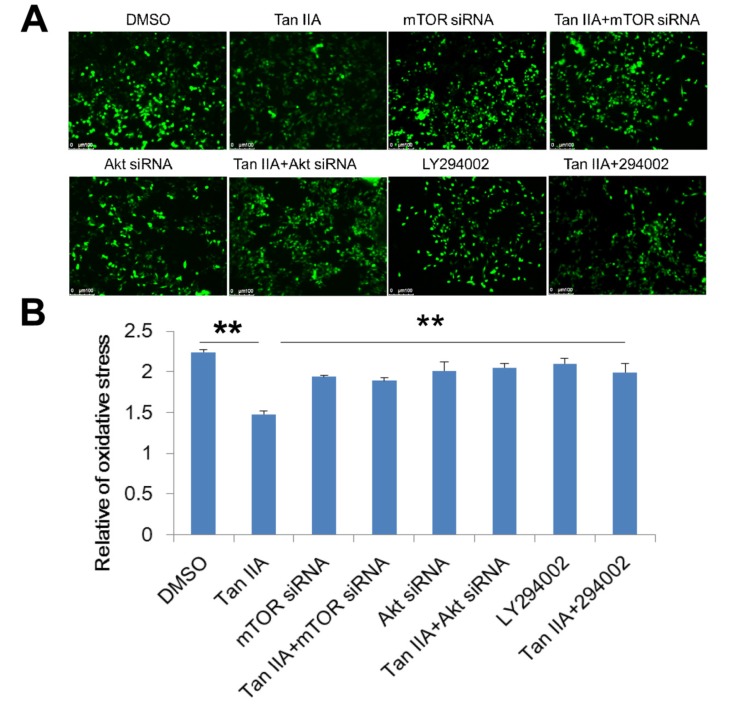
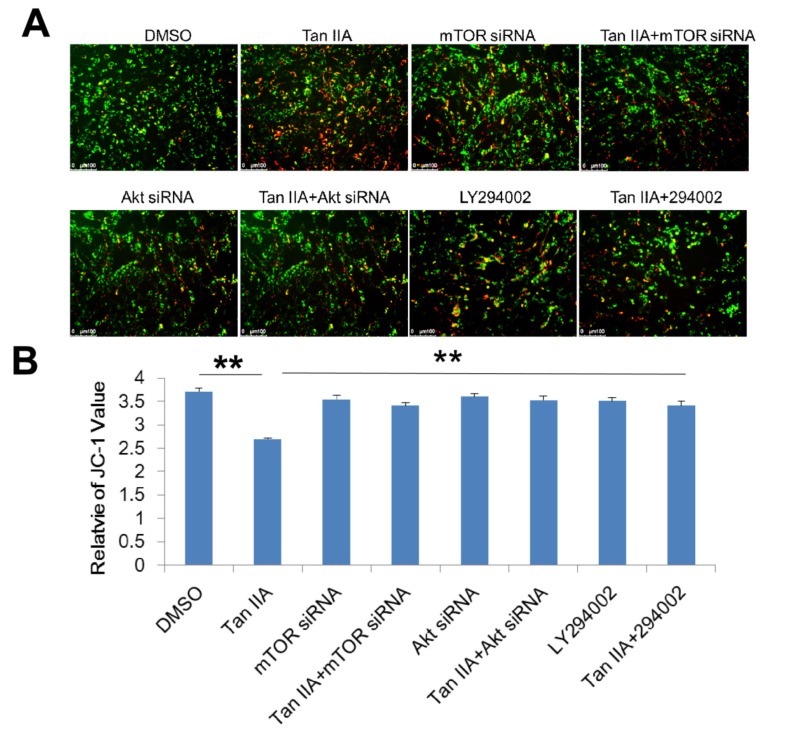
In order to determine whether PI3K/Akt/mTOR pathway participated in the OGD/R-associated changes of intra- cellular ROS levels and MMP, we interfered PI3K/Akt/ mTOR pathway using LY294002 treatment or transfected siRNAs of mTOR and Akt into HT-22 cells and stained them with CM-H2DCFDA and JC-1. As shown in Fig. (7), the HT-22 cells transfected siRNAs of mTOR and Akt, and LY294002 treatment showed significantly higher intracellular ROS production as compared to the cells with tanshinone IIA treatment (Fig. 7). Similarly, the HT-22 cells transfected siRNAs of mTOR and Akt, and LY294002 treatment also increased the tanshinone IIA-mediated reduction of the MMP (Fig. 8).
