Abstract
Background
Pharmaceuticals with targets in the cholinergic transmission have been used for decades and are still fundamental treatments in many diseases and conditions today. Both the transmission and the effects of the somatomotoric and the parasympathetic nervous systems may be targeted by such treatments. Irrespective of the knowledge that the effects of neuronal signalling in the nervous systems may include a number of different receptor subtypes of both the nicotinic and the muscarinic receptors, this complexity is generally overlooked when assessing the mechanisms of action of pharmaceuticals.
Methods
We have search of bibliographic databases for peer-reviewed research literature focused on the cholinergic system. Also, we have taken advantage of our expertise in this field to deduce the conclusions of this study.
Results
Presently, the life cycle of acetylcholine, muscarinic receptors and their effects are reviewed in the major organ systems of the body. Neuronal and non-neuronal sources of acetylcholine are elucidated. Examples of pharmaceuticals, in particular cholinesterase inhibitors, affecting these systems are discussed. The review focuses on salivary glands, the respiratory tract and the lower urinary tract, since the complexity of the interplay of different muscarinic receptor subtypes is of significance for physiological, pharmacological and toxicological effects in these organs.
Conclusion
Most pharmaceuticals targeting muscarinic receptors are employed at such large doses that no selectivity can be expected. However, some differences in the adverse effect profile of muscarinic antagonists may still be explained by the variation of expression of muscarinic receptor subtypes in different organs. However, a complex pattern of interactions between muscarinic receptor subtypes occurs and needs to be considered when searching for selective pharmaceuticals. In the development of new entities for the treatment of for instance pesticide intoxication, the muscarinic receptor selectivity needs to be considered. Reactivators generally have a muscarinic M2 receptor acting profile. Such a blockade may engrave the situation since it may enlarge the effect of the muscarinic M3 receptor effect. This may explain why respiratory arrest is the major cause for deaths by esterase blocking.
Keywords: Acetylcholine, acetylcholinesterase, muscarinic receptor subtypes, pharmacotherapy
INTRODUCTION
A large number of pharmaceuticals intervene with the cholinergic transmission, most commonly by a direct antagonism on muscarinic receptors [1]. Examples of drugs exerting effects via intervention of the muscarinic receptor are pharmaceuticals employed in the treatment of the overactive urinary bladder, in obstructive pulmonary diseases and in the treatment of eye diseases [2]. Also, the muscarinic receptor may be either directly or indirectly targeted in pharmacotherapies of the central nervous system [3]. Despite the general occurrence of a composite muscarinic receptor (muscarinic M1 – M5 receptors) population in the neuronal junction, this has generally been disregarded because the drugs exert almost no selectivity between the muscarinic receptor subtypes [4]. Furthermore, the muscarinic M3 receptor is considered to be the principal receptor subtype mediating the parasympathetic contractile response in smooth muscle tissues. As a consequence, any interactions via other muscarinic receptor subtypes in neuronal and neuro-effector junctions have been neglected in the assessment of drug effects.
Pharmaceuticals intervening indirectly with cholinergic transmission, such as in the treatment of Alzheimer’s disease (AD), generally act on acetylcholine esterase. In organophos- phorus intoxication, such as by pesticides blocking the esterase, antimuscarinic treatment is usually combined with reactivators of the acetylcholine esterase (AChE [5]). However, when examining the mechanism of action of the reactivators it has turned out to be complex [6]. One probable mechanism is an antagonism of acetylcholine effects exerted on muscarinic receptors [6c]. Also, this antimuscarinic mechanism of action of the reactivators shows varying degree of selectivity for muscarinic receptor subtypes [7]. Oximes, for example, bind to and antagonize cholinergic effects preferentially via muscarinic M2 receptors [8]. Due to the varying significance of the composite muscarinic receptor population in different organs, the functional implication of intervening at a specific level of the cholinergic transmission may be hard to predict in the whole body [9].
Currently, the cholinergic transmission and the interactions of muscarinic receptors in the synapse and at different levels of the reflex arc are reviewed. Divergent effects of pharmaceuticals due to composite cholinergic mechanisms are addressed from the perspective of functional implications of possible interplays between muscarinic receptor subtypes.
Cholinergic transmission
The transmitter acetylcholine is a phylogenetically old substance and almost all living organisms are able to synthesize the compound. The evolvement of the acetylcholine synthesizing systems occurred well ahead of the appearance of living organisms expressing a nervous system [10]. Over the years, the possibility of non-neuronal synthesis of acetylcholine has been overlooked in mammals. However, today two sources of acetylcholine synthesis are recognized – neurons and non-neuronal tissues [11]. The non-neuronal sources of acetylcholine have gained increased interest lately. Originally, non-neuronal release of acetylcholine was ascribed either cells correlated to pathology, e.g. cancer cells or immune cells, or tissue lining cells, e.g. keratinocytes, urothelial cells, airway epithelial cells or endothelial cells [12]. However, it has been found that also other types of cells may produce acetylcholine. One example is cardiomyocytes, in which the production is thought to be protective against hypertrophic stimuli [13].
Acetylcholine is mainly synthetized from acetyl coenzyme A and choline by the enzyme choline acetyltransferase (ChAT) [14]. ChAT mediates the transfer of an acetyl group from acetyl coenzyme A to choline in the nerve terminal. Neuronal ChAT uses choline that is accessible from the extracellular fluid by a high-affinity choline transporter (ChT). The transmitter is then accumulated in the synaptic vesicles by a vesicular acetylcholine transporter (VAChT). However, the mitochondrial enzyme carnitine acetyltransferase (CarAT) may also contribute to the synthesis of acetylcholine. The ChAT-related enzyme CarAT is expressed in all cells [10]. Besides enhancing the ChAT mediated acetylcholine synthesis by cooperative mechanisms, CarAT may by itself cause acetylcholine synthesis [15]. CarAT seems to have a significant role in cholinergic signalling in some tissues. For example, the measurement of the synthesis of acetylcholine in homogenates of the extensor digitorum longus muscles (in the normally sciatic nerve innervated and in the denervated muscle) revealed half of the acetylcholine to be synthetized by CarAT in the former case and almost all of it in the latter case [16]. However, CarAT may also contribute to acetylcholine synthesis in organs consisting of smooth muscle. Specifically, CarAT is the major ACh-synthesizing enzyme in the urothelium in the urinary bladder [17]. Here, as well as in other non-neuronal cells, the polyspecific organic cation transporter (OCT) has been shown to present choline intracellularly.
Acetylcholine release occurs in a quantal (vesicular) and a non-quantal (non-vesicular) way [18]. The quantal release was first described for the neuromuscular junction, but is typical for neuronal transmission [19]. The basis for quantal release is the exocytosis of acetylcholine containing vesicles in the neuron induced by impulse activity. The membrane depolarization causes voltage-gated Ca2+ channels to open and exocytosis of the vesicle content starts within sub-milliseconds after calcium influx. The delay of the release is minimized by a large number of calcium channels being accessible close to the active zone [20]. However, the vesicles docking with the presynaptic membrane of the active zone have to undergo a priming reaction before exocytosis [21]. The anchoring of the vesicle to the membrane and its exocytosis include interactions of a number of proteins (e.g., SNAREs, syntaxin and synaptotagmins), whose activation is Ca2+ dependent [22]. At the neuromuscular synapses, the content of an acetylcholine quantum is fairly constant (6000–10000 molecules) [23].
The less well-characterized non-quantal release, on the other hand, may occur independently of impulse activity either in neurons or in non-neuronal tissues and is not restricted to the quantal pulsatile nature of release [19c, 24]. One example of the latter is the urothelial release in the urinary bladder, and further, the OCT seems to be of particular importance in the non-neuronal release of acetylcholine [25, 26]. However, the neuronal non-quantal release may involve ChT as well as VAChT that remains in the plasma membrane after the vesicular fusion [24]. It has been speculated that the ChT may transport choline retrogradely and ACh orthogradely.
Acetylcholine may be hydrolyzed by either of two structurally similar enzymes in the body - acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). In the synaptic cleft, acetylcholine is metabolized by AChE within milliseconds after its release from the nerve, forming choline and acetic acid [27]. In non-neuronal cells both AChE and BuChE are expressed, while BuChE is particularly obvious in blood plasma, the kidneys and the liver. BuChE catalyzes acetylcholine hydrolysis as well [28]. However, it hydrolyses esters with larger moieties, like butyrylcholine or benzoylcholine, more efficiently. The full significance of BuChE is still unclear, but it probably plays a role in the metabolism of cellular intermediates.
In the periphery, acetylcholine is a classical transmitter in the somatomotoric and autonomic nervous systems. The nicotinergic contraction of skeletal muscle and the muscarinic effects mediated via the autonomic nervous system are well known [4b, 9, 29]. In the parasympathetic nervous system, acetylcholine transmits signals both in the ganglia and at the effector cell level. In conditions of excessive discharge of the parasympathetic nervous systems, typical signs of cholinergic effects appear [30]. These signs include salivation, lacrimation, urination, defecation, emesis and vomiting, miosis, bradycardia and bronchial obstruction. Pronounced AChE inhibition causes similar symptoms, but affects the neuromuscular signalling as well [31]. The signs of excessive parasympathetic discharges elucidate the functions that are regulated by the parasympathetic nervous system under physiological conditions.
In contrast to the periphery, acetylcholine primarily acts as a neuromodulator within the central nervous system [32]. Here, acetylcholine modulates a number of vital functions such as cognition, reward and motor control. The effects are exerted by activation of both major groups of cholinergic receptors, muscarinic and nicotinic, which are widely distributed throughout the central nervous system [29, 33]. One major pathway is the cholinergic projection from the nucleus basalis to cortical areas, which is particularly related to attention and memory. In dementia, such as AD, the failure of cholinergic transmission is one important cause for the symptoms.
Muscarinic receptors
Muscarinic receptors occur in five subtypes, which all belong to the family of G protein-coupled receptors [9]. The G protein consists of one α-, β- and γ-subunit. Depending on the primary sequence homology of the α -subunit, the G protein is classified as Gs, Gi/o, Gq or G12 [4b]. The muscarinic receptor subtypes couple differentially to the G proteins, and the subunits of G proteins activate distinct cellular pathways. The inhibitory muscarinic M2 and M4 receptors mainly couple to G i/o, whereas the excitatory muscarinic M1, M3 and M5 receptors most frequently couple to G q/11. The muscarinic M2 and M4 receptors mainly exert inhibitory effects by reducing the activity of adenylate cyclase, prolonging the opening of potassium, non-selective cation and transient receptor potential channels. Muscarinic M1, M3 and M5 receptors increase intracellular calcium by mobilizing phosphoinositides that generate inositol 1,4,5-trisphosphate and 1,2-diacylglycerol.
Nicotinic receptors
Nicotinic receptors belong to a family of ligand-gated ion channels, where binding of two molecules of acetylcholine results in a conformation change of the receptor and the subsequent formation of a pore between the subunits allows the permeation of cations through the receptor. Usually, sodium (Na+) ions flow inward and potassium (K+) outward, however, some neuronal nicotinic receptors have been shown to be permeable for calcium (Ca2+) ions, thereby affecting the release of other neurotransmitters [34]. Two types of nicotinic receptors occur – the muscular and the neuronal type. The muscle-type receptors are situated in the neuromuscular junctions and are composed of α1, β1, δ, ε or γ subunits. The adult and fetal isoforms differ by substitution of the γ subunit with the ε subunit. The stoichiometry of the muscle-type nicotinic receptor is always α2β(ε)γδ. The neuronal type, sometimes subdivided into a CNS type and a ganglionic type, is always composed of α- and β-subunits. Homomeric receptors consist of only α7-10 subunits, heteromeric of α2-6 and β2-4 subunits in a conserved 2:3 ratio [35].
Nicotinic receptors are localized both pre- and post-synaptically. The former ones serve as regulatory receptors modulating the release of acetylcholine and other neurotransmitters in the CNS. The nicotinic-evoked release of transmitter can be both positively and negatively modulated by interactions with metabotropic and ionotropic receptors [36]. At the motor neurons, presynaptic nicotinic receptors facilitate mobilization of a reserve pool of acetylcholine, thereby enhancing the cholinergic transmission [37]. The significance of postsynaptic nicotinic receptors is evident as they depolarise the membrane at the neuro- muscular endplate, leading to muscle contraction. Further, in the CNS they play a crucial role in maintaining cognitive function. Nicotinic receptors in the CNS are commonly linked to neurodegenerative diseases such as Alzheimer´s and Parkinson’s disease [38].
