Abstract
Background
Ageing can be simply defined as the process of becoming older, which is genetically determined but also environmentally modulated. With the continuous increase of life expectancy, quality of life during ageing has become one of the biggest challenges of developed countries. The quest for a healthy ageing has led to the extensive study of plant polyphenols with the aim to prevent age-associated deterioration and diseases, including neurodegenerative diseases. The world of polyphenols has fascinated researchers over the past decades, and in vitro, cell-based, animal and human studies have attempted to unravel the mechanisms behind dietary polyphenols neuroprotection.
Methods
In this review, we compiled some of the extensive and ever-growing research in the field, highlighting some of the most recent trends in the area.
Results
The main findings regarding polypolyphenols neuroprotective potential performed using in vitro, cellular and animal studies, as well as human trials are covered in this review. Concepts like bioavailability, polyphenols biotransformation, transport of dietary polyphenols across barriers, including the blood-brain barrier, are here explored.
Conclusion
The diversity and holistic properties of polypolyphenol present them as an attractive alternative for the treatment of multifactorial diseases, where a multitude of cellular pathways are disrupted. The underlying mechanisms of polypolyphenols for nutrition or therapeutic applications must be further consolidated, however there is strong evidence of their beneficial impact on brain function during ageing. Nevertheless, only the tip of the iceberg of nutritional and pharmacological potential of dietary polyphenols is hitherto understood and further research needs to be done to fill the gaps in pursuing a healthy ageing.
Keywords: Bioavailability, blood-brain barrier, healthy ageing, neurodegenerative disorders, neuroprotection, polyphenols
INTRODUCTION
Neurodegenerative disorders (NDs) collectively refer to debilitating, life-threatening conditions that affect brain cells. As chronic and progressive neurological syndromes, they are caused by nervous system dysfunction resulting from neuronal cell failure [1], leading to impaired mental functioning (dementia) or movement complications (ataxia). These diseases can arise from hereditary or sporadic conditions, having a complex pathogenesis that triggers atrophy of central or peripheral structures of the nervous system [2]. Disease-modifying therapies to delay or reverse disease progression are not yet available, there is only a paucity of pharmacotherapy strategies focused on symptomatic relief.
More than 600 disorders have been described to afflict the nervous system such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), brain cancer, degenerative nerve diseases, encephalitis, epilepsy, genetic brain disorders, head and brain malformations, hydro-cephalus, stroke, and prion diseases. They are heterogeneous and multifactorial pathologies, which affect different brain structures and have different etiologies. AD, PD and HD, as well as ALS, share the aggregation of misfolded proteins as common pathological processes, being collectively designated as protein conformational disorders [2, 3].
AD is a clinical syndrome characterized by the progressive degeneration of hippocampus and neocortical brain neurons [4], which is responsible for the major disease symptoms – memory loss and cognitive decline. AD pathological hallmarks include the accumulation of extracellular amyloid plaques, majorly composed by amyloid-β peptides (Aβ40 and Aβ42) [5], and intracellular aggregates of hyperphosphorylated tau microtubule-binding protein, designated neurofibrillary tangles [6].
PD is the most common motor neuron disease. Its clinical symptoms include muscle rigidity, bradykinesia, resting tremor and postural instability, caused by the loss of dopaminergic neurons in the substantia nigra pars compacta [7]. The cytoplasmic inclusions designated Lewy bodies (LBs), predominantly enclosing aggregated α-synuclein (αSyn) [8], are the major pathological hallmark of the disease. αSyn is highly expressed in the brain and its function is thought to be involved in the regulation of dopamine neurotransmission and synaptic function/plasticity [9-14].
HD displays a wide variety of symptoms including chorea, dementia, and emotional disturbance [15, 16]. It is characterized by neuronal demise especially in the striatal region of the basal ganglia. Genetic mutations causing HD are linked to the expression of N-terminal polyglutamine (polyQ)-expanded huntingtin (Htt) beyond a critical length of ~35 glutamine residues. The cleavage of these polyQ tails generates cytotoxic fragments with high propensity to cross-link and form protein aggregates in both neuronal and glial cells [17, 18].
ALS is a fatal motor neuron disease leading to death usually within 3-5 years after the disease onset, mostly due to respiratory failure. It is characterized by progressive muscle weakness, which frequently starts in the limbs, axial, bulbar, or respiratory muscles and afterwards generalizes relentlessly causing a gradual disability. The disease is caused by progressive loss of cortical, bulbar, and ventral cord motor neurons, with the major genetic risk factors being mutations in the genes encoding the superoxide dismutase SOD1 [19], the TAR-DNA-binding protein [20] and the fused in sarcoma or translocated in liposarcoma protein (FUS/TLS) [21, 22].
Epidemiology of Neurodegenerative Disorders
Dementia is responsible for the greatest burden of NDs, a recent estimation indicates that there is nearly 46.6 million people worldwide living with dementia [23]. The same study reveals that the number of new cases will almost double every 20 years, with 65.7 million by 2030 and 115-130 million cases foreseen in 2050 [23, 24]. Given the huge social and economic impact of dementia in our society, it becomes imperative to develop strategies to prevent cognitive decline and to improve life quality of patients suffering the devastating effects of dementia-associated disorders.
AD represents the primary cause of dementia accounting for nearly 70% of known dementia cases and being one of the leading causes of mortality worldwide. Disease prevalence is estimated at 5.3 million Americans in 2015 [25], ranging between 3-7% in Europe and the US [26]. The incidence rates vary between 5-8 per thousand persons–years, which corresponds to half of new dementia cases each year [27, 28]. Following AD, PD is the second most common ND. The Parkinson’s Disease Foundation predicts that PD affects 7-10 million people worldwide. The prevalence rates vary from circa 50-300 per 100,000 individuals whereas the incidence rates are about 10-20 new cases per 100,000 people annually [29, 30]. As a rare neurodegenerative condition, the global HD prevalence is 2.71 per 100,000 individuals, being higher in Europe, North America and Australia (5.70 per 100,000) than in Asia (0.40 per 100,000), and the worldwide disease incidence rates are estimated at 0.38 per 100,000 individuals-year [31]. Consistent with the very limited survival of patients suffering ALS, prevalence is quite low, ranging between 4-5 people out of 100,000 and accounting for 1/300 to 1/400 of all deaths in the US. The median annual incidence of the disease in Europe and the US is estimated at 0.7-2.5 per 100,000 individuals [29, 32].
Altogether, these data reinforces the concomitant burden that NDs present in the develop world and the trend to increase over time. The need for novel therapies assisting to retard and prevent the development of NDs is imperative and must be the focus of research in this area, rather than finding new alternatives to treat symptoms in later stages of disease progression.
Neurodegenerative Disorders and the Ageing of Population
Ageing is a central risk factor in the development of degenerative processes associated to NDs. According to the Organization for Economic Co-operation and Development [33], the likelihood of developing dementia increases from 0.002-0.010% before the age of 65 [34] to 50% over the 95s in Europe [33]. Demographic ageing, incremented by the advances in medical care and living conditions, has therefore a profound impact on the increase of NDs prevalence [35]. Indeed, the expected increase in dementia prevalence to about 115 million by 2050 is largely correlated to population ageing as it is projected that the number of individuals aged above 60 years will reach 1.25 billion in the same period, accounting for 22% of the world’s population [24], with a particularly rapid increase in over-80s population.
Though ageing is considered a primary process in neurodegeneration, it differently influences the onset of the various NDs. Advancing age is the leading risk factor for AD, and disease onset usually occurs at the age of 65 [25]. Reports indicate that the risk of developing this disease almost doubles every five years after this age, as inferred by the incidence rates from 3 to as much as 69 per thousand individuals-year between the age of 65-69 and over the 90s, respectively [27, 28]. Likewise, PD majorly afflicts individuals over the 60s with higher median prevalence rates over 65s (9.5 per 1,000 persons) than in the overall population (max. 3 per 1,000 persons) of US and European countries [29]. As a consequence, median PD incidence ratios are also much higher in individuals over the 65s (160 per 100,000 person-years) than in the overall population (14 per 100,000 person-years) [29]. On the other hand, HD is a midlife disease usually diagnosed in adults between 35-50 years of age. Age-specific prevalence and incidence studies considering the global population are scarce, being restricted to particular countries. For example, a study performed in Taiwan found that HD incidence peaked between 40-49 year-old age in men (0.23 per 100,000 per year) and 50-59 year-old age in women (0.24 per 100,000 per year) [36]. Epidemiologic reports on ALS indicate acceleration in the upward trend for prevalence and incidence rates in individuals over the 40s, with prevalence estimations around 5 per 100,000 population over 70s [29].
Socio-Economic Impact of Age-Related Disorders
The global cost of dementia, both in terms of financial costs and the burden of the disease, is huge and set to rise further as a consequence of demographic ageing, representing the fastest growing major cause of disability globally. Due to the close association of ageing and neurodegenerative disorders, ageing has been considered the greatest social and economic challenge of the 21st century and dementia has also become a key policy priority for countries worldwide.
According to Alzheimer’s Disease International, the burden of dementia affect society at the level of patient itself, its family and caregivers, and at last the wider society, majorly impacting patient’s welfare as well as the quality of life of caregivers [23]. Dementia has significant social and economic implications in terms of direct medical, social care as well as informal care spending. Direct medical costs include hospital/nursing-home care, diagnostic and medication; direct nonmedical costs are associated to in-home day care; and indirect or intangible costs take into account the psychological pain of the patient and the loss of productivity of both patient and caregivers [37, 38]. For these reasons, dementia, especially AD, has been considered one of the costliest diseases for society at least in Europe and the United States [39, 40]. The current global costs associated to dementia are estimated at US$ 818 billion, 35.4% higher compared to the 2010 estimations (US$ 604 billion), representing 1.09% of global gross domestic product (GDP) [23]. It was estimated that in 2015 AD direct costs to American society would total circa US$ 226 billion and, unless measures are taken, in 2050 AD is projected to cost over US$ 1.1 trillion [25]. Regarding PD, the annual direct and indirect costs are estimated to be nearly US$ 25 billion in the US alone. Unlike AD, direct medical costs represent a substantial portion of the costs associated to PD, according to Parkinson’s disease Foundation.
In almost all the cases, as neurodegenerative diseases advance, there is an increase in dementia severity accompanied by increasingly deeper behavioural disturbances. The worsening of symptoms leads to the increase of caregiving time required for the provision of physical care. Therefore, measures to decrease cognitive decline, delay institutionalization or reduce caregiver needs will certainly provide economic benefits. Thus, the long-term goal of dementia policy is to develop effective preventive treatments and, if possible, to find a cure for these devastating diseases. In a short-term, a special attention has been given to the improvement of the quality of life of patients. In this frame, dementia is one of the priority conditions in the World Health Organization – WHO – Mental Health Gap Action Program (mhGAP), whose objective is to scale up care for dementia-associated neurological disorders [33]. Indeed, it is estimated that the development of treatments reducing severe cognitive impairment in older people by just 1% per year would cancel out the projected increases in the long-term care costs due to our ageing population (Alzheimer’s Research Trust).
Healthy Ageing and Transition to Disease
Ageing is a complex, irreversible, progressive and natural process, which is characterized by morphological, psychological, functional and biochemical changes affecting the welfare and health of the individual. The WHO describes the quality of life associated with ageing as a “broad and subjective concept that integrates physical health, psychological state, level of independence, social relationships, personal beliefs and convictions and their relationship with important aspects of the environment”. The WHO also defines “Active Ageing” as the process of optimizing opportunities for health, participation and social security, to improve the quality of life with ageing. The maximization of the functional capacity and health of the elderly is conditioned by factors such as nutrition, physical and social activity, education, and genetic background (Fig. 1), which ultimately define healthy ageing. Some of these factors are not modifiable, such as genetic ones, while others (nutrition, physical and social activity, etc.) are subject to change (i.e. environmental, psychological, social and lifestyle). In this sense, nutrition and other environmental factors have a huge impact on health and wellbeing. The nutritional status of elderly people has been increasingly considered a key aspect for a healthy ageing and, therefore, nutrition emerges as a critical modifiable risk factor to be exploited in policy strategies to prevent or delay the onset of neurodegenerative disorders and dementia [41]. There are several evidences that a continuous and prolonged intake of fruit and vegetables, rich sources of compounds named polyphenols, may help in the prevention of several degenerative pathologies such as diabetes, cardiovascular diseases, neurodegenerative diseases and cancer, and to prevent symptoms associated to ageing and menopause [42, 43].
Fig. (1).
Determinants of healthy ageing. The quality of life associated with ageing integrates different variables such as nutrition, physical and social activity, education, genetic background, and their relationship with the environment. Except for the genetic background, which is not modifiable, all the other aspects are subject to change. Nutrition has been increasingly considered a key aspect for a healthy ageing and emerges as a critical modifiable risk factor to be explored in research.
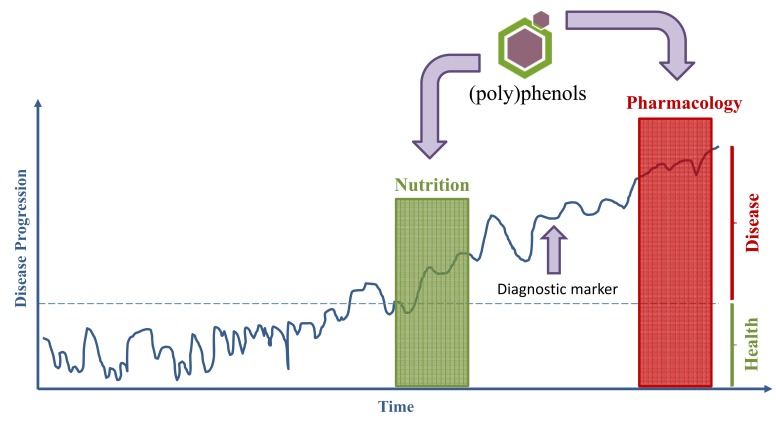
If we consider the normal process of evolution of a healthy state to a disease state, we may resume that the evidences indicate an active role of dietary polyphenols to homeostasis maintenance, delaying or even reversing the transition from a healthy to a pathological state (Fig. 2). Then, nutrition and in particular bioactive polyphenols identified in the diet, are strong contributors to the maintenance of a healthy condition. Moreover, polyphenols can also constitute lead compounds as basis for developing new drugs and therefore contribute to a pharmacology intervention (Fig. 2). Thus, pharmacological and nutritional approaches can be considered for the study of polyphenols. The next sections will be dedicated to review the evidences for both approaches on a neuroprotection perspective.
Fig. (2).
Polyphenols in a nutritional or pharmacological approach: disease progression and transition from a healthy to a disease state. Through nutrition polyphenols can act in the prevention of disease, or in the restoration of a healthy state in earlier disease stages, even before detection of diagnostic markers or drug administration. Polyphenols can also be the basis for developing new therapeutic compounds and therefore contribute to pharmacological interventions.
Polyphenols and neuroprotection
Phenolic compounds, commonly referred as polyphenols, constitute one of the most extensive and ubiquitous group of secondary metabolites in the plant kingdom. These compounds are characterized structurally by the presence of, at least, one hydroxyl functional group (-HO) linked to an aromatic ring [44]. Some compounds that do not present structural characteristics of polyphenols are commonly integrated in the group of polyphenols as “honorary”, such as phenolic acids or stilbenes. For this reason, recently the term “polyphenols” has been rewritten as “(poly)phenols” [45-47].
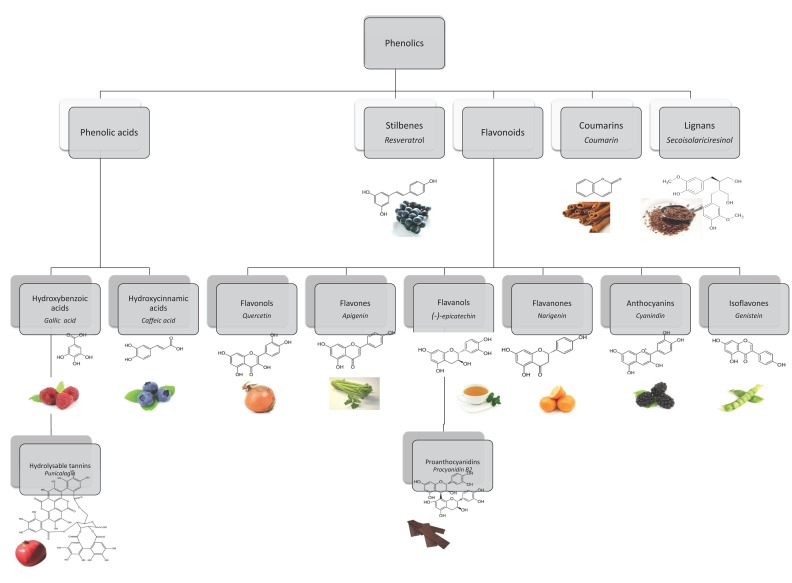
Polyphenols represent an extremely differentiated group not only in terms of chemical structure but also in terms of their biological activity. They occur conjugated with sugars, carboxylic and organic acids, amines, lipids and other phenols. Different groups are classified in terms of the number of phenol rings that they encompass and according to the structural elements binding these rings [48, 49]. The main classes are represented in Fig. (3) and include phenolic acids, stilbenes, flavonoids, coumarins and lignans.
Fig. (3).
Main polyphenol classes with structure, name of representative compounds (in italic) and examples of food sources.
Hydroxybenzoic and hydroxycinnamic acids represent the two different groups of phenolic acids. The first ones can be found in plants, both free and esterified. Examples are gallic acid, present in fruits, herbs, tea and wine, or more complex compounds, the hydrolysable tannins such as ellagic acid, gallotannins and ellagitannins [50]. Hydroxycinnamic acids are generally represented by p-coumaric, caffeic, ferulic and sinapic acids, usually found glycosylated or conjugated with quinic, shikimic and tartaric acids [48, 50]. Chlorogenic acid, an ester of caffeic and quinic acids can be found in several fruits and vegetables and is highly abundant in coffee. Ferulic acid is present in large amounts in cereal grains [50, 51]. Stilbenes frequently present in roots, barks, rhizomes and leaves, are not routinely consumed. By opposition we have a highly valued stilbene, resveratrol, present in grapes and in red wine [52].
The largest group of phenolic compounds in plants are flavonoids, with more than 10,000 different structures being identified [53]. The main classes of flavonoids are flavonols, flavones, isoflavones, flavanones, anthocyanins and flavanols. Monomers of flavanols (catechins), such as (+)-catechin and (-)-epicatechin, are relatively abundant in fruits, wine, chocolate and green tea [50, 54]. Proanthocyanidins, also known as condensed tannins, are constituted by dimers, oligomers and polymers of catechins. Proanthocyanidins can be found in fruits such as apples and grapes, in wine, cider, tea and beer, and also in cocoa [55]. Anthocyanins are glycosylated pigments, responsible for the colours of some flowers and fruits.
Cognitive Health and Dietary Polyphenols: Epidemiological and Population Based Studies
The beneficial effects resulting from polyphenol intake have been extensively studied, as in the studies concerning the Mediterranean Diet (MeDi). MeDi is characterized by a high consumption of fruits, vegetables, and grains, as well as sea-fish on regular bases. It also includes a modest consume of wine and olive oil as the principal source of fat, both highly enriched in polyphenols. Additionally, the intake of meat, dairy products, sweets and convenience food is rather low in the MeDi [56, 57].
A survey of studies reporting the effects of MeDi, carried out by WHO, revealed that it is a promising strategy to prevent diseases and to enhance quality of life (World Health Organization, 2009). Furthermore, epidemiologic studies over the last decades have supported the positive correlation between Mediterranean eating patterns and a large number of health benefits [58, 59], including (i) decreased risk of developing NDs; delayed AD and PD onset; (iii) lowered mortality in AD patients; and (iv) improved cognitive function [41, 60-62].
Polyphenols are abundant in MeDi and are believed to contribute to the beneficial effects of this diet when adopted in a regular basis, as revealed by studies showing that polyphenol-rich diets improve cognition, memory, learning, and vascular function in elderly people [63, 64]. The regular consumption of flavonoid rich-foods, representing the most common group of polyphenolic compounds in the human diet, has been associated to enhanced cognitive abilities and reduced risk of cognitive decline in aged individuals [65, 66]. Indeed, a large-scale population study indicated that flavonoids intake decreased dementia as well as premature death due to dementia [66]. Remarkably, it was found a correlation between high polyphenol concentrations in urine samples of older adults and lower risk of cognitive decline in global cognitive function in a prospective population-based study over a 3-year period [67].
Berries are a great source of polyphenols and wild blueberry diet supplementation was proved to improve cognitive function in older adults [68]. Increased consumption of berries and anthocyanins, as well as total flavonoids, was shown to be associated to a slower progression of cognitive decline in a large prospective cohort of older women [69]. Also, it was demonstrated that high anthocyanin consumption is associated with reduced risk of developing PD [70].
Collectively, these epidemiological and population based studies support the hypothesis that polyphenol-rich foods or supplements have a positive impact towards NDs. How it is processed and by which mechanisms diet alterations may exert protective effects is still a field of intensive research that, in the future, may change our perspective of an effective treatment for NDs.
Nutritional Relevance and Bioavailability of Polyphenols
Although polyphenols are not essential for humans, they have a positive impact on human nutrition. Polyphenols are widely spread in food, and the total polyphenols dietary intake could be as high as 100-150 mg per day, which is much higher than that of all other classes of phytochemicals [50]. Just for perspective, this is one order of magnitude higher than the intake of vitamin C and two orders of magnitude higher that the intake of vitamin E and carotenoids [71, 72]. Polyphenols main dietary sources are fruits and plant-derived beverages such as fruit juices, tea, coffee, and red wine. Vegetables, cereals, chocolate, and dry legumes also contribute to the total polyphenol intake [50]. The amount of research that has emerged in the past years in order to better understand polyphenols health benefits discloses a glimpse of the huge potential they may present [73-75]. However for a deeper understanding of the effect of polyphenols in human health, their absorption, distribution, metabolism and excretion in the human digestive tract needs to be studied.
The most common polyphenols present in the human diet are not necessarily the most active inside the body, either due to a low inherent activity or due to their poor absorption, extensively metabolization and rapid excretion [50]. Throughout digestion, polyphenols suffer several chemical modifications and metabolism, and the bioavailable metabolites found in blood and tissues may diverge from the native compounds in terms of biological role. Many different models are used to obtain a deeper knowledge about these mechanisms, ranging from in vitro enzymatic activities, cellular models, animal models or even the man himself. Although many differences can be seen between human and other animals’ digestive process, animal studies have been essential for the current understanding gathered so far on polyphenols bioavailability and effects.
One of the major difficulties on studying the bioavailability of polyphenols relies on their structural differences, resulting in different metabolic fates among compounds. Although some reactions could be common, several differences in the polyphenols metabolism can occur among classes or even within the same class.
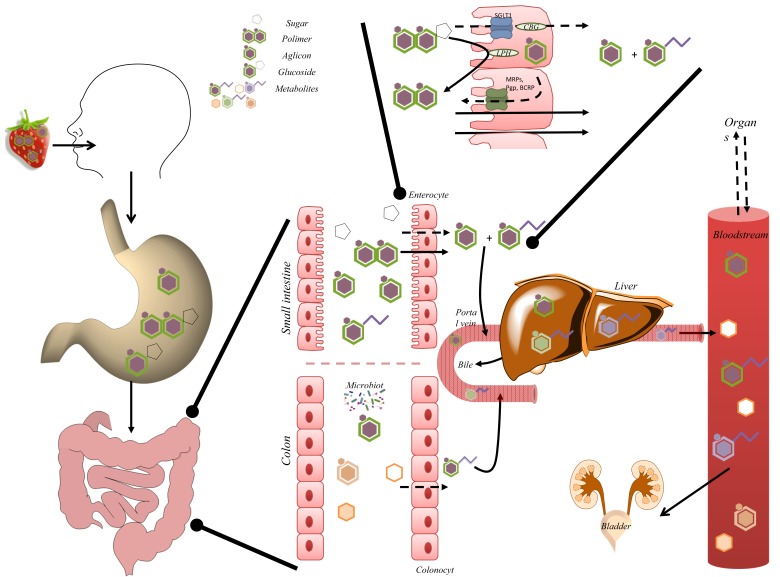
After ingestion, the availability of polyphenols, often associated with fibre or complex carbohydrates in the food matrix, can be modified in the oral cavity by amylase digestion and, perhaps, by particle size reduction [76]. Afterwards, the effective release of the phenolic compounds in the stomach maximizes the potential for absorption in the small intestine [76]. Absorption occurs mainly at the duodenum and at the proximal half of jejunum, where enterocytes are the predominant cells, being responsible for the absorption (Fig. 4). Being lipophilic compounds, most flavonoid aglycones and phenolic acids permeate intestinal cells by passive diffusion [42, 77, 78]. Polyphenols in the form of esters, glycosides or polymers, usually present in plants, cannot be directly absorbed and they probably resist to acid hydrolysis in the stomach, being able to reach the duodenum [50].
Fig. (4).
Schematic representation of absorption, biotransformation and excretion of polyphenols in the human body. Along the digestive process, polyphenol-rich food suffers transformations, starting in the mouth, stomach and throughout the entire gastrointestinal tract. The gastrointestinal tract is covered by the mucosa, which functions as a physical barrier, determining bioavailability of xenobiotics like polyphenols. This function is mediated by physical walls, metabolism and passive (solid arrows)/active (dashed arrows) transport mechanisms. Absorption occurs mainly at the duodenum and the proximal half of jejunum, in enterocytes. Enterocytes apical cell membranes contain microvilli, which increase the surface area of absorption. Passive intestinal permeability occurs mainly for aglycones and simple phenolic acids. Absorption of glycosylated compounds is usually preceded by release of aglycone through hydrolysis by lactase phloridzin hydrolase (LPH). Free aglycone can then enter the epithelial cells by passive diffusion. Alternatively, glycosylated compounds enter epithelial cells by the active sodium-dependent glucose transporter SGLT1 and are hydrolyzed by the cytosolic β-glucosidase (CBG). Once inside enterocytes, polyphenols can be extruded into the lumen by efflux transporters (P-gp, MRPs, BCRP). Compounds not absorbed reach the colon where they can be extensively metabolized by microbiota. Several transformations in polyphenols structure can occur. Most of the colonic metabolites are excreted in feces, although absorption can still take place. Then, polyphenols can undergo phase I and phase II reactions. Phase I reactions include oxidative and reductive reactions. Glucuronidation, sulfation and methylation are the most frequent phase II reactions. The conjugates, being more water soluble, are rapidly excreted through bile or urine [84]. Metabolites can then be transported into the bile (enterohepatic recirculation) and secreted back to the duodenum. Degradation of metabolites in the intestine generates catabolites available for reabsorption.
The absorption of glycosylated compounds is usually preceded by the hydrolysis of the glycoside and release of the aglycone (Fig. 4), by the enzyme lactase phloridzin hydrolase (LPH) in the brush-border of the small intestine epithelial cells. After hydrolysis, the free aglycone can enter the epithelial cells by passive diffusion [79]. Otherwise, glycosylated compounds are supposed to enter epithelial cells via the sodium-dependent glucose transporter, SGLT1, and be hydrolyzed by the cytosolic β-glucosidase (CBG), depending on the glycoside [80]. By opposition, the highly stable oligomeric and polymeric flavanols during digestion, are thought to have a very low absorption [81].
