Abstract
Introduction
Bisphosphonates are highly effective in reducing fracture risk yet some individuals treated with these agents still experience fracture. The goal of this study was to test the hypothesis that genotype influences the effect of zoledronate on bone mechanical properties.
Methods
Skeletally mature male mice from genetic backgrounds known to have distinct baseline post-yield properties (C57/B6, high post-yield displacement; A/J, low post-yield displacement) were treated for 8 weeks with saline (VEH) or zoledronate (ZOL, 0.06 mg/kg subcutaneously once every four weeks) in a 2×2 study design. Ex vivo µCT and mechanical testing (4-pt bending) were conducted on the femur to assess morphological and mechanical differences.
Results
Significant drug and/or genotype effects were found for several mechanical properties and significant drug × genotype interactions were found for measures of strength (ultimate force) and brittleness (total displacement, strain to failure). Treatment with ZOL affected bone biomechanical measures of brittleness (total displacement (−25%) and strain to failure (−23%)) in B6 mice significantly differently than in A/J mice. This was driven by unique drug × genotype effects on bone geometry in B6 animals yet likely also reflected changes to the tissue properties.
Conclusion
These data may support the concept that properties of the bone geometry and/or tissue at the time of treatment initiation play a role in determining the bone’s mechanical response to zoledronate treatment.
Keywords: bisphosphonate, toughness, mechanical properties, remodeling suppression
INTRODUCTION
Bisphosphonates have consistently been shown to be highly effective in reducing fracture risk [1]. While the majority of individuals experience the anti-fracture benefits of bisphosphonate treatment there is a small population of patients that fail to respond to treatment, defined as either not increasing BMD or experiencing a fracture [2–8]. Gaining a greater understanding of individual variation of response to drug treatment may lead to a more personalized treatment approach for fracture risk reduction.
Bone morphology and material properties exhibit great heterogeneity across the human population [9, 10]. Based on similar heterogeneity across genetic mouse strains [11–13], it has become clear that interactions between bone morphology and material properties (i.e. mineralization, remodeling, etc.) determine a bone’s mechanical properties. At far ends of the spectrum are bones referred to as slender – those with narrow widths relative to the length – and other referred to as robust – those with large widths relative to length. Slender bones (typical of some genetic mouse strains such as A/J) have higher cortical thickness, lower cross-sectional area moments of inertia, higher mineralization and lower remodeling – culminating in stiffer bones but with relatively lower post-yield behavior (i.e., brittle) [13]. Robust bones (typical of other genetic mouse strains such as B6) have lower cortical thickness, higher cross-sectional area moments of inertia, lower mineralization, and higher remodeling – culminating in a comparable whole bone stiffness as the slender mice but with relatively higher post-yield behavior (i.e., ductile)[13]. Despite significant work to understand interactions among morphological and material property traits, surprisingly little has been done to understand how these interactions affect the response to bone targeting drug treatments.
Bisphosphonates have well-described effects on bone remodeling, affecting bone morphology, mineralization, and mechanical properties [14]. Alterations in mechanical properties tend to manifest as improvements in ultimate force and stiffness, at the expense of reduced post-yield properties at the structural and/or material level [14]. In this study we aimed to test the hypothesis that the mechanical effects of zoledronate, one of several bisphosphonates used clinically, differ between inbred strains of mice that have divergent morphologies/mechanical properties (robust versus slender).
METHODS
Animals and bisphosphonate treatment
Prior to initiating these studies, all procedures were approved by the Indiana University School of Medicine Animal Care and Use Committee. Two strains of male mice were used in this study: C57Bl/6J (mouse strain with robust bones, n=40) and A/J (mouse strain with slender bones, n=40). Male mice were chosen as the majority of previous work in these strains used males [11–13]. Animals received a single injection of zoledronic acid (ZOL, subcutaneous injection of 0.06 mg/kg/BW, n=20/strain) or saline vehicle (VEH, n=20/strain) at 16 weeks of age, then another at 20 weeks of age. Treatment initiation at 16 weeks was based on the idea that skeletal growth has slowed by this age. The dose and duration of ZOL was chosen as it has shown efficacy in suppressing remodeling in mice [15]. At 24 weeks of age, animals were euthanized and femurs were removed, wrapped in saline-soaked gauze, and frozen. Four of the bones were damaged during dissection and thus not available for analysis.
