Abstract
Background
Shared connections between physical activity and neuroprotection have been studied for decades, but the mechanisms underlying this effect of specific exercise were only recently brought to light. Several evidences suggest that physical activity may be a reasonable and beneficial method to improve functional recovery in both peripheral and central nerve injuries and to delay functional decay in neurodegenerative diseases. In addition to improving cardiac and immune functions, physical activity may represent a multifunctional approach not only to improve cardiocirculatory and immune functions, but potentially modulating trophic factors signaling and, in turn, neuronal function and structure at times that may be critical for neurodegeneration and regeneration.
Methods
Research content related to the effects of physical activity and specific exercise programs in normal and injured nervous system have been reviewed.
Results
Sustained exercise, particularly if applied at moderate intensity and early after injury, exerts anti-inflammatory and pro-regenerative effects, and may boost cognitive and motor functions in aging and neurological disorders. However, newest studies show that exercise modalities can differently affect the production and function of brain-derived neurotrophic factor and other neurotrophins involved in the generation of neuropathic conditions. These findings suggest the possibility that new exercise strategies can be directed to nerve injuries with therapeutical benefits.
Conclusion
Considering the growing burden of illness worldwide, understanding of how modulation of neurotrophic factors contributes to exercise-induced neuroprotection and regeneration after peripheral nerve and spinal cord injuries is a relevant topic for research, and represents the beginning of a new non-pharmacological therapeutic approach for better rehabilitation of neural disorders.
Keywords: Exercise, inflammation, neurotrophic factors, neuropathic pain, nerve injury, nerve regeneration, survival
1. INTRODUCTION
Exercise is able to generate endogenous neuroprotection through the activation of multiple mechanisms, such as promoting neurogenesis, improving the neurovascular unit integrity, decreasing apoptosis, and modulating inflammation. Through these mechanisms, exercise can be used as a treatment to protect from nerve damage and stroke, and to promote regeneration of injured axons and reduce neuropathic pain. In general, exercise is conceived to provide substantial neuroprotection by means of several mechanisms that induce neuronal survival. This machinery is mainly focused on the neurovascular unit, consisting of neuronal, glial and vascular cells. Neurovascular unit is clearly reinforced by exercise training but its stability is compromised by nerve lesions. Excessive excitability, chronic inflammation and microgliosis, added to maladaptive plasticity of injured nerve pathways, are major contributors of neural disorders and neuropathic pain conditions. In this scenario, neuroprotection is conveyed by exercise partly through the upregulation of neurotrophins expression, including brain derived neurotrophic factor (BDNF), nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF). These important regulatory proteins increase neurogenesis, strengthening the neuronal network and potentiating the regenerative responses to nerve insult. Exercise modalities specific to the type of injury can convey more potent neuroprotection, and animal studies have recently started to tune up training paradigms of different intensities and at critical time windows for treating injuries to the peripheral and central nervous systems. This seems also to open a strategy for setting up an endo-pharmacology of pain and regeneration, based on the modulation of the endogenous levels of neurotrophins by means of different exercise protocols.
This may depend on the dual role that neurotrophic factors (particularly BDNF and NGF) play after nerve injury, and the opposite effects they can exert on stimulation of neurogenesis and regeneration but also on inhibition of chronic pain and maladaptive plasticity.
2. MECHANISMS OF EXERCISE-INDUCED NEURO-PROTECTION
2.1. Effects on the Neurovascular Unit
Exercise based therapies assume that repeated neuronal activity has to be supported by the neurovascular unit, then gradual training can induce an effective adaptation and reinforcement of its integrity as a whole. The blood brain barrier (BBB) constitutes a strong filtration mechanism where endothelial cells, the basal lamina and astroglial cells work together to preserve neurovascular integrity in case of a neuronal damage. Exercise induces reduction of physical damage to the neurovascular unit, and this effect is conveyed by strengthening the BBB [1, 2]. The integrity of the BBB is foremost to maintaining appropriate filtration of supplying and toxic molecules from the vascular system and in providing an indispensable structure to the unit.
Various proteins of the extracellular matrix compose the basal lamina, including collagen type IV as a major component, proteoglycan, heparan sulphate, laminin and fibronectin, which are provided by astrocytes and endothelial cells. When this membrane is damaged, its ability to selectively permeate nutrients in the neurovascular unit is compromised, this is diagnosable with a vasogenic edema. Under ischemic conditions, the loss of selective permeability and sustained edema lead to cellular swelling. In the brain, endothelial cells and astrocytes separate from the basal lamina to promote the discharge of vascular constituents into the cerebral interstitial space [2]. Exercise training enhances the basal lamina increasing both firmness and stability to the BBB [3]. Following CNS injury, these effects are combined to decrease cerebral stroke and edema volume, and enhance neuronal recovery. Collagen type IV is found to be upregulated in exercised rats, and exercise reduced its net loss after stroke [3]. The increase of collagen type IV in exercised animals was correlated also to a reduction of behavioral deficits after stroke [3].
Integrins add stability to the basal lamina and the BBB, and provide further reinforcement to the neurovascular unit. Composed of α and β heterodimers, these proteins act as cell adhesion molecules and make adherence within extracellular matrix and basal lamina, maintaining the integrity of these structures [4, 5]. Integrins serve as receptors for several proteins and ligands within the basal lamina matrix, mainly collagen and laminin [2, 5, 6]. They also serve as signaling receptors for anchoring endothelial cells and astrocytes together, allowing dynamic changes of the BBB in response to exercise, injury and pain. In this regard, exercised rats showed significantly higher integrins expression in astroglia and endothelium following muscle [7, 8] and CNS damage [6], correlating with a decrease in the behavioral deficit [9].
Astrocytes play a key role in maintaining the neuronal framework. Since chronic exercise induces astrocytosis, often observed both in brain [10] and in spinal cord [11], part of the neuroprotective function played by astrocytes is supposed to be increased by physical activity in order to strengthen the BBB and the neurovascular unit. First, astrocytes provide a rigid framework to withstand traumas of the neurovascular unit. Their expansions cover the 90% of the brain vascular surface with a primary function in restricting permeability of the insulted BBB [2, 12-14]. Exercise-induced astocytosis is associated to better outcomes in recovery from injuries [10]. It seems then that exercise training can transform the neurovascular system and develop a vital metabolic response to CNS injury.
Angiogenesis and endothelial cell proliferation are normally scant in the adult brain. Previous studies showed that locomotion on a treadmill can increase the density of blood vessel [15-18] and cortical and striatal angiogenesis [19, 20] in the brain. In addition exercise can increase arteriogenesis, which promotes cerebral and peripheral blood flow and attenuates neuronal injury and degeneration [21, 22]. By increasing the metabolic demand, exercise may swell up the blood supply allowing the enhanced delivery of nutrients to stimulated neurons and promoting further angiogenesis [16, 23]. These changes may reduce brain damage as well [19]. Exercise improves blood flow overall in the whole body, resulting in higher levels of neurotrophic factors and activation of Schwann cells in peripheral nerves [24, 25]. Neovascularization and increased peripheral blood flow induced by physical activity are also consequences of the increased expression of angiogenesis related genes in skeletal muscles [26]. The anatomical modifications seen in angiogenesis are compelled by regulatory growth factors and proteins, namely vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1) and angiopoietins (Ang) 1 and 2. VEGF and IGF-1 expression is increased in the periphery by physical activity stimulating these factors to cross the BBB and enter the brain [27-29]. Blocking either VEGF [28] or IGF-1 [27] peripheral entry to the brain prevented the proliferation of neural precursors in the hippocampus induced by exercise, along to reduced promotion of the survival signaling that exercise induces by means of neural precursors generation [27]. VEGF mRNA expression is exponentially higher with increasing duration of exercise training, and exercise angiogenesis is shown parallel to an increase of brain VEGF mRNA and protein [28, 29]. This effect of exercise stimulates potent mitotic activity on vascular endothelial cells, affecting proliferation, survival, adhesion, migration and formation of capillary tubes [30]. BDNF seems to participate in neurogenesis or angiogenesis during exercise, since the stimulation of BDNF participates also to increase proliferation and survival of new neurons and BDNF itself can regulate baseline neurogenesis in vivo [31].
2.2. Effects on the Inflammatory Response
The neuroprotective action of physical activity has been linked to the ability of preventing and modulating inflammatory conditions [32]. Several studies described fewer viral and bacterial infections [33, 34], and lower incidence of systemic low-grade inflammation [34, 35] as well as neurodegeneration and cognitive decline [36] in individuals who regularly practice physical activities. Experimental studies converge on demonstrating that sustained physical activity includes general enhancement of immune function and anti-inflammatory processes strongly enhancing neuroprotection. These effects seem to depend upon two critical parameters: intensity and duration. Through them, exercise can gradually mold and fix the inflammatory process, and lead to reduction in chronic inflammation if daily repeated and continuous. Conversely, acute exercise may promote or reduce inflammation by impacting the inflammatory cascade and increasing with intensity the release of pro-inflammatory or anti-inflammatory cytokines respectively [37]. Kohut et al. [38] followed older adults participating in aerobic or flexibility exercise along to 10 months, and found that physical activity induced significant reduction of interleukin 6 (IL-6), interleukin 8 (IL-8), C-reactive protein (CRP) and tumor necrosis factor α (TNFα) plasma levels, linking chronic exercise to anti-inflammatory effects. However, a meta-analysis [39] reported that a single bout of exercise enhanced the inflammatory response in patients diagnosed of type I diabetes mellitus, cystic fibrosis, and chronic obstructive pulmonary disease, whereas participation in chronic endurance training programs was associated to attenuation of the systemic inflammatory response in patients with chronic heart failure and type II diabetes mellitus. Thus, the analysis of exercise effects on particular inflammatory disorders should be conducted in relation to the mechanism by which inflammatory mediators and modulators are produced in the pathology and by the type of exercise.
2.2.1. Effects on Inflammatory Modulators
The enduring effects of chronic exercise could be attributed to the counteraction of inflammation played by acute bouts of exercise, which is partly mediated by IL-6 in addition to the neurovascular supply. IL-6 is a cytokine that may convey both pro- and anti-inflammatory effects, and is provided in peripheral tissue by T-cells, macrophages, fibroblasts, endothelial cells and osteoblasts [40]. IL-6 plays a clear role in the metabolic control of muscle cells and is released in response to eccentric muscle contraction [41]. Moderate aerobic exercise induces release of IL-6 from muscles, and its circulating levels can increase up to 100-fold during up to 1h following activity [42]. Other studies indicate that IL-6 similarly increases even after exhausting exercise, such as a marathon. Normal concentrations of IL-6 stimulate the circulation of anti-inflammatory cytokines IL-1α and IL-10, and may also inhibit the production of pro-inflammatory cytokine TNFα [42]. On the other side, IL-6 stimulates lipolysis and fat oxidation. The neuroprotective effects of exercise-induced pro-inflammatory IL-6 was first related to TNFα- insulin resistance. IL-6 mediated inhibition of TNFα production was suggested by in vitro [43], animal [44, 45] and human [46] studies. IL-6 easily crosses the BBB [47] and can introduce functional alterations on neurons and glial cells, with controversial effects on neuroprotection. Overexpression of IL-6 in mouse astrocytes has been linked to neurodegenerative alteration of age-dependent learning [48]. Strenuous exercise may result in IL-6 parallel brain inflammation; however a recent study demonstrated that a gradual resistance exercise combined with a protein enriched diet may reduce IL-6 expression [49], suggesting that the role of IL-6 and its dosing in exercise-induced neuro-protection requires further investigation.
Differently from IL-6, IL-8 or chemokine (CXC Motif) ligand 8 (CXCL8) is a chemokine with neuromodulatory effects. It is expressed in neurons, glia and endothelial cells. Neuroprotective effects of exercise-induced IL-8 may rely in promoting local angiogenesis in muscle [50]. IL-8 is produced in response to high [51] but not moderate intensity exercise [52, 53]. As will be discussed in paragraph 3.2, exercise intensity is a crucial parameter to modulate the expression and to determine the function of factors involved in inflammation, pain and regeneration following a nerve injury.
The link between exercise and the immune system implicates the modulation of various inflammatory processes that can be activated by factors implicated also in cognitive dysfunction and neurodegenerative diseases, that convey neuroprotection beyond the classical chemotactic reactions. For example, exercise is likely suppressing TNFα via pathways independent from IL-6, since TNFα decreases also in exercised IL-6 knockout mice [54]. Moreover, brief exercise can increase the expression of stromal cell-derived factor-1 (SDF-1), a pleiotropic chemokine also known as C-X-C motif chemokine ligand 12 (CXCL12). CXCL12 promotes adaptive immune responses and angiogenesis by recruiting endothelial progenitor cells from the bone marrow [55-57]. It has been shown that 3 weeks of free wheel running lead to higher CXCL12 gene and expression, along to enhanced learning and memory performances in the Tg2576 AD mouse model [58]. CXCL12 is extensively expressed in the CNS, and its presence demonstrated in cholinergic, dopaminergic and AVP-ergic brain neurons [59].
C-Reactive Protein (CRP) plays a central role in the human immune system. It is synthesized primarily in the liver, adipose tissue and vascular smooth muscle cells, and found in the blood during the acute phase of inflammation, where it is involved in activation of the complement system [60]. Hyppocampal neurons express CRP [60] as well as they show increased CRP levels in patients with neurodegenerative diseases [61]. In contrast, a number of cross-sectional studies demonstrated an inverse correlation between exercise and CRP expression [62]. However, clinical results are inconsistent since CRP levels were found significantly reduced in only half of the studies using aerobic exercise regimens in children, adult and elder subjects [63]. All these evidences confirm a direct action of physical activity on critical inflammatory players that may promote neuroprotection from nerve insults as well as from neuronal aging and degeneration.
2.2.2. Effects on Microglia and other Factors
The inhibition of microglial reaction seems to be one of the key effects of physical activity inducing neuroprotection after neural damage, as demonstrated in some recent studies [11, 65-67]. Microglial cells typically exist in a relatively quiescent form, however when detecting damage, they proliferate and form thick clusters, turn in an amoeboid morphology and start to phagocyte cellular debris and foreign material. Microglial activity is triggered by complex signaling cascades, and after nerve injury is regulated by activity-dependent mechanisms [68, 69]. The potential role of exercise as immunomodulatory treatment within the central nervous system is particularly relevant for neuroprotection against the age related progressive decline in cognitive function. Normal aging primes microglia towards the chronic inflammatory phenotype, but as demonstrated voluntary exercise can counteract the basal shift in microglia activation of aged mice, even if the effects may be different for age, sex, and brain region [65].
Microglial activation in the central nervous system depends on the presence of purines, cytokines and chemokines that can induce their activation and stimulate protective or toxic activity after peripheral or central injuries. In this scenario, chemokines such as CCL2 and CCL21, proteases such as MMP-9 and cathepsin-S (releasing further cytokines and chemokines, such as IL-1β and fraktalkine), growth factors such as neuregulin-1 (NR1), and purines such as adenosine triphosphate (ATP) have been described [70]. All these signals stimulate the proliferation and chemotaxis of microglia in regions of the CNS in proximity to damage or to which injured afferents project, can be enhanced by increased neuronal activity, and may be modulated by physical activity. Activity-dependent increase of ATP interacting with P2 purinergic receptors has been demonstrated to be a critical event inducing activation of microglial cells and generating pathologic inflammatory and painful states [70, 71]. In turn, activated microglia and astrocytes release IL-1β, TNFα, IL-6 and other cytokines and chemokines. In primary afferents cytokines contribute to enhance the neurotransmitter release and promote consequent actions associated with neuronal damage, including amplification of central microglial activation [72], infiltration of phagocytic neutrophils [73], and damage to Schwann cells and to axons [74]. After injury, a critical role seems to be played by BDNF released from microglia. Instead of having a neurotrophic effect as under normal conditions, BDNF induces hyperexcitability of nociceptive neurons [68] and is involved in the reduced expression of the potassium-chloride cotransporter 2 (KCC2), which showed to alter the anion gradient of GABAergic interneurons into an excitatory rather than an inhibitory action [75-77]. The overall effect is an enhanced excitability of central neurons, neurotoxicity and amplification of pain stimuli.
In this pathologic environment, specific exercise training could be effective in reducing or preventing the microglia activation and associated inflammatory and neuropathic mechanisms, promoting neuroprotection and resolution of the disease. An increasing-intensity treadmill training was effective in reducing microglia [11] and its expression of BDNF [64] in the dorsal horn after sciatic injury. BDNF mRNA also decreased in dorsal root ganglia (DRG) [78], indicating that increasing-intensity or intermediate intensity exercise is able to down-regulate specific neurotrophin signaling–dependent responses to injury which may generate maladaptive excitability of sensory neurons, without significantly altering the regeneration of injured axons [64, 78]. Downregulation of KCC2 transporter is a consequence of the increased BDNF-mediated TrKB activation, which suppresses the normal Cl-dependent fast inhibition in GABAergic neurons [79]. For this reason, after peripheral injury this reaction is responsible for the inversion of the anion flux from inhibitory to excitatory on activation of GABA receptors in the dorsal horn due to primary afferent depolarization [80, 81]. Contrary to what has been often shown after exercise, the expression of BDNF is significantly reduced in spinal microglia after increasing-intensity treadmill, suggesting that increased exercise activity can rescue KCC2 by changing microglia reactivity and ceasing excitation from central BDNF. Chronic exercise may reduce central microglial reactivity and inflammation through regulation of multiple metabolic and transcriptional processes that start in the skeletal muscle. The rate of both glucose uptake and glycogen synthesis are acutely raised in skeletal muscles during exercise. Glycogen synthase kinase 3 (GSK-3) is a major regulator of the balance between the pro- and anti-inflammatory mediators in immune cells, including microglia [82]. Chronic adaptation in skeletal muscle with long-term exercise training showed an inverse relation between GSK-3 and exercise levels [83-85]. GSK-3 stimulates the release of IL-1β, IL-6 and TNFα in activated microglia, and inhibits the release of anti-inflammatory cytokines like IL-10 [86-88]. GSK3 is involved in different neuronal functions, such as neurite outgrowth, synapse formation, neurotransmission and neurogenesis, and in the brain is a sensor for deciding the cells fate. Exercise may activate some extracellular signals that are known to inhibit GSK-3, including epidermal and platelet-derived growth factors [89, 90], α1A adrenergic receptor [91] and insulin [92]. It is noteworthy that significant/prolonged inactivation of GSK-3 in microglia and astrocytes shifts balance of secreted cytokines from pro-inflammatory to anti-inflammatory [86, 93, 94].
2.3. Effects on Neurotrophins Expression
Neurotrophins are polypeptides belonging to a family of closely related proteins that induce neural proliferation, survival, migration, and differentiation [95, 96], thus fundamental for neuroprotection [97, 98]. They include NGF, BDNF, neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT4/5). Synthesized by target neurons and glial cells, their biological action is mediated by two different classes of receptors, the low-affinity P75 neurotrophin receptor (P75NTR), a member of TNF receptor superfamily, and the tropomyosin related kinase (Trk) family of receptors tyrosine kinases [99, 100]. Neurotrophins perform their action by binding to their receptors and being internalized along with the receptor and retrogradely transported to the cell nucleus, where they can play multiple survival promoting effects [99]. The unique actions played by neutrophins however do not justify a different family of target receptors from mitogenic growth factors, such as platelet-derived growth factor (PDGF) or epidermal growth factor (EGF), whose receptors are known to be tyrosine kinases. During the last years, members of other neurotrophic factor families have also been shown to activate tyrosine kinases. About a third of primary sensory afferents do not express receptors for neurotrophins, but receptors for GDNF signaling (RET, GFRa-1, GFRa-2). GDNF is a member of the transforming growth factor-b (TGF-b) superfamily, and exerts effects similar to neurotrophins as promoting survival of brain dopaminergic, motor and sensory neurons.
Physical activity has a significant impact on neurotrophins production, as a few days of exercise increase the BDNF and NT-3 levels in muscles [101], dorsal root ganglia (DRG) [102] and spinal cord [103-105]. Following nerve injury, these effects were associated to enhanced survival and regeneration of injured axons. Other factors also participate in the exercise-dependent signaling for growth and innervation, including NGF [106, 107] and NT-4/5 [108]. In uninjured rats, chronic treadmill exercise locomotion of moderate intensity induced important changes of BDNF expression in muscle and brain, mostly in the grey matter. In parallel NT-4 expression increased in the white matter of the spinal cord, where astrocytes may be an additional source of neurotrophins. On the other side, GDNF expression in skeletal muscle seems to be directly produced by stretch, while membrane depolarization by acetylcholine (ACh) decreases its content [109]. Expression of GDNF increases in muscles and spinal cord after treadmill exercise [110, 111], and induces morphological changes at the neuromuscular junctions (NMJ) that are activity dependent [110, 112].