Compounds that are not absorbed in the proximal gastrointestinal tract can reach the large intestine where absorption can also happen (Fig. 4). However, colonic microbiota can degrade complex polyphenols into low molecular weight phenolics, aromatic acids [82], and also into oxaloacetate and CO2 [83].
As usually happens with xenobiotics, polyphenols can undergo enzymatic reactions known as ‘biotransformations’, phase I and phase II reactions [84] (Fig. 4). The resulting compounds are frequently less lipophilic so the body can more easily excrete them.
Phase III transporters are responsible for the final step in the elimination and/or detoxification of xenobiotics, resulting in the removal of the undesired compounds in faeces, urine and bile [85] (Fig. 4). These transporters are mainly constituted by the ABC protein family, including P-glycoprotein (P-gp), the multidrug resistance-associated proteins (MRP2, BCRP), and also by the solute carrier family (SLC) transporters [86, 87].
Importantly, most of the conjugated-polyphenols are excreted in urine. However, the mechanisms by which these conjugated-polyphenols can return into the intestinal lumen or to bile, by the action of intestinal cells or liver parenchymal cells, respectively, should also be considered (Fig. 4). Several polyphenols were shown to be considerably better absorbed in the presence of additional polyphenols, interfering with efflux transporters (P-gp, MRPs, BCRP), which normally reduce the intracellular concentration of such “xenobiotics” and, in that case, lead to their excretion to the apical side [88, 89]. On the other hand, transporters to the basolateral side are poorly understood. Compounds excreted into bile are eventually emptied in the duodenum. Usually, they are not absorbed in the small intestine, and reach the colon, where they can either be excreted into the faeces or be degraded by the colon microbiota, and be ultimately reabsorbed [90].
Bioavailability of polyphenols is thus a multi-stage process comprising de-conjugation and possible catabolism, absorption, conjugation and excretion (Fig. 4). Moreover, the possible sequestration of some polyphenol metabolites inside tissues has been recurrently undetermined and may contribute to underestimation of the their bioavailability. The accessibility of polyphenol metabolites to the central nervous system is an example where the presence of an additional barrier, the blood-brain barrier (BBB), reduces even more polyphenols bioavailability, in addition to the described metabolism. This aspect will be further discussed below. Therefore, controlled target delivery to central nervous system (CNS) is desirable to optimize polyphenols in terms of efficiency, specificity and safety.
Formulations and methods for enhancing polyphenols bioavailability, solubility and stability in the human body have been patented, creating new derivatives with improved biological activity and stability [91]. Encapsulating polyphenols in nanoparticles can be a promising solution by enhancing their bio-distribution, solubility, and stability in the human body, while reducing their extensive metabolism. Nanoparticles-loaded polyphenols constitute an active area of research, with the goal to increase the oral bioavailability of poorly absorbed phenolic compounds. Sahni and co-workers summarized the neurotherapeutic applications of nanoparticles loaded with curcumin, catechins or resveratrol for AD [92].
Neuroprotective potential of polyphenols
In addition to the epidemiological evidences already mentioned, neuroprotective evidences for polyphenols also result from in vitro, cellular, animal and clinical studies [63, 93]. Another aspect to consider is that polyphenols can be studied either in a pharmacological or nutritional perspective as valid strategies to supply them to humans. Besides the form of delivery/route of administration we can consider studies focusing on pure compounds in contrast to the use of mixtures or whole extracts. Target and nontargeted meta-bolomic profiling have evolved and allowing identification of new potential bioactive metabolites and the definition of absorption kinetics [94-97].
Neurodegeneration is a multifactorial process and polyphenols present pleiotropic effects (antioxidant, anti-inflammatory, immunomodulatory properties) [98] due to their ability to modulate the activity of multiple targets involved in pathogenesis, thereby halting the progression of these diseases. For many years, polyphenols were thought to protect cell constituents against oxidative damage through direct scavenging of free radicals. Such idea has become very popular leading to the appearance of several studies exploring extensively this property of polyphenols for NDs, since oxidative stress constitutes an important hallmark of these diseases. However, this concept now appears to be an oversimplified view of their mode of action. There is an emerging acceptance that polyphenols, as well as their metabolites, exert modulatory actions in proteins/enzymes through direct interaction with receptors or enzymes involved in signal transduction, such as protein kinase and lipid kinase signalling pathways [99]. Moreover, several neurochemical mechanisms underlying the protective action of plant polyphenols have been described: iron chelating properties [100]; modulation of signalling pathways related with neuronal survival and differentiation [99, 101]; inhibition of neuropathological processes [102, 103]; and regulation of mitochondrial function [104-106]. A recent review also addressed other positive effects of dietary polyphenols regarding brain health and cognition. Other mechanisms by which flavonoids can be neuroprotective include their positive role on peripheral and cerebrovascular blood flow, ultimately affecting synaptic plasticity processes and cognitive function [107].
Mixtures vs. Pure Compounds
The study of pure compounds is limited to their molecular mechanisms in neurodegeneration cell models, and their specific biological effects in animal models. On the other hand, studies using pure polyphenols miss the synergies among different polyphenols. The presence of synergism and prodrugs in an extract can be misleading by using pure compounds [108]. Total extract from G. biloba, a well-known matrix used for treating peripheral vascular diseases and cerebrovascular insufficiency in the elderly, is more active than its isolated polyphenols [109, 110]. St John’s Wort extract is another example, since no single compound or even a group of compounds has been found to be responsible for its activity [108, 111]. Therefore, studies with polyphenol-enriched fractions can be not only useful to identify sources to isolate pure bioactive compounds but also for identifying mixtures of bioactive compounds. Since polyphenols are emerging key compounds for the development of novel therapeutic agents for NDs, the identification of the molecular targets of isolated polyphenols or identification of novel sources of neuroactive polyphenols has become an important area of research.
In the next sections, it will be summarized the evidences pointing out the neuroprotective activities of polyphenols regarding different types of studies: in vitro; cellular models and primary cell cultures; in vivo animal assays; and human trials. The approach used, either nutritional or pharmacological, will be highlighted.
In Vitro Studies
The consensus fact that free radical-mediated reactions play an important role in both ageing and in the patho-physiology of most NDs lead to the extensive study of polyphenols chemical scavenging activity. One of the best studied source of plant polyphenols is G. biloba and its extract (EGb 761), which has been described to scavenge reactive nitrogen and oxygen species (RNOS) [112] and peroxyl radicals [113]. Direct scavenging of RNOS and peroxynitrite was also described for green tea [114] and for rosmarinic acid [115], respectively.
Substantial presynaptic cholinergic deficit is a feature registered in most NDs [116]. Malfunction of the cholinergic system may be tackled pharmacologically through inhibition of acetylcholinesterase [117], which catalyses the hydrolysis of the neurotransmitter acetylcholine (Ach) to choline. Therefore, AChE inhibition has been reported to ameliorate the symptoms of some NDs and has been used as a rationale to develop drugs to treat AD [118]. Green tea and white tea digested metabolites were described to inhibit AChE [119]. Additionally, a variety of plants with diverse phytochemistry features have been reported to exhibit AChE inhibitory activity [120] and, in some of these studies, polyphenols were the compounds associated with the plant extract activity [118, 121-126].
Another common pathological hallmark of many NDs is the generation of aberrant misfolded proteins with formation of intra- or extra-cellular high-ordered insoluble fibrils deposits [127, 128]. Potent activities towards the several steps of fibrils formation are modulated either by plant extracts or by isolated compounds. For instance, inhibition of Aβ-peptide aggregation and fibril formation was described for: isolated catechins and procyanidins and fractions of EGb761 extract containing these flavonoids [129]; blueberry anthocyanins enriched extract [130]; grape seed extract [131]; epigallocatechin gallate (EGCG) [132, 133]; myricetin [134]; resveratrol derivatives [135-139]; gallotannins and some derivatives [140]. Moreover, the ability to destabilize preformed fibrils in vitro was also described for curcumin [141], catechins and procyanidins [129]. Similarly, the protein αSyn is an amyloidogenic polypeptide that forms cytotoxic oligomers and quercetin was described to reduce αSyn fibrillization [142]. It was also shown that EGCG redirect the aggregation of αSyn monomers and remodel αSyn amyloid fibrils into disordered oligomers [132, 143]. Recent studies also reported that Corema album polyphenolic extract acts as inhibitor of αSyn fibrillization by the stabilization of non-toxic αSyn oligomers [144]. Furthermore, quercetin has raised interest as therapeutic for cerebral ischemia/reperfusion (I/R) injury due its predicted inhibitory effects on MMPs activation and acid sensing ion channel 1a channels mediated downstream survival/damage mechanisms [145].
In vitro studies are particularly relevant to determine the effect of polyphenols in specific pathological processes, without the interference of other cellular pathways. However, in vitro findings must be interpreted with caution, as some are not translated into cell and animal models. For instance, polyphenols present a very promising in vitro antioxidant capacity leading to the misconception that their cellular protection was mainly due to direct antioxidant scavenging. Studies with cellular and animal models were valuable to demystify this concept, and now we know that the mode of action of polyphenols go far beyond their antioxidant potential [146].
Cellular Models and Primary Cell Cultures
Cellular models, although less physiological than animal studies, are very important to unravel the molecular mechanisms underlying the polyphenol protective effects observed in animals. Cellular assays allow to study the cross-talk between pathways affected by polyphenols, giving a more integrated view of the metabolic pathways affected by these compounds. In contrast to in vitro studies, focused on a particular aspect or protein/enzyme function, in cellular models the whole cellular metabolism is evaluated. The complexity of these studies increases when analysing a mixture of compounds, as food extracts, in contrast to studies with pure compounds.
Several in vitro studies refer a general protection from oxidative stress effects either by direct scavenging activity or induction of antioxidant defences by plant polyphenolic extracts (see Table 1). Moreover, the effects of these extracts have also been explored for specific mechanisms hallmarks of NDs.
Table 1.
Neuroprotective evidences (in vitro, cellular models and animals) for the most representative plant polyphenolic extracts.
| Plant Extract | In Vitro | Cellular Models and Primary Cell Cultures | Animal Model |
|---|---|---|---|
|
Ginkgo biloba/ EGb 761 |
* Scavenged RNOS [112] and peroxyl radicals [113] * Inhibited of Aβ peptide aggregation and destabilized of preformed fibrils [129] |
* Protected from neurotoxicity induced by Aβ in N2a [147] and PC12 cells [148] and in hippocampal neurons [149] * ↓ Neuroinflammation [147] * Improved mitochondrial function [152] * Protection from oxidative stress [345] |
AD rodent models: * ↑ spatial learning and memory [178] * ↓decreased APP in the cortex [179] PD rodent models: * Potent neuropreventive/neurorecovery effects against neurotoxicity induced by MPTP/MPP+ [180, 181, 219] ALS rodent models: * Impart neuroprotective effects [182] Cerebral ischemia in rodents: * Protects neuronal destruction in the hippocampus [183] Hyperthermic brain injury: * ↓ Inducible nitric oxide synthase (iNOS) expression [346] |
| Green tea | * Scavenged RNOS [114] * Inhibited AChE [119] |
* Scavenging intracellular RNOS and induced endogenous antioxidant defences preventing DNA damage [114] * Protects primary rat cortical neurons against Aβ-induced cytotoxicity [150] |
Ageing and neurodegeneration models: * ↓ Protein/lipid oxidation [184] * ↑ Spatial learning [184] * Modulation of glutathione levels and antioxidant enzyme activities [185] * ↑ CREB activation [185] * ↑ BDNF and Bcl-2 levels [185] * ↑ Cognitive and behavioural capacities [186] * Protects against deltamethrin-induced neurotoxicity in rat [187] AD rodent models: * ↓ Aluminium chloride toxicity [220] PD non-human primates model: * Alleviate motor impairments, dopaminergic neuronal injury, and aSyn aggregation [188] |
| Grape seed extract and derivatives | * Inhibited Aβ aggregation and cytotoxicity [131] | * ↑ IL-6 and respective mRNAs in primary culture of astrocytes, which functions as a neuroprotective paracrine, protected neuronal cells from death by oxidative stress [347] * Protects neuronal cells against low extracellular Mg2+ concentration and oxygen glucose deprivation-induced neurotoxicity, in cultured rat hippocampal neurons mediated by inhibition of glutamate-induced calcium signaling and NO formation [348] |
AD rodent model: * ↓ cognitive deterioration [103] * ↑ cognitive function [189] * ↓ oligomerization of Aβ peptides and amyloid plaques [103] * ↓ microglial activation [190] * ↓ extracellular-signal-regulated kinases (ERK) 1 and 2 in the brain, suppressing tau neuropathy [192] * Interferes with the assembly of Aβ peptides into neurotoxic aggregates [194] * ↑ Spatial memory performance, ↓ cognitive deterioration and Aβ neuropathy [195] HD rodent model: * Neuroprotective following oral administration [193] |
| Berries and anthocyanin rich extracts | * Inhibit the formation of Aβ peptide fibrils [130, 137-139] | * ↓ Toxicity of Aβ aggregates toward Neuro2a cells [130] * ↑ Aβ aggregates clearance, ↓ fibrillization and supressed microglia activation, in murine cell cultures [153, 154] * Protection of neuronal cultures from oxidative stress [349] * ↓ Neuroinflammation [155] * ↓ Neuronal death in cells expressing Aβ by improving cellular metabolism [151] |
Ageing and neurodegeneration rodent models: * Reverse age related deficits in spatial working memory (↑CREB activity, ↑ BDNF, phosphorylation of hippocampal Akt, activation of TOR and ↑ expression of Arc/Arg3.1 [196] * Delayed age-related motor and cognitive behavioural deficits [200, 201, 350, 351] AD rodent model: * ↓ cognitive degeneration [130] * Reverses Aβ-induced effects on protein expression: mitochondrial apoptotic pathway (Bax, cytochrome C, caspase-9 and caspase-3) and AD markers (Aβ, APP, P-tau and BACE-1) [151] * Pomegranate juice oral intake improved spatial learning, and reduced Aβ plaques [202] |
Protection from neurotoxicity induced by Aβ was observed for: G. biloba EGb 761 extract, in the neuro-blastoma cell line Neuro2a [147], PC12 cells [148] and in hippocampal primary neurons [149]; green tea extracts, in primary rat cortical neurons [150]; blueberry anthocyanin-rich extract, in Neuro2a cells [130]; and Korean black soybeans anthocyanin-rich extract, in hippocampal HT22 cell line [151]. Other mechanisms impaired by Aβ were also described to be ameliorated by some of these plant extracts. For example, EGb 761 improves oxidative phosphorylation performance and restores from Aβ-induced mitochondrial dysfunction [152]. Also, blueberry anthocyanin-rich extract increase microglial Aβ clearance, inhibit its fibrillation and suppress microglial activation in murine cell culture [153]. Similarly, the rescue of αSyn aggregation toxicity was described for C. album polyphenols extract in human neuroglioma cells [144].
Neuroinflammation, another important hallmark of neurodegeneration, is also reduced by plant polyphenols extracts. Blueberry extracts attenuate inflammatory responses, inhibiting NO production and the release of the cytokines interleukin-1β and tumour necrosis factor-α in mouse brain BV-2 microglial cells [154]. Another anthocyanin-rich extract from açai reduce inflammatory stress signaling in BV-2 microglial cells by the down-regulation of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), p38 mitogen-activated protein kinase (p38-MAPK), tumour necrosis factor-α (TNFα), and nuclear factor κB (NF-κB) [155].
The studies described in this section have in common the use of complex mixture of compounds, most of them of dietary origin. However, these studies do not consider polyphenols metabolism and therefore they could only be considered in a pharmacological perspective with controlled target delivery to central nervous system (CNS). In fact, the described effects seen in these in vitro studies could be completely altered by metabolism. For example, blackberry extracts submitted to a simulated gastro-intestinal digestion reveal that neuronal protection to oxidative insult can be unrelated to the modulation of reactive oxygen species (ROS) and glutathione (GSH) levels, suggesting a pre-conditioning effect by the induction of caspase activity [156, 157]. On the other hand, Phellinus igniarius polyphenols also provided protection in a mouse stroke model [158].
Studies with pure isolated compounds also revealed more specific and targeted potential neuroprotective applications. Catechins increased neuronal viability by modulation of signal transduction pathways, cell survival genes and mitochondria functions [reviewed in [159, 160], reduction of neurotoxins, and Aβ toxicity in several cell lines and primary cultures (Table 2). EGCG, in particular, prevented Aβ fibril formation [161] and inhibited caspase activation mediated by Aβ in hippocampal neurons, leading to increased viability [162].
Table 2.
Neuroprotective evidences (in vitro, cellular models and animals) for the most representative pure polyphenols.
| Polyphenol | In Vitro | Cellular Models and Primary Cell Cultures | Animal Model | ||
|---|---|---|---|---|---|
| Cathecins (mainly EGCG) | * Prevent Aβ fibril formation [132] * Redirect the aggregation of αSyn monomers and remodel αSyn amyloid fibrils into disordered oligomers [132, 143] |
* ↑ neuronal viability, modulation of signal transduction pathways and mitochondria functions 16, [160] * ↓ neurotoxins and Aβ toxicity in cell lines and primary cultures [162, 173, 186, 352-354] * ↓ caspase activation mediated by Aβ in hippocampal neurons [162] |
Ageing and neurodegeneration rodent models: * Prevention of spatial learning and memory decline [203] * ↑ life span [204]; * Prevention brain inflammation [355] AD rodent model: * ↓ amyloidosis [102]; * Rescue memory impairment (↓ NF-кB pathway and ↓oxidative stress [161] * Restored mitochondria function in the brain hippocampus, cortex and striatum [206] MS rodent model: * Neuroprotective effects by modulating neuroinflammation and attenuating neural damage [205] Cerebral ischemia rodent model: * Ameliorated redox imbalance and limited inflammation [205] * EGCG improved age–related cognitive decline and protected against ischemia/reperfusion [221] |
||
| Resveratrol | * Inhibited the formation of Aβ peptide fibrils [130, 137-139] | * ↓ Aβ toxicity [163] * Modulation of NF-кB and SIRT1 pathways in cell models [163-165] |
AD rodent model: * ↓ formation of amyloid plaques, without affecting APP levels [209] * Protection from Aβ neurotoxicity by inhibiting iNOS [222] * ↓ hippocampal neurodegeneration [164] PD rodent model: * ↓ neural inflammation (↓ mRNA levels of COX-2 and TNF-α in the substantia nigra) [210] * ↓ oxidative stress, lipid peroxidation, and protein carbonyl [225] HD rodent model: * SIRT1 activation [214] MS rodent model: * ↓ neural damage (↑ SIRT1) [212] * Prevention neural loss without immunosuppression [213] Cerebral ischemia rodent model: * Improve brain energy metabolism [223] * Modulation of the release of neurotransmitters and neuromodulators [224] Non-human primate study: supplementation increased spatial memory performance [211] |
||
| Curcumin | * Destabilized preformed fibrils [141] | * Intracellular antioxidant activities and anti-amyloid activities [7, [168] and MPTP protective activity [169] * anti-inflammatory (↓COX-2) in both rat primary microglial and murine BV2 microglial cells [170] * ↓ iNOS and inhibition of NF-κB and AP-1 activation [171] |
AD rodent model: * ↓ Aβ plaques, oxidized proteins and Interleukin-1 beta (IL-1β) [141, 227] HD rodent model: * Counteract huntingtin aggregates formation and partial improvement of transcriptional deficits, as well as an amelioration of rearing deficits [215] |
||
| Polyphenol | In Vitro | Cellular Models and Primary Cell Cultures | Animal Model | ||
| Quercetin | * ↓ αSyn fibrillization [142] | * ↓ Aβ induced cytotoxicity, protein oxidation, lipid peroxidation and apoptosis in cultured neurons [174] * Protected cells from oxidative insults, IL-1β and PD related toxins [172] * Control immune response via modulation of IL-1β and TNF-α and reduced the proliferation of peripheral blood mononuclear cells isolated from MS patients [356]. |
Neurodegeneration rodent models: * ↑ memory and synaptic plasticity upon chronic lead exposure [232] * Protection against colchicine-induced cognitive impairment [216] * Improved motor function in a model of acute spinal cord injury [228] AD rodent model: * ↑ Performance on learning and spatial memory tasks and greater risk assessment behaviour [233] * ↓ Extracellular β-amyloidosis, tauopathy, astrogliosis and microgliosis in the hippocampus and the amygdala [233] * ↓ Plaque burden and mitochondrial dysfunction (↑AMPK activity) and ↑ cognitive impairment [357] PD rodent model: * Neuroprotective by inducing antioxidant defences and ATPases [217] Cerebral ischemia rodent model: * ↓ Lesion [229] * ↓ Hippocampal neuronal death [230] * ↓ Apoptosis (activation of BDNF-TrkB-PI3K/Akt signalling pathway) [231] |
||
| Rosmarinic acid | * Scavenging peroxynitrite [115] | * Protects neurons from oxidative stress [235, 358] | AD and ALS rodent model: * Alleviated memory impairment, delayed disease onset and ↑ lifespan [201, 234] |
||
| Hesperetin | * Cytoprotective effects in mouse primary neurons [117] * Cytoprotective in a cell model of PD induced by rotenone [177] |
AD rodent model: * Restore deficit in non-cognitive nesting ability and social interaction; attenuation on β-amyloid deposition, plaque associated APP expression, microglial activation and TGF-β immunoreactivity [218] |
|||
As a pure compound, the potential of resveratrol in NDs prevention have been extensively exploited. It was shown to reduce Aβ mediated accumulation of ROS and apoptosis in cell models [163], being its protection related with the modulation of NF-кB and SIRT1 pathways [163-165]. In yeast, resveratrol mimics caloric restriction, described to slow the pace of ageing, by stimulating Sir2 (the homologue of SIRT1), increasing DNA stability and extending life span by 70%, being therefore associated with a mitigation of age-related diseases, including neurodegeneration [166].
Curcumin has also been pointed as a polyphenol with a plethora of protective activities. It exhibits intracellular antioxidant activities, anti-amyloid activities [167, 168] and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) protective activity [169]. Moreover, studies reveal that curcumin also presented anti-inflammatory activity through inhibition of COX-2 in both rat primary microglial and murine BV2 microglial cells [170], suppressed iNOS and inhibited NF-κB and AP-1 activation [171].
The flavonoid quercetin is also among the well-characterized polyphenols in terms of biological activity. It increased cell survival upon treatment with H2O2, hydro-peroxide, tert-butyl hydroperoxide (TBHP), IL-1β and PD related toxins [reviewed in [172, 173]. Pre-treatment of primary hippocampal cultures with quercetin significantly attenuated Aβ-induced cytotoxicity, protein oxidation, lipid peroxidation and apoptosis [174].
A limited number of studies have revealed the protective activity of other phenolics (see Table 2). Chlorogenic acid, a polyphenol present in coffee, was shown to protect cortical primary neurons against glutamate neurotoxicity, with potential for ischemic stroke treatment [175]. The 3-O-caffeoylquinic acid reduced neuroinflammation by reducing microglia mediated ROS production and neuronal excitotoxicity [176], whereas hesperetin, a flavanone glycoside abundant in citrus fruits, have also revealed neurprotective effects in mouse primary neurons [117] and in a rotenone-induced apoptosis human neuroblastoma SK-N-SH cells, considered a cellular model of PD [177]. Other study detected protective activity by less representative flavonoids (chrysin, puerarin, naringenin, genestein) in mesencephalic cultures from injury by MPP+ also a model of PD [173].
Cellular models are indispensable to understand the molecular mechanisms of polyphenols and how they affect
cellular homeostasis. By studying pure polyphenols in cellular models specific mechanisms could be followed and attributed to the target compound. However, only by pharmacological approaches can be consider the scenario of the presence of these compounds in neuronal tissues. Studies are lacking to evaluate the effects of nutritional metabolites at the brain cells. Yet, in order to design nutritional or pharmaceutical approaches using polyphenols, it is mandatory to translate their benefits into animal models, and to study their effects at the level of the whole organism.
In Vivo Animal Assays
Most of the evidences for neuroprotection in animal studies come from studies were foods or plant extracts are given orally to animals. Those are defined as nutritional (nutraceutical) interventions since polyphenols will cross the gastro-intestinal barrier and will be metabolized as dietary compounds. Only encapsulated compounds, intra-venous or other forms of delivery were considered in a pharmacological perspective. The reason for that is related with the significant impact that digestion and metabolism impose to the biological effects of polyphenols as described in the previous sections.
Nutritional/Nutraceutical Interventions
EGb 761 has been shown to exert a protective effect in many models of neurodegenerative pathologies. In an AD transgenic mice, oral intake of G. biloba extract improved spatial learning and memory [178], and decreased APP in the cortex [179]. In PD animal models, EGb 761 exerted potent neuropreventive/neurorecovery effects against neurotoxic induced by MPTP/MPP+ [180, 181]. Oral administration of EGb 761 improved motor performance and survival, and protected against a loss of spinal-cord anterior motor horn neurons, in a mouse model of ALS carrying a mutation in SOD1 gene [182]. Protection was also observed in rodent models of cerebral ischemia and other neurodegenerative disorders [reviewed in [183].
Oral administration of green tea in animal models of ageing and of neurodegeneration prevented several dysfunctions, such as protein/lipid oxidation [184], improved spatial learning [184], modulated glutathione levels and antioxidant enzyme activities [185], increased cAMP response element-binding protein (CREB) activation [185], raised brain-derived neurotrophic factor (BDNF) and b-cell lymphoma 2 (Bcl-2) levels [185], as well as improved cognitive and behavioural capacities [186]. Oral ingestion of green tea extract also protected against deltamethrin-induced neurotoxicity in rat by improving the oxidative status and DNA fragmentation [187].
Interestingly, a recent study provided the first evidence that tea polyphenols administered by oral gavage alleviate motor impairments, dopaminergic neuronal injury, and cerebral αSyn aggregation in MPTP-intoxicated parkinsonian monkeys [188].
Another source of polyphenols in the stardom is grape and its derivatives. Oral ingestion of grape seed polyphenol extract improved cognitive function [189] and reduced cognitive deterioration [103], Aβ oligomerisation [103], amyloid plaques and microglial activation in animal models of AD [190]. Moreover, its incorporation in diet attenuated extracellular-signal-regulated kinases [191] 1 and 2 signalling in the brain, suppressing AD tau neuropathy [192]. Oral administration of grape seed also exhibited potential for HD treatment by improving lifespan in a Drosophila model and attenuating motor skill decay in a rodent model of HD [193]. Recent studies reported that rat intestinal microbial meta-bolites from grape seed extract accumulated in brains and interfered with the assembly of Aβ peptides into neurotoxic Aβ-amyloid aggregates, with relevance to AD pathogenesis [194]. A derivative of grape, red wine, was shown to increase spatial memory performance, reducing cognitive deterioration and Aβ neuropathy in an AD transgenic mouse model [195].
