Abstract
Background
Chronic pain is a major symptom that develops in cancer patients, most commonly emerging during advanced stages of the disease. The nature of cancer-induced pain is complex, and the efficacy of current therapeutic interventions is restricted by the dose-limiting side-effects that accompany common centrally targeted analgesics.
Methods
This review focuses on how up-regulated glutamate production and export by the tumour converge at peripheral afferent nerve terminals to transmit nociceptive signals through the transient receptor cation channel, TRPV1, thereby initiating central sensitization in response to peripheral disease-mediated stimuli.
Results
Cancer cells undergo numerous metabolic changes that include increased glutamine catabolism and over-expression of enzymes involved in glutaminolysis, including glutaminase. This mitochondrial enzyme mediates glutaminolysis, producing large pools of intracellular glutamate. Up-regulation of the plasma membrane cystine/glutamate antiporter, system xc-, promotes aberrant glutamate release from cancer cells. Increased levels of extracellular glutamate have been associated with the progression of cancer-induced pain and we discuss how this can be mediated by activation of TRPV1.
Conclusion
With a growing population of patients receiving inadequate treatment for intractable pain, new targets need to be considered to better address this largely unmet clinical need for improving their quality of life. A better understanding of the mechanisms that underlie the unique qualities of cancer pain will help to identify novel targets that are able to limit the initiation of pain from a peripheral source–the tumour.
Keywords: Cancer pain, glutamate, glutaminase, system xc-, TRPV1
INTRODUCTION
The central nervous system (CNS) senses diverse endogenous and environmental stimuli, transmitting responding signals to the brain for processing. Particularly intense stimuli have the potential to elicit acute pain, and recurring injury or tissue damage enhance both peripheral and central components that contribute to the transmission of pain signals, leading to hypersensitivity. Physiological initiation of protective responses, although beneficial, may lead to chronic pain when these changes persist. Within the peripheral nervous system, the dorsal root ganglia (DRG) are comprised of somatic sensory neurons that act as mechano-receptors, nociceptors, pruriceptors, and thermoreceptors [1, 2]. The majority of these DRG neurons are excitatory and glutamatergic, releasing glutamate, one of the most abundant neurotransmitters, onto postsynaptic neurons in the dorsal horn [3-5]. A subset of DRG neurons also release neuropeptides [6] such as substance P and calcitonin gene-related peptide (CGRP) [1, 4], among others. Glutamate also acts as a peripheral signalling molecule, with its receptors present in the spleen, pancreas, lung, heart, liver, and other organs of the digestive and reproductive systems (reviewed in [7]), as well as the bone microenvironment, where both osteoblasts and osteoclasts release glutamate [8, 9] and in turn respond to extracellular glutamate [10].
Aberrant glutamatergic signalling has been associated with various peripheral diseases, including cancer. As an example, breast cancer cells secrete significant levels of glutamate through the heterodimeric amino acid transporter, system xc- [11, 12], as a consequence of altered glutamine metabolism and changes in cellular redox balance. These cells frequently metastasize to bone [13], where excess glutamate can contribute to bone pathologies [14]. In the restricted bone microenvironment, glutamate acts as a paracrine mediator to coordinate intracellular communication, with even small changes in its levels significantly impacting the skeleton [15]. In addition, the periosteum, bone marrow, and, to a lesser extent, mineralized bone, are innervated by sensory and sympathetic nerve fibres [16]. Notably, these peripheral fibres express functional glutamate receptors and therefore actively respond to this ligand outside of the CNS [17-22].
The majority of breast cancer patients present with bone metastases, which are associated with severe, chronic, and often untreatable bone pain that significantly diminishes a patient’s quality of life [23]. Due to increased early detection and an expanding repertoire of clinically available treatment options, cancer deaths have decreased by 42% since peaking in 1986, although research is ongoing to identify tailored small molecules that target the growth and survival of specific cancer subtypes. Overall improvements in cancer management strategies have contributed to a significant proportion of patients living with cancer-induced morbidities including chronic pain, which has remained largely unaddressed. Available interventions such as non-steroidal anti-inflammatory drugs (NSAIDs) and opioids provide only limited analgesic relief, and are accompanied by significant side-effects that further affect patients’ overall quality of life [24].
Research is therefore focused on developing new strategies to better manage cancer-induced pain. Our laboratory recently conducted a high-throughput screen, identifying potential small molecule inhibitors of glutamate release from triple-negative breast cancer cells [25]. Efforts are underway to characterize the mode of action of a set of promising candidate molecules that demonstrate optimum inhibition of increased levels of extacellular glutamate derived from these cells. While potentially targeting the system xc- cystine/glutamate antiporter, the compounds that inhibit glutamate release from cancer cells do not definitively implicate this transporter, and may instead act through other mechanisms related to glutamine metabolism and calcium (Ca2+) signalling. Alternate targets include the potential inhibition of glutaminase (GA) activity or the transient receptor potential cation channel, subfamily V, member 1 (TRPV1). The benefit of blocking glutamate release from cancer cells, irrespective of the underlying mechanism(s), is to alleviate cancer-induced bone pain, potentially expanding the clinical application of “anti-cancer” small molecule inhibitors as analgesics. Furthermore, investigating these targets may reveal how tumour-derived glutamate propagates stimuli that elicit pain. The following review discusses 1. how dysregulated peripheral glutamate release from cancer cells may contribute to the processing of sensory information related to pain, and 2. methods of blocking peripheral glutamate release and signalling to alleviate pain symptoms.
GLUTAMATE PRODUCTION IN THE TUMOUR: THE ROLE OF GLUTAMINASE (GA)
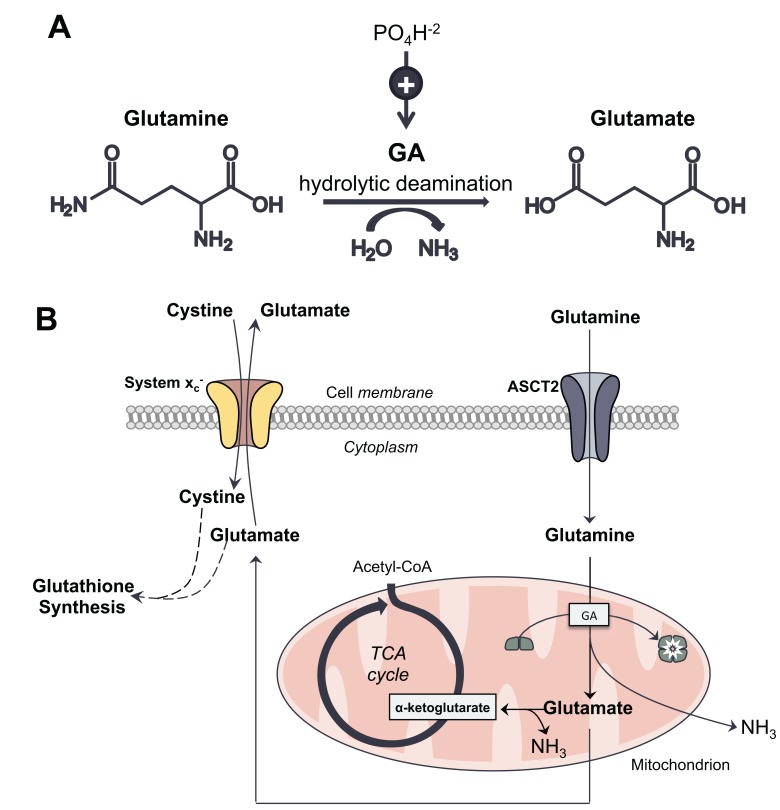
GA, also referred to as phosphate-activated GA, L-glutaminase, and glutamine aminohydrolase, is a mitochondrial enzyme that catalyzes the hydrolytic conversion of glutamine into glutamate, with the formation of ammonia (NH3) [26] (Fig. 1). Glutamate dehydrogenase subsequently converts glutamate into α-ketoglutarate, which is further metabolized in the tricarboxylic acid (TCA) cycle to produce adenosine triphosphate (ATP) and essential cellular building blocks. Glutamate also serves as one of the precursors for glutathione (GSH) synthesis. It is thought that NH3 diffuses from the mitochondria out of the cell, or is utilized to produce carbamoyl phosphate [27]. The enzymatic activity of GA serves to maintain normal tissue homeostasis, also contributing to the Warburg effect [28] by facilitating the “addiction” of cancer cells to glutamine as an alternative energy source [29]. The action of GA in a cancer cell is outlined in Fig. (1B).
Fig. (1).
A. Glutamine, the major circulating amino acid, undergoes hydrolytic deamidation through the enzymatic action of glutaminase (GA), producing glutamate and ammonia (NH3). GA is referred to as phosphate-activated, as the presence of phosphate can up-regulate its activity. B. In cancer cells, glutamine enters the cell through its membrane transporter, ASCT2. It is then metabolized in the mitochondria into glutamate through glutaminolysis, a process mediated by GA, which is converted from an inactive dimer into an active tetramer. Glutamate is subsequently transformed into α-ketoglutarate, which is further metabolized through the TCA cycle to produce pyruvate and NADPH, key cellular energy sources. The high rate of glutamine metabolism leads to excess levels of intracellular glutamate. At the plasma membrane, system xc- transports glutamate out of the cell while importing cystine, which is required for glutathione synthesis to maintain redox balance. NH3, a significant by-product of glutaminolysis, diffuses from the cell.
Structure and Expression Profile of GA
There are currently four structurally unique human isoforms of GA. The glutaminase 1 gene (GLS1) encodes two differentially spliced variants of “kidney-type”, with GLS2 encoding two variants of “liver-type” [29, 30] that arise due to alternative transcription initiation and the use of an alternate promoter [31]. The “kidney-type” GAs differ primarily in their C-terminal regions, with the longer isoform referred to as KGA and the shorter as glutaminase C (GAC) [32], collectively called GLS [33]. The two isoforms of “liver-type” GA include a long form, glutaminase B (GAB) [34], and short form, LGA, with the latter containing a domain in its C-terminus that mediates its association with proteins containing a PDZ domain [35]. The GA isoforms have unique kinetic properties and are expressed in distinct tissues [36]. Table 1 provides a summary of the various GA isoenzymes.
Table 1.
Glutaminase isoenzymes.
| GA | |||||||
|---|---|---|---|---|---|---|---|
| “Kidney-Type” | “Liver-Type” | ||||||
| Short Form | Long Form | Short Form Gene | Long Form | ||||
| Gene | Protein | Gene | Protein | Gene | Protein | Gene | Protein |
| GLS1 | GAC | GLS1 | KGA | GLS2 | LGA | GLS2 | GAB |
A tissue distribution profile of human GA expression revealed that GLS2 is primarily present in the liver, also being detected in the brain, pancreas, and breast cancer cells [37]. Both GLS1 transcripts (KGA and GAC) are expressed in the kidney, brain, heart, lung, pancreas, placenta, and breast cancer cells [32, 38]. GA has also been shown to localize to surface granules in human polymorphonuclear neutrophils [39], and both LGA and KGA proteins are expressed in human myeloid leukemia cells and medullar blood isolated from patients with acute lymphoblastic leukemia [40]. KGA is up-regulated in brain, breast, B cell, cervical, and lung cancers, with its inhibition slowing the proliferation of representative cancer cell lines in vitro [41-45], and GAC is also expressed in numerous cancer cell lines [41, 46]. Two or more GA isoforms may be coexpressed in one cell type (reviewed in [29]), suggesting that the mechanisms underlying this enzyme’s actions are likely complex. Given that the most significant differences between the GA isoforms map to domains that are important for protein-protein interactions and cellular localization, it is likely that each mediates distinct functions and undergoes differential regulation in a cell type-dependent manner [47].
The Functions of GA in Normal and Tissues and Disease
The Kidneys and Liver
In the kidneys, KGA plays a pivotal role in maintaining acid-base balance. As the major circulating amino acid in mammals, glutamine functions as a carrier of non-ionizable ammonia, which, unlike free NH3, does not induce alkalosis or neurotoxicity. Ammonia is thereby “safely” carried from peripheral tissues to the kidneys, where KGA hydrolyzes the nitrogen within glutamine, generating glutamate and NH3. The latter is secreted as free ammonium ion (NH4+) in the urine, thereby maintaining normal pH by reducing hydrogen ion (H+) concentrations. The liver scavenges NH3, incorporating it into urea as a means of clearing nitrogen waste. LGA localizes to distinct subpopulations of hepatocytes [30] and contributes to the urea cycle. During the onset of acidosis, the body diverts glutamine from the liver to the kidneys, where KGA catalyzes the generation of glutamate and NH3, with glutamate catabolism releasing additional NH3 during the formation of α-ketoglutarate. These pools of NH3 are then ionized to NH4+ for excretion.
The Central Nervous System (CNS)
In the CNS, the metabolism of glutamine, glutamate, and NH3 is closely regulated by the interaction between neurons, surrounding protective glial cells (astrocytes), and cerebral blood flow. This controlled metabolism, referred to as the glutamate-glutamine cycle, is essential for maintaining proper glutamate levels in the brain, with GA driving its synthesis [35]. The localization of GA to spinal and sensory neurons indicates that it also serves as a marker for glutamate neurotransmission in the CNS [48]. GA is active in the presynaptic terminals of CNS neurons, where it functions to convert astrocyte-derived glutamine into glutamate, which is then loaded into synaptic vesicles and released into the synapse. Glutamate subsequently undergoes rapid re-uptake by local astrocytes, which recycle it into glutamine, restarting the cycle. As a major neurotoxin, NH3 also factors into this process. Disorders resulting from elevated levels of circulating NH3, such as urea cycle disorders and liver dysfunction, can adversely affect the CNS and, in severe instances, cause death. The primary negative effects of hyperammonemia within the CNS are disruptions in astrocyte metabolism and neurotoxicity. Circulating NH3 that enters the brain reacts with glutamate through the activity of glutamine synthetase to form glutamine, and changes in this process can significantly alter glutamate levels in synaptic neurons, leading to pain and disease [49].
Cancer
The main functions of glutamine are storing nitrogen in the muscle and trafficking it through the circulation to different tissues [50, 51]. While mammals are able to synthesize glutamine, its supply may be surpassed by cellular demand during the onset and progression of disease, or in rapidly proliferating cells. Glutamine is utilized in metabolic reactions that require either its γ-nitrogen (for nucleotide and hexosamine synthesis) or its α-nitrogen/carbon skeleton, with glutamate acting as its intermediary metabolite. Although cancer cells generally have considerable intracellular glutamate reserves, adequate maintenance of these pools requires continuous metabolism of glutamine into glutamate. The GA-mediated conversion of glutamine into glutamate has been correlated with tumour growth rates in vivo [52, 53]. By limiting GA activity, the proliferation of cancer cells decreases, and growth rates of xenografts have been shown to be reduced [54, 55]. Human melanomas exhibit significantly higher GA activity compared to surrounding non-cancerous patient-matched skin [56]. In addition, the expression and activity of GA are up-regulated in various tumour types and cancer cell lines. While glutamine may contribute to cellular metabolism through other mechanisms, the activity of GA is essential for altered metabolic processes that support the rapid proliferation characteristic of cancer cells. Several cellular pathways related to amino acid synthesis, the TCA cycle, and redox balance are supported by glutamine-based metabolism through its intermediary, glutamate (Fig. 1), and metabolites derived from glutamate are directly relevant to tumour growth. These include nucleotide and hexosamine biosynthesis, glycosylation reactions, synthesis of nonessential amino acids, antioxidant synthesis (via GSH), production of respiratory substrates and reducing equivalents, and ammoniagenesis (reviewed in [57]).