Salivary glands
The increase in salivary flow evoked by activation of muscarinic receptors has generally been attributed to effects via muscarinic M3 receptors [39]. However, all subtypes have been described in salivary glands [40] and data from studies in several animal models have indicated that in particular muscarinic M1 receptors also contribute to the secretory response to muscarinic receptor stimulation [41]. In the rat sublingual and the ovine submandibular glands, concomitant activation of the different muscarinic receptor subtypes seems to be a necessity for maximum glandular responses [41a, 42].
Regarding the neuronal activation of salivary glands, prejunctional muscarinic receptors modulate the parasym- pathetic nerve-evoked response [43]. Here, muscarinic M1 receptors normally facilitate transmitter release during short, intense nerve activity. At low frequencies, on the other hand, muscarinic M2 receptors, or possibly muscarinic M4 receptors, inhibit the transmission, but only after some delay [42, 43b]. By blocking the prejunctional inhibitory muscarinic receptors the nerve-evoked secretory response may be threefold larger than in the absence of the inhibition in the rat parotid gland [43b]. This increase, however, does not only reflect cholinergic effects since neuropeptides, e.g., vasoactive intestinal peptide (VIP), are co-released from the parasympathetic nerve and markedly potentiate the cholinergic response. Also, in many species the neuro- peptides evoke atropine-resistant overt secretion (up to 50% of the parasympathetic response) [44]. In the concomitant parasympathetic vascular response, acetylcholine and VIP also interact [45]. Here, VIP is the major player, while acetylcholine, by acting on muscarinic M3 receptors, has additive effects [46].
The Urinary Bladder
In the urinary bladder, all five subtypes of the muscarinic receptor are expressed [47]. However, the expression varies in different bladder tissues. On the detrusor smooth muscle cells, muscarinic M2 receptors dominate in number, even though muscarinic M3 receptors have been shown to evoke the main cholinergic contractile response [43a, 48]. The other subtypes have been demonstrated to occur as well, but at a markedly lower number. In the urothelium, on the other hand, all muscarinic receptor subtypes are expressed in large numbers. The muscarinic M1 receptors occur on basal cells and muscarinic M2 receptors on umbrella cells, while muscarinic M3 and M4 receptors are homogenously distributed and muscarinic M5 receptors are distributed with a decreasing gradient from luminal to basal cells [49]. Traditionally, the urothelium has been regarded to be a passive barrier. However, during the last decade observations have accrued showing that the urothelium plays an important integrating role in the regulation of bladder function [50]. This indicates that the ways the muscarinic receptors may interact are numerous, which may be even more intricate when taking prejunctional receptors into consideration. Namely, muscarinic receptors are expressed on bladder neurons, afferent as well as efferent [43a, 51]. Muscarinic facilitatory and inhibitory receptors occur on efferent nerve terminals. While the former are of the muscarinic M1 receptor subtype, the latter has for long been considered to be of the M2 subtype. However, some reports indicate that the inhibitory subtype may be the muscarinic M4 receptor [47, 52]. Sensory neurons have been reported to express different subtypes of the muscarinic receptor (M2, M3 and M4 [53]), suggesting cholinergic influence on the processing of sensory information from the bladder. However, even though it is well known that the administration of muscarinic antagonists inhibits the neuronal activity in bladder afferents [54], the specific effects of the muscarinic receptor subtypes in the local bladder afferent activation are unclear.
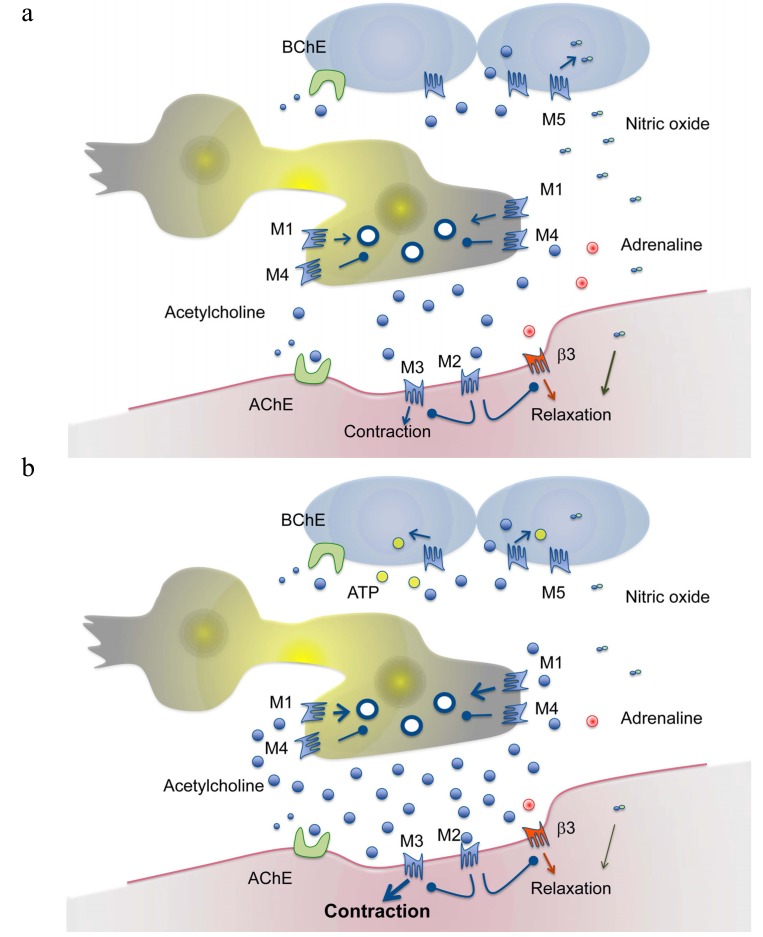
A number of substances may be released from the urothelium of which ATP seems to be of particular interest [55]. Stretch may release urothelial ATP that, directly or indirectly via inducing non-neuronal acetylcholine release, stimulates afferents within the micturition reflex [56]. Non-neuronal acetylcholine may, by acting on urothelial muscarinic M5 receptors, induce the release of nitric oxide that exerts inhibitory effects and that preserves low bladder pressures in spite of increasing urine volumes [57]. Any muscarinic M3 receptor effect on the effector smooth muscle cell evoked by low concentrations of acetylcholine, e.g. non-quantal release or release at low parasympathetic stimulation intensity, is counteracted by muscarinic M2 receptor hyper- polarization [58]. Also, muscarinic M2 receptors inhibit adenylate cyclase and by that the formation of cAMP [59]. This mechanism has been shown to prevent adrenergic and purinergic effects that might counteract the cholinergic contraction. Furthermore, the release of low amounts of acetylcholine upon parasympathetic activity exerts a prejunctional inhibition on its own release via muscarinic M4 (and/or M2) receptors. However, intense neuronal activity over short periods of time resulting in high concentrations of the transmitter stimulates, in resemblance with the glandular mechanism, facilitatory muscarinic M1 receptors on the nerve terminal [41c, 43b]. This eventually results in either larger (M1 effect) or smaller (M4 (and/or M2) effect) acetylcholine evoked detrusor contractions via the muscarinic M3 receptor. A schematic overview of the conceptual interactions of muscarinic receptor subtypes within the urinary bladder is presented in Fig. 1.
Fig. (1).
Schematic drawing of muscarinic receptor subtypes in the urinary bladder. The upper panel (a) indicates the filling phase of the urinary bladder. In this phase a limited amount of acetylcholine is released from neuronal (parasympathetic) and non-neuronal tissues (e.g. the urothelium). Adrenergic stimulation of relaxatory β3-adrenoceptors (by noradrenaline or adrenaline) is indicated. Relaxatory urothelial factors (e.g. nitric oxide released by activation of muscarinic receptors) stabilize the detrusor. Postsynaptic muscarinic M2 receptors inhibit the effects of contractile muscarinic M3 receptors. Presynaptic muscarinic M4 receptors inhibit the release of transmitter. The lower panel (b) indicates the emptying phase (detrusor contraction). In this phase a large number of vesicles release acetylcholine. Presynaptic muscarinic M1 receptors are activated and facilitate the release. The intense stimulation of contractile muscarinic M3 receptors overcomes any inhibitory effect of postsynaptic muscarinic M2 receptors. The muscarinic M2 receptors inhibit relaxatory effects via β3-adrenoceptors. Excitatory factors (e.g. ATP) are released from the urothelium. Both synaptic acetylcholinesterase and non-synaptic butyrylcholinesterase metabolizes acetylcholine.
The respiratory tract
The respiratory tract shows great resemblance with the urinary bladder regarding muscarinic receptors and the cholinergic system. All five subtypes are expressed in the lungs and both neuronal and non-neuronal release of acetylcholine occurs [60]. Muscarinic M3 receptors have a dominant role in the smooth muscle contraction. Furthermore, the muscarinic M2 receptors that outnumber the M3 receptors have indirect roles. These indirect roles are exerted by inhibition of relaxation evoked by β-adrenoceptors [61]. Both facilitatory and inhibitory prejunctional muscarinic receptors regulate the parasympathetic transmission [62]. In resemblance with the urinary bladder and salivary glands, prejunctional muscarinic M1 receptors facilitate the acetylcholine release [63]. However, most reports claim the inhibitory receptors to be of the M2 subtype, even though some animal studies propose the prejunctional receptors to be of the M4 subtype [64]. Nevertheless, the prejunctional inhibitory muscarinic receptor function has been suggested to be hampered in asthma [62]. In addition to the smooth muscle expression of muscarinic receptors, mucosal glands also exhibit muscarinic receptors and likewise the M3 subtype mediates the main secretory response [65].
The pulmonary arteries belong to the group of vessels, including those of salivary glands as well, that are influenced by parasympathetic nerve activity [66]. Namely, in the lung, stimulation of the vagal nerve induces vasodilatation via acetylcholine acting on muscarinic M3 receptors [67]. This may in turn cause endothelial release of nitric oxide [68]. The vascular response to acetylcholine appears biphasic, involving a contractile part as well. Muscarinic M1 receptors have been discussed in this context and have been proposed to have a key role in pulmonary vascular dysfunction [69]. However, the vagal transmitter release is not the only source of acetylcholine [70]. Non-neuronal release of acetylcholine from the endothelium has been proposed to participate in the vasodilator response due to local mechanical forces induced by blood flow changes [12].
The heart
The primary effect of the parasympathetic nervous system in the heart is to decrease the rate [71]. The source of acetylcholine release may be an atrial non-neuronal production [18a]. The classical view is that atrial muscarinic M2 receptors exclusively mediate this effect [72]. However, studies in a number of different species including man, indicate the expression of other subtypes, in particular in the ventricles [73]. Of these other subtypes, most interest has been given to muscarinic M1 and M3 receptors [74]. Whether functionally significant or not, all other subtypes are heavily outnumbered by the muscarinic M2 receptors [75].
Cholinergic stimulation of the atrial muscarinic M2 receptors slows the rate of spontaneous firing in the sinoatrial node [76]. Also, muscarinic M2 receptors influence the conductance of impulses in the atrioventricular node, which is slowed down by parasympathetic activity [77]. One way this effect of muscarinic M2 receptors is exerted is via potassium currents (IK(ACh)) that hyperpolarizes the membrane potential [76]. Another way is by inhibiting cAMP-dependent effects (i.e., β-adrenoceptor effects [78]). Several mechanisms have been suggested regarding the latter matter. For instance, muscarinic M2 receptors have been suggested to stimulate the production of cGMP via nitric oxide, which in turn inhibits cAMP-dependent responses.
In the heart, large concentrations of muscarinic agonists have been shown to increase automaticity and possibly contractility as well [74b, 79]. Muscarinic M1 and/or M3 receptors have been discussed in this context. A muscarinic M1 receptor effect has been suggested to be mediated via PLC-dependent mechanisms that enhance L-type Ca2+-channels [80]. The tentative muscarinic M3 receptor effect seems also to include PLC-dependent mechanisms, which activate potassium currents of a delayed rectifier type [81]. Thus, the most prominent and normal role of acetylcholine in the heart is to decrease the cardiac output via muscarinic M2 receptors. However, other subtypes may also affect cardiac function, particularly under pathological conditions such as in heart failure, ischemia and arrhythmias [82].
