Abstract
Objectives:
Cobicistat (COBI) enhances atazanavir (ATV) pharmacokinetic parameters similarly to ritonavir (RTV) in both healthy volunteers and HIV-infected adults. Primary efficacy and safety outcomes of this Phase 3, international, randomized, double-blind, double-dummy, active-controlled trial in HIV-1-infected treatment-naïve adults (GS-US-216-0114/NCT01108510) demonstrated that ATV+COBI was non-inferior to ATV+RTV, each in combination with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF), at Weeks 48 and 144, with high rates of virologic success for both regimens (85.2% and 87.4%, respectively, at Week 48; and 72.1% and 74.1% at Week 144), and with comparable safety and tolerability. Here, we describe virologic response and treatment discontinuation by a wider range of subgroups than previously presented.
Methods:
Subgroup analyses by baseline CD4 count (≤200, 201-350, >350 cells/mm3), baseline HIV-1 RNA level (≤100,000, >100,000 copies/mL), race, sex, and age (<40, ≥40 years) evaluated ATV+COBI versus ATV+RTV univariate odds ratios (ORs) for virologic success (viral load <50 copies/mL, intention-to-treat US Food and Drug Administration Snapshot algorithm) and discontinuation due to adverse events (AEs) at Weeks 48 and 144. Of 692 patients randomized, 344 received ATV+COBI and 348 ATV+RTV.
Results:
ATV+COBI versus ATV+RTV ORs for virologic success did not significantly differ by regimen overall at Weeks 48 and 144 (OR 0.90; 95% confidence interval [CI]: 0.64, 1.26) or within subgroups, except in females, for whom ATV+COBI was favored at Week 144 (OR 2.36; 95% CI: 1.02, 5.47). However, there were more discontinuations due to withdrawal of consent and pregnancies in females receiving ATV+RTV versus ATV+COBI. ORs for discontinuation due to AEs did not significantly differ by regimen overall at Weeks 48 and 144 (OR 0.98; 95% CI: 0.61, 1.58) or within subgroups.
Conclusion:
These findings indicate that both ATV+COBI and ATV+RTV, each with FTC/TDF, are effective and well-tolerated treatment options across a wide demographic range of HIV-infected patients.
Keywords: Atazanavir, cobicistat, ritonavir, subgroup analysis, HIV-1, pharmacokinetic, virologic
1. INTRODUCTION
Cobicistat (COBI) enhances all key pharmacokinetic parameters of atazanavir (ATV) similarly to ritonavir (RTV) in healthy volunteers and HIV-infected adults [1, 2].
Efficacy and safety outcomes of this Phase 3, international, randomized, double-blind, active-controlled trial in HIV-1-infected treatment-naïve adults (GS-US-216-0114/ NCT01108510) were previously published [3, 4]. ATV+
COBI was non-inferior to ATV+RTV, each in combination with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF), at Weeks 48 and 144. Both regimens achieved high rates of virologic success with comparable safety and tolerability.
With the introduction of a new antiretroviral pharmacoenhancer, in addition to establishing efficacy and safety in primary analyses, it is important to explore whether outcomes are consistent across patients with differing baseline demographic and disease characteristics.
A previous analysis of this study at 48 weeks by race, age, sex, baseline CD4 count (≤350 and >350 cells/mm3), HIV-1 RNA level, and drug adherence did not identify differences in virologic success between ATV+COBI and ATV+RTV within these subgroups [3].
Here, we sought to confirm the similarity of outcomes between ATV+COBI and ATV+RTV by examining odds ratios (ORs) across a wider range of outcomes (virologic success, virologic failure, and discontinuation due to adverse events [AEs]), and in patients with more advanced HIV disease (baseline CD4 count ≤200 cells/mm3), high viral load (baseline HIV-1 RNA ≥100,00 copies/mL [c/mL]) as well as in other relevant subgroups (race, age <40 vs. ≥40 years, and sex).
2. METHODS
A full description of study methods has been published previously [3].
2.1. Study Design
This Phase 3, international, randomized, double-blind, double-dummy, active-controlled clinical trial was approved by institutional review boards at all investigative centers. Study participants were HIV-1-infected adults with a plasma HIV-1 RNA ≥5000 c/mL and without prior use of antiretroviral agents. Key inclusion criteria included an estimated glomerular filtration rate of at least 70 mL/min (estimated using the Cockcroft-Gault formula) and genotypic sensitivity to ATV, FTC, and TDF at screening. Eligible patients were randomized 1:1 to receive either COBI 150 mg or RTV 100 mg and matching placebo, each administered once daily with ATV 300 mg in combination with FTC/TDF 200/300 mg.
2.2. Post Hoc Subgroup Analyses
The following subgroups were evaluated: baseline CD4 count, defined as ≤200, 201-350, and >350 cells/mm3; baseline viral load, defined as HIV-1 RNA <100,000 and ≥100,000 c/mL; race, defined as white, black/African American, Asian, and other; age, defined as <40 and ≥40 years; and sex.
The following outcomes were evaluated by subgroups at Weeks 48 and 144: virologic success, defined as the proportion of patients with virologic suppression (HIV-1 RNA <50 c/mL) using an intention-to-treat (ITT) algorithm and employing US Food and Drug Administration (FDA) Snapshot analysis; virologic failure, defined as the proportion of patients not achieving virologic suppression (HIV-1 RNA ≥50 c/mL) using ITT FDA Snapshot analysis; and discontinuation due to AEs.