The most disabling problem after peripheral nerve injury is the slow growth of injured axons, impairing functional recovery, along with significant misdirection of regenerating axons, that reinnervate inappropriate targets inducing changes of the central neural circuitry [113]. Peripheral nerves are able to regenerate and there is sufficient endogenous supply of neurotrophins in the axotomized motor and sensory neurons and in Schwann cells that can guide nerve regeneration. However the trophic support diminishes with time, so that in chronic lesions a therapeutical supplement may be beneficial when target reinnervation is delayed, particularly after severe nerve injuries requiring long time for regeneration [114]. Increased BDNF and NGF induced by exercise contribute to enhance axonal regeneration after nerve injury [115-117]. However, there are also evidences that an increased neurotrophic production by physical activity may be critical in long-term neuro-rehabilitation. Indeed, BDNF and GDNF administration did not significantly increase the number of regenerated axons in freshly cut and repaired rat nerves, but can do it after chronic axotomy [114]. Nevertheless, the same neurotrophic factors may promote excitability and maladaptive plasticity, such as excessive collateral sprouting, that in turn may exacerbate symptoms of pain in peripheral neuropathies. In this scenario, setting exercise protocols for decreasing instead of increasing neurotrophins signaling may be a more efficacious strategy for treating pathologies characterized by hyperexcitability of the nervous system.
Neurotrophins are well-known mediators not only of axonal regeneration, but also of pain, particularly NGF and BDNF [118], and play several roles in the development of neuropathic pain [119, 120]. Anti-NGF and anti-TrK receptor treatments demonstrated to significantly reduce neuropathic pain in sciatic nerve injury models, and the hypoalgesic effect has been associated to the prevention of nociceptive fibers collateral sprouting into the denervated skin [120-123]. Recently, swimming training was shown to significantly reduce mechanical allodynia that was associated to a normalization of increased BDNF, NGF and GDNF levels in DRG after partial sciatic nerve ligation [124]. These results are consistent with studies in our group using an increasing-intensity treadmill training (iTR) [11, 64], that produced reduction of neuropathic pain along with decreased BDNF, NGF and GDNF up-regulation in DRG [78], BDNF expression in the dorsal horn and NGF expression in the denervated skin [64]. We found these changes accompanied by the normalization of parallel mechanisms underlying neural excitability such as microgliosis in the spinal dorsal horn [64, 124], the disregulation of cation chloride cotrans-porters expression, and the collateral sprouting of nociceptors [64]. Elevated BDNF and NGF levels in sensory pathways are generally associated with neuropathic pain and inflammatory conditions. Activated microglia release BDNF, which increases excitability of nociceptive neurons [125] leading to allodynia [126]. Mechanical hyperalgesia as induced by delayed onset muscle soreness (DOMS) after unaccustomed strenuous exercise has been associated to elevated NGF and GDNF upregulation in muscles [127].
On the other side, spinal injuries oppositely determine a loss of neurotrophic factors that has been associated to neuropathic pain conditions. Hutchinson et al. [128] found that resolution of allodynia after spinal cord injury was concomitant to normal BDNF mRNA levels in both lumbar spinal cord and in the soleus muscle of treadmill trained rats, but not in swimming nor in standing upright trained rats. Upregulation of BDNF in the CNS is considered a principal effect of exercise for modulating spinal and brain circuitries, and promoting neuronal repair, memory and learning. Therefore, the effects of increased physical activity on functional recovery mediated by neurotrophins may be opposed in central and peripheral neural lesions.
In the last two decades, the study of exercise-induced neurotrophins expression in the brain showed a clear relationship between exercise and BDNF-NGF impact on brain functions. There is a positive correlation between the mean distance run on a running wheel and mRNA for BDNF in the hippocampus and caudal neocortex in rats, and the elevation of neurotrophins levels in brain circuits has been related to neuroprotection and cognitive improvement in neurodegenerative disorders. Some studies have identified correlations between decreased BDNF production and disorders such as depression, schizophrenia and dementia [129, 130]. Specifically, exercise has been used to delay neurodegeneration in aged AD transgenic mice [131-133], and humans [134-136]. In these pathologies, exercise reduces the levels of detrimental factors, for example oxidative stress and inflammation [58, 131-133], and increases the expression of neurotrophic factors, such as BDNF and IGF-1 [36, 137]. One of the mechanisms proposed for changes produced by exercise in the brain is the promotion of demethylation of BDNF promoter IV in the hippocampus, enhancing brain function and plasticity [138]. BDNF produced by exercise also induced long-term potentiation (LTP) by tetanic stimulation in the hippocampus, which showed only short-term potentiation (STP) in absence of BDNF [139]. The BDNF upregulation in the hippocampus induced by exercise was related with the improvement of cognitive function, including memory, in rodents [140], whereas downregulation of BDNF or TrkB impaired the formation of memory [141].
2.3.1. Conversion of Pro-neurotrophins into Mature Forms
Neurotrophins actions can change depending of their secretory pathway, and they can be altered not only at transcriptional mRNA level but also at protein level [142]. Neurotrophins are first synthesized as a precursor protein in the endoplasmic reticulum. These pro-neurotrophins are then transported to the Golgi apparatus for sorting into either constitutive or regulated secretory vesicles, and may be intracellularly converted into their mature form [143]. Recent studies support a “yin-yang” hypothesis that interaction of pro-neurotrophins or mature forms with p75NTR or Trk receptors, respectively, elicits different biological actions of neurotrophins, either degeneration or neuroprotection [144, 145]. For instance, proBDNF and mBDNF differently mediate apoptosis or survival by activation of p75NTR and TrkB receptors respectively [146]. Moreover, different frequency of neuronal activity may induce opposite functions of BDNF isoforms through induction of different types of receptors. Both low- (inducing long-term depression, LTD) and high- (inducing long-term potentiation, LTP) frequency of neuronal stimulation increases the extracellular proBDNF, however only high-frequency activity results in extracellular conversion of proBDNF to mBDNF [147]. Chronic voluntary exercise can increase brain BDNF concentration as a result of increasing the conversion of proBDNF into the mature form as reported by Berchtold et al. [148]. These results suggest that different mechanisms of plasticity may be activated by different types of neuronal stimulation, and can be induced as a result of different intensities of exercise in the intact and in the damaged neurons.
2.4. Effects on Neuronal Death and Survival Signaling Pathways Regulated by Neurotrophin Receptors
Despite the neuroprotective and pro-regenerative roles of neurotrophic factors have been extensively associated to physical activity, they also participate in apoptotic processes and may generate neurotoxic effects. The biological actions played by neurotrophins determining the fate of neurons are regulated by two different receptors: Tyrosine kinase receptors (Trk) and the p75NTR [149, 150]. NGF binds to TrkA, BDNF and NT4 to TrkB, and NT3 to TrkC. On the other hand, p75NTR can be activated by all neurotrophins, but is also able to regulate the affinity of Trk receptor for its cognate ligand. There are some evidences that Trk and p75NTR receptors may form complexes, and p75NTR increases the ligand selectivity of Trk receptors [151]. p75NTR also is component of other receptor complexes, prominently the Nogo complex [152], that participates in regulation of myelination and axonal regeneration. However, these two types of receptors are not allowed to bind each other directly. Thus by binding to p75NTR, neurotrophins can stimulate apoptosis in different cell types [153]. Proneurotrophins, as proNGF, have been demonstrated to exert this apoptotic function [154].
The biological effects of neurotrophins are determined by their proteolytic cleavage, as previously explained [144, 145]. Apoptosis by p75NTR is facilitated by binding to pro-neurotrophins and sortilin, which is a trafficking receptor. Cell death through p75NTR signaling has been observed under conditions of stress, injury or inflammation, when p75NTR has a pro-apoptotic function for developmental cell death and after nervous system injury [155]. The p75NTR activates three main signaling pathways: the NF-κB pathway, resulting in transcription of several genes involved in neuronal survival; the Jun kinase pathway, controlling other genes, some of which instead promote apoptosis; and the Rho signaling, controlling the motility of neurite growth cone. Moreover, it is known that pro-neurotrophins induce apoptosis through ligand engagement of p75NTR more effectively than mature NGF [151].
On the other side, neurotrophins boost neuronal survival and differentiation through activation of Trk receptors [150]. Each Trk controls three basic signaling pathways. After neurotrophin binding, Trks activate and recruit adaptor Src homology 2-containing proteins (Shc) and fibroblast growth factor receptor substrate 2 (FRS2), and effectors such as phosphoinositide 3-kinase (PI3K) and phospolipase C-γ (PLC-γ). Synaptic plasticity is promoted by the activation of PLC-γ1 which binds to Tyr790, and this interaction has been proposed to facilitate interactions with ion channels, such as the vanilloid receptor-1 (VR-1) channel, finally resulting in activation of Ca2+ and protein kinase C (PKC) regulated pathways. Moreover, Tyr490, Shc or FRS2 become tyrosine phosphorylated and provide a scaffold for other signaling proteins, then leading to activation of the Ras/MAPK (mitogen-activated protein kinase) pathway, which promotes neuronal differentiation and neurite outgrowth, or the PI3K/Akt pathway, which induces cell growth and survival [144, 145].
A number of neurotrophic factors have been associated to functions of survival and differentiation in brain and spinal neurons. Particularly, BDNF, NGF, NT-3 and IGF-1 have been involved in mediating neuroprotective effects of exercise against apoptosis [36, 156, 157]. Aerobic exercise has shown to modulate the β- Ca2+/calmodulin-dependent kinase II (β-CaMKII) and the PI3K/Akt pathways via neurotrophic factors activation [157, 158]. Akt pathway is more sensitive to activation of IGF-1 receptor [159], whereas β-CaMKII responses are more induced by TrkB activation [160]. Different authors showed that exercise-induced BDNF production leads to activation of survival signaling through PI3K and MAPK pathways [157, 161, 162]. Moreover, blockade of TrkB in the hippocampus abolishes the plastic changes associated to exercise, such as the stimulation of MAPK and CaMKII [163, 164]. BDNF also protects against brain damage induced by hypoxia–ischemia and inhibits apoptosis by blocking caspase 3 activation. Anti-apoptotic effects of treadmill exercise have been associated to modulation of Bcl-2 family molecules, which inhibit the expression of caspases after central injuries [157, 165, 166]. GDNF is also able to inhibit caspase-3 activation and antagonize neuronal apoptosis following hypoxic–ischemic injury [167, 168]. Neurotrophins and GDNF treatment was proposed as neuroprotective and regenerative therapy for brain neurons in neurodegenerative diseases [169-171].
2.5. Effects on Neurodegenerative Diseases
The protective impact of exercise on neurodegenerative disorders is still under evaluation, since the mechanisms underlying the potential benefits have not been determined yet. This is due to the heterogeneity of the causes and the course of degeneration in pathologies affecting mid to aged humans, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD), to devastating younger onset diseases like Amyotrophic Lateral Sclerosis (ALS). For PD and AD, pharmacological therapies are currently inconsistent and mostly offer palliative care to patients. There is growing evidence suggesting that physical activity can generally slow down aging and prevent degenerative diseases [172, 173].
PD is a neurodegenerative disease characterized by clinical symptoms such as resting tremors, rigidity and akinesia, usually associated to a reduction of dopaminergic neurons in the nigrostriatal system [174]. A meta-analysis of the effects of exercise on PD patients showed improvement of physical functions, health-related quality of life, strength, balance and gait speed [175]. Moderate exercise training exerted neuroprotective effects in a rat model of PD, enhancing neural progenitor cell proliferation and migration associated to increased BDNF and GDNF levels, with subsequent improvement in deteriorated motor function [176]. Usually these factors are both significantly reduced in the substantia nigra of PD patients [177]. In another animal model, systemic administration of lipopolysaccharide (LPS) induced nigral cell loss associated to parkinsonism; exercise counteracted these effects in proportion to the duration of training, this parallel to elevation of BDNF levels [178]. Although it is not yet clear how much these animal models relate to PD, the experimental studies suggest that neuroprotection and plasticity are stimulated by exercise in cortical circuits to delay the onset of degeneration. This may be achieved by modulation of neurochemical status in the striatum of rats, particularly increasing neurotrophic factors and possibly by improving oxidative stress. Treadmill exercise in PD rats increased the levels of BDNF and oxidative stress markers such as superoxide dismutase (SOD), sarcoplasmic reticulum Ca2+-ATPase (SERCA II) and catalase (CAT), thus decreasing oxidative damage of lipids and protein [179]. BDNF and GDNF levels were increased in substantia nigra and striatum, respectively, after 18 weeks of treadmill, with associated restoration of mitochondrial activity, and levels of SOD and ATP, along to improvement of motor behavior [180].
The role of oxidative stress in switching from normal to pathological brain aging, and the benefits of physical activity on AD, are corroborated by studies using animal models of AD, particularly the 3xTg and the Tg2576 mice [158, 181-183]. AD is typically characterized by memory disorders associated to presence of amyloid-beta plaques (Aβ), neurofibrillary tangles, inflammation and brain neuronal loss [184]. Demonstrated effects of exercise include enhancement of mitochondrial function and reduction of oxidative stress, but also stimulation of neurogenesis and angiogenesis over secretion of neurotrophic factors, strengthening of synaptic transmission, epigenetic changes, reduction of amyloid plaques formation, and reinvigoration of cardiocirculatory and immunoendocrine functions [158, 181-183, 185, 186]. These beneficial effects are also believed to occur in humans [36, 187]. Particularly, AD symptoms has been correlated to low NGF levels and parallel higher pro-NGF levels [188]. The accumulation of Aβ-peptide restrains the conversion to mature NGF, resulting in increased concentrations of proneurotrophin. Exercise oppositely increases mature NGF levels [107, 189-191]. Noteworthy, one of these studies [191] revealed that exercise training can boost BDNF and NGF by inducing long term potentiation (LTP) and counteract the age-associated neurotrophic decay.
Circulating BDNF can be considered as a marker of age-dependent memory performance [192], since vascular low levels of BDNF have been associate to patients predisposition to AD. Exercise stimulates BDNF mRNA and protein synthesis in the hippocampus and in the cortex, and overall brain contributes to about 70-80% of the circulating BDNF [193]. Exercise induction of NGF and BDNF can in turn enhance hippocampal neurogenesis, resulting in the enhancement of cognitive function. These effects may in part counteract the pathological course of AD. GDNF plays also an important role in neuronal plasticity and survival, and particularly in the hippocampal region [194]. Although there are no sufficient studies to determine the role of GDNF under exercise stimulation, some observations suggest that GDNF is produced as well as its neuroprotective effects [176, 195].
Additional neuroprotection in AD is conveyed by exercise through modulation of inflammatory factors, such as TNFα. Increased expression of TNFα is a prominent risk for AD in elders [196]. Some studies directly link TNFα levels to aggravation of dementia in AD [197-199]. On the other side, moderate chronic exercise reduces the levels of TNFα [200], resulting in a significant decrease of TNFα gene expression in muscles of aged individuals [201].
An active lifestyle continuously providing sufficient amounts of physical activity can be considered a non-pharmacological protective approach for averting neuronal degeneration as well as to avoid the excessive use of drugs. However, the benefits of exercise appear to be inverted in the case of ALS, or at least when physical activity is intense, as in high-performance athletes. A recent recollection of data from thousands professional Italian football players established rates of morbidity significantly increased for ALS disease, notably of young onset, with at the highest risk the footballers who played for more than 5 years [202]. Other studies showed a higher risk of ALS among long-distance race runners and rugby players. The exact mechanism that triggers motoneuronal degeneration in both sporadic and familial ALS is still under evaluation. Inheritance of familial ALS is usually autosomal dominant, often linked to mutations of the copper/zinc superoxide-dismutase-1 gene (SOD-1) resulting in the typical adult-onset ALS phenotype [203]. The SOD-1 enzyme is critically involved as free radical scavenger, since it catalyzes the conversion of the superoxide anion into molecular oxygen and hydrogen peroxide. The pathophysiology of ALS is multifactorial, since it involves complex genetic and molecular interactions, that result into damage of key target proteins and organelles within the motoneuron. Possible links between exercise and ALS comprise oxidative stress, production of free radicals and stimulation of glutamate release, all mechanisms that contribute to motoneuron death. These mechanisms may become neurotoxic as a result of excessive exercise and increased neuronal activation in susceptible individuals. On the other hand, recent studies are in favor of specific low-intensity exercise programs, particularly swimming [204] or moderate treadmill locomotion [205], that increased the lifespan and delayed the motor decline in the transgenic SOD1G93A mouse model of ALS. Recently a pilot study involved inspiratory muscle training in ALS patients and suggested a potential benefit of such exercise [206]. Moreover, flexibility, balance, strength and aerobic exercises are indicated in the management of ALS patients for postponing muscular loss, maintain aerobic responses and reduce spasticity [207-209].
3. DOSING NEUROPROTECTION BY TUNING UP EXERCISE PARAMETERS
It is still unclear to date by human studies as physical exercise can be used to make a proper device level of neuroprotection after nerve injury. Moreover, a number of training protocols have been applied for locomotion in experimental animal models of nerve injury, with conflicting evidences with regard to beneficial and deleterious effects on neuronal survival, pain and regeneration [210, 211]. Although physical activity could produce endogenous neuroprotection and cardioprotection [212, 213], the intensity, time and duration of different types of exercise have not been conclusively determined. Some studies reported that moderate or intermediate intensity and duration of exercise correlate with better outcomes as opposed to mild or exhaustive exercise [214]. Intermediate levels of exercise intensity seem to promote long-term adaptation and induce beneficial plasticity and neuroprotection. In this section we revise the evidence for several critical parameters in programming the protocol of exercise following nerve damage, focusing on the effects that each parameter may have on plasticity and recovery. The neurotrophic contribution can be modulated by tuning the parameters of training protocols in order to optimize the exercise for rehabilitation. For example, a day session of exercise is known to increase plasma and serum levels of BDNF in proportion to exercise intensity [215] in the same way of a shorter but intense bout of exercise. Longer training induces also an increase of resting BDNF levels, although ambiguity exists on the duration, type and intensity of training that are required to maintain this effect over time [216]. A better understanding of the possible neuroprotective versus neurotoxic effects of exercise on nerve injury can be traced back to the results of modulation of its main variables.
3.1. Forced Versus Voluntary Exercise
A first distinction in assessing the effects of activity treatments should compare the results of voluntary and forced exercise. Voluntary exercise can increase the expression of several molecules that stimulate with BDNF the synaptic activity and the neurite outgrowth in spinal cord and hindlimb muscles [103, 217]. On the other side, forced exercise like treadmill locomotion may produce responses of both transient and chronic stress, that after nerve injury can induce more robust neuroprotective effects, and also analgesic effects [218-222]. With regard to the effects on pain, prolonged exercise may generate chronic stress responses but nociceptive thresholds have been mostly measured after forced exercise-induced acute stress responses are resolved [218, 220]. Voluntary exercise may also elicit a stress-like response in acute phases [219] due to the increased physical activity; however, various studies showed that prolonged voluntary wheel running is anxiolytic when performed under mild to moderate stress [223-227].
Rodents models of stroke showed that forced exercise such treadmill running tends to induce better recovery than voluntary wheel running exercise [228]. Sheanan et al. [229] recently showed that, contrary to what demonstrated with forced exercise paradigms, voluntary short-duration running is not sufficient to induce hypoalgesia in peripheral nerve models of pain and acute inflammation. The discrepancy in the effects on neuroprotection of voluntary versus forced exercise seems to be due to the different behaviors. Even if slower, forced treadmill locomotion is more constant than voluntary running, the latter occurring in shorter spurts at faster speed, although an equal distance is covered by both the exercised groups [220]. Neuroprotection by forced exercise leads to increased neurogenesis and cerebral metabolism, decreased stroke volume and improved neurologic deficit, these also associated to the upregulation of heat shock proteins [218, 228, 230]. There seems to be sufficient evidence to support the conclusion that intermediate exercise intensities distributed over a longer time period, as provided by programming treadmill training, convey greater neuroprotection than further robust exercise over short time periods as in voluntary running.
A different response to exercise types seems to emerge in case of neurodegenerative diseases. Contrary to acute brain injury, which may require less intense but steady activity, in mouse models of AD simple voluntary exercise was sufficient to inhibit the progression of neuronal degeneration [182], and was more neuroprotective when compared to forced exercise [183]. It may be hypothetized that voluntary exercise enhances endogenous neuroprotective responses to progressive degeneration, while sustained and forced exercise can counteract more effectively the damage from acute injuries.
3.2. Intensity of Exercise
An important question for the preparation of an exercise schedule is how to establish the adequate intensity of exercise to activate neuroprotective and regenerative mechanisms, but also to prevent or inhibit maladaptive responses, such as pain and excessive excitability of motor and sensory neurons. Evidences that the neurotrophic factors expression may be dependent on the amount of activity performed during the training suggest a key role of the intensity parameter in the positive or negative modulation of neurotrophins. While some studies reported an increase [104, 105, 217, 231], others showed a decrease of neurotrophic factors following exercise [232, 233]. This discrepancy may rely on variations in the intensity of exercise applied. BDNF and NT-3 are known to increase in the lumbar spinal cord following low-to-moderate intensity exercise [101, 102, 107]. However, moderate-intensity running caused a depression of BDNF mRNA, but not of its protein, even if elevating blood lactate and corticosterone levels [234]. An increasing-intensity treadmill exercise protocol decreased BDNF, NGF and GDNF mRNA levels in the rat DRG [78], and BDNF expression in the spinal cord [64]. A similar effect was observed in the frontal cortex of mice performing high-intensity treadmill exercise, independently if continuous or intermittent [235, 236]. Thus, it seems that moderate-to-high intensities of exercise may trigger responses opposite to low intensity exercise on neurotrophins production, as a regulatory negative feedback similar to that observed under sustained neural activity and stress. Results from these studies are consistent with previous reporting decreased hyppocampal BDNF mRNA under stress and glucocorticoids stimulation, immobilization and strenuous exercise [237]. However more studies are needed to accurately understand the interplay between exercise intensity and neurotrophic factors production.
