Abstract
Background
At present, much attention has been focused on the beneficial effects of natural products on the human health due to their high efficacy and low adverse effects. Among them, polyphenolic compounds are known as one of the most important and common classes of natural products, which possess multiple range of health-promotion effects including anti-inflammatory and antioxidant activities. A plethora of scientific evidence has shown that polyphenolic compounds possess beneficial effects on the central nervous system.
Methods
Data were collected from Web of Science (ISI Web of Knowledge), Medline, Pubmed, Scopus, Embase, and BIOSIS Previews (from 1950 to 2015), through searching of these keywords: “chlorogenic acid and mental diseases” and “chlorogenic acid and neuroprotection”.
Results
Chlorogenic acid is known as one of the most common polyphenolic compounds, and is found in different types of fruits and vegetables, spices, wine, olive oil, as well as coffee. The potential neuroprotective effects of chlorogenic acid have been highlighted in several in vitro and in vivo studies. This review critically analyses the available scientific evidence regarding the neuroprotective effects of chlorogenic acid, and its neuropharmacological mechanisms of action. In addition, we also discuss its biosynthesis, sources, bioavailability and metabolism, to provide a broad perspective of the therapeutic implications of this compound in brain health and disease.
Conclusion
The present review showed that chlorogenic acid possesses neuroprotective effects under the both in vitro and in vivo models.
Keywords: Antioxidant, chlorogenic acid, inflammation, neuroprotective, oxidative stress, polyphenolic
INTRODUCTION
Polyphenols have favourable effects on human health, exhibiting several biological properties such as anti-inflammatory and antioxidant activities. The beneficial effects of polyphenols have been well established, acting against metabolic and cardiovascular alterations, cerebral ischemia, obesity and lipid metabolism [1-6]. Moreover, several studies have explored the neuroprotective effects of polyphenols [7, 8]. Polyphenolic compounds have been shown to modulate several molecular cascades, attenuate oxidative stress, and ameliorate neuroinflammation [9-13].
The pathobiologies of several neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), have been associated with chronic oxidative stress and proinflammatory mechanisms, which lead to neuronal damage [8, 14]. Therefore, compounds that reduce the production of reactive oxygen and nitrogen species may prevent oxidative damage to macromolecules and restore normal cellular homeostasis [15-17].
Numerous studies have reported that coffee can exert positive effects against depression [18, 19]. The beneficial effects of coffee intake have also been reported in several preclinical and clinical studies of AD and PD [20-22]. In fact, coffee has been widely studied due to its high content of polyphenols, which include chlorogenic acid.
Chlorogenic acid is a polyphenol that can be found in several plant tissues and foods such as fruits, spices, vegetables, wine, olive oil, and coffee [23-26]. In fact, chlorogenic acid and caffeic acid are the main polyphenolic representatives of hydroxycinnamic acids. While several studies have focused on caffeine as a neuroprotective agent against neurodegenerative disorders, chlorogenic acid is also an important active component of coffee. The literature describes this polyphenol as having several biological effects, including antioxidant, antibacterial, antihistaminic, anti-inflammatory, analgesic, hepatoprotective, anticancer, and neuroprotective properties [27-33]. Due to these properties, there are current studies focusing on the potential benefits of chlorogenic acid consumption. However, the exact mechanism(s) related to the neuroprotective effects of this acid have not yet been clearly established, though promising results are emerging in literature.
BIOSYNTHESIS AND SOURCES OF CHLOROGENIC ACID
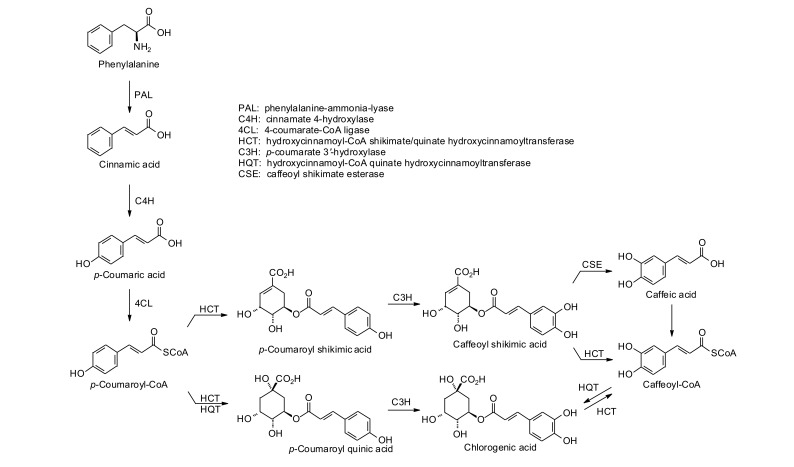
The biosynthesis of chlorogenic acid (3-O-caffeoylquinic acid) has been shown to involve the shikimic acid pathway (see Fig. 1) [34-37]. In brief, phenylalanine is converted to (E)-cinnamic acid by phenylalanine-ammonia-lyase [38], which is then hydroxylated by cinnamate 4-hydroxylase to p-coumaric acid. Conjugation of p-coumaric acid with coenzyme A is catalyzed by 4-coumarate-CoA ligase. Transesterification of p-coumaroyl-CoA with shikimic acid gives p-coumaroyl shikimic acid while quinic acid gives p-coumaroyl quinic acid [39]. Hydroxylation of p-coumaroyl quinic acid by p-coumarate 3ʹ-hydroxylase gives chlorogenic acid [40]. Alternatively, p-coumaroyl shikimic acid can be hydroxylated to caffeoyl shikimic acid, which can be converted to caffeic acid or to caffeoyl-CoA. Caffeoyl-CoA can then be transesterified with quinic acid to give chlorogenic acid [41].
Fig. (1).
Biosynthesis of chlorogenic acid in plants [37].
Important dietary sources of chlorogenic acid are coffee (Coffea arabica and Coffea canephora) beans [42, 43], potato (Solanum tuberosum) tubers [44], apple (Malus domestica) fruits [45], prune (Prunus domestica) fruits [46], sunflower (Helianthus annuus) seed kernels [47], Jerusalem artichoke (Helianthus tuberosus) leaves [48], and sweet potato (Ipomoea batatas) leaves [49].
Herbal medicinal sources of chlorogenic acid are the flowers and buds of Japanese honeysuckle (Lonicera japonica) [50, 51], South China honeysuckle (Lonicera confusa) [52], leaves of “guaco” (Mikania glomerata and Mikania laevigata) [53], Kuding tea (Ilex latifolia) [54], and zhi zi (Gardenia jasminoides) fruits [55].