The central nervous system
All five muscarinic receptor subtypes occur in the central nervous system. In contrast to the periphery, based on their frequency of occurrence, muscarinic M3 receptors seem to be of less significance [83]. Muscarinic M3 receptors are mainly expressed in cortical pyramidal cells and in glial cells. Muscarinic M1 receptors show a similar distribution and are found throughout the brain, but in much higher concentrations [84]. The highest concentrations occur in cortical regions and hippocampus. The cortical muscarinic M1 receptors are mostly located postsynaptically in excitatory synapses. Muscarinic M2 receptors occur both pre- and postsynaptically, and the receptor subtype occurs in high levels in the nucleus basalis, cerebellum, pons/medulla and thalamus/hypothalamus [85]. Muscarinic M2 receptors are expressed in other cortical areas also, such as hippo- campus and putamen. Muscarinic M4 receptors occur in the highest concentrations in basal ganglia, where they occur in association with dopaminergic receptors. Muscarinic M5 receptors, on the other hand, are sparsely expressed in the central nervous system [86]. However, some expression occurs in the hippocampus, substantia nigra and ventral tegmental area.
The cholinergic systems are affected in a number of disorders of the central nervous system. In particular in AD and other cognitive disorders, muscarinic receptors have been regarded to be potential targets when developing new pharmaceuticals. Muscarinic M1 receptors seem to be particularly important for mediating cognitive effects, and M1-selective agonists have been proposed to be suitable in the pharmacotherapy of AD. The favourable effects of treatment with acetylcholine esterase inhibitors support this notion.
Application of pharmaceuticals acting on the parasympathetic nervous system
A number of pharmaceuticals used in the clinic intervene with the cholinergic systems and because of the broad spectrum of parasympathetic effects in the body, adverse effects are numerous [1]. Historically, drugs affecting the cholinergic systems were often employed in the pharmacotherapy of diseases in the gastrointestinal tract e.g. gastric ulcer and intestine disorders [87]. Today, other treatments have substantially replaced the old therapies. However, the use of pharmaceuticals acting on the parasympathetic nervous system is still indicated in the pharmacotherapy, but then non-systemic administration approaches of the drugs may be employed [88].
Generally, systemic administration of muscarinic receptor antagonists is more common than of muscarinic receptor agonists. When agonists are used the indication often only requires a single dose administration. In cases of atonic gut or atonic bladder, a muscarinic agonist may be injected in order to trigger the activity of the smooth muscle [89]. However, in the treatment of glaucoma (local) or oral dryness as in Sjögren’s syndrome (systemic) pilocarpine may be prescribed for long-term use [90]. Acetylcholine esterase inhibitors are an alternative to the direct agonists and are indicated in the treatment of diseases in the central nervous system (e.g. AD; see below [91]). Outside of the central nervous system they may be used in the treatment of glaucoma, to increase bladder and intestine motility, to overcome neuromuscular blockade in anaesthetized patients and in the treatment of myasthenia gravis. Esterase inhibitors with strong and irreversible binding to the enzyme have been used in chemical warfare or misused for terroristic purposes. Also, pesticides have the same mechanism of action as the inhibitors used in warfare and may cause, likewise to the militarily used inhibitors, death mainly by respiratory arrest. In the treatment of esterase inhibition, so called esterase reactivators are given in addition to muscarinic antagonists [92]. The mechanism of action of the reactivators is via affinity to the enzyme and the ability to bind the organophosphate which is inhibiting its active site, thereby making the site accessible to acetylcholine [93]. In the absence of inhibition, the reactivators act as weak acetylcholine esterase inhibitors. The exact mechanism of action of the reactivators is not totally unraveled. However, muscarinic and nicotinic receptor antagonism has been suggested [92].
Even though muscarinic receptor antagonists may be administered locally, e.g. for inducing mydriasis, counteracting cycloplegia and as asthma inhalation treatment [88, 90b], they are applied systemically in the treatment of certain diseases and conditions such as for resuscitation purposes via inhibition of bradycardia and for relief of postprandial discomfort [82, 87, 94]. However, the most common use of drugs exerting antimuscarinic receptor effects is for urge incontinence. Also, in cases of organophosphate poisoning (chemical warfare or pesticides), muscarinic receptor antagonists are indicated as antidotes [92].
Treatments with anticholinergic drugs affecting the lower urinary tract
Today it is well accepted that bladder contraction is almost solely caused by activation of muscarinic M3-receptors, with slight influence of M2-receptors via indirect inhibition of adrenergic and purinergic induced detrusor relaxation [47]. However, when employing anticholinergic drugs in the treatment of the patient, the whole micturition reflex arc must be taken into consideration. Even though the rationale for anticholinergic treatment of overactive bladder is via blockade of muscarinic receptors, the effect is likely exerted at more levels than merely by antagonizing receptors in the detrusor [95]. For instance, one must take non-neuronally released acetylcholine into account, which can act on muscarinic M2, M3 and M4 receptors, yielding an excitatory or inhibitory effect on afferent activity in the micturition arc. An anticholinergic effect on such excitatory muscarinic receptors is suggested to at least partially improve the disease symptoms. When examining the affinity of the antimuscarinic drugs, most of them have greatest affinity for muscarinic M3 receptors with some exceptions such as imidafenacin and tolterodine [96]. Therefore, if one solely takes into account the detrusor overactivity in OAB patients to be caused by activation of muscarinic M3 receptors located either on the detrusor muscle [97] or on afferents [98], it is impossible to explain the positive clinical effects of the drugs.
Competitive inhibition of b[3H]NMS [149] and a[3H]imidafenacin [96d] binding by muscarinic antagonists in homogenates of human bladder and parotid gland and c of carbachol-induced Ca2+ mobilization in bladder and submandibular gland cells from Cynomolgus monkeys [150].
Over the past decade and a half, the concept of tissue selectivity has emerged which could be one possible approach to develop new entities showing less anticholinergic adverse effects. A number of studies have indicated that some antagonists exert this form of bladder selectivity, in comparison with salivary glands when examined in vivo [99]. The suggestion that these observations show general bladder selectivity may be an over-interpretation. No, or only small, differences appear when studying the tissue affinity of antagonists when comparing the bladder and salivary glands (Table 1a). The low degree of selectivity is also supported by results from binding experiments on cell lines expressing the specific muscarinic receptor subtypes (Table 1b). Data from one species must be assessed based on its particular physiology. In rat a pronounced non-adrenergic, non-cholinergic (NANC) salivary secretion occurs, while in cat the NANC transmitters (e.g., VIP) markedly potentiate the cholinergic response [9]. No such responses have been described in man. It may be argued that the same situation is valid in the urinary bladder. However, the NANC transmitters are different [100]. In the case of the occurrence of NANC responses, ATP (short-lasting effects) is the main transmitter in the bladder [101], while neuropeptides (substance P, VIP and calcitonin gene-related peptide; long-lasting effects) evoke the atropine-resistant responses in glands [102]. Therefore, it is hazardous stating tissue selectivity based on responses evoked by nerve stimulation. Also, carbachol has routinely been used as the muscarinic agonist of choice in studies on the urinary bladder. This may, likewise to acetylcholine, also create a confounding situation due to its pronounced effect on nicotinic receptors [103]. Thus, to conclude the occurrence of tissue/bladder selectivity, in vivo data could be faulty for various reasons. For one, bladder contraction and salivation are induced in different ways. This, in turn, is linked to the possibility that other factors may obscure the data and its interpretation. For instance, it seems likely that effects involving prejunctional inhibitory muscarinic receptors, i.e. M2 and/or M4, will affect measurements of salivation and bladder contraction [43, 52, 60, 64b, 104]. Further, it is still not known exactly how the various muscarinic receptor subtypes per se affect bladder contraction and salivation in different species, and to link findings in an animal species to possible effects in man is therefore hard. However, the fact remains that in vivo data of this kind are hard to interpret due the influence of prejunctional receptors and a non-complete understanding of bladder contraction in other species, as well as the various levels at which antimuscarinics affect bladder contraction.
Table 1a.
Inhibitory constants of muscarinic antagonists in urinary bladder and salivary gland tissue.
| Compound | Urinary Bladder (pKi) | Parotid Gland (pKi) | Submandibular Gland (pKi) |
|---|---|---|---|
| Imidafenacin | 9.2a | 9.2 a | |
| Tolterodine | 8.6 a, 8.7 b, 8.5 c | 8.5 a, 8.5 b | 8.7 c |
| Oxybutynin | 8.4 a, 7.8 b, 8.7 c | 8.8 a, 8.2 b | 9.0 c |
| Solifenacin | 7.4 a, 8.5 c | 7.7 a | 8.2 c |
| Darifenacin | 8.4 c, 7.7 b | 8.7 b | 8.8 c |
| Propiverine | 6.3 b | 6.6 b |
Table 1b.
Binding affinities of muscarinic antagonists in cell lines expressing human muscarinic receptor subtypes.
| Compound | M1 (pKi) | M2 (pKi) | M3 (pKi) | M4 (pKi) | M5 (pKi) |
|---|---|---|---|---|---|
| Tolterodine | 8.7, c 7.8 b | 8.4 c, 8.0 b | 8.4 c, 8.3 b | 8.4 c, 8.6 b | 8.8 c, 8.6 b |
| Oxybutynin | 8.6 c, 8.5 a | 8.1 c, 7.6 a | 8.8 c, 8.7 a | 8.7 c, 8.5 a | 8.8 c |
| Solifenacin | 7.6 c, 7.1b | 7.1 c, 7.0 b | 7.7 c, 7.7 b | 6.8 c, 7.7b | 7.2 c, 7.5 b |
| Darifenacin | 7.5 c, 7.2b | 7.2 c, 6.9 b | 8.6 c, 8.4b | 7.3 c, 7.5 b | 7.9 c, 7.6b |
| Fesoterodine | 7.8 b | 8.6 b | 7.3 b | 8.0 b | |
| Propiverine | 6.4 a | 5.7 a | 6.7 a | 6.5 a | |
| Trospium | 8.5 b | 9.0 b | 9.0 b | 8.8b | 8.2b |
References:
a=151 b=152 c=153
Treatments with anticholinergic drugs affecting the respiratory tract
Anticholinergic drugs are often employed in the treatment of chronic obstructive pulmonary diseases in which an increased vagal tone may occur [105]. Consequently, the drugs may exert a number of attractive effects, which in addition to counteracting the smooth muscle contraction, decreases mucus secretion and bronchial vasodilatation [106]. However, in cases of the anticholinergic drug reaching the circulation, adverse effects such as dry mouth, urine retention and tachycardia often occur. Anticholinergics may also be indicated in asthma therapy when the condition is associated with vagal activity. However, β2-agonists are generally superior in improving airflow in acute asthma when compared with anticholinergics [107].
Two types of muscarinic receptor antagonists are approved for marketing authorization; so called short-acting (e.g., ipratropium and oxitropium) and long-acting muscarinic receptor antagonists (e.g., tiotropium) [108]. Even though tiotropium is more potent than ipratropium as an antagonist at muscarinic receptors, its longer dissociation half-life at muscarinic M3 receptors provides the long-acting duration.
A number of new anticholinergic drugs are currently in phase II or III. One mutual focus when new anticholinergic drugs are developed is to create compounds with larger effects on muscarinic M3 receptors than on muscarinic M2 receptors [109]. This aims to reduce the cardiac adverse effects induced by the treatment. Namely, increased risks of cardiovascular death have been reported [110]. Furthermore, another positive result of this new selectivity profile could be that less effect occurs on the prejunctional inhibitory muscarinic receptors. However, it is still not clarified if these latter receptors are of the muscarinic M2 or the M4 receptor subtype in humans, even though animal experiments indicate the receptors to be of the latter subtype [111]. If they are of the muscarinic M4 receptor subtype, selectivity for muscarinic M3 over M2 receptors might be of no significance regarding the prejunctional effects. Furthermore, the selectivity for muscarinic M3 over M2 receptors may even be disadvantageous in view of the muscarinic M2 receptor inhibiting adrenergic relaxation [112]. Namely, if only the muscarinic M3 receptors are blocked, while leaving the muscarinic M2 receptors unaffected, acetylcholine can still exert its M2-mediated inhibition of β2-adrenoceptor induced relaxation.
Treatments with pharmaceuticals affecting AChE
As have been mentioned previously, AChE modulators can be used in the clinical treatment of several conditions. AChE modulators comprise of two different subgroups - widely used AChE inhibitors and specific AChE reactivators. One important characteristic of the latter subgroup is that they usually act as weak AChE inhibitors [7]. However, the specific name originates from the pharmacological action, which is reactivation and which is ensured by the oxime moiety in their structure. Therefore, the term oxime reactivator, or just plainly oxime, is commonly used. Both types of compounds affecting the esterase show composite mechanisms of action. A summary of AChE affecting compounds is presented in Table 2. While the AChE inhibition frequently is combined with effects on the nicotinic receptor, the effect on muscarinic receptors is particularly obvious for the reactivators.