In the muscles, neurotrophic factors expression may vary depending on the type of muscle that is more stimulated by specific activities. Moreover, the amount of neurotrophin protein production across different types of skeletal muscles may depend on the intensity of exercise training [238, 239]. Low to intermediate intensity locomotion likely activates more slow-twitch than fast-twitch muscle fibers. Treadmill training increased BDNF levels in the soleus muscle (containing slow-twitch fibers), but no changes in the gastrocnemius muscle (containing fast-twitch fibers) were observed [240]. GDNF protein content also increased in the soleus and decreased in the extensor digitorum longus muscle (with high proportion of fast-twitch fibers) following low-intensity exercise [109]. Moreover, NT-4 was much more expressed in the soleus muscle slow fibers if compared with the gastrocnemius muscle fast fibers [238].
Exercise intensity may also affect the neurotrophic response to injuries depending on their severity. Acute and prolonged passive cycling treatment in spinalyzed rats increased the mRNA of BDNF, GDNF and NT-4 in motoneurons compared with small mRNA increase following the injury [241], differently to what was observed after peripheral nerve injuries [242]. In contrast, mRNA and receptors of neurotrophic factors were largely unchanged in large DRG neurons after spinal cord injury either with or without exercise [241]. These results indicate that intensity of training should be taken in consideration and adapted to different types and grades of injury in planning rehabilitative exercise protocols.
The debate is open regarding how and to what extent the exercise intensity may be adapted to the type and degree of nervous injury, in order to generate an optimal neurotrophic contribution. Possible mechanisms for neurotrophins increase following moderate intensity exercise are considered the increased conversion of pro-neurotrophins to the mature form [243], the induction of IGF-1 [244, 245] and estrogens [246, 247], as well as the reduction of leptin [245] and corticosterone concentrations [246]. It is still unclear, however, how high intensity exercise may induce the opposite effect and, therefore, trigger a downregulation of neurotrophic factors. Both high [248] or low [246] intensity exercise training enhanced BDNF concentration in the brain due to the reduction of corticosterone concentration, suggesting that mechanisms for inducing neurotrophin downregulation may be more than just activation of a cortical stress response.
Observation of rodents preferences for training to continuous forced running on a treadmill at sustained speed led us to formulate an increasing-intensity treadmill protocol (iTR) [11, 64, 78]. iTR running in rats starts at normal locomotion speed (10 cm/sec) and gradually increases (2 cm/sec every 5 minutes) until 1 hour of training session. In this way injured animals [11, 78] do not refuse the final high speed running (32 cm/sec) if slowly adapted to it without stressors or electrical shocks. Resembling this protocol, intermediate levels of exercise intensity may be applied to patients by similarly starting with normal kinesis and gradually increasing velocity until elevated but sustainable levels of activity. Such protocols may induce the patient to activate more complex responses (for example, potentiating the use of slow-twitch muscle for the control end of the movement) and their strengthening by means of gradual increase of exercise intensity.
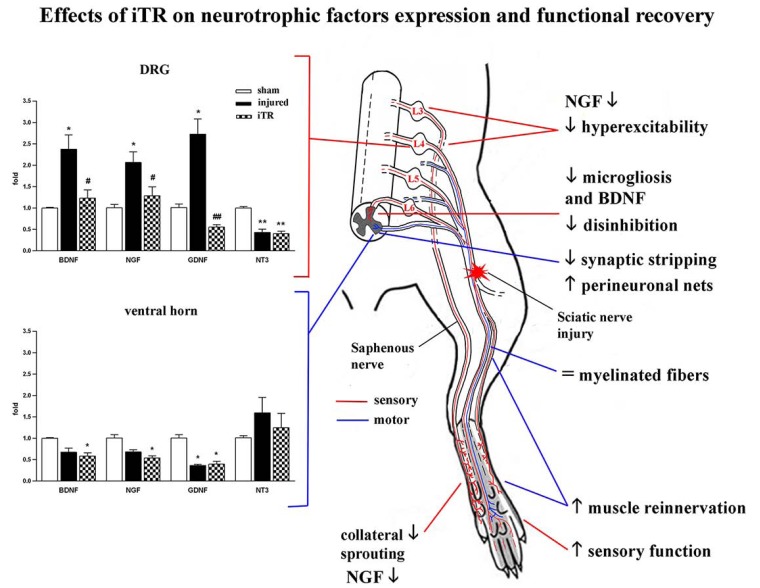
Significant reduction of mechanical allodynia was observed after early and short-lasting iTR in the same way if after chronic constriction injury or section and suture repair of sciatic nerve [11, 249]. Normal nerve regeneration was not impaired even if reduction of hyperalgesia was parallel to the decrease of BDNF and NGF levels in DRG and spinal cord, inhibition of microgliosis and normalization of chloride cotransporters homeostasis [64, 249]. Compared to a low-intensity protocol, the iTR protocol demonstrated neuro-protective effects by counteracting the destruction of perineuronal nets (PNN) and reducing the motoneuronal synaptic stripping, partially preventing the reduction of Vglut1 synaptic buttons, after sciatic nerve lesion [250] (Fig. 1). These findings suggest that gradually increasing the exercise intensity, such as in the iTR protocol described, may be a strategy to optimize neurotrophins actions on both sensory and motor neurons in order to reduce early neurotrophin-mediated hyper-excitability in sensory neurons but in parallel to stimulate neuroprotection of spinal motor circuitry.
Fig. (1).
Summary of the effects of the increasing-intensity treadmill (iTR) exercise protocol on the expression of neurotrophic factors and functional recovery from injury to the rat sciatic nerve. The mRNA expression of BDNF, NGF, GDNF and NT3 was assessed in sensory (dorsal root ganglia, DRG) and motor (spinal cord ventral horn) neurons of the injured paw at 8 days post-injury and after performing 5 days of training (from day 3 to day 7) (left panels). Sciatic nerve injury induced a peripheral increase of BDNF, NGF and GDNF in lumbar DRG, but their central expression tended to decrease. Conversely, NT3 expression was reduced in sensory neurons. iTR prevented the neurotrophic factors increase in DRG without significantly affecting central neurotrophin changes compared with injured untrained animals. Some effects of iTR on mechanisms of pain and regeneration are outlined (right side) and correlated to local neurotrophin reduction at the plantar paw skin, sciatic nerve, lumbar DRGs and spinal cord dorsal horn.
3.3. Duration of Exercise
Duration of exercise bouts following an injury is also an important variable for setting activity treatments. A greater duration of exercise seems to lead to greater stimulation of the neurotrophic system. Recently serum BDNF levels were measured in adult human males after a vigorous or a moderate intensity aerobic cycle exercise bout [251]. All the exercise types caused a substantial BDNF increase, even if significantly higher BDNF elevation was found after longer duration (40 min). Other studies showed that both acute and chronic aerobic exercise lead to an increase of BDNF levels, but this is not induced in case of strength training [252-254]. Based on these considerations, aerobic exercise programs for neuroprotection should take into account the volume of neurotrophin release over time.
Animal studies using chronic aerobic exercise have consistently reported the elevation of basal BDNF levels in the hippocampus, striatum and other cortical regions [19, 107, 163, 193, 255, 256]. Resistance exercise can also showed to increase hippocampal BDNF [257]. BDNF levels remained upregulated in the rat hippocampus after 28 consecutive days of exercise [148, 258], differently from other neurotrophic factors that showed tolerance to chronic exercise. Benefits of chronic exercise-induced BDNF that have been demonstrated in animal models of disease are cell survival [259], decreased depressive symptoms [260] and functional recovery after traumatic brain injury [261]. Furthermore, robust effects on cognition, learning and memory were described in healthy laboratory animals that performed either voluntary or forced exercise at disparate intensities and durations, whether assessed by Morris water maze [140, 237, 262], radial arm maze [263], Y-maze [264], object recognition tasks [265], or pain avoidance tests [191, 266].
Despite these results, there is controversy about the dependence of BDNF and GDNF expression on exercise duration. Some studies showed that short-term treadmill running (3, 7, 15 days training periods) resulted in the same BDNF levels in the hippocampus and basal forebrain of both control and exercise trained animals [267, 268]. On the other hand, a recent study showed that both high intensity interval training and continuous training increased rat brain BDNF and GDNF levels [269]. Different studies have shown that long-term exercise training at moderate intensity increased GDNF concentration in striatum, spinal cord and sciatic nerve [180, 270], while short-term exercise at low to moderate intensities did not influence the GDNF concentration as observed in the striatum and substantia nigra [178]. Therefore, production and activation of neurotrophins during exercise seem to be dependent on the duration of exercise protocols, and the time spent in performing the activity can be tuned up to gradually increase the intensity of a specific exercise. Duration of training also affects the maintenance of neurotrophic supply. Thus, after increasing long-term training, serum BDNF expression normalizes after 8 weeks of detraining [253].
If chronic exercise provides the injured nervous system with a higher neurotrophic supply, excessive neuromuscular activity may produce opposite harmful effects for muscle reinnervation [271], reducing axonal regeneration [115, 272] and the proliferation of Schwann cells [273], and also inhibiting collateral sprouting of axons in partially denervated muscles [274-276]. Activity treatments in the form of electrical stimulation or treadmill running may result detrimental for peripheral nerve regeneration, neuropathic pain and inflammation when they are exhaustive and continuous [11, 277]. The physiological responses of adaptation to increased duration of exercise should therefore always be monitored after injury.
Another critical variable for setting the rehabilitation exercise treatment is the time of administration with respect to the nerve injury. Application of exercise prior to injury or pre-training is thought to have a neuroprotective effect, for example priming neurons for increased axonal regeneration following injury [102]. Moreover emerging evidence suggests that better recovery may be achieved when exercise is applied at early times after injury [11]. Exercise, either acute or prolonged, may affect the early expression of genes that respond for plasticity and apoptosis as well as neurotrophic factor production in a cell specific manner (differently in motor than in sensory neurons) after injury [241]. The first week after injury seems to be a crucial time window, during which starting activity at different intensities may stimulate or inhibit the neuronal regenerative response. The activation of neurotrophin signal transduction pathways peaks within 7 days for NGF, and around 7-14 days for BDNF and GDNF in injured peripheral nerves; on the contrary, other neurotrophins such NT-3 and CNTF are downregulated after injury [5, 242, 278]. Thus application of exercise during the first days from nerve injury may be critical in regulating the neurotrophic response to stimulate survival and growth of sensory [102, 279] and motor [279, 280, 281] neurons, or to inhibit excessive hyperexcitability, maladaptive collateral sprouting and mechanisms of neuropathic pain [11, 64, 78, 241].
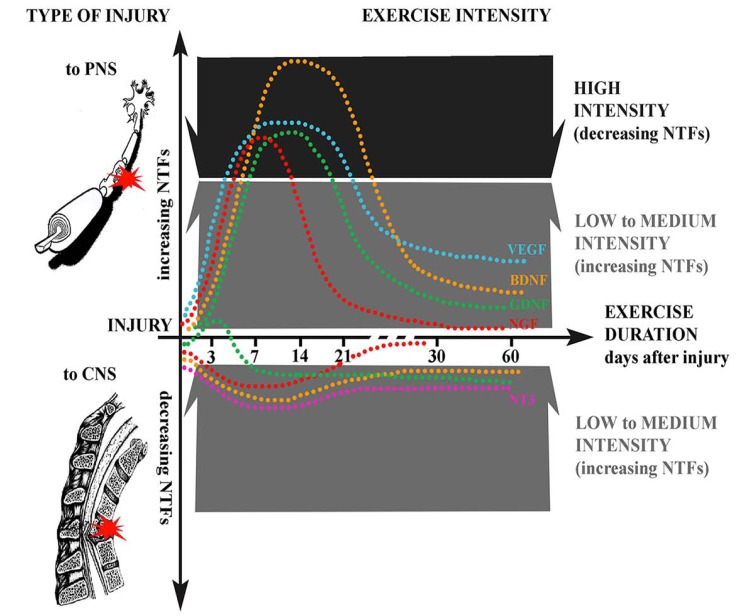
On the other side, central injuries, such as spinal cord injury, induce a loss of some neurotrophic factors support, so that early applied exercise may boost and normalize their levels. Hemisection of the spinal cord induced a decrease in BDNF and synapsin-I levels, that were restored by voluntary running exercise [282]. Of several neurotrophic factors analyzed, injury to spinal cord increased the early expression of mRNA for GDNF [283] and NT-3 and of its receptor TrkB [241]. However, cycling exercise increased the mRNA for both BDNF and GDNF, and attenuated the expression of those factors increased by spinal cord injury. Taken together, all these studies support the idea that starting exercise during the first days after injury may promote functional restoration by modulating the changes in neurotrophic factors induced by neural injuries (Fig. 2).
Fig. (2).
Schematic of the main effects of exercise on the expression of neurotrophic factors (NTFs) after nerve injury. Depending on the type of injury, NTFs expression may increase or decrease (positive/negative dotted lines), with different effects on neuroprotection. Injury to the peripheral nervous system (PNS, top) induces the activation of NTFs signal transduction pathways peaking within 7 days for NGF, and around 7-14 days for BDNF, GDNF and VEGF in the injured nerves; on the contrary, other neurotrophins such as NT-3 are downregulated. Injuries to the central nervous system (CNS, bottom), such as spinal cord injury, reduce neurotrophic factors support. Exercise can boost or reduce NTFs levels, depending on the intensity and duration. The efficacy of exercise intensity to induce functional recovery by modulation of NTFs is also represented. Low to intermediate intensity exercise generally increase NTFs expression in both PNS and CNS, stimulating neuroprotection and regeneration, while high intensity exercise may trigger NTFs reduction and induce opposite effects, similarly to a stress response, reducing hyperexcitability.
4. THE DOUBLE ROLE OF NEUROTROPHIC FACTORS IN EXERCISE
In previous sections we lined out both neuroprotective and excitotoxic effects of exercise that are mediated by endogenous neurotrophins. The dichotomy of exercise effects may be explained by the dual role that neurotrophic factors play under activity-dependent increasing stimulation following nerve injury and denervation. In this context, intensity and duration of stimulation may differently convey neuroprotection by producing an enhancement or a decrease of neurotrophic factors. The expression or inhibition of neurotrophic factors, such as BDNF, can promote or inhibit pain and regeneration, depending on whether exercise activates the neurotrophin signaling and excites neurons at peripheral or central levels.
4.1. Peripheral Versus Central Neurotrophin Expression
A systematic review [284] analyzing peripheral BDNF expression after exercise suggests that basal BDNF concentrations are increased by acute aerobic but not strength exercise, although the effect is transient. Circulating BDNF originates from both central and peripheral sources. Exercise temporarily elevates basal BDNF and upregulates BDNF synthesis, release, absorption and degradation. On this line, an acute exercise bout would result in higher BDNF synthesis if compared with untrained subjects, and if exercise is repeated in subsequent training, further BDNF could be released into the bloodstream and induce more efficient absorption by both central and peripheral tissues, finally producing a cascade of neurotrophic and neuro-protective effects.
Aerobic but not strength training produces an increase of peripheral BDNF levels [252-254]. This is a consequence that muscle BDNF is not released into systemic circulation and that muscle itself is probably not a source of peripheral BDNF during chronic exercise. In fact the main source of exercise-induced BDNF appears to be the brain, and peripheral blood mononuclear cells and endothelial cells may contribute to approximately 20–30% of peripheral levels [193, 285]. Moreover, aerobic exercise may be a potent stimulator of neurotrophins in the peripheral as well as in the central nervous system, but central expression may be different from peripheral. For example, voluntary wheel running increased mRNA levels of NT-3 and its receptor TrkC in the spinal cord and in the soleus muscle, but changes in NT-3 protein were found only in the spinal cord, suggesting that the central nervous system may produce its own neurotrophins in response to exercise [217].
Brain and spinal expression of neurotrophic factors can be modulated by direct activation of noradrenergic and serotonergic pathways, with relevant effects on neuro-protection and functional recovery after injuries. Exercise-induced noradrenergic stimulation via β-adrenergic receptors seems to be essential for regulation of BDNF [286], since β-adrenergic blockade significantly attenuates the elevation of BDNF mRNA as induced by exercise in the cortex [287]. Moreover, some cognitive advantages of exercise, such as the reinforcement of contextual fear conditioning, are also attenuated by β-adrenoreceptors blockage [288]. Moreover, it is known that exercise mediates increased BDNF expression by stimulating the PKA pathway following the activation of β-adrenergic G-protein-coupled receptor via the transcription factor CREB [289]. Ubiquitously expressed, CREB is a nuclear transcription factor implicated in all cell proliferation, differentiation, adaptation and survival [290]. Through β-adrenergic receptors physical activity may not only maintain muscle metabolism after injury but also activate important pathways, such as those of MAPKs and PI3K-Akt signaling [291-294]. Motor activity directly modulates also serotonergic activity in brain and spinal cord [295], and prominent effects are observed when exercise is applied after peripheral and central nervous injuries [296, 297]. For this reason, exercise-induced BDNF alterations via direct serotonergic activation may be more complicated. Blockade of 5-HT2A/C receptors alters exercise-induced BDNF mRNA levels [287]. Activation of 5HT2A receptors is relevant after injury for strengthening the locomotor alternating pattern and rescuing the lost post-synaptic inhibition by restoring the spinal chloride homeostasis and counteracting central disinhibition, spasticity and neuropathic pain [297]. On the contrary, 5-HT1A receptor blockage seems to do not affect exercise-induced BDNF, although it further enhances its levels in the anterior cingulate cortex [287]. These findings indicate that exercise may activate several neural descending pathways to differently modulate neurotrophin expression in peripheral and central neurons.
4.2. Positive and Negative Feedback for Neurotrophic Factors Expression
The delicate interplay that takes place between glutamate and neurotrophic factor signaling systems represents the functional core of the activity-dependent neuroplasticity during development as well as in the adult [298]. Neuro-trophic factors can directly modify the glutamate signaling by changing the expression of Ca2+-regulating proteins and glutamate receptor subunits, and also indirectly by engaging the production of antioxidant enzymes, energy-regulating proteins and anti-apoptotic Bcl2 family members [299]. On the other side, glutamate can stimulate the production of neurotrophic factors such as BDNF, promoting neurogenesis, synaptogenesis and neurite outgrowth. Mechanisms of activity-dependent survival may be triggered by exercise and intermittent fasting in prevention of neuronal death as revealed in experimental models of stroke [300]. It is usually accepted that low or intermediate intensities of exercise produce neuroprotection for brain neurons, while on the contrary high intensities may cause damage. In this case, intense and prolonged activation of glutamate receptors may provoke neuronal dysfunction and degeneration [301]. Indeed, harmful effects could appear in undue conditions of physical or psychological stress. These conditions may reverse neurotrophic factor production by providing a negative feedback regulation of the hypothalamo-pituitary-adrenocortical (HPA) axis, and trigger oxidative and stress responses [302].
Imbalance of glutamatergic neurotransmission could contribute to maladaptive serotonergic neurotransmission, and negative regulation of neurotrophic factors may be parallel also to extensive activation of noradrenergic and serotonergic pathways. However, after nerve injury, chronic exercise may affect the noradrenergic and serotonergic systems in similar ways to pharmacological interventions, increasing excitatory noradrenaline and serotonin activity in the brain [303-305]. Increased serotonergic activity after treadmill exercise [296] has shown to induce downregulation of BDNF expression in the brain [306]. We found reduced NGF, BDNF and GDNF expression in DRG and spinal cord after increasing-intensity treadmill locomotion in sciatic nerve injured rats [64, 78]. The reduction of neurotrophins expression is associated to an increased expression of β2-adrenergic and 5HT2A serotonergic receptors in lumbar spinal cord, which are reduced after sciatic nerve injury (unpublished data). These results indicate that the increased neurotrophic factor production observed after peripheral nerve injury can be modulated by exercise in order to preserve and maintain the central circuitry from maladaptive plastic changes typically associated to neuropathic conditions [64].
On the other side, sustained and excessive activation of glutamate receptors is known to be excitotoxic, particularly under conditions of reduced availability of energy and increased oxidative stress. The upregulation of NMDA receptors is a mechanism for long-term enhancement of glutamatergic activity by exercise [307]. In addition, prolonged activation of AMPA receptors can promote Ca2+-mediated BDNF release for signaling through TrkB receptor [308, 309]. High intensity exercise increases BDNF levels that may lead to neuronal hyperexcitability [310]. Consequently BDNF may further induce enhancement of synaptic plasticity by strengthening the excitatory glutamatergic transmission. This positive feedback loop between BDNF and glutamatergic activities may result in increased seizure vulnerability and effects of excitotoxicity that are often seen after experimental manipulations to stimulate BDNF function, as showed in the hippocampus [311-313]. Hyperexcitability following BDNF upregulation may also account for excitotoxicity as due to increased vulnerability to kainic acid as demonstrated after injection into the hippocampus of chronically exercised rats [314].