A blueberry-supplemented diet was also shown to reverse age-related deficits in spatial working memory through the increase of CREB activity, BDNF, the modulation of phosphorylation of hippocampal Akt, the activation of target of rapamycin (TOR), and the regulation of Arc/Arg3.1 [196]. To define the causal agents in the cognitive benefits of blueberry, the same authors tested diets with the pure flavanols, (−)-epicatechin and (+)-catechin and pure anthocyanins, and concluded that they were associated to the beneficial effects on memory in aged rats [197]. Furthermore, a diet containing blueberry anthocyanins prevented the cognitive degeneration in AD mice [130].
Intake of berries such as pomegranate, strawberry, blueberry and blackberry ameliorated several aspects of memory and learning [198], delayed age-related motor and cognitive behavioural deficits in rodent models [199-201]. Pomegranate juice oral intake also improved spatial learning, and reduced Aβ plaques in transgenic mice expressing Aβ-amyloid peptide [202]. In rats receiving anthocyanins orally, it was observed the reversion of Aβ-induced effects on the expression of proteins related with the mitochondrial apoptotic pathway (BCL2-associated X protein (Bax), cytochrome C, caspase-9 and caspase-3) and AD markers (Aβ, P-tau and Beta-secretase 1 (BACE-1) [151]. Administration of a cocoa polyphenolic extract orally to aged rats delayed the onset of age-related cognitive deficits and increased lifespan with improvements in cognitive performances [51].
Several animal studies have been also performed for individual polyphenols present in the most neuroprotective extracts described earlier (catechins from tea, resveratrol from grapes and wine products, etc.) through dietary interventions. For instance, oral administration of catechin prevented spatial learning and memory decline in aged mice [203] and increased the lifespan in a senescence-accelerated mice model [204]. It also ameliorated redox imbalance and limited inflammation in rats with cerebral ischemia [205]. Catechin oral administration also rescued memory impairment induced by Aβ, through inhibition of the NF-кB pathway, and mitigated oxidative stress in the brain [161]. Further-more, EGCG oral intake restored mitochondria function in the brain hippocampus, cortex and striatum of a transgenic mouse model of AD [206]. An EGCG diet rescued transgenic mice of trisomy from morphogenesis defects, low BDNF levels and mnemonic deficits [207]. It also exhibited neuroprotective effects by modulating neuroinflammation and attenuating neural damage in an in vivo model of multiple sclerosis (MS) (gavage administration) [208].
In an animal model overexpressing APP, a resveratrol rich diet reduced the formation of amyloid plaques, without affecting amyloid peptide levels [209]. Furthermore, oral administration of resveratrol exhibited a positive effect in PD animal models, as it reduced neural inflammation by lowering mRNA levels of COX-2 and tumour necrosis factor (TNF-α) mRNA in the substantia nigra [210]. In a non-human primate, diet supplementation with resveratrol for 18 months increased spatial memory performance compared to placebo [211]. In agreement with this, in animal models of MS, resveratrol oral intake was found to attenuate neural damage through SIRT1 activation [212] and prevented neural loss without immunosuppression [213]. Regarding HD, oral gavage of resveratrol also shown a beneficial effect via SIRT1 activation in a transgenic mouse model [214].
Curcumin also reduced Aβ plaques, oxidized proteins and Interleukin-1 beta (IL-1β) in AD transgenic mice, by administrating curcumin to diet [141], whereas it counteracted HD aggregates formation [215].
Quercetin protects against colchicine-induced memory impairment and oxidative damage in rats [216]. In PD in vivo models, quercetin oral administration showed to be neuroprotective by inducing antioxidant defences and ATPases [217].
Hesperidin, a flavanone glycoside found abundantly in citrus fruits, was orally given to a transgenic mouse model of cerebral amyloidosis for AD [218]. Fascinatingly, after a relatively short-term treatment of 10 days, hesperidin significantly restored deficits in non-cognitive nesting ability and social interaction. A significant attenuation on β-amyloid deposition, plaque associated amyloid peptide expression, microglial activation and transforming growth factor beta (TGF-β) immunoreactivity was observed in the brains of mice [218].
Pharmacological Interventions
Several attempts were done with a more pharmacological approach, by direct injection of bioactive polyphenols to the organism with the aim to circumvent its metabolism and improving their efficacy. Some examples are explored bellow in this section.
In a mice model of PD, EGb 761 administered by intraperitoneal injection before or after MPTP treatment protects against nigrostriatal dopaminergic neurotoxicity involving the inhibition of brain monoamine oxidase [219].
Green tea extract injected into the hippocampus region of rat brain reduced aluminium chloride toxicity, associated with AD [220]. In animal models, EGCG administered by intraperitoneal injection improved age–related cognitive decline and protected against ischemia/reperfusion [221] as well from amyloidosis in AD mice models [102].
In an AD mouse model injected with Aβ, intra-cerebroventricular injection of resveratrol protected from Aβ neurotoxicity by inhibiting iNOS [222]. Reduced hippocampal neurodegeneration has also been shown after intracerebroventricular injection of resveratrol in rodent models of AD/tauopathies [164]. In rat models of ischemic injury, intraperitoneal injection with resveratrol improved brain energy metabolism [223], along with modulation of the release of neurotransmitters and neuromodulators [224]. Resveratrol was also tested by intraperitoneal injection on a 6-hydroxydopamine (6-OHDA)-induced Parkinson's disease (PD) in rats and revealed attenuation of oxidative stress, lipid peroxidation, and protein carbonyl content [225]. Recently, resveratrol was shown to reduce cell death in ovariectomized female rats subjected to chronic cerebral hypoperfusion [226].
Notably, curcumin when injected in the tail vein of an AD rodent model was able to cross the BBB, target and disrupt existing plaques in [227] and also reduced Aβ plaques, oxidized proteins and IL-1β in AD transgenic mice [141].
Intraperitoneal administration of quercetin improved motor function in a model of acute spinal cord injury [228], reduced ischemic lesion [229], as well as hippocampal neuronal death [230], and cell apoptosis in a rat model of cerebral ischemia [231]. By the same route, quercetin improved memory and synaptic plasticity upon chronic lead exposure in rats [232]. In a triple transgenic AD model, quercetin was able to reverse the histopathological hallmarks of AD and to ameliorate cognitive and emotional impairments [233].
A rosemary phytochemical, rosemarinic acid, alleviated memory impairment mediated by Aβ, delayed disease onset and increased lifespan in AD mouse models [234]. Rosemary extract and rosmarinic acid also prevented ALS degeneration in mouse models of the disease after intraperitoneal injection [201]. Carnosic acid, also present in rosemary, crossed BBB and preserved GSH levels in mouse models of ischemia/reperfusion after intraperitoneal injection [235].
An integrated view of in vitro, cellular and animal model studies either with food or plant extracts (Table 1) or pure compounds (Table 2) reveals the whole panorama of knowledge gathered for polyphenols, and clearly reinforces their neuroprotective potential. Although human trials are more reliable and the closest approach to human physiology, they are time and cost consuming. The animal in vivo and cellular assays have constituted an unparalleled tool to dissect mechanisms of action, and for genetic and compound screening assays. On the other hand, the in vitro assays are crucial to understand the interaction of polyphenols with proteins, metals or other small molecules, by excluding cell components interference and serving as a fundamental tool to tune the overall picture revealed by cell studies.
Human Trials
Human clinical trials to ascertain the impact of polyphenols in neurodegenerative diseases and ageing are still very scarce. Although allusive of a protective role in neurodegeneration, there is still too many fragmented evidences to build an integrated picture with the mechanistic studies.
Most of the human trials with G. biloba were inconclusive [236] and its long-term oral intake did not affect the prevalence of cerebral Aβ deposition [237]. Nevertheless, recently, positive results were obtained with G. biloba extract in ischemic stroke [238] and in children with attention deficit hyperactivity disorder [239]. A recent systematic review and meta-analysis about the efficacy and adverse effects of G. biloba for cognitive impairment and dementia concluded that it stabilizes or decelerates the decline in cognition, function and behaviour, at 22-26 weeks administration, especially for patients with neuropsychiatric symptoms [240].
Concerning green tea extracts, human studies revealed the increase of brain activity in the dorsolateral prefrontal cortex, an area involved in memory processing [241]. Recently, consumption of green and black tea by healthy volunteers increased the brain theta waves as measured by a simplified electroencephalogram, suggesting a role in cognitive function, specifically alertness and attention [242]. Moreover, EGCG reduced cognitive deficits in a pilot study with Down syndrome individuals, with effects on memory recognition, working memory and quality of life [243].
Recently, Brickman and co-workers [65] reported that a high cocoa flavanol-containing diet over 3 months enhanced memory function and improved related activation in the dentate gyrus, the hippocampus region characterized by life-long neurogenesis, in comparison to a cocoa diet low in flavanol, in a randomized study with healthy old subjects using functional magnetic resonance imaging (MRI). In the Cocoa Cognition and Ageing (CoCoA) study, consumption of cocoa flavonols for 8 weeks improved cognitive performance in a group of cognitively intact older adults [244].
In an intervention study, blueberry juice supplementation improved memory in older adults [68]. Also, supplementation with Concord grape juice enhanced neurocognitive function in older adults with pre-described mild cognitive impairments, which was supported by studies on brain activity using functional magnetic resonance imaging [245].
There are several studies examining flavanols and anthocyanins neuroprotective properties in humans but little investigation into the flavonoid subclass known as flavanones. This is an incredible gap because orange juice, a rich source of flavanones, is one of the most-commonly consumed juices throughout the world. A recent study evaluated the consumption of flavanone-rich orange juice over 8 weeks and concluded that there was an association with benefits for global cognitive function in healthy older adults, in comparison to the consumption of a low-flavanone control juice [246]. Moreover, the same authors observed that ingestion of a flavonoid-rich orange juice promoted acute cognitive benefits over 6 h in healthy middle-aged adults [247].
Only a few placebo-controlled interventional studies are available to date concerning resveratrol cognitive improvements. Kennedy et al. (2010) [248] assessed the effects of oral administration of resveratrol on cognitive performance, in a randomized control trial crossover study in 22 healthy adults, with the result that even single doses of orally administered resveratrol could modulate cerebral blood flow variables, measured using MRI [248].
A recent study examined the effects of curcumin on cognition and mood in a healthy older population. Working memory and mood were significantly improved following 4 weeks treatment, confirming the potential psychological and cognitive benefits of curcumin in an older population [249].
On the other hand, a contradictory study in a large community sample, with a 12-week supplementation of quercetin, provided evidence that quercetin may not have an ergogenic effect on neurocognitive functioning [250], consistent with a growing body of literature raising concerns about the generalization of findings from in vitro and animal quercetin research to human populations.
Some human studies have been performed for dietary formulations that include mixtures of polyphenols or polyphenols with other compounds, highlighting possible synergies between constituents. For instance, in a double-blind, clinical trial with older adults by Small and colleagues [251], the intake of a pill-based nutraceutical that contained a proprietary formulation of blueberry, green tea, carnosine, vitamin D3 and biotin, resulted in significantly increased processing speed. Also, in healthy overweight older individuals, a daily intake of a formulation containing resveratrol and quercetin significantly improved memory performance [252].
However, for polyphenols to reach the brain they have to be able to cross BBB. An increasing body of evidence regarding this subject has been collected and discussed in the next chapter.
Polyphenols and restrains: interaction with barriers and beyond
The extensive metabolism to which dietary polyphenols are submitted once ingested, either in the intestine, liver and or in cells, leads to the arise of a broad range of polyphenol derivatives. Despite accumulating evidence concerning polyphenols neuroprotection (see Tables 1 and 2), and assuming the effective transport/distribution/delivery of dietary polyphenol metabolites to target tissues, we also need consider the existence of other barriers that must be crossed by these polyphenols metabolites or, at least, be able to alter the surrounding environment in order to induce responses in the target organs.
Blood-Brain Barrier
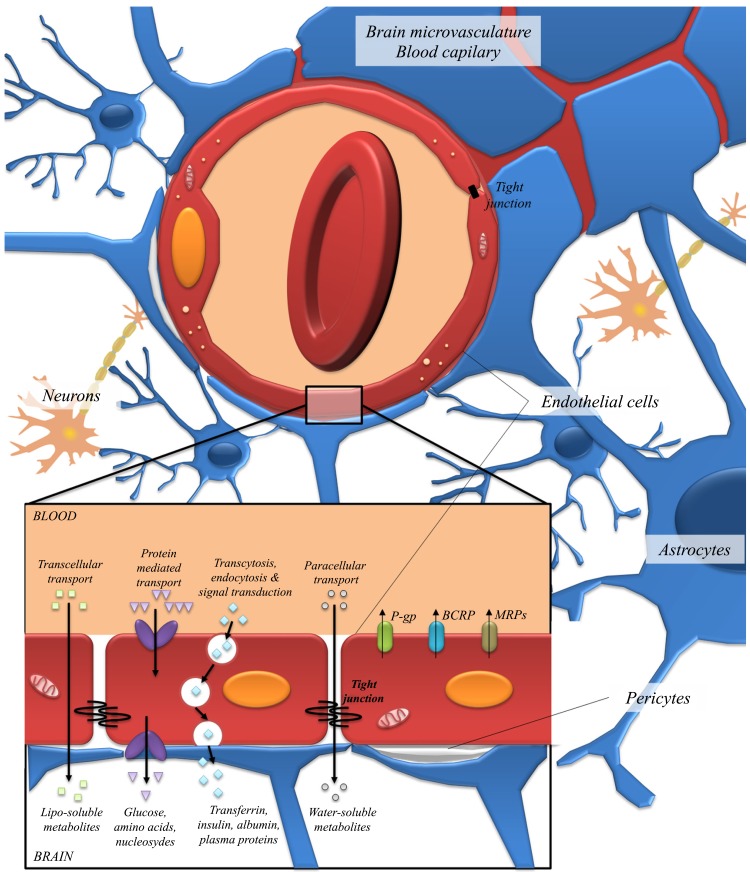
The existence of three layers of barrier at the CNS limits and regulates molecular exchanges between the blood and the neuronal tissue, or its fluidic spaces: i) the BBB, majorly constituted by cerebrovascular endothelial cells, located between the blood and the brain interstitial fluid; ii) the choroid plexus epithelium, located between the blood and ventricular cerebrospinal fluid; iii) and the arachnoid epithelium, located between the blood and subarachnoid cerebrospinal fluid [3]. In particular, the choroid plexus is responsible for the transport of vitamins, small peptides, amino acids, inorganic ions and hormones to the cerebrospinal fluid [253]. Among the different barriers of the CNS, the BBB exerts the highest control over the close microenvironment of brain cells, since its surface area is 5000 times larger than the blood-cerebrospinal fluid barrier, having a dominant role in providing nutrients for the brain, as well as controlling metabolites, like polyphenols, accessibility to neuronal cells [254].
The BBB is a dynamically selective and complex interface, protecting the CNS from toxic compounds and pathogens, acting as a border between the periphery and the brain [255]. Besides the microvascular endothelial cells that comprise the anatomical basis of the BBB, it is surrounded and interacts with perycites, astrocytic endfeet, microglia and neurons, which constitute the neurovascular unit (Fig. 5) [254, 256, 257]. The neurovascular unit complexity, together with the tight control exerted by the brain endothelium due to the presence of intracellular tight junctions, lack of fenestrations, low pinocytic activity and efflux pumps, controls the passage of the smallest polar molecules and macromolecules [254, 258, 259] (Fig. 5). Polyphenols are no exception, in order for them or their metabolites to access the brain, they must cross a tightly regulated, selectively permeable endothelial layer. As a growing number of biological effects have been attributed to these molecules in the past few years, attention to their role as modulators of the transport capacity of epithelial barriers has also received great attention.
Fig. (5).
The neurovascular unit. The BBB is composed by endothelial cells, astrocytes and perycites, where endothelial cells form a boundary between the blood and the CNS. The flux of nutrients and metabolites from the blood to the CNS is regulated by the BBB, which controls their availability by transport systems. Transport across the BBB occurs via several pathways: lipid-mediated diffusion (transcellular transport), paracellular diffusion, carrier-mediated transport through proteins, receptor-mediated transcytosis and absorptive-mediated transcytosis. Besides these pathways, active efflux transport through ABC transporters such as P-gp, BCRP and MRPs family also takes place, in order to prevent the entry and accumulation of harmful substances to the brain.
Youdim and co-workers have elucidated the polyphenols permeation through the BBB [260, 261]. They suggested that polyphenols transmembrane diffusion in vitro is related with its lipophilicity, where less polar derivatives (e.g. methylated derivatives) are capable of higher brain uptake than more polar metabolites (e.g. sulphated and glucuronides). Accumulating evidence of polyphenols uptake by the BBB reinforces their putative potential in a neurological context. Nevertheless, it is not yet totally clear whether the primary route by which polyphenols cross the BBB is simple diffusion or carrier-mediated transport [262]. To date, only a few compounds from each flavonoid subclasses have been studied, and there is limited knowledge on the effects of flavonoid structure on their bioavailability at the brain level. Therefore, the true mechanisms by which flavonoids and their circulating metabolites interact with the BBB remain a hot topic in neuroscience research.
Considering polyphenol and their metabolites permeability across the BBB, the existence of different specialized transporters in the plasma membranes of luminal (blood) and abluminal (brain) sides of the endothelial cells should be taken into account. The endothelial cells permeability to polyphenols is particularly influenced by the expression profile, functionality and precise location/orientation of efflux transporters, solute carriers and organic-anionic transporters [263]. Nevertheless, there are strong evidences of the effective capacity of polyphenols to reach the brain and exert neuroprotection, and it is an ever-growing field of investigation (Table 3).
Table 3.
Evidences of polyphenol transport at the BBB and neuroprotective potential, concerning interaction studies with ABC efflux transporters expressed in endothelial cells at the BBB. P-gp - P-glycoprotein, MRP - multidrug resistant protein, BCRP - breast cancer resistant protein, BMEC - brain microvascular endothelial cells, TR-rats - transport deficient rats.
| Polyphenol | Evidence of BBB Penetration | Evidence of Neuroprotection | Efflux Transporter Interaction | Experimental Setup | Refs. |
|---|---|---|---|---|---|
| Apigenin | Yes [307] | Yes [307] | P-gp inhibitor | Rat BMEC | [307] |
| P-gp inhibitor | CCRF-CEM, CEM/ADR5000 leukemia cells | [308] | |||
| BCRP inhibitor | MDA-MB-231-BCRP cells | [308] | |||
| Catechin / epicatechin | Yes [278] | Yes [309, 310] | P-gp activator | NIH-3T3-G185 cells | [189] |
| MRP2 substrate | Caco-2 cells | [311] | |||
| MRP1/MRP2 substrate | MDCKII/MRP1 cells & MDCKII/MRP2 cells | [312] | |||
| Chrysin | N. D. | Yes [313, 314] | P-gp inhibitor | Mouse BMEC | [315] |
| MRP2 substrate | Caco-2 cells | [316] | |||
| BCRP inhibitor | MCF-7 MX100 cells | [317] | |||
| Curcumin | Yes [227] | Yes [318] | P-gp inhibitor | MCF-7 cells | [319] |
| BCRP inhibitor | Rat brain capillaries | [320] | |||
| Fisetin | Yes [321] | Yes [321] | MRPs inhibitor | Caco-2 cells | [322] |
| Genistein | Yes [323] | Yes [324, 325] | P-gp inhibitor | MCF7/BC19-3, rats | [326, 327] |
| MRP2 inhibitor | TR-rats | [328] | |||
| BCRP inhibitor | K562/BCRP cells | [329] | |||
| Hesperitin | Yes [260] | Yes [330] | P-gp inhibitor | Mouse BMEC | [315] |
| BCRP inhibitor | ABCG2 over-expressing cells | [331] | |||
| Kaempferol | Yes [307] | Yes [332] | P-gp inhibitor | Mouse BMEC | [315] |
| MRPs inhibitor | Human glioblastoma cell line T98G | [333] | |||
| BCRP inhibitor | MDCK/Bcrp1 cells | [334] | |||
| Myricetin | Yes [335] | Yes [335] | P-gp inhibitor | MCF-7/ADR cells | [336] |
| BCRP inhibitor | HEK293/ABCG2 cells | [280] | |||
| Naringenin | Yes [287, 337] | Yes [338] | P-gp inhibitor | Mouse BMEC | [315] |
| MRPs inhibitor | HEK293/ABCG2 cells | [280] | |||
| Quercetin | Yes [261] | Yes [339] | P-gp inhibitor | Mouse BMEC | [315] |
| MRPs inhibitor | HEK293/MRP1, HEK/MRP4, HEK/MRP5 cells | [340] | |||
| BCRP inhibitor | ABCG2 over-expressing cells | [331] | |||
| Resveratrol | Yes [341] | Yes [342] | P-gp inhibitor | MCF-7/ADR cells | [343] |
| BCRP inhibitor | ABCG2 over-expressing cells | [331] | |||
| Rutin | Yes [307] | Yes [344] | P-gp inhibitor | Rat BMEC | [307] |
In terms of uptake, there is evidence suggesting that transport mediated by glucose transporter 1 (GLUT1), organic anion transporters (OATs) and organic anion-transporting polypeptide (OATP1A2) may affect the distribution of flavonoid glucoronides into the brain, in a similar fashion to other glucoronidated metabolites [264, 265]. In fact, the presence of these glucoronidated metabolites can be partially derived, due to the high expression of both GLUT1 and OAT1A2 on both luminal and abluminal membrane of
endothelial cells. In a recent study, the authors successfully localized quercetin-3-O-glucuronide, a major phase-II metabolite of quercetin, in human brain tissue, namely at epithelial cells of the choroid plexus and also in macrophages [266].
Microdialysis sampling in rats also showed that (+)-catechin and (-)-epicatechin could pass through the BBB [267]. In fact, several polyphenols have been identified in different brain regions of the rat [268, 269] and pig [270, 271], usually accumulating in a nonregion-specific manner [272]. Several animal studies also indicate that polyphenols are able to cross the BBB and co-localize within the brain tissues independently of their route of administration: epigallocatechin gallate [273], epicatechin [274], and anthocyanins [269, 275] were detected in the brain after oral administration; naringenin and its glucuronide were identified in the cerebral cortex, after intravenous administration [276], and intravenously administered hesperetin was also detected in the brain, especially in the striatum [277].
Another study has examined the permeability of flavonoids and their known circulating metabolites across an in vitro model of the BBB [260]. Hesperetin, naringenin, and their respective in vivo glucuronides, as well as the anthocyanins cyanidin-3-rutinoside and pelargonidin-3-glucoside, all showed measurable permeability. In another study, both catechin and epicatechin could cross a cellular model of BBB in a time-dependent and stereo-selective manner, with epicatechin presenting a substantially higher transport than catechin [278]. In vitro transmembrane transport of different flavonoids (flavonols, flavan-3-ols and anthocyanins) and some of their methylated and glucoronidated metabolites was also observed, where in most cases the metabolites exhibited higher transport efficiency than their parent compounds [279]. All these collected data strongly suggests the effective uptake of dietary polyphenols and their metabolites through the BBB endothelium, reinforcing their ultimate neuroprotective potential.
Polyphenols as Modulators of Membrane Transport
Importantly, polyphenols have also shown to be able to modulate the activity of some ATP-binding cassette transporters (ABC transporters). The ABC transporters are efflux pumps responsible for controlling the bioavailability of many xenobiotics, being vastly present in the epithelia of the gut, placenta and BBB, as well as in cancer cells. Cancer cells tend to overexpress these ABC transporters, which confers them bigger resistance to chemotherapy [280].
Several studies have demonstrated inhibition of ABC transporters by flavonoids (Table 3). The interaction between flavonoids and ABC-transporters could be advantageous for poorly absorbed drugs; otherwise, this interaction could also lead to drug intoxication, particularly in the context of drugs with a narrow therapeutic window. Additionally, some flavonoids are themselves substrates of the most phar-macologically relevant ABC transporters: P-gp, multidrug resistant protein type 2 (MRP2) and breast cancer resistant protein (BCRP) [281].
For example, it was shown that extracts from St John’s Wort upregulate the expression of P-gp at intestinal level, which could ultimately reduce the bioavailability of substrate pharmaceuticals [282]. On the other hand, diterpenes, triterpenes and carotenoids, naturally occurring lipophilic phytochemicals, were able to inhibit human P-gp in vitro at a low range, while other combinations had a synergistic activity [146, 283]. Quercetin, hypericin and kaempferol were shown to promote the cellular uptake of ritonavir on P-gp overexpressing cells [284]. Besides, both in vitro and in vivo assays (only with short-term exposure) with these polyphenols inhibited the action of efflux pumps and increased substrate bioavailability; in vivo assays of chronic exposure boosted the expression of P-gp and lead to a reducing bioavailability of substrate drugs [285, 286].
Besides their modulation of ABC transporter function, polyphenols can also be substrates of these transporters, a property that can dramatically limit their bioavailability. Youdim and co-workers have showed the limitations that quercetin has to BBB transposition: when co-administrated with a P-gp or BCRP inhibitor, this polyphenol was able to enter epithelial cells of the BBB, possibly by passive diffusion due to its hydrophobicity, but was then specifically exported by the BCRP [260]. Such phenomenon explains, at least in some extent, why quercetin and resveratrol presented very limited bioavailability, despite the amount ingested [287, 288].
Taking all this information into account, it could be interesting to explore the combination of an inhibitor of the ABC transporters and a beneficial polyphenol, in order to increase the accumulation of such polyphenol in the target region and potentiate its efficiency. The co-administration of a quercetin-rich product and an inhibitor of BCRP may increase quercetin’s access into the brain and then unleash novel bioactivities from this polyphenol [289]. In addition, if the inhibitors of ABC transporters themselves are orally bioavailable, they may assist polyphenols transport through the BBB [261].
Blood-Testis Barrier & Placenta
Besides brain integrity, mainly protected by the presence of the BBB, the insurance of life perpetuation and preservation must not be disrupted by foreign molecules in the human body. The presence of barriers protect germ cells and foetus, saving from harm the new life-to-be inside pharmacological sanctuaries. These pharmacological sanctuaries have highly restricted access to exogenous compounds, and where polyphenols, contrarily to other metabolites, may have an open door.
The blood-testis barrier (BTB) is one of the tightest blood-tissue barriers in the mammalian body and it separates the seminiferous epithelium into the basal and the apical compartments [290]. A specialized and unique micro-environment is safeguarded by the BTB, where meiosis, spermiogenisis and spermiation occur. BTB controls the entry of nutritional compounds, vital molecules (such as hormones and electrolytes) and also harmful toxicants, into the compartment in which post-meiotic germ cell development takes place [290].
On the other hand, the placenta constitutes the link between the mother and the developing foetus, performing several strict functions that are essential for the maintenance of pregnancy and normal foetal development. The placenta mediates the uptake of nutrients between the mother and the foetus, eliminating metabolic waste fetal products. This capacity is mediated due to the presence of transporters at the maternal-facing brush-border membrane and at the foetal-facing epithelial basal membrane. It is the activity of these specialized transporters that will largely determine the extent at which organic compounds will cross the placenta and enter the foetal blood circulation [291].