2.3. Statistical Analysis
The proportions of patients with events were summarized by treatment group, overall and by subgroup. ATV+COBI versus ATV+RTV univariate ORs and associated 95% confidence intervals (CIs) were calculated, overall and by subgroup using the PROC FREQ procedure in SAS version 9.2 (SAS Institute, Cary, NC, USA).
These post hoc analyses were not powered for between-subgroup comparisons, and no adjustments were made for multiple comparisons.
3. RESULTS
3.1. Patients
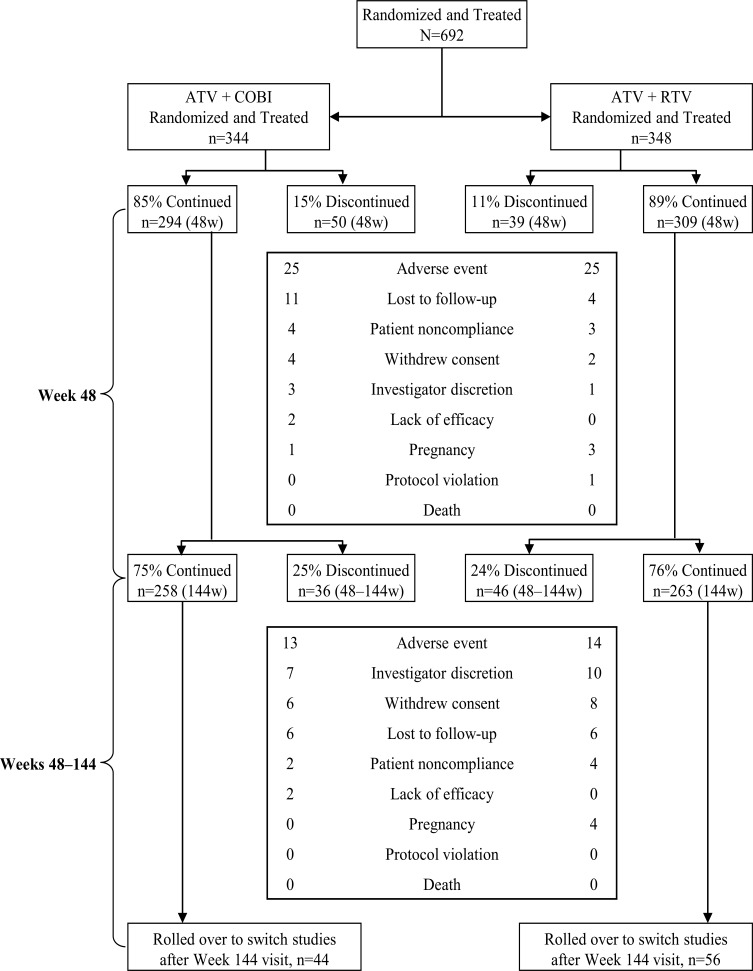
Of 692 patients randomized and treated, 87.1% completed 48 weeks and 75.3% completed 144 weeks of treatment (Fig. 1). Demographic and baseline characteristics were balanced across the 2 randomized treatment groups (Table 1).
Fig. (1).
Patient disposition at randomization, at Week 48, and at Week 144.
Table 1.
Demographic and baseline characteristics.
| ATV+COBI | ATV+RTV | |
|---|---|---|
| Overall number of patients | 344 | 348 |
| Age, n (%) | ||
| <40 years | 209 (60.8) | 205 (58.9) |
| ≥40 years | 135 (39.2) | 143 (41.1) |
| Sex, n (%) | ||
| Male | 287 (83.4) | 287 (82.5) |
| Female | 57 (16.6) | 61 (17.5) |
| Race, n (%) | ||
| White | 198 (57.6) | 215 (61.8) |
| Black/African American | 65 (18.9) | 63 (18.1) |
| Asian | 44 (12.8) | 37 (10.6) |
| Other | 37 (10.8) | 33 (9.5) |
| Baseline CD4 count, n (%) | ||
| ≤200 cells/mm3 | 60 (17.4) | 57 (16.4) |
| 201–350 cells/mm3 | 114 (33.1) | 126 (36.2) |
| >350 cells/mm3 | 170 (49.4) | 165 (47.4) |
| Baseline HIV-1 RNA, n (%) | ||
| <100,000 c/mL | 212 (61.6) | 205 (58.9) |
| ≥100,000 c/mL | 132 (38.4) | 143 (41.1) |
3.2. Virologic Success
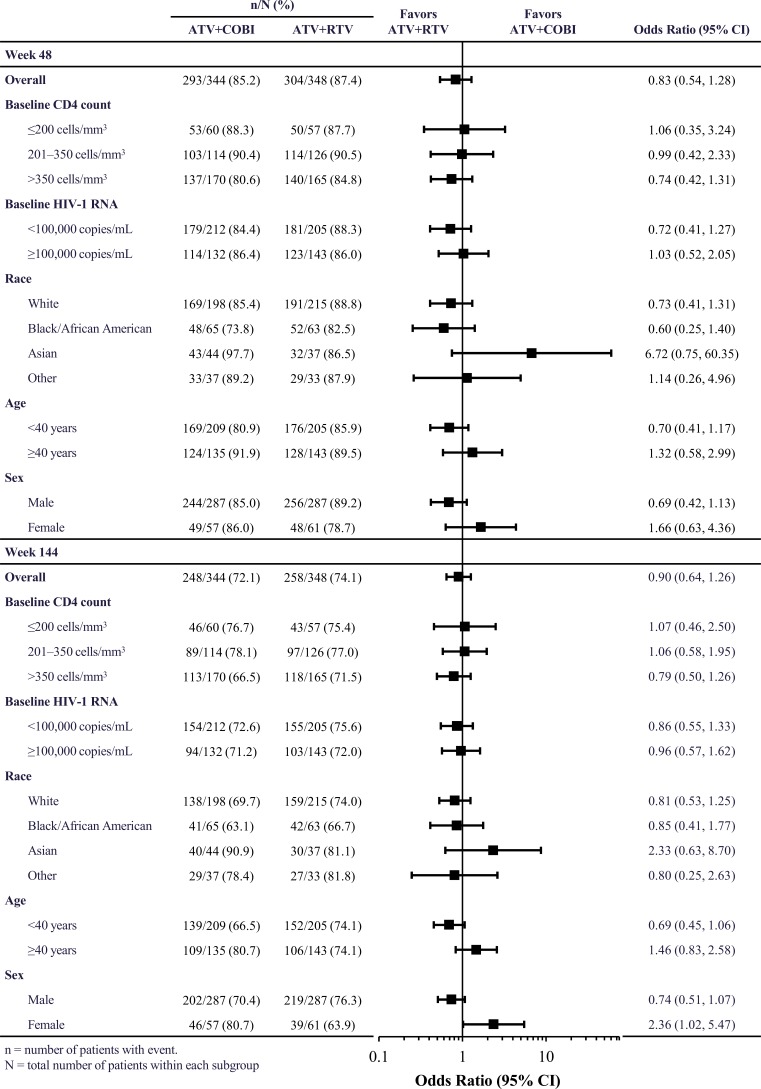
Overall, the proportions of patients achieving virologic success at Week 48 were 85.2% and 87.4% with ATV+COBI and ATV+RTV, respectively. Corresponding proportions at Week 144 were 72.1% and 74.1%, respectively.