BIOAVAILABILITY AND METABOLISM OF CHLOROGENIC ACID
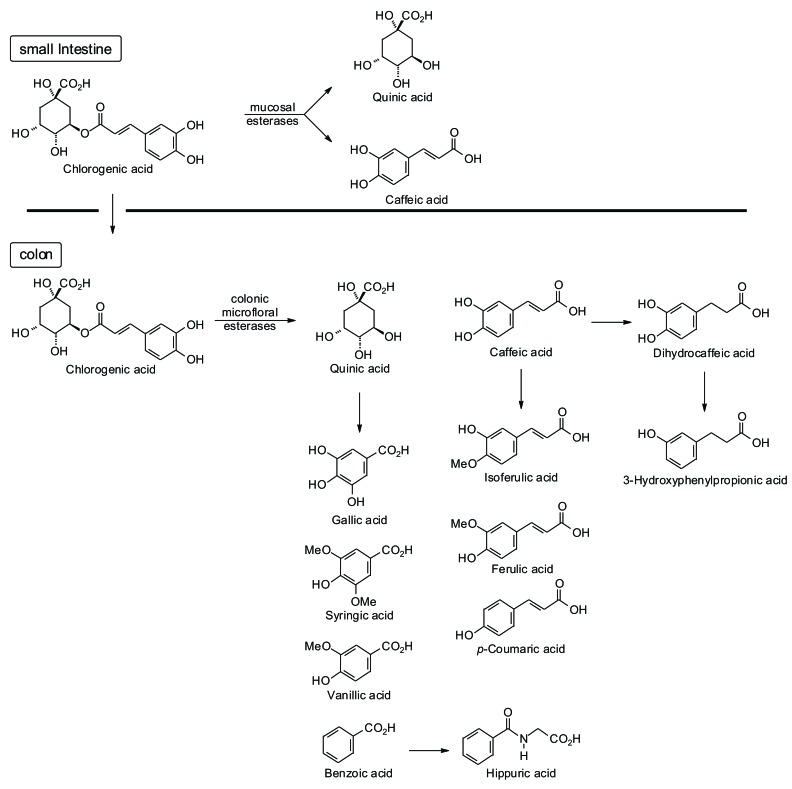
Most studies on chlorogenic acid have been performed using coffee, due to high amounts of this polyphenol (about 400 µmol/200 ml coffee bean cup), yielding evidence of its antioxidant capacity [18-22, 56, 57]. However, the bioavailability of chlorogenic acid is controversial, since its utilization and excretion are unclear. About one-third of ingested chlorogenic acid is absorbed by the small intestine in humans [58] (see Fig. 2). Most of the ingested chlorogenic acid reaches the colon, largely unaltered, where it is then hydrolyzed by esterases produced by colonic microflora (e.g., Escherichia coli, Bifidobacterium lactis, and Lactobacillus gasseri) [59]. Gastric esterases, most likely located on the apical membrane, release free caffeic acid which then passively diffuses across the cell surface [60]. The microfloral metabolites can then reach circulation [61].
Fig. (2).
Several clinical trials have studied the bioavailability of chlorogenic acid in urine, ileal fluid, and plasma of ileostomist volunteers, who drank different concentrations of chlorogenic acid in coffee. The results seem to indicate that the metabolism of these polyphenols is dependent on gastrointestinal transit time, and that its absorption occurs in the small intestine [56, 62]. However, another study has indicated the key role of the colon in the bioavailability of phenolic compounds in coffee [63]. These discrepancies may be attributed to the low number of subjects that volunteer to participate in these studies, or to differences in methodology.
However, one study has shown that chlorogenic acid is not well absorbed by the digestive tract in a rat model [64], and it was not detected in plasma or bile, suggesting that it may be absorbed and hydrolyzed in the small intestine with the uptake of caffeic acid taking place through the gut mucosa [65]. However, the microbial metabolites of chlorogenic acid represent major components of both urine and plasma of tested rats, indicating that the bioavailability of chlorogenic acid depends on its metabolism by gut microflora [66]. Likewise, urine does not appear to be a major excretion pathway of intact chlorogenic acid in humans [67].
REACTIONS OF CHLOROGENIC ACID
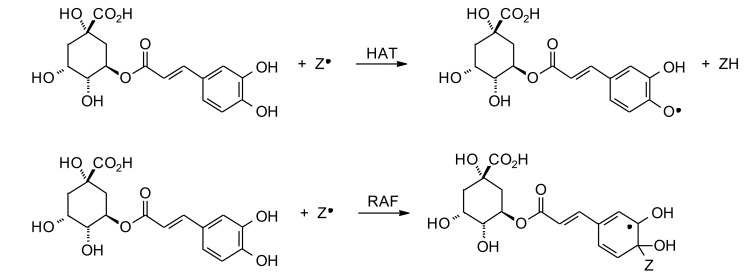
The antioxidant effects of chlorogenic acid are well established [68-74]. Chlorogenic acid can scavenge hydroxyl radicals (•OH) [75], superoxide radicals (•O2¯) [73], 2,2-diphenyl-1-picrylhydrazyl radicals (DPPH•) [69], and 2,2ʹ-azino-bis (3-ethylbenothiazoline-6-sulfonic acid) cation radicals (ABTS•+) [76] in a dose-dependent manner. There are two plausible mechanisms for the radical scavenging activity of chlorogenic acid: (1) a hydrogen-atom-transfer (HAT) reaction where the free radical abstracts a hydrogen atom from chlorogenic acid; (2) a radical adduct formation (RAF) mechanism where the free radical undergoes addition to chlorogenic acid to form a radical intermediate (Fig. 3) [77, 78]. Chlorogenic acid also reacts with the oxidizing agent peroxynitrite (ONOO¯), but the products of this reaction have not been studied [73].
Fig. (3).
Possible radical-scavenging mechanisms of chlorogenic acid.
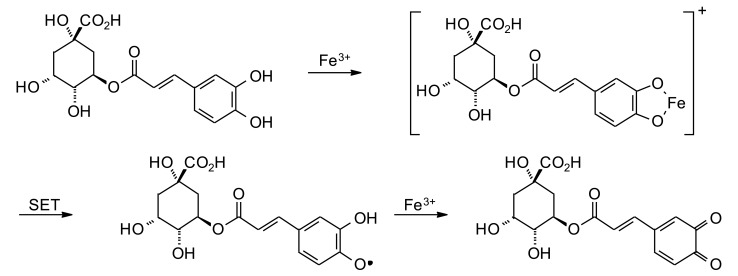
Chlorogenic acid also reacts with redox-active transition metals. In particular, chlorogenic acid reacts with iron(III) to form a chelate between the Fe(III) and the catechol moiety. Subsequent single electron transfer (SET) produces a semiquinone and Fe(II); which then reacts with another Fe(III), producing the corresponding quinone (Fig. 4) [79]. A similar reaction sequence has been proposed between chlorogenic acid and copper(II) [80]. Chlorogenic acid can be electrochemically oxidized to the corresponding o-quinone [81]. The catechol moiety of chlorogenic acid can be enzymatically oxidized to the corresponding quinone [82-84]. The electrophilic quinone can then undergo conjugate addition reactions with suitable nucleophiles such as amines and thiols [85], including glutathione [86].
Fig. (4).
Oxidation of chlorogenic acid by iron(III).
NEUROPROTECTIVE EFFECTS OF CHLORO-GENIC ACID
Ability to Cross the Blood-brain Barrier
It has been reported that chlorogenic acid or its metabolites may cross the blood-brain barrier and exert neuroprotective effects on brain tissue [87-89]. Decaffeinated coffee enriched with chlorogenic acid was administered to 39 healthy older participants, which reported positive effects on mood and cognition, including diminished headaches, and lower mental fatigue in older adults [90]. Similar results were found on mood in 60 healthy individuals following the ingestion of chlorogenic acid. However, no improvement was reported in some mood and cognitive function assessments [91].