µCT Imaging
Femora were scanned (Skyscan 1176, 9 micron resolution) prior to mechanical testing to obtain trabecular morphology (trabecular bone volume) of the distal femur (0.5 mm long segment of the metaphysis region) and the cortical geometry at mid-diaphysis (single slice ROI). Trabecular parameters included bone volume, tissue volume and bone volume/tissue volume (BV/TV). Cortical parameters included total cross-sectional area, bone area, marrow area, cross-sectional moment of inertia, cortical thickness and tissue mineral density. Nomenclature is reported in accordance with suggested guidelines [16].
Mechanical Testing
Whole bone mechanical properties were assessed in four-point bending [17]. As opposed to three point bending which places large shear stresses at the single loading point, four-point bending produces pure bending between the two loading point allowing fracture to occur at the weakest location in this region due to bending [18]. The anterior surface of the each femur was placed on two lower supports with a span length of 9 mm and an upper span length of 3 mm. Specimens were loaded to failure at a rate of 2 mm/min, producing a force-displacement curve for each sample. Structural properties (ultimate force, stiffness, displacement, energy absorption) were determined from the force-displacement curves. Apparent material properties were derived using cross-sectional moments of inertia and the distances from the centroid to the tensile surface using standard beam-bending equations for four-point bending. Yield points were defined using the 0.2% offset criterion. All mechanical analyses were performed using a custom MATLAB (Version 11) program [19].
Statistical Analysis
The 2×2 factor design (genotype × treatment) was assessed using a two-way ANOVA. Body weight values were assessed with a three-way ANOVA (genotype × treatment × time). When significant interactions were present (p ≤ 0.05) simple main effect analyses were conducted. All data are presented as means and standard deviations.
RESULTS
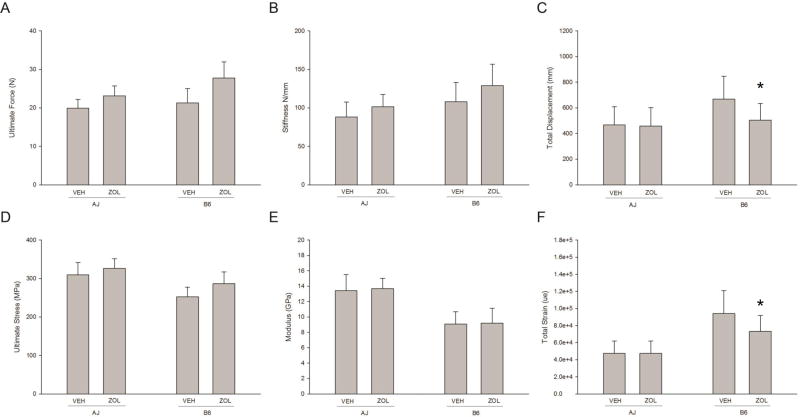
There was a significant effect of genotype on structural and mechanical material properties. B6 animals had significantly higher ultimate force, stiffness, and displacement at the structural level; tissue-level ultimate stress and modulus were significantly lower while total strain and toughness were significantly higher compared to A/J animals (Figure 1 and Table 1). At the structural level, threre was no significant interaction on stiffness, yet a significant effect of drug with ZOL-treated animals being higher than VEH (p=0.0016). There was a significant drug × genotype interaction for ultimate force with both strains having significantly higher values in ZOL treated animals compared to VEH (A/J: +16%, p<0.001 and B6: +30%, p<0.001). There was also a significant interaction for total displacement, with no effect of ZOL in A/J but having a significant negative effect in B6 animals (A/J: −2%, p=0.82 and B6: −25%, p < 0.01).
Figure 1.