No significant differences in ATV+COBI versus ATV+RTV univariate ORs for virologic success, overall or by subgroup, were observed at Week 48 (Fig. 2). However, at Week 144, ATV+COBI was favored for virologic success in females (Fig. 2); this was driven by more pregnancies in females receiving ATV+RTV (3 by Week 48 and an additional 4 during Weeks 48-144) versus in those receiving ATV+COBI (1 by Week 48), and by more discontinuations due to withdrawal of consent in females receiving ATV+RTV than in females receiving ATV+COBI (Table 2).
Fig. (2).
Proportions and univariate odds ratios (95% CI) for virologic success.
Table 2.
Reasons for discontinuation of study medication at Week 144 by treatment arm and by sex.
| ATV+COBI | ATV+RTV | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Number of patients | 57 | 287 | 61 | 287 |
| Reason for discontinuation, n (%) | ||||
| Adverse event | 5 (8.8) | 33 (11.5) | 7 (11.5) | 32 (11.1) |
| Lost to follow-up | 1 (1.8) | 16 (5.6) | 1 (1.6) | 9 (3.1) |
| Investigator discretion | 1 (1.8) | 9 (3.1) | 1 (1.6) | 10 (3.5) |
| Withdrew consent | 0 | 10 (3.5) | 5 (8.2) | 5 (1.7) |
| Patient noncompliance | 0 | 6 (2.1) | 1 (1.6) | 6 (2.1) |
| Lack of efficacy | 1 (1.8) | 3 (1.0) | 0 | 0 |
| Pregnancy | 1 (1.8) | 0 | 7 (11.5) | 0 |
| Protocol violation | 0 | 0 | 0 | 1 (0.3) |
| Death | 0 | 0 | 0 | 0 |
3.3. Virologic Failure
Overall, the proportions of patients with virologic failure at Week 48 were 5.8% and 4.0% with ATV+COBI and ATV+RTV, respectively. Corresponding proportions at Week 144 were 8.1% and 4.9%, respectively.
ATV+COBI versus ATV+RTV univariate ORs for virologic failure did not indicate significant differences overall or by subgroup at both Week 48 and Week 144 (Table 3).
Table 3.