CONCLUSION
Physical activity exerts neuroprotective effects that are conveyed by multiple mechanisms to peripheral and central neurons. The neuroprotection resulting from exercise training is an endogenous effect that may be best afforded by specific exercise protocols. Neuroprotective and pro-regenerative effects of exercise have been associated to modulation of neurotrophic factors. The enhancement of neurotrophic factors expression is connected to a number of responses, such the strengthening the neurovascular unit, the induction of astrocytosis, angiogenesis and arteriogenesis, the decrease of inflammatory response and microgliosis, and the decrease of apoptotis, that all together protect neurons from the consequences of nerve injuries and neuro-degenerative diseases. However, the effects of increasing neurotrophic factors are dichotomous, ranging from neuro-protection and nerve repair to cell death, hyperexcitability and induction of pain. Significant reduction of inflammation and neuropathic pain was recently achieved through moderate-to-high intensity exercise that was associated to a decrease of neurotrophic factors, particularly NGF, BDNF and GDNF levels in sensory neurons, after peripheral nerve injuries [64, 78, 124]. Furthermore, high intensity exercise reduces brain neurotrophins similarly to the activation of metabolic and glucocorticoid stress responses [235-237].
From a practical point of view, exercise protocols can be easily applied to induce neuroprotection in the setting of rehabilitation after nerve injuries but several critical parameters should take into account. First of all, the intensity of activity can be tuned up to reach more efficaciously the aimed outcome of training. It may be increased in order to enhance neurotrophic factor production (for example to stimulate motor and sensory nerve regeneration), or further increased till high levels to trigger the opposite effect and decrease neurotrophic factors (for example, to modulate plasticity, pain and hyperexcitability). The type of injury (peripheral versus central) is crucial to choose between different intensity exercises, since neurotrophic factor response to insult may be opposite: peripheral nerve injuries induce an increase of neurotrophic factors expression as spontaneous response to regenerate, but after spinal cord injuries a loss of neurotrophic factors has been observed and even associated to pain conditions. Moreover, exercise intensity is relevant in promoting neuroprotection at different sensory and motor modalities: for example, low-to-intermediate intensities of exercise activate slow-twitch muscle fibers to a greater extent than fast twitch muscle fibers, and stimulate sprouting and regeneration of sensory neurons, but contrarily adaptation of fast twitch muscles and reduction of sensory fiber sprouting were observed after high intensity exercise. The time window of exercise treatment after injury is another critical parameter. Since chronic and acute physical activity can have opposite effects on neuroprotection, this duality should be better assumed, particularly in individuals with underlying inflammatory conditions. The greater effects of exercise take place within
the time of higher vulnerability and plasticity of the damaged pathways. This seems to be the early time after the insult, when modulation of neurotrophic factors transcription and activation may play a stronger effect in protecting neurons from primary and secondary damage.
Physical activity may be considered an affordable and effective method to increase neuroprotection and improve functional recovery by acting on neurotrophic factors, representing an endogenous treatment. Although the application of different exercise treatments has uncovered the differences in cognitive and behavioral phenotypes in animal models of nerve injuries, more recent studies underscored the various levels by which the function of BDNF and other factors can be manipulated with activity-dependent therapies. The use of distinct intensities of activity, the preferential application at critical phases of neurotrophins expression, the regulation of pro-neuro-trophins to mature neurotrophin cleavage, and the design of paradigms to differentially stimulate peripheral versus central and sensory versus motor neurons, provide a multiple repertoire of regulatory mechanisms that may be optimized for each individual pathology.
ACKNOWLEDGEMENTS
This work was supported by TERCEL and CIBERNED funds from the Instituto de Salud Carlos III of Spain, and Grants BIOHYBRID (FP7-278612) and EPIONE (FP7-602547) from the European Commission (EC).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.del Zoppo G.J., Hallenbeck J.M. Advances in the vascular pathophysiology of ischemic stroke. Thromb. Res. 2000;98(3):73–81. doi: 10.1016/s0049-3848(00)00218-8. [http://dx.doi.org/10.1016/S0049-3848(00)00218-8]. [PMID: 10812160]. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo G.J., Mabuchi T. Cerebral microvessel responses to focal ischemia. J. Cereb. Blood Flow Metab. 2003;23(8):879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [http://dx.doi.org/10.1097/01.WCB.0000078322.96027.78]. [PMID: 12902832]. [DOI] [PubMed] [Google Scholar]
- 3.Davis W., Mahale S., Carranza A., Cox B., Hayes K., Jimenez D., Ding Y. Exercise pre-conditioning ameliorates blood-brain barrier dysfunction in stroke by enhancing basal lamina. Neurol. Res. 2007;29(4):382–387. doi: 10.1179/016164107X204701. [http://dx.doi.org/10.1179/ 016164107X204701]. [PMID: 17626734]. [DOI] [PubMed] [Google Scholar]
- 4.Dans M.J., Giancotti F.G. Guidebook to the extracellular matrix, anchor, and adhesion proteins. Oxford: Sambrook & Tooze; 1999. [Google Scholar]
- 5.Allodi I., Udina E., Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog. Neurobiol. 2012;98(1):16–37. doi: 10.1016/j.pneurobio.2012.05.005. [http://dx.doi.org/10.1016/j.pneurobio.2012.05.005]. [PMID: 22609046]. [DOI] [PubMed] [Google Scholar]
- 6.Tawil N.J., Wilson P., Carbonetto S. Expression and distribution of functional integrins in rat CNS glia. J. Neurosci. Res. 1994;39(4):436–447. doi: 10.1002/jnr.490390411. [http://dx.doi.org/10.1002/jnr.490390411]. [PMID: 7533845]. [DOI] [PubMed] [Google Scholar]
- 7.Boppart M.D., Volker S.E., Alexander N., Burkin D.J., Kaufman S.J. Exercise promotes alpha7 integrin gene transcription and protection of skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295(5):R1623–R1630. doi: 10.1152/ajpregu.00089.2008. [http://dx.doi.org/10. 1152/ajpregu.00089.2008]. [PMID: 18784336]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lueders T.N., Zou K., Huntsman H.D., Meador B., Mahmassani Z., Abel M., Valero M.C., Huey K.A., Boppart M.D. The α7β1-integrin accelerates fiber hypertrophy and myogenesis following a single bout of eccentric exercise. Am. J. Physiol. Cell Physiol. 2011;301(4):C938–C946. doi: 10.1152/ajpcell.00515.2010. [http://dx.doi.org/10.1152/ajpcell.00515. 2010]. [PMID: 21753185]. [DOI] [PubMed] [Google Scholar]
- 9.Ding Q., Vaynman S., Akhavan M., Ying Z., Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. [http://dx.doi.org/10.1016/j.neuroscience. 2006.02.084]. [PMID: 16650607]. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Ding Y.H., Rafols J.A., Lai Q., McAllister J.P., II, Ding Y. Increased astrocyte proliferation in rats after running exercise. Neurosci. Lett. 2005;386(3):160–164. doi: 10.1016/j.neulet.2005.06.009. [http://dx.doi.org/10.1016/j. neulet.2005.06.009]. [PMID: 16024173]. [DOI] [PubMed] [Google Scholar]
- 11.Cobianchi S., Marinelli S., Florenzano F., Pavone F., Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168(1):273–287. doi: 10.1016/j.neuroscience.2010.03.035. [http://dx.doi.org/10.1016/ j.neuroscience.2010.03.035]. [PMID: 20347016]. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi Y., Utsumi H., Chiba H., Yamada-Sasamori Y., Tobioka H., Kamimura Y., Furuuchi K., Kokai Y., Nakagawa T., Mori M., Sawada N. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem. Biophys. Res. Commun. 1999;261(1):108–112. doi: 10.1006/bbrc.1999.0992. [http://dx.doi.org/10.1006/bbrc.1999.0992]. [PMID: 10405331]. [DOI] [PubMed] [Google Scholar]
- 13.Janzer R.C., Raff M.C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–257. doi: 10.1038/325253a0. [http://dx.doi.org/10.1038/325253a0]. [PMID: 3543687]. [DOI] [PubMed] [Google Scholar]
- 14.Willis C.L., Nolan C.C., Reith S.N., Lister T., Prior M.J., Guerin C.J., Mavroudis G., Ray D.E. Focal astrocyte loss is followed by microvascular damage, with subsequent repair of the blood-brain barrier in the apparent absence of direct astrocytic contact. Glia. 2004;45(4):325–337. doi: 10.1002/glia.10333. [http://dx.doi.org/10.1002/glia. 10333]. [PMID: 14966864]. [DOI] [PubMed] [Google Scholar]
- 15.Black J.E., Isaacs K.R., Anderson B.J., Alcantara A.A., Greenough W.T. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. USA. 1990;87(14):5568–5572. doi: 10.1073/pnas.87.14.5568. [http://dx. doi.org/10.1073/pnas.87.14.5568]. [PMID: 1695380]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isaacs K.R., Anderson B.J., Alcantara A.A., Black J.E., Greenough W.T. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J. Cereb. Blood Flow Metab. 1992;12(1):110–119. doi: 10.1038/jcbfm.1992.14. [http://dx.doi.org/10.1038/jcbfm.1992.14]. [PMID: 1370068]. [DOI] [PubMed] [Google Scholar]
- 17.Kleim J.A., Cooper N.R., VandenBerg P.M. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934(1):1–6. doi: 10.1016/s0006-8993(02)02239-4. [http://dx.doi.org/10.1016/S0006-8993(02)02239-4]. [PMID: 11937064]. [DOI] [PubMed] [Google Scholar]
- 18.Swain R.A., Harris A.B., Wiener E.C., Dutka M.V., Morris H.D., Theien B.E., Konda S., Engberg K., Lauterbur P.C., Greenough W.T. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [http://dx.doi.org/10.1016/ S0306-4522(02)00664-4]. [PMID: 12654355]. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y., Li J., Luan X., Ding Y.H., Lai Q., Rafols J.A., Phillis J.W., Clark J.C., Diaz F.G. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004;124(3):583–591. doi: 10.1016/j.neuroscience.2003.12.029. [a]. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P., Yu H., Zhou N., Zhang J., Wu Y., Zhang Y., Bai Y., Jia J., Zhang Q., Tian S., Wu J., Hu Y. Early exercise improves cerebral blood flow through increased angiogenesis in experimental stroke in rat model. J. Neuroeng. Rehabil. 2013;10:43. doi: 10.1186/1743-0003-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd P.G., Prior B.M., Yang H.T., Terjung R.L. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am. J. Physiol. Heart Circ. Physiol. 2003;284(5):H1668–H1678. doi: 10.1152/ajpheart.00743.2002. [http://dx.doi.org/10.1152/ajpheart.00743. 2002]. [PMID: 12543634]. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd P.G., Prior B.M., Li H., Yang H.T., Terjung R.L. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am. J. Physiol. Heart Circ. Physiol. 2005;288(2):H759–H768. doi: 10.1152/ajpheart.00786.2004. [http://dx.doi.org/10.1152/ajpheart.00786.2004]. [PMID: 15471974]. [DOI] [PubMed] [Google Scholar]
- 23.Vissing J., Andersen M., Diemer N.H. Exercise-induced changes in local cerebral glucose utilization in the rat. J. Cereb. Blood Flow Metab. 1996;16(4):729–736. doi: 10.1097/00004647-199607000-00025. [http://dx.doi.org/10.1097/ 00004647-199607000-00025]. [PMID: 8964814]. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm J.C., Xu M., Cucoranu D., Chmielewski S., Holmes T., Lau K.S., Bassell G.J., English A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012;32(14):5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [http://dx.doi.org/10.1523/JNEUROSCI.1411-11.2012]. [PMID: 22492055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng F.C., Sheu M.L., Su H.L., Chen Y.J., Chen C.J., Chiu W.T., Sheehan J., Pan H.C. The effect of exercise on mobilization of hematopoietic progenitor cells involved in the repair of sciatic nerve crush injury. J. Neurosurg. 2013;118(3):594–605. doi: 10.3171/2012.8.JNS111580. [http://dx.doi.org/10.3171/2012.8.JNS111580]. [PMID: 23176341]. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson T., Puntschart A., Kaijser L., Jansson E., Sundberg C.J. Exercise-induced expression of angiogenesis-related trans- cription and growth factors in human skeletal muscle. Am. J. Physiol. 1999;276(2 Pt 2):H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [PMID: 9950871]. [DOI] [PubMed] [Google Scholar]
- 27.Trejo J.L., Carro E., Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21(5):1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [PMID: 11222653]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabel K., Fabel K., Tam B., Kaufer D., Baiker A., Simmons N., Kuo C.J., Palmer T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [http://dx.doi.org/10.1111/j.1460-9568.2003. 03041.x]. [PMID: 14656329]. [DOI] [PubMed] [Google Scholar]
- 29.Tang K., Xia F.C., Wagner P.D., Breen E.C. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir. Physiol. Neurobiol. 2010;170(1):16–22. doi: 10.1016/j.resp.2009.10.007. [http://dx.doi.org/10.1016/j.resp.2009.10.007]. [PMID: 19853064]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [http://dx.doi.org/10.1210/edrv.18.1.0287]. [PMID: 9034784]. [DOI] [PubMed] [Google Scholar]
- 31.Lee J., Duan W., Mattson M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [http://dx.doi.org/10.1046/j.1471-4159.2002.01085.x]. [PMID: 12354284]. [DOI] [PubMed] [Google Scholar]
- 32.DiPenta J.M., Johnson J.G., Murphy R.J. Natural killer cells and exercise training in the elderly: a review. Can. J. Appl. Physiol. 2004;29(4):419–443. doi: 10.1139/h04-027. [http://dx.doi.org/10.1139/h04-027]. [PMID: 15317983]. [DOI] [PubMed] [Google Scholar]
- 33.Kohut M.L., Senchina D.S. Reversing age-associated immuno- senescence via exercise. Exerc. Immunol. Rev. 2004;10:6–41. [PMID: 15633584]. [PubMed] [Google Scholar]
- 34.Stewart K.J. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288(13):1622–1631. doi: 10.1001/jama.288.13.1622. [http://dx.doi.org/10.1001/jama.288.13.1622]. [PMID: 12350193]. [DOI] [PubMed] [Google Scholar]
- 35.Colbert L.H., Visser M., Simonsick E.M., Tracy R.P., Newman A.B., Kritchevsky S.B., Pahor M., Taaffe D.R., Brach J., Rubin S., Harris T.B. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004;52(7):1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [http://dx.doi.org/10.1111/j.1532-5415.2004.52307.x]. [PMID: 15209647]. [DOI] [PubMed] [Google Scholar]
- 36.Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. [http://dx.doi.org/10.1016/ j.tins.2007.06.011]. [PMID: 17765329]. [DOI] [PubMed] [Google Scholar]
- 37.Petersen A.M., Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [http://dx.doi.org/10.1152/japplphysiol.00164.2004]. [PMID: 15772055]. [DOI] [PubMed] [Google Scholar]
- 38.Kohut M.L., McCann D.A., Russell D.W., Konopka D.N., Cunnick J.E., Franke W.D., Castillo M.C., Reighard A.E., Vanderah E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006;20(3):201–209. doi: 10.1016/j.bbi.2005.12.002. [http://dx.doi.org/10.1016/ j.bbi.2005.12.002]. [PMID: 16504463]. [DOI] [PubMed] [Google Scholar]
- 39.Ploeger H.E., Takken T., de Greef M.H., Timmons B.W. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc. Immunol. Rev. 2009;15:6–41. [PMID: 19957870]. [PubMed] [Google Scholar]
- 40.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [http://dx.doi.org/10.1016/j.bbamcr.2011.01.034]. [PMID: 21296109]. [DOI] [PubMed] [Google Scholar]
- 41.Febbraio M.A., Pedersen B.K. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16(11):1335–1347. doi: 10.1096/fj.01-0876rev. [http://dx.doi.org/10.1096/fj.01-0876rev]. [PMID: 12205025]. [DOI] [PubMed] [Google Scholar]
- 42.Steensberg A., Fischer C.P., Keller C., Møller K., Pedersen B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285(2):E433–E437. doi: 10.1152/ajpendo.00074.2003. [http://dx.doi.org/10.1152/ajpendo.00074.2003]. [PMID: 12857678]. [DOI] [PubMed] [Google Scholar]
- 43.Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [http://dx.doi.org/10.1016/0014-5793(91)80803-B]. [PMID: 1649771]. [DOI] [PubMed] [Google Scholar]
- 44.Matthys P., Mitera T., Heremans H., Van Damme J., Billiau A. Anti-gamma interferon and anti-interleukin-6 antibodies affect staphylococcal enterotoxin B-induced weight loss, hypoglycemia, and cytokine release in D-galactosamine-sensitized and unsensitized mice. Infect. Immun. 1995;63(4):1158–1164. doi: 10.1128/iai.63.4.1158-1164.1995. [PMID: 7890366]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuhara H., ONeill E., Seki N., Ogawa T., Kusunoki C., Otsuka K., Satoh S., Niwa M., Senoh H., Fujiwara H. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J. Exp. Med. 1994;179(5):1529–1537. doi: 10.1084/jem.179.5.1529. [http://dx.doi.org/10.1084/jem.179.5.1529]. [PMID: 8163936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starkie R., Ostrowski S.R., Jauffred S., Febbraio M., Pedersen B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [PMID: 12626436]. [DOI] [PubMed] [Google Scholar]
- 47.Banks W.A., Kastin A.J., Gutierrez E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994;179(1-2):53–56. doi: 10.1016/0304-3940(94)90933-4. [http://dx.doi.org/10.1016/0304-3940(94) 90933-4]. [PMID: 7845624]. [DOI] [PubMed] [Google Scholar]
- 48.Heyser C.J., Masliah E., Samimi A., Campbell I.L., Gold L.H. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. USA. 1997;94(4):1500–1505. doi: 10.1073/pnas.94.4.1500. [http://dx.doi.org/10.1073/pnas.94.4.1500]. [PMID: 9037082]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daly R.M., OConnell S.L., Mundell N.L., Grimes C.A., Dunstan D.W., Nowson C.A. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am. J. Clin. Nutr. 2014;99(4):899–910. doi: 10.3945/ajcn.113.064154. [http://dx.doi.org/10.3945/ajcn.113.064154]. [PMID: 24477043]. [DOI] [PubMed] [Google Scholar]
- 50.Akerstrom T., Steensberg A., Keller P., Keller C., Penkowa M., Pedersen B.K. Exercise induces interleukin-8 expression in human skeletal muscle. J. Physiol. 2005;563(Pt 2):507–516. doi: 10.1113/jphysiol.2004.077610. [http://dx. doi.org/10.1113/jphysiol.2004.077610]. [PMID: 15618276]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Nieman D.C. Current perspective on exercise immunology. Curr. Sports Med. Rep. 2003;2(5):239–242. doi: 10.1249/00149619-200310000-00001. [http://dx.doi.org/10.1249/ 00149619-200310000-00001]. [PMID: 12959703]. [DOI] [PubMed] [Google Scholar]
- 52.Nieman D.C., Custer W.F., Butterworth D.E., Utter A.C., Henson D.A. Psychological response to exercise training and/or energy restriction in obese women. J. Psychosom. Res. 2000;48(1):23–29. doi: 10.1016/s0022-3999(99)00066-5. [http://dx.doi.org/10.1016/S0022-3999(99)00066-5]. [PMID: 10750626]. [DOI] [PubMed] [Google Scholar]