Whole bone mechanical properties from femoral four-point bending. (A) Ultimate Force (Genotype p = 0.0001; Drug p = 0.0001; Interaction = 0.0021), (B) Stiffness (Genotype p = 0.0001; Drug p = 0.0016; Interaction = 0.441), (C) Total Displacement (Genotype p = 0.0001; Drug p = 0.0137; Interaction = 0.0304), (D) Ultimate Stress (Genotype p = 0.0001; Drug p = 0.0003; Interaction = 0.1779), (E) Modulus (Genotype p = 0.0001; Drug p = 0.6261; Interaction = 0.9058), (F) Total Strain (Genotype p = 0.0001; Drug p = 0.0242; Interaction = 0.0217), * denotes a significant interaction between genotype and drug existed and within genotype there was a significant drug effect. All data presented as means and standard deviations.
Table 1.
Whole bone structural and estimated material mechanical properties from femoral four-point bending.
| AJ-VEH (N=20) |
AJ-ZOL (N-19) |
B6-VEH (N=20) |
B6-ZOL (N=17) |
Genotype effect |
Drug effect |
Interaction effect |
|
|---|---|---|---|---|---|---|---|
| Yield Force (N) | 17.33 ± 2.83 | 18.72 ± 3.58 | 10.73 ± 3.96 | 17.16 ± 3.1 * | 0.0001 | 0.0001 | 0.0021 |
| Displacement to Yield (mm) | 221.4 ± 48.6 | 205.6 ± 33.0 | 114.9 ± 35.5 | 153.3 ± 45.0 * | 0.0001 | 0.2355 | 0.0055 |
| Post-yield Displacement (mm) | 246.8 ± 148.8 | 251.5 ± 148.4 | 553. ± 187.6 | 350.8 ± 148.0 * | 0.0001 | 0.0092 | 0.0065 |
| Work to Yield (mJ) | 2.13 ± 0.65 | 2.11 ± 0.62 | 0.74 ± 0.39 | 1.48 ± 0.63 * | 0.0001 | 0.0085 | 0.0059 |
| Post-yield Work (mJ) | 4.12 ± 2.4 | 4.65 ± 2.33 | 9.57 ± 3.54 | 8.3 ± 3.64 | 0.0001 | 0.5982 | 0.2062 |
| Total Work (mJ) | 6.25 ± 2.25 | 6.76 ± 2.3 | 10.31 ± 3.41 | 9.79 ± 3.33 | 0.0001 | 0.9929 | 0.4405 |
| Yield Stress (MPa) | 270.3 ± 48.3 | 263.2 ± 42.5 | 130.6 ± 52.9 | 180.5 ± 44.4 * | 0.0001 | 0.0606 | 0.0115 |
| Strain to Yield (µε) | 22337 ± 4435 | 21503 ± 3925 | 16123 ± 4803 | 22293 ± 6657 * | 0.022 | 0.0241 | 0.0035 |
| Resilience (MPa) | 3.37 ± 1.03 | 3.13 ± 0.96 | 1.28 ± 0.77 | 2.28 ± 1.02 * | 0.0001 | 0.0874 | 0.0064 |
| Toughness (MPa) | 10.01 ± 4.1 | 10.04 ± 3.65 | 17.24 ± 5.22 | 14.94 ± 5.29 | 0.0001 | 0.2989 | 0.2772 |
Data presented as means and standard deviations. Two-way ANOVA was performed and the genotype, drug and interaction effects are displayed.
When interactions were significant, simple main effects were evaluated and are noted as * vs VEH within genotpye.
Estimation of material properties based on bone geometry normalization revealed that the different response of the two mouse strains to ZOL treatment was due to both geometry and material properties (Figure 1 and Table 1). There was a significant yet similar effect of ZOL on ultimate stress in both genotypes compared to VEH-treated animals (A/J: +5%, p<0.001 and B6: +14%, p<0.001). Modulus was not affected by ZOL in either genotype. Total strain, the material level equivalent of displacement, displayed a significant interaction with A/J animals being unaffected but with B6 animals showing significantly lower values with ZOL treatment (−23% compared to VEH; p = 0.0016).