Antioxidant Effects in the Brain
Several studies have been performed using animals and human cell lines as models for assessing the protective effects of the chlorogenic acid against oxidative damage in brain tissue [92-94]. The antioxidant effects of chlorogenic acid have also been reported in rats [89], mice [95] and rabbits [96]. Oboh and collaborators [97] observed that chlorogenic acid reduces oxidative stress induced by Fe2+ and by sodium nitroprusside in a dose dependent manner. The reduction of ROS production and the inhibition of DNA fragmentation and caspase-3 cleavage have also been reported in PC12 cells following incubation with chlorogenic acid [94].
In contrast, a previous study has shown that chlorogenic acid (100 ng/ml) increases astrocyte viability in cultured cells, but has no direct effect on hydrogen peroxide toxicity in astrocytes. Apart from the free-radical scavenging properties of chlorogenic acid, antioxidant effects have been attributed to the increased expression of neuroprotective ribosomal proteins (PEP-1-rpS3) [98]. Differences in the methodological procedures of different studies may be responsible for the variability of the results reported in the literature.
Antiapoptotic Effects
Despite its antioxidant effects, chlorogenic acid may also inhibit apoptotic processes. For instance, proteins from the B-cell lymphoma 2 (Bcl-2) family act as antiapoptotic molecules, and the inhibition of Bcl-XL by hydrogen peroxide was found to be strongly reduced following pretreatment with chlorogenic acid [94].
Effects on Signaling Cascade
It is well known that ROS can activate protein kinase cascades [93]. Cho and collaborators [94] observed that pretreatment with chlorogenic acid stimulated JNK and p38 MAPK activation in the PC12 cell line.
Antiamyloidogenic Effects
A study using a human neuroblastoma cell line (MC65) revealed diminished cell viability when cells were treated with β-amyloid proteins (which form the typical extracellular plaques of AD). However, this effect can be prevented by pretreatment with chlorogenic acid. This induces an overexpression of the glycolytic enzyme phosphoglycerate kinase-1, which is associated with ATP production and regulation of neuronal apoptosis [99]. This study agrees with another study conducted using the extracts derived from the plant Centella asiatica, which has a high content of chlorogenic acid [100].
Effects Against α-synuclein
PC12 is a useful cell line used to monitor the expression levels of α-synuclein, a pathogenic molecule that induces the overexpression of Parkinson genes and the loss of dopaminergic neurons. Pretreatment with of PC12 cells with chlorogenic acid induces a protective effect against toxicity induced by α-synuclein. Incubation with chlorogenic acid provides protection against oxidative stress which, leads to increased cell viability [101]. In addition, negative effects caused by the oxidized forms of dopamine, and α-synuclein were inhibited by chlorogenic acid [102].
Cognitive Effects
The effects of chlorogenic acid on cognitive performance have been investigated in senescence-accelerated-prone mice 8, by means of the Morris water maze test. Improvements in the execution of this test and diminished latency in reaching the platform were reported in mice treated with chlorogenic acid, compared to control animals [99]. Chlorogenic acid can also protect against anxiolytic and depressive processes by means of reducing the oxidative status in a mouse model [92].
Cholinergic Effects
Chlorogenic acid has been shown to induce neuro-protection in rat brain homogenates by lowering the activities of acetylcholinesterase and butyrylcholinesterase [97]. These enzymatic activities are increased in neuro-degenerative diseases such as AD. Inhibition of these enzymatic activities can result in increased levels of acetylcholine in the synapses leading to an increased communication between neurons [97].
Protective Effects Against Ischemia
In another study of focal cerebral ischemia in rats, animals were treated with different doses of chlorogenic acid in order to assess its potential neuroprotective effects against ischemia. The authors reported that the treated animals had lower brain infarction than the control rats. Moreover, sensory-motor function was improved by the high dose used (30 mg/kg i.p.). Additionally, chlorogenic acid treatment was found to reduce the pressure and compression on brain tissue, and attenuate lipid peroxidation in the brain [89]. Lee and others [89] also described a reduction in lipid peroxidation levels after chlorogenic acid treatment in ischemic rats. The free radical scavenging properties of the acid and inhibition of matrix metalloproteinase 2 and 9 activities are thought to a play a role in the neuroprotective effects of chlorogenic acid. Likewise, another study showed that chlorogenic acid (100 μg/kg i.p.) increased the signal of ribosomal proteins in the hippocampus (CA1 region), and slightly protected neuronal cells in the CA1 region when transient ischemia was induced in Mongolian gerbils [98].
CONCLUSION AND FUTURE PROSPECTS
This review summarises the neuroprotective effects of chlorogenic acid in both in vitro and in vivo models. The molecular mechanisms of the neuroprotective effects of chlorogenic acid are complex, and its bioavailability and clinical relevance remain unclear. However, it can be hypothesized that its beneficial effects are due to high antioxidant and anti-inflammatory activities. A search of the US governmental clinical trials database (https://clinicaltrials.gov/) with the keyword “chlorogenic acid” (accessed July 15, 2015) showed that there is only one recruited clinical trial regarding the clinical impacts of chlorogenic acid. In view of this, it may be difficult to make a clear decision about its clinical efficacy and to ascertain the most effective therapeutic dose for the treatment and management of diseases of the central nervous system. We recommend that future studies should focus on ascertaining (1) the bioavailability and metabolisms of chlorogenic acid; (2) molecular pathways underlying the neuroprotective effects of chlorogenic acid; (3) toxicological studies to determine the maximum non-fatal doses of chlorogenic acid; and finally (4) clinical studies to examine the efficacy of chlorogenic acid in cognitively impaired humans.