- 53.Chan M.H., Carey A.L., Watt M.J., Febbraio M.A. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(2):R322–R327. doi: 10.1152/ajpregu.00030.2004. [http://dx.doi.org/10.1152/ ajpregu.00030.2004]. [PMID: 15072962]. [DOI] [PubMed] [Google Scholar]
- 54.Keller C., Keller P., Giralt M., Hidalgo J., Pedersen B.K. Exercise normalises overexpression of TNF-alpha in knockout mice. Biochem. Biophys. Res. Commun. 2004;321(1):179–182. doi: 10.1016/j.bbrc.2004.06.129. [http://dx.doi.org/10.1016/j.bbrc.2004.06.129]. [PMID: 15358232]. [DOI] [PubMed] [Google Scholar]
- 55.Salcedo R., Oppenheim J.J. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10(3-4):359–370. doi: 10.1038/sj.mn.7800200. [http://dx.doi.org/10.1080/mic.10.3-4.359.370]. [PMID: 12851652]. [DOI] [PubMed] [Google Scholar]
- 56.Hill W.D., Hess D.C., Martin-Studdard A., Carothers J.J., Zheng J., Hale D., Maeda M., Fagan S.C., Carroll J.E., Conway S.J. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 2004;63(1):84–96. doi: 10.1093/jnen/63.1.84. [http://dx.doi.org/10.1093/jnen/63.1.84]. [PMID: 14748564]. [DOI] [PubMed] [Google Scholar]
- 57.Zaldivar F., Eliakim A., Radom-Aizik S., Leu S.Y., Cooper D.M. The effect of brief exercise on circulating CD34+ stem cells in early and late pubertal boys. Pediatr. Res. 2007;61(4):491–495. doi: 10.1203/pdr.0b013e3180332d36. [http://dx.doi.org/10.1203/pdr.0b013e3180332d36]. [PMID: 17515877]. [DOI] [PubMed] [Google Scholar]
- 58.Parachikova A., Nichol K.E., Cotman C.W. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol. Dis. 2008;30(1):121–129. doi: 10.1016/j.nbd.2007.12.008. [http://dx.doi.org/10.1016/j.nbd.2007.12.008]. [PMID: 18258444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callewaere C., Fernette B., Raison D., Mechighel P., Burlet A., Calas A., Kitabgi P., Parsadaniantz S.M., Rostène W. Cellular and subcellular evidence for neuronal interaction between the chemokine stromal cell-derived factor-1/CXCL 12 and vasopressin: regulation in the hypothalamo-neurohypophysial system of the Brattleboro rats. Endocrinology. 2008;149(1):310–319. doi: 10.1210/en.2007-1097. [http://dx. doi.org/10.1210/en.2007-1097]. [PMID: 17901225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasojima K., Schwab C., McGeer E.G., McGeer P.L. Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimers disease. Brain Res. 2000;887(1):80–89. doi: 10.1016/s0006-8993(00)02970-x. [http://dx. doi.org/10.1016/S0006-8993(00)02970-X]. [PMID: 11134592]. [DOI] [PubMed] [Google Scholar]
- 61.Bi B.T., Lin H.B., Cheng Y.F., Zhou H., Lin T., Zhang M.Z., Li T.J., Xu J.P. Promotion of β-amyloid production by C-reactive protein and its implications in the early pathogenesis of Alzheimers disease. Neurochem. Int. 2012;60(3):257–266. doi: 10.1016/j.neuint.2011.12.007. [http://dx.doi.org/10.1016/j.neuint.2011.12.007]. [PMID: 22202667]. [DOI] [PubMed] [Google Scholar]
- 62.Woods J.A., Vieira V.J., Keylock K.T. Exercise, inflammation, and innate immunity. Neurol. Clin. 2006;24(3):585–599. doi: 10.1016/j.ncl.2006.03.008. [http://dx.doi.org/10.1016/j.ncl.2006.03.008]. [PMID: 16877125]. [DOI] [PubMed] [Google Scholar]
- 63.Michigan A., Johnson T.V., Master V.A. Review of the relationship between C-reactive protein and exercise. Mol. Diagn. Ther. 2011;15(5):265–275. doi: 10.1007/BF03256418. [http://dx.doi.org/10.1007/ BF03256418]. [PMID: 22047154]. [DOI] [PubMed] [Google Scholar]
- 64.López-Álvarez V.M., Mòdol L., Navarro X., Cobianchi S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156(9):1812–1825. doi: 10.1097/j.pain.0000000000000268. [http://dx.doi.org/10.1097/j.pain. 0000000000000268]. [PMID: 26090759]. [DOI] [PubMed] [Google Scholar]
- 65.Kohman R.A., Bhattacharya T.K., Wojcik E., Rhodes J.S. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J. Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [http://dx.doi.org/10.1186/1742-2094-10-114]. [PMID: 24044641]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sung Y.H., Kim S.C., Hong H.P., Park C.Y., Shin M.S., Kim C.J., Seo J.H., Kim D.Y., Kim D.J., Cho H.J. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinsons disease mice. Life Sci. 2012;91(25-26):1309–1316. doi: 10.1016/j.lfs.2012.10.003. [http://dx.doi.org/10.1016/j.lfs.2012.10.003]. [PMID: 23069581]. [DOI] [PubMed] [Google Scholar]
- 67.Yoo D.Y., Chae J., Jung H.Y., Yim H.S., Kim J.W., Nam S.M., Kim D.W., Choi J.H., Seong J.K., Yoon Y.S., Hwang I.K. Treadmill exercise is associated with reduction of reactive micro- gliosis and pro-inflammatory cytokine levels in the hippocampus of type 2 diabetic rats. Neurol. Res. 2015;37(8):732–738. doi: 10.1179/1743132815Y.0000000015. [http://dx. doi.org/10.1179/1743132815Y.0000000015]. [PMID: 25797150]. [DOI] [PubMed] [Google Scholar]
- 68.Ren K., Dubner R. Activity-triggered tetrapartite neuron-glial interactions following peripheral injury. Curr. Opin. Pharmacol. 2016;26:16–25. doi: 10.1016/j.coph.2015.09.006. [http://dx.doi.org/10.1016/j.coph.2015.09.006]. [PMID: 26431645]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scholz J., Woolf C.J. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [http://dx.doi.org/10.1038/nn1992]. [PMID: 17965656]. [DOI] [PubMed] [Google Scholar]
- 70.Calvo M., Dawes J.M., Bennett D.L. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11(7):629–642. doi: 10.1016/S1474-4422(12)70134-5. [http://dx.doi.org/10.1016/S1474-4422(12)70134-5]. [PMID: 22710756]. [DOI] [PubMed] [Google Scholar]
- 71.Trang T., Beggs S., Salter M.W. ATP receptors gate microglia signaling in neuropathic pain. Exp. Neurol. 2012;234(2):354–361. doi: 10.1016/j.expneurol.2011.11.012. [http://dx.doi.org/10.1016/j.expneurol.2011.11.012]. [PMID: 22116040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou L.J., Yang T., Wei X., Liu Y., Xin W.J., Chen Y., Pang R.P., Zang Y., Li Y.Y., Liu X.G. Brain-derived neurotrophic factor contributes to spinal long-term potentiation and mechanical hypersensitivity by activation of spinal microglia in rat. Brain Behav. Immun. 2011;25(2):322–334. doi: 10.1016/j.bbi.2010.09.025. [http://dx.doi.org/10.1016/ j.bbi.2010.09.025]. [PMID: 20933591]. [DOI] [PubMed] [Google Scholar]
- 73.Nadeau S., Filali M., Zhang J., Kerr B.J., Rivest S., Soulet D., Iwakura Y., de Rivero Vaccari J.P., Keane R.W., Lacroix S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J. Neurosci. 2011;31(35):12533–12542. doi: 10.1523/JNEUROSCI.2840-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.2840-11.2011]. [PMID: 21880915]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schäfers M., Lee D.H., Brors D., Yaksh T.L., Sorkin L.S. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-α after spinal nerve ligation. J. Neurosci. 2003;23(7):3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [PMID: 12684490]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coull J.A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M.W., De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [http://dx.doi.org/10.1038/nature04223]. [PMID: 16355225]. [DOI] [PubMed] [Google Scholar]
- 76.Coull J.A., Boudreau D., Bachand K., Prescott S.A., Nault F., Sík A., De Koninck P., De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [http://dx.doi.org/10.1038/nature01868]. [PMID: 12931188]. [DOI] [PubMed] [Google Scholar]
- 77.Ferrini F., De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013 doi: 10.1155/2013/429815. 429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cobianchi S., Casals-Díaz L., Jaramillo J., Navarro X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp. Neurol. 2013;240:157–167. doi: 10.1016/j.expneurol.2012.11.023. [http://dx.doi.org/10.1016/ j.expneurol.2012.11.023]. [PMID: 23201096]. [DOI] [PubMed] [Google Scholar]
- 79.Rivera C., Li H., Thomas-Crusells J., Lahtinen H., Viitanen T., Nanobashvili A., Kokaia Z., Airaksinen M.S., Voipio J., Kaila K., Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 2002;159(5):747–752. doi: 10.1083/jcb.200209011. [http://dx.doi.org/10.1083/jcb. 200209011]. [PMID: 12473684]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beggs S., Trang T., Salter M.W. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 2012;15(8):1068–1073. doi: 10.1038/nn.3155. [http://dx.doi.org/10.1038/nn.3155]. [PMID: 22837036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Price T.J., Cervero F., de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr. Top. Med. Chem. 2005;5(6):547–555. doi: 10.2174/1568026054367629. [http://dx.doi.org/10.2174/ 1568026054367629]. [PMID: 16022677]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuskaitis C.J., Jope R.S. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell. Signal. 2009;21(2):264–273. doi: 10.1016/j.cellsig.2008.10.014. [http://dx.doi.org/10.1016/j.cellsig.2008.10.014]. [PMID: 19007880]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Markuns J.F., Wojtaszewski J.F., Goodyear L.J. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J. Biol. Chem. 1999;274(35):24896–24900. doi: 10.1074/jbc.274.35.24896. [http://dx.doi.org/10.1074/jbc.274.35.24896]. [PMID: 10455163]. [DOI] [PubMed] [Google Scholar]
- 84.Sakamoto K., Arnolds D.E., Ekberg I., Thorell A., Goodyear L.J. Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem. Biophys. Res. Commun. 2004;319(2):419–425. doi: 10.1016/j.bbrc.2004.05.020. [http://dx.doi.org/10.1016/ j.bbrc.2004.05.020]. [PMID: 15178423]. [DOI] [PubMed] [Google Scholar]
- 85.Wojtaszewski J.F., Nielsen P., Kiens B., Richter E.A. Regulation of glycogen synthase kinase-3 in human skeletal muscle: effects of food intake and bicycle exercise. Diabetes. 2001;50(2):265–269. doi: 10.2337/diabetes.50.2.265. [http://dx.doi.org/10.2337/diabetes.50.2.265]. [PMID: 11272135]. [DOI] [PubMed] [Google Scholar]
- 86.Beurel E., Michalek S.M., Jope R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010;31(1):24–31. doi: 10.1016/j.it.2009.09.007. [http://dx.doi.org/10.1016/j.it.2009. 09.007]. [PMID: 19836308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaidanovich-Beilin O., Woodgett J.R. GSK-3: functional insights from cell biology and animal models. Front. Mol. Neurosci. 2011;4:40. doi: 10.3389/fnmol.2011.00040. [http://dx.doi.org/10.3389/fnmol.2011.00040]. [PMID: 22110425]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maixner D.W., Weng H.R. The role of glycogen synthase kinase 3 beta in neuroinflammation and pain. . J. Pharm. Pharmacol. 2013;1(1) doi: 10.13188/2327-204X.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stambolic V., Ruel L., Woodgett J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6(12):1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [http://dx.doi.org/10. 1016/S0960-9822(02)70790-2]. [PMID: 8994831]. [DOI] [PubMed] [Google Scholar]
- 90.Shaw M., Cohen P. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 1999;461(1-2):120–124. doi: 10.1016/s0014-5793(99)01434-9. [http://dx.doi.org/10.1016/S0014-5793(99)01434-9]. [PMID: 10561508]. [DOI] [PubMed] [Google Scholar]
- 91.Bailou L.M., Tian P.Y., Lin H.Y., Jiang Y.P., Lin R.Z. Dual regulation of glycogen synthases kinase-3 beta by the alpha 1A-adrenergic receptor. J. Biol. Chem. 2001;274:40910–40916. doi: 10.1074/jbc.M103480200. [http://dx.doi.org/10.1074/jbc.M103480200]. [DOI] [PubMed] [Google Scholar]
- 92.Markuns J.F., Wojtaszewski J.F., Goodyear L.J. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J. Biol. Chem. 1999;274(35):24896–24900. doi: 10.1074/jbc.274.35.24896. [http://dx.doi.org/10.1074/jbc.274.35.24896]. [PMID: 10455163]. [DOI] [PubMed] [Google Scholar]
- 93.Wang M.J., Huang H.Y., Chen W.F., Chang H.F., Kuo J.S. Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. J. Neuroinflammation. 2010;7:99–116. doi: 10.1186/1742-2094-7-99. [http://dx.doi.org/10.1186/1742-2094-7-99]. [PMID: 21194439]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Green H.F., Nolan Y.M. GSK-3 mediates the release of IL-1β, TNF-α and IL-10 from cortical glia. Neurochem. Int. 2012;61(5):666–671. doi: 10.1016/j.neuint.2012.07.003. [http://dx.doi.org/10.1016/j.neuint.2012.07.003]. [PMID: 22796213]. [DOI] [PubMed] [Google Scholar]
- 95.Salehi A., Delcroix J.D., Swaab D.F. Alzheimers disease and NGF signaling. J. Neural. Transm (Vienna) 2004;111(3):323–345. doi: 10.1007/s00702-003-0091-x. [http://dx.doi.org/10.1007/s00702-003-0091-x]. [PMID: 14991458]. [DOI] [PubMed] [Google Scholar]
- 96.Salehi A., Delcroix J.D., Mobley W.C. Traffic at the intersection of neurotrophic factor signaling and neurodegeneration. Trends Neurosci. 2003;26(2):73–80. doi: 10.1016/S0166-2236(02)00038-3. [http://dx.doi.org/10.1016/S0166-2236(02)00038-3]. [PMID: 12536130]. [DOI] [PubMed] [Google Scholar]
- 97.Kim K.H., Kim M.A., Moon E., Kim S.Y., Choi S.Z., Son M.W., Lee K.R. Furostanol saponins from the rhizomes of Dioscorea japonica and their effects on NGF induction. Bioorg. Med. Chem. Lett. 2011;21(7):2075–2078. doi: 10.1016/j.bmcl.2011.02.003. [http://dx.doi.org/10.1016/j.bmcl.2011.02.003]. [PMID: 21353549]. [DOI] [PubMed] [Google Scholar]
- 98.Konar A., Shah N., Singh R., Saxena N., Kaul S.C., Wadhwa R., Thakur M.K. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6(11):e27265. doi: 10.1371/journal.pone.0027265. [http://dx.doi.org/10.1371/journal.pone.0027265]. [PMID: 22096544]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curtis R., Adryan K.M., Stark J.L., Park J.S., Compton D.L., Weskamp G., Huber L.J., Chao M.V., Jaenisch R., Lee K.F. Differential role of the low affinity neurotrophin receptor (p75) in retrograde axonal transport of the neurotrophins. Neuron. 1995;14(6):1201–1211. doi: 10.1016/0896-6273(95)90267-8. [http://dx.doi.org/10.1016/0896-6273(95)90267-8]. [PMID: 7541633]. [DOI] [PubMed] [Google Scholar]
- 100.Villanueva R. Neurobiology of major depressive disorder. NeuralPlast. 2013 doi: 10.1155/2013/873278. 873278. [http://dx.doi.org/10.1155/2013/873278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gómez-Pinilla F., Ying Z., Opazo P., Roy R.R., Edgerton V.R. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur. J. Neurosci. 2001;13(6):1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [http://dx.doi.org/10.1046/j.0953-816x.2001.01484.x]. [PMID: 11285004]. [DOI] [PubMed] [Google Scholar]
- 102.Molteni R., Zheng J.Q., Ying Z., Gómez-Pinilla F., Twiss J.L. Voluntary exercise increases axonal regeneration from sensory neurons. Proc. Natl. Acad. Sci. USA. 2004;101(22):8473–8478. doi: 10.1073/pnas.0401443101. [http://dx.doi.org/10.1073/pnas.0401443101]. [PMID: 15159540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gómez-Pinilla F., Ying Z., Roy R.R., Molteni R., Edgerton V.R. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002;88(5):2187–2195. doi: 10.1152/jn.00152.2002. [http://dx.doi.org/10.1152/jn.00152.2002]. [PMID: 12424260]. [DOI] [PubMed] [Google Scholar]
- 104.Macias M., Fehr S., Dwornik A., Sulejczak D., Wiater M., Czarkowska-Bauch J., Skup M., Schachner M. Exercise increases mRNA levels for adhesion molecules N-CAM and L1 correlating with BDNF response. Neuroreport. 2002;13(18):2527–2530. doi: 10.1097/00001756-200212200-00029. [http://dx.doi.org/10.1097/00001756-200212200-00029]. [PMID: 12499861]. [DOI] [PubMed] [Google Scholar]
- 105.Skup M., Dwornik A., Macias M., Sulejczak D., Wiater M., Czarkowska-Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp. Neurol. 2002;176(2):289–307. doi: 10.1006/exnr.2002.7943. [http://dx.doi.org/10.1006/exnr.2002.7943]. [PMID: 12359171]. [DOI] [PubMed] [Google Scholar]
- 106.Chae C.H., Kim H.T. Forced, moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochem. Int. 2009;55(4):208–213. doi: 10.1016/j.neuint.2009.02.024. [http://dx.doi.org/10.1016/j.neuint.2009.02.024]. [PMID: 19524110]. [DOI] [PubMed] [Google Scholar]
- 107.Neeper S.A., Gómez-Pinilla F., Choi J., Cotman C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1-2):49–56. [http://dx.doi.org/10.1016/0006-8993(96)00273-9]. [PMID: 8836544]. [PubMed] [Google Scholar]
- 108.English A.W., Wilhelm J.C., Sabatier M.J. Enhancing recovery from peripheral nerve injury using treadmill training. Ann. Anat. 2011;193(4):354–361. doi: 10.1016/j.aanat.2011.02.013. [http://dx.doi.org/10.1016/j.aanat.2011.02. 013]. [PMID: 21498059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCullough M.J., Peplinski N.G., Kinnell K.R., Spitsbergen J.M. Glial cell line-derived neurotrophic factor protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience. 2011;174:234–244. doi: 10.1016/j.neuroscience.2010.11.016. [http://dx.doi.org/10.1016/j.neuroscience.2010.11.016]. [PMID: 21081155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wehrwein E.A., Roskelley E.M., Spitsbergen J.M. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve. 2002;26(2):206–211. doi: 10.1002/mus.10179. [http://dx.doi.org/10.1002/ mus.10179]. [PMID: 12210384]. [DOI] [PubMed] [Google Scholar]
- 111.McCullough M.J., Gyorkos A.M., Spitsbergen J.M. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience. 2013;240:258–268. doi: 10.1016/j.neuroscience.2013.02.063. [http://dx.doi.org/10.1016/j.neuroscience.2013.02.063]. [PMID: 23500094]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gyorkos A.M., McCullough M.J., Spitsbergen J.M. Glial cell line-derived neurotrophic factor (GDNF) expression and NMJ plasticity in skeletal muscle following endurance exercise. Neuroscience. 2014;257:111–118. doi: 10.1016/j.neuroscience.2013.10.068. [http://dx.doi.org/10.1016/ j.neuroscience.2013.10.068]. [PMID: 24215980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Navarro X., Vivó M., Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog. Neurobiol. 2007;82(4):163–201. doi: 10.1016/j.pneurobio.2007.06.005. [http://dx.doi.org/10.1016/j.pneurobio.2007.06.005]. [PMID: 17643733]. [DOI] [PubMed] [Google Scholar]
- 114.Gordon T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J. Commun. Disord. 2010;43(4):265–273. doi: 10.1016/j.jcomdis.2010.04.003. [http://dx.doi.org/10.1016/j.jcomdis.2010.04.003]. [PMID: 20451212]. [DOI] [PubMed] [Google Scholar]
- 115.Sabatier M.J., Redmon N., Schwartz G., English A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008;211(2):489–493. doi: 10.1016/j.expneurol.2008.02.013. [http://dx.doi.org/10.1016/ j.expneurol.2008.02.013]. [PMID: 18420199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mika J.K., Schwarz K., Wanzenboeck H.D., Scholze P., Bertagnolli E. Investigation of neurotrophic factor concentrations with a novel in vitro concept for peripheral nerve regeneration. J. Neurosci. Res. 2015;93(11):1631–1640. doi: 10.1002/jnr.23598. [http://dx.doi.org/10. 1002/jnr.23598]. [PMID: 26214267]. [DOI] [PubMed] [Google Scholar]
- 117.Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg. Focus. 2009;26(2):E3. doi: 10.3171/FOC.2009.26.2.E3. [http://dx.doi.org/10.3171/ FOC.2009.26.2.E3]. [PMID: 19228105]. [DOI] [PubMed] [Google Scholar]