Table 2.
IC50 values (in µM) representing affinity of different AChE inhibitors to cholinergic enzymes (human acetylcholinesterase (hAChE) and human butyrylcholinesterase (hBAChE), cholinergic receptors (muscarinic; Musc) and nicotinic (Nic) receptors.
| Compound | hAChE | hBChE | Musc | Nic |
|---|---|---|---|---|
| Pralidoxime | >1000a | >1000b | N/A | N/A |
| Trimedoxime | 215a | >1000b | N/A | N/A |
| Methoxime | >1000a | >1000b | N/A | N/A |
| Obidoxime | >1000a | >1000b | 3.5i | 140-250j |
| HI-6 | 281a | >1000b | 167i | 140-250j |
| K027 | >1000a | >1000b | 18i | >600j |
| K203 | >1000a | >1000b | 4.7i | 140-250j |
| Physostigmine | 0.072g | 0.035g | 24000q | Direct agonisms Potentiation(1-100 µM)p Antagonism (IC50=70 µM)s |
| Pyridostigmine | 40c | 16000c | >10v | Potentiation(200-400 µM)x Antagonism (IC50=2000 µM) x |
| Neostigmine | 0.1c | 0.8c | No affinityr,v | 100t |
| Ambenonium | 0.0007c | 6.82c | 0.37u | Direct agonismw |
| Distigmine | 0.25g | 0.27g | Ki=2u | Ki=23u |
| Edrophonium | 5.17c | 1370c | >10v | Direct agonism (<300 µM] k IC50=44k |
| Tacrine | 0.5d | 0.02d | 12.6d | 3.6d |
| Donepezil | 0.022e | 4.15 e | 21m | Antagonism (10-100 µM)n |
| Rivastigmine | 4.15f | 0.037f | No affinityo | 15-30p |
| Galantamine | 0.8f | 73f | >10v | Potentiation (1-10 µM) Antagonism (>10 µM)l |
Reactivators of AChE
Reactivators are used in a specific case - the organophosporus (OP) poisoning caused by either nerve agents like soman, sarin tabun etc. or by OP pesticides [92]. The former cause is nowadays rather rare, however, recent misuse of sarin in Syria testifies in favor of maintaining readiness against OP terroristic threats. Moreover, their place in the army gear is unquestionable. Reactivators, together with atropine, represent a necessary first aid for OP intoxication. Every year, hundreds of thousands of people die due to pesticide poisoning, either by mistake or rather commonly by committing suicide [113]. Reactivators represent the causal treatment whereas atropine is merely symptomatic [114]. Pralidoxime, trimedoxime, methoxime, obidoxime and HI-6 are reactivators that are currently used against OP poisoning. Unfortunately, the reactivators differ in their efficacy against individual nerve agents, which means that the source of poisoning must be identified before application of a proper oxime. The most versatile oxime, HI-6, lacks efficacy against tabun for which obidoxime or trimedoxime must be used. On the contrary, those compounds are ineffective against soman poisoning (for review see [115]). Soman poisoning is also complicated due to the process called “aging”, which is a time-dependent loss of AChE ability to be reactivated [116]. Regarding soman, aging occurs within a few minutes. Pesticide poisoning is easier to treat, since aging may take a couple of days. Obidoxime is the drug of choice here. However, due to its higher toxicity, a replacement of obidoxime would be beneficial and the oxime K027 seems to be a rather promising candidate [117]. Reactivators also exhibit varying degree of affinity for cholinergic receptors [7]. Obidoxime, for instance, exerts significant antagonism on the muscarinic M2 receptor [8]. At first, this mechanism of protecting cholinergic receptors may seem advantageous. However, in the light of what has been discussed above regarding the physiological function of this particular receptor, it may explain its higher toxicity [58]. Another common feature of all reactivators is a low blood-brain barrier penetration due to the charged nitrogen in their structure [114b]. Therefore, the use of non-quaternary and monoquaternary reactivators or other approaches targeting blood-brain barrier penetration are currently being thoroughly investigated. An oxime moiety seems to be essential for the effects of reactivators. Attempts with compounds lacking this moiety have been made, but these compounds are not better than oxime-based reactivators regarding efficacy against OP compounds (for review see [118].
Acetylcholinesterase inhibitors
Inhibitors of AChE have found their use in a wide area of applications. Reversible inhibitors can serve as prophylaxis for OP poisoning. The resistance to OP irreversible inhibitors is ensured by their higher affinity to AChE than organophosphate, and after being spontaneously hydrolyzed AChE can fulfill its physiological tasks. Carbamates (pyridostigmine, physostigmine) belong to this group of reversible AChE inhibitors [114a].
The use of AChE inhibitors is wide, for instance as symptomatic treatment in Alzheimer´s disease (AD) and other types of dementia (e.g. Lewy body dementia), glaucoma, postural tachycardia and myasthenia gravis [119]. Especially AD represents an increasing health problem [120], and the massive worldwide pursuit for finding an effective cure has been fruitless so far. In the treatment of AD, the fact that inhibitors of AChE might also be inhibitors of BuChE should be considered. It has been reported that non-selective inhibitors of cholinesterases would be a more nuanced and logical treatment, since BuChE activity rises in the AD brain [121]. As expected, selective AChE inhibitors have been reported to be more effective than inhibitors of BuChE due to the fact that AChE outnumbers BuChE in the brain [119a]. Since one area of frequent employment of cholinesterase inhibitors is AD, the pharmacology of some key inhibitors are reviewed with this particular focus.
The first nonselective AChE inhibitor was tacrine (1,2,3,4-tetrahydro-9-aminoacridine), commercially named (Cognex®), which was approved by the FDA in 1993. However, the use of tacrine was limited by its poor oral bioavailability, the necessity of four-times daily dosing, and considerable adverse effects (mainly hepatotoxicity) [122]. Due to the adverse effects, the compound was eventually withdrawn from the market. Interestingly, tacrine is a relatively weak AChE inhibitor, as compared to donepezil or rivastigmine, with a complex mechanism of action involving other cholinergic structures, ion channels and monoaminergic systems [123]. Furthermore, tacrine reduces the levels of total β-amyloid peptide (Aβ), suggesting its involvement in the amyloidogenic pathway [124]. Even though tacrine itself is an obsolete molecule, it is a parent compound for many derivatives due to its multi-target directed ligand (MTDL) mode of action. Such compounds have recently been reported to combine inhibition of cholinesterases with antioxidant activity, chelating of metal ions, interaction with cholinergic receptors, neuroprotection and the inhibition of Aβ turnover [125].
Later, rivastigmine (Excelon®), another non-selective cholinesterase inhibitor, was approved for the treatment of mild to moderate AD. Rivastigmine is a pseudo-irreversible selective AChE inhibitor [126]. The pseudo-irreversibility refers to its binding to AChE, which cleaves the rivastigmine molecule resulting in the release of an inert phenolic product while the carbamate moiety remains bound to the esteratic site of the AChE [127]. Similarly to tacrine, rivastigmine seems to possess additional mechanisms of action of importance in the treatment of AD, even though probably not to same extent. Nevertheless, it has been reported to beneficially modulate the glutaminergic pathway [128] and shift Aβ processing towards the more beneficial α-secretase cleavage pathway [129]. The latter effect on the Aβ cascade might be a common feature of all BChE inhibitors since the locally neurotoxic Aβ plaque development is driven by the increase of BChE at the plaque level [128].
Galantamine, an alkaloid from the family Amaryliidacae, represents the weakest AChE inhibitor among the approved compounds. However, it also has additional cholinergic effects. Galantamine acts as a positive allosteric modulator on nicotinic receptors in the central nervous system, which are down regulated in AD patients [130]. Besides the direct and indirect cholinomimetic effect, galantamine modulates the nicotinic receptor-induced release of glutamate and monoamines, such as serotonin and norepinephrine, which also play a role in the pathogenesis of AD [131].
The last, but probably most widely used and most effective, AChE inhibitor is donepezil. Its efficacy is ensured by its simultaneous binding to the active site and to the peripheral anionic site of the enzyme [132], which has been reported to enhance Aβ aggregation [133]. Thus, donepezil stimulates the cholinergic pathway and inhibits Aβ turnover in the AD brain.
Despite the variations in the mode of action of the three cholinesterase inhibitors, which do notonly pertain AChE inhibition, there is no evidence of any major differences between them in respect to efficacy against AD. However, there appears to be less adverse effects associated with donepezil [134]. Newly developed derivatives of currently used AChE inhibitors have been reviewed recently [135].
Another disease in which AChE inhibitors are prescribed is myasthenia gravis. Myasthenia gravis is a rare disease affecting 20 out of 100 000 people [119b, 136]. Since the target is the motoric synapse at the neuromuscular junction, the AChE inhibitors of choice, in contrary to those used in the treatment of AD, are charged molecules. The anticipated clinical effect is simply an enhancement of cholinergic transmission, which is defected by an antibody-dependent attack on the postsynaptic membrane in the neuromuscular junction [137]. Antibodies may either affect the muscular nicotinic receptor (seropositive myasthenia gravis) or other components in the neuromuscular junction (seronegative myasthenia gravis) [138]. It has been shown that many seronegative patients have antibodies that bind to the muscle-specific tyrosine kinase that anchors and clusters the nicotinic receptor at the postsynaptic membrane [139].
Similarly to the treatment of AD, tAChE inhibitors employed in the treatment of myasthenia gravis only have symptomatic effects, by increasing acetylcholine and thereby facilitating neuromuscular transmission. The carbamates neostigmine, pyridostigmine and the extremely effective ambenonium are commonly used drugs [140]. Furthermore, edrophonium is a rapidly acting and reversible AChE inhibitor used to differentiate between myasthenia gravis, Lambert-Eaton myasthenic syndrome and cholinergic crisis (the so called Tensilon test). Edrophonium, which also possesses a direct nicotinic effect, improves the muscular weakness in patients with myasthenia gravis, whereas it has no effect or worsens weakness in the other conditions.
AChE inhibitors are usually employed in the early stage of myasthenia gravis, when an adequate number of nicotinic receptors still exists [141]. Even though no randomised controlled trial has been conducted on the use of the inhibitors, pyridostigmine seems to be better tolerated and more widely used than neostigmine [142]. Although possessing one quarter of the potency of neostigmine, pyridostigmine is the drug of choice also due to its long duration of action (6 h) and fewer gastrointestinal side effects [143]. Besides, physostigmine is sometimes used in the treatment of primary and secondary glaucoma. It facilitates the drainage of aqueous humour, thus lowering intraocular pressure. Neostigmine is used mainly for reversing neuromuscular blockade, but may be used in other conditions as well such as in colonic pseudo-obstructive conditions [144]. Ambenonium is an interesting compound with the highest affinity to the AChE, despite the fact that it forms only non-covalent bounds [145]. Ambenonium, due to its efficacy, became a parent structure for the derivatives capable to cross the blood brain barrier and which are useful pharmaceuticals in the treatment of AD [146]. Sometimes, the long lasting distigmine, which additionally and directly interacts with muscarinic and nicotinic receptors, may also be used; e.g. for the treatment of detrusor under-activity [147]. Quaternary derivatives used in the treatment of myasthenia gravis was reviewed by Komloova et al. [148].
Concluding remarks
Most pharmaceuticals targeting muscarinic receptors are employed at such large doses that no selectivity can be expected. However, some differences in the adverse effect profile of muscarinic antagonists may still be explained by the variation of expression of muscarinic receptor subtypes in different organs; e.g., an antagonist with a muscarinic M3 receptor selective profile affects urinary bladder contraction more than salivation which is exerted by activation of both M1 and M3 receptors. However, a complex pattern of interactions between muscarinic receptor subtypes occurs, as indicated in Fig. 1, that needs to be considered when searching for selective pharmaceuticals. In the development of new entities for the treatment of for instance pesticide intoxication, the muscarinic receptor selectivity needs to be considered. Reactivators generally have a muscarinic M2 receptor acting profile. Such a blockade may engrave the situation since it may enlarge the effect of the muscarinic M3 receptor effect. This may explain why respiratory arrest is the major cause for deaths by esterase blocking. Thus, in future drug development, muscarinic receptor subtype effects and interactions ought to be considered to a greater degree.