ACKNOWLEDGEMENTS
A. Sureda was supported by Programme of Promotion of Biomedical Research and Health Sciences, Project CIBEROBN CB12/03/30038.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Nabavi S.M., Nabavi S.F., Eslami S., Moghaddam A.H. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012;132(2):931–935. [http://dx.doi.org/10.1016/j.foodchem.2011.11.070]. [Google Scholar]
- 2.Nabavi S.F., Daglia M., Moghaddam A.H., Habtemariam S., Nabavi S.M. Curcumin and liver disease: from chemistry to medicine. Compr. Rev. Food Sci. Food Saf. 2014;13(1):62–77. doi: 10.1111/1541-4337.12047. [http://dx.doi.org/10.1111/1541-4337.12047]. [DOI] [PubMed] [Google Scholar]
- 3.Curti V., Capelli E., Boschi F., Nabavi S.F., Bongiorno A.I., Habtemariam S., Nabavi S.M., Daglia M. Modulation of human miR-173p expression by methyl 3-O-methyl gallate as explanation of its in vivo protective activities. Mol. Nutr. Food Res. 2014;58(9):1776–1784. doi: 10.1002/mnfr.201400007. [http://dx.doi.org/10.1002/mnfr.201400007]. [PMID: 24975036]. [DOI] [PubMed] [Google Scholar]
- 4.Nabavi S.F., Russo G.L., Daglia M., Nabavi S.M. Role of quercetin as an alternative for obesity treatment: you are what you eat. Food Chem. 2015;179:305–310. doi: 10.1016/j.foodchem.2015.02.006. [http://dx.doi.org/10.1016/ j.foodchem.2015.02.006]. [PMID: 25722169]. [DOI] [PubMed] [Google Scholar]
- 5.Nabavi S.F., Nabavi S.M., Mirzaei M., Moghaddam A.H. Protective effect of quercetin against sodium fluoride induced oxidative stress in rats heart. Food Funct. 2012;3(4):437–441. doi: 10.1039/c2fo10264a. [http://dx.doi.org/10.1039/c2fo10264a]. [PMID: 22314573]. [DOI] [PubMed] [Google Scholar]
- 6.Nabavi S.F., Nabavi S.M., Habtemariam S., Moghaddam A.H., Sureda A., Jafari M., Latifi A.M. Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress. Ind. Crops Prod. 2013;44:50–55. [http://dx.doi.org/10.1016/j.indcrop.2012.10.024]. [Google Scholar]
- 7.Pathak L., Agrawal Y., Dhir A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs. 2013;22(7):863–880. doi: 10.1517/13543784.2013.794783. [http://dx.doi.org/10.1517/13543784.2013. 794783]. [PMID: 23642183]. [DOI] [PubMed] [Google Scholar]
- 8.Orhan I.E., Daglia M., Nabavi S.F., Loizzo M.R., Sobarzo-Sánchez E., Nabavi S.M. Flavonoids and dementia: an update. Curr. Med. Chem. 2015;22(8):1004–1015. doi: 10.2174/0929867322666141212122352. [http://dx.doi.org/10. 2174/0929867322666141212122352]. [PMID: 25515512]. [DOI] [PubMed] [Google Scholar]
- 9.Russo M., Russo G.L., Daglia M., Kasi P.D., Ravi S., Nabavi S.F., Nabavi S.M. Understanding genistein in cancer: The good and the bad effects: A review. Food Chem. 2016;196:589–600. doi: 10.1016/j.foodchem.2015.09.085. [http://dx.doi.org/10.1016/j.foodchem.2015.09.085]. [PMID: 26593532]. [DOI] [PubMed] [Google Scholar]
- 10.Gasperotti M., Passamonti S., Tramer F., Masuero D., Guella G., Mattivi F., Vrhovsek U. Fate of microbial metabolites of dietary polyphenols in rats: is the brain their target destination? ACS Chem. Neurosci. 2015;6(8):1341–1352. doi: 10.1021/acschemneuro.5b00051. [http://dx.doi.org/10.1021/acschemneuro.5b00051]. [PMID: 25891864]. [DOI] [PubMed] [Google Scholar]
- 11.Devi K.P., Rajavel T., Nabavi S.F., Setzer W.N., Ahmadi A., Mansouri K., Nabavi S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015;76:582–589. [http://dx.doi.org/10.1016/j.indcrop.2015.07.051]. [Google Scholar]
- 12.Pasinetti G.M. Novel role of red wine-derived polyphenols in the prevention of Alzheimers disease dementia and brain pathology: experimental approaches and clinical implications. Planta Med. 2012;78(15):1614–1619. doi: 10.1055/s-0032-1315377. [http://dx.doi.org/10.1055/s-0032-1315377]. [PMID: 23023952]. [DOI] [PubMed] [Google Scholar]
- 13.Devi K.P., Malar D.S., Nabavi S.F., Sureda A., Xiao J., Nabavi S.M., Daglia M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015;99:1–10. doi: 10.1016/j.phrs.2015.05.002. [http://dx.doi.org/10. 1016/j.phrs.2015.05.002]. [PMID: 25982933]. [DOI] [PubMed] [Google Scholar]
- 14.Daglia M., Di Lorenzo A., Nabavi S.F., Talas Z.S., Nabavi S.M. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Curr. Pharm. Biotechnol. 2014;15(4):362–372. doi: 10.2174/138920101504140825120737. [http://dx.doi.org/10.2174/138920101504140825120737]. [PMID: 24938889]. [DOI] [PubMed] [Google Scholar]
- 15.Nabavi S.M., Habtemariam S., Daglia M., Braidy N., Loizzo M.R., Tundis R., Nabavi S.F. Neuroprotective effects of ginkgolide B against ischemic stroke: A review of current literature. Curr. Top. Med. Chem. 2015;15(21):2222–2232. doi: 10.2174/1568026615666150610142647. [http://dx.doi.org/10. 2174/1568026615666150610142647]. [PMID: 26059355]. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed T., Gilani A.U., Abdollahi M., Daglia M., Nabavi S.F., Nabavi S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Rep. 2015;67(5):970–979. doi: 10.1016/j.pharep.2015.03.002. [http://dx.doi.org/10.1016/ j.pharep.2015.03.002]. [PMID: 26398393]. [DOI] [PubMed] [Google Scholar]
- 17.Nabavi S.F., Sureda A., Habtemariam S., Nabavi S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J. Ginseng Res. 2015;39(4):299–303. doi: 10.1016/j.jgr.2015.02.002. [http://dx.doi.org/10.1016/ j.jgr.2015.02.002]. [PMID: 26869821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park R.J., Moon J.D. Coffee and depression in Korea: the fifth Korean National Health and Nutrition Examination Survey. Eur. J. Clin. Nutr. 2015;69(4):501–504. doi: 10.1038/ejcn.2014.247. [http://dx.doi.org/10.1038/ejcn. 2014.247]. [PMID: 25469468]. [DOI] [PubMed] [Google Scholar]
- 19.Park S.H., Sim Y.B., Han P.L., Lee J.K., Suh H.W. Antidepressant-like effect of chlorogenic acid isolated from Artemisia capillaris Thunb. Anim. Cells Syst. 2010;14(4):253–259. [http://dx.doi.org/10.1080/19768354.2010.528192]. [Google Scholar]
- 20.Ascherio A., Chen H., Schwarzschild M.A., Zhang S.M., Colditz G.A., Speizer F.E. Caffeine, postmenopausal estrogen, and risk of Parkinsons disease. Neurology. 2003;60(5):790–795. doi: 10.1212/01.wnl.0000046523.05125.87. [http://dx.doi.org/10.1212/01.WNL.0000046523.05125.87]. [PMID: 12629235]. [DOI] [PubMed] [Google Scholar]