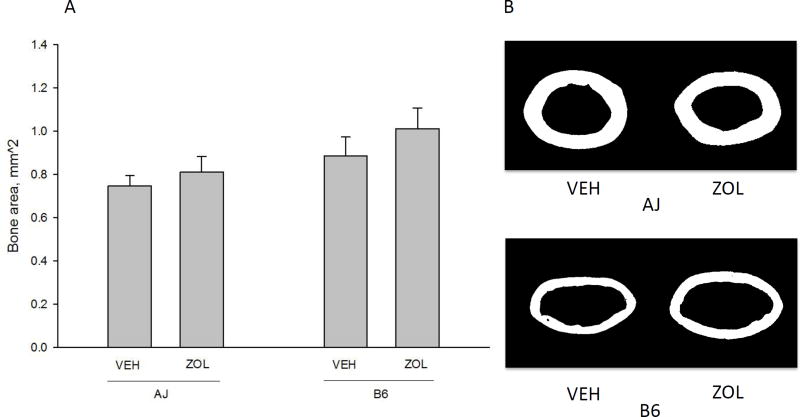
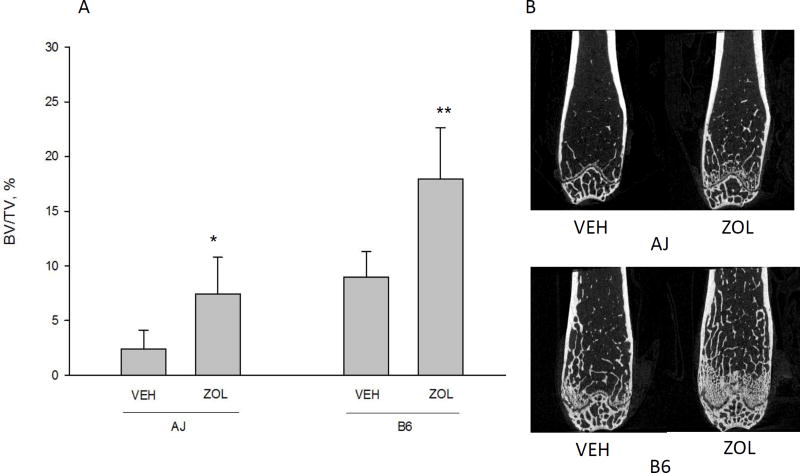
All of the assessed cortical bone geometry variables were significantly different between genotypes (Table 2). Further, total cross-sectional area, bone area (Figure 2), and cross-sectional moment of inertia were all affected similarly by ZOL in the two genotypes. There were no significant interactions for any of the cortical geometry variables (Table 2). B6 animals had significantly higher cortical total cross-sectional area and cortical bone area compared to A/J animals (p<0.0001) and significantly lower tissue material density. ZOL did not alter cortical thickness or tissue material density in either strain but resulted in significantly higher cortical bone area in A/J animals by 8% (p=0.0001) and B6 animals by 14% (p=0.0001). Animals within both genotypes had significantly higher trabecular bone volume/tissue volume (BV/TV) when treated with ZOL (Figure 3). A/J animals had a 207% higher BV/TV (p<0.0001) and B6 animals had a 100% higher BV/TV (p<0.0001) compared to VEH within genotype.
Table 2.
Cortical geometry by microCT
| AJ-VEH (N=20) |
AJ-ZOL (N-19) |
B6-VEH (N=20) |
B6-ZOL (N=17) |
Genotyoe effect |
Drug effect |
Interaction effect |
|
|---|---|---|---|---|---|---|---|
| Total cross sectional area (mm2) | 1.25 ± 0.094 | 1.32 ± 0.118 | 2.018 ± 0.228 | 2.171 ± 0.218 | 0.0001 | 0.0064 | 0.304 |
| Marrow area (mm^2) | 0.502 ± 0.049 | 0.509 ± 0.054 | 1.131 ± 0.153 | 1.158 ± 0.138 | 0.0001 | 0.495 | 0.697 |
| Cross-sectional moment of inertia (mm4) | 0.074 ± 0.011 | 0.084 ± 0.013 | 0.136 ± 0.03 | 0.163 ± 0.033 | 0.0001 | 0.001 | 0.109 |
| Cortical thickness (mm) | 0.208 ± 0.005 | 0.217 ± 0.008 | 0.151 ± 0.011 | 0.155 ± 0.028 | 0.0001 | 0.0756 | 0.428 |
| Tissue mineral density (0–255 scale) | 201 ± 33 | 203 ± 32 | 184 ± 29 | 180 ± 30 | 0.0075 | 0.9145 | 0.749 |
Data presented as means and standard deviations. Two-way ANOVA was performed and the genotype, drug and interaction effects are displayed.