Fig. (7).
Effects of PI3K/Akt/mTOR pathway on the tanshinone IIA-suppressed intracellular ROS. The mTOR siRNA, Akt siRNA transfection or LY294002 treatment disrupted the inhibitory role of tanshinone IIA in OGD/R-mediated ROS production. Representative photographs of fluorescence (A). The fluorescence is determined at 485/528 nm (B). Scale bar represents 50 µm. **p<0.01.
Fig. (8).
Roles of PI3K/Akt/mTOR pathway on the tanshinone IIA-decreased MMP. The mTOR siRNA, Akt siRNA transfection or LY294002 treatment abrogated the effects of tanshinone IIA on OGD/R-mediated MMP decreasing. Representative photographs of fluorescence (A). Quantification of the fluorescence (B) Scale bar represents 50 µm. **p<0.01.
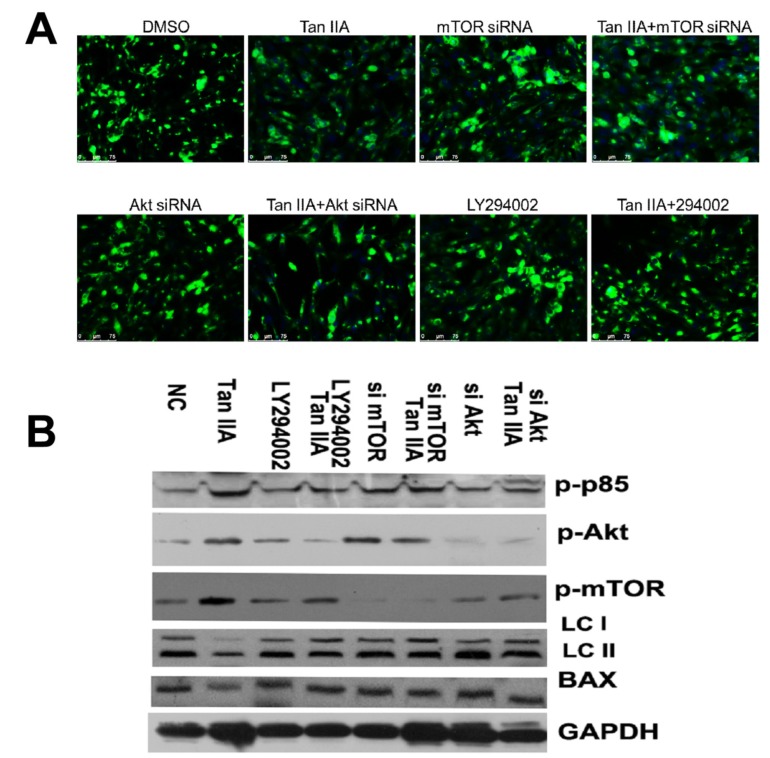
3.7. Tanshinone IIA Inhibits OGD/R-Caused Autophagy in HT-22 Cells via Activating PI3K/Akt/mTOR Pathway
We here investigated whether PI3K/Akt/mTOR pathway activation is vital to tanshinone IIA-mediated inhibitory role in OGD/R-caused autophagy in HT-22 cells. As shown Fig. (9), the the HT-22 cells transfected siRNAs of mTOR and Akt, and LY294002 treatment abrogated the effects that tanshinone IIA-mediated reduction OGD/R-mediated LC3 II expression (Fig. 9). The results of western-blots showed that tanshinone IIA treatment could activate the PI3K/ Akt/mTOR pathway by increased expression of pp85, p-akt and p-mTOR, and repressed the LC3 II expression (Fig. 9).
Fig. (9).
Effect of PI3K/Akt/mTOR pathway on tanshinone IIA-reduced autophagy in HT-22 cells. The mTOR siRNA, Akt siRNA transfection or LY294002 treatment increased expression of LC3 II in the presence of tanshinone IIA, which suggests tanshinone IIA inhibited autophagy was dependent on the PI3K/Akt/mTOR pathway. Representative photographs of fluorescence (A). Western-blots analysis (B) Scale bar represents 50 µm.