Relevance of GA in Other Diseases
In addition to the up-regulation of KGA and GAC in various cancers, which contributes to an altered metabolic state associated with a more aggressive cancer phenotype, GA also contributes to other diseases, some of which are associated with pain. During chronic acidosis, GLS1 expression is up-regulated in the kidneys, and it has been observed that in cultured renal epithelial cells, KGA mRNA levels increase significantly as a means to counter pH changes [58]. Active lesions in multiple sclerosis (MS) express higher than normal levels of GA in macrophages and microglia that closely localize to dystrophic axons [59]. Hyperammonemia within the brain, a typical secondary complication of primary liver disease known as hepatic encephalopathy, affects glutamate/glutamine cycling [60]. Intestinal GA may play a possible role in the pathogenesis of hepatic encephalopathy and has been suggested as a target for novel therapeutic interventions [61]. In hippocampal samples collected from patients with Alzheimer’s disease (AD), the number of pyramidal glutamate- and GA-positive neurons are reduced, with remaining neurons displaying shortened, irregular dendritic fields that are consistent with neurofibrillary tangles typically associated with AD [62]. Post-mortem studies of AD patients have indicated loss of GA activity coupled with reduced glutamate levels and a lower number of pyramidal cell perikarya, which are generally correlated with the severity of dementia [63]. Cortical GA has also been linked with AD [64]. In addition, the activity of GA is lower in other neurologically-linked pathological conditions, including Huntington's disease [65].
GA and Pain
Upon injection into human skin or muscle, glutamate causes acute pain, and painful conditions such as arthritis, myalgia, and tendonitis (reviewed in [66]), as well as MS, are associated with increased glutamate levels in affected tissues. Human chronic pain has been studied using animal models and through the injection of inflammatory agents such as complete Freund’s adjuvant [67]. During inflammation, various neurotransmitters, including glutamate, as well as stimuli such as ATP, cations such as hydrogen ions (H+), and prostaglandins, sensitize afferent primary neurons by lowering their activation threshold, increasing spontaneous neural activity, and increasing and/or prolonging neural firing [66]. One mechanism by which sensory neurons alter their responses to inflammation, noxious stimulation, or tissue damage is to increase the expression and availability of neurotransmitters. Indeed, the levels of glutamate are higher in inflamed tissues, and during inflammation, glutamate sensitizes the axons of primary afferent neurons by decreasing their firing threshold and inducing a hyper-excitable state [68]. The primary afferent neuron may act as a significant possible source of glutamate, and in both humans and animal models, antagonism of glutamate receptors that are expressed on axons of primary afferent neurons during inflammation lessens pain [66]. It has been shown that the peripheral inhibition of GA using 6-diazo-5-oxo-l-norleucine (DON) relieves inflammatory pain, which is supported by work in rats demonstrating that GA itself may act as a peripheral inflammatory mediator [69]. Inflammation also up-regulates the expression of substance P and CGRP in the DRG [70, 71] and the spinal dorsal horn [72], as well as in the joints and skin [73, 74], with these changes providing a marker of pain-sensing neurons. Neurons that release substance P and CGRP are also glutamatergic [75, 76] and produce glutamate through enhanced GA activity [66, 77]. However, how chronic glutamate production is regulated in pain models remains understudied.
It is known that in response to noxious stimuli, acute glutamate release from primary afferent terminals [78-81], occurring concomitant with the release of substance P and CGRP, drives spinal neuron sensitization, which has been associated with chronic changes [82]. Induced inflammation in the simian knee joint increases fibers in the spinal cord that are immunoreactive for glutamate by approximately 30% at 4 hours and 40% at 8 hours, consistent with a sustained effect [83]. Indeed, in rat spinal cords, extracellular glutamate levels are 150% higher than controls at 24 hours [80], further supporting that glutamate release from central primary afferent neurons is prolonged and activity-dependent during inflammation. These findings indicate that the production and release of glutamate are altered in response to pain, most likely due to modified flux control and local changes in the GA-mediated glutamate-glutamine cycle [84]. In support of this latter notion, persistent inflammation, which was experimentally induced by complete Freund’s adjuvant in a rat model of arthritis, was shown to increase GA expression and enzymatic activity in DRG neurons [85]. It was hypothesized that elevated GA in primary sensory neurons could increase the production of glutamate in spinal primary afferent terminals, thereby either directly contributing to central or peripheral sensitization [85]. In an animal model of MS, GA was found to be highly expressed and correlated with axonal damage in macrophages and microglial cells associated with active lesions [59]. A comparison of white matter from various inflammatory neurologic diseases, including MS, with non-inflammatory conditions revealed high GA reactivity only during inflammation [59]. It is likely that dysregulated glutamate homeostasis contributes to axonal dystrophy in MS, and that manipulating the imbalanced glutamate-glutamine cycle may be of therapeutic relevance. GA, as an important regulator of glutamate production, could therefore be targeted for the development of novel therapeutics aimed at treating pain, including cancer-induced pain.
The Regulation of GA
GA activity is regulated through several mechanisms. In vitro, the enzyme may be stimulated by adding inorganic phosphate, and it is therefore often referred to as phosphate-activated (Fig. 1). While exposure to low phosphate levels activates LGA, a response that is not inhibited by glutamate, KGA activity is dependent on high levels of phosphate and can be inhibited by glutamate [36]. In particular, GAC transitions from a dimer to an active tetramer in vitro following the addition of 50 to 100 mM of inorganic phosphate [36, 86].
The conditions above suggest that LGA and KGA are differentially regulated. One activator of GLS2/LGA is adenosine diphosphate (ADP), which lowers the enzymatic Km, with the opposite effect occurring in the presence of ATP, and both effects dependent on mitochondrial integrity [87]. GLS2 is linked with increased metabolism, decreased levels of intracellular reactive oxygen species (ROS), and decreased DNA oxidation in both normal and stressed cells. It has been suggested that the control of ROS levels by GLS2 is mediated by p53 as a means of protecting cells from DNA damage, also supporting cell survival in response to genotoxic stress [27]. Depending on the cell type, as well as the level and type of stress, the extent of GLS2 transcriptional up-regulation by p53 differs in normal and cancer cells [27].
Positive Regulators
Relative to healthy tissue, the levels of GLS protein are increased in breast tumours [41]. In particular, increased GAC levels have been associated with a higher grade of invasive ductal breast carcinoma [33]. The oncogene c-Myc positively affects glutamine metabolism, as its up-regulation is sufficient to drive mitochondrial glutaminolysis [88, 89]. Of the two GLS isoforms, mitochondrial GAC is stimulated by c-Myc in transformed fibroblasts and breast cancer cells [41]. c-Myc also indirectly influences GLS expression through its action on microRNA (miR) 23a and 23b [54]. Under normal conditions, miR23a and b bind to the 3’ untranslated region of GLS transcripts, thereby preventing translation. c-Myc transcriptionally suppresses miR-23a/b expression, de-repressing the block on GLS translation and thereby facilitating glutamine metabolism [54]. Interestingly, acting through its p65 subunit, NF-κB also positively regulates GLS expression by inhibiting miR-23a [90]. NF-κB is the common intermediary that modulates GA activation downstream of Rho GTPase signalling [2]. Another protein regulating glutamine metabolism is signal transducer and activator of transcription (STAT) 1, the phosphorylated/activated form of which binds within the GLS1 promoter region, with interferon alpha (IFNα) -stimulated STAT1 activation up-regulating GLS1 expression [91]. Mitogen-activated protein kinase (MAPK) signaling and changes in GA expression are also linked based on a report demonstrating that KGA binds directly to MEK-ERK [92]. Activation of the MEK-ERK pathway in response to epidermal growth factor (EGF) treatment, or pathway inactivation by the selective MEK1/2 inhibitorU0126, activates or represses KGA activity, respectively, suggesting a phosphorylation-dependent mode of regulation [92]. This latter point is in line with alkaline phosphatase exposure completely blocking basal GAC activity [41].
Negative Regulators
There are several mechanisms by which GA is negatively regulated. Anaphase-promoting complex/cyclosome (APC/C) associates with cadherin 1 (CDH1), acting as a ubiquitin ligase to down-regulate GA [93]. The APC/C–CDH1 complex targets proteins with either a destruction box (D box; [RH]xxLxx[LIVM]) or KEN box (Lys-Glu-Asn) for ubiquitination, followed by targeted proteosomal degradation. Of the two GLS1 splice variants, only KGA has both boxes in its C terminus [93], making the APC/C-CDH1 pathway a potential target for down-regulating KGA in cancer cells. Another negative GA regulator is Lon protease, which localizes to the mitochondrial matrix and preferentially targets misfolded or unassembled proteins [94]. Diphenylarsinic acid (DPAAV) rapidly promotes Lon protease-mediated GAC tetramer dissociation and subsequent proteosomal degradation in a human hepatocarcinoma cell line without affecting GAC mRNA levels or translation [94].
GLUTAMATE RELEASE FROM THE TUMOUR: SYSTEM xc-
Glutamate release from cancer cells has been associated with over-expression of the system xc- cystine/glutamate antiporter [95, 96], which is up-regulated as an antioxidant defense mechanism to counter high levels of ROS associated with altered glutamine metabolism. The primary role of system xc- in the tumour is to acquire cystine for the intracellular synthesis of GSH [97]. In addition to GSH synthesis within the cell, cystine reduction to cysteine across the plasma membrane also confers antioxidant potential by mitigating extracellular levels of ROS [98]. As an obligatory antiporter, import of cystine through system xc- must be coupled to the release of glutamate. Increased levels of glutamate are ultimately a by-product of the dysregulated, malignancy-associated metabolic changes that promote the rapid growth and continuous survival of cancer cells. This phenomenon has been well documented [99, 100]. System xc- activity may be regulated through several mechanisms, including by glutamate itself [101], as well feedback from changes in cellular redox balance. Its expression at the mRNA level is affected by ROS in MCF-7 human breast cancer cells via the KEAP-1/NRF2 pathway [102], nutrient sensing as mediated by ATF4 in human T24 bladder carcinoma cells [103], STAT3 and/or STAT5-mediated signalling in human breast cancer cells [104], and in response to the RNA-binding protein huR in primary mouse astrocytes [105]. We have shown that system xc- contributes to cancer-induced bone pain, as inhibition of glutamate release with sulfasalazine [13] attenuates mechanical allodynia in an animal model [11]. Importantly, glutamate transport through system xc- represents an intermediate mechanism linking the dysregulated production of glutamate at the tumour site with its detrimental extracellular effects (reviewed by [106]), including the glutamate-promoted migration and invasion potential of aggressive cancer cells [107] and increased cancer-induced pain. Having implicated this particular transporter in in vivo pain models, the focus of this review is to discuss the possible mechanisms by which excess glutamate initiates nociceptive responses in cancer.
PERCEPTION OF EXTRACELLULAR GLUTAMATE IN THE PERIPHERY: TRPV1 AND ITS INTER-ACTION WITH GLUTAMATE RECEPTORS
TRVP1 was first identified based on its response to heat and vanilloids such as capsaicin [108]. It is a gated, non-selective cation channel of the transient receptor potential family composed of identical tetramers comprised of six transmembrane domains [109]. TRPV1 is permeable to Ca2+ and localizes to both spinal nociceptive afferent fibres [110-112] and supraspinal structures where they can also play a role in central sensitization [113, 114], enabling it to modulate membrane potential and to transduce sensory signals along excitable cells. Cation permeability of TRPV1 is not static and can vary its ionic selectivity based on both the type and concentration of agonist [115]. Therefore, this channel plays a major role in integrating a variety of noxious stimuli [112] with pain perception by initiating and propagating nociceptive signalling cascades along small, unmyelinated primary afferent fibres [108].
Regulation of TRPV1
TRPV1 is subject to sensitization and desensitization by a diverse range of factors that can both directly and indirectly activate channel activity through recognition and/or phosphorylation sites on TRPV1.
Positive Regulators of TRPV1
Commonly described as a thermoreceptor, TRPV1 is physiologically activated at temperatures greater than 43°C. It is also directly gated by protons that initiate signaling at a non-physiological change in pH below 5.9. Endogenous TRPV1 ligands include the fatty acid-like molecule anandamide, as well as N-arachidonoyl dopamine (NADA) and N-oleoyldopamine (OLDA), which are both metabolites of arachidonic acid [116]. Interestingly, diacylglycerol (DAG) can directly activate TRPV1, linking it to G-protein coupled receptor (GPCR) signalling [117]. In this manner, TRPV1 is sensitized by downstream signalling mediators that include phospholipase C (PLC), protein kinase A (PKA), and protein kinase C (PKC). This channel can also be activated by exogenous vanilloids such as capsaicin, the pungent component of chilli peppers, and resiniferatoxin (RTX), a naturally occurring capsaicin analog found in the Euphorbia plant [112]. TRPV1 agonists constitute a diverse population of small molecule ligands that have been extensively reviewed [118].
In response to tissue injury and inflammation, endogenous factors are modulated in order to increase the response to pain, whereby pain-transducing factors are up-regulated in sensory nerve endings, heightening their ability to perceive noxious stimuli associated with pathological changes. Translocation of TRPV1 to the cell membrane is essential for its activity and is mediated by a variety of factors, including bradykinin, insulin-like growth factor (IGF-1) [119], and nerve growth factor (NGF) [120]. Ultimately, TRPV1 activation is voltage dependent, relying on membrane depolarization. The specific factors that initiate channel activation also, in part, shift the membrane potential to a voltage that sensitizes the channel to temperature [121]. Therefore, persistent depolarization of neurons would be expected to reduce the threshold for temperature-mediated activation of TRPV1, allowing it to propagate allodynia and hyperalgesia in response to physiological changes in temperature [121].
Negative Regulators of TRPV1
Due to its role in pain signalling, TRPV1 is an attractive pharmacological target for the development of analgesics. Capsazepine was the first competitive antagonist developed against TRPV1 [122]. A more potent antagonist was created by modifying the agonist, Resiniferatoxin (RTX), generating 5-iodo-RTX (IRTX), which has a forty times higher affinity for TRPV1 compared to capsazepine [123]. Interestingly, TRPV1 is susceptible to desensitization by agonists such as capsaicin, where prolonged exposure decreases the receptor’s ligand-mediated response, thereby providing long-lasting but reversible analgesia in a complex process reviewed by Touska et al. [124]. A heterogenous population of TRPV1 antagonists and their therapeutic potential have also been comprehensively reviewed [125]. Phosphatidylinositol 4,5-bisphosphate (PIP2) has also been shown to tonically inhibit TRPV1 at the membrane in lieu of PLC activity [126].
The Function of TRPV1 in Cancer
TRPV1 expression has been documented in colon [127], pancreatic [128], and prostate [129] cancers. Interestingly, the effects of capsaicin vary between cancer cell types, possibly due to off-target effects or the level of channel expression. Also, the role of TRPV1 in cell proliferation varies, which could be due to the degree of Ca2+ signalling induced by channel activation. For example, it has been shown that capsaicin does not affect the proliferation of TRPV1-expressing MCF-7 breast cancer cells, but does induce apoptosis [130]. The latter effect has recently been associated with a rise in intracellular free Ca2+ concentrations upon TRPV1 activation [131]. The same anti-tumour activity has been observed in gliomas, in which TRPV1 gene expression is inversely correlated to tumour grade [132]. However, due to the heterogeneity of responses elicited by TRPV1 activation in cancer cells, therapeutically targeting this channel may present a risky strategy, as its inhibition has been reported to promote proliferation in some cancers [133]. Expression levels of TRP family proteins, including TRPV1, can be used as a marker of cancer progression [134]. In addition, TRPV1 expression levels in peripheral cancers have been correlated to pain scores [128], suggesting that channels not directly localizing to afferent nerve terminals may initiate a pain response, possibly by inducing the release of mediators such as glutamate from these terminals [135]. In an osteosarcoma model of bone cancer-induced pain, TRPV1 expression increased in the DRG [136], and TRPV1 antagonists inhibit both central [113] and peripheral [137] nociceptive transmission.