ACKNOWLEDGEMENTS
The work was supported by The Colliander Foundation, the Long Term Development plan – 1011, MH CZ - DRO (University Hospital Hradec Kralove, No. 00179906), National Institute of Mental Health (NIMH - CZ), grant number ED2.1.00/03.0078 and the European Regional Development Fund.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Heller B.J., Laiken N. In Goodman Gilman's The Pharmacological Basis of Therapeutics; New York: The McGraw-Hill Companies; 2011. Muscarinic Receptor Agonists and Antagonists. pp. 219–238. [Google Scholar]
- 2.Whitson J.T. Glaucoma: a review of adjunctive therapy and new management strategies. Expert Opin. Pharmacother. 2007;8(18):3237–3249. doi: 10.1517/14656566.8.18.3237. [DOI] [PubMed] [Google Scholar]; Prat M., GavaldA A., Fonquerna S., Miralpeix M. Inhaled muscarinic antagonists for respiratory diseases: a review of patents and current developments (2006 - 2010). Expert Opin. Ther. Pat. 2011;21(10):1543–1573. doi: 10.1517/13543776.2011.596528. [DOI] [PubMed] [Google Scholar]; Holley A.D., Boots R.J. Review article: management of acute severe and nearfatal asthma. Emerg. Med. Australas. 2009;21(4):259–268. doi: 10.1111/j.1742-6723.2009.01195.x. [DOI] [PubMed] [Google Scholar]; Dmochowski R.R., Gomelsky A. Update on the treatment of overactive bladder. Curr. Opin. Urol. 2011;21(4):286–290. doi: 10.1097/MOU.0b013e3283468da3. [DOI] [PubMed] [Google Scholar]
- 3.Langmead C.J., Watson J., Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008;117(2):232–243. doi: 10.1016/j.pharmthera.2007.09.009. [http://dx.doi.org/10.1016/j.pharmthera.2007.09.009]. [PMID: 18082893]. [DOI] [PubMed] [Google Scholar]
- 4.Abrams P., Andersson K.E., Buccafusco J.J., Chapple C., de Groat W.C., Fryer A.D., Kay G., Laties A., Nathanson N.M., Pasricha P.J., Wein A.J. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006;148(5):565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]; Eglen R. M. Overview of muscarinic receptor subtypes. Handb Exp. Pharmacol. 2012;208:3–28. doi: 10.1007/978-3-642-23274-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Marrs T.C. Organophosphate poisoning. Pharmacol. Ther. 1993;58(1):51–66. doi: 10.1016/0163-7258(93)90066-m. [http://dx.doi.org/10.1016/0163-7258(93)90066-M]. [PMID: 8415873]. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton M.G., Lundy P.M. HI-6 therapy of soman and tabun poisoning in primates and rodents. Arch. Toxicol. 1989;63(2):144–149. doi: 10.1007/BF00316437. [DOI] [PubMed] [Google Scholar]; Tattersall J.E. Ion channel blockade by oximes and recovery of diaphragm muscle from soman poisoning in vitro. Br. J. Pharmacol. 1993;108(4):1006–1015. doi: 10.1111/j.1476-5381.1993.tb13498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; van Helden H.P., Busker R.W., Melchers B.P., Bruijnzeel P.L. Pharmacological effects of oximes: how relevant are they? Arch. Toxicol. 1996;70(12):779–786. doi: 10.1007/s002040050340. [DOI] [PubMed] [Google Scholar]; van Helden H.P., van der Wiel H.J., de Lange J., Busker R.W., Melchers B.P., Wolthuis O.L. Therapeutic efficacy of HI-6 in soman-poisoned marmoset monkeys. Toxicol. Appl. Pharmacol. 1992;115(1):50–56. doi: 10.1016/0041-008x(92)90366-z. [DOI] [PubMed] [Google Scholar]
- 7.Soukup O., Jun D., Tobin G., Kuca K. The summary on non-reactivation cholinergic properties of oxime reactivators: the interaction with muscarinic and nicotinic receptors. 2013 doi: 10.1007/s00204-012-0977-1. [DOI] [PubMed] [Google Scholar]
- 8.Soukup O., Tobin G., Kumar U.K., Jun D., Fusek J., Kuca K. Characterization of the anticholinergic properties of obidoxime; functional examinations of the rat atria and the urinary bladder. Toxicol. Mech. Methods. 2010;20(7):428–433. doi: 10.3109/15376516.2010.497974. [http://dx.doi.org/ 10.3109/15376516.2010.497974]. [PMID: 20602545]. [DOI] [PubMed] [Google Scholar]
- 9.Tobin G., Giglio D., Lundgren O. Muscarinic receptor subtypes in the alimentary tract. J. Physiol. Pharmacol. 2009;60(1):3–21. [PMID: 19439804]. [PubMed] [Google Scholar]
- 10.Horiuchi Y., Kimura R., Kato N., Fujii T., Seki M., Endo T., Kato T., Kawashima K. Evolutional study on acetylcholine expression. Life Sci. 2003;72(15):1745–1756. doi: 10.1016/s0024-3205(02)02478-5. [http://dx.doi.org/ 10.1016/S0024-3205(02)02478-5]. [PMID: 12559395]. [DOI] [PubMed] [Google Scholar]
- 11.Wessler I.K., Kirkpatrick C.J. Activation of muscarinic receptors by non-neuronal acetylcholine. Handbook Exp. Pharmacol. 2012;208:469–491. doi: 10.1007/978-3-642-23274-9_20. [DOI] [PubMed] [Google Scholar]
- 12.Eglen R.M. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton. Autacoid Pharmacol. 2006;26(3):219–233. doi: 10.1111/j.1474-8673.2006.00368.x. [http://dx.doi.org/10.1111/j.1474-8673.2006.00368.x]. [PMID: 16879488]. [DOI] [PubMed] [Google Scholar]
- 13.Roy A., Fields W.C., Rocha-Resende C., Resende R.R., Guatimosim S., Prado V.F., Gros R., Prado M.A. Cardiomyocytesecreted acetylcholine is required for maintenance of homeostasis in the heart. FASEB J. 2013;27(12):5072–5082. doi: 10.1096/fj.13-238279. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rocha-Resende C., Roy A., Resende R., Ladeira M.S., Lara A., de Morais Gomes E.R., Prado V.F., Gros R., Guatimosim C., Prado M.A., Guatimosim S. Non-neuronal cholinergic machinery present in cardiomyocytes offsets hypertrophic signals. J. Mol. Cell. Cardiol. 2012;53(2):206–216. doi: 10.1016/j.yjmcc.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rand J. B. WormBook . 2007. Acetylcholine. pp. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White H.L., Wu J.C. Choline and carnitine acetyltransferases of heart. Biochemistry. 1973;12(5):841–846. doi: 10.1021/bi00729a009. [http://dx.doi.org/10. 1021/bi00729a009]. [PMID: 4686801]. [DOI] [PubMed] [Google Scholar]
- 16.Tucek S. The synthesis of acetylcholine in skeletal muscles of the rat. J. Physiol. 1982;322:53–69. doi: 10.1113/jphysiol.1982.sp014022. [http://dx.doi.org/10.1113/ jphysiol.1982.sp014022]. [PMID: 7069630]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna-Mitchell A.T., Beckel J.M., Barbadora S., Kanai A.J., de Groat W.C., Birder L.A. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80(24-25):2298–2302. doi: 10.1016/j.lfs.2007.02.010. [http://dx.doi.org/10.1016/j.lfs.2007.02.010]. [PMID: 17363007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramochkin D.V., Borodinova A.A., Rosenshtraukh L.V., Nikolsky E.E. Both neuronal and non-neuronal acetylcholine take part in non-quantal acetylcholine release in the rat atrium. Life Sci. 2012;91(21-22):1023–1026. doi: 10.1016/j.lfs.2012.03.031. [DOI] [PubMed] [Google Scholar]; Thesleff S. Functional aspects of quantal and non-quantal release of acetylcholine at the neuromuscular junction. Prog. Brain Res. 1990;84:93–99. doi: 10.1016/s0079-6123(08)60892-4. [DOI] [PubMed] [Google Scholar]
- 19.Katz B., Miledi R. Suppression of transmitter release at the neuromuscular junction. Proc. R. Soc. Lond. B Biol. Sci. 1977;196(1125):465–469. doi: 10.1098/rspb.1977.0051. [DOI] [PubMed] [Google Scholar]; Straughan D.W. The release of acetylcholine from mammalian motor nerve endings. Br. Pharmacol. Chemother. 1960;15(3):417–424. doi: 10.1111/j.1476-5381.1960.tb01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mitchell J.F., Silver A. The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J. Physiol. 1963;165(1):117–129. doi: 10.1113/jphysiol.1963.sp007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaumont M., BatA(c)jat D., Coste O., Doireau P., Chauffard F., Enslen M., Lagarde D., Pierard C. Recovery after prolonged sleep deprivation: residual effects of slow-release caffeine on recovery sleep, sleepiness and cognitive functions. Neuropsychobiology. 2005;51(1):16–27. doi: 10.1159/000082851. [DOI] [PubMed] [Google Scholar]; Sabatini B.L., Regehr W.G. Timing of synaptic transmission. Annu. Rev. Physiol. 1999;61:521–542. doi: 10.1146/annurev.physiol.61.1.521. [DOI] [PubMed] [Google Scholar]
- 21.Geppert M., Sudhof T.C. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu. Rev. Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [http://dx.doi.org/10.1146/annurev.neuro.21.1.75]. [PMID: 9530492]. [DOI] [PubMed] [Google Scholar]
- 22.Whyte J.R., Munro S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 2002;115(Pt 13):2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]; Shi L., Shen Q.T., Kiel A., Wang J., Wang H.W., Melia T.J., Rothman J.E., Pincet F. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335(6074):1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dai H., Shen N., AraA D., Rizo J. A quaternary SNARE-synaptotagmin-Ca2+- phospholipid complex in neurotransmitter release. J. Mol. Biol. 2007;367(3):848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kuffler S.W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J. Physiol. 1975;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuffler S.W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J. Physiol. 1975;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [http://dx.doi.org/10.1113/jphysiol.1975.sp011103]. [PMID: 171380]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyskocil F., Malomouzh A.I., Nikolsky E.E. Non-quantal acetylcholine release at the neuromuscular junction. Physiol. Res. 2009;58(6):763–784. doi: 10.33549/physiolres.931865. [PMID: 20059289]. [DOI] [PubMed] [Google Scholar]
- 25.Wessler I., Roth E., Deutsch C., Brockerhoff P., Bittinger F., Kirkpatrick C.J., Kilbinger H. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br. J. Pharmacol. 2001;134(5):951–956. doi: 10.1038/sj.bjp.0704335. [http://dx.doi.org/10.1038/sj.bjp.0704335]. [PMID: 11682442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kummer W., Wiegand S., Akinci S., Wessler I., Schinkel A.H., Wess J., Koepsell H., Haberberger R.V., Lips K.S. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir. Res. 2006;7(1):65. doi: 10.1186/1465-9921-7-65. [http://dx.doi.org/10.1186/1465-9921-7-65]. [PMID: 16608531]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girard E., Bernard V., Minic J., Chatonnet A., Krejci E., Molgo J. Butyrylcholinesterase and the control of synaptic responses in acetylcholinesterase knockout mice. Life Sci. 2007;80(24-25):2380–2385. doi: 10.1016/j.lfs.2007.03.011. [http://dx.doi.org/10.1016/j.lfs.2007.03.011]. [PMID: 17467011]. [DOI] [PubMed] [Google Scholar]
- 28.Greig N.H., Reale M., Tata A.M. New pharmacological approaches to the cholinergic system: an overview on muscarinic receptor ligands and cholinesterase inhibitors. Recent Patents CNS Drug Discov. 2013;8(2):123–141. doi: 10.2174/1574889811308020003. [http://dx.doi.org/10.2174/ 1574889811308020003]. [PMID: 23597304]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurst R., Rollema H., Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013;137(1):22–54. doi: 10.1016/j.pharmthera.2012.08.012. [http://dx.doi.org/10.1016/j.pharmthera.2012. 08.012]. [PMID: 22925690]. [DOI] [PubMed] [Google Scholar]