Figure 2.
Cortical bone area of the femoral diaphysis. There was a significant main effect of genotype (B6 had higher bone area compared to A/J, p = 0.0001) and drug (zoledronate (ZOL) led to significantly higher bone area compared to vehicle (VEH), p = .0001). There was no significant interaction between variables (p = 0.076). Representative images (from the animal within each group closest to the group mean) of diaphyseal bone for each genotype/drug condition are shown for comparison. All data presented as means and standard deviations.
Figure 3.
Distal femoral trabecular bone volume/tissue volume (BV/TV). There was a significant main effect of genotype (B6 had higher BV/TV compared to A/J, p = 0.001)) and drug (zoledronate (ZOL) led to significantly higher BV/TV compared to vehicle (VEH), p = 0.0001). There was a significant interaction between genotype and drug (p = 0.0087) indicating that although both genotypes responded positively to ZOL, the response to was significantly greater in B6 compared to A/J. * denotes a significant interaction between genotype and drug existed and within genotype there was a significant drug effect. ** indicates that the ZOL effect was significantly greater for B6 than A/J. Representative images (from the animal within each group closest to the group mean) of distal femur bone for each genotype/drug condition are shown for comparison. All data presented as means and standard deviations.
Three-way ANOVA (genotype, time, treatment) on body weight data revealed significant genotype and time effects but no significant treatment effects or interactions among any of the variables. B6 animals weighed significantly more than A/J animals at baseline (26.6 g vs 25.4 g, respectively) and at the end of the experiment (28.5 g vs 26.8 g, respectively) and body weights were significantly higher at the end of the experiment than baseline.
DISCUSSION
Bisphosphonates play an important role in the management of osteoporosis and have been consistently shown to reduce fracture risk [20]. Several reports have shown that there are a small number of individuals that exhibit poor response to bisphosphonate treatment [2–8]. Potential explanations for this heterogeneous response include differences in patient compliance [21], underlying cause of bone loss/fracture risk, or intrinsic differences in the skeleton. Given the known continuum of geometric and material properties across individuals [9, 10] we aimed to test the hypothesis that zoledronate, one of several commonly used bisphosphonates, had different effects on bone mechanics in bones with disparate geometries/material properties. The results of this work, utilizing two commonly described inbred mouse strains (A/J and B6), support the hypothesis and suggest interactions between bone geometry/material properties and the response to zoledronate.
We chose to study two commonly used inbred strains of mice, A/J and B6, due to their well-described contrasting bone morphology and material properties [11–13]. Our VEH-treated animals confirmed previous work showing that A/J animals have bones with relatively low post-yield behavior while B6 animals have bones with higher post-yield properties (Figure 1). The difference in post-yield properties between the genotypes is due in part to differences in the degree of mineralization arising from an intrinsic adaptive feature of the skeletal system to coordinately match material-level properties with bone external size to establish mechanical homeostasis across a population [11–13]. A similar coordinated regulation between bone external morphology and tissue-level mechanical properties has also been observed for human long bone [10, 22]. Despite differences in mineralization and geometry between the two mouse strains, the effects of zoledronate were quite similar for many parameters. Trabecular bone volume and mechanical properties such as ultimate force were higher in ZOL-treated animals compared to those treated with VEH within genotype. These represent clear benefits of zoledronate, confirming both previous animal work and the majority of clinical reports. Interestingly, although both genotypes responded similar to ZOL for trabecular bone, the response was actually 2× higher for A/J mice compared to B6 (representing a significant interaction). The underpinnings of these different responses deserve more exploration.