Proportions and univariate ORs (95% CI) for virologic failure.*
| Week 48 | Week 144 | |||||
|---|---|---|---|---|---|---|
| ATV+COBI | ATV+RTV | ATV+COBI vs. ATV+RTV | ATV+COBI | ATV+RTV | ATV+COBI vs. ATV+RTV | |
| n/N (%) | n/N (%) | OR (95% CI) | n/N (%) | n/N (%) | OR (95% CI) | |
| Overall | 20/344 (5.8) | 14/348 (4.0) | 1.47 (0.73, 2.97) | 28/344 (8.1) | 17/348 (4.9) | 1.73 (0.93, 3.21) |
| Baseline CD4 count | ||||||
| ≤200 cells/mm3 | 5/60 (8.3) | 2/57 (3.5) | 2.50 (0.47, 13.44) | 8/60 (13.3) | 3/57 (5.3) | 2.77 (0.70, 11.01) |
| 201–350 cells/mm3 | 7/114 (6.1) | 3/126 (2.4) | 2.68 (0.68, 10.63) | 10/114 (8.8) | 4/126 (3.2) | 2.93 (0.89, 9.63) |
| >350 cells/mm3 | 8/170 (4.7) | 9/165 (5.5) | 0.86 (0.32, 2.28) | 10/170 (5.9) | 10/165 (6.1) | 0.97 (0.39, 2.39) |
| Baseline HIV-1 RNA | ||||||
| <100,000 c/mL | 10/212 (4.7) | 7/205 (3.4) | 1.40 (0.52, 3.75) | 14/212 (6.6) | 10/205 (4.9) | 1.38 (0.60, 3.18) |
| ≥100,000 c/mL | 10/132 (7.6) | 7/143 (4.9) | 1.59 (0.59, 4.31) | 14/132 (10.6) | 7/143 (4.9) | 2.31 (0.90, 5.90) |
| Race | ||||||
| White | 10/198 (5.1) | 5/215 (2.3) | 2.23 (0.75, 6.65) | 14/198 (7.1) | 6/215 (2.8) | 2.65 (1.00, 7.04) |
| Black/African American | 7/65 (10.8) | 6/63 (9.5) | 1.15 (0.36, 3.62) | 10/65 (15.4) | 7/63 (11.1) | 1.45 (0.52, 4.10) |
| Asian | 1/44 (2.3) | 1/37 (2.7) | 0.84 (0.05, 13.86) | 3/44 (6.8) | 2/37 (5.4) | 1.28 (0.20, 8.10) |
| Other | 2/37 (5.4) | 2/33 (6.1) | 0.89 (0.12, 6.67) | 1/37 (2.7) | 2/33 (6.1) | 0.43 (0.04, 4.98) |
| Age | ||||||
| <40 years | 15/209 (7.2) | 10/205 (4.9) | 1.51 (0.66, 3.44) | 20/209 (9.6) | 11/205 (5.4) | 1.87 (0.87, 4.00) |
| ≥40 years | 5/135 (3.7) | 4/143 (2.8) | 1.34 (0.35, 5.09) | 8/135 (5.9) | 6/143 (4.2) | 1.44 (0.49, 4.26) |
| Sex | ||||||
| Male | 16/287 (5.6) | 10/287 (3.5) | 1.64 (0.73, 3.67) | 22/287 (7.7) | 14/287 (4.9) | 1.62 (0.81, 3.23) |
| Female | 4/57 (7.0) | 4/61 (6.6) | 1.08 (0.26, 4.52) | 6/57 (10.5) | 3/61 (4.9) | 2.27 (0.54, 9.56) |
n = number of patients with event. N = total number of patients within each subgroup. *HIV-1 RNA ≥50 c/mL using intention-to-treat FDA Snapshot analysis.
3.4. Discontinuation Due to Adverse Events
Overall, the proportions of patients discontinuing due to AEs at Week 48 were 7.3% and 7.2% with ATV+COBI and ATV+RTV, respectively. Corresponding proportions at Week 144 were 11.0% and 11.2%, respectively.
Discontinuation due to AEs did not significantly differ by regimen within each subgroup at either Week 48 or at Week 144 (Table 4).
Table 4.
Proportions and univariate ORs (95% CI) for discontinuation due to AEs.
| Week 48 | Week 144 | |||||
|---|---|---|---|---|---|---|
| ATV+COBI | ATV+RTV | ATV+COBI vs. ATV+RTV | ATV+COBI | ATV+RTV | ATV+COBI vs. ATV+RTV | |
| n/N (%) | n/N (%) | OR (95% CI) | n/N (%) | n/N (%) | OR (95% CI) | |
| Overall | 25/344 (7.3) | 25/348 (7.2) | 1.01 (0.57, 1.80) | 38/344 (11.0) | 39/348 (11.2) | 0.98 (0.61, 1.58) |
| Baseline CD4 count | ||||||
| ≤200 cells/mm3 | 1/60 (1.7) | 3/57 (5.3) | 0.31 (0.03, 3.02) | 3/60 (5.0) | 6/57 (10.5) | 0.45 (0.11, 1.88) |
| 201–350 cells/mm3 | 6/114 (5.3) | 9/126 (7.1) | 0.72 (0.25, 2.10) | 11/114 (9.6) | 12/126 (9.5) | 1.01 (0.43, 2.40) |
| >350 cells/mm3 | 18/170 (10.6) | 13/165 (7.9) | 1.38 (0.66, 2.93) | 24/170 (14.1) | 21/165 (12.7) | 1.13 (0.60, 2.12) |
| Baseline HIV-1 RNA | ||||||
| <100,000 c/mL | 17/212 (8.0) | 11/205 (5.4) | 1.54 (0.70, 3.37) | 23/212 (10.8) | 18/205 (8.8) | 1.26 (0.66, 2.42) |
| ≥100,000 c/mL | 8/132 (6.1) | 14/143 (9.8) | 0.59 (0.24, 1.47) | 15/132 (11.4) | 21/143 (14.7) | 0.74 (0.37, 1.51) |
| Race | ||||||
| White | 17/198 (8.6) | 16/215 (7.4) | 1.17 (0.57, 2.38) | 28/198 (14.1) | 28/215 (13.0) | 1.10 (0.63, 1.93) |
| Black/African American | 4/65 (6.2) | 3/63 (4.8) | 1.31 (0.28, 6.11) | 6/65 (9.2) | 5/63 (7.9) | 1.18 (0.34, 4.08) |
| Asian | 1/44 (2.3) | 4/37 (10.8) | 0.19 (0.02, 1.80) | 1/44 (2.3) | 4/37 (10.8) | 0.19 (0.02, 1.80) |
| Other | 3/37 (8.1) | 2/33 (6.1) | 1.37 (0.21, 8.74) | 3/37 (8.1) | 2/33 (6.1) | 1.37 (0.21, 8.74) |
| Age | ||||||
| <40 years | 18/209 (8.6) | 15/205 (7.3) | 1.19 (0.59, 2.44) | 25/209 (12.0) | 19/205 (9.3) | 1.33 (0.71, 2.50) |
| ≥40 years | 7/135 (5.2) | 10/143 (7.0) | 0.73 (0.27, 1.97) | 13/135 (9.6) | 20/143 (14.0) | 0.66 (0.31, 1.38) |
| Sex | ||||||
| Male | 20/287 (7.0) | 20/287 (7.0) | 1.00 (0.53, 1.90) | 33/287 (11.5) | 32/287 (11.1) | 1.04 (0.62, 1.74) |
| Female | 5/57 (8.8) | 5/61 (8.2) | 1.08 (0.30, 3.94) | 5/57 (8.8) | 7/61 (11.5) | 0.74 (0.22, 2.49) |
n = number of patients with event. N = total number of patients within each subgroup.