TRPV1 Activation in Response to Inflammation
TRPV1 levels in DRG and spinal neurons increase in response to inflammation [120] and the presence of tumour-secreted factors [138] through signal transduction pathways that overlap with those engaged by lipopolysaccharide (LPS) [139, 140]. Peripheral inflammation induces the MAPK signalling cascade in nociceptive neurons, which increases both TRPV1 levels in the DRG and the subsequent transfer of these channels to peripheral terminals of nociceptive neurons, thereby promoting hypersensitivity [120]. Initiation of the MAPK cascade lies downstream of Toll-like receptor 4 (TLR4) activation in trigeminal sensory neurons [141]. Cancer cells secrete damage associated molecular patterns (DAMPs) [142-144] which can activate TLR4 receptors on peripheral sensory neurons proximal to tumour. Therefore, the role of TLR4 extends beyond that of the innate immune response and plays a role in non-infectious excitation of primary sensory neurons (Reviewed in [145]), including sensitization of TRPV1 on sensory nociceptive fibres (Fig. 2) [139]. Furthermore, TLR4/MAPK signalling also induces the release of pro-inflammatory cytokines such as interleukin 1-beta (IL-1β) and tumour necrosis factor-alpha (TNF-α) from tumour-infiltrating immune cells, and by cancer cells themselves [146], which can in turn activate/sensitize TRPV1 channels [147, 148]. MAPKs also influence PKA and PKC activity in modulating neuronal excitability [149], which are both known regulators of TRPV1 activity [150-152].
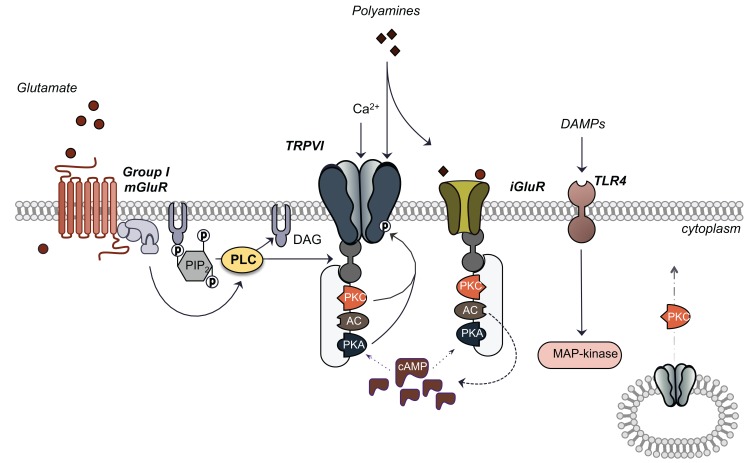
Fig. (2).
TRPV1 located on peripheral afferent terminals of sensory neurons indirectly responds to increased local levels of extracellular glutamate secreted from the tumour. Glutamate-mediated activation of TRPV1 occurs via metabotropic glutamate receptors of the group I class as well as ionotropic glutamate receptors that integrate downstream signalling kinase-mediated signalling cascades. Protein Kinase C (PKC) and Protein Kinase A (PKA) phosphorylate and therefore sensitize and/or activate TRPV1. Activation of these kinases lies downstream of mGluR-coupled phospholipase C (PLC) activation which promotes the association of anchoring kinase association protein 79/150 (AKAP 79/150) to TRPV1 where it localizes kinase activity proximal to the channel. AKAP 79/150 also tethers to iGluRs which can mediate activity of anchored kinases. This scaffold system also includes adenylyl cyclase (AC) which promotes cAMP production aiding in activation of PKA. PKC also mediates the translocation of cytoplasmic TRPV1 to the plasma membrane in response to stimuli. In addition to glutamate, exogenous, tumour-secreted factors initiate TRPV1 activation through iGluR polyamine recognition sites and danger associated molecular pattern-induced toll-like receptor 4 (TLR4) activation.
Role for TRPV1 in Cancer-Induced Pain
Numerous studies have documented the role of TRPV1 in nociception in diverse tissues, including those composed of non-excitable cells. The potential role of TRPV1 in the propagation of cancer-induced pain will therefore be discussed with a focus on its peripheral effects and how the channel functions in conjunction with glutamatergic signalling to evoke a nociceptive response from peripheral (tumour-secreted) mediators.
In the periphery, glutamate, a mediator of inflammation and tissue injury, plays a role in physiological nociceptive transmission [153] through both ionotropic [154-156] and metabotropic [157, 158] glutamate receptor activation. Several studies have shown that in both humans [159, 160] and animal models [19, 161, 162], glutamate is released from peripheral terminals of C-fiber neurons, increasing its local concentration. This excitatory amino acid is then able to stimulate neighboring glutamate receptors in an autocrine fashion, promoting not only the development, but also the maintenance and propagation, of pain. Many of these nociceptive responses can be blocked by local, peripheral administration of ionotropic glutamate receptor antagonists [20, 154, 156].
The transmission of sensory information by glutamate and glutamate receptor activation is potentiated by TRPV1 phosphorylation. TRPV1 contains phosphorylation sites on its cytoplasmic N- and C-termini, and its phosphorylation status underlies its ability to respond to noxious stimuli [163]. Extracellular glutamate in the periphery promotes phosphorylation of TRPV1 on the terminals of primary afferents, resulting in channel sensitization. Group I metabotropic glutamate receptors (mGluRs; R1 and R5) are also expressed on the peripheral termini of unmyelinated nociceptive afferents, propagating glutamate-induced hyper- and thermal sensitivity [17]. Activation of group I mGluRs by peripheral glutamate induces DAG production via PLC. DAG can then activate TRPV1 directly [117] or through downstream activation of protein kinases [150]. In addition, PKC [151, 164, 165] and PKA [166] have both been shown to phosphorylate and activate TRPV1 activity downstream of glutamate receptor activation. In this manner, increases in local extracellular glutamate levels can initiate a nociceptive response. This nociceptive processing can be amplified by increasing the number of TRPV1 receptors that are available on peripheral afferents.
Interestingly, PKC signalling also initiates TRPV1 translocation from vesicular pools to the plasma membrane of sensory neurons (Fig. 2) [119, 165], enhancing neural transmission in response to noxious stimuli, as well as the maintenance of hyperalgesia. Transport of TRPV1 from the dorsal root ganglion to peripheral nerve terminals has also been observed in response to peripheral inflammation via retrograde transport of NGF from a peripheral site of inflammation to the DRG. In the DRG, NGF induces sustained MAPK activation, increasing TRPV1 translation and its transport to peripheral terminals [120]. In addition to its signalling in the DRG, NGF also plays a role in sensitizing the peripheral TRPV1 channels, again through a PKC-mediated mechanism [167, 168]. Together, these observations illustrate a mechanism by which peripheral glutamate engages TRPV1 in a nociceptive response and promotes ongoing nociceptive signalling.
Pro-inflammatory agents are also able to activate the TRPV1 channel through second messenger signalling cascades [112] that lead to the development of inflammatory hyperalgesia through PLC activation [169]. Extracellular agonists of TRPV1 increase during inflammation and in response to cancer [170, 171]. In particular, polyamines are often produced during inflammation, and increased pools of these organic cations have also been observed in tumour cells. As by-products of amino acid metabolism, the synthesis and catabolism of polyamines may contribute to tumourigenesis (reviewed by [172]). Therefore, TRPV1 activation by tumour-derived polyamines provides another potential mechanism that propagates cancer-induced pain signals. Polyamines are able to directly sensitize and activate TRPV1 channels and to induce pain behaviours [170, 173, 174]. The pain responses induced by polyamines can also be mediated indirectly by glutamatergic input independent of substance P [174]. In this case, glutamate mediates polyamine-induced activation of TRPV1 through N-methyl-D-aspartate (NMDA) ionotropic glutamate receptors (iGluR). NMDA receptors are responsible for increased synaptic strength and long-term potentiation of C-fiber synapses [175, 176]. They modulate TRPV1 activity through protein kinase-directed phosphorylation mechanisms (Fig. 2) [177-180]. Similar to mGluR expression, NMDA receptors localize along the length of DRG neurons, including their peripheral processes [18], where they would be proximal to TRPV1 channels. The functional localization of these glutamate receptors on peripheral afferent terminals has been further confirmed by the induction of allodynia and hyperalgesia following peripheral administration of agonists against this class of ionotropic receptor [21].
Scaffolding proteins mediate the interactions between protein kinases and TRPV1 to promote ion channel phosphorylation. The A kinase anchoring protein 79 (human)/150 (rat) (AKAP 79/150) facilitates the targeting of PKC and PKA to TRPV1, and underlies the NMDA-induced activation of TRPV1 via PKC in sensory neurons [177]. AKAP150 (rat) mediates the formation of a signalling complex that initiates nociceptor sensitization [177]. AKAP79/150 also facilitates cyclic AMP (cAMP)-dependent PKA phosphorylation of TRPV1 by coupling this signalling complex to adenylyl cyclase. This increases the local concentration of cAMP around TRPV1 and the co-anchored PKA, enhancing phosphorylation and sensitization of TRPV1 (Fig. 2) [181]. Similarly, peripheral mGluR-induced TRPV1 activation is dependent on AKAP150 [182, 183]. Therefore, formation of organized signalling complexes on AKAP ensures rapid phosphorylation of TRPV1 in response to local signals, including cancer-secreted glutamate, revealing another mechanism by which this neurotransmitter can induce sensitization of peripheral sensory neurons and induce pain behaviours. Interestingly, NMDA receptors contain polyamine recognition sites, which potentiate NMDA activation only in the presence of glutamate and glycine [184]. Taken together, it can be hypothesized that excess tumour-derived glutamate initiates a pain response via paracrine activation of glutamate receptors on peripheral afferent terminals of nociceptive fibres, propagating a central pain response via subsequent activation of proximal TRPV1 receptors.
When applying these interconnected processed to the bone microenvironment, it becomes evident how these mechanisms are able to play a role in propagating cancer-induced bone pain signals. TRPV1 is expressed on sensory fibres that innervate mineralized bone [185]. This channel can therefore respond to local changes in the bone microenvironment that arise in response to dysregulated glutamate signalling, which is known to occur in the presence of cancer [186-189]. Given the multiple chemical mediators, including those involved in inflammation and bone degradation (acid), as well as tumour-secreted factors (glutamate), TRPV1 may become sensitized and activated under physiological conditions by its endogenous agonists. Indeed, subcutaneous injection of capsaicin has been shown to induce peripheral glutamate release, which could be inhibited by pre-administration of capsazepine, a TRPV1 antagonist [149, 190].
Development of Therapeutic Interven-tions
The role of glutamate-induced nociception in cancer-induced pain implicates several targets that, when blocked, may inhibit the production and release of glutamate from the tumour, also potentially affecting the perception of glutamate as a noxious stimulus by peripheral primary afferent nerve endings.
Inhibition of GA
DON, a non-selective irreversible glutamine-competitive inhibitor with several other targets [191], has been used to effectively block KGA activity [192]. Other glutamine analogs with diazo groups, including azaserine and acivicin, irreversibly bind to the active site of GA, but also affect various other enzymes that utilize glutamine as a substrate, as well as glutamine transporters [191]. DON and azotomycin showed promising antitumour activity against human cell lines implanted into nude mice, although clinical trials with these small-molecule inhibitors were less efficacious and associated with toxicities [193]. New small molecules have been discovered that inhibit both KGA and its GAC splice variant. Molecule 968, although resisting enzyme co-crystallization, allosterically regulates GAC without competing with glutamine [41, 194]. Its inhibitory potential has been described in various cancer cell lines in vitro and in a mouse xenograft model [41], although its hydrophobic nature has made it difficult to apply in vivo. The effects of 968 on metabolically sensitive epigenetic markers and their effects on cancer-related genes were also examined. In this context, GA inhibition enhanced histone acetylation at H4 while down-regulating the expression of AKT and ERBB2, suggesting that 968 could potentially be applied as an effective epigenetic therapeutic agent [195, 196]. In addition, 968 has been used to test whether GA-driven glutamine metabolism has evolved in cancer cells more as a means to control intracellular pH through the release of NH3 than to provide metabolites to fuel the TCA cycle [43]. Although not in line with established doctrine, this study presents evidence that modulating cellular acidity is an important component of glutamine metabolism. Glutamine withdrawal elicits less drastic effects on the viability of HeLa or MCF-7 cells when their growth media is maintained at a neutral pH 7.3 rather than under acidic conditions (corresponding to pH 6.3), with 968 treatment inhibiting cell proliferation only at the lower pH. However, cell lines resistant to glutamine withdrawal have been shown to regain sensitivity to this amino acid when exposed to glutamine synthetase inhibitors, and glutamine synthetase, through its production of glutamine, consumes NH3, thereby potentially acidifying the cellular microenvironment, which were not considered in the study [197]. Nevertheless, these findings present an intriguing secondary consequence of glutamine metabolism in cancer cells, meriting further investigation into acid/base balance.
Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) has emerged as an important allosteric GA inhibitor that specifically targets KGA over LGA. BPTES binds at the interface where two KGA dimers join to form a tetramer, stabilizing a region near its active site and controlling access to its catalytic pocket, thereby inactivating the enzyme [198-201]. Similar to 968, BPTES inhibits KGA activity in various tumour types [44, 45], but, unlike 968, BPTES remains effective even in the presence of inorganic phosphate. BPTES analogs have been designed to improve upon its poor metabolic stability and low aqueous solubility [201]. Other small molecules have been described that inhibit KGA and/or GAC [202], including thiourea molecules designed to function as farnesyl diphosphate mimetics that haven been proven to be efficacious against GA activity [203]. However, even the most potent novel compound was less efficacious than 968, BPTES, or DON.
Recently, CB-839, a novel, orally bioavailable inhibitor selective for KGA and GAC, has been developed and characterized, which potently blocks the proliferation of HCC-1806 triple-negative breast cancer cells in vitro while also decreasing glutamine catabolism and the levels of glutamate, GSH, and intermediates of the TCA cycle [204]. Interestingly, in the T47D estrogen receptor-positive breast cancer cell line, no anti-proliferative effect was observed in response to CB-839, although its administration did modestly down-regulate glutamine metabolism and the levels of its metabolites. A screen of 23 breast cancer cell lines revealed that while expression of LGA, KGA, and GAC could be detected at some level in most cells, GAC protein levels were high, primarily in triple-negative cell lines compared to estrogen-receptor positive cells. In addition, the triple-negative subtype was associated with increased GA activity and was also most sensitive to CB-839 treatment. In two xenograft models, CB-839 mediated significant anti-tumour activity. CB-839 may therefore be a promising novel therapeutic molecule for targeting glutamine-dependent tumours in patients, as well for treating cancer-induced pain or inflammatory pain linked to increased glutamate levels in the CNS, meriting further investigation and clinical testing.
Inhibition of TRPV1
TRPV1 has emerged as an attractive target for pharmacological intervention in pathological conditions associated with pain, including cancer-induced bone pain [185, 205]. Desensitization of TRPV1 on peripheral afferent terminals renders these termini insensitive to a wide range of agonists that induce nociception through channel activation, including glutamate. TRPV1 antagonism has been an active area of medicinal chemistry, resulting in the synthesis of novel antagonists (reviewed in [206]). Some of these compounds display only modest efficacy in reducing nociceptive behaviours associated with chronic pain, potentially due to the multi-modal nature of TRPV1 sensitization [207]. However, A-425619, AMG 9810, AMG 517, and AMG 8163 display antagonism against heat-, proton- and capsaicin-induced TRPV1 activation, demonstrating enhanced abilities to reduce pain [206]. JNJ-17203212 has been shown to relieve pain symptoms in an osteolytic sarcoma model, specifically implicating TRPV1 antagonism with reduced cancer-induced bone pain [185].