The most distinct divergence in the response of the two genetic mouse strains was in bone displacement, specifically in the post-yield region, during mechanical testing. All measures of bone brittleness (total displacement, post-yield displacement, and total strain) displayed a significant interaction between genotype and drug treatment. VEH-treated B6 animals had higher values than VEH-A/J animals and while A/J animals were unaffected by zoledronate, B6 animals had significantly lower values with treatment. This reduction in post-yield displacement/strain represents an increase in tissue and structural brittleness. Previous work by our group and others have documented reductions, most specifically in post-yield properties, following bisphosphonate treatment (alendronate, risedronate, and zoledronate) [14, 23, 24].
Post-yield properties are known to be negatively affected by several tissue-level properties including mineralization [25], microdamage [26], collagen cross-linking [27], and hydration [28]. The absence of significant change in tissue-level density (from microCT) suggest some other tissue-level change – such as microdamage, collagen, or hydration – is likely playing a significant role in these B6 mice treated with zoledronate. Since, in our personal experience, microdamage is quite rare in mice without some external stimulus, altered hydration and/or collagen crosslinking are the lead candidates that will necessitate further exploration in B6 mice treated with zoledronate.
There are several limitations to the current work. We utilized only one bisphosphonate, zoledronate, and thus do not know if this effect is common across the whole drug class. We also used a single dose and dosing schedule, as well as a single time point of assessment (8 weeks of dosing). Our study utilized male mice with normal bone mass, building on previous work in these mouse strains; future studies will aim to study this strain/drug interaction in more clinically relevant models such as ovariectomized females. Finally, the mechanical testing of mouse bone violates assumptions of the beam bending equations used in the calculations of parameters, although this is true of all papers describing the mechanics of mouse bone.
In conclusion we have documented genotype-specific mechanical property changes in response to zoledronate treatment. Understanding the underpinnings of these differences will be important to help determine if these effects are relevant to humans.
Acknowledgments
This work was supported by NIH grants AR62002 (MRA), DK108554 (F32 support for EM), and AR65971 (T32 support for MA). The microCT utilized in this experiment was purchased through a NIH S10 grant (OD 016208).
Footnotes
COI: M Aref, E McNerny, D Brown, K Jepsen, and M Allen declare that they have no conflict of interest.
References
- 1.Reid IR. Short-term and long-term effects of osteoporosis therapies. Nature Reviews Endocrinology. 2015;11:418–28. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- 2.Lewiecki EM. Nonresponders to Osteoporosis Therapy. Journal of Clinical Densitometry. 2003;6:307–314. doi: 10.1385/jcd:6:4:307. [DOI] [PubMed] [Google Scholar]
- 3.Chapurlat RD, Cummings SR. Does follow-up of osteoporotic women treated with antiresorptive therapies improve effectiveness? Osteoporos Int. 2002;13:738–744. doi: 10.1007/s001980200101. [DOI] [PubMed] [Google Scholar]
- 4.Sebba AI, Bonnick SL, Kagan R, et al. Response to therapy with once-weekly alendronate 70 mg compared to once-weekly risedronate 35 mg in the treatment of postmenopausal osteoporosis. Curr Med Res Opin. 2004;20:2031–2041. doi: 10.1185/030079904x16768. [DOI] [PubMed] [Google Scholar]
- 5.Nakano T, Yamamoto M, Hashimoto J, et al. Higher response with bone mineral density increase with monthly injectable ibandronate 1 mg compared with oral risedronate in the MOVER study. J Bone Miner Metab. 2015 doi: 10.1007/s00774-015-0717-8. [DOI] [PubMed] [Google Scholar]
- 6.Carmel AS, Shieh A, Bang H, Bockman RS. The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/ml. Osteoporos Int. 2012;23:2479–2487. doi: 10.1007/s00198-011-1868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter I, Rogers A, Eastell R, Peel N. Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos Int. 2012;24:941–947. doi: 10.1007/s00198-012-2097-4. [DOI] [PubMed] [Google Scholar]