- 21.Maia L., de Mendonça A. Does caffeine intake protect from Alzheimers disease? Eur. J. Neurol. 2002;9(4):377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [http://dx.doi.org/10.1046/j.1468-1331.2002.00421.x]. [PMID: 12099922]. [DOI] [PubMed] [Google Scholar]
- 22.Arendash G.W., Schleif W., Rezai-Zadeh K., Jackson E.K., Zacharia L.C., Cracchiolo J.R., Shippy D., Tan J. Caffeine protects Alzheimers mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience. 2006;142(4):941–952. doi: 10.1016/j.neuroscience.2006.07.021. [http://dx.doi.org/10.1016/j.neuroscience.2006.07.021]. [PMID: 16938404]. [DOI] [PubMed] [Google Scholar]
- 23.Kendir G., Dinç E., Güvenç A.K. Ultra-performance liquid chromatography for the simultaneous quantification of rutin and chlorogenic acid in leaves of Ribes L. species by conventional and chemometric calibration approaches. J. Chromatogr. Sci. 2015;53(9):1577–1587. doi: 10.1093/chromsci/bmv060. [http://dx.doi.org/10.1093/chromsci/bmv060]. [PMID: 26014965]. [DOI] [PubMed] [Google Scholar]
- 24.Moreira A.S., Coimbra M.A., Nunes F.M., Passos C.P., Santos S.A., Silvestre A.J., Silva A.M., Rangel M., Domingues M.R. Chlorogenic acid-arabinose hybrid domains in coffee melanoidins: Evidences from a model system. Food Chem. 2015;185:135–144. doi: 10.1016/j.foodchem.2015.03.086. [http://dx.doi.org/10.1016/j.foodchem.2015.03.086]. [PMID: 25952851]. [DOI] [PubMed] [Google Scholar]
- 25.Wianowska D., Typek R., Dawidowicz A.L. Chlorogenic acid stability in pressurized liquid extraction conditions. J. AOAC Int. 2015;98(2):415–421. doi: 10.5740/jaoacint.14-200. [http://dx.doi.org/10.5740/jaoacint.14-200]. [PMID: 25905748]. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Wen J., Zheng W., Zhao L., Fu X., Wang Z., Xiong Z., Li F., Xiao W. Simultaneous determination of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid and geniposide in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study after administration of Reduning injection. Biomed. Chromatogr. 2015;29(1):68–74. doi: 10.1002/bmc.3241. [http://dx.doi.org/10.1002/bmc.3241]. [PMID: 24842397]. [DOI] [PubMed] [Google Scholar]
- 27.Hao M.L., Pan N., Zhang Q.H., Wang X.H. Therapeutic efficacy of chlorogenic acid on cadmium-induced oxidative neuropathy in a murine model. Exp. Ther. Med. 2015;9(5):1887–1894. doi: 10.3892/etm.2015.2367. [PMID: 26136910]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang R., Hodgson J.M., Mas E., Croft K.D., Ward N.C. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J. Nutr. Biochem. 2016;27:53–60. doi: 10.1016/j.jnutbio.2015.08.017. [http://dx.doi.org/10.1016/ j.jnutbio.2015.08.017]. [PMID: 26386740]. [DOI] [PubMed] [Google Scholar]
- 29.Khan S., Baboota S., Ali J., Narang R.S., Narang J.K. Chlorogenic acid stabilized nanostructured lipid carriers (NLC) of atorvastatin: formulation, design and in vivo evaluation. Drug Dev. Ind. Pharm. 2016;42(2):209–220. doi: 10.3109/03639045.2015.1040414. [http://dx.doi.org/10.3109/ 03639045.2015.1040414]. [PMID: 26016780]. [DOI] [PubMed] [Google Scholar]
- 30.Xing L.N., Zhou M.M., Li Y., Shi X.W., Jia W. Recent progress of potential effects and mechanisms of chlorogenic acid and its intestinal metabolites on central nervous system diseases. Zhongguo Zhong yao za zhi. 2015;40(6):1044–1047. [PubMed] [Google Scholar]
- 31.Xu R., Kang Q., Ren J., Li Z., Xu X. Corrigendum to Antitumor Molecular Mechanism of Chlorogenic Acid on Inducting Genes GSK-3beta and APC and Inhibiting Gene beta-Catenin. . J. Anal. Methods Chem. 2013;2013:951319.. doi: 10.1155/2013/951319. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Yan Y., Li J., Han J., Hou N., Song Y., Dong L. Chlorogenic acid enhances the effects of 5-fluorouracil in human hepatocellular carcinoma cells through the inhibition of extracellular signal-regulated kinases. Anticancer Drugs. 2015;26(5):540–546. doi: 10.1097/CAD.0000000000000218. [http://dx.doi.org/10.1097/CAD.0000000000000218]. [PMID: 25714249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Z., Sheng Y., Lu B., Ji L. The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem. Biol. Interact. 2015;238:93–101. doi: 10.1016/j.cbi.2015.05.023. [http://dx.doi.org/10.1016/j.cbi.2015.05.023]. [PMID: 26079055]. [DOI] [PubMed] [Google Scholar]
- 34.Levy C.C., Zucker M. Cinnamyl and p-coumaryl esters as intermediates in the biosynthesis of chlorogenic acid. J. Biol. Chem. 1960;235(8):2418–2425. [PMID: 14416373]. [PubMed] [Google Scholar]
- 35.Hanson K.R., Zucker M. The biosynthesis of chlorogenic acid and related conjugates of the hydroxycinnamic acids. Chromatographic separation and characterization. J. Biol. Chem. 1963;238(3):1105–1115. [PMID: 13952639]. [PubMed] [Google Scholar]
- 36.Lepelley M., Cheminade G., Tremillon N., Simkin A., Caillet V., McCarthy J. Chlorogenic acid synthesis in coffee: An analysis of CGA content and real-time RT-PCR expression of HCT, HQT, C3H1, and CCoAOMT1 genes during grain development in C. canephora. Plant Sci. 2007;172(5):978–996. [http://dx.doi.org/10.1016/j.plantsci.2007.02.004]. [Google Scholar]
- 37.Valiñas M.A., Lanteri M.L., ten Have A., Andreu A.B. Chlorogenic acid biosynthesis appears linked with suberin production in potato tuber (Solanum tuberosum). J. Agric. Food Chem. 2015;63(19):4902–4913. doi: 10.1021/jf505777p. [http://dx.doi.org/10.1021/jf505777p]. [PMID: 25921651]. [DOI] [PubMed] [Google Scholar]
- 38.Lamb C.J., Rubery P.H. Photocontrol of chlorogenic acid biosynthesis in potato tuber discs. Phytochemistry. 1976;15(5):665–668. [http://dx.doi.org/10.1016/S0031-9422(00)94416-9]. [Google Scholar]
- 39.Clé C., Hill L.M., Niggeweg R., Martin C.R., Guisez Y., Prinsen E., Jansen M.A. Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum; consequences for phenolic accumulation and UV-tolerance. Phytochemistry. 2008;69(11):2149–2156. doi: 10.1016/j.phytochem.2008.04.024. [http://dx.doi.org/10.1016/j.phytochem.2008.04.024]. [PMID: 18513762]. [DOI] [PubMed] [Google Scholar]
- 40.Mahesh V., Million-Rousseau R., Ullmann P., Chabrillange N., Bustamante J., Mondolot L., Morant M., Noirot M., Hamon S., de Kochko A., Werck-Reichhart D., Campa C. Functional characterization of two p-coumaroyl ester 3-hydroxylase genes from coffee tree: evidence of a candidate for chlorogenic acid biosynthesis. Plant Mol. Biol. 2007;64(1-2):145–159. doi: 10.1007/s11103-007-9141-3. [http://dx. doi.org/10.1007/s11103-007-9141-3]. [PMID: 17333503]. [DOI] [PubMed] [Google Scholar]