- 118.Pezet S., McMahon S.B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [http://dx.doi.org/10.1146/annurev.neuro.29.051605.112929]. [PMID: 16776595]. [DOI] [PubMed] [Google Scholar]
- 119.Obata K., Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci. Res. 2006;55(1):1–10. doi: 10.1016/j.neures.2006.01.005. [http://dx.doi.org/10.1016/j. neures.2006.01.005]. [PMID: 16516994]. [DOI] [PubMed] [Google Scholar]
- 120.Ro L.S., Chen S.T., Tang L.M., Jacobs J.M. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79(2-3):265–274. doi: 10.1016/s0304-3959(98)00164-x. [http://dx.doi.org/10.1016/S0304-3959(98)00164-X]. [PMID: 10068172]. [DOI] [PubMed] [Google Scholar]
- 121.Diamond J., Coughlin M., Macintyre L., Holmes M., Visheau B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc. Natl. Acad. Sci. USA. 1987;84(18):6596–6600. doi: 10.1073/pnas.84.18.6596. [http://dx.doi.org/10.1073/pnas.84.18.6596]. [PMID: 3306683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ugolini G., Marinelli S., Covaceuszach S., Cattaneo A., Pavone F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc. Natl. Acad. Sci. USA. 2007;104(8):2985–2990. doi: 10.1073/pnas.0611253104. [http://dx.doi.org/10.1073/pnas. 0611253104]. [PMID: 17301229]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wild K.D., Bian D., Zhu D., Davis J., Bannon A.W., Zhang T.J., Louis J.C. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J. Pharmacol. Exp. Ther. 2007;322(1):282–287. doi: 10.1124/jpet.106.116236. [http://dx.doi.org/10.1124/jpet.106.116236]. [PMID: 17431136]. [DOI] [PubMed] [Google Scholar]
- 124.Almeida C., DeMaman A., Kusuda R., Cadetti F., Ravanelli M.I., Queiroz A.L., Sousa T.A., Zanon S., Silveira L.R., Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156(3):504–513. doi: 10.1097/01.j.pain.0000460339.23976.12. [http://dx.doi.org/10.1097/01.j.pain.0000460339. 23976.12]. [PMID: 25687543]. [DOI] [PubMed] [Google Scholar]
- 125.Ren K., Dubner R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010;16(11):1267–1276. doi: 10.1038/nm.2234. [http://dx. doi.org/10.1038/nm.2234]. [PMID: 20948535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kerr B.J., Bradbury E.J., Bennett D.L., Trivedi P.M., Dassan P., French J., Shelton D.B., McMahon S.B., Thompson S.W. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J. Neurosci. 1999;19(12):5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [PMID: 10366647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murase S., Terazawa E., Hirate K., Yamanaka H., Kanda H., Noguchi K., Ota H., Queme F., Taguchi T., Mizumura K. Upregulated glial cell line-derived neurotrophic factor through cyclooxygenase-2 activation in the muscle is required for mechanical hyperalgesia after exercise in rats. J. Physiol. 2013;591(12):3035–3048. doi: 10.1113/jphysiol.2012.249235. [http://dx.doi.org/10.1113/jphysiol.2012.249235]. [PMID: 23587883]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hutchinson K.J., Gómez-Pinilla F., Crowe M.J., Ying Z., Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(Pt 6):1403–1414. doi: 10.1093/brain/awh160. [http://dx.doi.org/10.1093/brain/awh160]. [PMID: 15069022]. [DOI] [PubMed] [Google Scholar]
- 129.Pezet S., Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin. Ther. Targets. 2004;8(5):391–399. doi: 10.1517/14728222.8.5.391. [http://dx.doi.org/10.1517/14728222.8.5.391]. [PMID: 15469390]. [DOI] [PubMed] [Google Scholar]
- 130.Schulte-Herbrüggen O., Braun A., Rochlitzer S., Jockers-Scherübl M.C., Hellweg R. Neurotrophic factorsa tool for therapeutic strategies in neurological, neuropsychiatric and neuro- immunological diseases? Curr. Med. Chem. 2007;14(22):2318–2329. doi: 10.2174/092986707781745578. [http://dx.doi.org/10.2174/092986707781745578]. [PMID: 17896980]. [DOI] [PubMed] [Google Scholar]
- 131.Ke H.C., Huang H.J., Liang K.C., Hsieh-Li H.M. Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise. Brain Res. 2011;1403:1–11. doi: 10.1016/j.brainres.2011.05.056. [http://dx.doi.org/10.1016/j.brainres.2011. 05.056]. [PMID: 21689809]. [DOI] [PubMed] [Google Scholar]
- 132.Liu H.L., Zhao G., Cai K., Zhao H.H., Shi L.D. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav. Brain Res. 2011;218(2):308–314. doi: 10.1016/j.bbr.2010.12.030. [http://dx.doi.org/10.1016/j.bbr.2010.12.030]. [PMID: 21192984]. [DOI] [PubMed] [Google Scholar]
- 133.Um H.S., Kang E.B., Koo J.H., Kim H.T., Jin Lee, Kim E.J., Yang C.H., An G.Y., Cho I.H., Cho J.Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimers disease. Neurosci. Res. 2011;69(2):161–173. doi: 10.1016/j.neures.2010.10.004. [http://dx.doi.org/10.1016/j.neures.2010.10.004]. [PMID: 20969897]. [DOI] [PubMed] [Google Scholar]
- 134.Abe K. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;79(10):1071. doi: 10.1212/WNL.0b013e31826bd5cf. [http://dx.doi.org/10.1212/WNL.0b013e31826bd5cf]. [PMID: 22946116]. [DOI] [PubMed] [Google Scholar]
- 135.Hamer M., Chida Y. Physical activity and risk of neuro- degenerative disease: a systematic review of prospective evidence. Psychol. Med. 2009;39(1):3–11. doi: 10.1017/S0033291708003681. [http://dx.doi.org/10.1017/ S0033291708003681]. [PMID: 18570697]. [DOI] [PubMed] [Google Scholar]
- 136.Liang K.Y., Mintun M.A., Fagan A.M., Goate A.M., Bugg J.M., Holtzman D.M., Morris J.C., Head D. Exercise and Alzheimers disease biomarkers in cognitively normal older adults. Ann. Neurol. 2010;68(3):311–318. doi: 10.1002/ana.22096. [http://dx.doi.org/10.1002/ana. 22096]. [PMID: 20818789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cotman C.W., Berchtold N.C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [http://dx.doi.org/10.1016/S0166-2236(02)02143-4]. [PMID: 12086747]. [DOI] [PubMed] [Google Scholar]
- 138.Gomez-Pinilla F., Zhuang Y., Feng J., Ying Z., Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011;33(3):383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [http://dx.doi.org/10.1111/j.1460-9568.2010.07508. x]. [PMID: 21198979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Figurov A., Pozzo-Miller L.D., Olafsson P., Wang T., Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381(6584):706–709. doi: 10.1038/381706a0. [http://dx.doi.org/10.1038/381706a0]. [PMID: 8649517]. [DOI] [PubMed] [Google Scholar]
- 140.Adlard P.A., Perreau V.M., Engesser-Cesar C., Cotman C.W. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci. Lett. 2004;363(1):43–48. doi: 10.1016/j.neulet.2004.03.058. [http://dx.doi.org/10. 1016/j.neulet.2004.03.058]. [PMID: 15157993]. [DOI] [PubMed] [Google Scholar]
- 141.Gomez-Pinilla F., Vaynman S., Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur. J. Neurosci. 2008;28(11):2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [http://dx.doi.org/10.1111/j.1460-9568.2008.06524.x]. [PMID: 19046371]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Greenberg M.E., Xu B., Lu B., Hempstead B.L. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI.3566-09.2009]. [PMID: 19828787]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Teng H.K., Teng K.K., Lee R., Wright S., Tevar S., Almeida R.D., Kermani P., Torkin R., Chen Z.Y., Lee F.S., Kraemer R.T., Nykjaer A., Hempstead B.L. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005;25(22):5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [http://dx.doi.org/10.1523/JNEUROSCI.5123-04.2005]. [PMID: 15930396]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chao M.V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [http://dx.doi.org/10.1038/nrn1078]. [PMID: 12671646]. [DOI] [PubMed] [Google Scholar]
- 145.Chao M.V., Rajagopal R., Lee F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006;110(2):167–173. doi: 10.1042/CS20050163. [http://dx.doi.org/10.1042/CS20050163]. [PMID: 16411893]. [DOI] [PubMed] [Google Scholar]
- 146.Woo N.H., Teng H.K., Siao C.J., Chiaruttini C., Pang P.T., Milner T.A., Hempstead B.L., Lu B. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 2005;8(8):1069–1077. doi: 10.1038/nn1510. [http://dx.doi.org/10.1038/ nn1510]. [PMID: 16025106]. [DOI] [PubMed] [Google Scholar]
- 147.Nagappan G., Zaitsev E., Senatorov V.V., Jr, Yang J., Hempstead B.L., Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc. Natl. Acad. Sci. USA. 2009;106(4):1267–1272. doi: 10.1073/pnas.0807322106. [http://dx.doi.org/10.1073/ pnas.0807322106]. [PMID: 19147841]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berchtold N.C., Kesslak J.P., Cotman C.W. Hippocampal brain-derived neurotrophic factor gene regulation by exercise and the medial septum. J. Neurosci. Res. 2002;68(5):511–521. doi: 10.1002/jnr.10256. [http://dx. doi.org/10.1002/jnr.10256]. [PMID: 12111841]. [DOI] [PubMed] [Google Scholar]
- 149.Chao M.V., Calissano P. Rita Levi-Montalcini: in memoriam. Neuron. 2013;77(3):385–387. doi: 10.1016/j.neuron.2013.01.019. [http://dx.doi.org/10.1016/j.neuron. 2013.01.019]. [PMID: 23527384]. [DOI] [PubMed] [Google Scholar]
- 150.Huang E.J., Reichardt L.F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [http://dx.doi.org/10.1146/annurev.biochem.72.121801.161629]. [PMID: 12676795]. [DOI] [PubMed] [Google Scholar]
- 151.Lee F.S., Chao M.V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. USA. 2001;98(6):3555–3560. doi: 10.1073/pnas.061020198. [http://dx.doi.org/10.1073/pnas.061020198]. [PMID: 11248116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [http://dx.doi.org/10.1098/rstb.2006.1894]. [PMID: 16939974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y., Cui H., Schroering A., Ding J.L., Lane W.S., McGill G., Fisher D.E., Ding H.F. NF-kappa B2 p100 is a pro-apoptotic protein with anti-oncogenic function. Nat. Cell Biol. 2002;4(11):888–893. doi: 10.1038/ncb872. [http://dx.doi.org/10.1038/ncb872]. [PMID: 12389034]. [DOI] [PubMed] [Google Scholar]
- 154.Feng D., Kim T., Ozkan E., Light M., Torkin R., Teng K.K., Hempstead B.L., Garcia K.C. Molecular and structural insight into proNGF engagement of p75NTR and sortilin. J. Mol. Biol. 2010;396(4):967–984. doi: 10.1016/j.jmb.2009.12.030. [http://dx.doi.org/10.1016/j.jmb.2009. 12.030]. [PMID: 20036257]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roux P.P., Barker P.A. Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 2002;67(3):203–233. doi: 10.1016/s0301-0082(02)00016-3. [http://dx.doi.org/10.1016/S0301-0082(02)00016-3]. [PMID: 12169297]. [DOI] [PubMed] [Google Scholar]
- 156.Kim D.H., Ko I.G., Kim B.K., Kim T.W., Kim S.E., Shin M.S., Kim C.J., Kim H., Kim K.M., Baek S.S. Treadmill exercise inhibits traumatic brain injury-induced hippocampal apoptosis. Physiol. Behav. 2010;101(5):660–665. doi: 10.1016/j.physbeh.2010.09.021. [http://dx.doi.org/10. 1016/j.physbeh.2010.09.021]. [PMID: 20888848]. [DOI] [PubMed] [Google Scholar]
- 157.Jung S.Y., Kim D.Y., Yune T.Y., Shin D.H., Baek S.B., Kim C.J. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp. Ther. Med. 2014;7(3):587–593. doi: 10.3892/etm.2013.1451. [PMID: 24520250]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen M.J., Russo-Neustadt A.A. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res. Mol. Brain Res. 2005;135(1-2):181–193. doi: 10.1016/j.molbrainres.2004.12.001. [http://dx.doi.org/10.1016/j.molbrainres. 2004.12.001]. [PMID: 15857681]. [DOI] [PubMed] [Google Scholar]
- 159.Dudek H., Datta S.R., Franke T.F., Birnbaum M.J., Yao R., Cooper G.M., Segal R.A., Kaplan D.R., Greenberg M.E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275(5300):661–665. doi: 10.1126/science.275.5300.661. [http://dx.doi.org/10.1126/science.275.5300.661]. [PMID: 9005851]. [DOI] [PubMed] [Google Scholar]
- 160.Cassilhas R.C., Lee K.S., Fernandes J., Oliveira M.G., Tufik S., Meeusen R., de Mello M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [http://dx.doi.org/10.1016/j.neuroscience.2011.11.029]. [PMID: 22155655]. [DOI] [PubMed] [Google Scholar]
- 161.Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D.R. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [http://dx. doi.org/10.1016/S0092-8674(03)00035-7]. [PMID: 12553913]. [DOI] [PubMed] [Google Scholar]
- 162.Mathai A.S., Bonen A., Benton C.R., Robinson D.L., Graham T.E. Rapid exercise-induced changes in PGC-1α mRNA and protein in human skeletal muscle. J. Appl. Physiol. 2008;105(4):1098–1105. doi: 10.1152/japplphysiol.00847.2007. [http://dx.doi.org/10.1152/japplphysiol.00847.2007]. [PMID: 18653753]. [DOI] [PubMed] [Google Scholar]
- 163.Vaynman S., Ying Z., Gómez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J. Neurosci. Res. 2004;76(3):356–362. doi: 10.1002/jnr.20077. [a]. [DOI] [PubMed] [Google Scholar]
- 164.Ding Y.H., Li J., Zhou Y., Rafols J.A., Clark J.C., Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc. Res. 2006;3(1):15–23. doi: 10.2174/156720206775541787. [http://dx.doi.org/10.2174/156720206775541787]. [PMID: 16472122]. [DOI] [PubMed] [Google Scholar]
- 165.Liu G., Keeler B.E., Zhukareva V., Houlé J.D. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp. Neurol. 2010;226(1):200–206. doi: 10.1016/j.expneurol.2010.08.032. [http://dx.doi.org/10.1016/j.expneurol.2010.08.032]. [PMID: 20816819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim D.H., Ko I.G., Kim B.K., Kim T.W., Kim S.E., Shin M.S., Kim C.J., Kim H., Kim K.M., Baek S.S. Treadmill exercise inhibits traumatic brain injury-induced hippocampal apoptosis. Physiol. Behav. 2010;101(5):660–665. doi: 10.1016/j.physbeh.2010.09.021. [http://dx.doi.org/10.1016/ j.physbeh.2010.09.021]. [PMID: 20888848]. [DOI] [PubMed] [Google Scholar]
- 167.Li S.J., Liu W., Wang J.L., Zhang Y., Zhao D.J., Wang T.J., Li Y.Y. The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur. Rev. Med. Pharmacol. Sci. 2014;18(6):905–909. [PMID: 24706318]. [PubMed] [Google Scholar]
- 168.Wang X., Guo S., Lu S., Zhou J., Li J., Xia S. Ultrasound-induced release of GDNF from lipid coated microbubbles injected into striatum reduces hypoxic-ischemic injury in neonatal rats. Brain Res. Bull. 2012;88(5):495–500. doi: 10.1016/j.brainresbull.2012.05.001. [http://dx.doi.org/10.1016/ j.brainresbull.2012.05.001]. [PMID: 22579834]. [DOI] [PubMed] [Google Scholar]
- 169.Clarkson E.D., Zawada W.M., Freed C.R. GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell Tissue Res. 1997;289(2):207–210. doi: 10.1007/s004410050867. [http://dx.doi.org/10.1007/s004410050867]. [PMID: 9211823]. [DOI] [PubMed] [Google Scholar]
- 170.Revilla S., Ursulet S., Álvarez-López M.J., Castro-Freire M., Perpiñá U., García-Mesa Y., Bortolozzi A., Giménez-Llort L., Kaliman P., Cristòfol R., Sarkis C., Sanfeliu C. Lenti-GDNF gene therapy protects against Alzheimers disease-like neuro- pathology in 3xTg-AD mice and MC65 cells. CNS Neurosci. Ther. 2014;20(11):961–972. doi: 10.1111/cns.12312. [http://dx.doi.org/10.1111/cns.12312]. [PMID: 25119316]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.d'Anglemont de Tassigny X., Pascual A., López-Barneo J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergicnigrostriatal pathway. Implications for Parkinson's disease. Front. Neuroanat. 2015;13(9):10. doi: 10.3389/fnana.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mattson M.P. Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Res. 2000;886(1-2):47–53. doi: 10.1016/s0006-8993(00)02790-6. [http://dx.doi.org/10.1016/S0006-8993(00)02790-6]. [PMID: 11119686]. [DOI] [PubMed] [Google Scholar]
- 173.Haskell W.L., Lee I.M., Pate R.R., Powell K.E., Blair S.N., Franklin B.A., Macera C.A., Heath G.W., Thompson P.D., Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [http://dx.doi.org/10.1161/CIRCULATIONAHA.107.185649]. [PMID: 17671237]. [DOI] [PubMed] [Google Scholar]
- 174.Sethi K.D. Clinical aspects of Parkinson disease. Curr. Opin. Neurol. 2002;15(4):457–460. doi: 10.1097/00019052-200208000-00009. [http://dx.doi.org/10.1097/ 00019052-200208000-00009]. [PMID: 12151843]. [DOI] [PubMed] [Google Scholar]
- 175.Goodwin V.A., Richards S.H., Taylor R.S., Taylor A.H., Campbell J.L. The effectiveness of exercise interventions for people with Parkinsons disease: a systematic review and meta-analysis. Mov. Disord. 2008;23(5):631–640. doi: 10.1002/mds.21922. [http://dx.doi.org/10.1002/mds.21922]. [PMID: 18181210]. [DOI] [PubMed] [Google Scholar]
- 176.Tajiri N., Yasuhara T., Shingo T., Kondo A., Yuan W., Kadota T., Wang F., Baba T., Tayra J.T., Morimoto T., Jing M., Kikuchi Y., Kuramoto S., Agari T., Miyoshi Y., Fujino H., Obata F., Takeda I., Furuta T., Date I. Exercise exerts neuroprotective effects on Parkinsons disease model of rats. Brain Res. 2010;1310(1310):200–207. doi: 10.1016/j.brainres.2009.10.075. [http://dx.doi.org/10.1016/j. brainres.2009.10.075]. [PMID: 19900418]. [DOI] [PubMed] [Google Scholar]
- 177.Chauhan N.B., Siegel G.J., Lee J.M. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinsons disease brain. J. Chem. Neuroanat. 2001;21(4):277–288. doi: 10.1016/s0891-0618(01)00115-6. [http://dx.doi.org/10.1016/S0891-0618(01)00115-6]. [PMID: 11429269]. [DOI] [PubMed] [Google Scholar]
- 178.Wu S.Y., Wang T.F., Yu L., Jen C.J., Chuang J-I., Wu F.S., Wu C.W., Kuo Y.M. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav. Immun. 2011;25(1):135–146. doi: 10.1016/j.bbi.2010.09.006. [http://dx.doi.org/10.1016/ j.bbi.2010.09.006]. [PMID: 20851176]. [DOI] [PubMed] [Google Scholar]
- 179.Tuon T., Valvassori S.S., Lopes-Borges J., Luciano T., Trom C.B., Silva L.A., Quevedo J., Souza C.T., Lira F.S., Pinho R.A. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinsons disease. Neuroscience. 2012;227:305–312. doi: 10.1016/j.neuroscience.2012.09.063. [http://dx.doi.org/10.1016/ j.neuroscience.2012.09.063]. [PMID: 23041759]. [DOI] [PubMed] [Google Scholar]
- 180.Lau Y.S., Patki G., Das-Panja K., Le W.D., Ahmad S.O. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinsons disease with moderate neuro- degeneration. Eur. J. Neurosci. 2011;33(7):1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [http://dx. doi.org/10.1111/j.1460-9568.2011.07626.x]. [PMID: 21375602]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.García-Mesa Y., López-Ramos J.C., Giménez-Llort L., Revilla S., Guerra R., Gruart A., Laferla F.M., Cristòfol R., Delgado-García J-M., Sanfeliu C. Physical exercise protects against Alzheimers disease in 3xTg-AD mice. J. Alzheimers Dis. 2011;24(3):421–454. doi: 10.3233/JAD-2011-101635. [PMID: 21297257]. [DOI] [PubMed] [Google Scholar]
- 182.Adlard P.A., Perreau V.M., Pop V., Cotman C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimers disease. J. Neurosci. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.0496-05.2005]. [PMID: 15858047]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yuede C.M., Zimmerman S.D., Dong H., Kling M.J., Bero A.W., Holtzman D.M., Timson B.F., Csernansky J.G. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimers disease. Neurobiol. Dis. 2009;35(3):426–432. doi: 10.1016/j.nbd.2009.06.002. [http://dx.doi.org/10.1016/j.nbd.2009.06.002]. [PMID: 19524672]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Obisesan T.O., Gillum R.F., Johnson S., Umar N., Williams D., Bond V., Kwagyan J. Neuroprotection and neurodegeneration in Alzheimer's disease: role of cardiovascular disease risk factors, implications for dementia rates, and prevention with aerobic exercise in African Americans. . Int. J. Alzheimers Dis. 2012 doi: 10.1155/2012/568382. 568382. [http://dx.doi.org/10.1155/2012/568382] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Revilla S., Suñol C., García-Mesa Y., Giménez-Llort L., Sanfeliu C., Cristòfol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. [http://dx.doi.org/10.1016/j.neuropharm.2014.01.037]. [PMID: 24486380]. [DOI] [PubMed] [Google Scholar]