Recently, it was demonstrated that prenatal stress can reduce mitochondrial content and antioxidative capacity, while increasing oxidative stress in the hippocampus of offspring, contributes to decreased neurogenesis and cognitive function. Impressively, maternal hydroxytyrosol administration successfully promoted cognitive function, through modulation of mitochondrial content and phase II enzymes in the progeny [292]. Such observations evidence that the drastic alterations that polyphenol supplementation induce are more dramatic than what was initially thought: the effects of polyphenols are transversal, affecting not only the animal itself but also its offspring, leading to benefits that can only be explained by its capacity to cross the placenta.
Neuroprotective effects of resveratrol on prenatal stress were also investigated in the brain of neonatal rats. Administration of resveratrol during pregnancy inverted the memory impairment caused by prenatal stress, as well as the oxidative damage and loss of neurons at the dentate gyrus [293].
Already in 2005 such kind of neuroprotective potential in neonatal brains was assessed. It was seen that maternal dietary supplementation with pomegranate juice was neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury [294]. This dietary supplementation resulted in markedly decreased brain tissue loss, with the highest pomegranate juice dose having substantial statistical significance (p ≤ 0.001). Pomegranate juice also diminished caspase-3 activation in the hippocampus and in the cortex. Ellagic acid, a polyphenolic component in pomegranate juice, was detected in plasma from treated pups but not in control pups [294].
Contrarily, other authors showed that flavanol metabolites were abundant in maternal placenta but were detected at low levels in foetuses and amniotic fluid. This study was performed in pregnant rats subjected to acute intake of a grape seed proanthocyanidin extract [295]. Additionally, flavanol metabolism appears to be less active in the liver during pregnancy, and altogether the data suggests that transport across the placenta is not efficient for flavanols and their metabolites, where the placenta seems to act as a barrier. However, these compounds target the foetus and are excreted in the amniotic fluid [295].
Beyond the putative neuroprotective effects of polyphenols to the offspring, in a study of Keating and co-workers [296] it was evidenced the benefits on folic acid absorption, once folic acid is critically important for normal foetal development. The authors suggested a detrimental effect of short-term exposure to epicatechin and isoxanthohumol on placental folic acid absorption and, on the other hand, a beneficial effect of a long-term exposure to xanthohumol, isoxanthohumol and quercetin on folic acid absorption at the placental level [296]. The effect of isolated polyphenols on placental transport of glucose was studied as well: several polyphenolics compounds were found to affect the apical uptake of deoxy-D-glucose in a trophoblast cell model. Rutin, catechin and epicatechin increased deoxy-D-glucose uptake and, when tested in a long-term exposure (48 h) rutin and myricetin increased apical uptake of deoxy-D-glucose. This observation was true for the isolated compounds or the combination of both. Chrysin and quercetin decreased deoxy-D-glucose uptake in a concentration dependent manner [297].
Moreover, several studies highlighted the protective potential of polyphenols in ensuring the integrity of germ cells. It was found that, in irradiated tests, EGCG ameliorates several parameters related to spermatogenesis in mice [298]. This study determined the effects before and after radiation treatment on spermatogenesis in the testicles: pre-treatment seems to be crucial to protect spermatogenesis against radiation, while post-treatment appears to be able to support spermatogenic rescue from radiation [298]. Altogether, EGCG seems to prevent germ cells from radiation-induced cell death by multiple mechanisms, and the use of this bioactive polyphenol can present an attractive strategy to preserve fertility in males exposed to conventional radiation therapy [298].
In another study, the administration of 10 μmol [3H]epicatechin to rats resulted in major amounts of radioactivity in the large intestine and caecum at 24 h, and little radioactivity was observed in the stomach, plasma, small intestine, blood and testes [299]. The relatively high concentration of epicatechin metabolites found in the testes of rats subjected to a cocoa-enriched diet (mainly epicatechin glucuronide), highlighted the BTB putative permeability to this polyphenol metabolite, and shows that its accumulation could occur [300].
Beyond Barriers
In the light of today’s knowledge, novel mechanisms of action for polyphenols are proposed far beyond the classical direct antioxidant radical scavenging power. In fact, polyphenol concentration at cerebrospinal fluid rarely exceeds 1-5 μmol. Therefore, it is highly improbable that polyphenol intracellular concentrations in neurons and glial cells would exceed the micromolar-nanomolar range, which is not enough to exert significant direct antioxidant effects. Nevertheless, such concentrations are in fact beneficial, pointing out that polyphenols and their metabolites modulate other cellular parameters and pathways at the CNS level, even at low concentrations [262].
The noteworthy effect of polyphenols and their meta-bolites in cellular signalling pathways at concentrations as low as 1 μmol has been reported already [301], reinforcing the idea of indirect mechanisms that could be involved in polyphenols neuroprotection. The activation of hormetic responses and effects on peripheral systems of the body, that culminate in changes in the CNS function are the proposed indirect mechanisms of action of polyphenols and degradation metabolites. Hormesis describes a process in which exposure to a low dose of an agent, that is toxic at higher doses, induces a beneficial effect on the cell or organism [301]. Direct evidence for hormesis induction in vivo has already been reported in mammals: pre-treatment of mice with epicatechin significantly reduced the negative impact of stroke induction in wild-type but not nuclear factor erythroid 2 (Nrf2) knock-out animals, as the transcription factor Nrf2 is one of the key regulators responsible for the induction of antioxidant and cell protective genes [302]. Additionally, the regulation of brain integrity and function must not be seen as an isolated process, but instead as strongly dependent on feedback information (in the form of hormones, nutrients, metabolites and, of course, sensory neuron signalling of the body periphery).
Furthermore, evidences of the metabolism of polyphenols at the BBB level has already been reported with the detection of the compounds conjugated with glucuronic acid [278]. The hypothesis that polyphenol metabolites resulting from biotransformation in endothelial cells, are the key actors in neuroprotection, arises as a novel creed in a nutritional/nutraceutical perspective. The physiological role of conjugated-flavonoids is discussible, but there is the possibility of their presence in the extracellular fluid in the CNS, and exerting biological effects. In fact, some reports have suggested that flavonoid metabolites possess more biological activity than the intact form [303, 304]. Noteworthy, biotransformation processes may constitute an essential step to convert polyphenols into more water-soluble metabolites, and subsequently facilitate its elimination from the body or, oppositely, allow an easier xenobiotic transport and delivery around the body.
In a pioneer pharmacokinetics study, 23 polyphenols microbial metabolites were administered intravenously to rats, to reliably reproduce a physiological post absorption situation [305]. Remarkably, the brain was found to be a specific target organ for 10 of the 23 polyphenol metabolites injected, which significantly increased in the treated animals and most compounds were excreted into the urine [305].
In that sense, it is imperative to adjust the study of polyphenols beyond the reductionist dogma of polyphenol-effect: research should focus not only in the study of polyphenols but also the metabolites arising from digestion and metabolism. Dietary and colonic metabolites derived from polyphenols present in foods and drinks can reach considerable concentrations in circulation, usually higher than their parent compounds, and such physiologically-relevant concentrations must be studied, taking into account the multiplicity of interactions inside the human body as an overall beneficial outcome to the CNS.
CONCLUSION
The diversity and holistic properties of polyphenol present them as an attractive alternative for the treatment of multifactorial diseases, where a multitude of cellular pathways are disrupted. The main findings regarding polyphenols neuroprotective potential performed using in vitro, cellular and animal studies, as well as human trials are covered in this review. The underlying mechanisms of polyphenols for nutrition or therapeutic applications must be further consolidated, however there is strong evidence of their beneficial impact on brain function during ageing. Nevertheless, there is an unmet need for clinical trials regarding polyphenols in the context of neurodegenerative diseases [63, 306]. Pandora’s box is ajar and new evidence are revealing polyphenols impact in the brain beyond barriers. Offspring cognitive improvements after maternal ingestion of polyphenols is a new amazing unexplored route of effects. Understanding how the presence of polyphenols during embryonic development impacts in infancy, adult and advanced life is still an enigma to solve.
ACKNOWLEDGEMENTS
iNOVA4Health Research Unit (LISBOA-01-0145-FEDER-007344), which is cofunded by Fundação para a Ciência e Tecnologia / Ministério da Ciência e do Ensino Superior, through national funds, and by FEDER under the PT2020 Partnership Agreement, is acknowledged. Authors would like to acknowledge to FCT for financial support of CNS (IF/01097/2013), IF (SFRH/BD/86584/2012), and DM (SFRH/BD/73429/2010). CNS, RM and IC acknowledge funding via BacHBerry (Project No. FP7-613793; www.bachberry.eu).
LIST OF ABBREVIATIONS
- ABC
ATP-binding cassette
- Ach
Acetylcholine
- AChE
Acetylcholinesterase
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- ASIC1A
Acid sensing ion channel 1A
- BACE-1
Beta-secretase 1
- Bax
BCL2-associated X protein
- BBB
Blood-brain barrier
- Bcl-2
B-cell lymphoma 2 (Bcl-2)
- BCRP
Breast cancer resistant protein
- BDNF
Brain-derived neurotrophic factor
- BTB
Blood-testis barrier
- CBG
Cytosolic β-glucosidase
- CNS
Central nervous system
- CoCoA
Cocoa, cognition, and ageing
- COX-2
Cyclooxygenase-2
- CREB
cAMP response element-binding protein
- EGCG
Epigallocatechin gallate
- ERK
Extracellular-signal-regulated kinases
- FUS/TLS
Fused in sarcoma/translocated in Liposarcoma
- GDP
Gross domestic product
- GLUT1
Glucose transporter 1
- GSH
Glutathione
- HD
Huntington’s disease
- Htt
Huntingtin
- IL-1β
Interleukin-1 beta
- iNOS
Inducible nitric oxide synthase
- LPH
Lactase phloridzin hydrolase
- MeDi
Mediterranean diet
- mhGAP
Mental health gap action program
- MMPs
Metalloproteinases
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine
- MRI
Magnetic resonance imaging
- MRP2
Multidrug resistant protein type 2
- MS
Multiple sclerosis
- NDs
Neurodegenerative disorders
- NF-kB
Nuclear factor kappa B
- Nrf2
Nuclear factor erythroid 2
- OATP
Organic anion-transporting polypeptide
- OATs
Organic anion transporters
- P-gp
P-glycoprotein
- PD
Parkinson’s disease
- PolyQ
Polyglutamine
- RNOS
Reactive nitrogen and oxygen species
- ROS
Reactive oxygen species
- SIRT1
Silent mating type information regulation 2 homolog 1
- SLC
Solute carrier
- TBHP
Tert-butyl hydroperoxide
- TGF-β
Transforming growth factor beta
- TNF-α
Tumor necrosis factor
- TOR
Target of rapamycin
- WHO
World health organization
- αSyn
α-Synuclein
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Brettschneider J., Del Tredici K., Lee V.M., Trojanowski J.Q. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat. Rev. Neurosci. 2015;16(2):109–120. doi: 10.1038/nrn3887. [http://dx.doi.org/10.1038/nrn3887]. [PMID: 25588378]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soto C. Unfolding the role of protein misfolding in neuro- degenerative diseases. Nat. Rev. Neurosci. 2003;4(1):49–60. doi: 10.1038/nrn1007. [http://dx.doi.org/10.1038/nrn1007]. [PMID: 12511861]. [DOI] [PubMed] [Google Scholar]
- 3.Quist A., Doudevski I., Lin H., Azimova R., Ng D., Frangione B., Kagan B., Ghiso J., Lal R. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA. 2005;102(30):10427–10432. doi: 10.1073/pnas.0502066102. [http://dx.doi.org/10.1073/pnas. 0502066102]. [PMID: 16020533]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H., Braak E., Yilmazer D., de Vos R.A., Jansen E.N., Bohl J. Pattern of brain destruction in Parkinsons and Alzheimers diseases. J. Neural Transm. (Vienna) 1996;103(4):455–490. doi: 10.1007/BF01276421. [http://dx.doi.org/10.1007/BF01276421]. [PMID: 9617789]. [DOI] [PubMed] [Google Scholar]
- 5.OBrien R.J., Wong P.C. Amyloid precursor protein processing and Alzheimers disease. Annu. Rev. Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [http://dx.doi.org/10.1146/annurev-neuro-061010-113613]. [PMID: 21456963]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [http://dx. doi.org/10.1146/annurev.neuro.24.1.1121]. [PMID: 11520930]. [DOI] [PubMed] [Google Scholar]
- 7.Lees A.J. Unresolved issues relating to the shaking palsy on the celebration of James Parkinsons 250th birthday. Mov. Disord. 2007;22(Suppl. 17):S327–S334. doi: 10.1002/mds.21684. [http://dx.doi.org/10.1002/mds. 21684]. [PMID: 18175393]. [DOI] [PubMed] [Google Scholar]
- 8.Shults C.W. Lewy bodies. Proc. Natl. Acad. Sci. USA. 2006;103(6):1661–1668. doi: 10.1073/pnas.0509567103. [http://dx.doi.org/10.1073/pnas.0509567103]. [PMID: 16449387]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellani S., Sousa V.L., Ronzitti G., Valtorta F., Meldolesi J., Chieregatti E. The regulation of synaptic function by α-synuclein. Commun. Integr. Biol. 2010;3(2):106–109. doi: 10.4161/cib.3.2.10964. [http://dx.doi.org/10. 4161/cib.3.2.10964]. [PMID: 20585500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauvet B., Mbefo M.K., Fares M.B., Desobry C., Michael S., Ardah M.T., Tsika E., Coune P., Prudent M., Lion N., Eliezer D., Moore D.J., Schneider B., Aebischer P., El-Agnaf O.M., Masliah E., Lashuel H.A. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 2012;287(19):15345–15364. doi: 10.1074/jbc.M111.318949. [http://dx.doi.org/10.1074/jbc.M111.318949]. [PMID: 22315227]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George J.M. The synucleins. Genome Biol. 2002;3(1) doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein C., Lohmann-Hedrich K. Impact of recent genetic findings in Parkinsons disease. Curr. Opin. Neurol. 2007;20(4):453–464. doi: 10.1097/WCO.0b013e3281e6692b. [http://dx.doi.org/10.1097/WCO.0b013e3281e6692b]. [PMID: 17620882]. [DOI] [PubMed] [Google Scholar]
- 13.Maroteaux L., Campanelli J.T., Scheller R.H. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [PMID: 3411354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menezes R., Tenreiro S., Macedo D., Santos C.N., Outeiro T.F. From the baker to the bedside: yeast models of Parkinson's disease. Microb. Cell. 2015;2(8):18. doi: 10.15698/mic2015.08.219. [http://dx.doi.org/10.15698/mic2015. 08.219]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin J.B., Gusella J.F. Huntingtons disease. Pathogenesis and management. N. Engl. J. Med. 1986;315(20):1267–1276. doi: 10.1056/NEJM198611133152006. [http://dx.doi.org/10.1056/NEJM198611133152006]. [PMID: 2877396]. [DOI] [PubMed] [Google Scholar]
- 16.Walker F.O. Huntingtons disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [http://dx.doi.org/10.1016/S0140-6736(07)60111-1]. [PMID: 17240289]. [DOI] [PubMed] [Google Scholar]
- 17.Tydlacka S., Wang C-E., Wang X., Li S., Li X-J. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. J. Neurosci. 2008;28(49):13285–13295. doi: 10.1523/JNEUROSCI.4393-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.4393-08.2008]. [PMID: 19052220]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bugg C.W., Isas J.M., Fischer T., Patterson P.H., Langen R. Structural features and domain organization of huntingtin fibrils. J. Biol. Chem. 2012;287(38):31739–31746. doi: 10.1074/jbc.M112.353839. [http://dx.doi.org/10. 1074/jbc.M112.353839]. [PMID: 22801429]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., ORegan J.P., Deng H.X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [http://dx.doi.org/10.1038/362059a0]. [PMID: 8446170]. [DOI] [PubMed] [Google Scholar]
- 20.Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E., Baralle F., de Belleroche J., Mitchell J.D., Leigh P.N., Al-Chalabi A., Miller C.C., Nicholson G., Shaw C.E. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [http://dx.doi.org/10.1126/ science.1154584]. [PMID: 18309045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vance C., Rogelj B., Hortobágyi T., De Vos K.J., Nishimura A.L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., Ganesalingam J., Williams K.L., Tripathi V., Al-Saraj S., Al-Chalabi A., Leigh P.N., Blair I.P., Nicholson G., de Belleroche J., Gallo J.M., Miller C.C., Shaw C.E. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942. [http://dx. doi.org/10.1126/science.1165942]. [PMID: 19251628]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiatkowski T.J., Jr, Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., Valdmanis P., Rouleau G.A., Hosler B.A., Cortelli P., de Jong P.J., Yoshinaga Y., Haines J.L., Pericak-Vance M.A., Yan J., Ticozzi N., Siddique T., McKenna-Yasek D., Sapp P.C., Horvitz H.R., Landers J.E., Brown R.H., Jr Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [http://dx.doi.org/10.1126/science.1166066]. [PMID: 19251627]. [DOI] [PubMed] [Google Scholar]
- 23.Prince M., Wilmo A., Guerchet M., Ali G-C., Wu Y-T., Prina M. International, A. S. D. World Alzheimer Report 2015: The Global Impact of Dementia, An Analysis of Prevalence. London: Incidence, Cost and Trends; 2015. [Google Scholar]
- 24.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: a systematic review and metaanalysis. . Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Alzheimer’sAssociation . Alzheimer’s Disease Facts and Figures. Chicago: 2015. [Google Scholar]
- 26.Takizawa C., Thompson P.L., van Walsem A., Faure C., Maier W.C. Epidemiological and economic burden of Alzheimers disease: a systematic literature review of data across Europe and the United States of America. J. Alzheimers Dis. 2015;43(4):1271–1284. doi: 10.3233/JAD-141134. [PMID: 25159675]. [DOI] [PubMed] [Google Scholar]
- 27.Di Carlo A., Baldereschi M., Amaducci L., Lepore V., Bracco L., Maggi S., Bonaiuto S., Perissinotto E., Scarlato G., Farchi G., Inzitari D., Group I.W. Incidence of dementia, Alzheimers disease, and vascular dementia in Italy. The ILSA Study. J. Am. Geriatr. Soc. 2002;50(1):41–48. doi: 10.1046/j.1532-5415.2002.50006.x. [http://dx.doi.org/10.1046/j. 1532-5415.2002.50006.x]. [PMID: 12028245]. [DOI] [PubMed] [Google Scholar]
- 28.Bermejo-Pareja F., Benito-León J., Vega S., Medrano M.J., Román G.C. Incidence and subtypes of dementia in three elderly populations of central Spain. J. Neurol. Sci. 2008;264(1-2):63–72. doi: 10.1016/j.jns.2007.07.021. [http://dx.doi.org/10.1016/j.jns.2007.07.021]. [PMID: 17727890]. [DOI] [PubMed] [Google Scholar]
- 29.Hirtz D., Thurman D.J., Gwinn-Hardy K., Mohamed M., Chaudhuri A.R., Zalutsky R. How common are the common neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [http://dx. doi.org/10.1212/01.wnl.0000252807.38124.a3]. [PMID: 17261678]. [DOI] [PubMed] [Google Scholar]
- 30.Lo R.Y., Tanner C.M. Handbook of Parkinson’s Disease. 5th ed. CRC Press Taylor & Francis Group; 2013. Epidemiology. [Google Scholar]
- 31.Pringsheim T., Wiltshire K., Day L., Dykeman J., Steeves T., Jette N. The incidence and prevalence of Huntingtons disease: a systematic review and meta-analysis. Mov. Disord. 2012;27(9):1083–1091. doi: 10.1002/mds.25075. [http://dx.doi.org/10.1002/mds.25075]. [PMID: 22692795]. [DOI] [PubMed] [Google Scholar]
- 32.Worms P.M. The epidemiology of motor neuron diseases: a review of recent studies. J. Neurol. Sci. 2001;191(1-2):3–9. doi: 10.1016/s0022-510x(01)00630-x. [http://dx. doi.org/10.1016/S0022-510X(01)00630-X]. [PMID: 11676986]. [DOI] [PubMed] [Google Scholar]
- 33.OECD . Addressing Dementia: The OECD Response. Paris: OECD Publishing; 2015. The growing human and financial cost of dementia: The case for policy action. In: OECD. [Google Scholar]
- 34.WHO-WorldHealthOrganization Dementia Fact sheet N°362. 2015 [Google Scholar]
- 35.Niccoli T., Partridge L. Ageing as a risk factor for disease. Curr. Biol. 2012;22(17):R741–R752. doi: 10.1016/j.cub.2012.07.024. [http://dx.doi.org/10.1016/j.cub. 2012.07.024]. [PMID: 22975005]. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.Y., Lai C.H. Nationwide population-based epidemiologic study of Huntingtons Disease in Taiwan. Neuroepidemiology. 2010;35(4):250–254. doi: 10.1159/000319462. [http://dx.doi.org/10.1159/000319462]. [PMID: 20881427]. [DOI] [PubMed] [Google Scholar]
- 37.Colucci L., Bosco M., Fasanaro A.M., Gaeta G.L., Ricci G., Amenta F. Alzheimers disease costs: what we know and what we should take into account. J. Alzheimers Dis. 2014;42(4):1311–1324. doi: 10.3233/JAD-131556. [PMID: 25024334]. [DOI] [PubMed] [Google Scholar]
- 38.Caravau H., Martín I. Direct costs of dementia in nursing homes. Front. Aging Neurosci. 2015;7:146. doi: 10.3389/fnagi.2015.00146. [http://dx.doi.org/10.3389/ fnagi.2015.00146]. [PMID: 26283959]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonin-Guillaume S., Zekry D., Giacobini E., Gold G., Michel J.P. Presse Med. 2005;34(1):35–41. doi: 10.1016/s0755-4982(05)83882-5. [The economical impact of dementia]. [The economical impact of dementia]. [http://dx.doi.org/10.1016/S0755-4982(05)83882-5]. [PMID: 15685097]. [DOI] [PubMed] [Google Scholar]
- 40.Costa N., Ferlicoq L., Derumeaux-Burel H., Rapp T., Garnault V., Gillette-Guyonnet S., Andrieu S., Vellas B., Lamure M., Grand A., Molinier L. Comparison of informal care time and costs in different age-related dementias: a review. Biomed Res. Int., 2013 doi: 10.1155/2013/852368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Rest O., Berendsen A.A., Haveman-Nies A., de Groot L.C. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv. Nutr. 2015;6(2):154–168. doi: 10.3945/an.114.007617. [http://dx.doi.org/10.3945/an.114.007617]. [PMID: 25770254]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Del Rio D., Rodriguez-Mateos A., Spencer J.P., Tognolini M., Borges G., Crozier A. Dietary polyphenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18(14):1818–1892. doi: 10.1089/ars.2012.4581. [http://dx.doi.org/10.1089/ars.2012.4581]. [PMID: 22794138]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vauzour D., Rodriguez-Mateos A., Corona G., Oruna-Concha M.J., Spencer J.P. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2(11):1106–1131. doi: 10.3390/nu2111106. [http://dx.doi.org/10.3390/nu2111106]. [PMID: 22254000]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi: 10.3390/nu2121231. [http://dx.doi.org/10.3390/ nu2121231]. [PMID: 22254006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diederich M. Live longer, drink polyphenols. Cell Cycle. 2012;11(22):4109–4109. doi: 10.4161/cc.22603. [http://dx.doi.org/10.4161/cc.22603]. [PMID: 23099919]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Mateos A., Heiss C., Borges G., Crozier A. Berry polyphenols and cardiovascular health. J. Agric. Food Chem. 2014;62(18):3842–3851. doi: 10.1021/jf403757g. [http://dx.doi.org/10.1021/jf403757g]. [PMID: 24059851]. [DOI] [PubMed] [Google Scholar]