4. DISCUSSION
This study is the only Phase 3, randomized, double-blind comparison of the use of COBI and RTV as pharmacoenhancers of a protease inhibitor (PI) in HIV-1-infected patients. High and durable virologic success rates were achieved with ATV+COBI and ATV+RTV (85.2% and 87.4% at Week 48, and 72.1% and 74.1% at Week 144, respectively) and virologic failure rates were low in each arm (5.8% and 4.0% at Week 48, and 8.1% and 4.9% at Week 144, respectively). In this study, ATV and COBI were dosed as separate agents, but are now available as a Fixed-Dose Combination (FDC) [5, 6].
These results compare favorably with responses obtained in similar patients initiating therapy with the PI darunavir (DRV) plus COBI. In the single-arm, open-label Phase 3b GS-US-216-0130 study in patients receiving DRV+COBI (dosed as separate agents) with FTC/TDF, virologic success (FDA Snapshot) and protocol-defined virologic failure (FDA Snapshot) in the treatment-naïve subgroup were 83% and 8% at Week 48, respectively [7]. In the randomized, double-blind, Phase 2 GS-US-299-0102 study in treatment-naïve patients receiving DRV+COBI (dosed as separate agents) plus FTC/TDF versus DRV/COBI/FTC/tenofovir alafenamide (as a single-tablet regimen), virologic success (FDA Snapshot) was achieved in 84.0% and 76.7%, and protocol-defined virologic failure (FDA Snapshot) occurred in 12% and 16% at Week 48, respectively [8].
Given that high baseline HIV-1 RNA and low baseline CD4 count have been associated with poorer treatment responses with regimens containing older PIs such as saquinavir and lopinavir (LPV) [9-12], it is reassuring that in patients with a baseline HIV-1 RNA ≥100,000 c/mL, both ATV+COBI and ATV+RTV were associated with high proportions of patients achieving virologic success at Week 48 (86.4% and 86.0%, respectively) and Week 144 (71.2% and 72.0%, respectively), and with low proportions experiencing virologic failure at Week 48 (7.6% and 4.9%, respectively) and Week 144 (10.6% and 4.9%, respectively). A similar response pattern was observed in patients with a baseline CD4 count ≤200 cells/mm3.
The high level of virologic responses with both ATV+COBI and ATV+RTV in treatment-naïve patients with high viral load at baseline compares favorably with responses obtained in similar patients initiating therapy with other commonly used boosted PIs. In the Phase 3 open-label CASTLE study, the proportion of patients with a baseline HIV-1 RNA ≥100,000 c/mL who achieved an HIV-1 RNA <50 c/mL (confirmed virologic response, non-comple-ter=failure) with ATV+RTV + FTC/TDF and with LPV/ RTV + FTC/TDF was 74% and 72%, respectively, at Week 48 (overall proportions regardless of viral load, 78% and 76%, respectively) [11], and 74% and 66%, respectively, at Week 96 (overall proportions regardless of viral load, 74% and 68%, respectively) [12]. Similar findings were evident in CASTLE for patients with CD4 counts <200 cells/mm3 at baseline [11, 12]. In the Phase 3 open-label ARTEMIS trial, the proportion of patients with a baseline HIV-1 RNA ≥100,000 c/mL who achieved an HIV-1 RNA <50 c/mL (ITT-time to loss of virological response) with DRV/RTV + FTC/TDF and with LPV/RTV + FTC/TDF was 79% and 67%, respectively, at Week 48 (overall proportions regardless of viral load, 84% and 78%, respectively) [13], 76% and 63%, respectively at Week 96 (overall proportions regardless of viral load, 79% and 71%, respectively) [14], and 67.5% and 51.7%, respectively, at Week 192 (overall proportions regardless of viral load, 69% and 57%, respectively) [15]. Similar findings were observed in ARTEMIS for patients with CD4 counts <200 cells/mm3 at baseline [13-15]. In the overall population of GS-US-216-0130 (94.2% treatment-naïve and 5.8% treatment-experienced without DRV resistance-associated mutations), proportions with virologic success and failure at Week 48 with DRV+COBI + FTC/TDF in patients with a baseline HIV-1 RNA >100,000 c/mL were 80% and 15%, respectively (overall proportions regardless of viral load, 81% and 11%, respectively) [7]. In this same study, virologic success at Week 48 with DRV+COBI + FTC/TDF in treatment-naïve patients with a baseline HIV-1 RNA >100,000 c/mL was 81% (overall proportion regardless of viral load, 83%) [7]. In the Phase 3b open-label FLAMINGO trial, the proportion of patients with a baseline HIV-1 RNA >100,000 c/mL who achieved an HIV-1 RNA <50 c/mL (FDA Snapshot) at Week 48 was 35/49 (71%) in those receiving DRV/RTV + FTC/TDF and 45/48 (94%) in those recei-ving dolutegravir (DTG) + FTC/TDF (overall proportions