- 186.Intlekofer K.A., Cotman C.W. Exercise counteracts declining hippocampal function in aging and Alzheimers disease. Neurobiol. Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [http://dx.doi.org/10.1016/j.nbd.2012. 06.011]. [PMID: 22750524]. [DOI] [PubMed] [Google Scholar]
- 187.Radak Z., Hart N., Sarga L., Koltai E., Atalay M., Ohno H., Boldogh I. Exercise plays a preventive role against Alzheimers disease. J. Alzheimers Dis. 2010;20(3):777–783. doi: 10.3233/JAD-2010-091531. [PMID: 20182027]. [DOI] [PubMed] [Google Scholar]
- 188.Bruno M.A., Leon W.C., Fragoso G., Mushynski W.E., Almazan G., Cuello A.C. Amyloid beta-induced nerve growth factor dysmetabolism in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009;68(8):857–869. doi: 10.1097/NEN.0b013e3181aed9e6. [http://dx.doi.org/10.1097/NEN. 0b013e3181aed9e6]. [PMID: 19606067]. [DOI] [PubMed] [Google Scholar]
- 189.Chae C.H., Jung S.L., An S.H., Park B.Y., Wang S.W., Cho I.H., Cho J.Y., Kim H.T. Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience. 2009;164(4):1665–1673. doi: 10.1016/j.neuroscience.2009.09.075. [http://dx.doi.org/10. 1016/j.neuroscience.2009.09.075]. [PMID: 19800940]. [DOI] [PubMed] [Google Scholar]
- 190.OCallaghan R.M., Griffin E.W., Kelly A.M. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19(10):1019–1029. doi: 10.1002/hipo.20591. [http://dx.doi.org/10.1002/hipo.20591]. [PMID: 19309034]. [DOI] [PubMed] [Google Scholar]
- 191.Radak Z., Toldy A., Szabo Z., Siamilis S., Nyakas C., Silye G., Jakus J., Goto S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem. Int. 2006;49(4):387–392. doi: 10.1016/j.neuint.2006.02.004. [http://dx.doi.org/10.1016/ j.neuint.2006.02.004]. [PMID: 16564605]. [DOI] [PubMed] [Google Scholar]
- 192.Komulainen P., Pedersen M., Hänninen T., Bruunsgaard H., Lakka T.A., Kivipelto M., Hassinen M., Rauramaa T.H., Pedersen B.K., Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: the DRs EXTRA Study. Neurobiol. Learn. Mem. 2008;90(4):596–603. doi: 10.1016/j.nlm.2008.07.014. [http://dx.doi.org/10.1016/ j.nlm.2008.07.014]. [PMID: 18707012]. [DOI] [PubMed] [Google Scholar]
- 193.Rasmussen P., Brassard P., Adser H., Pedersen M.V., Leick L., Hart E., Secher N.H., Pedersen B.K., Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [http://dx.doi.org/10.1113/expphysiol.2009.048512]. [PMID: 19666694]. [DOI] [PubMed] [Google Scholar]
- 194.Lindvall O., Kokaia Z., Bengzon J., Elmér E., Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17(11):490–496. doi: 10.1016/0166-2236(94)90139-2. [http://dx.doi.org/10.1016/0166-2236(94)90139-2]. [PMID: 7531892]. [DOI] [PubMed] [Google Scholar]
- 195.Kleim J.A., Jones T.A., Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem. Res. 2003;28(11):1757–1769. doi: 10.1023/a:1026025408742. [http://dx.doi.org/10.1023/A: 1026025408742]. [PMID: 14584829]. [DOI] [PubMed] [Google Scholar]
- 196.Tan Z.S., Beiser A.S., Vasan R.S., Roubenoff R., Dinarello C.A., Harris T.B., Benjamin E.J., Au R., Kiel D.P., Wolf P.A., Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68(22):1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [http://dx.doi.org/10.1212/01.wnl.0000263217.36439.da]. [PMID: 17536046]. [DOI] [PubMed] [Google Scholar]
- 197.Guerreiro R.J., Santana I., Brás J.M., Santiago B., Paiva A., Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimers disease and mild cognitive impairment. Neurodegener. Dis. 2007;4(6):406–412. doi: 10.1159/000107700. [http://dx.doi.org/10.1159/000107700]. [PMID: 17934323]. [DOI] [PubMed] [Google Scholar]
- 198.Bonotis K., Krikki E., Holeva V., Aggouridaki C., Costa V., Baloyannis S. Systemic immune aberrations in Alzheimers disease patients. J. Neuroimmunol. 2008;193(1-2):183–187. doi: 10.1016/j.jneuroim.2007.10.020. [http://dx. doi.org/10.1016/j.jneuroim.2007.10.020]. [PMID: 18037502]. [DOI] [PubMed] [Google Scholar]
- 199.Culpan D., Palmer J., Miners J.S., Love S., Kehoe P.G. The influence of tumour necrosis factor- α (TNF-α) on amyloid-β (Aβ)-degrading enzymes in vitro. Int. J. Mol. Epidemiol. Genet. 2011;2(4):409–415. [PMID: 22200003]. [PMC free article] [PubMed] [Google Scholar]
- 200.Ambarish V., Chandrashekara S., Suresh K.P. Moderate regular exercises reduce inflammatory response for physical stress. Indian J. Physiol. Pharmacol. 2012;56(1):7–14. [PMID: 23029958]. [PubMed] [Google Scholar]
- 201.Lambert C.P., Wright N.R., Finck B.N., Villareal D.T. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 2008;105(2):473–478. doi: 10.1152/japplphysiol.00006.2008. [http://dx.doi.org/10.1152/ japplphysiol.00006.2008]. [PMID: 18535122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chiò A., Benzi G., Dossena M., Mutani R., Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(Pt 3):472–476. doi: 10.1093/brain/awh373. [http://dx.doi.org/10.1093/brain/awh373]. [PMID: 15634730]. [DOI] [PubMed] [Google Scholar]
- 203.Vucic S., Kiernan M.C. Pathophysiology of neurodegeneration in familial amyotrophic lateral sclerosis. Curr. Mol. Med. 2009;9(3):255–272. doi: 10.2174/156652409787847173. [http://dx.doi.org/10.2174/156652409787847173]. [PMID: 19355908]. [DOI] [PubMed] [Google Scholar]
- 204.Deforges S., Branchu J., Biondi O., Grondard C., Pariset C., Lécolle S., Lopes P., Vidal P.P., Chanoine C., Charbonnier F. Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 2009;587(Pt 14):3561–3572. doi: 10.1113/jphysiol.2009.169748. [http://dx.doi.org/10.1113/jphysiol.2009.169748]. [PMID: 19491245]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Carreras I., Yuruker S., Aytan N., Hossain L., Choi J.K., Jenkins B.G., Kowall N.W., Dedeoglu A. Moderate exercise delays the motor performance decline in a transgenic model of ALS. Brain Res. 2010;1313:192–201. doi: 10.1016/j.brainres.2009.11.051. [http://dx.doi.org/10. 1016/j.brainres.2009.11.051]. [PMID: 19968977]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cheah B.C., Boland R.A., Brodaty N.E., Zoing M.C., Jeffery S.E., McKenzie D.K., Kiernan M.C. INSPIRATIonAL-INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2009;10(5-6):384–392. doi: 10.3109/17482960903082218. [http://dx.doi.org/10.3109/ 17482960903082218]. [PMID: 19922129]. [DOI] [PubMed] [Google Scholar]
- 207.de Almeida J.P., Silvestre R., Pinto A.C., de Carvalho M. Exercise and amyotrophic lateral sclerosis. Neurol. Sci. 2012;33(1):9–15. doi: 10.1007/s10072-011-0921-9. [http://dx.doi.org/10.1007/s10072-011-0921-9]. [PMID: 22228269]. [DOI] [PubMed] [Google Scholar]
- 208.Anziska Y., Sternberg A. Exercise in neuromuscular disease. Muscle Nerve. 2013;48(1):3–20. doi: 10.1002/mus.23771. [http://dx.doi.org/10.1002/mus. 23771]. [PMID: 23695822]. [DOI] [PubMed] [Google Scholar]
- 209.Majmudar S., Wu J., Paganoni S. Rehabilitation in amyotrophic lateral sclerosis: why it matters. Muscle Nerve. 2014;50(1):4–13. doi: 10.1002/mus.24202. [http://dx.doi.org/10.1002/mus.24202]. [PMID: 24510737]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.van Meeteren N.L., Brakkee J.H., Hamers F.P., Helders P.J., Gispen W.H. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch. Phys. Med. Rehabil. 1997;78(1):70–77. doi: 10.1016/s0003-9993(97)90013-7. [http://dx. doi.org/10.1016/S0003-9993(97)90013-7]. [PMID: 9014961]. [DOI] [PubMed] [Google Scholar]
- 211.Udina E., Cobianchi S., Allodi I., Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann. Anat. 2011;193(4):347–353. doi: 10.1016/j.aanat.2011.02.012. [http://dx. doi.org/10.1016/j.aanat.2011.02.012]. [PMID: 21514121]. [DOI] [PubMed] [Google Scholar]
- 212.Hu G., Sarti C., Jousilahti P., Silventoinen K., Barengo N.C., Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 2005;36(9):1994–1999. doi: 10.1161/01.STR.0000177868.89946.0c. [http://dx.doi.org/10.1161/01.STR.0000177868.89946.0c]. [PMID: 16081862]. [DOI] [PubMed] [Google Scholar]
- 213.Kloner R.A. Preinfarct angina and exercise: yet another reason to stay physically active. J. Am. Coll. Cardiol. 2001;38(5):1366–1368. doi: 10.1016/s0735-1097(01)01559-5. [http://dx.doi.org/10.1016/S0735-1097(01)01559-5]. [PMID: 11691509]. [DOI] [PubMed] [Google Scholar]
- 214.Larson E.B., Wang L., Bowen J.D., McCormick W.C., Teri L., Crane P., Kukull W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [http://dx.doi.org/10.7326/0003-4819-144-2-200601170-00004]. [PMID: 16418406]. [DOI] [PubMed] [Google Scholar]
- 215.Ferris L.T., Williams J.S., Shen C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007;39(4):728–734. doi: 10.1249/mss.0b013e31802f04c7. [http://dx. doi.org/10.1249/mss.0b013e31802f04c7]. [PMID: 17414812]. [DOI] [PubMed] [Google Scholar]
- 216.Zoladz J.A., Pilc A., Majerczak J., Grandys M., Zapart-Bukowska J., Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. Am. J. Physiol. 1975;229(3):721–730. [PMID: 2015]. [PubMed] [Google Scholar]
- 217.Ying Z., Roy R.R., Edgerton V.R., Gómez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987(1):93–99. doi: 10.1016/s0006-8993(03)03258-x. [http://dx.doi.org/10.1016/S0006-8993(03)03258-X]. [PMID: 14499950]. [DOI] [PubMed] [Google Scholar]
- 218.Leasure J.L., Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–465. doi: 10.1016/j.neuroscience.2008.07.041. [http://dx.doi.org/10.1016/j.neuroscience.2008.07. 041]. [PMID: 18721864]. [DOI] [PubMed] [Google Scholar]
- 219.Ploughman M., Granter-Button S., Chernenko G., Attwood Z., Tucker B.A., Mearow K.M., Corbett D. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [http://dx.doi.org/10.1016/j.brainres.2007.02.065]. [PMID: 17382914]. [DOI] [PubMed] [Google Scholar]
- 220.Noble E.G., Moraska A., Mazzeo R.S., Roth D.A., Olsson M.C., Moore R.L., Fleshner M. Differential expression of stress proteins in rat myocardium after free wheel or treadmill run training. J. Appl. Physiol. 1999;86(5):1696–1701. doi: 10.1152/jappl.1999.86.5.1696. [PMID: 10233137]. [DOI] [PubMed] [Google Scholar]
- 221.Butler R.K., Finn D.P. Stress-induced analgesia. Prog. Neurobiol. 2009;88(3):184–202. doi: 10.1016/j.pneurobio.2009.04.003. [http://dx.doi.org/10.1016/ j.pneurobio.2009.04.003]. [PMID: 19393288]. [DOI] [PubMed] [Google Scholar]
- 222.Carmody J., Cooper K. Swim stress reduces chronic pain in mice through an opioid mechanism. Neurosci. Lett. 1987;74(3):358–363. doi: 10.1016/0304-3940(87)90324-7. [http://dx.doi.org/10.1016/0304-3940(87)90324-7]. [PMID: 3561889]. [DOI] [PubMed] [Google Scholar]
- 223.Dubreucq S., Marsicano G., Chaouloff F. Emotional consequences of wheel running in mice: which is the appropriate control? Hippocampus. 2011;21(3):239–242. doi: 10.1002/hipo.20778. [http://dx.doi.org/10.1002/ hipo.20778]. [PMID: 20232385]. [DOI] [PubMed] [Google Scholar]
- 224.Sciolino N.R., Holmes P.V. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev. 2012;36(9):1965–1984. doi: 10.1016/j.neubiorev.2012.06.005. [http://dx.doi.org/10.1016/j.neubiorev.2012.06.005]. [PMID: 22771334]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Binder E., Droste S.K., Ohl F., Reul J.M. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav. Brain Res. 2004;155(2):197–206. doi: 10.1016/j.bbr.2004.04.017. [http://dx.doi.org/10.1016/j.bbr.2004.04.017]. [PMID: 15364478]. [DOI] [PubMed] [Google Scholar]
- 226.Duman C.H., Schlesinger L., Russell D.S., Duman R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [http://dx.doi.org/10.1016/j.brainres.2007.12.047]. [PMID: 18267317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Salam J.N., Fox J.H., Detroy E.M., Guignon M.H., Wohl D.F., Falls W.A. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav. Brain Res. 2009;197(1):31–40. doi: 10.1016/j.bbr.2008.07.036. [http://dx.doi.org/10.1016/j.bbr.2008.07.036]. [PMID: 18722480]. [DOI] [PubMed] [Google Scholar]
- 228.Hayes K., Sprague S., Guo M., Davis W., Friedman A., Kumar A., Jimenez D.F., Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115(3):289–296. doi: 10.1007/s00401-008-0340-z. [http://dx.doi.org/10.1007/s00401-008-0340-z]. [PMID: 18210137]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sheahan T.D., Copits B.A., Golden J.P., Gereau R.W. IV Voluntary exercise training: analysis of mice in uninjured, inflammatory, and nerve-injured pain states. PLoS One. 2015;10(7):e0133191. doi: 10.1371/journal.pone.0133191. [http://dx.doi.org/10.1371/journal.pone.0133191]. [PMID: 26196858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kinni H., Guo M., Ding J.Y., Konakondla S., Dornbos D., III, Tran R., Guthikonda M., Ding Y. Cerebral metabolism after forced or voluntary physical exercise. Brain Res. 2011;1388:48–55. doi: 10.1016/j.brainres.2011.02.076. [http://dx.doi.org/10.1016/j.brainres.2011.02.076]. [PMID: 21396919]. [DOI] [PubMed] [Google Scholar]
- 231.Ferraiuolo L., De Bono J.P., Heath P.R., Holden H., Kasher P., Channon K.M., Kirby J., Shaw P.J. Transcriptional response of the neuromuscular system to exercise training and potential implications for ALS. J. Neurochem. 2009;109(6):1714–1724. doi: 10.1111/j.1471-4159.2009.06080.x. [http://dx.doi.org/10.1111/j.1471-4159.2009.06080.x]. [PMID: 19344372]. [DOI] [PubMed] [Google Scholar]
- 232.Engesser-Cesar C., Anderson A.J., Cotman C.W. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144(3):1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [http://dx.doi.org/10.1016/j.neuroscience.2006.10.016]. [PMID: 17137724]. [DOI] [PubMed] [Google Scholar]
- 233.Siamilis S., Jakus J., Nyakas C., Costa A., Mihalik B., Falus A., Radak Z. The effect of exercise and oxidant-antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord. 2009;47(6):453–457. doi: 10.1038/sc.2008.125. [http://dx.doi.org/10.1038/sc.2008.125]. [PMID: 18936770]. [DOI] [PubMed] [Google Scholar]
- 234.Soya H., Nakamura T., Deocaris C.C., Kimpara A., Iimura M., Fujikawa T., Chang H., McEwen B.S., Nishijima T. BDNF induction with mild exercise in the rat hippocampus. Biochem. Biophys. Res. Commun. 2007;358(4):961–967. doi: 10.1016/j.bbrc.2007.04.173. [http://dx.doi.org/10.1016/j.bbrc.2007.04.173]. [PMID: 17524360]. [DOI] [PubMed] [Google Scholar]
- 235.Aguiar A.S., Jr, Tuon T., Pinho C.A., Silva L.A., Andreazza A.C., Kapczinski F., Quevedo J., Streck E.L., Pinho R.A. Mitochondrial IV complex and brain neurothrophic derived factor responses of mice brain cortex after downhill training. Neurosci. Lett. 2007;426(3):171–174. doi: 10.1016/j.neulet.2007.08.058. [http://dx.doi.org/10.1016/j.neulet. 2007.08.058]. [PMID: 17904742]. [DOI] [PubMed] [Google Scholar]
- 236.Aguiar A.S., Jr, Tuon T., Pinho C.A., Silva L.A., Andreazza A.C., Kapczinski F., Quevedo J., Streck E.L., Pinho R.A. Intense exercise induces mitochondrial dysfunction in mice brain. Neurochem. Res. 2008;33(1):51–58. doi: 10.1007/s11064-007-9406-x. [http://dx.doi.org/10.1007/ s11064-007-9406-x]. [PMID: 17619145]. [DOI] [PubMed] [Google Scholar]
- 237.Huang A.M., Jen C.J., Chen H.F., Yu L., Kuo Y.M., Chen H.I. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J. Neural. Transm (Vienna) 2006;113(7):803–811. doi: 10.1007/s00702-005-0359-4. [http://dx.doi.org/10.1007/s00702-005-0359-4]. [PMID: 16252072]. [DOI] [PubMed] [Google Scholar]
- 238.Funakoshi H., Belluardo N., Arenas E., Yamamoto Y., Casabona A., Persson H., Ibáñez C.F. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268(5216):1495–1499. doi: 10.1126/science.7770776. [http://dx.doi.org/10.1126/science. 7770776]. [PMID: 7770776]. [DOI] [PubMed] [Google Scholar]
- 239.Wang X.H., Poo M.M. Potentiation of developing synapses by postsynaptic release of neurotrophin-4. Neuron. 1997;19(4):825–835. doi: 10.1016/s0896-6273(00)80964-2. [http://dx.doi.org/10.1016/S0896-6273(00)80964-2]. [PMID: 9354329]. [DOI] [PubMed] [Google Scholar]
- 240.Ogborn D.I., Gardiner P.F. Effects of exercise and muscle type on BDNF, NT-4/5, and TrKB expression in skeletal muscle. Muscle Nerve. 2010;41(3):385–391. doi: 10.1002/mus.21503. [http://dx.doi.org/10.1002/mus. 21503]. [PMID: 19813200]. [DOI] [PubMed] [Google Scholar]
- 241.Keeler B.E., Liu G., Siegfried R.N., Zhukareva V., Murray M., Houlé J.D. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;1438:8–21. doi: 10.1016/j.brainres.2011.12.015. [http://dx.doi.org/10. 1016/j.brainres.2011.12.015]. [PMID: 22244304]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Höke A., Redett R., Hameed H., Jari R., Zhou C., Li Z.B., Griffin J.W., Brushart T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006;26(38):9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [http://dx.doi.org/10.1523/JNEUROSCI. 1620-06.2006]. [PMID: 16988035]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Berchtold N.C., Chinn G., Chou M., Kesslak J.P., Cotman C.W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [http://dx.doi.org/10.1016/ j.neuroscience.2005.03.026]. [PMID: 15896913]. [DOI] [PubMed] [Google Scholar]
- 244.Ma X., Hamadeh M.J., Christie B.R., Foster J.A., Tarnopolsky M.A. Impact of treadmill running and sex on hippocampal neurogenesis in the mouse model of amyotrophic lateral sclerosis. PLoS One. 2012;7(4):e36048. doi: 10.1371/journal.pone.0036048. [http://dx.doi.org/10.1371/journal. pone.0036048]. [PMID: 22558322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Seifert T., Brassard P., Wissenberg M., Rasmussen P., Nordby P., Stallknecht B., Adser H., Jakobsen A.H., Pilegaard H., Nielsen H.B., Secher N.H. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298(2):R372–R377. doi: 10.1152/ajpregu.00525.2009. [http://dx.doi.org/10.1152/ ajpregu.00525.2009]. [PMID: 19923361]. [DOI] [PubMed] [Google Scholar]
- 246.Marosi K., Felszeghy K., Mehra R.D., Radák Z., Nyakas C. Are the neuroprotective effects of estradiol and physical exercise comparable during ageing in female rats? Biogerontology. 2012;13(4):413–427. doi: 10.1007/s10522-012-9386-3. [http://dx.doi.org/10.1007/s10522-012-9386-3]. [PMID: 22722983]. [DOI] [PubMed] [Google Scholar]
- 247.Berchtold N.C., Kesslak J.P., Pike C.J., Adlard P.A., Cotman C.W. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur. J. Neurosci. 2001;14(12):1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [http://dx.doi.org/10.1046/j.0953-816x.2001.01825.x]. [PMID: 11860494]. [DOI] [PubMed] [Google Scholar]
- 248.Ogonovszky H., Berkes I., Kumagai S., Kaneko T., Tahara S., Goto S., Radák Z. The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair, and memory, in rat brain. Neurochem. Int. 2005;46(8):635–640. doi: 10.1016/j.neuint.2005.02.009. [http://dx.doi.org/10.1016/j.neuint.2005.02.009]. [PMID: 15863241]. [DOI] [PubMed] [Google Scholar]