4. DISCUSSION
OGD/R is a classical model of cerebral ischemia in vitro [20, 21]. In this study, we have shown that tanshinone IIA can reduce oxidative stress, apoptosis and autophagy that induced by OGD/R treatment in HT-22 cells. We also demonstrated that tanshinone IIA provided neuronal protection partly through the activation of PI3K/Akt/mTOR signal pathway.
Danshen is a very important traditional Chinese medicinal herb that has been commonly used in traditional Chinese medicine practice for many years in the treatment of coronary artery disease and cerebrovascular diseases including stroke [4, 22]. In recent decades, the tanshinone IIA has been widely used in research for the treatment of cerebrovascular disease and has limited or no adverse effects [12, 23, 24]. In this study, we found that tanshinone IIA could suppress the OGD/R-caused cell death via the inhibition of apoptosis and autophagy.
ROS are generated during I/R which are associated with the burst of ROS generation after reperfusion [25]. In this study, the data we presented here demonstrated that tanshinone IIA significantly inhibited OGD/R-caused ROS production in hippocampal neural HT-22 cells. In addition to, an increase in ROS production could cause a reduction of mitochondrial membrane potential and autophagy after exposure to OGD6/R. The PI3K/Akt signaling pathway plays a central role in cell growth and survival. Signaling by PI3K downstream of growth factor receptors is required to suppress apoptosis and to stimulate cell proliferation in most mammalian cell type. Tanshinone IIA protects the different organs or tissues from oxidative stress injury through the PI3K/Akt pathway [13, 26-31]. We here showed that tanshinone IIA could protect HT-22 cell from oxidative stress injury by activating the PI3K/Akt signaling.
Downstream components of PI3K/Akt signaling include the protein kinase mTOR, which is a key regulator of autophagy [32, 33]. Autophagy is a highly conserved metabolic process that permits the degradation and recycling of cellular constituents. The induction of autophagy is seen in mouse striatum and cortex after hypoxic-ischemic injury, and oxidative stress following cerebral hypoxia-ischemia may induce autophagy, which initially aborts apoptosis by eliminating damaged mitochondria and preventing necrosis via catabolic energy production [26, 34-36]. Upstream of mTOR the survival PI3K/AKT pathway modulates mTOR activity that is altered in neurodegenerative diseases and cancer [37]. However, it is unknown whether tanshinone IIA can inhibit OGD/R-caused autophagy through inhibiting PI3K/Akt/mTOR pathway in neural cells. The study clearly demonstrated that tanshinone IIA activated the PI3K/Akt/ mTOR pathway, resulting in the reduction of autophagy marker LC3 II expression and cell death in OGD/R model.
CONCLUSION
Taken together, the present study indicates that the tanshinone IIA might protect neurons through inhibiting apoptosis and autophagy evoked by oxidative stress. These findings have partially revealed the molecular mechanisms underlying neuroprotection of tanshinone IIA. However, we could not exclude the possibility that tanshinone IIA may undergo neuroprotective activities through other pathways. Other supportive experiments need to be performed for further investigation.