The effectiveness of a potential TRPV1-targeted therapeutic agent for treating pain may vary given the array of stimuli that modulate TRPV1 activity. Targeting TRPV1 also poses the risk of impairing the perception of noxious stimuli to such an extent as to evoke pathological changes in core body temperature and increasing the risk of burn-related injuries [208, 209]. Recently, a study aimed at elucidating the mechanism controlling the physical opening of the TRPV1 channel in response to extracellular stimuli has implicated its hydrophobic interaction with lipid rafts [210]. Novel pharmacological developments could potentially aim to target this particular interaction in an effort to better regulate TRPV1 activity.
SUMMARY
The uncontrolled proliferation of cancer cells is promoted by significant metabolic adaptations that accommodate an increased demand for energy and metabolic intermediates. This is reflected by GA up-regulation in cancer cells, promoting the production of glutamate, an essential metabolic substrate. With the energetic requirements in place to support rapid growth, cancer cells must be able to clear increased levels of ROS that accompany elevated metabolic rates, which otherwise would impair their survival due to oxidative stress. The need to maintain redox balance is met by up-regulating the system xc- cystine/glutamate antiporter, which provides cancer cells with a source of cysteine, the rate-limiting substrate of GSH synthesis. In order to produce adequate levels of GSH, high levels of glutamate are exported by system xc- in exchange for cystine across the plasma membrane. The increasing extracellular levels of glutamate may be what ultimately drive the propagation of noxious stimuli through the activity of receptors located on the terminal ends of primary afferent sensory neurons. Metabotropic and ionotropic glutamate receptors relay sensory information through proximal TRPV1 receptors, thereby linking peripheral sensory information to the CNS and initiating a pain response. This hypothesis is depicted in Fig. (3). Together, GA, system xc-, and TRPV1 underlie the pathological role of dysregulated glutamatergic signalling associated with cancer-induced pain. Each acts as a potential therapeutic target to diminish the production of and response to glutamate in the periphery. This offers the potential for developing novel interventions that limit dysregulated glutamatergic signalling and the associated debilitating side-effects related to cancer-induced pain that compromise patient quality of life.
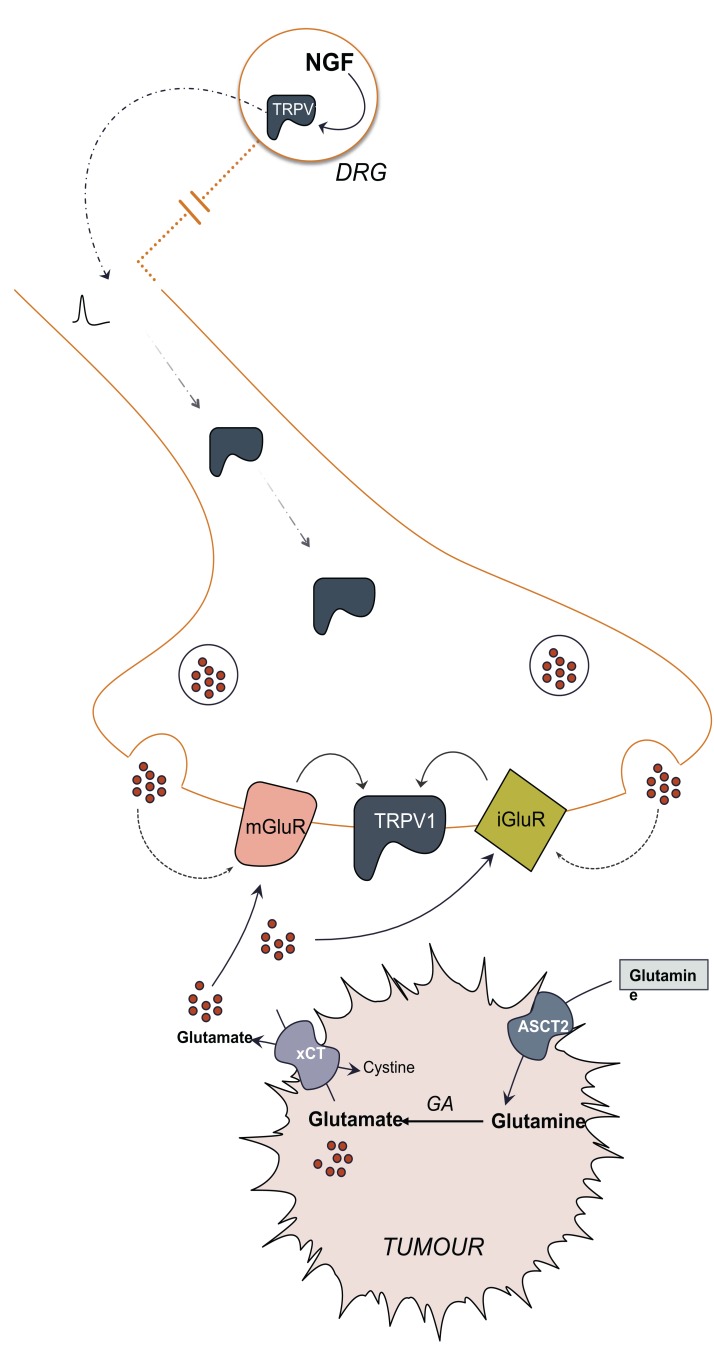
Fig. (3).
Overview of peripheral nociception induced by tumour-derived glutamate. Dysregulated cancer cell metabolism promotes glutamine uptake by ASCT2 transporter and production of large intracellular glutamate pools that drive the activity of the cystine/glutamate transporter, xCT to accommodate the intracellular demand for cysteine, the limiting reagent in glutathione synthesis. Upregulation of glutaminase (GA) and system xc- increases the extracellular concentration of glutamate that can be perceived by proximal nociceptive terminals that can translate glutamate into a nociceptive signal by integrating the activities of glutamate receptors with TRPV1. These terminals also release glutamate that can act in an autocrine fashion also activating these same glutamate receptors. TRPV1 translocation to these terminals increases in response to peripheral noxious stimuli through the action of NGF in the dorsal root ganglion.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [http://dx.doi.org/10.1016/j.cell.2009.09.028]. [PMID: 19837031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delmas P., Hao J., Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 2011;12(3):139–153. doi: 10.1038/nrn2993. [http://dx.doi.org/10.1038/ nrn2993]. [PMID: 21304548]. [DOI] [PubMed] [Google Scholar]
- 3.Broman J., Anderson S., Ottersen O.P. Enrichment of glutamate-like immunoreactivity in primary afferent terminals throughout the spinal cord dorsal horn. Eur. J. Neurosci. 1993;5(8):1050–1061. doi: 10.1111/j.1460-9568.1993.tb00958.x. [http://dx.doi.org/10.1111/j.1460-9568.1993.tb00958.x]. [PMID: 7904222]. [DOI] [PubMed] [Google Scholar]
- 4.De Biasi S., Rustioni A. Glutamate and substance P coexist in primary afferent terminals in the superficial laminae of spinal cord. Proc. Natl. Acad. Sci. USA. 1988;85(20):7820–7824. doi: 10.1073/pnas.85.20.7820. [http://dx. doi.org/10.1073/pnas.85.20.7820]. [PMID: 2459717]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider S.P., Perl E.R. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. J. Neurosci. 1988;8(6):2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [PMID: 2898513]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hökfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7(6):867–879. doi: 10.1016/0896-6273(91)90333-u. [http://dx.doi.org/10.1016/0896-6273(91) 90333-U]. [PMID: 1684901]. [DOI] [PubMed] [Google Scholar]
- 7.Gill S.S., Pulido O.M. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol. Pathol. 2001;29(2):208–223. doi: 10.1080/019262301317052486. [http://dx.doi.org/10.1080/ 019262301317052486]. [PMID: 11421488]. [DOI] [PubMed] [Google Scholar]
- 8.Mysona B., Dun Y., Duplantier J., Ganapathy V., Smith S.B. Effects of hyperglycemia and oxidative stress on the glutamate transporters GLAST and system xc- in mouse retinal Müller glial cells. Cell Tissue Res. 2009;335(3):477–488. doi: 10.1007/s00441-008-0742-1. [http://dx.doi.org/10. 1007/s00441-008-0742-1]. [PMID: 19156441]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akerboom T.P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [http://dx.doi.org/10.1016/ S0076-6879(81)77050-2]. [PMID: 7329314]. [DOI] [PubMed] [Google Scholar]
- 10.Patel S.A., Warren B.A., Rhoderick J.F., Bridges R.J. Differentiation of substrate and non-substrate inhibitors of transport system xc(-): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology. 2004;46(2):273–284. doi: 10.1016/j.neuropharm.2003.08.006. [http://dx.doi.org/10.1016/j.neuropharm.2003.08.006]. [PMID: 14680765]. [DOI] [PubMed] [Google Scholar]
- 11.Ungard R.G., Seidlitz E.P., Singh G. Inhibition of breast cancer-cell glutamate release with sulfasalazine limits cancer-induced bone pain. Pain. 2014;155(1):28–36. doi: 10.1016/j.pain.2013.08.030. [http://dx.doi.org/10.1016/ j.pain.2013.08.030]. [PMID: 23999057]. [DOI] [PubMed] [Google Scholar]
- 12.Timmerman L.A., Holton T., Yuneva M., Louie R.J., Padró M., Daemen A., Hu M., Chan D.A., Ethier S.P., van’t Veer L.J., Polyak K., McCormick F., Gray J.W. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24(4):450–465. doi: 10.1016/j.ccr.2013.08.020. [http://dx.doi.org/10.1016/j.ccr.2013.08.020]. [PMID: 24094812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gout P.W., Buckley A.R., Simms C.R., Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001;15(10):1633–1640. doi: 10.1038/sj.leu.2402238. [http://dx.doi.org/10. 1038/sj.leu.2402238]. [PMID: 11587223]. [DOI] [PubMed] [Google Scholar]
- 14.Shih A.Y., Murphy T.H. xCt cystine transporter expression in HEK293 cells: pharmacology and localization. Biochem. Biophys. Res. Commun. 2001;282(5):1132–1137. doi: 10.1006/bbrc.2001.4703. [http://dx.doi.org/10. 1006/bbrc.2001.4703]. [PMID: 11302733]. [DOI] [PubMed] [Google Scholar]
- 15.Murphy T.H., Schnaar R.L., Coyle J.T. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4(6):1624–1633. [PMID: 2180770]. [PubMed] [Google Scholar]
- 16.Mach D.B., Rogers S.D., Sabino M.C., Luger N.M., Schwei M.J., Pomonis J.D., Keyser C.P., Clohisy D.R., Adams D.J., OLeary P., Mantyh P.W. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–166. doi: 10.1016/s0306-4522(02)00165-3. [http://dx.doi.org/10.1016/S0306-4522(02)00165-3]. [PMID: 12123694]. [DOI] [PubMed] [Google Scholar]
- 17.Bhave G., Karim F., Carlton S.M., Gereau R.W., IV Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat. Neurosci. 2001;4(4):417–423. doi: 10.1038/86075. [http://dx.doi.org/10. 1038/86075]. [PMID: 11276233]. [DOI] [PubMed] [Google Scholar]
- 18.Carlton S.M., Hargett G.L., Coggeshall R.E. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci. Lett. 1995;197(1):25–28. doi: 10.1016/0304-3940(95)11889-5. [http://dx.doi.org/10.1016/0304-3940(95)11889-5]. [PMID: 8545047]. [DOI] [PubMed] [Google Scholar]
- 19.Davidson E.M., Coggeshall R.E., Carlton S.M. Peripheral NMDA and non-NMDA glutamate receptors contribute to nociceptive behaviors in the rat formalin test. Neuroreport. 1997;8(4):941–946. doi: 10.1097/00001756-199703030-00025. [http://dx.doi.org/10.1097/00001756-199703030-00025]. [PMID: 9141069]. [DOI] [PubMed] [Google Scholar]
- 20.Lawand N.B., Willis W.D., Westlund K.N. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur. J. Pharmacol. 1997;324(2-3):169–177. doi: 10.1016/s0014-2999(97)00072-1. [http://dx.doi.org/10. 1016/S0014-2999(97)00072-1]. [PMID: 9145768]. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S., Bonasera L., Carlton S.M. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;7(4):895–900. doi: 10.1097/00001756-199603220-00012. [http://dx.doi.org/10.1097/ 00001756-199603220-00012]. [PMID: 8724668]. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S., Komak S., Du J., Carlton S.M. Metabotropic glutamate 1alpha receptors on peripheral primary afferent fibers: their role in nociception. Brain Res. 2001;913(1):18–26. doi: 10.1016/s0006-8993(01)02747-0. [http://dx.doi.org/10. 1016/S0006-8993(01)02747-0]. [PMID: 11532243]. [DOI] [PubMed] [Google Scholar]
- 23.Mercadante S., Serretta R., Sapio M., Villari P., Calderone L. When all else fails: stepwise multiple solutions for a complex cancer pain syndrome. Support. Care Cancer. 1999;7(1):47–50. doi: 10.1007/s005200050223. [http://dx.doi.org/10.1007/s005200050223]. [PMID: 9926975]. [DOI] [PubMed] [Google Scholar]
- 24.Nersesyan H., Slavin K.V. Current aproach to cancer pain management: Availability and implications of different treatment options. Ther. Clin. Risk Manag. 2007;3(3):381–400. [PMID: 18488078]. [PMC free article] [PubMed] [Google Scholar]
- 25.Fazzari J., Lin H., Murphy C., Ungard R., Singh G. Inhibitors of glutamate release from breast cancer cells; new targets for cancer-induced bone-pain. Sci. Rep. 2015;5:8380. doi: 10.1038/srep08380. [http://dx.doi.org/10.1038/srep08380]. [PMID: 25670024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matés J.M., Segura J.A., Campos-Sandoval J.A., Lobo C., Alonso L., Alonso F.J., Márquez J. Glutamine homeostasis and mitochondrial dynamics. Int. J. Biochem. Cell Biol. 2009;41(10):2051–2061. doi: 10.1016/j.biocel.2009.03.003. [http://dx.doi.org/10.1016/j.biocel.2009.03.003]. [PMID: 19703661]. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S., Tanaka T., Poyurovsky M.V., Nagano H., Mayama T., Ohkubo S., Lokshin M., Hosokawa H., Nakayama T., Suzuki Y., Sugano S., Sato E., Nagao T., Yokote K., Tatsuno I., Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl. Acad. Sci. USA. 2010;107(16):7461–7466. doi: 10.1073/pnas.1002459107. [http://dx.doi.org/10.1073/pnas.1002459107]. [PMID: 20351271]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [http://dx.doi.org/10.1126/science.123.3191. 309]. [PMID: 13298683]. [DOI] [PubMed] [Google Scholar]
- 29.Szeliga M., Obara-Michlewska M. Glutamine in neoplastic cells: focus on the expression and roles of glutaminases. Neurochem. Int. 2009;55(1-3):71–75. doi: 10.1016/j.neuint.2009.01.008. [http://dx.doi.org/10.1016/j.neuint.2009.01. 008]. [PMID: 19428809]. [DOI] [PubMed] [Google Scholar]
- 30.Botman D., Tigchelaar W., Van Noorden C.J. Determination of phosphate-activated glutaminase activity and its kinetics in mouse tissues using metabolic mapping (quantitative enzyme histochemistry). J. Histochem. Cytochem. 2014;62(11):813–826. doi: 10.1369/0022155414551177. [http://dx.doi.org/10.1369/0022155414551177]. [PMID: 25163927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martín-Rufián M., Tosina M., Campos-Sandoval J.A., Manzanares E., Lobo C., Segura J.A., Alonso F.J., Matés J.M., Márquez J. Mammalian glutaminase Gls2 gene encodes two functional alternative transcripts by a surrogate promoter usage mechanism. PLoS One. 2012;7(6):e38380. doi: 10.1371/journal.pone.0038380. [http://dx.doi.org/10.1371/journal.pone.0038380]. [PMID: 22679499]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elgadi K.M., Meguid R.A., Qian M., Souba W.W., Abcouwer S.F. Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol. Genomics. 1999;1(2):51–62. doi: 10.1152/physiolgenomics.1999.1.2.51. [PMID: 11015561]. [DOI] [PubMed] [Google Scholar]
- 33.Cassago A., Ferreira A.P., Ferreira I.M., Fornezari C., Gomes E.R., Greene K.S., Pereira H.M., Garratt R.C., Dias S.M., Ambrosio A.L. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc. Natl. Acad. Sci. USA. 2012;109(4):1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Rosa V., Campos-Sandoval J.A., Martín-Rufián M., Cardona C., Matés J.M., Segura J.A., Alonso F.J., Márquez J. A novel glutaminase isoform in mammalian tissues. Neurochem. Int. 2009;55(1-3):76–84. doi: 10.1016/j.neuint.2009.02.021. [http://dx.doi.org/10.1016/j.neuint.2009. 02.021]. [PMID: 19428810]. [DOI] [PubMed] [Google Scholar]