- 47.Zanotti I., DallAsta M., Mena P., Mele L., Bruni R., Ray S., Del Rio D. Atheroprotective effects of polyphenols: a focus on cell cholesterol metabolism. Food Funct. 2015;6(1):13–31. doi: 10.1039/c4fo00670d. [http://dx.doi.org/10.1039/C4FO00670D]. [PMID: 25367393]. [DOI] [PubMed] [Google Scholar]
- 48.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [http://dx.doi.org/10.4161/oxim.2.5.9498]. [PMID: 20716914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pimpão R.C. Exploring the bioavailability of polyphenols from berries and their potential activities in humans. Oeiras: PhD, Universidade Nova de Lisboa; 2014. [Google Scholar]
- 50.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [PMID: 15113710]. [DOI] [PubMed] [Google Scholar]
- 51.Bisson J-F., Nejdi A., Rozan P., Hidalgo S., Lalonde R., Messaoudi M. Effects of long-term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats. Br. J. Nutr. 2008;100(1):94–101. doi: 10.1017/S0007114507886375. [http://dx.doi.org/10.1017/S0007114507886375]. [PMID: 18179729]. [DOI] [PubMed] [Google Scholar]
- 52.Cassidy A., Hanley B., Lamuela-Raventos R.M. Isoflavones, lignans and stilbenes – origins, metabolism and potential importance to human health. J. Sci. Food Agric. 2000;80(7):1044–1062. [http://dx.doi.org/10.1002/(SICI)1097-0010(20000515)80:7<1044: AID-JSFA586>3.0.CO;2-N]. [Google Scholar]
- 53.Cheynier V., Comte G., Davies K.M., Lattanzio V., Martens S. Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [http://dx.doi.org/10.1016/j.plaphy.2013.05.009]. [PMID: 23774057]. [DOI] [PubMed] [Google Scholar]
- 54.Hollman P.C., Arts I.C. Flavonols, flavones and flavanols – nature, occurrence and dietary burden. J. Sci. Food Agric. 2000;80(7):1081–1093. [http://dx.doi.org/10.1002/(SICI)1097-0010 (20000515)80:7<1081:AID-JSFA566>3.0.CO;2-G]. [Google Scholar]
- 55.Santos-Buelga C., Scalbert A. Proanthocyanidins and tannin-like compounds – nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000;80(7):1094–1117. [http://dx.doi.org/10.1002/(SICI)1097-0010(20000515)80:7<1094: AID-JSFA569>3.0.CO;2-1]. [Google Scholar]
- 56.Gotsis E., Anagnostis P., Mariolis A., Vlachou A., Katsiki N., Karagiannis A. Health benefits of the Mediterranean Diet: an update of research over the last 5 years. Angiology. 2015;66(4):304–318. doi: 10.1177/0003319714532169. [http://dx.doi.org/10.1177/0003319714532169]. [PMID: 24778424]. [DOI] [PubMed] [Google Scholar]
- 57.Trichopoulou A., Kouris-Blazos A., Wahlqvist M.L., Gnardellis C., Lagiou P., Polychronopoulos E., Vassilakou T., Lipworth L., Trichopoulos D. Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–1460. doi: 10.1136/bmj.311.7018.1457. [http://dx.doi.org/10.1136/bmj.311. 7018.1457]. [PMID: 8520331]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keys A. Coronary heart disease in seven countries. Circulation. 1970;41(1):186–195. [PubMed] [Google Scholar]
- 59.Keys A., Menotti A., Karvonen M.J., Aravanis C., Blackburn H., Buzina R., Djordjevic B.S., Dontas A.S., Fidanza F., Keys M.H., Kromhout D., Nedeljkovic S., Punsar S., Seccareccia F., Toshima H. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986;124(6):903–915. doi: 10.1093/oxfordjournals.aje.a114480. [PMID: 3776973]. [DOI] [PubMed] [Google Scholar]
- 60.Alcalay R.N., Gu Y., Mejia-Santana H., Cote L., Marder K.S., Scarmeas N. The association between Mediterranean diet adherence and Parkinsons disease. Mov. Disord. 2012;27(6):771–774. doi: 10.1002/mds.24918. [http://dx.doi.org/10.1002/mds.24918]. [PMID: 22314772]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lourida I., Soni M., Thompson-Coon J., Purandare N., Lang I.A., Ukoumunne O.C., Llewellyn D.J. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24(4):479–489. doi: 10.1097/EDE.0b013e3182944410. [http://dx.doi.org/10.1097/ EDE.0b013e3182944410]. [PMID: 23680940]. [DOI] [PubMed] [Google Scholar]
- 62.Scarmeas N., Stern Y., Mayeux R., Manly J.J., Schupf N., Luchsinger J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009;66(2):216–225. doi: 10.1001/archneurol.2008.536. [http://dx.doi.org/10.1001/ archneurol.2008.536]. [PMID: 19204158]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhullar K. S., Rupasinghe H. P. V. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxidative Medicine and Cellular Longevity. 2013 doi: 10.1155/2013/891748. 891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45(4):287–306. doi: 10.1080/1040869059096. [http://dx.doi.org/10.1080/ 1040869059096]. [PMID: 16047496]. [DOI] [PubMed] [Google Scholar]
- 65.Brickman A.M., Khan U.A., Provenzano F.A., Yeung L-K., Suzuki W., Schroeter H., Wall M., Sloan R.P., Small S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014;17(12):1798–1803. doi: 10.1038/nn.3850. [http://dx.doi.org/10.1038/nn.3850]. [PMID: 25344629]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nurk E., Refsum H., Drevon C.A., Tell G.S., Nygaard H.A., Engedal K., Smith A.D. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J. Nutr. 2009;139(1):120–127. doi: 10.3945/jn.108.095182. [http://dx.doi.org/10.3945/jn.108.095182]. [PMID: 19056649]. [DOI] [PubMed] [Google Scholar]
- 67.Rabassa M., Cherubini A., Zamora-Ros R., Urpi-Sarda M., Bandinelli S., Ferrucci L., Andres-Lacueva C. Low levels of a urinary biomarker of dietary polyphenol are associated with substantial cognitive decline over a 3-year period in older adults: The invecchiare in chianti study. J. Am. Geriatr. Soc. 2015;63(5):938–946. doi: 10.1111/jgs.13379. [http://dx.doi.org/10.1111/jgs.13379]. [PMID: 25919574]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krikorian R., Shidler M.D., Nash T.A., Kalt W., Vinqvist-Tymchuk M.R., Shukitt-Hale B., Joseph J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010;58(7):3996–4000. doi: 10.1021/jf9029332. [http://dx.doi.org/10.1021/jf9029332]. [PMID: 20047325]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Devore E.E., Kang J.H., Breteler M.M., Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012;72(1):135–143. doi: 10.1002/ana.23594. [http://dx.doi.org/10.1002/ana. 23594]. [PMID: 22535616]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao X., Cassidy A., Schwarzschild M.A., Rimm E.B., Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology. 2012;78(15):1138–1145. doi: 10.1212/WNL.0b013e31824f7fc4. [http://dx.doi.org/10.1212/WNL.0b013e31824f7fc4]. [PMID: 22491871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81(1) Suppl.:230S–242S. doi: 10.1093/ajcn/81.1.230S. [PMID: 15640486]. [DOI] [PubMed] [Google Scholar]
- 72.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 2005;81(1) Suppl.:215S–217S. doi: 10.1093/ajcn/81.1.215S. [PMID: 15640483]. [DOI] [PubMed] [Google Scholar]
- 73.Parr A.J., Bolwell G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000;80(7):985–1012. [http://dx.doi.org/10.1002/(SICI)1097-0010 (20000515)80:7<985:AID-JSFA572>3.0.CO;2-7]. [Google Scholar]
- 74.Peterson J.J., Dwyer J.T., Jacques P.F., McCullough M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr. Rev. 2012;70(9):491–508. doi: 10.1111/j.1753-4887.2012.00508.x. [http://dx.doi.org/10.1111/j.1753-4887.2012. 00508.x]. [PMID: 22946850]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81(1) Suppl.:317S–325S. doi: 10.1093/ajcn/81.1.317S. [PMID: 15640497]. [DOI] [PubMed] [Google Scholar]
- 76.Alminger M., Aura A.M., Bohn T., Dufour C., El S.N., Gomes A., Karakaya S., Martínez-Cuesta M.C., McDougall G.J., Requena T., Santos C.N. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Comprehensive Reviews in Food Science and Food Safety. 2014;13(4):413–436. doi: 10.1111/1541-4337.12081. [http://dx.doi.org/10.1111/1541-4337.12081]. [DOI] [PubMed] [Google Scholar]
- 77.Farrell T.L., Poquet L., Dew T.P., Barber S., Williamson G. Predicting phenolic acid absorption in Caco-2 cells: a theoretical permeability model and mechanistic study. Drug Metab. Dispos. 2012;40(2):397–406. doi: 10.1124/dmd.111.041665. [http://dx.doi.org/10.1124/dmd.111.041665]. [PMID: 22096083]. [DOI] [PubMed] [Google Scholar]
- 78.Tian X-J., Yang X-W., Yang X., Wang K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009;367(1-2):58–64. doi: 10.1016/j.ijpharm.2008.09.023. [http://dx.doi.org/10.1016/ j.ijpharm.2008.09.023]. [DOI] [PubMed] [Google Scholar]
- 79.Day A.J. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468(2-3):166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 80.Gee J.M., DuPont M.S., Day A.J., Plumb G.W., Williamson G., Johnson I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000;130(11):2765–2771. doi: 10.1093/jn/130.11.2765. [PMID: 11053519]. [DOI] [PubMed] [Google Scholar]
- 81.Serra A., Macià A., Romero M-P., Valls J., Bladé C., Arola L., Motilva M-J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010;103(7):944–952. doi: 10.1017/S0007114509992741. [http://dx.doi.org/10.1017/ S0007114509992741]. [PMID: 20003617]. [DOI] [PubMed] [Google Scholar]
- 82.Borges G., Lean M.E., Roberts S.A., Crozier A. Bioavailability of dietary polyphenols: a study with ileostomists to discriminate between absorption in small and large intestine. Food Funct. 2013;4(5):754–762. doi: 10.1039/c3fo60024f. [http://dx.doi.org/10.1039/c3fo60024f]. [PMID: 23471276]. [DOI] [PubMed] [Google Scholar]
- 83.Williamson G., Clifford M.N. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br. J. Nutr. 2010;104(Suppl. 3):S48–S66. doi: 10.1017/S0007114510003946. [http://dx.doi.org/10.1017/ S0007114510003946]. [PMID: 20955650]. [DOI] [PubMed] [Google Scholar]
- 84.Nebbia C. Biotransformation enzymes as determinants of xenobiotic toxicity in domestic animals. Vet. J. 2001;161(3):238–252. doi: 10.1053/tvjl.2000.0561. [http://dx.doi.org/10.1053/tvjl.2000.0561]. [PMID: 11352482]. [DOI] [PubMed] [Google Scholar]
- 85.Döring B., Petzinger E. Phase 0 and phase III transport in various organs: combined concept of phases in xenobiotic transport and metabolism. Drug Metab. Rev. 2014;46(3):261–282. doi: 10.3109/03602532.2014.882353. [http://dx. doi.org/10.3109/03602532.2014.882353]. [PMID: 24483608]. [DOI] [PubMed] [Google Scholar]
- 86.Xu C., Li C.Y., Kong A-N. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005;28(3):249–268. doi: 10.1007/BF02977789. [http://dx.doi.org/10.1007/BF02977789]. [PMID: 15832810]. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida K., Maeda K., Sugiyama Y. Hepatic and intestinal drug transporters: prediction of pharmacokinetic effects caused by drug-drug interactions and genetic polymorphisms. Annu. Rev. Pharmacol. Toxicol. 2013;53:581–612. doi: 10.1146/annurev-pharmtox-011112-140309. [http://dx.doi.org/10.1146/ annurev-pharmtox-011112-140309]. [PMID: 23140240]. [DOI] [PubMed] [Google Scholar]
- 88.Bohn T., McDougall G.J., Alegría A., Alminger M., Arrigoni E., Aura A-M., Brito C., Cilla A., El S.N., Karakaya S., Martínez-Cuesta M.C., Santos C.N. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phyto- chemicals and their metabolitesa position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015;59(7):1307–1323. doi: 10.1002/mnfr.201400745. [http://dx.doi.org/10.1002/mnfr.201400745]. [PMID: 25988374]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zumdick S., Deters A., Hensel A. In vitro intestinal transport of oligomeric procyanidins (DP 2 to 4) across monolayers of Caco-2 cells. Fitoterapia. 2012;83(7):1210–1217. doi: 10.1016/j.fitote.2012.06.013. [http://dx.doi.org/10.1016/j.fitote.2012.06.013]. [PMID: 22776719]. [DOI] [PubMed] [Google Scholar]
- 90.DeSesso J.M., Jacobson C.F. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem. Toxicol. 2001;39(3):209–228. doi: 10.1016/s0278-6915(00)00136-8. [http://dx.doi.org/10.1016/S0278-6915(00)00136-8]. [PMID: 11278053]. [DOI] [PubMed] [Google Scholar]
- 91.Marzocchella L., Fantini M., Benvenuto M., Masuelli L., Tresoldi I., Modesti A., Bei R. Dietary flavonoids: molecular mechanisms of action as anti- inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011;5(3):200–220. doi: 10.2174/187221311797264937. [http://dx. doi.org/10.2174/187221311797264937]. [PMID: 21827399]. [DOI] [PubMed] [Google Scholar]
- 92.Sahni J.K., Doggui S., Ali J., Baboota S., Dao L., Ramassamy C. Neurotherapeutic applications of nanoparticles in Alzheimers disease. J. Control. Release. 2011;152(2):208–231. doi: 10.1016/j.jconrel.2010.11.033. [http://dx.doi.org/10.1016/j.jconrel.2010.11.033]. [PMID: 21134407]. [DOI] [PubMed] [Google Scholar]
- 93.Mdel R., Torres-Ramos M.A., Campos-Esparza Neuroprotection by natural polyphenols: molecular mechanisms. Cent. Nerv. Syst. Agents Med. Chem. 2010;10(4):269–277. doi: 10.2174/187152410793429728. [http://dx.doi.org/10.2174/187152410793429728]. [PMID: 20868360]. [DOI] [PubMed] [Google Scholar]
- 94.Aura A-M., Mattila I., Hyötyläinen T., Gopalacharyulu P., Cheynier V., Souquet J-M., Bes M., Le Bourvellec C., Guyot S., Orešič M. Characterization of microbial metabolism of Syrah grape products in an in vitro colon model using targeted and non-targeted analytical approaches. Eur. J. Nutr. 2013;52(2):833–846. doi: 10.1007/s00394-012-0391-8. [http://dx.doi.org/10.1007/s00394-012-0391-8]. [PMID: 22699306]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grün C.H., van Dorsten F.A., Jacobs D.M., Le Belleguic M., van Velzen E.J., Bingham M.O., Janssen H-G., van Duynhoven J.P. GC-MS methods for metabolic profiling of microbial fermentation products of dietary polyphenols in human and in vitro intervention studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;871(2):212–219. doi: 10.1016/j.jchromb.2008.04.039. [http://dx.doi.org/10.1016/j.jchromb. 2008.04.039]. [PMID: 18502705]. [DOI] [PubMed] [Google Scholar]
- 96.Jacobs D.M., Deltimple N., van Velzen E., van Dorsten F.A., Bingham M., Vaughan E.E., van Duynhoven J. (1)H NMR metabolite profiling of feces as a tool to assess the impact of nutrition on the human microbiome. NMR Biomed. 2008;21(6):615–626. doi: 10.1002/nbm.1233. [http://dx.doi.org/10.1002/nbm.1233]. [PMID: 18085514]. [DOI] [PubMed] [Google Scholar]
- 97.Pimpão R.C., Dew T., Figueira M.E., McDougall G.J., Stewart D., Ferreira R.B., Santos C.N., Williamson G. Urinary metabolite profiling identifies novel colonic metabolites and conjugates of phenolics in healthy volunteers. Mol. Nutr. Food Res. 2014;58(7):1414–1425. doi: 10.1002/mnfr.201300822. [http://dx.doi.org/10.1002/mnfr.201300822]. [PMID: 24740799]. [DOI] [PubMed] [Google Scholar]
- 98.Kimura Y., Ito H., Ohnishi R., Hatano T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010;48(1):429–435. doi: 10.1016/j.fct.2009.10.041. [http://dx.doi.org/10.1016/j.fct.2009.10.041]. [PMID: 19883715]. [DOI] [PubMed] [Google Scholar]
- 99.Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [http://dx.doi.org/10.1016/j.freeradbiomed.2004. 01.001]. [PMID: 15019969]. [DOI] [PubMed] [Google Scholar]
- 100.Griffioen G., Duhamel H., Van Damme N., Pellens K., Zabrocki P., Pannecouque C., van Leuven F., Winderickx J., Wera S. A yeast-based model of α-synucleinopathy identifies compounds with therapeutic potential. Biochim. Biophys. Acta. 2006;1762(3):312–318. doi: 10.1016/j.bbadis.2005.11.009. [http://dx.doi.org/10.1016/j.bbadis.2005. 11.009]. [PMID: 16413174]. [DOI] [PubMed] [Google Scholar]
- 101.Spencer J.P. Beyond antioxidants: the cellular and molecular interactions of flavonoids and how these underpin their actions on the brain. Proc. Nutr. Soc. 2010;69(2):244–260. doi: 10.1017/S0029665110000054. [http://dx. doi.org/10.1017/S0029665110000054]. [PMID: 20158941]. [DOI] [PubMed] [Google Scholar]
- 102.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., Hardy J., Town T., Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25(38):8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.1521-05.2005]. [PMID: 16177050]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J., Ho L., Zhao W., Ono K., Rosensweig C., Chen L., Humala N., Teplow D.B., Pasinetti G.M. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimers disease. J. Neurosci. 2008;28(25):6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.0364-08.2008]. [PMID: 18562609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mandel S., Youdim M.B. Catechin polyphenols: neuro- degeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004;37(3):304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [http://dx.doi.org/10.1016/j.freeradbiomed.2004.04.012]. [PMID: 15223064]. [DOI] [PubMed] [Google Scholar]
- 105.Skupień K., Oszmiański J., Kostrzewa-Nowak D., Tarasiuk J. In vitro antileukaemic activity of extracts from berry plant leaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006;236(2):282–291. doi: 10.1016/j.canlet.2005.05.018. [http://dx.doi.org/10.1016/j.canlet.2005. 05.018]. [PMID: 16039042]. [DOI] [PubMed] [Google Scholar]
- 106.Surh Y.J., Chun K.S., Cha H.H., Han S.S., Keum Y.S. [Google Scholar]; Park K.K., Lee S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001;480-481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [http://dx.doi.org/10.1016/S0027-5107(01)00183-X]. [PMID: 11506818]. [DOI] [PubMed] [Google Scholar]
- 107.Rendeiro C., Rhodes J.S., Spencer J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015;89:126–139. doi: 10.1016/j.neuint.2015.08.002. [http://dx.doi.org/10.1016/ j.neuint.2015.08.002]. [PMID: 26260546]. [DOI] [PubMed] [Google Scholar]
- 108.Verpoorte R., Choi Y.H., Kim H.K. Ethnopharmacology and systems biology: a perfect holistic match. J. Ethnopharmacol. 2005;100(1-2):53–56. doi: 10.1016/j.jep.2005.05.033. [http://dx.doi.org/10.1016/j.jep.2005.05. 033]. [PMID: 16026949]. [DOI] [PubMed] [Google Scholar]
- 109.DeFeudis F.V., Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr. Drug Targets. 2000;1(1):25–58. doi: 10.2174/1389450003349380. [http://dx.doi.org/10.2174/ 1389450003349380]. [PMID: 11475535]. [DOI] [PubMed] [Google Scholar]
- 110.Kleijnen J., Knipschild P. Ginkgo biloba. Lancet. 1992;340(8828):1136–1139. doi: 10.1016/0140-6736(92)93158-j. [http://dx.doi.org/10.1016/0140-6736(92) 93158-J]. [PMID: 1359218]. [DOI] [PubMed] [Google Scholar]
- 111.Li P., Qi L-W., Liu E.H., Zhou J-L., Wen X-D. Analysis of Chinese herbal medicines with holistic approaches and integrated evaluation models. Trends Analyt. Chem. 2008;27(1):66–77. [http://dx.doi.org/10.1016/j.trac.2007.11.005]. [Google Scholar]
- 112.Marcocci L., Packer L., Droy-Lefaix M.T., Sekaki A., Gardès-Albert M. Antioxidant action of Ginkgo biloba extract EGb 761. Methods Enzymol. 1994;234:462–475. doi: 10.1016/0076-6879(94)34117-6. [http://dx.doi.org/10.1016/ 0076-6879(94)34117-6]. [PMID: 7808320]. [DOI] [PubMed] [Google Scholar]
- 113.Maitra I., Marcocci L., Droy-Lefaix M.T., Packer L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem. Pharmacol. 1995;49(11):1649–1655. doi: 10.1016/0006-2952(95)00089-i. [http://dx.doi.org/10.1016/0006-2952(95)00089-I]. [PMID: 7786306]. [DOI] [PubMed] [Google Scholar]
- 114.Singh M., Arseneault M., Sanderson T., Murthy V., Ramassamy C. Challenges for research on polyphenols from foods in Alzheimers disease: bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008;56(13):4855–4873. doi: 10.1021/jf0735073. [http://dx.doi.org/10.1021/jf0735073]. [PMID: 18557624]. [DOI] [PubMed] [Google Scholar]
- 115.Choi H.R., Choi J.S., Han Y.N., Bae S.J., Chung H.Y. Peroxynitrite scavenging activity of herb extracts. Phytother. Res. 2002;16(4):364–367. doi: 10.1002/ptr.904. [http://dx.doi.org/10.1002/ptr.904]. [PMID: 12112294]. [DOI] [PubMed] [Google Scholar]
- 116.Bachurin S.O. Medicinal chemistry approaches for the treatment and prevention of Alzheimers disease. Med. Res. Rev. 2003;23(1):48–88. doi: 10.1002/med.10026. [http://dx.doi.org/10.1002/med.10026]. [PMID: 12424753]. [DOI] [PubMed] [Google Scholar]
- 117.Rainey-Smith S., Schroetke L-W., Bahia P., Fahmi A., Skilton R., Spencer J.P., Rice-Evans C., Rattray M., Williams R.J. Neuroprotective effects of hesperetin in mouse primary neurones are independent of CREB activation. Neurosci. Lett. 2008;438(1):29–33. doi: 10.1016/j.neulet.2008.04.056. [http://dx.doi.org/10.1016/j.neulet.2008.04.056]. [PMID: 18467030]. [DOI] [PubMed] [Google Scholar]
- 118.Williams P., Sorribas A., Howes M-J. Natural products as a source of Alzheimers drug leads. Nat. Prod. Rep. 2011;28(1):48–77. doi: 10.1039/c0np00027b. [http://dx.doi.org/10.1039/C0NP00027B]. [PMID: 21072430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okello E.J., Leylabi R., McDougall G.J. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct. 2012;3(6):651–661. doi: 10.1039/c2fo10174b. [http://dx.doi.org/10.1039/c2fo10174b]. [PMID: 22418730]. [DOI] [PubMed] [Google Scholar]
- 120.Mukherjee P.K., Kumar V., Mal M., Houghton P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14(4):289–300. doi: 10.1016/j.phymed.2007.02.002. [http://dx.doi.org/10.1016/j.phymed.2007.02.002]. [PMID: 17346955]. [DOI] [PubMed] [Google Scholar]
- 121.Gomes A., Fortalezas S., Pimpão R., Figueira I., Maroco J., Aguiar C., Ferreira R.B., Miguel C., Santos C.N. Valuing the Endangered Species Antirrhinum lopesianum: Neuroprotective Activities and Strategies for in vitro Plant Propagation. Antioxidants (Basel) 2013;2(4):273–292. doi: 10.3390/antiox2040273. [http://dx.doi.org/10.3390/antiox 2040273]. [PMID: 26784465]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gomes A., Pimpão R.C., Fortalezas S., Figueira I., Miguel C.l., Aguiar C., Salgueiro L., Cavaleiro C., Gonçalves M.J., Clemente A., Costa C., Martins-Loução M.A., Ferreira R.B., Santos C.N. Chemical characterization and bioactivity of phytochemicals from Iberian endemic Santolina semidentata and strategies for ex situ propagation. Ind. Crops Prod. 2015;74:505–513. [http://dx.doi.org/10.1016/j.indcrop.2015.04.037]. [Google Scholar]
- 123.Kubínová R., Švajdlenka E., Jankovská D. Anticholinesterase, antioxidant activity and phytochemical investigation into aqueous extracts from five species of Agrimonia genus. Nat. Prod. Res. 2015;30(10):1174–1177. doi: 10.1080/14786419.2015.1043552. [PMID: 26235662]. [DOI] [PubMed] [Google Scholar]
- 124.Tavares L., Fortalezas S., Tyagi M., Barata D., Serra A.T., Duarte C.M., Duarte R.O., Feliciano R.P., Bronze M.R., Espírito-Santo M.D., Ferreira R.B., Santos C.N. Bioactive compounds from endemic plants of Southwest Portugal: inhibition of acetylcholinesterase and radical scavenging activities. Pharm. Biol. 2012;50(2):239–246. doi: 10.3109/13880209.2011.596209. [http://dx.doi.org/10.3109/13880209. 2011.596209]. [PMID: 22074503]. [DOI] [PubMed] [Google Scholar]
- 125.Tavares L., McDougall G.J., Fortalezas S., Stewart D., Ferreira R.B., Santos C.N. The neuroprotective potential of phenolic-enriched fractions from four Juniperus species found in Portugal. Food Chem. 2012;135(2):562–570. doi: 10.1016/j.foodchem.2012.05.023. [http://dx.doi.org/10.1016/ j.foodchem.2012.05.023]. [PMID: 22868129]. [DOI] [PubMed] [Google Scholar]
- 126.Ado M.A., Abas F., Ismail I.S., Ghazali H.M., Shaari K. Chemical profile and antiacetylcholinesterase, antityrosinase, antioxidant and α-glucosidase inhibitory activity of Cynometra cauliflora L. leaves. J. Sci. Food Agric. 2015;95(3):635–642. doi: 10.1002/jsfa.6832. [http://dx.doi.org/10.1002/jsfa.6832]. [PMID: 25048579]. [DOI] [PubMed] [Google Scholar]
- 127.Herczenik E., Gebbink M.F. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 2008;22(7):2115–2133. doi: 10.1096/fj.07-099671. [http://dx.doi.org/10.1096/fj.07-099671]. [PMID: 18303094]. [DOI] [PubMed] [Google Scholar]
- 128.Woulfe J.M. Abnormalities of the nucleus and nuclear inclusions in neurodegenerative disease: a work in progress. Neuropathol. Appl. Neurobiol. 2007;33(1):2–42. doi: 10.1111/j.1365-2990.2006.00819.x. [http://dx.doi.org/10.1111/ j.1365-2990.2006.00819.x]. [PMID: 17239006]. [DOI] [PubMed] [Google Scholar]
- 129.Xie H., Wang J-R., Yau L-F., Liu Y., Liu L., Han Q-B., Zhao Z., Jiang Z-H. Catechins and procyanidins of Ginkgo biloba show potent activities towards the inhibition of β-amyloid peptide aggregation and destabilization of preformed fibrils. Molecules. 2014;19(4):5119–5134. doi: 10.3390/molecules19045119. [http://dx.doi.org/10.3390/molecules 19045119]. [PMID: 24759072]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamakawa M.Y., Uchino K., Watanabe Y., Adachi T., Nakanishi M., Ichino H., Hongo K., Mizobata T., Kobayashi S., Nakashima K., Kawata Y. Anthocyanin suppresses the toxicity of Abeta deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimers disease. Nutr. Neurosci. 2015 doi: 10.1179/1476830515Y.0000000042. [PMID: 26304685]. [DOI] [PubMed] [Google Scholar]