ACKNOWLEDGEMENTS
This work was funded by the National Natural Science Foundation (No.81573807).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Paraskevas K.I. Secondary prevention of stroke. Lancet. 2008;372(9643):1036. doi: 10.1016/S0140-6736(08)61438-5. [DOI] [PubMed] [Google Scholar]
- 3.Genovese T., Mazzon E., Paterniti I., Esposito E., Bramanti P., Cuzzocrea S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011;1372:92–102. doi: 10.1016/j.brainres.2010.11.088. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L., Bondy S.C., Jian L., et al. Tanshinone IIA attenuates the cerebral ischemic injury-induced increase in levels of GFAP and of caspases-3 and -8. Neuroscience. 2015;288:105–111. doi: 10.1016/j.neuroscience.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 5.Mao X.Y., Zhou H.H., Li X., Liu Z.Q. Huperzine A alleviates oxidative glutamate toxicity in hippocampal HT22 cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway. Cell. Mol. Neurobiol. 2016;36(6):915–925. doi: 10.1007/s10571-015-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H., Zhong R., Xia Z., Song J., Feng L. Neuroprotective effects of rhynchophylline against ischemic brain injury via regulation of the Akt/mTOR and TLRs signaling pathways. Molecules. 2014;19(8):11196–11210. doi: 10.3390/molecules190811196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J., Li J., Zhang S., et al. IGF-1 induces hypoxia-inducible factor 1alpha-mediated GLUT3 expression through PI3K/Akt/mTOR dependent pathways in PC12 cells. Brain Res. 2012;1430:18–24. doi: 10.1016/j.brainres.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 8.Dong K., Xu W., Yang J., Qiao H., Wu L. Neuroprotective effects of Tanshinone IIA on permanent focal cerebral ischemia in mice. Phytother. Res. 2009;23(5):608–613. doi: 10.1002/ptr.2615. [DOI] [PubMed] [Google Scholar]
- 9.Adams J.D., Wang R., Yang J., Lien E.J. Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin. Med. 2006;1:3. doi: 10.1186/1749-8546-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C., Xue H., Bai C., Fu R., Wu A. The effects of Tanshinone IIA on blood-brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine: Int J Phytother Phytopharmacol. 2010;17(14):1145–1149. doi: 10.1016/j.phymed.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Liu L., Zhang X., Wang L., et al. The neuroprotective effects of Tanshinone IIA are associated with induced nuclear translocation of TORC1 and upregulated expression of TORC1, pCREB and BDNF in the acute stage of ischemic stroke. Brain Res. Bull. 2010;82(3-4):228–233. doi: 10.1016/j.brainresbull.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Wu X., Yu S., et al. Neuroprotective capabilities of Tanshinone IIA against cerebral ischemia/reperfusion injury via anti-apoptotic pathway in rats. Biol. Pharm. Bull. 2012;35(2):164–170. doi: 10.1248/bpb.35.164. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y., Liang Z., Wang H., et al. Tanshinone IIA protects H9c2 cells from oxidative stress-induced cell death via microRNA-133 upregulation and Akt activation. Experiment Ther Med. 2016;12(2):1147–1152. doi: 10.3892/etm.2016.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu H., Zhai C., Qian G., et al. Protective effects of tanshinone IIA on myocardial ischemia reperfusion injury by reducing oxidative stress, HMGB1 expression, and inflammatory reaction. Pharm. Biol. 2015;53(12):1752–1758. doi: 10.3109/13880209.2015.1005753. [DOI] [PubMed] [Google Scholar]
- 15.Yang P., Jia Y.H., Li J., Li L.J., Zhou F.H. Study of anti-myocardial cell oxidative stress action and effect of tanshinone IIA on prohibitin expression. Chung i tsa chih ying wen pan. J. Tradit. Chin. Med. 2010;30(4):259–264. doi: 10.1016/s0254-6272(10)60053-2. [DOI] [PubMed] [Google Scholar]
- 16.Yang R., Liu A., Ma X., Li L., Su D., Liu J. Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J. Cardiovasc. Pharmacol. 2008;51(4):396–401. doi: 10.1097/FJC.0b013e3181671439. [DOI] [PubMed] [Google Scholar]
- 17.Wei R., Zhang R., Xie Y.S. hen L, Chen F. Hydrogen suppresses hypoxia/reoxygenation-induced cell death in hippocampal neurons through reducing oxidative stress. Cell. Physiol. Biochem. 2015;36(2):585–598. doi: 10.1159/000430122. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y., Zhou Y., Chen Y., et al. The expression of epithelial-mesenchymal transition-related proteins in biliary epithelial cells is associated with liver fibrosis in biliary atresia. Pediatr. Res. 2015;77(2):310–315. doi: 10.1038/pr.2014.181. [DOI] [PubMed] [Google Scholar]