- 41.Stöckigt J., Zenk M.H. Enzymatic synthesis of chlorogenic acid from caffeoyl coenzyme A and quinic acid. FEBS Lett. 1974;42(2):131–134. doi: 10.1016/0014-5793(74)80769-6. [http://dx.doi.org/10.1016/0014-5793(74)80769-6]. [PMID: 4851874]. [DOI] [PubMed] [Google Scholar]
- 42.Preedy V.R. Coffee in Health and Disease Prevention. Academic Press; 2014. [Google Scholar]
- 43.Preedy V.R. Processing and Impact on Active Components in Food. Elsevier Science; 2014. [Google Scholar]
- 44.Dao L., Friedman M. Chlorogenic acid content of fresh and processed potatoes determined by ultraviolet spectrophotometry. J. Agric. Food Chem. 1992;40(11):2152–2156. [http://dx.doi.org/10. 1021/jf00023a022]. [Google Scholar]
- 45.Awad M.A., de Jager A., van Westing L.M. Flavonoid and chlorogenic acid levels in apple fruit: characterisation of variation. Sci. Hortic. (Amsterdam) 2000;83(3):249–263. [http://dx.doi.org/10.1016/S0304-4238(99)00124-7]. [Google Scholar]
- 46.Nakatani N., Kayano S., Kikuzaki H., Sumino K., Katagiri K., Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000;48(11):5512–5516. doi: 10.1021/jf000422s. [http://dx.doi.org/10.1021/jf000422s]. [PMID: 11087511]. [DOI] [PubMed] [Google Scholar]
- 47.Milić B., Stojanović S., Vučurević N., Turčić M. Chlorogenic and quinic acids in sunflower meal. J. Sci. Food Agric. 1968;19(2):108–113. [http://dx.doi.org/10.1002/jsfa.2740190211]. [Google Scholar]
- 48.Sun P.C., Liu Y., Yi Y.T., Li H.J., Fan P., Xia C.H. Preliminary enrichment and separation of chlorogenic acid from Helianthus tuberosus L. leaves extract by macroporous resins. Food Chem. 2015;168:55–62. doi: 10.1016/j.foodchem.2014.07.038. [http://dx.doi.org/10.1016/ j.foodchem.2014.07.038]. [PMID: 25172683]. [DOI] [PubMed] [Google Scholar]
- 49.Zheng W., Clifford M.N. Profiling the chlorogenic acids of sweet potato (Ipomoea batatas) from China. Food Chem. 2008;106(1):147–152. [http://dx.doi.org/10.1016/j.foodchem.2007.05.053]. [Google Scholar]
- 50.Wang T., Jiang X., Yang L., Wu S. pH-gradient counter-current chromatography isolation of natural antioxidant chlorogenic acid from Lonicera japonica Thumb. using an upright coil planet centrifuge with three multi-layer coils connected in series. J. Chromatogr. A. 2008;1180(1-2):53–58. doi: 10.1016/j.chroma.2007.11.112. [http://dx.doi.org/10.1016/ j.chroma.2007.11.112]. [PMID: 18160073]. [DOI] [PubMed] [Google Scholar]
- 51.Zhang B., Yang R., Liu C.Z. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Separ. Purif. Tech. 2008;62(2):480–483. [http://dx.doi.org/10. 1016/j.seppur.2008.02.013]. [Google Scholar]
- 52.Xiang Z., Ning Z. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT-. Food Sci. Technol. (Campinas.) 2008;41(7):1189–1203. [Google Scholar]
- 53.de Melo L.V., Sawaya A.C. UHPLC–MS quantification of coumarin and chlorogenic acid in extracts of the medicinal plants known as guaco (Mikania glomerata and Mikania laevigata). Rev. Bras. Farmacogn. 2015;25(2):105–110. [http://dx.doi.org/10. 1016/j.bjp.2015.02.005]. [Google Scholar]
- 54.Wang Z., Clifford M.N., Sharp P. Analysis of chlorogenic acids in beverages prepared from Chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured Caco-2 cells. Food Chem. 2008;108(1):369–373. [http://dx.doi.org/10.1016/ j.foodchem.2007.10.083]. [Google Scholar]
- 55.Clifford M.N., Wu W., Kirkpatrick J., Jaiswal R., Kuhnert N. Profiling and characterisation by liquid chromatography/multi-stage mass spectrometry of the chlorogenic acids in Gardeniae Fructus. Rapid Commun. Mass Spectrom. 2010;24(21):3109–3120. doi: 10.1002/rcm.4751. [http://dx.doi.org/10.1002/rcm.4751]. [PMID: 20941757]. [DOI] [PubMed] [Google Scholar]
- 56.Stalmach A., Williamson G., Crozier A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014;5(8):1727–1737. doi: 10.1039/c4fo00316k. [http://dx.doi.org/10.1039/C4FO00316K]. [PMID: 24947504]. [DOI] [PubMed] [Google Scholar]
- 57.Stalmach A., Mullen W., Barron D., Uchida K., Yokota T., Cavin C., Steiling H., Williamson G., Crozier A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009;37(8):1749–1758. doi: 10.1124/dmd.109.028019. [http://dx.doi.org/10.1124/dmd.109.028019]. [PMID: 19460943]. [DOI] [PubMed] [Google Scholar]
- 58.Olthof M.R., Hollman P.C., Katan M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001;131(1):66–71. doi: 10.1093/jn/131.1.66. [PMID: 11208940]. [DOI] [PubMed] [Google Scholar]
- 59.Couteau D., McCartney A.L., Gibson G.R., Williamson G., Faulds C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001;90(6):873–881. doi: 10.1046/j.1365-2672.2001.01316.x. [http://dx.doi.org/10.1046/j.1365-2672.2001. 01316.x]. [PMID: 11412317]. [DOI] [PubMed] [Google Scholar]
- 60.Farrell T.L., Dew T.P., Poquet L., Hanson P., Williamson G. Absorption and metabolism of chlorogenic acids in cultured gastric epithelial monolayers. Drug Metab. Dispos. 2011;39(12):2338–2346. doi: 10.1124/dmd.111.040147. [http://dx.doi.org/10.1124/dmd.111.040147]. [PMID: 21937734]. [DOI] [PubMed] [Google Scholar]
- 61.Olthof M.R., Hollman P.C., Buijsman M.N., van Amelsvoort J.M., Katan M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003;133(6):1806–1814. doi: 10.1093/jn/133.6.1806. [PMID: 12771321]. [DOI] [PubMed] [Google Scholar]
- 62.Erk T., Williamson G., Renouf M., Marmet C., Steiling H., Dionisi F., Barron D., Melcher R., Richling E. Dose-dependent absorption of chlorogenic acids in the small intestine assessed by coffee consumption in ileostomists. Mol. Nutr. Food Res. 2012;56(10):1488–1500. doi: 10.1002/mnfr.201200222. [http://dx.doi.org/10.1002/mnfr.201200222]. [PMID: 22945604]. [DOI] [PubMed] [Google Scholar]
- 63.Renouf M., Marmet C., Giuffrida F., Lepage M., Barron D., Beaumont M., Williamson G., Dionisi F. Dose-response plasma appearance of coffee chlorogenic and phenolic acids in adults. Mol. Nutr. Food Res. 2014;58(2):301–309. doi: 10.1002/mnfr.201300349. [http://dx.doi.org/10.1002/ mnfr.201300349]. [PMID: 24039147]. [DOI] [PubMed] [Google Scholar]
- 64.Azuma K., Ippoushi K., Nakayama M., Ito H., Higashio H., Terao J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000;48(11):5496–5500. doi: 10.1021/jf000483q. [http://dx.doi.org/10.1021/jf000483q]. [PMID: 11087508]. [DOI] [PubMed] [Google Scholar]