- 30.Schecter W.P. Cholinergic symptoms due to nerve agent attack: a strategy for management. Anesthesiol. Clin. North America. 2004;22(3):579–590. doi: 10.1016/j.atc.2004.04.005. [viii.]. [DOI] [PubMed] [Google Scholar]
- 31.Namba T. Cholinesterase inhibition by organophosphorus compounds and its clinical effects. Bull. World Health Organ. 1971;44(1-3):289–307. [PMID: 4941660]. [PMC free article] [PubMed] [Google Scholar]
- 32.Perry E., Walker M., Grace J., Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273–280. doi: 10.1016/s0166-2236(98)01361-7. [http://dx.doi.org/10.1016/S0166-2236(98) 01361-7]. [PMID: 10354606]. [DOI] [PubMed] [Google Scholar]
- 33.Scarr E. Muscarinic receptors: their roles in disorders of the central nervous system and potential as therapeutic targets. CNS Neurosci. Ther. 2012;18(5):369–379. doi: 10.1111/j.1755-5949.2011.00249.x. [http://dx.doi.org/10.1111/j.1755-5949.2011.00249.x]. [PMID: 22070219]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itier V., Bertrand D. Neuronal nicotinic receptors: from protein structure to function. FEBS Lett. 2001;504(3):118–125. doi: 10.1016/s0014-5793(01)02702-8. [http://dx.doi.org/10.1016/S0014-5793(01)02702-8]. [PMID: 11532443]. [DOI] [PubMed] [Google Scholar]
- 35.Broad L.M., Zwart R., Pearson K.H., Lee M., Wallace L., McPhie G.I., Emkey R., Hollinshead S.P., Dell C.P., Baker S.R., Sher E. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J. Pharmacol. Exp. Ther. 2006;318(3):1108–1117. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]; Jürgensen S., Ferreira S.T. Nicotinic receptors, amyloid-beta, and synaptic failure in Alzheimers disease. J. Mol. Neurosci. 2010;40(1-2):221–229. doi: 10.1007/s12031-009-9237-0. [DOI] [PubMed] [Google Scholar]
- 36.Marchi M., Grilli M. Presynaptic nicotinic receptors modulating neurotransmitter release in the central nervous system: functional interactions with other coexisting receptors. Prog. Neurobiol. 2010;92(2):105–111. doi: 10.1016/j.pneurobio.2010.06.004. [http://dx.doi.org/10.1016/j.pneurobio.2010. 06.004]. [PMID: 20558239]. [DOI] [PubMed] [Google Scholar]
- 37.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20(2):92–98. doi: 10.1016/s0166-2236(96)10073-4. [http://dx.doi.org/10.1016/S0166-2236(96)10073-4]. [PMID: 9023878]. [DOI] [PubMed] [Google Scholar]
- 38.Takada-Takatori Y., Kume T., Izumi Y., Ohgi Y., Niidome T., Fujii T., Sugimoto H., Akaike A. Roles of nicotinic receptors in acetylcholinesterase inhibitor-induced neuroprotection and nicotinic receptor up-regulation. Biol. Pharm. Bull. 2009;32(3):318–324. doi: 10.1248/bpb.32.318. [http://dx.doi.org/10.1248/bpb.32.318]. [PMID: 19252271]. [DOI] [PubMed] [Google Scholar]
- 39.Baum B.J., Wellner R.B. Receptors in salivary glands. In: Neuronal Mechanisms of Salivary Secretion; In: Garrett J.R., Ekstrom J., Anderson L.C., editors. Karger: Basel. Vol. 3. 1999. pp. 44–58. [Google Scholar]; Caulfield M.P. Muscarinic receptorscharacterization, coupling and function. Pharmacol. Ther. 1993;58(3):319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 40.Dai Y.S., Ambudkar I.S., Horn V.J., Yeh C.K., Kousvelari E.E., Wall S.J., Li M., Yasuda R.P., Wolfe B.B., Baum B.J. Evidence that M3 muscarinic receptors in rat parotid gland couple to two second messenger systems. Am. J. Physiol. 1991;261(6 Pt 1):C1063–C1073. doi: 10.1152/ajpcell.1991.261.6.C1063. [DOI] [PubMed] [Google Scholar]; Bockman C.S., Bradley M.E., Dang H.K., Zeng W., Scofield M.A., Dowd F.J. Molecular and pharmacological characterization of muscarinic receptor subtypes in a rat parotid gland cell line: comparison with native parotid gland. J. Pharmacol. Exp. Ther. 2001;297(2):718–726. [PubMed] [Google Scholar]
- 41.Culp D.J., Luo W., Richardson L.A., Watson G.E., Latchney L.R. Both M1 and M3 receptors regulate exocrine secretion by mucous acini. Am. J. Physiol. 1996;271(6 Pt 1):C1963–C1972. doi: 10.1152/ajpcell.1996.271.6.C1963. [DOI] [PubMed] [Google Scholar]; Gautam D., Heard T.S., Cui Y., Miller G., Bloodworth L., Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol. Pharmacol. 2004;66(2):260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]; Tobin G. Muscarinic receptor subtypes in the submandibular gland and the urinary bladder of the rabbit: in vivo and in vitro functional comparisons of receptor antagonists. J. Auton. Pharmacol. 1995;15(6):451–463. doi: 10.1111/j.1474-8673.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]; EkstrAm J., Godoy T., Riva A. Clozapine: agonistic and antagonistic salivary secretory actions. J. Dent. Res. 2010;89(3):276–280. doi: 10.1177/0022034509356055. [DOI] [PubMed] [Google Scholar]
- 42.Tobin G., Ryberg A.T., Gentle S., Edwards A.V. Distribution and function of muscarinic receptor subtypes in the ovine submandibular gland. J. Appl. Physiol. 2006;100(4):1215–1223. doi: 10.1152/japplphysiol.00779.2005. [http://dx.doi.org/10.1152/japplphysiol.00779.2005]. [PMID: 16322368]. [DOI] [PubMed] [Google Scholar]
- 43.Tobin G., Sjogren C. In vivo and in vitro effects of muscarinic receptor antagonists on contractions and release of [3H]acetylcholine in the rabbit urinary bladder. Eur. J. Pharmacol. 1995;281(1):1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]; Tobin G. Presynaptic muscarinic M1 and M2 receptor modulation of auriculotemporal nerve transmission in the rat. J. Auton. Nerv. Syst. 1998;72(1):61–71. doi: 10.1016/s0165-1838(98)00088-5. [DOI] [PubMed] [Google Scholar]; Tobin G. Presynaptic muscarinic receptor mechanisms and submandibular responses to stimulation of the parasympathetic innervation in bursts in rats. Auton. Neurosci. 2002;99(2):111–118. doi: 10.1016/s1566-0702(02)00094-2. [DOI] [PubMed] [Google Scholar]
- 44.EkstrAm J., Garrett J.R., Mansson B., Tobin G. The effects of atropine and chronic sympathectomy on maximal parasympathetic stimulation of parotid saliva in rats. J. Physiol. 1988;403:105–116. doi: 10.1113/jphysiol.1988.sp017241. [http://dx.doi.org/10.1113/jphysiol.1988.sp017241]. [PMID: 2473192]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobin G., EkstrAm J., Bloom S.R., Edwards A.V. Atropine-resistant submandibular responses to stimulation of the parasympathetic innervation in the anaesthetized ferret. J. Physiol. 1991;437:327–339. doi: 10.1113/jphysiol.1991.sp018598. [http://dx.doi.org/10.1113/jphysiol.1991. sp018598]. [PMID: 1890638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryberg A.T., Selberg H., Soukup O., Gradin K., Tobin G. Cholinergic submandibular effects and muscarinic receptor expression in blood vessels of the rat. Arch. Oral Biol. 2008;53(7):605–616. doi: 10.1016/j.archoralbio.2008.01.016. [http://dx.doi.org/10.1016/j.archoralbio.2008.01.016]. [PMID: 18329001]. [DOI] [PubMed] [Google Scholar]
- 47.Giglio D., Tobin G. Muscarinic receptor subtypes in the lower urinary tract. Pharmacology. 2009;83(5):259–269. doi: 10.1159/000209255. [http://dx.doi. org/10.1159/000209255]. [PMID: 19295256]. [DOI] [PubMed] [Google Scholar]
- 48.Mansfield K.J., Liu L., Mitchelson F.J., Moore K.H., Millard R.J., Burcher E. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in ageing. Br. J. Pharmacol. 2005;144(8):1089–1099. doi: 10.1038/sj.bjp.0706147. [http://dx.doi.org/10.1038/ sj.bjp.0706147]. [PMID: 15723094]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarghooni S., Wunsch J., Bodenbenner M., BrA1/4ggmann D., Grando S.A., Schwantes U., Wess J., Kummer W., Lips K.S. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci. 2007;80(24-25):2308–2313. doi: 10.1016/j.lfs.2007.01.046. [http://dx.doi.org/10.1016/j.lfs.2007.01.046]. [PMID: 17337281]. [DOI] [PubMed] [Google Scholar]
- 50.Birder L.A. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am. J. Physiol. Renal Physiol. 2005;289(3):F489–F495. doi: 10.1152/ajprenal.00467.2004. [http://dx.doi.org/10.1152/ajprenal.00467. 2004]. [PMID: 16093424]. [DOI] [PubMed] [Google Scholar]
- 51.Kanai A.J. Afferent mechanism in the urinary tract. Handbook Exp. Pharmacol. 2011;202:171–205. doi: 10.1007/978-3-642-16499-6_9. [DOI] [PubMed] [Google Scholar]
- 52.Alberts P. Classification of the presynaptic muscarinic receptor subtype that regulates 3H-acetylcholine secretion in the guinea pig urinary bladder in vitro. J. Pharmacol. Exp. Ther. 1995;274(1):458–468. [PMID: 7616431]. [PubMed] [Google Scholar]
- 53.Nandigama R., Bonitz M., Papadakis T., Schwantes U., Bschleipfer T., Kummer W. Muscarinic acetylcholine receptor subtypes expressed by mouse bladder afferent neurons. Neuroscience. 2010;168(3):842–850. doi: 10.1016/j.neuroscience.2010.04.012. [http://dx.doi.org/10.1016/j. neuroscience.2010.04.012]. [PMID: 20394802]. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y., Yoshimura N., Masuda H., de Miguel F., Chancellor M.B. Antimuscarinic agents exhibit local inhibitory effects on muscarinic receptors in bladder-afferent pathways. Urology. 2005;65(2):238–242. doi: 10.1016/j.urology.2004.11.021. [http://dx.doi.org/10.1016/j.urology.2004.11.021]. [PMID: 15708029]. [DOI] [PubMed] [Google Scholar]
- 55.Birder L.A. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul. Pharmacol. 2006;45(4):221–226. doi: 10.1016/j.vph.2005.08.027. [http://dx.doi.org/10.1016/j.vph.2005.08.027]. [PMID: 16891158]. [DOI] [PubMed] [Google Scholar]
- 56.Sun Y., Keay S., De Deyne P.G., Chai T.C. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J. Urol. 2001;166(5):1951–1956. [PubMed] [Google Scholar]; Buckner S.A., Milicic I., Daza A.V., Coghlan M.J., Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. J. Pharmacol. 2002;135(3):639–648. doi: 10.1038/sj.bjp.0704499. [DOI] [PMC free article] [PubMed] [Google Scholar]; Giglio D., Ryberg A.T., To K., Delbro D.S., Tobin G. Altered muscarinic receptor subtype expression and functional responses in cyclophosphamide induced cystitis in rats. Auton. Neurosci. 2005;122(1-2):9–20. doi: 10.1016/j.autneu.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Andersson M., Aronsson P., Doufish D., Lampert A., Tobin G. Muscarinic receptor subtypes involved in urothelium-derived relaxatory effects in the inflamed rat urinary bladder. Auton. Neurosci.: basic & clinical, 2012, 170, 5 -11.b. Andersson, M.C.; Tobin, G.; Giglio, D. Cholinergic nitric oxide release from the urinary bladder mucosa in cyclophosphamide-induced cystitis of the anaesthetized rat. Br. J. Pharmacol. 2008;153(7):1438–1444. doi: 10.1016/j.autneu.2012.06.004. [http://dx.doi.org/10.1038/bjp.2008.6]. [PMID: 18246091]. [DOI] [PubMed] [Google Scholar]