- 131.Ono K., Condron M.M., Ho L., Wang J., Zhao W., Pasinetti G.M., Teplow D.B. Effects of grape seed-derived polyphenols on amyloid β-protein self-assembly and cytotoxicity. J. Biol. Chem. 2008;283(47):32176–32187. doi: 10.1074/jbc.M806154200. [http://dx.doi.org/10.1074/jbc. M806154200]. [PMID: 18815129]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ehrnhoefer D.E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15(6):558–566. doi: 10.1038/nsmb.1437. [http://dx.doi.org/10.1038/nsmb.1437]. [PMID: 18511942]. [DOI] [PubMed] [Google Scholar]
- 133.Palhano F.L., Lee J., Grimster N.P., Kelly J.W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013;135(20):7503–7510. doi: 10.1021/ja3115696. [http://dx.doi.org/10.1021/ja3115696]. [PMID: 23611538]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeToma A.S., Choi J-S., Braymer J.J., Lim M.H. Myricetin: a naturally occurring regulator of metal-induced amyloid-β aggregation and neurotoxicity. ChemBioChem. 2011;12(8):1198–1201. doi: 10.1002/cbic.201000790. [http://dx.doi.org/10.1002/cbic.201000790]. [PMID: 21538759]. [DOI] [PubMed] [Google Scholar]
- 135.Pasinetti G.M., Wang J., Ho L., Zhao W., Dubner L. Roles of resveratrol and other grape-derived polyphenols in Alzheimers disease prevention and treatment. Biochim. Biophys. Acta. 2015;1852(6):1202–1208. doi: 10.1016/j.bbadis.2014.10.006. [http://dx.doi.org/10.1016/j.bbadis.2014.10. 006]. [PMID: 25315300]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pasinetti G.M., Wang J., Marambaud P., Ferruzzi M., Gregor P., Knable L.A., Ho L. Neuroprotective and metabolic effects of resveratrol: therapeutic implications for Huntingtons disease and other neurodegenerative disorders. Exp. Neurol. 2011;232(1):1–6. doi: 10.1016/j.expneurol.2011.08.014. [http://dx.doi.org/10.1016/j.expneurol.2011.08.014]. [PMID: 21907197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Richard T., Pawlus A.D., Iglésias M-L., Pedrot E., Waffo-Teguo P., Mérillon J-M., Monti J-P. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 2011;1215(1):103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [http://dx.doi.org/10.1111/j.1749-6632.2010.05865.x]. [PMID: 21261647]. [DOI] [PubMed] [Google Scholar]
- 138.Richard T., Poupard P., Nassra M., Papastamoulis Y., Iglésias M-L., Krisa S., Waffo-Teguo P., Mérillon J-M., Monti J-P. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg. Med. Chem. 2011;19(10):3152–3155. doi: 10.1016/j.bmc.2011.04.001. [http://dx.doi.org/10.1016/ j.bmc.2011.04.001]. [PMID: 21524590]. [DOI] [PubMed] [Google Scholar]
- 139.Rivière C., Richard T., Quentin L., Krisa S., Mérillon J-M., Monti J-P. Inhibitory activity of stilbenes on Alzheimers β-amyloid fibrils in vitro. Bioorg. Med. Chem. 2007;15(2):1160–1167. doi: 10.1016/j.bmc.2006.09.069. [http://dx.doi.org/10.1016/j.bmc.2006.09.069]. [PMID: 17049256]. [DOI] [PubMed] [Google Scholar]
- 140.Sylla T., Pouységu L., Da Costa G., Deffieux D., Monti J-P., Quideau S. Gallotannins and tannic acid: first chemical syntheses and in vitro inhibitory activity on alzheimers amyloid β-peptide aggregation. Angew. Chem. Int. Ed. Engl. 2015;54(28):8217–8221. doi: 10.1002/anie.201411606. [http://dx.doi.org/10.1002/anie.201411606]. [PMID: 26013280]. [DOI] [PubMed] [Google Scholar]
- 141.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., Cole G.M. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [http://dx.doi.org/10. 1074/jbc.M404751200]. [PMID: 15590663]. [DOI] [PubMed] [Google Scholar]
- 142.Zhu M., Han S., Fink A.L. Oxidized quercetin inhibits α-synuclein fibrillization. Biochim. Biophys. Acta. 2013;1830(4):2872–2881. doi: 10.1016/j.bbagen.2012.12.027. [http://dx.doi.org/10.1016/j.bbagen.2012.12.027]. [PMID: 23295967]. [DOI] [PubMed] [Google Scholar]
- 143.Lorenzen N., Nielsen S.B., Yoshimura Y., Vad B.S., Andersen C.B., Betzer C., Kaspersen J.D., Christiansen G., Pedersen J.S., Jensen P.H., Mulder F.A., Otzen D.E. How epigallocatechin gallate can inhibit α-synuclein oligomer toxicity in vitro. J. Biol. Chem. 2014;289(31):21299–21310. doi: 10.1074/jbc.M114.554667. [http://dx.doi.org/10.1074/ jbc.M114.554667]. [PMID: 24907278]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Macedo D., Tavares L., McDougall G.J., Vicente Miranda H., Stewart D., Ferreira R.B., Tenreiro S., Outeiro T.F., Santos C.N. Polyphenols protect from α-synuclein toxicity by reducing oxidative stress and promoting autophagy. Hum. Mol. Genet. 2015;24(6):1717–1732. doi: 10.1093/hmg/ddu585. [http://dx.doi.org/10.1093/hmg/ddu585]. [PMID: 25432533]. [DOI] [PubMed] [Google Scholar]
- 145.Pandey A.K., Verma S., Bhattacharya P., Paul S., Mishra A., Patnaik R. An in-silico strategy to explore neuroprotection by quercetin in cerebral ischemia: a novel hypothesis based on inhibition of matrix metalloproteinase (MMPs) and acid sensing ion channel 1a (ASIC1a). Med. Hypotheses. 2012;79(1):76–81. doi: 10.1016/j.mehy.2012.04.005. [http://dx.doi.org/10.1016/j.mehy.2012.04.005]. [PMID: 22543073]. [DOI] [PubMed] [Google Scholar]
- 146.Scheepens A., Tan K., Paxton J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010;5(1):75–87. doi: 10.1007/s12263-009-0148-z. [http://dx.doi.org/10.1007/s12263-009-0148-z]. [PMID: 19841960]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Longpré F., Garneau P., Christen Y., Ramassamy C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free Radic. Biol. Med. 2006;41(12):1781–1794. doi: 10.1016/j.freeradbiomed.2006.08.015. [http://dx.doi.org/10.1016/j.freeradbiomed.2006.08.015]. [PMID: 17157181]. [DOI] [PubMed] [Google Scholar]
- 148.Yao Z-x., Drieu K., Papadopoulos V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from β-amyloid-induced cell death by inhibiting the formation of β-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001;889(1-2):181–190. doi: 10.1016/s0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]
- 149.Bastianetto S., Ramassamy C., Doré S., Christen Y., Poirier J., Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur. J. Neurosci. 2000;12(6):1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [http://dx.doi.org/10.1046/j. 1460-9568.2000.00069.x]. [PMID: 10886329]. [DOI] [PubMed] [Google Scholar]
- 150.Qin X.Y., Cheng Y., Yu L.C. Potential protection of green tea polyphenols against intracellular amyloid beta-induced toxicity on primary cultured prefrontal cortical neurons of rats. Neurosci. Lett. 2012;513(2):170–173. doi: 10.1016/j.neulet.2012.02.029. [http://dx.doi.org/10.1016/j.neulet.2012.02. 029]. [PMID: 22381400]. [DOI] [PubMed] [Google Scholar]
- 151.Badshah H., Kim T.H., Kim M.O. Protective effects of anthocyanins against amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015;80:51–59. doi: 10.1016/j.neuint.2014.10.009. [http://dx.doi.org/10. 1016/j.neuint.2014.10.009]. [PMID: 25451757]. [DOI] [PubMed] [Google Scholar]
- 152.Rhein V., Giese M., Baysang G., Meier F., Rao S., Schulz K.L., Hamburger M., Eckert A. Ginkgo biloba extract ameliorates oxidative phosphorylation performance and rescues abeta-induced failure. PLoS One. 2010;5(8):e12359. doi: 10.1371/journal.pone.0012359. [http://dx.doi.org/10.1371/ journal.pone.0012359]. [PMID: 20808761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu Y., Bickford P.C., Sanberg P., Giunta B., Tan J. Blueberry opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activation protein kinase. Rejuvenation Res. 2008;11(5):891–901. doi: 10.1089/rej.2008.0757. [http://dx.doi.org/10.1089/ rej.2008.0757]. [PMID: 18789000]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lau F.C., Bielinski D.F., Joseph J.A. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J. Neurosci. Res. 2007;85(5):1010–1017. doi: 10.1002/jnr.21205. [http://dx.doi.org/10.1002/jnr.21205]. [PMID: 17265471]. [DOI] [PubMed] [Google Scholar]
- 155.Poulose S.M., Fisher D.R., Larson J., Bielinski D.F., Rimando A.M., Carey A.N., Schauss A.G., Shukitt-Hale B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012;60(4):1084–1093. doi: 10.1021/jf203989k. [http://dx.doi.org/10.1021/jf203989k]. [PMID: 22224493]. [DOI] [PubMed] [Google Scholar]
- 156.Tavares L., Figueira I., Macedo D., McDougall G.J., Leitão M.C., Vieira H.L., Stewart D., Alves P.M., Ferreira R.B., Santos C.N. Neuroprotective effect of blackberry (Rubus sp.) polyphenols is potentiated after simulated gastrointestinal digestion. Food Chem. 2012;131(4):1443–1452. [http://dx.doi.org/10.1016/ j.foodchem.2011.10.025]. [Google Scholar]
- 157.Tavares L., Figueira I., McDougall G.J., Vieira H.L., Stewart D., Alves P.M., Ferreira R.B., Santos C.N. Neuroprotective effects of digested polyphenols from wild blackberry species. Eur. J. Nutr. 2013;52(1):225–236. doi: 10.1007/s00394-012-0307-7. [http://dx.doi.org/10.1007/s00394-012-0307-7]. [PMID: 22314351]. [DOI] [PubMed] [Google Scholar]
- 158.Suabjakyong P., Saiki R., Van Griensven L.J., Higashi K., Nishimura K., Igarashi K., Toida T. Polyphenol extract from Phellinus igniarius protects against acrolein toxicity in vitro and provides protection in a mouse stroke model. PLoS One. 2015;10(3):e0122733. doi: 10.1371/journal.pone.0122733. [http://dx.doi.org/10.1371/journal.pone.0122733]. [PMID: 25811373]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Amit T., Avramovich-Tirosh Y., Youdim M.B., Mandel S. Targeting multiple Alzheimers disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J. 2008;22(5):1296–1305. doi: 10.1096/fj.07-8627rev. [http://dx.doi.org/10.1096/fj.07-8627rev]. [PMID: 18048580]. [DOI] [PubMed] [Google Scholar]
- 160.Mandel S.A., Amit T., Weinreb O., Reznichenko L., Youdim M.B. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci. Ther. 2008;14(4):352–365. doi: 10.1111/j.1755-5949.2008.00060.x. [http://dx.doi.org/10.1111/j.1755-5949.2008.00060.x]. [PMID: 19040558]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee J.W., Lee Y.K., Ban J.O., Ha T.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Green tea (-)-epigallocatechin-3-gallate inhibits β-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009;139(10):1987–1993. doi: 10.3945/jn.109.109785. [http://dx.doi.org/10.3945/jn.109.109785]. [PMID: 19656855]. [DOI] [PubMed] [Google Scholar]
- 162.Choi Y-T., Jung C-H., Lee S-R., Bae J-H., Baek W-K., Suh M-H., Park J., Park C-W., Suh S-I. The green tea polyphenol (-)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70(5):603–614. doi: 10.1016/s0024-3205(01)01438-2. [http://dx.doi.org/10.1016/S0024-3205(01)01438-2]. [PMID: 11811904]. [DOI] [PubMed] [Google Scholar]
- 163.Jang J.H., Surh Y.J. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic. Biol. Med. 2003;34(8):1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [http://dx.doi.org/10.1016/S0891-5849(03)00062-5]. [PMID: 12684095]. [DOI] [PubMed] [Google Scholar]
- 164.Kim D., Nguyen M.D., Dobbin M.M., Fischer A., Sananbenesi F., Rodgers J.T., Delalle I., Baur J.A., Sui G., Armour S.M., Puigserver P., Sinclair D.A., Tsai L-H. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimers disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–3179. doi: 10.1038/sj.emboj.7601758. [http://dx.doi.org/10.1038/sj.emboj.7601758]. [PMID: 17581637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang B.L. Resveratrol is neuroprotective because it is not a direct activator of Sirt1-A hypothesis. Brain Res. Bull. 2010;81(4-5):359–361. doi: 10.1016/j.brainresbull.2009.12.007. [http://dx.doi.org/10.1016/j.brainresbull.2009.12.007]. [PMID: 20026255]. [DOI] [PubMed] [Google Scholar]
- 166.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., Scherer B., Sinclair D.A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [http://dx.doi.org/10.1038/nature01960]. [PMID: 12939617]. [DOI] [PubMed] [Google Scholar]
- 167.Aggarwal B., Surh Y-J., Shishodia S., Menon V., Sudheer A. In: The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, Springer US;: 2007. Antioxidant and anti-inflammatory properties of curcumin. pp. 105–125. [Google Scholar]
- 168.Kim D.S., Park S-Y., Kim J.K. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(142) insult. Neurosci. Lett. 2001;303(1):57–61. doi: 10.1016/s0304-3940(01)01677-9. [http://dx. doi.org/10.1016/S0304-3940(01)01677-9]. [PMID: 11297823]. [DOI] [PubMed] [Google Scholar]
- 169.Chen J., Tang X.Q., Zhi J.L., Cui Y., Yu H.M., Tang E.H., Sun S.N., Feng J.Q., Chen P.X. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11(6):943–953. doi: 10.1007/s10495-006-6715-5. [http://dx.doi.org/10.1007/s10495-006-6715-5]. [PMID: 16547587]. [DOI] [PubMed] [Google Scholar]
- 170.Aggarwal B., Surh Y-J., Shishodia S., Rao C. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, Springer. Vol. 595. US: Springer; 2007. Regulation of Cox and Lox by Curcumin. pp. 213–226. [Google Scholar]
- 171.Aggarwal B., Surh Y-J., Shishodia S., Singh T., Chaturvedi M. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, Springer. Vol. 595. US: Springer; 2007. Modulation of Transcription Factors by Curcumin. pp. 127–148. [DOI] [PubMed] [Google Scholar]
- 172.Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012;143(2):383–396. doi: 10.1016/j.jep.2012.07.005. [http://dx. doi.org/10.1016/j.jep.2012.07.005]. [PMID: 22820241]. [DOI] [PubMed] [Google Scholar]
- 173.Mercer L.D., Kelly B.L., Horne M.K., Beart P.M. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem. Pharmacol. 2005;69(2):339–345. doi: 10.1016/j.bcp.2004.09.018. [http://dx.doi.org/10. 1016/j.bcp.2004.09.018]. [PMID: 15627486]. [DOI] [PubMed] [Google Scholar]
- 174.Ansari M.A., Abdul H.M., Joshi G., Opii W.O., Butterfield D.A. Protective effect of quercetin in primary neurons against Abeta(142): relevance to Alzheimers disease. J. Nutr. Biochem. 2009;20(4):269–275. doi: 10.1016/j.jnutbio.2008.03.002. [http://dx.doi.org/10.1016/j.jnutbio.2008.03. 002]. [PMID: 18602817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mikami Y., Yamazawa T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015;139:69–74. doi: 10.1016/j.lfs.2015.08.005. [http://dx.doi.org/10.1016/j.lfs.2015.08.005]. [PMID: 26285175]. [DOI] [PubMed] [Google Scholar]
- 176.Socodato R., Portugal C.C., Canedo T., Domith I., Oliveira N.A., Paes-de-Carvalho R., Relvas J.B., Cossenza M. c-Src deactivation by the polyphenol 3-O-caffeoylquinic acid abrogates reactive oxygen species-mediated glutamate release from microglia and neuronal excitotoxicity. Free Radic. Biol. Med. 2015;79:45–55. doi: 10.1016/j.freeradbiomed.2014.11.019. [http://dx.doi.org/10.1016/j.freeradbiomed.2014.11.019]. [PMID: 25486178]. [DOI] [PubMed] [Google Scholar]
- 177.Tamilselvam K., Braidy N., Manivasagam T., Essa M. M., Prasad N. R., Karthikeyan S., Thenmozhi A. J., Selvaraju S., Guillemin G. J. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for parkinson's disease. Oxidative Med. Cell. Longevity. 2013;2013 doi: 10.1155/2013/102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stackman R.W., Eckenstein F., Frei B., Kulhanek D., Nowlin J., Quinn J.F. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimers disease by chronic Ginkgo biloba treatment. Exp. Neurol. 2003;184(1):510–520. doi: 10.1016/s0014-4886(03)00399-6. [http://dx.doi.org/10.1016/S0014-4886(03)00399-6]. [PMID: 14637120]. [DOI] [PubMed] [Google Scholar]
- 179.Augustin S., Rimbach G., Augustin K., Schliebs R., Wolffram S., Cermak R. Effect of a short- and long-term treatment with Ginkgo biloba extract on amyloid precursor protein levels in a transgenic mouse model relevant to Alzheimers disease. Arch. Biochem. Biophys. 2009;481(2):177–182. doi: 10.1016/j.abb.2008.10.032. [http://dx.doi.org/10.1016/j.abb.2008.10.032]. [PMID: 18996078]. [DOI] [PubMed] [Google Scholar]
- 180.Ramassamy C., Cloetre F., Christen Y., Costentin J. In vivo Ginkgo biloba extract (EGB 761) protects against neurotoxic effects induced by MPTP: Investigations into its mechanism(s) of action. In: Christen Y., Costentin J., Lacour M., editors. Advances in Ginkgo biloba Extract Research. 1992. pp. 27–36. [Google Scholar]
- 181.Rojas P., Montes P., Rojas C., Serrano-García N., Rojas-Castañeda J.C. Effect of a phytopharmaceutical medicine, Ginko biloba extract 761, in an animal model of Parkinson's disease: Therapeutic perspectives. Nutrition. 2012;28(11-12):1081–1088. doi: 10.1016/j.nut.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 182.Ferrante R.J., Klein A.M., Dedeoglu A., Beal M.F. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J. Mol. Neurosci. 2001;17(1):89–96. doi: 10.1385/jmn:17:1:89. [http://dx.doi.org/10.1385/JMN:17:1:89]. [PMID: 11665866]. [DOI] [PubMed] [Google Scholar]
- 183.Christen Y. Ginkgo biloba and neurodegenerative disorders. Front. Biosci. 2004;9:3091–3104. doi: 10.2741/1462. [http://dx.doi.org/10.2741/1462]. [PMID: 15353340]. [DOI] [PubMed] [Google Scholar]
- 184.Assunção M., Santos-Marques M.J., Carvalho F., Lukoyanov N.V., Andrade J.P. Chronic green tea consumption prevents age-related changes in rat hippocampal formation. Neurobiol. Aging. 2011;32(4):707–717. doi: 10.1016/j.neurobiolaging.2009.03.016. [http://dx.doi.org/10.1016/j.neurobiolaging. 2009.03.016]. [PMID: 19411127]. [DOI] [PubMed] [Google Scholar]
- 185.Assunção M., Santos-Marques M.J., Carvalho F., Andrade J.P. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free Radic. Biol. Med. 2010;48(6):831–838. doi: 10.1016/j.freeradbiomed.2010.01.003. [http://dx.doi.org/10.1016/j. freeradbiomed.2010.01.003]. [PMID: 20064606]. [DOI] [PubMed] [Google Scholar]
- 186.Mandel S.A., Weinreb O., Amit T., Youdim M.B. The importance of the multiple target action of green tea polyphenols for neuroprotection. Front. Biosci. 2012;4:581–598. doi: 10.2741/S286. [http://dx. doi.org/10.2741/s286]. [DOI] [PubMed] [Google Scholar]
- 187.Ogaly H.A., Khalaf A.A., Ibrahim M.A., Galal M.K., Abd-Elsalam R.M. Influence of green tea extract on oxidative damage and apoptosis induced by deltamethrin in rat brain. Neurotoxicol. Teratol. 2015;50:23–31. doi: 10.1016/j.ntt.2015.05.005. [http://dx.doi.org/10.1016/j.ntt.2015.05. 005]. [PMID: 26013673]. [DOI] [PubMed] [Google Scholar]
- 188.Chen M., Wang T., Yue F., Li X., Wang P., Li Y., Chan P., Yu S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience. 2015;286:383–392. doi: 10.1016/j.neuroscience.2014.12.003. [http://dx.doi.org/10.1016/j.neuroscience.2014.12.003]. [PMID: 25498223]. [DOI] [PubMed] [Google Scholar]
- 189.Wang J., Ferruzzi M.G., Ho L., Blount J., Janle E.M., Gong B., Pan Y., Gowda G.A., Raftery D., Arrieta-Cruz I., Sharma V., Cooper B., Lobo J., Simon J.E., Zhang C., Cheng A., Qian X., Ono K., Teplow D.B., Pavlides C., Dixon R.A., Pasinetti G.M. Brain-targeted proanthocyanidin metabolites for Alzheimers disease treatment. J. Neurosci. 2012;32(15):5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [http://dx.doi.org/10.1523/JNEUROSCI.6437-11.2012]. [PMID: 22496560]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Y.J., Thomas P., Zhong J.H., Bi F.F., Kosaraju S., Pollard A., Fenech M., Zhou X.F. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimers disease mouse. Neurotox. Res. 2009;15(1):3–14. doi: 10.1007/s12640-009-9000-x. [http://dx.doi.org/10.1007/s12640-009-9000-x]. [PMID: 19384583]. [DOI] [PubMed] [Google Scholar]
- 191.Knaup B., Kahle K., Erk T., Valotis A., Scheppach W., Schreier P., Richling E. Human intestinal hydrolysis of phenol glycosides - a study with quercetin and p-nitrophenol glycosides using ileostomy fluid. Mol. Nutr. Food Res. 2007;51(11):1423–1429. doi: 10.1002/mnfr.200700036. [http://dx.doi.org/10.1002/mnfr.200700036]. [PMID: 17966139]. [DOI] [PubMed] [Google Scholar]
- 192.Wang J., Santa-Maria I., Ho L., Ksiezak-Reding H., Ono K., Teplow D.B., Pasinetti G.M. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimers disease. J. Alzheimers Dis. 2010;22(2):653–661. doi: 10.3233/JAD-2010-101074. [PMID: 20858961]. [DOI] [PubMed] [Google Scholar]
- 193.Wang J., Pfleger C.M., Friedman L., Vittorino R., Zhao W., Qian X., Conley L., Ho L., Pasinetti G.M. Potential application of grape derived polyphenols in huntingtons disease. Transl. Neurosci. 2010;1(2):95–100. doi: 10.2478/v10134-010-0022-y. [http://dx.doi.org/10.2478/v10134-010-0022-y]. [PMID: 21331299]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang D., Ho L., Faith J., Ono K., Janle E.M., Lachcik P.J., Cooper B.R., Jannasch A.H., DArcy B.R., Williams B.A., Ferruzzi M.G., Levine S., Zhao W., Dubner L., Pasinetti G.M. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimers disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015;59(6):1025–1040. doi: 10.1002/mnfr.201400544. [http://dx.doi.org/10.1002/mnfr.201400544]. [PMID: 25689033]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang J., Ho L., Zhao Z., Seror I., Humala N., Dickstein D.L., Thiyagarajan M., Percival S.S., Talcott S.T., Pasinetti G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimers disease. FASEB J. 2006;20(13):2313–2320. doi: 10.1096/fj.06-6281com. [http://dx.doi.org/10.1096/ fj.06-6281com]. [PMID: 17077308]. [DOI] [PubMed] [Google Scholar]
- 196.Williams C.M., El Mohsen M.A., Vauzour D., Rendeiro C., Butler L.T., Ellis J.A., Whiteman M., Spencer J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008;45(3):295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [http://dx.doi.org/10.1016/j.freeradbiomed.2008. 04.008]. [PMID: 18457678]. [DOI] [PubMed] [Google Scholar]
- 197.Rendeiro C., Vauzour D., Rattray M., Waffo-Téguo P., Mérillon J.M., Butler L.T., Williams C.M., Spencer J.P. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS One. 2013;8(5):e63535. doi: 10.1371/journal.pone.0063535. [http://dx.doi.org/10.1371/journal.pone. 0063535]. [PMID: 23723987]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seeram N.P. Berry fruits: compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008;56(3):627–629. doi: 10.1021/jf071988k. [http://dx.doi.org/10.1021/jf071988k]. [PMID: 18211023]. [DOI] [PubMed] [Google Scholar]
- 199.Joseph J.A., Shuckitt-Hale B., Denisova N.A., Prior R., Cao G., Bickford P.C. Strawberry, spinach or vitamin E supplementation retards age-related CNS and cognitive behavioral changes. FASEB J. 1998;12(5):A878–A878. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Joseph J.A., Shukitt-Hale B., Denisova N.A., Bielinski D., Martin A., McEwen J.J., Bickford P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999;19(18):8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [PMID: 10479711]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shukitt-Hale B., Cheng V., Joseph J.A. Effects of blackberries on motor and cognitive function in aged rats. Nutr. Neurosci. 2009;12(3):135–140. doi: 10.1179/147683009X423292. [http://dx.doi.org/10.1179/147683009X423292]. [PMID: 19356316]. [DOI] [PubMed] [Google Scholar]
- 202.Hartman R.E., Shah A., Fagan A.M., Schwetye K.E., Parsadanian M., Schulman R.N., Finn M.B., Holtzman D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimers disease. Neurobiol. Dis. 2006;24(3):506–515. doi: 10.1016/j.nbd.2006.08.006. [http://dx.doi.org/10.1016/j.nbd.2006.08.006]. [PMID: 17010630]. [DOI] [PubMed] [Google Scholar]
- 203.Li Q., Zhao H.F., Zhang Z.F., Liu Z.G., Pei X.R., Wang J.B., Li Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta142 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience. 2009;163(3):741–749. doi: 10.1016/j.neuroscience.2009.07.014. [http://dx.doi.org/10.1016/ j.neuroscience.2009.07.014]. [PMID: 19596052]. [DOI] [PubMed] [Google Scholar]
- 204.Kumari M.V., Yoneda T., Hiramatsu M. Effect of beta CATECHIN on the life span of senescence accelerated mice (SAM-P8 strain). Biochem. Mol. Biol. Int. 1997;41(5):1005–1011. doi: 10.1080/15216549700202071. [PMID: 9137832]. [DOI] [PubMed] [Google Scholar]
- 205.Ashafaq M., Raza S.S., Khan M.M., Ahmad A., Javed H., Ahmad M.E., Tabassum R., Islam F., Siddiqui M.S., Safhi M.M., Islam F. Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochem. Res. 2012;37(8):1747–1760. doi: 10.1007/s11064-012-0786-1. [http://dx.doi.org/10.1007/s11064-012-0786-1]. [PMID: 22570178]. [DOI] [PubMed] [Google Scholar]