- 65.Lafay S., Morand C., Manach C., Besson C., Scalbert A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006;96(1):39–46. doi: 10.1079/bjn20061714. [http://dx.doi.org/10.1079/BJN20061714]. [PMID: 16869989]. [DOI] [PubMed] [Google Scholar]
- 66.Gonthier M.P., Verny M.A., Besson C., Rémésy C., Scalbert A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003;133(6):1853–1859. doi: 10.1093/jn/133.6.1853. [PMID: 12771329]. [DOI] [PubMed] [Google Scholar]
- 67.Monteiro M., Farah A., Perrone D., Trugo L.C., Donangelo C. Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J. Nutr. 2007;137(10):2196–2201. doi: 10.1093/jn/137.10.2196. [PMID: 17884997]. [DOI] [PubMed] [Google Scholar]
- 68.Naso L.G., Valcarcel M., Roura-Ferrer M., Kortazar D., Salado C., Lezama L., Rojo T., González-Baró A.C., Williams P.A., Ferrer E.G. Promising antioxidant and anticancer (human breast cancer) oxidovanadium(IV) complex of chlorogenic acid. Synthesis, characterization and spectroscopic examination on the transport mechanism with bovine serum albumin. J. Inorg. Biochem. 2014;135:86–99. doi: 10.1016/j.jinorgbio.2014.02.013. [http://dx.doi.org/10.1016/j.jinorgbio. 2014.02.013]. [PMID: 24681549]. [DOI] [PubMed] [Google Scholar]
- 69.Xu J.G., Hu Q.P., Liu Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012;60(46):11625–11630. doi: 10.1021/jf303771s. [http://dx.doi.org/10.1021/jf303771s]. [PMID: 23134416]. [DOI] [PubMed] [Google Scholar]
- 70.Sato Y., Itagaki S., Kurokawa T., Ogura J., Kobayashi M., Hirano T., Sugawara M., Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011;403(1-2):136–138. doi: 10.1016/j.ijpharm.2010.09.035. [http://dx.doi.org/10.1016/j.ijpharm.2010. 09.035]. [PMID: 20933071]. [DOI] [PubMed] [Google Scholar]
- 71.Andre C.M., Oufir M., Guignard C., Hoffmann L., Hausman J.F., Evers D., Larondelle Y. Antioxidant profiling of native Andean potato tubers (Solanum tuberosum L.) reveals cultivars with high levels of beta-carotene, alpha-tocopherol, chlorogenic acid, and petanin. J. Agric. Food Chem. 2007;55(26):10839–10849. doi: 10.1021/jf0726583. [http://dx.doi.org/10.1021/jf0726583]. [PMID: 18044831]. [DOI] [PubMed] [Google Scholar]
- 72.Jung H.A., Park J.C., Chung H.Y., Kim J., Choi J.S. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arch. Pharm. Res. 1999;22(2):213–218. doi: 10.1007/BF02976549. [http://dx.doi.org/10.1007/BF02976549]. [PMID: 10230515]. [DOI] [PubMed] [Google Scholar]
- 73.Kono Y., Kobayashi K., Tagawa S., Adachi K., Ueda A., Sawa Y., Shibata H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta. 1997;1335(3):335–342. doi: 10.1016/s0304-4165(96)00151-1. [http://dx.doi.org/10.1016/S0304-4165 (96)00151-1]. [PMID: 9202196]. [DOI] [PubMed] [Google Scholar]
- 74.Kim S.S., Lee C.K., Kang S.S., Jung H.A., Choi J.S. Chlorogenic acid, an antioxidant principle from the aerial parts of Artemisia iwayomogi that acts on 1,1-diphenyl-2-picrylhydrazyl radical. Arch. Pharm. Res. 1997;20(2):148–154. doi: 10.1007/BF02974002. [http://dx.doi.org/10.1007/BF02974002]. [PMID: 18975193]. [DOI] [PubMed] [Google Scholar]
- 75.Zang L.Y., Cosma G., Gardner H., Castranova V., Vallyathan V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003;247(1-2):205–210. doi: 10.1023/a:1024103428348. [http://dx.doi.org/10.1023/ A:1024103428348]. [PMID: 12841649]. [DOI] [PubMed] [Google Scholar]
- 76.Nenadis N., Wang L.F., Tsimidou M., Zhang H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS(*+) assay. J. Agric. Food Chem. 2004;52(15):4669–4674. doi: 10.1021/jf0400056. [http://dx. doi.org/10.1021/jf0400056]. [PMID: 15264898]. [DOI] [PubMed] [Google Scholar]
- 77.Bakalbassis E.G., Chatzopoulou A., Melissas V.S., Tsimidou M., Tsolaki M., Vafiadis A. Ab initio and density functional theory studies for the explanation of the antioxidant activity of certain phenolic acids. Lipids. 2001;36(2):181–190. doi: 10.1007/s11745-001-0705-9. [http://dx.doi.org/10.1007/s11745-001-0705-9]. [PMID: 11269699]. [DOI] [PubMed] [Google Scholar]
- 78.Leopoldini M., Chiodo S.G., Russo N., Toscano M. Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J. Chem. Theory Comput. 2011;7(12):4218–4233. doi: 10.1021/ct200572p. [http://dx.doi.org/10.1021/ct200572p]. [PMID: 26598362]. [DOI] [PubMed] [Google Scholar]
- 79.Hynes M.J. OCoinceanainn, M. The kinetics and mechanisms of reactions of iron(III) with caffeic acid, chlorogenic acid, sinapic acid, ferulic acid and naringin. J. Inorg. Biochem. 2004;98(8):1457–1464. doi: 10.1016/j.jinorgbio.2004.05.009. [http://dx.doi.org/10.1016/j.jinorgbio.2004.05.009]. [PMID: 15271524]. [DOI] [PubMed] [Google Scholar]
- 80.Fan G.J., Jin X.L., Qian Y.P., Wang Q., Yang R.T., Dai F., Tang J.J., Shang Y.J., Cheng L.X., Yang J. Hydroxycinnamic acids as DNA cleaving agents in the presence of cuii ions: Mechanism, structure–activity relationship, and biological implications. Chemistry-A Eur. J. 2009;15(46):12889–12889. doi: 10.1002/chem.200901627. [DOI] [PubMed] [Google Scholar]
- 81.Namazian M., Zare H.R. Electrochemistry of chlorogenic acid: experimental and theoretical studies. Electrochim. Acta. 2005;50(22):4350–4355. [http://dx.doi.org/10.1016/j.electacta.2005.01.043]. [Google Scholar]
- 82.Oszmianski J., Lee C.Y. Enzymic oxidative reaction of catechin and chlorogenic acid in a model system. J. Agric. Food Chem. 1990;38(5):1202–1204. [http://dx.doi.org/10.1021/jf00095a009]. [Google Scholar]
- 83.Richard-Forget F.C., Gauillard F.A. Oxidation of chlorogenic acid, catechins, and 4-methylcatechol in model solutions by combinations of pear (Pyrus communis cv. Williams) polyphenol oxidase and peroxidase: a possible involvement of peroxidase in enzymatic browning. J. Agric. Food Chem. 1997;45(7):2472–2476. [http://dx.doi.org/10.1021/jf970042f]. [Google Scholar]
- 84.Richard-Forget F.C., Rouet-Mayer M.A., Goupy P.M., Philippon J., Nicolas J.J. Oxidation of chlorogenic acid, catechins, and 4-methylcatechol in model solutions by apple polyphenol oxidase. J. Agric. Food Chem. 1992;40(11):2114–2122. [http://dx.doi.org/10.1021/jf00023a015]. [Google Scholar]