- 35.Olalla L., Aledo J.C., Bannenberg G., Márquez J. The C-terminus of human glutaminase L mediates association with PDZ domain-containing proteins. FEBS Lett. 2001;488(3):116–122. doi: 10.1016/s0014-5793(00)02373-5. [http://dx.doi.org/10.1016/S0014-5793(00)02373-5]. [PMID: 11163757]. [DOI] [PubMed] [Google Scholar]
- 36.Curthoys N.P., Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [http://dx.doi.org/10.1146/annurev.nu.15.070195.001025]. [PMID: 8527215]. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Fabre P.M., Aledo J.C., Del Castillo-Olivares A., Alonso F.J., Núñez De Castro I., Campos J.A., Márquez J. Molecular cloning, sequencing and expression studies of the human breast cancer cell glutaminase. Biochem. J. 2000;345(Pt 2):365–375. [http://dx.doi.org/10.1042/bj3450365]. [PMID: 10620514]. [PMC free article] [PubMed] [Google Scholar]
- 38.Aledo J.C., Gómez-Fabre P.M., Olalla L., Márquez J. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm. Genome. 2000;11(12):1107–1110. doi: 10.1007/s003350010190. [http://dx.doi.org/10.1007/s003350010190]. [PMID: 11130979]. [DOI] [PubMed] [Google Scholar]
- 39.Castell L., Vance C., Abbott R., Marquez J., Eggleton P. Granule localization of glutaminase in human neutrophils and the consequence of glutamine utilization for neutrophil activity. J. Biol. Chem. 2004;279(14):13305–13310. doi: 10.1074/jbc.M309520200. [http://dx.doi.org/10.1074/ jbc.M309520200]. [PMID: 14722097]. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Gómez C., Campos-Sandoval J.A., Alonso F.J., Segura J.A., Manzanares E., Ruiz-Sánchez P., González M.E., Márquez J., Matés J.M. Co-expression of glutaminase K and L isoenzymes in human tumour cells. Biochem. J. 2005;386(Pt 3):535–542. doi: 10.1042/BJ20040996. [http://dx.doi.org/10.1042/BJ20040996]. [PMID: 15496140]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J-B., Erickson J.W., Fuji R., Ramachandran S., Gao P., Dinavahi R., Wilson K.F., Ambrosio A.L., Dias S.M., Dang C.V., Cerione R.A. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18(3):207–219. doi: 10.1016/j.ccr.2010.08.009. [http://dx.doi.org/10.1016/j.ccr.2010.08.009]. [PMID: 20832749]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Heuvel A.P., Jing J., Wooster R.F., Bachman K.E. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol. Ther. 2012;13(12):1185–1194. doi: 10.4161/cbt.21348. [http://dx.doi.org/10.4161/cbt.21348]. [PMID: 22892846]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang W., Choi W., Chen Y., Zhang Q., Deng H., He W., Shi Y. A proposed role for glutamine in cancer cell growth through acid resistance. Cell Res. 2013;23(5):724–727. doi: 10.1038/cr.2013.15. [http://dx.doi.org/10.1038/cr.2013.15]. [PMID: 23357849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le A., Lane A.N., Hamaker M., Bose S., Gouw A., Barbi J., Tsukamoto T., Rojas C.J., Slusher B.S., Zhang H., Zimmerman L.J., Liebler D.C., Slebos R.J., Lorkiewicz P.K., Higashi R.M., Fan T.W., Dang C.V. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15(1):110–121. doi: 10.1016/j.cmet.2011.12.009. [http://dx.doi.org/10.1016/j.cmet. 2011.12.009]. [PMID: 22225880]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seltzer M.J., Bennett B.D., Joshi A.D., Gao P., Thomas A.G., Ferraris D.V., Tsukamoto T., Rojas C.J., Slusher B.S., Rabinowitz J.D., Dang C.V., Riggins G.J. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–8987. doi: 10.1158/0008-5472.CAN-10-1666. [http://dx.doi.org/10.1158/ 0008-5472.CAN-10-1666]. [PMID: 21045145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szeliga M., Matyja E., Obara M., Grajkowska W., Czernicki T., Albrecht J. Relative expression of mRNAS coding for glutaminase isoforms in CNS tissues and CNS tumors. Neurochem. Res. 2008;33(5):808–813. doi: 10.1007/s11064-007-9507-6. [http://dx.doi.org/10.1007/s11064-007-9507-6]. [PMID: 17940881]. [DOI] [PubMed] [Google Scholar]
- 47.Márquez J., Tosina M., de la Rosa V., Segura J.A., Alonso F.J., Matés J.M., Campos-Sandoval J.A. New insights into brain glutaminases: beyond their role on glutamatergic transmission. Neurochem. Int. 2009;55(1-3):64–70. doi: 10.1016/j.neuint.2009.02.022. [http://dx.doi.org/10.1016/ j.neuint.2009.02.022]. [PMID: 19428808]. [DOI] [PubMed] [Google Scholar]
- 48.Cangro C.B., Sweetnam P.M., Neale J.H., Haser W.G., Curthoys N.P. Selective localization of glutaminase in spinal and sensory nerve cells. A potential marker for glutamate neuro- transmission. JAMA. 1984;251(6):797. [http://dx.doi.org/10.1001/ jama.1984.03340300087037]. [PMID: 6141300]. [PubMed] [Google Scholar]
- 49.Bosoi C.R., Rose C.F. Identifying the direct effects of ammonia on the brain. Metab. Brain Dis. 2009;24(1):95–102. doi: 10.1007/s11011-008-9112-7. [http://dx.doi.org/10.1007/s11011-008-9112-7]. [PMID: 19104924]. [DOI] [PubMed] [Google Scholar]
- 50.Bergström J., Fürst P., Norée L.O., Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J. Appl. Physiol. 1974;36(6):693–697. doi: 10.1152/jappl.1974.36.6.693. [PMID: 4829908]. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn K.S., Schuhmann K., Stehle P., Darmaun D., Fürst P. Determination of glutamine in muscle protein facilitates accurate assessment of proteolysis and de novo synthesis-derived endogenous glutamine production. Am. J. Clin. Nutr. 1999;70(4):484–489. doi: 10.1093/ajcn/70.4.484. [PMID: 10500016]. [DOI] [PubMed] [Google Scholar]
- 52.Knox W.E., Horowitz M.L., Friedell G.H. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969;29(3):669–680. [PMID: 5251291]. [PubMed] [Google Scholar]
- 53.Linder-Horowitz M., Knox W.E., Morris H.P. Glutaminase activities and growth rates of rat hepatomas. Cancer Res. 1969;29(6):1195–1199. [PMID: 4307782]. [PubMed] [Google Scholar]
- 54.Gao P., Tchernyshyov I., Chang T-C., Lee Y-S., Kita K., Ochi T., Zeller K.I., De Marzo A.M., Van Eyk J.E., Mendell J.T., Dang C.V. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–765. doi: 10.1038/nature07823. [http://dx.doi.org/10.1038/ nature07823]. [PMID: 19219026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lobo C., Ruiz-Bellido M.A., Aledo J.C., Márquez J., Núñez De Castro I., Alonso F.J. Inhibition of glutaminase expression by antisense mRNA decreases growth and tumourigenicity of tumour cells. Biochem. J. 2000;348(Pt 2):257–261. [http://dx.doi.org/10.1042/bj3480257]. [PMID: 10816417]. [PMC free article] [PubMed] [Google Scholar]
- 56.Zacharias D.P., Lima M.M., Souza A.L., Jr, de Abranches Oliveira Santos I.D., Enokiara M., Michalany N., Curi R. Human cutaneous melanoma expresses a significant phosphate-dependent glutaminase activity: a comparison with the surrounding skin of the same patient. Cell Biochem. Funct. 2003;21(1):81–84. doi: 10.1002/cbf.997. [http://dx.doi.org/10.1002/cbf.997]. [PMID: 12579526]. [DOI] [PubMed] [Google Scholar]
- 57.DeBerardinis R.J., Cheng T. Qs next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–324. doi: 10.1038/onc.2009.358. [http://dx.doi.org/10.1038/onc.2009.358]. [PMID: 19881548]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaiser S., Curthoys N.P. Effect of pH and bicarbonate on phosphoenolpyruvate carboxykinase and glutaminase mRNA levels in cultured renal epithelial cells. J. Biol. Chem. 1991;266(15):9397–9402. [PMID: 1851745]. [PubMed] [Google Scholar]
- 59.Werner P., Pitt D., Raine C.S. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann. Neurol. 2001;50(2):169–180. doi: 10.1002/ana.1077. [http://dx.doi.org/10.1002/ana.1077]. [PMID: 11506399]. [DOI] [PubMed] [Google Scholar]
- 60.Bak L.K., Schousboe A., Waagepetersen H.S. The glutamate/ GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006;98(3):641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.03913.x]. [PMID: 16787421]. [DOI] [PubMed] [Google Scholar]
- 61.Romero-Gómez M., Ramos-Guerrero R., Grande L., de Terán L.C., Corpas R., Camacho I., Bautista J.D. Intestinal glutaminase activity is increased in liver cirrhosis and correlates with minimal hepatic encephalopathy. J. Hepatol. 2004;41(1):49–54. doi: 10.1016/j.jhep.2004.03.021. [http://dx.doi.org/10.1016/j.jhep.2004.03.021]. [PMID: 15246207]. [DOI] [PubMed] [Google Scholar]
- 62.Kowall N.W., Beal M.F. Glutamate-, glutaminase-, and taurine-immunoreactive neurons develop neurofibrillary tangles in Alzheimers disease. Ann. Neurol. 1991;29(2):162–167. doi: 10.1002/ana.410290208. [http://dx. doi.org/10.1002/ana.410290208]. [PMID: 1672808]. [DOI] [PubMed] [Google Scholar]
- 63.Palmer A.M., Gershon S. Is the neuronal basis of Alzheimers disease cholinergic or glutamatergic? FASEB J. 1990;4(10):2745–2752. doi: 10.1096/fasebj.4.10.2165009. [PMID: 2165009]. [DOI] [PubMed] [Google Scholar]
- 64.McGeer E.G., McGeer P.L., Akiyama H., Harrop R. Cortical glutaminase, beta-glucuronidase and glucose utilization in Alzheimers disease. Can. J. Neurol. Sci. 1989;16(4) Suppl.:511–515. doi: 10.1017/s0317167100029851. [http://dx.doi.org/10.1017/S0317167100029851]. [PMID: 2804813]. [DOI] [PubMed] [Google Scholar]
- 65.Butterworth J., Yates C.M., Simpson J. Phosphate-activated glutaminase in relation to Huntingtons disease and agonal state. J. Neurochem. 1983;41(2):440–447. doi: 10.1111/j.1471-4159.1983.tb04761.x. [http://dx.doi.org/10.1111/ j.1471-4159.1983.tb04761.x]. [PMID: 6223989]. [DOI] [PubMed] [Google Scholar]
- 66.Miller K.E., Hoffman E.M., Sutharshan M., Schechter R. Glutamate pharmacology and metabolism in peripheral primary afferents: physiological and pathophysiological mechanisms. Pharmacol. Ther. 2011;130(3):283–309. doi: 10.1016/j.pharmthera.2011.01.005. [http://dx.doi.org/10. 1016/j.pharmthera.2011.01.005]. [PMID: 21276816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Besson J.M. The neurobiology of pain. Lancet. 1999;353(9164):1610–1615. doi: 10.1016/s0140-6736(99)01313-6. [http://dx.doi.org/10.1016/S0140-6736(99)01313-6]. [PMID: 10334274]. [DOI] [PubMed] [Google Scholar]
- 68.Kidd B.L., Urban L.A. Mechanisms of inflammatory pain. Br. J. Anaesth. 2001;87(1):3–11. doi: 10.1093/bja/87.1.3. [http://dx.doi.org/10.1093/bja/87.1.3]. [PMID: 11460811]. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman E.M., Miller K.E. Peripheral inhibition of glutaminase reduces carrageenan-induced Fos expression in the superficial dorsal horn of the rat. Neurosci. Lett. 2010;472(3):157–160. doi: 10.1016/j.neulet.2010.01.066. [http://dx.doi.org/10.1016/j.neulet.2010.01.066]. [PMID: 20132864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanesch U., Pfrommer U., Grubb B.D., Heppelmann B., Schaible H.G. The proportion of CGRP-immunoreactive and SP-mRNA containing dorsal root ganglion cells is increased by a unilateral inflammation of the ankle joint of the rat. Regul. Pept. 1993;46(1-2):202–203. doi: 10.1016/0167-0115(93)90033-5. [http://dx.doi.org/10.1016/0167-0115(93) 90033-5]. [PMID: 7692491]. [DOI] [PubMed] [Google Scholar]
- 71.Bulling D.G., Kelly D., Bond S., McQueen D.S., Seckl J.R. Adjuvant-induced joint inflammation causes very rapid transcription of beta-preprotachykinin and alpha-CGRP genes in innervating sensory ganglia. J. Neurochem. 2001;77(2):372–382. doi: 10.1046/j.1471-4159.2001.00175.x. [http://dx.doi.org/10.1046/j.1471-4159.2001.00175.x]. [PMID: 11299299]. [DOI] [PubMed] [Google Scholar]
- 72.Marlier L., Poulat P., Rajaofetra N., Privat A. Modifications of serotonin-, substance P- and calcitonin gene-related peptide-like immunoreactivities in the dorsal horn of the spinal cord of arthritic rats: a quantitative immunocytochemical study. Exp. Brain Res. 1991;85(3):482–490. doi: 10.1007/BF00231731. [http://dx.doi.org/10.1007/BF00231731]. [PMID: 1717303]. [DOI] [PubMed] [Google Scholar]
- 73.Nahin R.L., Byers M.R. Adjuvant-induced inflammation of rat paw is associated with altered calcitonin gene-related peptide immunoreactivity within cell bodies and peripheral endings of primary afferent neurons. J. Comp. Neurol. 1994;349(3):475–485. doi: 10.1002/cne.903490311. [http://dx.doi.org/10.1002/cne.903490311]. [PMID: 7852637]. [DOI] [PubMed] [Google Scholar]
- 74.Lee M., Kim B-J., Lim E.J., Back S.K., Lee J-H., Yu S-W., Hong S-H., Kim J.H., Lee S-H., Jung W-W., Sul D., Na H.S. Complete Freunds adjuvant-induced intervertebral discitis as an animal model for discogenic low back pain. Anesth. Analg. 2009;109(4):1287–1296. doi: 10.1213/ane.0b013e3181b31f39. [http://dx.doi.org/10.1213/ane.0b013e3181b31f39]. [PMID: 19762759]. [DOI] [PubMed] [Google Scholar]
- 75.Battaglia G., Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J. Comp. Neurol. 1988;277(2):302–312. doi: 10.1002/cne.902770210. [http://dx.doi.org/10.1002/cne. 902770210]. [PMID: 2466061]. [DOI] [PubMed] [Google Scholar]
- 76.Miller K.E., Douglas V.D., Kaneko T. Glutaminase immuno- reactive neurons in the rat dorsal root ganglion contain calcitonin gene-related peptide (CGRP). Neurosci. Lett. 1993;160(1):113–116. doi: 10.1016/0304-3940(93)90926-c. [http://dx.doi.org/10.1016/0304-3940(93)90926-C]. [PMID: 8247321]. [DOI] [PubMed] [Google Scholar]
- 77.Miller K.E., Richards B.A., Kriebel R.M. Glutamine, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carboxylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002;945(2):202–211. doi: 10.1016/s0006-8993(02)02802-0. [http://dx.doi.org/10. 1016/S0006-8993(02)02802-0]. [PMID: 12126882]. [DOI] [PubMed] [Google Scholar]