- 206.Dragicevic N., Smith A., Lin X., Yuan F., Copes N., Delic V., Tan J., Cao C., Shytle R.D., Bradshaw P.C. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimers amyloid-induced mitochondrial dysfunction. J. Alzheimers Dis. 2011;26(3):507–521. doi: 10.3233/JAD-2011-101629. [PMID: 21694462]. [DOI] [PubMed] [Google Scholar]
- 207.Guedj F., Sébrié C., Rivals I., Ledru A., Paly E., Bizot J.C., Smith D., Rubin E., Gillet B., Arbones M., Delabar J.M. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS One. 2009;4(2):e4606. doi: 10.1371/journal.pone.0004606. [http://dx.doi.org/10. 1371/journal.pone.0004606]. [PMID: 19242551]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Herges K., Millward J.M., Hentschel N., Infante-Duarte C., Aktas O., Zipp F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuro- inflammation. PLoS One. 2011;6(10):e25456. doi: 10.1371/journal.pone.0025456. [http://dx.doi.org/10.1371/journal.pone.0025456]. [PMID: 22022398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Karuppagounder S.S., Pinto J.T., Xu H., Chen H.L., Beal M.F., Gibson G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimers disease. Neurochem. Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [http://dx.doi.org/10.1016/ j.neuint.2008.10.008]. [PMID: 19041676]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jin F., Wu Q., Lu Y.F., Gong Q.H., Shi J.S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinsons disease in rats. Eur. J. Pharmacol. 2008;600(1-3):78–82. doi: 10.1016/j.ejphar.2008.10.005. [http://dx.doi.org/10.1016/j.ejphar.2008.10.005]. [PMID: 18940189]. [DOI] [PubMed] [Google Scholar]
- 211.Dal-Pan A., Pifferi F., Marchal J., Picq J-L., Aujard F. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One. 2011;6(1):e16581. doi: 10.1371/journal.pone.0016581. [http://dx.doi.org/10.1371/journal. pone.0016581]. [PMID: 21304942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shindler K.S., Ventura E., Dutt M., Elliott P., Fitzgerald D.C., Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. 2010;30(4):328–339. doi: 10.1097/WNO.0b013e3181f7f833. [http://dx.doi.org/10.1097/WNO.0b013e3181f7f833]. [PMID: 21107122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fonseca-Kelly Z., Nassrallah M., Uribe J., Khan R.S., Dine K., Dutt M., Shindler K.S. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front. Neurol. 2012;3:84. doi: 10.3389/fneur.2012.00084. [http://dx.doi.org/10.3389/fneur.2012.00084]. [PMID: 22654783]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ho D.J., Calingasan N.Y., Wille E., Dumont M., Beal M.F. Resveratrol protects against peripheral deficits in a mouse model of Huntingtons disease. Exp. Neurol. 2010;225(1):74–84. doi: 10.1016/j.expneurol.2010.05.006. [http://dx.doi.org/10.1016/j.expneurol.2010.05.006]. [PMID: 20561979]. [DOI] [PubMed] [Google Scholar]
- 215.Hickey M.A., Zhu C., Medvedeva V., Lerner R.P., Patassini S., Franich N.R., Maiti P., Frautschy S.A., Zeitlin S., Levine M.S., Chesselet M.F. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntingtons disease. Mol. Neurodegener. 2012;7:12. doi: 10.1186/1750-1326-7-12. [http://dx.doi.org/10.1186/1750-1326-7-12]. [PMID: 22475209]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kumar A., Sehgal N., Kumar P., Padi S.S., Naidu P.S. Protective effect of quercetin against ICV colchicine-induced cognitive dysfunctions and oxidative damage in rats. Phytother. Res. 2008;22(12):1563–1569. doi: 10.1002/ptr.2454. [http://dx.doi.org/10.1002/ptr.2454]. [PMID: 18980205]. [DOI] [PubMed] [Google Scholar]
- 217.Lv C., Hong T., Yang Z., Zhang Y., Wang L., Dong M., Zhao J., Mu J., Meng Y. Effect of Quercetin in the 1-Methyl-4-phenyl- 1, 2, 3, 6-tetrahydropyridine-induced mouse model of parkinson's disease. Evid Based Complement. Alternat. Med. 2012 doi: 10.1155/2012/928643. 928643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li C., Zug C., Qu H., Schluesener H., Zhang Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015;281:32–42. doi: 10.1016/j.bbr.2014.12.012. [http://dx.doi.org/10.1016/j.bbr.2014.12.012]. [PMID: 25510196]. [DOI] [PubMed] [Google Scholar]
- 219.Wu W-R., Zhu X-Z. Involvement of monoamine oxidase inhibition in neuroprotective and neurorestorative effects of Ginkgo biloba extract against MPTP-induced nigrostriatal dopaminergic toxicity in C57 mice. Life Sci. 1999;65(2):157–164. doi: 10.1016/s0024-3205(99)00232-5. [http://dx. doi.org/10.1016/S0024-3205(99)00232-5]. [PMID: 10416821]. [DOI] [PubMed] [Google Scholar]
- 220.Jelenković A., Jovanović M.D., Stevanović I., Petronijević N., Bokonjić D., Zivković J., Igić R. Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother. Res. 2014;28(1):82–87. doi: 10.1002/ptr.4962. [http://dx.doi.org/10.1002/ptr.4962]. [PMID: 23494944]. [DOI] [PubMed] [Google Scholar]
- 221.Sutherland B.A., Shaw O.M., Clarkson A.N., Jackson D.N., Sammut I.A., Appleton I. Neuroprotective effects of (-)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB J. 2005;19(2):258–260. doi: 10.1096/fj.04-2806fje. [PMID: 15569775]. [DOI] [PubMed] [Google Scholar]
- 222.Huang T.C., Lu K.T., Wo Y.Y., Wu Y.J., Yang Y.L. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One. 2011;6(12):e29102. doi: 10.1371/journal.pone.0029102. [http://dx.doi.org/10.1371/journal.pone. 0029102]. [PMID: 22220203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li H., Yan Z., Zhu J., Yang J., He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011;60(2-3):252–258. doi: 10.1016/j.neuropharm.2010.09.005. [http://dx.doi.org/10. 1016/j.neuropharm.2010.09.005]. [PMID: 20868700]. [DOI] [PubMed] [Google Scholar]
- 224.Li C., Yan Z., Yang J., Chen H., Li H., Jiang Y., Zhang Z. Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats. Neurochem. Int. 2010;56(3):495–500. doi: 10.1016/j.neuint.2009.12.009. [http://dx.doi.org/10.1016/j.neuint.2009.12.009]. [PMID: 20026214]. [DOI] [PubMed] [Google Scholar]
- 225.Khan M.M., Ahmad A., Ishrat T., Khan M.B., Hoda M.N., Khuwaja G., Raza S.S., Khan A., Javed H., Vaibhav K., Islam F. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinsons disease. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [http://dx.doi.org/10.1016/ j.brainres.2010.02.031]. [PMID: 20167206]. [DOI] [PubMed] [Google Scholar]
- 226.Ozacmak V.H., Sayan-Ozacmak H., Barut F. Chronic treatment with resveratrol, a natural polyphenol found in grapes, alleviates oxidative stress and apoptotic cell death in ovariectomized female rats subjected to chronic cerebral hypoperfusion. Nutr. Neurosci. 2016;19(4):176–186. doi: 10.1179/1476830515Y.0000000027. [PMID: 26005194]. [DOI] [PubMed] [Google Scholar]
- 227.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102(4):1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.04613.x]. [PMID: 17472706]. [DOI] [PubMed] [Google Scholar]
- 228.Schültke E., Kendall E., Kamencic H., Ghong Z., Griebel R.W., Juurlink B.H. Quercetin promotes functional recovery following acute spinal cord injury. J. Neurotrauma. 2003;20(6):583–591. doi: 10.1089/089771503767168500. [http://dx.doi.org/10.1089/089771503767168500]. [PMID: 12906742]. [DOI] [PubMed] [Google Scholar]
- 229.Dajas F., Rivera F., Blasina F., Arredondo F., Echeverry C., Lafon L., Morquio A., Heinzen H. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox. Res. 2003;5(6):425–432. doi: 10.1007/BF03033172. [http://dx.doi.org/10.1007/BF03033172]. [PMID: 14715446]. [DOI] [PubMed] [Google Scholar]
- 230.Pu F., Mishima K., Irie K., Motohashi K., Tanaka Y., Orito K., Egawa T., Kitamura Y., Egashira N., Iwasaki K., Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J. Pharmacol. Sci. 2007;104(4):329–334. doi: 10.1254/jphs.fp0070247. [http://dx.doi.org/10.1254/jphs.FP0070247]. [PMID: 17666865]. [DOI] [PubMed] [Google Scholar]
- 231.Yao R-Q., Qi D-S., Yu H-L., Liu J., Yang L-H., Wu X-X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 2012;37(12):2777–2786. doi: 10.1007/s11064-012-0871-5. [http://dx.doi.org/10.1007/s11064-012-0871-5]. [PMID: 22936120]. [DOI] [PubMed] [Google Scholar]
- 232.Hu P., Wang M., Chen W.H., Liu J., Chen L., Yin S.T., Yong W., Chen J.T., Wang H.L., Ruan D.Y. Quercetin relieves chronic lead exposure-induced impairment of synaptic plasticity in rat dentate gyrus in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 2008;378(1):43–51. doi: 10.1007/s00210-008-0301-z. [http://dx.doi.org/10.1007/s00210-008-0301-z]. [PMID: 18458876]. [DOI] [PubMed] [Google Scholar]
- 233.Sabogal-Guáqueta A.M., Muñoz-Manco J.I., Ramírez-Pineda J.R., Lamprea-Rodriguez M., Osorio E., Cardona-Gómez G.P. The flavonoid quercetin ameliorates Alzheimers disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimers disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [http://dx.doi.org/10.1016/j.neuropharm.2015. 01.027]. [PMID: 25666032]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Alkam T., Nitta A., Mizoguchi H., Itoh A., Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(2535). Behav. Brain Res. 2007;180(2):139–145. doi: 10.1016/j.bbr.2007.03.001. [http://dx.doi.org/10.1016/ j.bbr.2007.03.001]. [PMID: 17420060]. [DOI] [PubMed] [Google Scholar]
- 235.Satoh T., Kosaka K., Itoh K., Kobayashi A., Yamamoto M., Shimojo Y., Kitajima C., Cui J., Kamins J., Okamoto S., Izumi M., Shirasawa T., Lipton S.A. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J. Neurochem. 2008;104(4):1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.05039.x]. [PMID: 17995931]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dodge H.H., Zitzelberger T., Oken B.S., Howieson D., Kaye J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008;70(19 Pt 2):1809–1817. doi: 10.1212/01.wnl.0000303814.13509.db. [http://dx.doi.org/10.1212/01.wnl.0000303814.13509.db]. [PMID: 18305231]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mathis C.A., Kuller L.H., Klunk W.E., Snitz B.E., Price J.C., Weissfeld L.A., Rosario B.L., Lopresti B.J., Saxton J.A., Aizenstein H.J., McDade E.M., Kamboh M.I., DeKosky S.T., Lopez O.L. In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Ann. Neurol. 2013;73(6):751–761. doi: 10.1002/ana.23797. [http://dx.doi.org/10.1002/ana.23797]. [PMID: 23596051]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Oskouei D.S., Rikhtegar R., Hashemilar M., Sadeghi-Bazargani H., Sharifi-Bonab M., Sadeghi-Hokmabadi E., Zarrintan S., Sharifipour E. The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J. Stroke Cerebrovasc. Dis. 2013;22(8):e557–e563. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.010. [http://dx.doi.org/10.1016/j. jstrokecerebrovasdis.2013.06.010]. [PMID: 23871729]. [DOI] [PubMed] [Google Scholar]
- 239.Uebel-von Sandersleben H., Rothenberger A., Albrecht B., Rothenberger L.G., Klement S., Bock N. Ginkgo biloba extract EGb 761® in children with ADHD. Z. Kinder Jugendpsychiatr. Psychother. 2014;42(5):337–347. doi: 10.1024/1422-4917/a000309. [http://dx.doi.org/10.1024/1422-4917/a000309]. [PMID: 25163996]. [DOI] [PubMed] [Google Scholar]
- 240.Tan M.S., Yu J.T., Tan C.C., Wang H.F., Meng X.F., Wang C., Jiang T., Zhu X.C., Tan L. Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J. Alzheimers Dis. 2015;43(2):589–603. doi: 10.3233/JAD-140837. [PMID: 25114079]. [DOI] [PubMed] [Google Scholar]
- 241.Borgwardt S., Hammann F., Scheffler K., Kreuter M., Drewe J., Beglinger C. Neural effects of green tea extract on dorsolateral prefrontal cortex. Eur. J. Clin. Nutr. 66(11):1187–1192. doi: 10.1038/ejcn.2012.105. [http://dx.doi.org/10.1038/ejcn.2012.105] [PMID:22929964]. [DOI] [PubMed] [Google Scholar]
- 242.Okello E.J., Abadi A.M., Abadi S.A. Effects of green and black tea consumption on brain wave activities in healthy volunteers as measured by a simplified Electroencephalogram (EEG): A feasibility study. Nutr. Neurosci. 2015 doi: 10.1179/1476830515Y.0000000008. [PMID: 25714035]. [DOI] [PubMed] [Google Scholar]
- 243.De la Torre R., De Sola S., Pons M., Duchon A., de Lagran M.M., Farré M., Fitó M., Benejam B., Langohr K., Rodriguez J., Pujadas M., Bizot J.C., Cuenca A., Janel N., Catuara S., Covas M.I., Blehaut H., Herault Y., Delabar J.M., Dierssen M. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol. Nutr. Food Res. 2014;58(2):278–288. doi: 10.1002/mnfr.201300325. [http://dx.doi.org/10.1002/ mnfr.201300325]. [PMID: 24039182]. [DOI] [PubMed] [Google Scholar]
- 244.Mastroiacovo D., Kwik-Uribe C., Grassi D., Necozione S., Raffaele A., Pistacchio L., Righetti R., Bocale R., Lechiara M.C., Marini C., Ferri C., Desideri G. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Studya randomized controlled trial. Am. J. Clin. Nutr. 2015;101(3):538–548. doi: 10.3945/ajcn.114.092189. [http://dx.doi.org/10.3945/ajcn.114. 092189]. [PMID: 25733639]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Krikorian R., Boespflug E.L., Fleck D.E., Stein A.L., Wightman J.D., Shidler M.D., Sadat-Hossieny S. Concord grape juice supplementation and neurocognitive function in human aging. J. Agric. Food Chem. 2012;60(23):5736–5742. doi: 10.1021/jf300277g. [http://dx.doi.org/10.1021/jf300277g]. [PMID: 22468945]. [DOI] [PubMed] [Google Scholar]
- 246.Kean R.J., Lamport D.J., Dodd G.F., Freeman J.E., Williams C.M., Ellis J.A., Butler L.T., Spencer J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: an 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015;101(3):506–514. doi: 10.3945/ajcn.114.088518. [http://dx.doi.org/10.3945/ajcn.114.088518]. [PMID: 25733635]. [DOI] [PubMed] [Google Scholar]
- 247.Alharbi M., Lamport D., Dodd G., Saunders C., Harkness L., Butler L., Spencer J.E. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016;55:2021–2029. doi: 10.1007/s00394-015-1016-9. [PMID: 26280945]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kennedy D.O., Wightman E.L., Reay J.L., Lietz G., Okello E.J., Wilde A., Haskell C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010;91(6):1590–1597. doi: 10.3945/ajcn.2009.28641. [http://dx.doi.org/10.3945/ ajcn.2009.28641]. [PMID: 20357044]. [DOI] [PubMed] [Google Scholar]
- 249.Cox K.H., Pipingas A., Scholey A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. (Oxford) 2015;29(5):642–651. doi: 10.1177/0269881114552744. [http://dx.doi.org/10.1177/0269881114552744]. [PMID: 25277322]. [DOI] [PubMed] [Google Scholar]
- 250.Broman-Fulks J.J., Canu W.H., Trout K.L., Nieman D.C. The effects of quercetin supplementation on cognitive functioning in a community sample: a randomized, placebo-controlled trial. Ther. Adv. Psychopharmacol. 2012;2(4):131–138. doi: 10.1177/2045125312445894. [http://dx.doi.org/10.1177/2045125312445894]. [PMID: 23983966]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Small B.J., Rawson K.S., Martin C., Eisel S.L., Sanberg C.D., McEvoy C.L., Sanberg P.R., Shytle R.D., Tan J., Bickford P.C. Nutraceutical intervention improves older adults cognitive functioning. Rejuvenation Res. 2014;17(1):27–32. doi: 10.1089/rej.2013.1477. [http://dx.doi.org/10.1089/rej.2013.1477]. [PMID: 24134194]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Witte A.V., Kerti L., Margulies D.S., Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014;34(23):7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [http://dx.doi.org/10.1523/ JNEUROSCI.0385-14.2014]. [PMID: 24899709]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Strazielle N., Ghersi-Egea J.F. Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol. Pharm. 2013;10(5):1473–1491. doi: 10.1021/mp300518e. [http://dx.doi.org/10.1021/mp300518e]. [PMID: 23298398]. [DOI] [PubMed] [Google Scholar]
- 254.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [http://dx.doi.org/10.1038/nrn1824]. [PMID: 16371949]. [DOI] [PubMed] [Google Scholar]
- 255.Abbott N.J., Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia. 2012;53(Suppl. 6):1–6. doi: 10.1111/j.1528-1167.2012.03696.x. [http://dx.doi.org/10.1111/j.1528-1167.2012.03696.x]. [PMID: 23134489]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cardoso F.L., Brites D., Brito M.A. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res. Brain Res. Rev. 2010;64(2):328–363. doi: 10.1016/j.brainresrev.2010.05.003. [http://dx.doi.org/10.1016/j.brainresrev.2010.05.003]. [PMID: 20685221]. [DOI] [PubMed] [Google Scholar]
- 257.Faria A., Mateus N., Calhau C. Flavonoid transport across blood-brain barrier: implication for their direct neuroprotective actions. Nutr. Ageing. 2012;1:89–97. [Google Scholar]
- 258.Campos-Bedolla P., Walter F.R., Veszelka S., Deli M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014;45(8):610–638. doi: 10.1016/j.arcmed.2014.11.018. [http://dx.doi.org/10.1016/j.arcmed.2014.11.018]. [PMID: 25481827]. [DOI] [PubMed] [Google Scholar]
- 259.Youdim K.A., Shukitt-Hale B., Joseph J.A. Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004;37(11):1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [http://dx.doi.org/10.1016/j.freeradbiomed. 2004.08.002]. [PMID: 15528027]. [DOI] [PubMed] [Google Scholar]
- 260.Youdim K.A., Dobbie M.S., Kuhnle G., Proteggente A.R., Abbott N.J., Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J. Neurochem. 2003;85(1):180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [http://dx.doi.org/10.1046/j.1471-4159.2003.01652.x]. [PMID: 12641740]. [DOI] [PubMed] [Google Scholar]
- 261.Youdim K.A., Qaiser M.Z., Begley D.J., Rice-Evans C.A., Abbott N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004;36(5):592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [http://dx.doi.org/10.1016/j.freeradbiomed.2003.11.023]. [PMID: 14980703]. [DOI] [PubMed] [Google Scholar]
- 262.Schaffer S., Halliwell B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012;7(2):99–109. doi: 10.1007/s12263-011-0255-5. [http://dx.doi.org/10.1007/s12263-011-0255-5]. [PMID: 22012276]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ziberna L., Fornasaro S., Čvorović J., Tramer F., Passamonti S., Watson R.R., Preedy V.R., Zibadi S. Polyphenols in Human Health and Disease. San Diego: Academic Press; 2014. Bioavailability of Flavonoids: The Role of Cell Membrane Transporters. pp. 489–511. [http://dx.doi.org/10.1016/B978-0-12-398456-2.00037-2] [Google Scholar]
- 264.Aasmundstad T.A., Mørland J., Paulsen R.E. Distribution of morphine 6-glucuronide and morphine across the blood-brain barrier in awake, freely moving rats investigated by in vivo microdialysis sampling. J. Pharmacol. Exp. Ther. 1995;275(1):435–441. [PMID: 7562582]. [PubMed] [Google Scholar]
- 265.Bourasset F., Cisternino S., Temsamani J., Scherrmann J-M. Evidence for an active transport of morphine-6-β-d-glucuronide but not P-glycoprotein-mediated at the blood-brain barrier. J. Neurochem. 2003;86(6):1564–1567. doi: 10.1046/j.1471-4159.2003.01990.x. [http://dx.doi.org/10.1046/ j.1471-4159.2003.01990.x]. [PMID: 12950465]. [DOI] [PubMed] [Google Scholar]
- 266.Ishisaka A., Mukai R., Terao J., Shibata N., Kawai Y. Specific localization of quercetin-3-O-glucuronide in human brain. Arch. Biochem. Biophys. 2014;557:11–17. doi: 10.1016/j.abb.2014.05.025. [http://dx.doi.org/10.1016/ j.abb.2014.05.025]. [PMID: 24893148]. [DOI] [PubMed] [Google Scholar]
- 267.Wu L., Zhang Q-L., Zhang X-Y., Lv C., Li J., Yuan Y., Yin F-X. Pharmacokinetics and blood-brain barrier penetration of (+)-catechin and (-)-epicatechin in rats by microdialysis sampling coupled to high-performance liquid chromatography with chemiluminescence detection. J. Agric. Food Chem. 2012;60(37):9377–9383. doi: 10.1021/jf301787f. [http://dx.doi.org/10.1021/jf301787f]. [PMID: 22953747]. [DOI] [PubMed] [Google Scholar]
- 268.Passamonti S., Vrhovsek U., Vanzo A., Mattivi F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005;53(18):7029–7034. doi: 10.1021/jf050565k. [http://dx.doi.org/10.1021/jf050565k]. [PMID: 16131107]. [DOI] [PubMed] [Google Scholar]
- 269.Talavéra S., Felgines C., Texier O., Besson C., Gil-Izquierdo A., Lamaison J-L., Rémésy C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005;53(10):3902–3908. doi: 10.1021/jf050145v. [http://dx.doi.org/10.1021/ jf050145v]. [PMID: 15884815]. [DOI] [PubMed] [Google Scholar]
- 270.Kalt W., Blumberg J.B., McDonald J.E., Vinqvist-Tymchuk M.R., Fillmore S.A., Graf B.A., OLeary J.M., Milbury P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008;56(3):705–712. doi: 10.1021/jf071998l. [http://dx.doi.org/10.1021/jf071998l]. [PMID: 18211026]. [DOI] [PubMed] [Google Scholar]
- 271.Milbury P.E., Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J. Agric. Food Chem. 2010;58(7):3950–3956. doi: 10.1021/jf903529m. [http://dx.doi.org/10.1021/jf903529m]. [PMID: 20128604]. [DOI] [PubMed] [Google Scholar]
- 272.Janle E.M., Lila M.A., Grannan M., Wood L., Higgins A., Yousef G.G., Rogers R.B., Kim H., Jackson G.S., Ho L., Weaver C.M. Pharmacokinetics and tissue distribution of 14C-labeled grape polyphenols in the periphery and the central nervous system following oral administration. J. Med. Food. 2010;13(4):926–933. doi: 10.1089/jmf.2009.0157. [http://dx.doi.org/10.1089/jmf.2009.0157]. [PMID: 20673061]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Suganuma M., Okabe S., Oniyama M., Tada Y., Ito H., Fujiki H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19(10):1771–1776. doi: 10.1093/carcin/19.10.1771. [http://dx.doi.org/10.1093/carcin/19.10.1771]. [PMID: 9806157]. [DOI] [PubMed] [Google Scholar]
- 274.Abd El Mohsen M.M., Kuhnle G., Rechner A.R., Schroeter H., Rose S., Jenner P., Rice-Evans C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002;33(12):1693–1702. doi: 10.1016/s0891-5849(02)01137-1. [http://dx.doi.org/10.1016/S0891-5849(02)01137-1]. [PMID: 12488137]. [DOI] [PubMed] [Google Scholar]
- 275.El Mohsen M.A., Marks J., Kuhnle G., Moore K., Debnam E., Kaila Srai S., Rice-Evans C., Spencer J.P. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br. J. Nutr. 2006;95(1):51–58. doi: 10.1079/bjn20051596. [http://dx.doi.org/10.1079/BJN20051596]. [PMID: 16441916]. [DOI] [PubMed] [Google Scholar]
- 276.Peng H.W., Cheng F.C., Huang Y.T., Chen C.F., Tsai T.H. Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromato- graphy. J. Chromatogr. B Biomed. Sci. Appl. 1998;714(2):369–374. doi: 10.1016/s0378-4347(98)00204-7. [http://dx.doi.org/10.1016/S0378-4347(98)00204-7]. [PMID: 9766878]. [DOI] [PubMed] [Google Scholar]
- 277.Tung-Hu T., YenFei C. Determination of unbound hesperetin in rat blood and brain by microdialysis coupled to microbore liquid chromatography. J. Food Drug Anal. 2000;8(4):331–336. [Google Scholar]
- 278.Faria A., Pestana D., Teixeira D., Couraud P-O., Romero I., Weksler B., de Freitas V., Mateus N., Calhau C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011;2(1):39–44. doi: 10.1039/c0fo00100g. [http://dx.doi.org/10.1039/ C0FO00100G]. [PMID: 21773584]. [DOI] [PubMed] [Google Scholar]
- 279.Faria A., Meireles M., Fernandes I., Santos-Buelga C., Gonzalez-Manzano S., Dueñas M., de Freitas V., Mateus N., Calhau C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014;149:190–196. doi: 10.1016/j.foodchem.2013.10.095. [http://dx.doi.org/10.1016/j.foodchem. 2013.10.095]. [PMID: 24295694]. [DOI] [PubMed] [Google Scholar]
- 280.Tan K.W., Li Y., Paxton J.W., Birch N.P., Scheepens A. Identification of novel dietary phytochemicals inhibiting the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Food Chem. 2013;138(4):2267–2274. doi: 10.1016/j.foodchem.2012.12.021. [http://dx.doi.org/10.1016/j. foodchem.2012.12.021]. [PMID: 23497885]. [DOI] [PubMed] [Google Scholar]
- 281.Alvarez A.I., Real R., Pérez M., Mendoza G., Prieto J.G., Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010;99(2):598–617. doi: 10.1002/jps.21851. [http://dx.doi.org/10.1002/jps. 21851]. [PMID: 19544374]. [DOI] [PubMed] [Google Scholar]
- 282.Perloff M.D., von Moltke L.L., Störmer E., Shader R.I., Greenblatt D.J. Saint Johns wort: an in vitro analysis of P-glycoprotein induction due to extended exposure. Br. J. Pharmacol. 2001;134(8):1601–1608. doi: 10.1038/sj.bjp.0704399. [http://dx.doi.org/10.1038/ sj.bjp.0704399]. [PMID: 11739235]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Molnár J., Gyémánt N., Tanaka M., Hohmann J., Bergmann-Leitner E., Molnár P., Deli J., Didiziapetris R., Ferreira M.J. Inhibition of multidrug resistance of cancer cells by natural diterpenes, triterpenes and carotenoids. Curr. Pharm. Des. 2006;12(3):287–311. doi: 10.2174/138161206775201893. [http://dx.doi.org/10.2174/138161206775201893]. [PMID: 16454745]. [DOI] [PubMed] [Google Scholar]