regardless of viral load, 132/162 [81%] and 146/163 [90%], respectively) [16; Supplementary Appendix Fig. 2]. At Week 96, proportions with virologic success were 52% and 82% in patients with a baseline HIV-1 RNA >100,000 c/mL receiving DRV/RTV + FTC/TDF or abacavir (ABC)/lamivudine (3TC) and DTG + FTC/TDF or ABC/3TC, respectively (overall proportions regardless of viral load, 68% and 80%, respectively) [17]. In the AIDS Clinical Trial Group (ACTG) A5257 Phase 3, open-label, randomized study of ATV/RTV versus raltegravir (RAL) versus DRV/RTV, each in combination with FTC/TDF, the cumulative probabilities of virologic failure at Week 96 (defined as time to a confirmed HIV-1 RNA level > 1000 c/mL at or after 16 weeks and before 24 weeks or > 200 c/mL at or after 24 weeks) in patients with a baseline HIV-1 RNA >100,000 c/mL were not significantly different between treatment groups [18]. Overall, regardless of viral load, the cumulative probabilities of virologic failure at Week 96 were 12.6% in the ATV+RTV group, 9.0% in the RAL group, and 14.9% in the DRV+RAL group; for all pairwise treatment comparisons, the 97.5% CIs fell within the prespecified equivalence bound of ±10%, indicating equivalence of the 3 regimens for virologic failure. However, tolerability failure was higher for ATV+RTV versus RAL or versus DRV+RTV, which was driven by a higher rate of study discontinuation due to hyperbilirubinemia in the ATV+RTV group [18].
Although caution is necessary when making comparisons between studies with differing designs and study populations, the higher rate of virologic success with ATV+RTV at Week 48 in the current study (86%) versus that with DRV+RTV in ARTEMIS (79%) and FLAMINGO (71%) in patients with baseline HIV-1 RNA >100,000 c/mL could potentially be accounted for by the differing pharmacokinetic profiles of ATV+RTV and DRV+RTV. Patients with high viral load would be particularly vulnerable to episodes of subtherapeutic drug concentration. In healthy volunteers, on cessation of therapy, the proportions with drug levels below target concentrations at 30, 36, 40, and 48 hours post-dose were 0%, 47%, 71%, and 88%, respectively, for ATV+RTV (n=17) and 6%, 47%, 82%, and 94% respectively, for DRV+RTV (n=17) [19]. These data suggest a greater pharmacokinetic forgiveness with ATV+RTV than DRV+RTV. Furthermore, if RTV is missed, DRV exposure is poor, whereas ATV can be dosed once daily unboosted, potentially resulting in less consequence to partial non-adherence.
With similar caveats about cross-study comparisons, the higher rate of virologic success with ATV+COBI at Week 48 in the current study (86.4%) versus that with DRV+COBI in GS-US-216-0130 (81%) could potentially be accounted for by the differing pharmacokinetic profiles of ATV+COBI and DRV+COBI. In a pharmacokinetic substudy of GS-US-216-0114, ATV+COBI versus ATV+RTV pharmacokinetic parameters were comparable; specifically, the mean ratio for ATV Cτ (concentration at the end of the dosing interval) was 0.94 [6]. In contrast, DRV minimum concentrations were reduced when DRV was administered with COBI as separate agents (geometric mean ratio 0.69; 90% CI: 0.59, 0.82) [20] or as an FDC (least squares mean ratio 0.74; 90% CI: 0.63, 0.86) [21] versus administration with RTV in healthy volunteers. Although there are no direct comparative pharmacokinetic data with DRV+COBI versus DRV+RTV in patients with HIV infection, these data suggest that DRV concentrations are lower when DRV is administered with COBI versus RTV [22].
In the current study, ATV+COBI was favored for virologic success in females at Week 144 only. However, this appeared to be driven by more discontinuations due to withdrawal of consent and more pregnancies in females receiving ATV+RTV versus ATV+COBI. Females of childbearing potential were required to utilize 2 forms of highly effective contraception, one of which had to be an effective barrier method, from screening through to 30 days after the last dose of study drug. Pregnancy status was monitored at each study visit with urinary pregnancy tests. In GS-US-236-0103, which compared elvitegravir/COBI/FTC/TDF with ATV+RTV plus FTC/TDF, there were similar numbers of pregnancies in each treatment arm by 144 weeks [23]. Additionally, drug-drug interactions of ATV+RTV with hormonal contraceptives are well described and dosing recommendations exist [1]. Therefore, the excess of pregnancies in the ATV+RTV arm of the current study is likely to have occurred by chance. Thus, it is unlikely that ATV+COBI is more effective than ATV+RTV in females.