- 78.Skilling S.R., Smullin D.H., Beitz A.J., Larson A.A. Extracellular amino acid concentrations in the dorsal spinal cord of freely moving rats following veratridine and nociceptive stimulation. J. Neurochem. 1988;51(1):127–132. doi: 10.1111/j.1471-4159.1988.tb04845.x. [http://dx.doi.org/10.1111/ j.1471-4159.1988.tb04845.x]. [PMID: 2898001]. [DOI] [PubMed] [Google Scholar]
- 79.Sorkin L.S., Westlund K.N., Sluka K.A., Dougherty P.M., Willis W.D. Neural changes in acute arthritis in monkeys. IV. Time-course of amino acid release into the lumbar dorsal horn. Brain Res. Brain Res. Rev. 1992;17(1):39–50. doi: 10.1016/0165-0173(92)90005-7. [http://dx.doi.org/10.1016/0165-0173(92)90005-7]. [PMID: 1638274]. [DOI] [PubMed] [Google Scholar]
- 80.Yang L.C., Marsala M., Yaksh T.L. Characterization of time course of spinal amino acids, citrulline and PGE2 release after carrageenan/kaolin-induced knee joint inflammation: a chronic microdialysis study. Pain. 1996;67(2-3):345–354. doi: 10.1016/0304-3959(96)03106-5. [http://dx.doi.org/10.1016/0304-3959(96)03106-5]. [PMID: 8951928]. [DOI] [PubMed] [Google Scholar]
- 81.Dmitrieva N., Rodríguez-Malaver A.J., Pérez J., Hernández L. Differential release of neurotransmitters from superficial and deep layers of the dorsal horn in response to acute noxious stimulation and inflammation of the rat paw. Eur. J. Pain. 2004;8(3):245–252. doi: 10.1016/j.ejpain.2003.09.001. [http://dx.doi.org/10.1016/j.ejpain.2003.09.001]. [PMID: 15109975]. [DOI] [PubMed] [Google Scholar]
- 82.Woolf C.J., Ma Q. Nociceptorsnoxious stimulus detectors. Neuron. 2007;55(3):353–364. doi: 10.1016/j.neuron.2007.07.016. [http://dx.doi.org/10.1016/j.neuron. 2007.07.016]. [PMID: 17678850]. [DOI] [PubMed] [Google Scholar]
- 83.Sluka K.A., Dougherty P.M., Sorkin L.S., Willis W.D., Westlund K.N. Neural changes in acute arthritis in monkeys. III. Changes in substance P, calcitonin gene-related peptide and glutamate in the dorsal horn of the spinal cord. Brain Res. Brain Res. Rev. 1992;17(1):29–38. doi: 10.1016/0165-0173(92)90004-6. [http://dx.doi.org/10.1016/0165-0173(92)90004-6]. [PMID: 1379098]. [DOI] [PubMed] [Google Scholar]
- 84.Kvamme E., Svenneby G., Torgner I.A. Calcium stimulation of glutamine hydrolysis in synaptosomes from rat brain. Neurochem. Res. 1983;8(1):25–38. doi: 10.1007/BF00965651. [http://dx.doi.org/10.1007/BF00965651]. [PMID: 6856016]. [DOI] [PubMed] [Google Scholar]
- 85.Miller K.E., Balbás J.C., Benton R.L., Lam T.S., Edwards K.M., Kriebel R.M., Schechter R. Glutaminase immunoreactivity and enzyme activity is increased in the rat dorsal root ganglion following peripheral inflammation. Pain Res. Treat. 2012 doi: 10.1155/2012/414697. [http://dx.doi.org/10.1155/2012/414697] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kvamme E., Torgner I.A., Roberg B. Kinetics and localization of brain phosphate activated glutaminase. J. Neurosci. Res. 2001;66(5):951–958. doi: 10.1002/jnr.10041. [http://dx.doi.org/10.1002/jnr.10041]. [PMID: 11746423]. [DOI] [PubMed] [Google Scholar]
- 87.Masola B., Ngubane N.P. The activity of phosphate-dependent glutaminase from the rat small intestine is modulated by ADP and is dependent on integrity of mitochondria. Arch. Biochem. Biophys. 2010;504(2):197–203. doi: 10.1016/j.abb.2010.09.002. [http://dx.doi.org/10.1016/j.abb. 2010.09.002]. [PMID: 20831857]. [DOI] [PubMed] [Google Scholar]
- 88.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X-Y., Pfeiffer H.K., Nissim I., Daikhin E., Yudkoff M., McMahon S.B., Thompson C.B. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA. 2008;105(48):18782–18787. doi: 10.1073/pnas.0810199105. [http://dx.doi.org/10.1073/pnas.0810199105]. [PMID: 19033189]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuneva M., Zamboni N., Oefner P., Sachidanandam R., Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 2007;178(1):93–105. doi: 10.1083/jcb.200703099. [http://dx.doi.org/10.1083/jcb.200703099]. [PMID: 17606868]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rathore M.G., Saumet A., Rossi J-F., de Bettignies C., Tempé D., Lecellier C-H., Villalba M. The NF-κB member p65 controls glutamine metabolism through miR-23a. Int. J. Biochem. Cell Biol. 2012;44(9):1448–1456. doi: 10.1016/j.biocel.2012.05.011. [http://dx.doi.org/10.1016/j.biocel.2012. 05.011]. [PMID: 22634383]. [DOI] [PubMed] [Google Scholar]
- 91.Zhao L., Huang Y., Tian C., Taylor L., Curthoys N., Wang Y., Vernon H., Zheng J. Interferon-α regulates glutaminase 1 promoter through STAT1 phosphorylation: relevance to HIV-1 associated neurocognitive disorders. PLoS One. 2012;7(3):e32995. doi: 10.1371/journal.pone.0032995. [http://dx.doi.org/10.1371/journal.pone.0032995]. [PMID: 22479354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thangavelu K., Pan C.Q., Karlberg T., Balaji G., Uttamchandani M., Suresh V., Schüler H., Low B.C., Sivaraman J. Structural basis for the allosteric inhibitory mechanism of human kidney-type glutaminase (KGA) and its regulation by Raf-Mek-Erk signaling in cancer cell metabolism. Proc. Natl. Acad. Sci. USA. 2012;109(20):7705–7710. doi: 10.1073/pnas.1116573109. [http://dx.doi.org/10.1073/pnas.1116573109]. [PMID: 22538822]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Colombo S.L., Palacios-Callender M., Frakich N., Carcamo S., Kovacs I., Tudzarova S., Moncada S. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc. Natl. Acad. Sci. USA. 2011;108(52):21069–21074. doi: 10.1073/pnas.1117500108. [http://dx.doi.org/10.1073/pnas. 1117500108]. [PMID: 22106309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kita K., Suzuki T., Ochi T. Diphenylarsinic acid promotes degradation of glutaminase C by mitochondrial Lon protease. J. Biol. Chem. 2012;287(22):18163–18172. doi: 10.1074/jbc.M112.362699. [http://dx.doi.org/10. 1074/jbc.M112.362699]. [PMID: 22493432]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ye Z.C., Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59(17):4383–4391. [PMID: 10485487]. [PubMed] [Google Scholar]
- 96.Sharma M.K., Seidlitz E.P., Singh G. Cancer cells release glutamate via the cystine/glutamate antiporter. Biochem. Biophys. Res. Commun. 2010;391(1):91–95. doi: 10.1016/j.bbrc.2009.10.168. [http://dx.doi.org/10.1016/ j.bbrc.2009.10.168]. [PMID: 19896463]. [DOI] [PubMed] [Google Scholar]
- 97.Bannai S., Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J. Cell. Physiol. 1982;112(2):265–272. doi: 10.1002/jcp.1041120216. [http://dx.doi.org/10.1002/jcp.1041120216]. [PMID: 6126484]. [DOI] [PubMed] [Google Scholar]
- 98.Banjac A., Perisic T., Sato H., Seiler A., Bannai S., Weiss N., Kölle P., Tschoep K., Issels R.D., Daniel P.T., Conrad M., Bornkamm G.W. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27(11):1618–1628. doi: 10.1038/sj.onc.1210796. [http://dx.doi.org/10.1038/sj.onc.1210796]. [PMID: 17828297]. [DOI] [PubMed] [Google Scholar]
- 99.Conrad M., Sato H. The oxidative stress-inducible cystine/ glutamate antiporter, system x (c) (-): cystine supplier and beyond. Amino Acids. 2012;42(1):231–246. doi: 10.1007/s00726-011-0867-5. [http://dx.doi.org/10.1007/ s00726-011-0867-5]. [PMID: 21409388]. [DOI] [PubMed] [Google Scholar]
- 100.Lewerenz J., Hewett S.J., Huang Y., Lambros M., Gout P.W., Kalivas P.W., Massie A., Smolders I., Methner A., Pergande M., Smith S.B., Ganapathy V., Maher P. The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013;18(5):522–555. doi: 10.1089/ars.2011.4391. [http://dx.doi.org/10.1089/ars.2011. 4391]. [PMID: 22667998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J. Biol. Chem. 1986;261(5):2256–2263. [PMID: 2868011]. [PubMed] [Google Scholar]
- 102.Habib E., Linher-Melville K., Lin H-X., Singh G. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33–42. doi: 10.1016/j.redox.2015.03.003. [http://dx.doi.org/10.1016/j.redox.2015.03.003]. [PMID: 25827424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ye P., Mimura J., Okada T., Sato H., Liu T., Maruyama A., Ohyama C., Itoh K. Nrf2- and ATF4-dependent up-regulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol. Cell. Biol. 2014 doi: 10.1128/MCB.00221-14. MCB.00221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Linher-Melville K., Haftchenary S., Gunning P., Singh G. Signal transducer and activator of transcription 3 and 5 regulate system Xc- and redox balance in human breast cancer cells. Mol. Cell. Biochem. 2015;405(1-2):205–221. doi: 10.1007/s11010-015-2412-4. [http://dx.doi.org/10.1007/ s11010-015-2412-4]. [PMID: 25896132]. [DOI] [PubMed] [Google Scholar]
- 105.Shi J., He Y., Hewett S.J., Hewett J.A. Interleukin 1β regulation of the system Xc-substrate-specific subunit, xCT, in primary mouse astrocytes involves the RNA-binding protein HuR. J. Biol. Chem. 2015 doi: 10.1074/jbc.M115.697821. [PMID: 26601945]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reissner K.J. The cystine/glutamate antiporter: when too much of a good thing goes bad. J. Clin. Invest. 2014;124(8):3279–3281. doi: 10.1172/JCI76627. [http://dx.doi.org/10.1172/JCI76627]. [PMID: 25036700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Herner A., Sauliunaite D., Michalski C.W., Erkan M., De Oliveira T., Abiatari I., Kong B., Esposito I., Friess H., Kleeff J. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int. J. Cancer. 2011;129(10):2349–2359. doi: 10.1002/ijc.25898. [http://dx.doi.org/10.1002/ijc.25898]. [PMID: 21207374]. [DOI] [PubMed] [Google Scholar]
- 108.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [http://dx.doi.org/10.1038/39807]. [PMID: 9349813]. [DOI] [PubMed] [Google Scholar]
- 109.Laing R.J., Dhaka A. ThermoTRPs and Pain. Neuroscientist. 2016;22(2):171–187. doi: 10.1177/1073858414567884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo A., Vulchanova L., Wang J., Li X., Elde R. Immuno- cytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999;11(3):946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [http://dx. doi.org/10.1046/j.1460-9568.1999.00503.x]. [PMID: 10103088]. [DOI] [PubMed] [Google Scholar]
- 111.Maione S., Starowicz K., Cristino L., Guida F., Palazzo E., Luongo L., Rossi F., Marabese I., de Novellis V., Di Marzo V. Functional interaction between TRPV1 and mu-opioid receptors in the descending antinociceptive pathway activates glutamate transmission and induces analgesia. J. Neurophysiol. 2009;101(5):2411–2422. doi: 10.1152/jn.91225.2008. [http://dx.doi.org/10.1152/jn.91225.2008]. [PMID: 19297510]. [DOI] [PubMed] [Google Scholar]
- 112.Tominaga M., Caterina M.J., Malmberg A.B., Rosen T.A., Gilbert H., Skinner K., Raumann B.E., Basbaum A.I., Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [http://dx.doi.org/10.1016/ S0896-6273(00)80564-4]. [PMID: 9768840]. [DOI] [PubMed] [Google Scholar]
- 113.Cui M., Honore P., Zhong C., Gauvin D., Mikusa J., Hernandez G., Chandran P., Gomtsyan A., Brown B., Bayburt E.K., Marsh K., Bianchi B., McDonald H., Niforatos W., Neelands T.R., Moreland R.B., Decker M.W., Lee C-H., Sullivan J.P., Faltynek C.R. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J. Neurosci. 2006;26(37):9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [http://dx.doi.org/10.1523/ JNEUROSCI.1246-06.2006]. [PMID: 16971522]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Starowicz K., Cristino L., Di Marzo V. TRPV1 receptors in the central nervous system: potential for previously unforeseen therapeutic applications. Curr. Pharm. Des. 2008;14(1):42–54. doi: 10.2174/138161208783330790. [http://dx.doi.org/10.2174/138161208783330790]. [PMID: 18220817]. [DOI] [PubMed] [Google Scholar]
- 115.Chung M-K., Güler A.D., Caterina M.J. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat. Neurosci. 2008;11(5):555–564. doi: 10.1038/nn.2102. [http://dx.doi.org/10.1038/nn.2102]. [PMID: 18391945]. [DOI] [PubMed] [Google Scholar]
- 116.Huang S.M., Bisogno T., Trevisani M., Al-Hayani A., De Petrocellis L., Fezza F., Tognetto M., Petros T.J., Krey J.F., Chu C.J., Miller J.D., Davies S.N., Geppetti P., Walker J.M., Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA. 2002;99(12):8400–8405. doi: 10.1073/pnas.122196999. [http://dx.doi.org/10.1073/pnas.122196999]. [PMID: 12060783]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Woo D.H., Jung S.J., Zhu M.H., Park C-K., Kim Y.H., Oh S.B., Lee C.J. Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG). Mol. Pain. 2008;4:42. doi: 10.1186/1744-8069-4-42. [http://dx.doi.org/10.1186/1744-8069-4-42]. [PMID: 18826653]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Appendino G., Muñoz E., Fiebich B.L. TRPV1 vanilloid receptor, capsaicin receptor agonists and antagonists. Expert Opin. Ther. Pat. 2003;13:1825–1837. [http://dx.doi.org/10.1517/13543776. 13.12.1825]. [Google Scholar]
- 119.Van Buren J.J., Bhat S., Rotello R., Pauza M.E., Premkumar L.S. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol. Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [http://dx.doi.org/10.1186/1744-8069-1-17]. [PMID: 15857517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ji R-R., Samad T.A., Jin S-X., Schmoll R., Woolf C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/s0896-6273(02)00908-x. [http://dx.doi.org/10.1016/S0896-6273(02)00908-X]. [PMID: 12367506]. [DOI] [PubMed] [Google Scholar]
- 121.Voets T., Droogmans G., Wissenbach U., Janssens A., Flockerzi V., Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430(7001):748–754. doi: 10.1038/nature02732. [http://dx.doi.org/10.1038/nature02732]. [PMID: 15306801]. [DOI] [PubMed] [Google Scholar]