- 284.Patel J., Buddha B., Dey S., Pal D., Mitra A.K. In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: changes in P-gp and CYP3A4 activity. Am. J. Ther. 2004;11(4):262–277. doi: 10.1097/01.mjt.0000101827.94820.22. [http://dx.doi.org/10.1097/01.mjt.0000101827. 94820.22]. [PMID: 15266218]. [DOI] [PubMed] [Google Scholar]
- 285.Dürr D., Stieger B., Kullak-Ublick G.A., Rentsch K.M., Steinert H.C., Meier P.J., Fattinger K. St Johns Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin. Pharmacol. Ther. 2000;68(6):598–604. doi: 10.1067/mcp.2000.112240. [http://dx.doi.org/10. 1067/mcp.2000.112240]. [PMID: 11180019]. [DOI] [PubMed] [Google Scholar]
- 286.Hennessy M., Kelleher D., Spiers J.P., Barry M., Kavanagh P., Back D., Mulcahy F., Feely J. St Johns wort increases expression of P-glycoprotein: implications for drug interactions. Br. J. Clin. Pharmacol. 2002;53(1):75–82. doi: 10.1046/j.0306-5251.2001.01516.x. [http://dx.doi.org/10.1046/j.0306-5251.2001.01516.x]. [PMID: 11849198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Murakami T., Takano M. Intestinal efflux transporters and drug absorption. Expert Opin. Drug Metab. Toxicol. 2008;4(7):923–939. doi: 10.1517/17425255.4.7.923. [http://dx.doi.org/10.1517/17425255.4.7.923]. [PMID: 18624680]. [DOI] [PubMed] [Google Scholar]
- 288.van de Wetering K., Burkon A., Feddema W., Bot A., de Jonge H., Somoza V., Borst P. Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol. Pharmacol. 2009;75(4):876–885. doi: 10.1124/mol.108.052019. [http://dx.doi.org/10.1124/mol.108.052019]. [PMID: 19114588]. [DOI] [PubMed] [Google Scholar]
- 289.Brand W., Schutte M.E., Williamson G., van Zanden J.J., Cnubben N.H., Groten J.P., van Bladeren P.J., Rietjens I.M. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed. Pharmacother. 2006;60(9):508–519. doi: 10.1016/j.biopha.2006.07.081. [http://dx.doi.org/10.1016/j.biopha.2006.07.081]. [PMID: 16978825]. [DOI] [PubMed] [Google Scholar]
- 290.Cheng C.Y., Mruk D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012;64(1):16–64. doi: 10.1124/pr.110.002790. [http://dx.doi.org/10.1124/pr.110.002790]. [PMID: 22039149]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Martel F., Monteiro R., Calhau C. Effect of polyphenols on the intestinal and placental transport of some bioactive compounds. Nutr. Res. Rev. 2010;23(1):47–64. doi: 10.1017/S0954422410000053. [http://dx.doi.org/10.1017/ S0954422410000053]. [PMID: 20392307]. [DOI] [PubMed] [Google Scholar]
- 292.Zheng A., Li H., Cao K., Xu J., Zou X., Li Y., Chen C., Liu J., Feng Z. Maternal hydroxytyrosol administration improves neuro- genesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015;26(2):190–199. doi: 10.1016/j.jnutbio.2014.10.006. [http://dx.doi.org/10.1016/ j.jnutbio.2014.10.006]. [PMID: 25442671]. [DOI] [PubMed] [Google Scholar]
- 293.Sahu S.S. Effects of resveratrol on neuroanatomical, neuro- behavioral and biochemical recovery against prenatal restraint stress in rats. Karnataka: Manipal University; 2015. [Google Scholar]
- 294.Loren D.J., Seeram N.P., Schulman R.N., Holtzman D.M. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr. Res. 2005;57(6):858–864. doi: 10.1203/01.PDR.0000157722.07810.15. [http://dx.doi.org/10.1203/01.PDR.0000157722.07810.15]. [PMID: 15774834]. [DOI] [PubMed] [Google Scholar]
- 295.Arola-Arnal A., Oms-Oliu G., Crescenti A., del Bas J.M., Ras M.R., Arola L., Caimari A. Distribution of grape seed flavanols and their metabolites in pregnant rats and their fetuses. Mol. Nutr. Food Res. 2013;57(10):1741–1752. doi: 10.1002/mnfr.201300032. [PMID: 23728968]. [DOI] [PubMed] [Google Scholar]
- 296.Keating E., Lemos C., Gonçalves P., Martel F. Acute and chronic effects of some dietary bioactive compounds on folic acid uptake and on the expression of folic acid transporters by the human trophoblast cell line BeWo. J. Nutr. Biochem. 2008;19(2):91–100. doi: 10.1016/j.jnutbio.2007.01.007. [http://dx.doi.org/10.1016/j.jnutbio.2007.01.007]. [PMID: 17531458]. [DOI] [PubMed] [Google Scholar]
- 297.Araújo J.R., Gonçalves P., Martel F. Journal of Biochemistry. Vol. 144. USA: Oxford University Press; 2008. Modulation of Glucose Uptake in a Human Choriocarcinoma Cell Line (BeWo) by Dietary Bioactive Compounds and Drugs of Abuse. pp. 177–186. [http://dx.doi.org/10.1093/jb/mvn054] [DOI] [PubMed] [Google Scholar]
- 298.Ding J., Wang H., Wu Z-B., Zhao J., Zhang S., Li W. Protection of Murine Spermatogenesis Against Ionizing Radiation-Induced Testicular Injury by a Green Tea Polyphenol. Biol. Reprod. 2014;92(1):1–13. doi: 10.1095/biolreprod.114.122333. [DOI] [PubMed] [Google Scholar]
- 299.Abrahamse S.L., Kloots W.J., van Amelsvoort J.M. Absorption, distribution, and secretion of epicatechin and quercetin in the rat. Nutr. Res. 2005;25(3):305–317. [http://dx.doi.org/10.1016/ j.nutres.2004.10.013]. [Google Scholar]
- 300.Urpi-Sarda M., Ramiro-Puig E., Khan N., Ramos-Romero S., Llorach R., Castell M., Gonzalez-Manzano S., Santos-Buelga C., Andres-Lacueva C. Distribution of epicatechin metabolites in lymphoid tissues and testes of young rats with a cocoa-enriched diet. Br. J. Nutr. 2010;103(10):1393–1397. doi: 10.1017/S0007114509993473. [http://dx.doi.org/10.1017/S0007114509993473]. [PMID: 20100378]. [DOI] [PubMed] [Google Scholar]
- 301.Mattson M.P., Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29(11):632–639. doi: 10.1016/j.tins.2006.09.001. [http://dx.doi.org/10.1016/ j.tins.2006.09.001]. [PMID: 17000014]. [DOI] [PubMed] [Google Scholar]
- 302.Shah Z.A., Li R.C., Ahmad A.S., Kensler T.W., Yamamoto M., Biswal S., Doré S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010;30(12):1951–1961. doi: 10.1038/jcbfm.2010.53. [http://dx.doi.org/10.1038/jcbfm. 2010.53]. [PMID: 20442725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.di Gesso J.L., Kerr J.S., Zhang Q., Raheem S., Yalamanchili S.K., OHagan D., Kay C.D., OConnell M.A. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol. Nutr. Food Res. 2015;59(6):1143–1154. doi: 10.1002/mnfr.201400799. [http://dx.doi.org/10.1002/mnfr.201400799]. [PMID: 25801720]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Natsume M., Osakabe N., Yasuda A., Osawa T., Terao J. Inhibitory effects of conjugated epicatechin metabolites on peroxynitrite-mediated nitrotyrosine formation. J. Clin. Biochem. Nutr. 2008;42(1):50–53. doi: 10.3164/jcbn.2008008. [http://dx.doi.org/10.3164/jcbn.2008008]. [PMID: 18231630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gasperotti M., Passamonti S., Tramer F., Masuero D., Guella G., Mattivi F., Vrhovsek U. Fate of microbial metabolites of dietary polyphenols in rats: is the brain their target destination? ACS Chem. Neurosci. 2015;6(8):1341–1352. doi: 10.1021/acschemneuro.5b00051. [http://dx.doi.org/10.1021/acschemneuro.5b00051]. [PMID: 25891864]. [DOI] [PubMed] [Google Scholar]
- 306.Mecocci P., Tinarelli C., Schulz R.J., Polidori M.C. Nutraceuticals in cognitive impairment and Alzheimers disease. Front. Pharmacol. 2014;5:147. doi: 10.3389/fphar.2014.00147. [http://dx.doi.org/10.3389/ fphar.2014.00147]. [PMID: 25002849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yang Y., Bai L., Li X., Xiong J., Xu P., Guo C., Xue M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: an evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol. In Vitro. 2014;28(3):388–396. doi: 10.1016/j.tiv.2013.12.002. [http://dx.doi.org/10.1016/j.tiv.2013.12.002]. [PMID: 24362044]. [DOI] [PubMed] [Google Scholar]
- 308.Saeed M., Kadioglu O., Khalid H., Sugimoto Y., Efferth T. Activity of the dietary flavonoid, apigenin, against multidrug-resistant tumor cells as determined by pharmacogenomics and molecular docking. J. Nutr. Biochem. 2015;26(1):44–56. doi: 10.1016/j.jnutbio.2014.09.008. [http://dx.doi.org/10.1016/j.jnutbio.2014.09.008]. [PMID: 25459885]. [DOI] [PubMed] [Google Scholar]
- 309.Bitu Pinto N., da Silva Alexandre B., Neves K.R.T., Silva A. H., Leal L. K. A. M., Viana G. S. B. Neuroprotective properties of the standardized extract from camellia sinensis (Green Tea) and its main bioactive components, epicatechin and epigallocatechin gallate, in the 6-OHDA model of parkinson's disease. Evid Based Complement Alternat Med.,. 2015:161092. doi: 10.1155/2015/161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Syed Hussein S.S., Kamarudin M.N., Kadir H.A. (+)-Catechin attenuates NF-κB activation through regulation of Akt, MAPK, and AMPK signaling pathways in LPS-Induced BV-2 microglial cells. Am. J. Chin. Med. 2015;43(5):927–952. doi: 10.1142/S0192415X15500548. [http://dx.doi.org/10. 1142/S0192415X15500548]. [PMID: 26227399]. [DOI] [PubMed] [Google Scholar]
- 311.Vaidyanathan J.B., Walle T. Transport and metabolism of the tea flavonoid (-)-epicatechin by the human intestinal cell line Caco-2. Pharm. Res. 2001;18(10):1420–1425. doi: 10.1023/a:1012200805593. [http://dx.doi.org/10.1023/ A:1012200805593]. [PMID: 11697467]. [DOI] [PubMed] [Google Scholar]
- 312.Hong J., Lambert J.D., Lee S-H., Sinko P.J., Yang C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003;310(1):222–227. doi: 10.1016/j.bbrc.2003.09.007. [http://dx.doi.org/10.1016/j.bbrc.2003.09.007]. [PMID: 14511674]. [DOI] [PubMed] [Google Scholar]
- 313.He X-L., Wang Y-H., Bi M-G., Du G-H. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur. J. Pharmacol. 2012;680(1-3):41–48. doi: 10.1016/j.ejphar.2012.01.025. [http://dx.doi.org/10.1016/j.ejphar.2012.01.025]. [PMID: 22314218]. [DOI] [PubMed] [Google Scholar]
- 314.Souza L.C., Antunes M.S., Filho C.B., Del Fabbro L., de Gomes M.G., Goes A.T., Donato F., Prigol M., Boeira S.P., Jesse C.R. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015;134:22–30. doi: 10.1016/j.pbb.2015.04.010. [http://dx.doi.org/10.1016/j.pbb.2015.04.010]. [PMID: 25931267]. [DOI] [PubMed] [Google Scholar]
- 315.Mitsunaga Y., Takanaga H., Matsuo H., Naito M., Tsuruo T., Ohtani H., Sawada Y. Effect of bioflavonoids on vincristine transport across blood-brain barrier. Eur. J. Pharmacol. 2000;395(3):193–201. doi: 10.1016/s0014-2999(00)00180-1. [http://dx.doi.org/10.1016/S0014-2999(00)00180-1]. [PMID: 10812049]. [DOI] [PubMed] [Google Scholar]
- 316.Walle U.K., Galijatovic A., Walle T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 1999;58(3):431–438. doi: 10.1016/s0006-2952(99)00133-1. [http://dx.doi.org/10.1016/S0006-2952(99)00133-1]. [PMID: 10424761]. [DOI] [PubMed] [Google Scholar]
- 317.Zhang S., Wang X., Sagawa K., Morris M.E. Flavonoids chrysin and benzoflavone, potent breast cancer resistance protein inhibitors, have no significant effect on topotecan pharmacokinetics in rats or mdr1a/1b (-/-) mice. Drug Metab. Dispos. 2005;33(3):341–348. doi: 10.1124/dmd.104.002501. [http://dx.doi.org/10.1124/dmd.104.002501]. [PMID: 15608138]. [DOI] [PubMed] [Google Scholar]
- 318.Wu J.X., Zhang L.Y., Chen Y.L., Yu S.S., Zhao Y., Zhao J. Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation. Neural Regen. Res. 2015;10(3):481–489. doi: 10.4103/1673-5374.153700. [http://dx.doi.org/10. 4103/1673-5374.153700]. [PMID: 25878600]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wang S., Chen R., Zhong Z., Shi Z., Chen M., Wang Y. Epigallocatechin-3-gallate potentiates the effect of curcumin in inducing growth inhibition and apoptosis of resistant breast cancer cells. Am. J. Chin. Med. 2014;42(5):1279–1300. doi: 10.1142/S0192415X14500803. [http://dx.doi.org/10.1142/S0192415X14500803]. [PMID: 25242081]. [DOI] [PubMed] [Google Scholar]
- 320.Shukla S., Zaher H., Hartz A., Bauer B., Ware J.A., Ambudkar S.V. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm. Res. 2009;26(2):480–487. doi: 10.1007/s11095-008-9735-8. [http://dx.doi.org/10.1007/s11095-008-9735-8]. [PMID: 18841445]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lapchak P.A. Drug-like property profiling of novel neuroprotective compounds to treat acute ischemic stroke: guidelines to develop pleiotropic molecules. Transl. Stroke Res. 2013;4(3):328–342. doi: 10.1007/s12975-012-0200-y. [http://dx.doi.org/10.1007/s12975-012-0200-y]. [PMID: 23687519]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Shin Y., Kentaro K., Ryusuke M., Narumi S., Koji F. Inhibitory effect of flavonoids on the efflux of N-Acetyl 5-aminosalicylic acid intracellularly formed in Caco-2 cells. J. Biomed. Biotechnol. 2009:467489. doi: 10.1155/2009/467489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Huang Y-H., Zhang Q-H. Genistein reduced the neural apoptosis in the brain of ovariectomised rats by modulating mitochondrial oxidative stress. Br. J. Nutr. 2010;104(9):1297–1303. doi: 10.1017/S0007114510002291. [http://dx.doi.org/10.1017/S0007114510002291]. [PMID: 20579403]. [DOI] [PubMed] [Google Scholar]
- 324.Ding B., Yuan L., Yu H., Li L., Ma W., Bi Y., Feng J., Xiao R. Genistein and folic acid prevent oxidative injury induced by β-amyloid peptide. Basic Clin. Pharmacol. Toxicol. 2011;108(5):333–340. doi: 10.1111/j.1742-7843.2010.00661.x. [http://dx.doi.org/10.1111/j.1742-7843.2010.00661.x]. [PMID: 21205217]. [DOI] [PubMed] [Google Scholar]
- 325.Yu H-L., Li L., Zhang X-H., Xiang L., Zhang J., Feng J-F., Xiao R. Neuroprotective effects of genistein and folic acid on apoptosis of rat cultured cortical neurons induced by β-amyloid 3135. Br. J. Nutr. 2009;102(5):655–662. doi: 10.1017/S0007114509243042. [http://dx.doi.org/10.1017/S0007114509243042]. [PMID: 19331699]. [DOI] [PubMed] [Google Scholar]
- 326.Castro A.F., Altenberg G.A. Inhibition of drug transport by genistein in multidrug-resistant cells expressing P-glycoprotein. Biochem. Pharmacol. 1997;53(1):89–93. doi: 10.1016/s0006-2952(96)00657-0. [http://dx.doi.org/10.1016/S0006-2952(96)00657-0]. [PMID: 8960067]. [DOI] [PubMed] [Google Scholar]
- 327.Li X., Choi J-S. Effect of genistein on the pharmacokinetics of paclitaxel administered orally or intravenously in rats. J. Pharm. 2007;337(1-2):188–193. doi: 10.1016/j.ijpharm.2007.01.002. [http://dx.doi.org/10.1016/j.ijpharm.2007. 01.002]. [DOI] [PubMed] [Google Scholar]
- 328.Jäger W., Winter O., Halper B., Salamon A., Sartori M., Gajdzik L., Hamilton G., Theyer G., Graf J., Thalhammer T. Modulation of liver canalicular transport processes by the tyrosine-kinase inhibitor genistein: implications of genistein metabolism in the rat. Hepatology. 1997;26(6):1467–1476. doi: 10.1002/hep.510260613. [http://dx.doi.org/10.1002/hep.510260613]. [PMID: 9397986]. [DOI] [PubMed] [Google Scholar]
- 329.Imai Y., Tsukahara S., Asada S., Sugimoto Y. Phytoestrogens/ flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res. 2004;64(12):4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [http://dx.doi.org/10.1158/0008-5472.CAN-04-0078]. [PMID: 15205350]. [DOI] [PubMed] [Google Scholar]
- 330.Choi E.J., Ahn W.S. Neuroprotective effects of chronic hesperetin administration in mice. Arch. Pharm. Res. 2008;31(11):1457–1462. doi: 10.1007/s12272-001-2130-1. [http://dx.doi.org/10.1007/s12272-001-2130-1]. [PMID: 19023542]. [DOI] [PubMed] [Google Scholar]
- 331.Cooray H.C., Janvilisri T., van Veen H.W., Hladky S.B., Barrand M.A. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun. 2004;317(1):269–275. doi: 10.1016/j.bbrc.2004.03.040. [http://dx.doi.org/10.1016/j.bbrc.2004.03.040]. [PMID: 15047179]. [DOI] [PubMed] [Google Scholar]
- 332.Lagoa R., Lopez-Sanchez C., Samhan-Arias A.K., Gañan C.M., Garcia-Martinez V., Gutierrez-Merino C. Kaempferol protects against rat striatal degeneration induced by 3-nitropropionic acid. J. Neurochem. 2009;111(2):473–487. doi: 10.1111/j.1471-4159.2009.06331.x. [http://dx.doi.org/10.1111/ j.1471-4159.2009.06331.x]. [PMID: 19682208]. [DOI] [PubMed] [Google Scholar]
- 333.Nakatsuma A., Fukami T., Suzuki T., Furuishi T., Tomono K., Hidaka S. Effects of kaempferol on the mechanisms of drug resistance in the human glioblastoma cell line T98G. Die Pharmazie - An Int. J. Pharm. Sci. 2010;65(5):379–383. [PubMed] [Google Scholar]
- 334.An G., Gallegos J., Morris M.E. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab. Dispos. 2011;39(3):426–432. doi: 10.1124/dmd.110.035212. [http://dx.doi.org/10.1124/dmd.110.035212]. [PMID: 21139040]. [DOI] [PubMed] [Google Scholar]
- 335.Lei Y., Chen J., Zhang W., Fu W., Wu G., Wei H., Wang Q., Ruan J. In vivo investigation on the potential of galangin, kaempferol and myricetin for protection of D-galactose-induced cognitive impairment. Food Chem. 2012;135(4):2702–2707. doi: 10.1016/j.foodchem.2012.07.043. [http://dx.doi.org/10.1016/j.foodchem.2012.07.043]. [PMID: 22980861]. [DOI] [PubMed] [Google Scholar]
- 336.Choi S-J., Shin S-C., Choi J-S. Effects of myricetin on the bioavailability of doxorubicin for oral drug delivery in rats: possible role of CYP3A4 and P-glycoprotein inhibition by myricetin. Arch. Pharm. Res. 2011;34(2):309–315. doi: 10.1007/s12272-011-0217-x. [http://dx.doi.org/10. 1007/s12272-011-0217-x]. [PMID: 21380815]. [DOI] [PubMed] [Google Scholar]
- 337.Sarkar A., Angeline M.S., Anand K., Ambasta R.K., Kumar P. Naringenin and quercetin reverse the effect of hypobaric hypoxia and elicit neuroprotective response in the murine model. Brain Res. 2012;1481:59–70. doi: 10.1016/j.brainres.2012.08.036. [http://dx.doi.org/10.1016/j.brainres.2012. 08.036]. [PMID: 22981402]. [DOI] [PubMed] [Google Scholar]
- 338.Zbarsky V., Datla K.P., Parkar S., Rai D.K., Aruoma O.I., Dexter D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinsons disease. Free Radic. Res. 2005;39(10):1119–1125. doi: 10.1080/10715760500233113. [http://dx.doi.org/10.1080/10715760500233113]. [PMID: 16298737]. [DOI] [PubMed] [Google Scholar]
- 339.Lei X., Chao H., Zhang Z., Lv J., Li S., Wei H., Xue R., Li F., Li Z. Neuroprotective effects of quercetin in a mouse model of brain ischemic/reperfusion injury via anti-apoptotic mechanisms based on the Akt pathway. Mol. Med. Rep. 2015;12(3):3688–3696. doi: 10.3892/mmr.2015.3857. [PMID: 26016839]. [DOI] [PubMed] [Google Scholar]
- 340.Wu C-P., Calcagno A.M., Hladky S.B., Ambudkar S.V., Barrand M.A. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J. 2005;272(18):4725–4740. doi: 10.1111/j.1742-4658.2005.04888.x. [http://dx.doi.org/10.1111/j.1742-4658.2005.04888.x]. [PMID: 16156793]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J.E., Janle E.M., Lobo J., Ferruzzi M.G., Davies P., Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010;285(12):9100–9113. doi: 10.1074/jbc.M109.060061. [http://dx.doi.org/10.1074/jbc. M109.060061]. [PMID: 20080969]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Narayanan S.V., Dave K.R., Saul I., Perez-Pinzon M.A. Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke. 2015;46(6):1626–1632. doi: 10.1161/STROKEAHA.115.008921. [http://dx.doi.org/10.1161/STROKEAHA.115.008921]. [PMID: 25908459]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Choi J-S., Choi B-C., Kang K.W. Effect of resveratrol on the pharmacokinetics of oral and intravenous nicardipine in rats: possible role of P-glycoprotein inhibition by resveratrol. Die Pharmazie - An Int. J. Pharm. Sci. 2009;64(1):49–52. [PubMed] [Google Scholar]
- 344.Park S-E., Sapkota K., Choi J-H., Kim M-K., Kim Y.H., Kim K.M., Kim K.J., Oh H-N., Kim S-J., Kim S. Rutin from Dendropanax morbifera Leveille protects human dopaminergic cells against rotenone induced cell injury through inhibiting JNK and p38 MAPK signaling. Neurochem. Res. 2014;39(4):707–718. doi: 10.1007/s11064-014-1259-5. [http://dx.doi.org/10.1007/s11064-014-1259-5]. [PMID: 24549762]. [DOI] [PubMed] [Google Scholar]
- 345.Eckert A., Keil U., Kressmann S., Schindowski K., Leutner S., Leutz S., Müller W.E. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36(Suppl. 1):S15–S23. doi: 10.1055/s-2003-40449. [http://dx.doi.org/10.1055/s-2003-40449]. [PMID: 13130384]. [DOI] [PubMed] [Google Scholar]
- 346.Sharma H.S., Drieu K., Alm P., Westman J. Role of nitric oxide in blood-brain barrier permeability, brain edema and cell damage following hyperthermic brain injury. An experimental study using EGB-761 and Gingkolide B pretreatment in the rat. Acta Neurochir. Suppl. (Wien) 2000;76:81–86. doi: 10.1007/978-3-7091-6346-7_17. [PMID: 11450097]. [DOI] [PubMed] [Google Scholar]
- 347.Fujishita K., Ozawa T., Shibata K., Tanabe S., Sato Y., Hisamoto M., Okuda T., Koizumi S. Grape seed extract acting on astrocytes reveals neuronal protection against oxidative stress via interleukin-6-mediated mechanisms. Cell. Mol. Neurobiol. 2009;29(8):1121–1129. doi: 10.1007/s10571-009-9403-5. [http://dx.doi.org/10.1007/s10571-009-9403-5]. [PMID: 19381798]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Kim H.J., Yang J.S., Yoon S.H. Brief low [Mg(2+)]o-induced Ca(2+) spikes inhibit subsequent prolonged exposure-induced excitotoxicity in cultured rat hippocampal neurons. Korean J. Physiol. Pharmacol. 2016;20(1):101–109. doi: 10.4196/kjpp.2016.20.1.101. [http://dx.doi.org/10.4196/kjpp.2016.20.1.101]. [PMID: 26807029]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Vuong T., Matar C., Ramassamy C., Haddad P.S. Biotransformed blueberry juice protects neurons from hydrogen peroxide-induced oxidative stress and mitogen-activated protein kinase pathway alterations. Br. J. Nutr. 2010;104(5):656–663. doi: 10.1017/S0007114510001170. [http://dx.doi.org/10.1017/S0007114510001170]. [PMID: 20459875]. [DOI] [PubMed] [Google Scholar]
- 350.Joseph J.A., Denisova N.A., Arendash G., Gordon M., Diamond D., Shukitt-Hale B., Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr. Neurosci. 2003;6(3):153–162. doi: 10.1080/1028415031000111282. [http://dx.doi.org/10.1080/1028415031000111282]. [PMID: 12793519]. [DOI] [PubMed] [Google Scholar]
- 351.Joseph J.A., Shukitt-Hale B., Denisova N.A., Prior R.L., Cao G., Martin A., Taglialatela G., Bickford P.C. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J. Neurosci. 1998;18(19):8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [PMID: 9742171]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ban J.Y., Jeon S-Y., Bae K., Song K-S., Seong Y.H. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid β protein (2535)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006;79(24):2251–2259. doi: 10.1016/j.lfs.2006.07.021. [http://dx.doi.org/10.1016/j.lfs.2006. 07.021]. [PMID: 16978655]. [DOI] [PubMed] [Google Scholar]
- 353.Levites Y., Amit T., Youdim M.B., Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 2002;277(34):30574–30580. doi: 10.1074/jbc.M202832200. [http://dx. doi.org/10.1074/jbc.M202832200]. [PMID: 12058035]. [DOI] [PubMed] [Google Scholar]
- 354.Mandel S., Reznichenko L., Amit T., Youdim M.B. Green tea polyphenol (-)-epigallocatechin-3-gallate protects rat PC12 cells from apoptosis induced by serum withdrawal independent of P13-Akt pathway. Neurotox. Res. 2003;5(6):419–424. doi: 10.1007/BF03033171. [http://dx.doi.org/10.1007/BF03033171]. [PMID: 14715445]. [DOI] [PubMed] [Google Scholar]
- 355.Aktas O., Prozorovski T., Smorodchenko A., Savaskan N.E., Lauster R., Kloetzel P.M., Infante-Duarte C., Brocke S., Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004;173(9):5794–5800. doi: 10.4049/jimmunol.173.9.5794. [http://dx.doi.org/10.4049/jimmunol.173.9.5794]. [PMID: 15494532]. [DOI] [PubMed] [Google Scholar]
- 356.Sternberg Z., Chadha K., Lieberman A., Hojnacki D., Drake A., Zamboni P., Rocco P., Grazioli E., Weinstock-Guttman B., Munschauer F. Quercetin and interferon-beta modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J. Neuroimmunol. 2008;205(1-2):142–147. doi: 10.1016/j.jneuroim.2008.09.008. [http://dx.doi.org/10.1016/j.jneuroim.2008.09.008]. [PMID: 18926575]. [DOI] [PubMed] [Google Scholar]
- 357.Wang D-M., Li S-Q., Wu W-L., Zhu X-Y., Wang Y., Yuan H-Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimers disease. Neurochem. Res. 2014;39(8):1533–1543. doi: 10.1007/s11064-014-1343-x. [http://dx.doi.org/10. 1007/s11064-014-1343-x]. [PMID: 24893798]. [DOI] [PubMed] [Google Scholar]
- 358.Lee H.J., Cho H-S., Park E., Kim S., Lee S-Y., Kim C-S., Kim D.K., Kim S-J., Chun H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology. 2008;250(2-3):109–115. doi: 10.1016/j.tox.2008.06.010. [http://dx. doi.org/10.1016/j.tox.2008.06.010]. [DOI] [PubMed] [Google Scholar]