Discontinuation of study therapy due to AEs through Week 48 and Week 144 was similar between the ATV+COBI and ATV+RTV arms, regardless of subgroup. Notably, discontinuation due to AEs with ATV in the current study at Week 144 (11.1%), which included discontinuation due to hyperbilirubinemia, was lower than that reported in ACTG A5257 at Week 96 (15.7%) [18].
Limitations of this post hoc analysis were that it was not powered for between-subgroup comparisons, and no adjustments were made for multiple comparisons.
CONCLUSION
In this post hoc subgroup analysis, virologic success was high and virologic failure was low, and both were similar between the ATV+COBI and ATV+RTV groups at Weeks 48 and 144, regardless of race, sex, age, and baseline CD4 count or HIV-1 RNA level. The efficacy, safety, and tolerability (overall and by subgroups) of both ATV+COBI and ATV+RTV, each in combination with FTC/TDF, support their use as effective treatment options for HIV-infected patients.
ACKNOWLEDGEMENTS
JG, GM, JB, PS, HC, and JS contributed to the performance, analysis, and reporting of the work and were involved in the article drafting. Y-PL and LY conducted statistical analyses and were involved in the article drafting. JM and LR contributed to the analysis and reporting of the work and were involved in the article drafting.
Medical writing assistance was provided by Julian Martins of inScience Communications, Springer Healthcare, which was funded by Bristol-Myers Squibb.
CONFLICT OF INTEREST
JG has received consulting fees or advisory board honoraria from Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Theratechnologies, and ViiV Healthcare. His institution has received support for research he conducts from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Therapeutics, Merck & Co., Sangamo Biosciences, and ViiV Healthcare. GM has received consulting fees, advisory board honoraria, or speaker fees from Bristol-Myers Squibb, Gilead Sciences, Tobira Therapeutics, and Merck & Co (including MSD). He has acted as a consultant to Generics UK LTD (a subsidiary of Mylan), TEVA, and Trio Health. JB has received research grants from Gilead, MSD, and ViiV Healthcare and honoraria for advisory boards and talks from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, MSD, and ViiV Healthcare. PS has received consulting fees, advisory board honoraria, or speaker fees from Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck & Co (including MSD), and Viiv Healthcare. He has received research grants from Gilead Sciences, Janssen, and Viiv Healthcare. HC, Y-PL, and JS are employees of Gilead Sciences, Inc. JM, LR, and LY are employees of Bristol-Myers Squibb.
REFERENCES
- 1.Bristol-Myers Squibb Company Prescribing information for REYATAZ® (atazanavir). [updated: September 2015; cited: 21 September 2016]; Available from: http://packageinserts.bms.com/ pi/pi_reyataz.pdf.
- 2.Deeks E.D. Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs. 2014;74(2):195–206. doi: 10.1007/s40265-013-0160-x. [DOI] [PubMed] [Google Scholar]
- 3.Gallant J.E., Koenig E., Andrade-Villanueva J., Chetchotisakd P., DeJesus E., Antunes F., Arastéh K., Moyle G., Rizzardini G., Fehr J., Liu Y., Zhong L., Callebaut C., Szwarcberg J., Rhee M.S., Cheng A.K. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: week 48 results. J. Infect. Dis. 2013;208(1):32–39. doi: 10.1093/infdis/jit122. [DOI] [PubMed] [Google Scholar]
- 4.Gallant J.E., Koenig E., Andrade-Villanueva J.F., Chetchotisakd P., DeJesus E., Antunes F., Arastéh K., Rizzardini G., Fehr J., Liu H.C., Abram M.E., Cao H., Szwarcberg J. Brief report: Cobicistat compared with ritonavir as a pharmacoenhancer for atazanavir in combination with emtricitabine/tenofovir disoproxil fumarate: week 144 results. J. Acquir. Immune Defic. Syndr. 2015;69(3):338–340. doi: 10.1097/QAI.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration US Food and Drug Administration. New Drug Application approval for EVOTAZ® (atazanavir and cobicistat) tablet, 300 mg and 150 mg. [updated: 29 January 2015; cited: 14 March 2016]; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/ appletter/2015/206353Orig1s000ltr.pdf.
- 6.Bristol-Myers Squibb Company Prescribing information for EVOTAZ® (atazanavir and cobicistat). [updated: May 2015; cited: 23 November 2015]; Available from: http://packageinserts.bms. com/pi/pi_evotaz.pdf.