- 8.Obermayer-Pietsch BM, Marín F, Mccloskey EV, et al. Effects of Two Years of Daily Teriparatide Treatment on BMD in Postmenopausal Women With Severe Osteoporosis With and Without Prior Antiresorptive Treatment. J Bone Miner Res. 2008;23:1591–1600. doi: 10.1359/jbmr.080506. [DOI] [PubMed] [Google Scholar]
- 9.Goldman HM, Hampson NA, Guth JJ, et al. Intracortical remodeling parameters are associated with measures of bone robustness. Anat Rec (Hoboken) 2014;297:1817–1828. doi: 10.1002/ar.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jepsen KJ, Centi A, Duarte GF, et al. Biological constraints that limit compensation of a common skeletal trait variant lead to inequivalence of tibial function among healthy young adults. J Bone Miner Res. 2011;26:2872–2885. doi: 10.1002/jbmr.497. [DOI] [PubMed] [Google Scholar]
- 11.Jepsen KJ, Akkus OJ, Majeska RJ, Nadeau JH. Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome. 2003;14:97–104. doi: 10.1007/s00335-002-3045-y. [DOI] [PubMed] [Google Scholar]
- 12.Price C, Herman BC, Lufkin T, et al. Genetic Variation in Bone Growth Patterns Defines Adult Mouse Bone Fragility. J Bone Miner Res. 2005;20:1983–1991. doi: 10.1359/JBMR.050707. [DOI] [PubMed] [Google Scholar]
- 13.Jepsen KJ, Hu B, Tommasini SM, et al. Genetic randomization reveals functional relationships among morphologic and tissue-quality traits that contribute to bone strength and fragility. Mamm Genome. 2007;18:492–507. doi: 10.1007/s00335-007-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don't know. Bone. 2011;49:56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 15.Kubek D, Burr D, Allen M. Ovariectomy stimulates and bisphosphonates inhibit intracortical remodeling in the mouse mandible. Orthodontics & Craniofacial Research. 2010;13:214–222. doi: 10.1111/j.1601-6343.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 17.Jepsen KJ, Silva MJ, Vashishth D, et al. Establishing Biomechanical Mechanisms in Mouse Models: Practical Guidelines for Systematically Evaluating Phenotypic Changes in the Diaphyses of Long Bones. J Bone Miner Res. 2015;30:951–66. doi: 10.1002/jbmr.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace JM. Chapter 6 - Skeletal Hard Tissue Biomechanics. In: Burr David B, Allen Matthew R., editors. Basic and Applied Bone Biology. Academic Press; San Diego: 2014. pp. 115–130. [Google Scholar]
- 19.Berman AG, Wallace JM, Bart ZR, Allen MR. Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta. Matrix Biology. 2015;52–54:19–28. doi: 10.1016/j.matbio.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Delmas PD, Eastell R, Reid IR. Once-Yearly Zoledronic Acid for Treatment of Postmenopausal Osteoporosis. New England Journal of Medicine. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 21.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 22.Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. Relationship Between Bone Morphology and Bone Quality in Male Tibias: Implications for Stress Fracture Risk. J Bone Miner Res. 2005;20:1372–1380. doi: 10.1359/JBMR.050326. [DOI] [PubMed] [Google Scholar]
- 23.Acevedo C, Bale H, Gludovatz B, et al. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone. 2015;81:352–363. doi: 10.1016/j.bone.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone. 2015;71:58–62. doi: 10.1016/j.bone.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currey John D. Role of collagen and other organics in the mechanical properties of bone. Osteoporos Int. 2003;14(Suppl 5):S29–36. doi: 10.1007/s00198-003-1470-8. [DOI] [PubMed] [Google Scholar]
- 26.Reilly GC, Currey JD. The effects of damage and microcracking on the impact strength of bone. Journal of Biomechanics. 2000;33:337–343. doi: 10.1016/s0021-9290(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 27.Vashishth D, Gibson GJ, Khoury JI, et al. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 28.Granke M, Does MD, Nyman JS. The Role of Water Compartments in the Material Properties of Cortical Bone. Calcif Tissue Int. 2015;97:292–307. doi: 10.1007/s00223-015-9977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]