- 58.Killi U.K., Wsol V., Soukup O., Kuca K., Winder M., Tobin G. In vitro functional interactions of acetylcholine esterase inhibitors and muscarinic receptor antagonists in the urinary bladder of the rat. Clin. Exp. Pharmacol. Physiol. 2014;41(2):139–146. doi: 10.1111/1440-1681.12191. [http://dx.doi.org/10.1111/1440-1681.12191]. [PMID: 24341923]. [DOI] [PubMed] [Google Scholar]
- 59.Giglio D., Delbro D.S., Tobin G. Postjunctional modulation by muscarinic M2 receptors of responses to electrical field stimulation of rat detrusor muscle preparations. Auton. Autacoid Pharmacol. 2005;25(3):113–120. doi: 10.1111/j.1474-8673.2005.00340.x. [DOI] [PubMed] [Google Scholar]; Hegde S.S., Choppin A., Bonhaus D., Briaud S., Loeb M., Moy T.M., Loury D., Eglen R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120(8):1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DAgostino G., Bolognesi M.L., Lucchelli A., Vicini D., Balestra B., Spelta V., Melchiorre C., Tonini M. Prejunctional muscarinic inhibitory control of acetylcholine release in the human isolated detrusor: involvement of the M4 receptor subtype. Br. J. Pharmacol. 2000;129(3):493–500. doi: 10.1038/sj.bjp.0703080. [http://dx.doi.org/10.1038/ sj.bjp.0703080]. [PMID: 10711347]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehlert F.J. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal, airway and urinary bladder smooth muscle. Life Sci. 2003;74(2-3):355–366. doi: 10.1016/j.lfs.2003.09.023. [DOI] [PubMed] [Google Scholar]; Hirshman C.A., Lande B., Croxton T.L. Role of M2 muscarinic receptors in airway smooth muscle contraction. Life Sci. 1999;64(6-7):443–448. doi: 10.1016/s0024-3205(98)00586-4. [DOI] [PubMed] [Google Scholar]
- 62.Zaagsma J., Roffel A.F., Meurs H. Muscarinic control of airway function. Life Sci. 1997;60(13-14):1061–1068. doi: 10.1016/s0024-3205(97)00048-9. [http://dx.doi.org/ 10.1016/S0024-3205(97)00048-9]. [PMID: 9121348]. [DOI] [PubMed] [Google Scholar]
- 63.Killingsworth C.R., Robinson N.E. The role of muscarinic M1 and M2 receptors in airway constriction in the cat. Eur. J. Pharmacol. 1992;210(3):231–238. doi: 10.1016/0014-2999(92)90409-w. [http://dx.doi.org/10.1016/ 0014-2999(92)90409-W]. [PMID: 1612100]. [DOI] [PubMed] [Google Scholar]
- 64.Aas P., Maclagan J. Evidence for prejunctional M2 muscarinic receptors in pulmonary cholinergic nerves in the rat. Br. J. Pharmacol. 1990;101(1):73–76. doi: 10.1111/j.1476-5381.1990.tb12091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; DAgostino G., Barbieri A., Chiossa E., Tonini M. M4 muscarinic autoreceptormediated inhibition of -3H-acetylcholine release in the rat isolated urinary bladder. J. Pharmacol. Exp. Ther. 1997;283(2):750–756. [PubMed] [Google Scholar]
- 65.Pieper M.P. The non-neuronal cholinergic system as novel drug target in the airways. Life Sci. 2012;91(21-22):1113–1118. doi: 10.1016/j.lfs.2012.08.030. [http://dx.doi.org/10.1016/j.lfs.2012.08.030]. [PMID: 22982180]. [DOI] [PubMed] [Google Scholar]
- 66.van der Velden V.H., Hulsmann A.R. Autonomic innervation of human airways: structure, function, and pathophysiology in asthma. Neuroimmunomodulation, 1999;6(3):145–159. doi: 10.1159/000026376. [DOI] [PubMed] [Google Scholar]; Edwards A.V. Garrett J.R., EkstrAm J., Anderson L.C., editors. Autonomic Control of Salivary Blood Flow. In: Glandular Mechanisms of Salivary Secretion; Karger: Basel, . 1998;10:101–117. [Google Scholar]
- 67.Matran R. Neural control of lower airway vasculature. Involvement of classical transmitters and neuropeptides. Acta Physiol. Scand. Suppl. 1991;601:1–54. [PubMed] [Google Scholar]; Orii R., Sugawara Y., Sawamura S., Yamada Y. M(3) muscarinic receptors mediate acetylcholine-induced pulmonary vasodilation in pulmonary hypertension. Biosci. Trends. 2010;4(5):260–266. [PubMed] [Google Scholar]
- 68.Sasaki F., ParA(c) P., Ernest D., Bai T., Verburgt L., March R., Baile E. Endogenous nitric oxide influences acetylcholine-induced bronchovascular dilation in sheep. J. Appl. Physiol. 1995;78(2):539–545. doi: 10.1152/jappl.1995.78.2.539. [PMID: 7759423]. [DOI] [PubMed] [Google Scholar]
- 69.Peyter A.C., Muehlethaler V., Liaudet L., Marino M., Di Bernardo S., Diaceri G., Tolsa J.F. Muscarinic receptor M1 and phosphodiesterase 1 are key determinants in pulmonary vascular dysfunction following perinatal hypoxia in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295(1):L201–L213. doi: 10.1152/ajplung.00264.2007. [http://dx.doi. org/10.1152/ajplung.00264.2007]. [PMID: 18469116]. [DOI] [PubMed] [Google Scholar]
- 70.Kummer W., Lips K.S., Pfeil U. The epithelial cholinergic system of the airways. Histochem. Cell Biol. 2008;130(2):219–234. doi: 10.1007/s00418-008-0455-2. [http://dx.doi.org/10.1007/s00418-008-0455-2]. [PMID: 18566825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higgins C.B., Vatner S.F., Braunwald E. Parasympathetic control of the heart. Pharmacol. Rev. 1973;25(1):119–155. [PMID: 4348231]. [PubMed] [Google Scholar]
- 72.Brodde O.E., Bruck H., Leineweber K., Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Res. Cardiol. 2001;96(6):528–538. doi: 10.1007/s003950170003. [http://dx.doi.org/10.1007/s003950170003]. [PMID: 11770070]. [DOI] [PubMed] [Google Scholar]
- 73.Myslivecek J., Klein M., Novakova M., Ricny J. The detection of the non-M2 muscarinic receptor subtype in the rat heart atria and ventricles. Naunyn Schmiedebergs Arch. Pharmacol. 2008;378(1):103–116. doi: 10.1007/s00210-008-0285-8. [DOI] [PubMed] [Google Scholar]; Perez C. C., Tobar I. D., Jimenez E., Castaneda D., Rivero M. B., Concepcion J. L., Chiurillo M. A., Bonfante-Cabarcas R. Kinetic and molecular evidences that human cardiac muscle express non-M2 muscarinic receptor subtypes that are able to interact themselves. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2006;54(5):345–55. doi: 10.1016/j.phrs.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Abramochkin D.V., Tapilina S.V., Sukhova G.S., Nikolsky E.E., Nurullin L.F. Functional M3 cholinoreceptors are present in pacemaker and working myocardium of murine heart. . Pflugers Arch. 2012;463(4):523–529. doi: 10.1007/s00424-012-1075-1. [DOI] [PubMed] [Google Scholar]; Woo S.H., Lee B.H., Kwon K.I., Lee C.O. Excitatory effect of M1 muscarinic acetylcholine receptor on automaticity of mouse heart. Arch. Pharm. Res. 2005;28(8):930–935. doi: 10.1007/BF02973879. [DOI] [PubMed] [Google Scholar]; Hardouin S.N., Richmond K.N., Zimmerman A., Hamilton S.E., Feigl E.O., Nathanson N.M. Altered cardiovascular responses in mice lacking the M(1) muscarinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 2002;301(1):129–137. doi: 10.1124/jpet.301.1.129. [DOI] [PubMed] [Google Scholar]
- 75.Krejc A.A., Tucek S. Quantitation of mRNAs for M(1) to M(5) subtypes of muscarinic receptors in rat heart and brain cortex. Mol. Pharmacol. 2002;61(6):1267–1272. doi: 10.1124/mol.61.6.1267. [http://dx.doi.org/10.1124/ mol.61.6.1267]. [PMID: 12021386]. [DOI] [PubMed] [Google Scholar]
- 76.Dobrzynski H., Marples D.D., Musa H., Yamanushi T.T., Henderson Z., Takagishi Y., Honjo H., Kodama I., Boyett M.R. Distribution of the muscarinic K+ channel proteins Kir3.1 and Kir3.4 in the ventricle, atrium, and sinoatrial node of heart. J. Histochem. Cytochem. Off. Soc. 2001;49(10):1221–1234. doi: 10.1177/002215540104901004. [DOI] [PubMed] [Google Scholar]
- 77.Sasaki S., Motomura S. Comparison of anti-M2-muscarinic effect of AF-DX 116 on atrioventricular nodal conduction with those of pirenzepine and atropine as antibradyarrhythmic drugs. J. Cardiovasc. Pharmacol. 1999;33(6):912–921. doi: 10.1097/00005344-199906000-00012. [http://dx.doi.org/ 10.1097/00005344-199906000-00012]. [PMID: 10367595]. [DOI] [PubMed] [Google Scholar]
- 78.Nascimento J.H., SallA(c) L., Hoebeke J., Argibay J., Peineau N. cGMP-mediated inhibition of cardiac L-type Ca(2+) current by a monoclonal antibody against the M(2) ACh receptor. Am. J. Physiol. Cell Physiol. 2001;281(4):C1251–C1258. doi: 10.1152/ajpcell.2001.281.4.C1251. [PMID: 11546662]. [DOI] [PubMed] [Google Scholar]
- 79.Harvey R.D., Belevych A.E. Muscarinic regulation of cardiac ion channels. Br. J. Pharmacol. 2003;139(6):1074–1084. doi: 10.1038/sj.bjp.0705338. [http://dx. doi.org/10.1038/sj.bjp.0705338]. [PMID: 12871825]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallo M.P., Alloatti G., Eva C., Oberto A., Levi R.C. M1 muscarinic receptors increase calcium current and phosphoinositide turnover in guinea-pig ventricular cardiocytes. J. Physiol. 1993;471:41–60. doi: 10.1113/jphysiol.1993.sp019890. [http://dx.doi.org/10.1113/jphysiol.1993.sp019890]. [PMID: 8120813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitazawa T., Asakawa K., Nakamura T., Teraoka H., Unno T., Komori S., Yamada M., Wess J. M3 muscarinic receptors mediate positive inotropic responses in mouse atria: a study with muscarinic receptor knockout mice. J. Pharmacol. Exp. Ther. 2009;330(2):487–493. doi: 10.1124/jpet.109.153304. [http://dx.doi.org/10.1124/jpet.109.153304]. [PMID: 19429792]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harvey R.D. Muscarinic receptor agonists and antagonists: effects on cardiovascular function. Handbook Exp. Pharmacol. 2012;208:299–316. doi: 10.1007/978-3-642-23274-9_13. [DOI] [PubMed] [Google Scholar]
- 83.Levey A.I., Edmunds S.M., Heilman C.J., Desmond T.J., Frey K.A. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63(1):207–221. doi: 10.1016/0306-4522(94)90017-5. [http://dx.doi.org/10.1016/0306-4522(94)90017-5]. [PMID: 7898649]. [DOI] [PubMed] [Google Scholar]
- 84.Flynn D.D., Ferrari-DiLeo G., Mash D.C., Levey A.I. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimers disease. J. Neurochem. 1995;64(4):1888–1891. doi: 10.1046/j.1471-4159.1995.64041888.x. [http://dx.doi.org/10.1046/j.1471-4159.1995.64041888.x]. [PMID: 7891119]. [DOI] [PubMed] [Google Scholar]
- 85.Li M., Yasuda R.P., Wall S.J., Wellstein A., Wolfe B.B. Distribution of m2 muscarinic receptors in rat brain using antisera selective for m2 receptors. Mol. Pharmacol. 1991;40(1):28–35. [PMID: 1857338]. [PubMed] [Google Scholar]
- 86.Vilaro M.T., Palacios J.M., Mengod G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci. Lett. 1990;114(2):154–159. doi: 10.1016/0304-3940(90)90064-g. [http://dx.doi.org/10.1016/0304-3940(90)90064-G]. [PMID: 2395528]. [DOI] [PubMed] [Google Scholar]
- 87.Ehlert F.J., Pak K.J., Griffin M.T. Muscarinic agonists and antagonists: effects on gastrointestinal function. Handbook Exp. Pharmacol. 2012;208:343–374. doi: 10.1007/978-3-642-23274-9_15. [DOI] [PubMed] [Google Scholar]
- 88.Buels K.S., Fryer A.D. Muscarinic receptor antagonists: effects on pulmonary function. Handbook Exp. Pharmacol. 2012;208:317–341. doi: 10.1007/978-3-642-23274-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McEvoy G.K. AHFS Drug information 2008. Bethesda, MD: American Society of Health-System Pharmacists; 2008. Bethanecol. pp. 1240–1241. [Google Scholar]