- 7.Tashima K., Crofoot G., Tomaka F.L., Kakuda T.N., Brochot A., Van de Casteele T., Opsomer M., Garner W., Margot N., Custodio J.M., Fordyce M.W., Szwarcberg J. Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a Phase IIIb, open-label single-arm trial. AIDS Res. Ther. 2014;11:39. doi: 10.1186/1742-6405-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills A., Crofoot G., Jr, McDonald C., Shalit P., Flamm J.A., Gathe J., Jr, Scribner A., Shamblaw D., Saag M., Cao H., Martin H., Das M., Thomas A., Liu H.C., Yan M., Callebaut C., Custodio J., Cheng A., McCallister S. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: A randomized phase 2 study. J. Acquir. Immune Defic. Syndr. 2015;69(4):439–445. doi: 10.1097/QAI.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 9.Casado J.L., Perez-Elías M.J., Antela A., Sabido R., Martí-Belda P., Dronda F., Blazquez J., Quereda C. Predictors of long-term response to protease inhibitor therapy in a cohort of HIV-infected patients. AIDS. 1998;12(11):F131–F135. doi: 10.1097/00002030-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Grabar S., Pradier C., Le Corfec E., Lancar R., Allavena C., Bentata M., Berlureau P., Dupont C., Fabbro-Peray P., Poizot-Martin I., Costagliola D. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS. 2000;14(2):141–149. doi: 10.1097/00002030-200001280-00009. [DOI] [PubMed] [Google Scholar]
- 11.Molina J.M., Andrade-Villanueva J., Echevarria J., Chetchotisakd P., Corral J., David N., Moyle G., Mancini M., Percival L., Yang R., Thiry A., McGrath D., CASTLE Study Team Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372(9639):646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 12.Molina J.M., Andrade-Villanueva J., Echevarria J., Chetchotisakd P., Corral J., David N., Moyle G., Mancini M., Percival L., Yang R., Wirtz V., Lataillade M., Absalon J., McGrath D., CASTLE Study Team Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J. Acquir. Immune Defic. Syndr. 2010;53(3):323–332. doi: 10.1097/QAI.0b013e3181c990bf. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz R., Dejesus E., Khanlou H., Voronin E., van Lunzen J., Andrade-Villanueva J., Fourie J., De Meyer S., De Pauw M., Lefebvre E., Vangeneugden T., Spinosa-Guzman S. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389–1397. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 14.Mills A.M., Nelson M., Jayaweera D., Ruxrungtham K., Cassetti I., Girard P.M., Workman C., Dierynck I., Sekar V., Abeele C.V., Lavreys L. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23(13):1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 15.Orkin C., DeJesus E., Khanlou H., Stoehr A., Supparatpinyo K., Lathouwers E., Lefebvre E., Opsomer M., Van de Casteele T., Tomaka F. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59. doi: 10.1111/j.1468-1293.2012.01060.x. [DOI] [PubMed] [Google Scholar]
- 16.Clotet B., Feinberg J., van Lunzen J., Khuong-Josses M.A., Antinori A., Dumitru I., Pokrovskiy V., Fehr J., Ortiz R., Saag M., Harris J., Brennan C., Fujiwara T., Min S., ING114915 Study Team Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–2231. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
- 17.Molina J.M., Clotet B., van Lunzen J., Lazzarin A., Cavassini M., Henry K., Kulagin V., Givens N., de Oliveira C.F., Brennan C., FLAMINGO study team Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2(4):e127–e136. doi: 10.1016/S2352-3018(15)00027-2. [DOI] [PubMed] [Google Scholar]
- 18.Lennox J.L., Landovitz R.J., Ribaudo H.J., Ofotokun I., Na L.H., Godfrey C., Kuritzkes D.R., Sagar M., Brown T.T., Cohn S.E., McComsey G.A., Aweeka F., Fichtenbaum C.J., Presti R.M., Koletar S.L., Haas D.W., Patterson K.B., Benson C.A., Baugh B.P., Leavitt R.Y., Rooney J.F., Seekins D., Currier J.S., ACTG A5257 Team Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann. Intern. Med. 2014;161(7):461–471. doi: 10.7326/M14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boffito M., Jackson A., Amara A., Back D., Khoo S., Higgs C., Seymour N., Gazzard B., Moyle G. Pharmacokinetics of once-daily darunavir-ritonavir and atazanavir-ritonavir over 72 hours following drug cessation. Antimicrob. Agents Chemother. 2011;55(9):4218–4223. doi: 10.1128/AAC.01747-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathias A., Liu H.C., Warren D., Sekar V., Kearney B.P. Relative bioavailability and pharmacokinetics of darunavir when boosted with the pharmacoenhancer GS-9350 versus RTV [Abstract 28]. 11th International Workshop on Clinical Pharmacology of HIV Therapy, 7–9 April 2010, Sorrento, Italy. Available from: http://www.natap.org/2010/PK/PK_10.htm. 2010.
- 21.Kakuda T.N., Opsomer M., Timmers M., Iterbeke K., Van De Casteele T., Hillewaert V., Petrovic R., Hoetelmans R.M. Pharmacokinetics of darunavir in fixed-dose combination with cobicistat compared with coadministration of darunavir and ritonavir as single agents in healthy volunteers. J. Clin. Pharmacol. 2014;54(8):949–957. doi: 10.1002/jcph.290. [DOI] [PubMed] [Google Scholar]
- 22.Kakuda T.N., Brochot A., Tomaka F.L., Vangeneugden T., Van De Casteele T., Hoetelmans R.M. Pharmacokinetics and pharmacodynamics of boosted once-daily darunavir. J. Antimicrob. Chemother. 2014;69(10):2591–2605. doi: 10.1093/jac/dku193. [DOI] [PubMed] [Google Scholar]
- 23.Clumeck N., Molina J-M., Henry K., et al. Elvitegravir/ cobicistat/emtricitabine/tenofovir df (stb) has durable efficacy and differentiated safety compared to atazanavir boosted by ritonavir plus emtricitabine/tenofovir df at week 144 in treatment-naive HIV-1 infected patients. EACS Online Library. Oct 18, 2013; 39247. . [cited: 22 September 2016]; Available from: http://eacs. multilearning.com/eacs/2013/14th/ 39247/nathan.clumeck. elvitegravir. cobicistat.emtricitabine.tenofovir.df.28stb29.has.html?f=m1e 674. 2013.