- 122.Walpole C.S., Bevan S., Bovermann G., Boelsterli J.J., Breckenridge R., Davies J.W., Hughes G.A., James I., Oberer L., Winter J. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J. Med. Chem. 1994;37(13):1942–1954. doi: 10.1021/jm00039a006. [http://dx.doi.org/10.1021/jm00039a006]. [PMID: 8027976]. [DOI] [PubMed] [Google Scholar]
- 123.Wahl P., Foged C., Tullin S., Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59(1):9–15. doi: 10.1124/mol.59.1.9. [PMID: 11125018]. [DOI] [PubMed] [Google Scholar]
- 124.Touska F., Marsakova L., Teisinger J., Vlachova V. A cute desensitization of TRPV1. Curr. Pharm. Biotechnol. 2011;12(1):122–129. doi: 10.2174/138920111793937826. [http://dx.doi.org/10.2174/138920111793937826]. [PMID: 20932251]. [DOI] [PubMed] [Google Scholar]
- 125.Szallasi A., Cortright D.N., Blum C.A., Eid S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007;6(5):357–372. doi: 10.1038/nrd2280. [http://dx.doi.org/10.1038/nrd2280]. [PMID: 17464295]. [DOI] [PubMed] [Google Scholar]
- 126.Chuang H.H., Prescott E.D., Kong H., Shields S., Jordt S.E., Basbaum A.I., Chao M.V., Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi: 10.1038/35082088. [http://dx. doi.org/10.1038/35082088]. [PMID: 11418861]. [DOI] [PubMed] [Google Scholar]
- 127.Dömötör A., Peidl Z., Vincze A., Hunyady B., Szolcsányi J., Kereskay L., Szekeres G., Mózsik G. Immunohistochemical distribution of vanilloid receptor, calcitonin-gene related peptide and substance P in gastrointestinal mucosa of patients with different gastrointestinal disorders. Inflammopharmacology. 2005;13(1-3):161–177. doi: 10.1163/156856005774423737. [http://dx.doi.org/10.1163/156856005774423737]. [PMID: 16259736]. [DOI] [PubMed] [Google Scholar]
- 128.Hartel M., di Mola F.F., Selvaggi F., Mascetta G., Wente M.N., Felix K., Giese N.A., Hinz U., Di Sebastiano P., Büchler M.W., Friess H. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. 2006;55(4):519–528. doi: 10.1136/gut.2005.073205. [http://dx.doi.org/10.1136/gut.2005.073205]. [PMID: 16174661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Czifra G., Varga A., Nyeste K., Marincsák R., Tóth B.I., Kovács I., Kovács L., Bíró T. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 2009;135(4):507–514. doi: 10.1007/s00432-008-0482-3. [http://dx.doi.org/10.1007/s00432-008-0482-3]. [PMID: 18830626]. [DOI] [PubMed] [Google Scholar]
- 130.Wu T.T., Peters A.A., Tan P.T., Roberts-Thomson S.J., Monteith G.R. Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium. 2014;56(2):59–67. doi: 10.1016/j.ceca.2014.04.006. [http://dx.doi.org/10.1016/j.ceca.2014.04.006]. [PMID: 24889371]. [DOI] [PubMed] [Google Scholar]
- 131.Koşar P.A., Nazıroğlu M., Övey İ.S., Çiğ B. Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: Involvement of TRPV1 channels. J. Membr. Biol. 2015 doi: 10.1007/s00232-015-9855-0. [PMID: 26525975]. [DOI] [PubMed] [Google Scholar]
- 132.Amantini C., Mosca M., Nabissi M., Lucciarini R., Caprodossi S., Arcella A., Giangaspero F., Santoni G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007;102(3):977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.04582.x]. [PMID: 17442041]. [DOI] [PubMed] [Google Scholar]
- 133.Capiod T. Cell proliferation, calcium influx and calcium channels. Biochimie. 2011;93(12):2075–2079. doi: 10.1016/j.biochi.2011.07.015. [http://dx.doi.org/10.1016/ j.biochi.2011.07.015]. [PMID: 21802482]. [DOI] [PubMed] [Google Scholar]
- 134.Bödding M. TRP proteins and cancer. Cell. Signal. 2007;19(3):617–624. doi: 10.1016/j.cellsig.2006.08.012. [http://dx.doi.org/10.1016/j.cellsig.2006.08.012]. [PMID: 17029734]. [DOI] [PubMed] [Google Scholar]
- 135.Szallasi A., Blumberg P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51(2):159–212. [PMID: 10353985]. [PubMed] [Google Scholar]
- 136.Niiyama Y., Kawamata T., Yamamoto J., Omote K., Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience. 2007;148(2):560–572. doi: 10.1016/j.neuroscience.2007.05.049. [http://dx.doi.org/10.1016/j.neuroscience.2007.05.049]. [PMID: 17656027]. [DOI] [PubMed] [Google Scholar]
- 137.Seabrook G.R., Sutton K.G., Jarolimek W., Hollingworth G.J., Teague S., Webb J., Clark N., Boyce S., Kerby J., Ali Z., Chou M., Middleton R., Kaczorowski G., Jones A.B. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J. Pharmacol. Exp. Ther. 2002;303(3):1052–1060. doi: 10.1124/jpet.102.040394. [http://dx.doi.org/10.1124/jpet.102.040394]. [PMID: 12438527]. [DOI] [PubMed] [Google Scholar]
- 138.Han Y., Li Y., Xiao X., Liu J., Meng X-L., Liu F-Y., Xing G-G., Wan Y. Formaldehyde up-regulates TRPV1 through MAPK and PI3K signaling pathways in a rat model of bone cancer pain. Neurosci. Bull. 2012;28(2):165–172. doi: 10.1007/s12264-012-1211-0. [http://dx.doi.org/10. 1007/s12264-012-1211-0]. [PMID: 22466127]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y., Adamek P., Zhang H., Tatsui C.E., Rhines L.D., Mrozkova P., Li Q., Kosturakis A.K., Cassidy R.M., Harrison D.S., Cata J.P., Sapire K., Zhang H., Kennamer-Chapman R.M., Jawad A.B., Ghetti A., Yan J., Palecek J., Dougherty P.M. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J. Neurosci. 2015;35(39):13487–13500. doi: 10.1523/JNEUROSCI.1956-15.2015. [http://dx.doi.org/10.1523/ JNEUROSCI.1956-15.2015]. [PMID: 26424893]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diogenes A., Ferraz C.C., Akopian A.N., Henry M.A., Hargreaves K.M. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dent. Res. 2011;90(6):759–764. doi: 10.1177/0022034511400225. [http://dx.doi.org/10.1177/0022034511400225]. [PMID: 21393555]. [DOI] [PubMed] [Google Scholar]
- 141.Li Y., Zhang H., Zhang H., Kosturakis A.K., Jawad A.B., Dougherty P.M. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J. Pain. 2014;15(7):712–725. doi: 10.1016/j.jpain.2014.04.001. [http://dx.doi.org/10.1016/j.jpain.2014.04.001]. [PMID: 24755282]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jube S., Rivera Z.S., Bianchi M.E., Powers A., Wang E., Pagano I., Pass H.I., Gaudino G., Carbone M., Yang H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72(13):3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [http://dx.doi.org/10.1158/0008-5472.CAN-11-3481]. [PMID: 22552293]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun S., Zhang W., Cui Z., Chen Q., Xie P., Zhou C., Liu B., Peng X., Zhang Y. High mobility group box-1 and its clinical value in breast cancer. Onco Targets Ther. 2015;8:413–419. doi: 10.2147/OTT.S73366. [PMID: 25709474]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amornsupak K., Insawang T., Thuwajit P., O-Charoenrat P., Eccles S.A., Thuwajit C. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer. 2014;14:955. doi: 10.1186/1471-2407-14-955. [http://dx.doi.org/10.1186/1471-2407-14-955]. [PMID: 25512109]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu T., Gao Y-J., Ji R-R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012;28(2):131–144. doi: 10.1007/s12264-012-1219-5. [http://dx.doi.org/10.1007/s12264-012-1219-5]. [PMID: 22466124]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zaks-Zilberman M., Zaks T.Z., Vogel S.N. Induction of proinflammatory and chemokine genes by lipopolysaccharide and paclitaxel (Taxol) in murine and human breast cancer cell lines. Cytokine. 2001;15(3):156–165. doi: 10.1006/cyto.2001.0935. [http://dx.doi.org/10.1006/cyto. 2001.0935]. [PMID: 11554785]. [DOI] [PubMed] [Google Scholar]
- 147.Spicarova D., Palecek J. Tumor necrosis factor alpha sensitizes spinal cord TRPV1 receptors to the endogenous agonist N-oleoyldopamine. J. Neuroinflammation. 2010;7:49. doi: 10.1186/1742-2094-7-49. [http://dx. doi.org/10.1186/1742-2094-7-49]. [PMID: 20796308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou C., Qi C., Zhao J., Wang F., Zhang W., Li C., Jing J., Kang X., Chai Z. Interleukin-1β inhibits voltage-gated sodium currents in a time- and dose-dependent manner in cortical neurons. Neurochem. Res. 2011;36(6):1116–1123. doi: 10.1007/s11064-011-0456-8. [http://dx.doi.org/10.1007/s11064-011-0456-8]. [PMID: 21448594]. [DOI] [PubMed] [Google Scholar]
- 149.Hu H-J., Gereau R.W., IV ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J. Neurophysiol. 2003;90(3):1680–1688. doi: 10.1152/jn.00341.2003. [http://dx.doi.org/10.1152/jn.00341.2003]. [PMID: 12750418]. [DOI] [PubMed] [Google Scholar]
- 150.Hu H-J., Bhave G., Gereau R.W., IV Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J. Neurosci. 2002;22(17):7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [PMID: 12196566]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bhave G., Hu H-J., Glauner K.S., Zhu W., Wang H., Brasier D.J., Oxford G.S., Gereau R.W., IV Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc. Natl. Acad. Sci. USA. 2003;100(21):12480–12485. doi: 10.1073/pnas.2032100100. [http://dx. doi.org/10.1073/pnas.2032100100]. [PMID: 14523239]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Numazaki M., Tominaga T., Toyooka H., Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J. Biol. Chem. 2002;277(16):13375–13378. doi: 10.1074/jbc.C200104200. [http://dx.doi.org/10.1074/ jbc.C200104200]. [PMID: 11884385]. [DOI] [PubMed] [Google Scholar]
- 153.Jin Y.H., Nishioka H., Wakabayashi K., Fujita T., Yonehara N. Effect of morphine on the release of excitatory amino acids in the rat hind instep: Pain is modulated by the interaction between the peripheral opioid and glutamate systems. Neuroscience. 2006;138(4):1329–1339. doi: 10.1016/j.neuroscience.2005.12.049. [http://dx.doi.org/10.1016/j.neuroscience.2005. 12.049]. [PMID: 16473472]. [DOI] [PubMed] [Google Scholar]
- 154.Jackson D.L., Graff C.B., Richardson J.D., Hargreaves K.M. Glutamate participates in the peripheral modulation of thermal hyperalgesia in rats. Eur. J. Pharmacol. 1995;284(3):321–325. doi: 10.1016/0014-2999(95)00449-u. [http://dx.doi.org/10.1016/0014-2999(95)00449-U]. [PMID: 8666015]. [DOI] [PubMed] [Google Scholar]
- 155.Lawand N.B., McNearney T., Westlund K.N. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. 2000;86(1-2):69–74. doi: 10.1016/s0304-3959(99)00311-5. [http://dx.doi.org/10.1016/S0304-3959(99) 00311-5]. [PMID: 10779662]. [DOI] [PubMed] [Google Scholar]
- 156.Carlton S.M., Zhou S., Coggeshall R.E. Evidence for the interaction of glutamate and NK1 receptors in the periphery. Brain Res. 1998;790(1-2):160–169. doi: 10.1016/s0006-8993(97)01471-6. [http://dx.doi.org/10.1016/S0006-8993(97)01471-6]. [PMID: 9593874]. [DOI] [PubMed] [Google Scholar]
- 157.Jin Y-H., Yamaki F., Takemura M., Koike Y., Furuyama A., Yonehara N. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J. Pharmacol. Sci. 2009;109(2):233–241. doi: 10.1254/jphs.08262fp. [http://dx.doi.org/10.1254/jphs.08262FP]. [PMID: 19202316]. [DOI] [PubMed] [Google Scholar]
- 158.Masuoka T., Ishibashi T., Nishio M. Biphasic modulation of noxious heat sensitivity in sensory neurons by peripheral metabotropic glutamate receptors. Inflamm. Cell Signal. 2015 [Google Scholar]
- 159.Nordlind K., Johansson O., Lidén S., Hökfelt T. Glutamate- and aspartate-like immunoreactivities in human normal and inflamed skin. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993;64(2):75–82. doi: 10.1007/BF02915098. [http://dx.doi.org/10.1007/BF02915098]. [PMID: 8106099]. [DOI] [PubMed] [Google Scholar]
- 160.Warncke T., Stubhaug A., Jørum E. Preinjury treatment with morphine or ketamine inhibits the development of experimentally induced secondary hyperalgesia in man. Pain. 2000;86(3):293–303. doi: 10.1016/S0304-3959(00)00260-8. [http://dx.doi.org/10.1016/S0304-3959(00)00260-8]. [PMID: 10812259]. [DOI] [PubMed] [Google Scholar]
- 161.Cairns B.E., Sessle B.J., Hu J.W. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J. Neurosci. 1998;18(19):8056–8064. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [PMID: 9742172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davidson E.M., Carlton S.M. Intraplantar injection of dextrorphan, ketamine or memantine attenuates formalin-induced behaviors. Brain Res. 1998;785(1):136–142. doi: 10.1016/s0006-8993(97)01396-6. [http://dx.doi.org/10. 1016/S0006-8993(97)01396-6]. [PMID: 9526066]. [DOI] [PubMed] [Google Scholar]
- 163.Planells-Cases R., Garcìa-Sanz N., Morenilla-Palao C., Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005;451(1):151–159. doi: 10.1007/s00424-005-1423-5. [http://dx.doi.org/10.1007/s00424-005-1423-5]. [PMID: 15909179]. [DOI] [PubMed] [Google Scholar]
- 164.Premkumar L.S., Ahern G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408(6815):985–990. doi: 10.1038/35050121. [http://dx.doi.org/10.1038/35050121]. [PMID: 11140687]. [DOI] [PubMed] [Google Scholar]
- 165.Morenilla-Palao C., Planells-Cases R., García-Sanz N., Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J. Biol. Chem. 2004;279(24):25665–25672. doi: 10.1074/jbc.M311515200. [http://dx.doi.org/10.1074/jbc.M311515200]. [PMID: 15066994]. [DOI] [PubMed] [Google Scholar]
- 166.Bhave G., Zhu W., Wang H., Brasier D.J., Oxford G.S., Gereau R.W., IV cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–731. doi: 10.1016/s0896-6273(02)00802-4. [http://dx.doi.org/10.1016/S0896-6273(02) 00802-4]. [PMID: 12194871]. [DOI] [PubMed] [Google Scholar]