- 19.Wu L., Li H.H., Wu Q., et al. Lipoxin A4 activates Nrf2 pathway and ameliorates cell damage in cultured cortical astrocytes exposed to oxygen-glucose deprivation/reperfusion insults. J. Mol. Neurosci. 2015;56(4):848–857. doi: 10.1007/s12031-015-0525-6. [DOI] [PubMed] [Google Scholar]
- 20.Tian X., Peng J., Zhong J., et al. Beta-caryophyllene protects in vitro neurovascular unit against oxygen-glucose deprivation and re-oxygenation-induced injury. J. Neurochem. 2016;56(4):848–857. doi: 10.1111/jnc.13833. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y., Zhang L., Sun S., Yi Z., Jiang X., Jia D. Neuroprotective effects of syringic acid against OGD/R-induced injury in cultured hippocampal neuronal cells. Int. J. Mol. Med. 2016;38(2):567–573. doi: 10.3892/ijmm.2016.2623. [DOI] [PubMed] [Google Scholar]
- 22.Deng Y., Ng E.S., Kwan Y.W., et al. Cerebral vasodilator properties of Danshen and Gegen: A study of their combined efficacy and mechanisms of actions. Phytomedicine: Int J Phytother Phytopharmacol. 2014;21(4):391–399. doi: 10.1016/j.phymed.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Park J.H., Park O., Cho J.H., et al. Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem. Res. 2014;39(7):1300–1312. doi: 10.1007/s11064-014-1312-4. [DOI] [PubMed] [Google Scholar]
- 24.Tang Q., Han R., Xiao H., Shen J., Luo Q., Li J. Neuroprotective effects of tanshinone IIA and/or tetramethylpyrazine in cerebral ischemic injury in vivo and in vitro. Brain Res. 2012;1488:81–91. doi: 10.1016/j.brainres.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Levraut J., Iwase H., Shao Z.H., Vanden Hoek T.L., Schumacker P.T. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am. J. Physiol. Heart Circ. Physiol. 2003;284(2):549–558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 26.Heras-Sandoval D., Perez-Rojas J.M., Hernandez-Damian J., Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014;26(12):2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Li Q., Shen L., Wang Z., Jiang H.P., Liu L.X. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2016;84:106–114. doi: 10.1016/j.biopha.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Yuan X., Jing S., Wu L., Chen L., Fang J. Pharmacological postconditioning with tanshinone IIA attenuates myocardial ischemia-reperfusion injury in rats by activating the phosphatidylinositol 3-kinase pathway. Experiment Ther Med. 2014;8(3):973–977. doi: 10.3892/etm.2014.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su C.C., Chiu T.L. Tanshinone IIA decreases the protein expression of EGFR, and IGFR blocking the PI3K/Akt/mTOR pathway in gastric carcinoma AGS cells both in vitro and in vivo. Oncol. Rep. 2016;36(2):1173–1179. doi: 10.3892/or.2016.4857. [DOI] [PubMed] [Google Scholar]
- 30.Ding L., Wang S., Wang W., et al. Tanshinone IIA affects autophagy and apoptosis of glioma cells by inhibiting phosphatidylinositol 3-kinase/akt/mammalian target of Rapamycin signaling pathway. Pharmacology. 2016;99(3-4):188–195. doi: 10.1159/000452340. [DOI] [PubMed] [Google Scholar]
- 31.Feng J., Li S., Chen H. Tanshinone IIA inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: Possible role of silent information regulator 1. Eur. J. Pharmacol. 2016;791:632–639. doi: 10.1016/j.ejphar.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 32.Hu Z., Yang B., Mo X., Xiao H. Mechanism and regulation of autophagy and its role in neuronal diseases. Mol. Neurobiol. 2015;52(3):1190–1209. doi: 10.1007/s12035-014-8921-4. [DOI] [PubMed] [Google Scholar]
- 33.Zoncu R., Efeyan A., Sabatini D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhami F., Schloemer A., Kuan C.Y. The roles of autophagy in cerebral ischemia. Autophagy. 2007;3(1):42–44. doi: 10.4161/auto.3412. [DOI] [PubMed] [Google Scholar]
- 35.Cao J., Ying M., Xie N., et al. The oxidation states of DJ-1 dictate the cell fate in response to oxidative stress triggered by 4-hpr: autophagy or apoptosis? Antioxid. Redox Signal. 2014;21(10):1443–1459. doi: 10.1089/ars.2013.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu C., Wang X., Xu F., et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12(2):162–176. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]
- 37.Wu J.C., Lai C.S., Badmaev V., Nagabhushanam K., Ho C.T., Pan M.H. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/ Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol. Nutr. Food Res. 2011;55(11):1646–1654. doi: 10.1002/mnfr.201100454. [DOI] [PubMed] [Google Scholar]