- 85.Pierpoint W.S. The enzymic oxidation of chlorogenic acid and some reactions of the quinone produced. Biochem. J. 1966;98(2):567–580. doi: 10.1042/bj0980567. [http://dx.doi.org/10.1042/bj0980567]. [PMID: 5941350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moridani M.Y., Scobie H., Jamshidzadeh A., Salehi P., OBrien P.J. Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: glutathione conjugate formation. Drug Metab. Dispos. 2001;29(11):1432–1439. [PMID: 11602518]. [PubMed] [Google Scholar]
- 87.de Paulis T., Schmidt D.E., Bruchey A.K., Kirby M.T., McDonald M.P., Commers P., Lovinger D.M., Martin P.R. Dicinnamoylquinides in roasted coffee inhibit the human adenosine transporter. Eur. J. Pharmacol. 2002;442(3):215–223. doi: 10.1016/s0014-2999(02)01540-6. [http://dx. doi.org/10.1016/S0014-2999(02)01540-6]. [PMID: 12065074]. [DOI] [PubMed] [Google Scholar]
- 88.Ohnishi R., Ito H., Iguchi A., Shinomiya K., Kamei C., Hatano T., Yoshida T. Effects of chlorogenic acid and its metabolites on spontaneous locomotor activity in mice. Biosci. Biotechnol. Biochem. 2006;70(10):2560–2563. doi: 10.1271/bbb.60243. [http://dx.doi.org/10.1271/ bbb.60243]. [PMID: 17031047]. [DOI] [PubMed] [Google Scholar]
- 89.Lee K., Lee J.S., Jang H.J., Kim S.M., Chang M.S., Park S.H., Kim K.S., Bae J., Park J.W., Lee B., Choi H.Y., Jeong C.H., Bu Y. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012;689(1-3):89–95. doi: 10.1016/j.ejphar.2012.05.028. [http://dx.doi.org/10.1016/j.ejphar.2012.05.028]. [PMID: 22659584]. [DOI] [PubMed] [Google Scholar]
- 90.Cropley V., Croft R., Silber B., Neale C., Scholey A., Stough C., Schmitt J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology (Berl.) 2012;219(3):737–749. doi: 10.1007/s00213-011-2395-0. [http://dx.doi.org/10.1007/s00213-011-2395-0]. [PMID: 21773723]. [DOI] [PubMed] [Google Scholar]
- 91.Camfield D.A., Silber B.Y., Scholey A.B., Nolidin K., Goh A., Stough C. A randomised placebo-controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS One. 2013;8(12):e82897. doi: 10.1371/journal.pone.0082897. [http://dx. doi.org/10.1371/journal.pone.0082897]. [PMID: 24349389]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouayed J., Rammal H., Dicko A., Younos C., Soulimani R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007;262(1-2):77–84. doi: 10.1016/j.jns.2007.06.028. [http://dx.doi.org/10.1016/j.jns.2007.06.028]. [PMID: 17698084]. [DOI] [PubMed] [Google Scholar]
- 93.Petersen R.B., Nunomura A., Lee H.G., Casadesus G., Perry G., Smith M.A., Zhu X. Signal transduction cascades associated with oxidative stress in Alzheimers disease. J. Alzheimers Dis. 2007;11(2):143–152. doi: 10.3233/jad-2007-11202. [PMID: 17522439]. [DOI] [PubMed] [Google Scholar]
- 94.Cho E.S., Jang Y.J., Hwang M.K., Kang N.J., Lee K.W., Lee H.J. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat. Res. 2009;661(1-2):18–24. doi: 10.1016/j.mrfmmm.2008.10.021. [http://dx.doi.org/10.1016/j.mrfmmm.2008.10.021]. [PMID: 19028509]. [DOI] [PubMed] [Google Scholar]
- 95.Wu C., Luan H., Zhang X., Wang S., Zhang X., Sun X., Guo P. Chlorogenic acid protects against atherosclerosis in ApoE-/- mice and promotes cholesterol efflux from RAW264.7 macrophages. PLoS One. 2014;9(9):e95452. doi: 10.1371/journal.pone.0095452. [http://dx.doi.org/10.1371/journal. pone.0095452]. [PMID: 25187964]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lapchak P.A. The phenylpropanoid micronutrient chlorogenic acid improves clinical rating scores in rabbits following multiple infarct ischemic strokes: synergism with tissue plasminogen activator. Exp. Neurol. 2007;205(2):407–413. doi: 10.1016/j.expneurol.2007.02.017. [http://dx.doi.org/10.1016/ j.expneurol.2007.02.017]. [PMID: 17439814]. [DOI] [PubMed] [Google Scholar]
- 97.Oboh G., Agunloye O.M., Akinyemi A.J., Ademiluyi A.O., Adefegha S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimers disease and some pro-oxidant induced oxidative stress in rats brain-in vitro. Neurochem. Res. 2013;38(2):413–419. doi: 10.1007/s11064-012-0935-6. [http://dx.doi.org/10.1007/s11064-012-0935-6]. [PMID: 23184188]. [DOI] [PubMed] [Google Scholar]
- 98.Ahn E.H., Kim D.W., Shin M.J., Kwon S.W., Kim Y.N., Kim D.S., Lim S.S., Kim J., Park J., Eum W.S., Hwang H.S., Choi S.Y. Chlorogenic acid improves neuroprotective effect of PEP-1-ribosomal protein S3 against ischemic insult. Exp. Neurobiol. 2011;20(4):169–175. doi: 10.5607/en.2011.20.4.169. [http://dx.doi.org/10.5607/en.2011.20.4.169]. [PMID: 22355261]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han J., Miyamae Y., Shigemori H., Isoda H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience. 2010;169(3):1039–1045. doi: 10.1016/j.neuroscience.2010.05.049. [http://dx.doi.org/10.1016/j.neuroscience.2010.05.049]. [PMID: 20570715]. [DOI] [PubMed] [Google Scholar]
- 100.Gray N.E., Morré J., Kelley J., Maier C.S., Stevens J.F., Quinn J.F., Soumyanath A. Caffeoylquinic acids in Centella asiatica protect against amyloid-β toxicity. J. Alzheimers Dis. 2014;40(2):359–373. doi: 10.3233/JAD-131913. [PMID: 24448790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teraoka M., Nakaso K., Kusumoto C., Katano S., Tajima N., Yamashita A., Zushi T., Ito S., Matsura T. Cytoprotective effect of chlorogenic acid against α-synuclein-related toxicity in catecholaminergic PC12 cells. J. Clin. Biochem. Nutr. 2012;51(2):122–127. doi: 10.3164/jcbn.D-11-00030. [http://dx.doi.org/10.3164/jcbn.D-11-00030]. [PMID: 22962530]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Masuda M., Hasegawa M., Nonaka T., Oikawa T., Yonetani M., Yamaguchi Y., Kato K., Hisanaga S., Goedert M. Inhibition of α-synuclein fibril assembly by small molecules: analysis using epitope-specific antibodies. FEBS Lett. 2009;583(4):787–791. doi: 10.1016/j.febslet.2009.01.037. [http://dx.doi.org/10.1016/j.febslet.2009.01.037]. [PMID: 19183551]. [DOI] [PubMed] [Google Scholar]
- 103.Konishi Y., Kobayashi S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004;52(9):2518–2526. doi: 10.1021/jf035407c. [http://dx.doi.org/10.1021/jf035407c]. [PMID: 15113150]. [DOI] [PubMed] [Google Scholar]
- 104.Farah A., Monteiro M., Donangelo C.M., Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008;138(12):2309–2315. doi: 10.3945/jn.108.095554. [http://dx.doi.org/10.3945/ jn.108.095554]. [PMID: 19022950]. [DOI] [PubMed] [Google Scholar]