- 167.Bonnington J.K., McNaughton P.A. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J. Physiol. 2003;551(Pt 2):433–446. doi: 10.1113/jphysiol.2003.039990. [http://dx.doi.org/10.1113/jphysiol.2003.039990]. [PMID: 12815188]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang X., Huang J., McNaughton P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [http://dx.doi.org/10.1038/sj.emboj. 7600893]. [PMID: 16319926]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [http://dx.doi.org/10.1146/annurev-cellbio-101011-155833]. [PMID: 24099085]. [DOI] [PubMed] [Google Scholar]
- 170.Ahern G.P., Wang X., Miyares R.L. Polyamines are potent ligands for the capsaicin receptor TRPV1. J. Biol. Chem. 2006;281(13):8991–8995. doi: 10.1074/jbc.M513429200. [http://dx.doi.org/10.1074/jbc.M513429200]. [PMID: 16431906]. [DOI] [PubMed] [Google Scholar]
- 171.Babbar N., Gerner E.W. Targeting polyamines and inflammation for cancer prevention. Recent Results Cancer Res. 2011;188:49–64. doi: 10.1007/978-3-642-10858-7_4. [http://dx.doi.org/10.1007/978-3-642-10858-7_4]. [PMID: 21253788]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gerner E.W., Meyskens F.L., Jr Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4(10):781–792. doi: 10.1038/nrc1454. [http://dx.doi.org/10.1038/nrc1454]. [PMID: 15510159]. [DOI] [PubMed] [Google Scholar]
- 173.Ahern G.P., Brooks I.M., Miyares R.L., Wang X.B. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J. Neurosci. 2005;25(21):5109–5116. doi: 10.1523/JNEUROSCI.0237-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.0237-05.2005]. [PMID: 15917451]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tan-No K., Taira A., Wako K., Niijima F., Nakagawasai O., Tadano T., Sakurada C., Sakurada T., Kisara K. Intrathecally administered spermine produces the scratching, biting and licking behaviour in mice. Pain. 2000;86(1-2):55–61. doi: 10.1016/s0304-3959(99)00312-7. [http://dx.doi.org/10.1016/S0304-3959(99)00312-7]. [PMID: 10779660]. [DOI] [PubMed] [Google Scholar]
- 175.Hrabetova S., Serrano P., Blace N., Tse H.W., Skifter D.A., Jane D.E., Monaghan D.T., Sacktor T.C. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J. Neurosci. 2000;20(12):RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [PMID: 10827202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu X.G., Sandkühler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-D-aspartic acid receptor blockage. Neurosci. Lett. 1995;191(1-2):43–46. doi: 10.1016/0304-3940(95)11553-0. [http://dx.doi.org/10.1016/0304-3940(95)11553-0]. [PMID: 7659287]. [DOI] [PubMed] [Google Scholar]
- 177.Lee J., Chung M-K., Ro J.Y. Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia. Biochem. Biophys. Res. Commun. 2012;424(2):358–363. doi: 10.1016/j.bbrc.2012.07.008. [http://dx.doi.org/10.1016/j.bbrc.2012.07.008]. [PMID: 22789851]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Studer M., McNaughton P.A. Modulation of single-channel properties of TRPV1 by phosphorylation. J. Physiol. 2010;588(Pt 19):3743–3756. doi: 10.1113/jphysiol.2010.190611. [http://dx.doi.org/10.1113/jphysiol.2010.190611]. [PMID: 20693293]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jung J., Shin J.S., Lee S-Y., Hwang S.W., Koo J., Cho H., Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 2004;279(8):7048–7054. doi: 10.1074/jbc.M311448200. [http://dx.doi.org/10.1074/jbc. M311448200]. [PMID: 14630912]. [DOI] [PubMed] [Google Scholar]
- 180.Lee J., Saloman J.L., Weiland G., Auh Q-S., Chung M-K., Ro J.Y. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain. 2012;153(7):1514–1524. doi: 10.1016/j.pain.2012.04.015. [http://dx.doi.org/10.1016/j.pain.2012.04.015]. [PMID: 22609428]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Efendiev R., Bavencoffe A., Hu H., Zhu M.X., Dessauer C.W. Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J. Biol. Chem. 2013;288(6):3929–3937. doi: 10.1074/jbc.M112.428144. [http://dx.doi.org/10.1074/jbc. M112.428144]. [PMID: 23264624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Szteyn K., Rowan M.P., Gomez R., Du J., Carlton S.M., Jeske N.A. A-kinase anchoring protein 79/150 coordinates metabotropic glutamate receptor sensitization of peripheral sensory neurons. Pain. 2015;156(11):2364–2372. doi: 10.1097/j.pain.0000000000000295. [http://dx.doi.org/10.1097/j.pain. 0000000000000295]. [PMID: 26172554]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chung M-K., Lee J., Joseph J., Saloman J., Ro J.Y. Peripheral group I metabotropic glutamate receptor activation leads to muscle mechanical hyperalgesia through TRPV1 phosphorylation in the rat. J. Pain. 2015;16(1):67–76. doi: 10.1016/j.jpain.2014.10.008. [http://dx.doi.org/10.1016/j.jpain. 2014.10.008]. [PMID: 25451626]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ransom R.W., Stec N.L. Cooperative modulation of [3H]MK-801 binding to the N-methyl-D-aspartate receptor-ion channel complex by L-glutamate, glycine, and polyamines. J. Neurochem. 1988;51(3):830–836. doi: 10.1111/j.1471-4159.1988.tb01818.x. [http://dx.doi.org/10.1111/j.1471-4159.1988.tb01818.x]. [PMID: 2457653]. [DOI] [PubMed] [Google Scholar]
- 185.Ghilardi J.R., Röhrich H., Lindsay T.H., Sevcik M.A., Schwei M.J., Kubota K., Halvorson K.G., Poblete J., Chaplan S.R., Dubin A.E., Carruthers N.I., Swanson D., Kuskowski M., Flores C.M., Julius D., Mantyh P.W. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005;25(12):3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [http://dx.doi.org/10.1523/ JNEUROSCI.3815-04.2005]. [PMID: 15788769]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bloom A.P., Jimenez-Andrade J.M., Taylor R.N., Castañeda-Corral G., Kaczmarska M.J., Freeman K.T., Coughlin K.A., Ghilardi J.R., Kuskowski M.A., Mantyh P.W. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain. 2011;12(6):698–711. doi: 10.1016/j.jpain.2010.12.016. [http://dx.doi.org/10.1016/j.jpain.2010.12.016]. [PMID: 21497141]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brakspear K.S., Mason D.J. Glutamate signaling in bone. Front. Endocrinol. (Lausanne) 2012;3:97. doi: 10.3389/fendo.2012.00097. [PMID: 22888325]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chenu C., Serre C.M., Raynal C., Burt-Pichat B., Delmas P.D. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22(4):295–299. doi: 10.1016/s8756-3282(97)00295-0. [http://dx.doi.org/10.1016/S8756-3282(97)00295-0]. [PMID: 9556127]. [DOI] [PubMed] [Google Scholar]
- 189.Genever P.G., Skerry T.M. Regulation of spontaneous glutamate release activity in osteoblastic cells and its role in differentiation and survival: evidence for intrinsic glutamatergic signaling in bone. FASEB J. 2001;15(9):1586–1588. doi: 10.1096/fj.00-0594fje. [PMID: 11427494]. [DOI] [PubMed] [Google Scholar]
- 190.Jin Y-H., Takemura M., Furuyama A., Yonehar N. Interactions Between Glutamate Receptors and TRPV1 Involved in Nociceptive Processing at Peripheral Endings of Primary Afferent Fibers. In: Gallelli L., editor. Pharmacology. InTech; 2012. [http://dx.doi.org/10.5772/34100] [Google Scholar]
- 191.Kisner D.L., Catane R., Muggia F.M. The Rediscovery of DON (6-Diazo-5-Oxo-L-Norleucine). Recent Results Cancer Res. 1980;74:258–263. doi: 10.1007/978-3-642-81488-4_30. [DOI] [PubMed] [Google Scholar]
- 192.Pinkus L.M. Glutamine binding sites. Methods Enzymol. 1977;46:414–427. doi: 10.1016/s0076-6879(77)46049-x. [http://dx.doi.org/10.1016/S0076-6879(77)46049-X]. [PMID: 909432]. [DOI] [PubMed] [Google Scholar]
- 193.Catane R., Von Hoff D.D., Glaubiger D.L., Muggia F.M. Azaserine, DON, and azotomycin: three diazo analogs of L-glutamine with clinical antitumor activity. Cancer Treat. Rep. 1979;63(6):1033–1038. [PMID: 380801]. [PubMed] [Google Scholar]
- 194.Katt W.P., Ramachandran S., Erickson J.W., Cerione R.A. Dibenzophenanthridines as inhibitors of glutaminase C and cancer cell proliferation. Mol. Cancer Ther. 2012;11(6):1269–1278. doi: 10.1158/1535-7163.MCT-11-0942. [http://dx.doi.org/10.1158/1535-7163.MCT-11-0942]. [PMID: 22496480]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Simpson N.E., Tryndyak V.P., Beland F.A., Pogribny I.P. An in vitro investigation of metabolically sensitive biomarkers in breast cancer progression. Breast Cancer Res. Treat. 2012;133(3):959–968. doi: 10.1007/s10549-011-1871-x. [http://dx.doi.org/10.1007/s10549-011-1871-x]. [PMID: 22101407]. [DOI] [PubMed] [Google Scholar]
- 196.Simpson N.E., Tryndyak V.P., Pogribna M., Beland F.A., Pogribny I.P. Modifying metabolically sensitive histone marks by inhibiting glutamine metabolism affects gene expression and alters cancer cell phenotype. Epigenetics. 2012;7(12):1413–1420. doi: 10.4161/epi.22713. [http://dx.doi.org/10.4161/epi.22713]. [PMID: 23117580]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tardito S., Chiu M., Uggeri J., Zerbini A., Da Ros F., DallAsta V., Missale G., Bussolati O. L-Asparaginase and inhibitors of glutamine synthetase disclose glutamine addiction of β-catenin-mutated human hepatocellular carcinoma cells. Curr. Cancer Drug Targets. 2011;11(8):929–943. doi: 10.2174/156800911797264725. [http://dx.doi.org/10.2174/ 156800911797264725]. [PMID: 21834755]. [DOI] [PubMed] [Google Scholar]
- 198.Cassago A., Ferreira A.P., Ferreira I.M., Fornezari C., Gomes E.R., Greene K.S., Pereira H.M., Garratt R.C., Dias S.M., Ambrosio A.L. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc. Natl. Acad. Sci. USA. 2012;109(4):1092–1097. doi: 10.1073/pnas.1112495109. [http://dx.doi.org/10.1073/pnas.1112495109]. [PMID: 22228304]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Robinson M.M., McBryant S.J., Tsukamoto T., Rojas C., Ferraris D.V., Hamilton S.K., Hansen J.C., Curthoys N.P. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem. J. 2007;406(3):407–414. doi: 10.1042/BJ20070039. [http://dx.doi.org/10.1042/ BJ20070039]. [PMID: 17581113]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.DeLaBarre B., Gross S., Fang C., Gao Y., Jha A., Jiang F., Song J.J., Wei W., Hurov J.B. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry (Mosc.) 2011;50:10764–10770. doi: 10.1021/bi201613d. [http://dx.doi.org/10.1021/bi201613d]. [DOI] [PubMed] [Google Scholar]
- 201.Hartwick E.W., Curthoys N.P. BPTES inhibition of hGA (124551), a truncated form of human kidney-type glutaminase. J. Enzyme Inhib. Med. Chem. 2012;27(6):861–867. doi: 10.3109/14756366.2011.622272. [http://dx.doi.org/10.3109/14756366.2011.622272]. [PMID: 21999665]. [DOI] [PubMed] [Google Scholar]
- 202.Erdmann N., Zhao J., Lopez A.L., Herek S., Curthoys N., Hexum T.D., Tsukamoto T., Ferraris D., Zheng J. Glutamate production by HIV-1 infected human macrophage is blocked by the inhibition of glutaminase. J. Neurochem. 2007;102(2):539–549. doi: 10.1111/j.1471-4159.2007.04594.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.04594.x]. [PMID: 17596215]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vega-Pérez J.M., Periñán I., Argandoña M., Vega-Holm M., Palo-Nieto C., Burgos-Morón E., López-Lázaro M., Vargas C., Nieto J.J., Iglesias-Guerra F. Isoprenyl-thiourea and urea derivatives as new farnesyl diphosphate analogues: synthesis and in vitro antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2012;58:591–612. doi: 10.1016/j.ejmech.2012.10.042. [http://dx.doi.org/10.1016/j.ejmech.2012.10.042]. [PMID: 23174318]. [DOI] [PubMed] [Google Scholar]
- 204.Gross M.I., Demo S.D., Dennison J.B., Chen L., Chernov-Rogan T., Goyal B., Janes J.R., Laidig G.J., Lewis E.R., Li J., Mackinnon A.L., Parlati F., Rodriguez M.L., Shwonek P.J., Sjogren E.B., Stanton T.F., Wang T., Yang J., Zhao F., Bennett M.K. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 2014;13(4):890–901. doi: 10.1158/1535-7163.MCT-13-0870. [http://dx.doi.org/10.1158/1535-7163.MCT-13-0870]. [PMID: 24523301]. [DOI] [PubMed] [Google Scholar]
- 205.Asai H., Ozaki N., Shinoda M., Nagamine K., Tohnai I., Ueda M., Sugiura Y. Heat and mechanical hyperalgesia in mice model of cancer pain. Pain. 2005;117(1-2):19–29. doi: 10.1016/j.pain.2005.05.010. [http://dx.doi.org/10.1016/j.pain.2005.05.010]. [PMID: 16043290]. [DOI] [PubMed] [Google Scholar]
- 206.Jara-Oseguera A., Simon S.A., Rosenbaum T. TRPV1: on the road to pain relief. Curr. Mol. Pharmacol. 2008;1(3):255–269. doi: 10.2174/1874467210801030255. [http://dx.doi.org/10.2174/1874467210801030255]. [PMID: 20021438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Menéndez L., Juárez L., García E., García-Suárez O., Hidalgo A., Baamonde A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci. Lett. 2006;393(1):70–73. doi: 10.1016/j.neulet.2005.09.046. [http://dx.doi.org/10.1016/j.neulet.2005.09.046]. [PMID: 16243435]. [DOI] [PubMed] [Google Scholar]
- 208.Kort M.E., Kym P.R. TRPV1 antagonists: clinical setbacks and prospects for future development. Prog. Med. Chem. 2012;51:57–70. doi: 10.1016/B978-0-12-396493-9.00002-9. [http://dx.doi.org/10.1016/B978-0-12-396493-9.00002-9]. [PMID: 22520471]. [DOI] [PubMed] [Google Scholar]
- 209.Szolcsányi J., Sándor Z. Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol. Sci. 2012;33(12):646–655. doi: 10.1016/j.tips.2012.09.002. [http://dx.doi.org/10.1016/j.tips.2012.09.002]. [PMID: 23068431]. [DOI] [PubMed] [Google Scholar]
- 210.Sághy É., Szőke É., Payrits M., Helyes Z., Börzsei R., Erostyák J., Jánosi T.Z., Sétáló G., Jr, Szolcsányi J. Evidence for the role of lipid rafts and sphingomyelin in Ca2+-gating of Transient Receptor Potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacol. Res. 2015;100:101–116. doi: 10.1016/j.phrs.2015.07.028. [http://dx.doi.org/10.1016/j.phrs.2015.07.028]. [PMID: 26238178]. [DOI] [PubMed] [Google Scholar]