- 249.Cobianchi S., de Cruz J., Navarro X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp. Neurol. 2014;255:1–11. doi: 10.1016/j.expneurol.2014.02.008. [http://dx.doi.org/10.1016/j.expneurol.2014.02.008]. [PMID: 24552688]. [DOI] [PubMed] [Google Scholar]
- 250.Arbat-Plana A., Torres-Espín A., Navarro X., Udina E. Activity dependent therapies modulate the spinal changes that motoneurons suffer after a peripheral nerve injury. Exp. Neurol. 2015;263:293–305. doi: 10.1016/j.expneurol.2014.10.009. [http://dx.doi.org/10.1016/j.expneurol.2014.10.009]. [PMID: 25448160]. [DOI] [PubMed] [Google Scholar]
- 251.Schmolesky M.T., Webb D.L., Hansen R.A. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J. Sports Sci. Med. 2013;12(3):502–511. [PMID: 24149158]. [PMC free article] [PubMed] [Google Scholar]
- 252.Correia P.R., Pansani A., Machado F., Andrade M., Silva A.C., Scorza F.A., Cavalheiro E.A., Arida R.M. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clinics (Sao Paulo) 2010;65(11):1123–1126. doi: 10.1590/S1807-59322010001100012. [http://dx.doi.org/10.1590/ S1807-59322010001100012]. [PMID: 21243284]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Goekint M., Roelands B., De Pauw K., Knaepen K., Bos I., Meeusen R. Does a period of detraining cause a decrease in serum brain-derived neurotrophic factor? Neurosci. Lett. 2010;486(3):146–149. doi: 10.1016/j.neulet.2010.09.032. [http://dx.doi.org/10.1016/j.neulet.2010.09.032]. [PMID: 20854879]. [DOI] [PubMed] [Google Scholar]
- 254.Huang F.F., Zhang L., Wu D.S., Yuan X.Y., Yu Y.H., Zhao X.L., Chen F.P., Zeng H. PTEN regulates BCRP/ABCG2 and the side population through the PI3K/Akt pathway in chronic myeloid leukemia. PLoS One. 2014;9(3):e88298. doi: 10.1371/journal.pone.0088298. [http://dx.doi.org/10.1371/journal.pone.0088298]. [PMID: 24603487]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Oliff H.S., Berchtold N.C., Isackson P., Cotman C.W. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res. Mol. Brain Res. 1998;61(1-2):147–153. doi: 10.1016/s0169-328x(98)00222-8. [http://dx.doi.org/10.1016/S0169-328X(98)00222-8]. [PMID: 9795193]. [DOI] [PubMed] [Google Scholar]
- 256.Widenfalk J., Olson L., Thorén P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci. Res. 1999;34(3):125–132. doi: 10.1016/s0168-0102(99)00051-6. [http://dx.doi.org/10.1016/ S0168-0102(99)00051-6]. [PMID: 10515254]. [DOI] [PubMed] [Google Scholar]
- 257.Suijo K., Inoue S., Ohya Y., Odagiri Y., Takamiya T., Ishibashi H., Itoh M., Fujieda Y., Shimomitsu T. Resistance exercise enhances cognitive function in mouse. Int. J. Sports Med. 2013;34(4):368–375. doi: 10.1055/s-0032-1323747. [PMID: 23041964]. [DOI] [PubMed] [Google Scholar]
- 258.Molteni R., Ying Z., Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur. J. Neurosci. 2002;16(6):1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [http://dx.doi.org/10.1046/j.1460-9568.2002. 02158.x]. [PMID: 12383240]. [DOI] [PubMed] [Google Scholar]
- 259.Ang E.T., Wong P.T., Moochhala S., Ng Y.K. Neuroprotection associated with running: is it a result of increased endogenous neurotrophic factors? Neuroscience. 2003;118(2):335–345. doi: 10.1016/s0306-4522(02)00989-2. [http://dx.doi.org/10.1016/S0306-4522(02)00989-2]. [PMID: 12699770]. [DOI] [PubMed] [Google Scholar]
- 260.Marais L., Stein D.J., Daniels W.M. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab. Brain Dis. 2009;24(4):587–597. doi: 10.1007/s11011-009-9157-2. [http://dx.doi.org/10.1007/s11011-009-9157-2]. [PMID: 19844781]. [DOI] [PubMed] [Google Scholar]
- 261.Griesbach G.S., Hovda D.A., Molteni R., Wu A., Gómez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. doi: 10.1016/j.neuroscience.2004.01.030. [http://dx.doi.org/10.1016/ j.neuroscience.2004.01.030]. [PMID: 15051152]. [DOI] [PubMed] [Google Scholar]
- 262.Vaynman S., Ying Z., Gómez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004;20(10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [b]. [DOI] [PubMed] [Google Scholar]
- 263.Anderson B.J., Rapp D.N., Baek D.H., McCloskey D.P., Coburn-Litvak P.S., Robinson J.K. Exercise influences spatial learning in the radial arm maze. Physiol. Behav. 2000;70(5):425–429. doi: 10.1016/s0031-9384(00)00282-1. [http://dx.doi.org/10.1016/S0031-9384(00)00282-1]. [PMID: 11110995]. [DOI] [PubMed] [Google Scholar]
- 264.Van der Borght K., Havekes R., Bos T., Eggen B.J., Van der Zee E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav. Neurosci. 2007;121(2):324–334. doi: 10.1037/0735-7044.121.2.324. [http://dx.doi.org/10. 1037/0735-7044.121.2.324]. [PMID: 17469921]. [DOI] [PubMed] [Google Scholar]
- 265.OCallaghan R.M., Ohle R., Kelly Á.M. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav. Brain Res. 2007;176(2):362–366. doi: 10.1016/j.bbr.2006.10.018. [http://dx.doi.org/10.1016/j.bbr.2006.10.018]. [PMID: 17113656]. [DOI] [PubMed] [Google Scholar]
- 266.Liu Y.F., Chen H.I., Yu L., Kuo Y.M., Wu F.S., Chuang J.I., Liao P.C., Jen C.J. Upregulation of hippocampal TrkB and synaptotagmin is involved in treadmill exercise-enhanced aversive memory in mice. Neurobiol. Learn. Mem. 2008;90(1):81–89. doi: 10.1016/j.nlm.2008.02.005. [http://dx.doi.org/10.1016/j.nlm.2008.02.005]. [PMID: 18374609]. [DOI] [PubMed] [Google Scholar]
- 267.Albeck D.S., Sano K., Prewitt G.E., Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav. Brain Res. 2006;168(2):345–348. doi: 10.1016/j.bbr.2005.11.008. [http://dx.doi.org/10.1016/ j.bbr.2005.11.008]. [PMID: 16388860]. [DOI] [PubMed] [Google Scholar]
- 268.Ferreira A.F., Real C.C., Rodrigues A.C., Alves A.S., Britto L.R. Short-term, moderate exercise is capable of inducing structural, BDNF-independent hippocampal plasticity. Brain Res. 2011;1425:111–122. doi: 10.1016/j.brainres.2011.10.004. [http://dx.doi.org/10.1016/j.brainres.2011. 10.004]. [PMID: 22035567]. [DOI] [PubMed] [Google Scholar]
- 269.Afzalpour M.E., Chadorneshin H.T., Foadoddini M., Eivari H.A. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol. Behav. 2015;147:78–83. doi: 10.1016/j.physbeh.2015.04.012. [http://dx.doi.org/10.1016/j.physbeh.2015.04.012]. [PMID: 25868740]. [DOI] [PubMed] [Google Scholar]
- 270.Groover A.L., Ryals J.M., Guilford B.L., Wilson N.M., Christianson J.A., Wright D.E. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013;154(12):2658–2667. doi: 10.1016/j.pain.2013.07.052. [http://dx.doi.org/10.1016/j.pain.2013. 07.052]. [PMID: 23932909]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Herbison G.J., Jaweed M.M., Ditunno J.F. Effect of activity and inactivity on reinnervating rat skeletal muscle contractility. Exp. Neurol. 1980;70(3):498–506. doi: 10.1016/0014-4886(80)90176-4. [http://dx.doi.org/10.1016/0014-4886(80)90176-4]. [PMID: 7439289]. [DOI] [PubMed] [Google Scholar]
- 272.van Meeteren N.L., Brakkee J.H., Helders P.J., Gispen W.H. The effect of exercise training on functional recovery after sciatic nerve crush in the rat. J. Peripher. Nerv. Syst. 1998;3(4):277–282. [PMID: 10970128]. [PubMed] [Google Scholar]
- 273.Seo T.B., Oh M.J., You B.G., Kwon K.B., Chang I.A., Yoon J.H., Lee C.Y., Namgung U. ERK1/2-mediated Schwann cell proliferation in the regenerating sciatic nerve by treadmill training. J. Neurotrauma. 2009;26(10):1733–1744. doi: 10.1089/neu.2008.0711. [http://dx.doi.org/10. 1089/neu.2008.0711]. [PMID: 19257802]. [DOI] [PubMed] [Google Scholar]
- 274.Love F.M., Son Y.J., Thompson W.J. Activity alters muscle reinnervation and terminal sprouting by reducing the number of Schwann cell pathways that grow to link synaptic sites. J. Neurobiol. 2003;54(4):566–576. doi: 10.1002/neu.10191. [http://dx.doi.org/10.1002/neu. 10191]. [PMID: 12555269]. [DOI] [PubMed] [Google Scholar]
- 275.Tam S.L., Archibald V., Jassar B., Tyreman N., Gordon T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J. Neurosci. 2001;21(2):654–667. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [PMID: 11160444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tam S.L., Gordon T. Neuromuscular activity impairs axonal sprouting in partially denervated muscles by inhibiting bridge formation of perisynaptic Schwann cells. J. Neurobiol. 2003;57(2):221–234. doi: 10.1002/neu.10276. [http://dx.doi.org/10.1002/neu.10276]. [PMID: 14556287]. [DOI] [PubMed] [Google Scholar]
- 277.Asensio-Pinilla E., Udina E., Jaramillo J., Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009;219(1):258–265. doi: 10.1016/j.expneurol.2009.05.034. [http://dx.doi.org/10.1016/j.expneurol.2009.05.034]. [PMID: 19500575]. [DOI] [PubMed] [Google Scholar]
- 278.Michalski B., Bain J.R., Fahnestock M. Long-term changes in neurotrophic factor expression in distal nerve stump following denervation and reinnervation with motor or sensory nerve. J. Neurochem. 2008;105(4):1244–1252. doi: 10.1111/j.1471-4159.2008.05224.x. [http://dx.doi.org/10.1111/ j.1471-4159.2008.05224.x]. [PMID: 18194437]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Goulart C.O., Jürgensen S., Souto A., Oliveira J.T., de Lima S., Tonda-Turo C., Marques S.A., de Almeida F.M., Martinez A.M. A combination of Schwann-cell grafts and aerobic exercise enhances sciatic nerve regeneration. PLoS One. 2014;9(10):e110090. doi: 10.1371/journal.pone.0110090. [http://dx.doi.org/10.1371/journal.pone.0110090]. [PMID: 25333892]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.English A.W., Cucoranu D., Mulligan A., Sabatier M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J. Comp. Neurol. 2009;517(2):245–255. doi: 10.1002/cne.22149. [http://dx.doi.org/10.1002/cne. 22149]. [PMID: 19731339]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Park J.S., Höke A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS One. 2014;9(3):e90245. doi: 10.1371/journal.pone.0090245. [http://dx. doi.org/10.1371/journal.pone.0090245]. [PMID: 24618564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ying Z., Roy R.R., Edgerton V.R., Gómez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005;193(2):411–419. doi: 10.1016/j.expneurol.2005.01.015. [http://dx.doi.org/10.1016/j.expneurol.2005.01.015]. [PMID: 15869943]. [DOI] [PubMed] [Google Scholar]
- 283.Satake K., Matsuyama Y., Kamiya M., Kawakami H., Iwata H., Adachi K., Kiuchi K. Up-regulation of glial cell line-derived neurotrophic factor (GDNF) following traumatic spinal cord injury. Neuroreport. 2000;11(17):3877–3881. doi: 10.1097/00001756-200011270-00054. [http://dx.doi.org/10. 1097/00001756-200011270-00054]. [PMID: 11117507]. [DOI] [PubMed] [Google Scholar]
- 284.Knaepen K., Goekint M., Heyman E.M., Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40(9):765–801. doi: 10.2165/11534530-000000000-00000. [http://dx.doi.org/10.2165/11534530-000000000-00000]. [PMID: 20726622]. [DOI] [PubMed] [Google Scholar]
- 285.Nakahashi T., Fujimura H., Altar C.A., Li J., Kambayashi J., Tandon N.N., Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470(2):113–117. doi: 10.1016/s0014-5793(00)01302-8. [http://dx.doi.org/10.1016/S0014-5793(00)01302-8]. [PMID: 10734218]. [DOI] [PubMed] [Google Scholar]
- 286.Garcia C., Chen M.J., Garza A.A., Cotman C.W., Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119(3):721–732. doi: 10.1016/s0306-4522(03)00192-1. [http://dx.doi.org/10.1016/S0306-4522(03)00192-1]. [PMID: 12809693]. [DOI] [PubMed] [Google Scholar]
- 287.Ivy A.S., Rodriguez F.G., Garcia C., Chen M.J., Russo-Neustadt A.A. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol. Biochem. Behav. 2003;75(1):81–88. doi: 10.1016/s0091-3057(03)00044-3. [http://dx. doi.org/10.1016/S0091-3057(03)00044-3]. [PMID: 12759116]. [DOI] [PubMed] [Google Scholar]
- 288.Van Hoomissen J.D., Holmes P.V., Zellner A.S., Poudevigne A., Dishman R.K. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav. Neurosci. 2004;118(6):1378–1390. doi: 10.1037/0735-7044.118.6.1378. [http://dx.doi.org/10.1037/0735-7044. 118.6.1378]. [PMID: 15598146]. [DOI] [PubMed] [Google Scholar]
- 289.Russo-Neustadt A. Handbook of the neuroscience of aging. USA: Elsevier; 1999. Exercise: optimizing function and survival at the cellular level. pp. 603–608. [Google Scholar]
- 290.Mayr B., Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2001;2(8):599–609. doi: 10.1038/35085068. [http://dx.doi.org/10.1038/35085068]. [PMID: 11483993]. [DOI] [PubMed] [Google Scholar]
- 291.Lynch G.S., Ryall J.G. Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev. 2008;88(2):729–767. doi: 10.1152/physrev.00028.2007. [http://dx.doi.org/10.1152/ physrev.00028.2007]. [PMID: 18391178]. [DOI] [PubMed] [Google Scholar]
- 292.Sato S., Shirato K., Tachiyashiki K., Imaizumi K. Muscle plasticity and β2-adrenergic receptors: adaptive responses of β2-adrenergic receptor expression to muscle hypertrophy and atrophy. J. Biomed. Biotech. 2011 doi: 10.1155/2011/729598. 729598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Daaka Y., Luttrell L.M., Lefkowitz R.J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390(6655):88–91. doi: 10.1038/36362. [http://dx. doi.org/10.1038/36362]. [PMID: 9363896]. [DOI] [PubMed] [Google Scholar]
- 294.Murga C., Fukuhara S., Gutkind J.S. A novel role for phosphatidylinositol 3-kinase β in signaling from G protein-coupled receptors to Akt. J. Biol. Chem. 2000;275(16):12069–12073. doi: 10.1074/jbc.275.16.12069. [http://dx.doi.org/10.1074/jbc.275.16.12069]. [PMID: 10766839]. [DOI] [PubMed] [Google Scholar]
- 295.Jacobs B.L., Martín-Cora F.J., Fornal C.A. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. Brain Res. Rev. 2002;40(1-3):45–52. doi: 10.1016/s0165-0173(02)00187-x. [http://dx.doi.org/10.1016/S0165-0173(02)00187-X]. [PMID: 12589905]. [DOI] [PubMed] [Google Scholar]
- 296.Korb A., Bonetti L.V., da Silva S.A., Marcuzzo S., Ilha J., Bertagnolli M., Partata W.A., Faccioni-Heuser M.C. Effect of treadmill exercise on serotonin immunoreactivity in medullary raphe nuclei and spinal cord following sciatic nerve transection in rats. Neurochem. Res. 2010;35(3):380–389. doi: 10.1007/s11064-009-0066-x. [http://dx.doi.org/10.1007/s11064-009-0066-x]. [PMID: 19774460]. [DOI] [PubMed] [Google Scholar]
- 297.Gackière F., Vinay L. Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord. Front. Neural Circuits. 2014;8:102. doi: 10.3389/fncir.2014.00102. [PMID: 25221477]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen. Pharmacol. 1998;31(5):667–674. doi: 10.1016/s0306-3623(98)00190-6. [http://dx.doi.org/10.1016/S0306-3623(98) 00190-6]. [PMID: 9809461]. [DOI] [PubMed] [Google Scholar]
- 299.Mattson M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [http://dx.doi.org/10.1196/annals.1418.005]. [PMID: 19076369]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sim Y.J., Kim H., Kim J.Y., Yoon S.J., Kim S.S., Chang H.K., Lee T.H., Lee H.H., Shin M.C., Shin M.S., Kim C.J. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiol. Behav. 2005;84(5):733–738. doi: 10.1016/j.physbeh.2005.02.019. [http://dx.doi.org/10.1016/j.physbeh.2005.02.019]. [PMID: 15885249]. [DOI] [PubMed] [Google Scholar]
- 301.Mattson M.P. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neuro- degenerative disorders. Neuromol. Med. 2003;3(2):65–94. doi: 10.1385/NMM:3:2:65. [http://dx.doi.org/10.1385/NMM:3:2:65]. [PMID: 12728191]. [DOI] [PubMed] [Google Scholar]
- 302.Smith M.A., Makino S., Kvetnansky R., Post R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [PMID: 7891134]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Dunn A.L., Reigle T.G., Youngstedt S.D., Armstrong R.B., Dishman R.K. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med. Sci. Sports Exerc. 1996;28(2):204–209. doi: 10.1097/00005768-199602000-00008. [http://dx.doi.org/10.1097/00005768-199602000-00008]. [PMID: 8775155]. [DOI] [PubMed] [Google Scholar]
- 304.Dishman R.K., Renner K.J., White-Welkley J.E., Burke K.A., Bunnell B.N. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Res. Bull. 2000;52(5):337–342. doi: 10.1016/s0361-9230(00)00271-9. [http://dx.doi.org/10.1016/S0361-9230(00)00271-9]. [PMID: 10922511]. [DOI] [PubMed] [Google Scholar]
- 305.Dishman R.K., Renner K.J., Youngstedt S.D., Reigle T.G., Bunnell B.N., Burke K.A., Yoo H.S., Mougey E.H., Meyerhoff J.L. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res. Bull. 1997;42(5):399–406. doi: 10.1016/s0361-9230(96)00329-2. [http://dx.doi.org/10.1016/S0361-9230(96)00329-2]. [PMID: 9092882]. [DOI] [PubMed] [Google Scholar]
- 306.Vaidya V.A., Terwilliger R.M., Duman R.S. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci. Lett. 1999;262(1):1–4. doi: 10.1016/s0304-3940(99)00006-3. [http://dx.doi.org/10.1016/S0304-3940(99) 00006-3]. [PMID: 10076858]. [DOI] [PubMed] [Google Scholar]
- 307.van Praag H. Neurogenesis and exercise: past and future directions. Neuromol. Med. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [http://dx.doi.org/10.1007/s12017-008-8028-z]. [PMID: 18286389]. [DOI] [PubMed] [Google Scholar]
- 308.Ota K.T., Duman R.S. Environmental and pharmacological modulations of cellular plasticity: role in the pathophysiology and treatment of depression. Neurobiol. Dis. 2013;57:28–37. doi: 10.1016/j.nbd.2012.05.022. [http://dx.doi.org/10.1016/j.nbd.2012.05.022]. [PMID: 22691453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Koleske A.J. Molecular mechanisms of dendrite stability. Nat. Rev. Neurosci. 2013;14(8):536–550. doi: 10.1038/nrn3486. [http://dx.doi.org/10.1038/ nrn3486]. [PMID: 23839597]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Murray P.S., Holmes P.V. A novel view of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus. Int. J. Pept., 2011 doi: 10.1155/2011/654085. 654085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Binder D.K., Croll S.D., Gall C.M., Scharfman H.E. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24(1):47–53. doi: 10.1016/s0166-2236(00)01682-9. [http://dx.doi.org/10.1016/S0166-2236(00)01682-9]. [PMID: 11163887]. [DOI] [PubMed] [Google Scholar]
- 312.Papaleo F., Silverman J.L., Aney J., Tian Q., Barkan C.L., Chadman K.K., Crawley J.N. Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learn. Mem. 2011;18(8):534–544. doi: 10.1101/lm.2213711. [http://dx.doi.org/10.1101/lm.2213711]. [PMID: 21791566]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Scharfman H.E., Goodman J.H., Sollas A.L., Croll S.D. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp. Neurol. 2002;174(2):201–214. doi: 10.1006/exnr.2002.7869. [http://dx.doi.org/10.1006/exnr.2002.7869]. [PMID: 11922662]. [DOI] [PubMed] [Google Scholar]
- 314.Ramsden M., Berchtold N.C., Patrick Kesslak J., Cotman C.W., Pike C.J. Exercise increases the vulnerability of rat hippocampal neurons to kainate lesion. Brain Res. 2003;971(2):239–244. doi: 10.1016/s0006-8993(03)02365-5. [http://dx.doi.org/10.1016/S0006-8993(03)02365-5]. [PMID: 12706240]. [DOI] [PubMed] [Google Scholar]