- 90.Craddock T.J., Fritsch P., Rice M.A., del Rosario R.M., Miller D.B., Fletcher M.A., Klimas N.G., Broderick G. A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf War Illness and chronic fatigue syndrome. PLoS One. 2014;9(1):e84839. doi: 10.1371/journal.pone.0084839. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mitchelson F. Muscarinic receptor agonists and antagonists: effects on ocular function. Handb. Exp. Pharmacol. 2012;208:263–298. doi: 10.1007/978-3-642-23274-9_12. [DOI] [PubMed] [Google Scholar]; Ramos-Casals M., Tzioufas A.G., Stone J.H., SisA3 A., Bosch X. Treatment of primary SjAgren syndrome: a systematic review. JAMA. 2010;304(4):452–460. doi: 10.1001/jama.2010.1014. [DOI] [PubMed] [Google Scholar]
- 91.Giacobini E. Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacol. Res. Off. J. Ital. Soc. 2004;50(4):433–440. doi: 10.1016/j.phrs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 92.Soukup O., Tobin G., Kumar U.K., Binder J., Proska J., Jun D., Fusek J., Kuca K. Interaction of nerve agent antidotes with cholinergic systems. Curr. Med. Chem. 2010;17(16):1708–1718. doi: 10.2174/092986710791111260. [http://dx.doi.org/10.2174/092986710791111260]. [PMID: 20345348]. [DOI] [PubMed] [Google Scholar]
- 93.Marrs T.C. Organophosphate poisoning. Pharmacol. Ther. 1993;58(1):51–66. doi: 10.1016/0163-7258(93)90066-m. [http://dx.doi.org/10.1016/0163-7258(93)90066-M]. [PMID: 8415873]. [DOI] [PubMed] [Google Scholar]
- 94.Sellers D.J., Chess-Williams R. Muscarinic agonists and antagonists: effects on the urinary bladder. Handbook Exp. Pharmacol. 2012;208:375–400. doi: 10.1007/978-3-642-23274-9_16. [DOI] [PubMed] [Google Scholar]
- 95.Yamaguchi O. Antimuscarinics and overactive bladder: other mechanism of action. Neurourol. Urodyn. 2010;29(1):112–115. doi: 10.1002/nau.20796. [PMID: 19693952]. [DOI] [PubMed] [Google Scholar]
- 96.Nelson C.P., Gupta P., Napier C.M., Nahorski S.R., Challiss R.A. Functional selectivity of muscarinic receptor antagonists for inhibition of M3-mediated phosphoinositide responses in guinea pig urinary bladder and submandibular salivary gland. J. Pharmacol. Exp. Ther. 2004;310(3):1255–1265. doi: 10.1124/jpet.104.067140. [DOI] [PubMed] [Google Scholar]; Gillberg P.G., Sundquist S., Nilvebrant L. Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists. Eur. J. Pharmacol. 1998;349(2-3):285–292. doi: 10.1016/s0014-2999(98)00214-3. [DOI] [PubMed] [Google Scholar]; Mansfield K.J., Chandran J.J., Vaux K.J., Millard R.J., Christopoulos A., Mitchelson F.J., Burcher E. Comparison of receptor binding characteristics of commonly used muscarinic antagonists in human bladder detrusor and mucosa. J. Pharmacol. Exp. Ther. 2009;328(3):893–899. doi: 10.1124/jpet.108.145508. [DOI] [PubMed] [Google Scholar]; Yoshida A., Kuraoka S., Ito Y., Okura T., Deguchi Y., Otsuka A., Ozono S., Takeda M., Masuyama K., Araki I., Yamada S. Muscarinic receptor binding of the novel radioligand, [3h]imidafenacin in the human bladder and parotid gland. J. Pharmacol. Sci. 2014;124(1):40–46. doi: 10.1254/jphs.13193fp. [DOI] [PubMed] [Google Scholar]; Masumori N. Long-term safety, efficacy, and tolerability of imidafenacin in the treatment of overactive bladder: a review of the Japanese literature. Patient Prefer. Adherence. 2013;7:111–120. doi: 10.2147/PPA.S28160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersson K.E. Advances in the pharmacological control of the bladder. Exp. Physiol. 1999;84(1):195–213. doi: 10.1111/j.1469-445x.1999.tb00083.x. [http://dx.doi.org/ 10.1111/j.1469-445X.1999.tb00083.x]. [PMID: 10081718]. [DOI] [PubMed] [Google Scholar]
- 98.Andersson K-E., Yoshida M. Antimuscarinics and the overactive detrusorwhich is the main mechanism of action? Eur. Urol. 2003;43(1):1–5. doi: 10.1016/s0302-2838(02)00540-7. [http://dx.doi.org/10.1016/S0302-2838(02)00540-7]. [PMID: 12507537]. [DOI] [PubMed] [Google Scholar]
- 99.Kobayashi F., Yageta Y., Yamazaki T., Wakabayashi E., Inoue M., Segawa M., Matsuzawa S. Pharmacological effects of imidafenacin (KRP-197/ONO-8025), a new bladder selective anticholinergic agent, in rats. Comparison of effects on urinary bladder capacity and contraction, salivary secretion and performance in the Morris water maze task. Arzneimittelforschung. 2007;57(3):147–154. doi: 10.1055/s-0031-1296598. [DOI] [PubMed] [Google Scholar]; Yamazaki T., Muraki Y., Anraku T. In vivo bladder selectivity of imidafenacin, a novel antimuscarinic agent, assessed by using an effectiveness index for bladder capacity in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2011;384(3):319–329. doi: 10.1007/s00210-011-0675-1. [DOI] [PubMed] [Google Scholar]; Nilvebrant L., Hallen B., Larsson G. Tolterodinea new bladder selective muscarinic receptor antagonist: preclinical pharmacological and clinical data. Life Sci. 1997;60(13-14):1129–1136. doi: 10.1016/s0024-3205(97)00057-x. [DOI] [PubMed] [Google Scholar]; Ikeda K., Kobayashi S., Suzuki M., Miyata K., Takeuchi M., Yamada T., Honda K. M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366(2):97–103. doi: 10.1007/s00210-002-0554-x. [DOI] [PubMed] [Google Scholar]
- 100.Vesela R., Aronsson P., Andersson M., Wsol V., Tobin G. The potential of non-adrenergic, non-cholinergic targets in the treatment of interstitial cystitis/painful bladder syndrome. J. Physiol. Pharmacol. 2012;63(3):209–216. [PMID: 22791634]. [PubMed] [Google Scholar]
- 101.Burnstock G., Satchell D.G., Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br. J. Pharmacol. 1972;46(2):234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [http://dx.doi.org/10.1111/ j.1476-5381.1972.tb06868.x]. [PMID: 4631338]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ekstrom J., Asztely A., Tobin G. Parasympathetic non-adrenergic, non-cholinergic mechanisms in salivary glands and their role in reflex secretion. Eur. J. Morphol. 1998;36(Suppl.):208–212. [PMID: 9825924]. [PubMed] [Google Scholar]
- 103.Sokilde B., Mikkelsen I., Stensbol T.B., Andersen B., Ebdrup S., Krogsgaard-Larsen P., Falch E. Analogues of carbacholine: synthesis and relationship between structure and affinity for muscarinic and nicotinic acetylcholine receptors. Arch. Pharm. (Weinheim) 1996;329(2):95–104. doi: 10.1002/ardp.19963290207. [http://dx.doi.org/10.1002/ ardp.19963290207]. [PMID: 8851473]. [DOI] [PubMed] [Google Scholar]
- 104.Tobin G., Sjogren C. Prejunctional facilitatory and inhibitory modulation of parasympathetic nerve transmission in the rabbit urinary bladder. J. Auton. Nerv. Syst. 1998;68(3):153–156. doi: 10.1016/s0165-1838(97)00128-8. [http:// dx.doi.org/10.1016/S0165-1838(97)00128-8]. [PMID: 9626942]. [DOI] [PubMed] [Google Scholar]
- 105.Gordon E., Lazarus S.C. Management of chronic obstructive pulmonary disease: moving beyond the asthma algorithm. J. Allergy Clin. Immunol. 2009;124(5):873–880. doi: 10.1016/j.jaci.2009.09.040. [http://dx.doi.org/10.1016/ j.jaci.2009.09.040]. [PMID: 19895979]. [DOI] [PubMed] [Google Scholar]
- 106.Barnes P.J. The role of anticholinergics in chronic obstructive pulmonary disease. Am. J. Med. 2004;117(Suppl. 12A):24S–32S. doi: 10.1016/j.amjmed.2004.10.018. [DOI] [PubMed] [Google Scholar]; Gross N.J. The influence of anticholinergic agents on treatment for bronchitis and emphysema. Am. J. Med. 1991;91(4A):11S–12S. doi: 10.1016/0002-9343(91)90255-v. [DOI] [PubMed] [Google Scholar]
- 107.Rodrigo G.J., Rodrigo C. Triple inhaled drug protocol for the treatment of acute severe asthma. Chest. 2003;123(6):1908–1915. doi: 10.1378/chest.123.6.1908. [http://dx.doi.org/10.1378/chest.123.6.1908]. [PMID: 12796167]. [DOI] [PubMed] [Google Scholar]
- 108.Cazzola M., Page C.P., Calzetta L., Matera M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012;64(3):450–504. doi: 10.1124/pr.111.004580. [http://dx.doi.org/10.1124/pr.111.004580]. [PMID: 22611179]. [DOI] [PubMed] [Google Scholar]
- 109.Alabaster V.A. Discovery & development of selective M3 antagonists for clinical use. Life Sci. 1997;60(13-14):1053–1060. doi: 10.1016/s0024-3205(97)00047-7. [http://dx. doi.org/10.1016/S0024-3205(97)00047-7]. [PMID: 9121347]. [DOI] [PubMed] [Google Scholar]
- 110.Anthonisen N.R., Connett J.E., Enright P.L., Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am. J. Respir. Crit. Care Med. 2002;166(3):333–339. doi: 10.1164/rccm.2110093. [http://dx.doi.org/ 10.1164/rccm.2110093]. [PMID: 12153966]. [DOI] [PubMed] [Google Scholar]
- 111.Kilbinger H., von Bardeleben R.S., Siefken H., Wolf D. Prejunctional muscarinic receptors regulating neurotransmitter release in airways. Life Sci. 1995;56(11-12):981–987. doi: 10.1016/0024-3205(95)00037-7. [http://dx. doi.org/10.1016/0024-3205(95)00037-7]. [PMID: 10188802]. [DOI] [PubMed] [Google Scholar]
- 112.Sarria B., Naline E., Zhang Y., Cortijo J., Molimard M., Moreau J., Therond P., Advenier C., Morcillo E.J. Muscarinic M2 receptors in acetylcholine-isoproterenol functional antagonism in human isolated bronchus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283(5):L1125–L1132. doi: 10.1152/ajplung.00084.2002. [http://dx.doi.org/10.1152/ ajplung.00084.2002]. [PMID: 12376367]. [DOI] [PubMed] [Google Scholar]
- 113.Saw L., Shumway J., Ruckart P. Surveillance data on pesticide and agricultural chemical releases and associated public health consequences in selected US states, 20032007. J. Med. Toxicol. 2011;7(2):164–171. doi: 10.1007/s13181-011-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Patel V., Ramasundarahettige C., Vijayakumar L., Thakur J.S., Gajalakshmi V., Gururaj G., Suraweera W., Jha P. Suicide mortality in India: a nationally representative survey. Lancet. 2012;379(9834):2343–2351. doi: 10.1016/S0140-6736(12)60606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]; Korabecny J., Soukup O., Dolezal R., Spilovska K., Nepovimova E., Andrs M., Nguyen T.D., Jun D., Musilek K., Kucerova-Chlupacova M., Kuca K. From pyridinium-based to centrally active acetylcholinesterase reactivators. Mini Rev. Med. Chem. 2014;14(3):215–221. doi: 10.2174/1389557514666140219103138. [DOI] [PubMed] [Google Scholar]
- 115.Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J. Toxicol. Clin. Toxicol. 2002;40(6):803–816. doi: 10.1081/clt-120015840. [http://dx.doi.org/10.1081/CLT-120015840]. [PMID: 12475193]. [DOI] [PubMed] [Google Scholar]
- 116.Chandar N.B., Ganguly B. A first principles investigation of aging processes in soman conjugated AChE. Chem. Biol. Interact. 2013;204(3):185–190. doi: 10.1016/j.cbi.2013.05.013. [http://dx.doi.org/10.1016/j.cbi.2013.05.013]. [PMID: 23747845]. [DOI] [PubMed] [Google Scholar]
- 117.Kuca K., Musilek K., Jun D., Pohanka M., Ghosh K.K., Hrabinova M. Oxime K027: novel low-toxic candidate for the universal reactivator of nerve agent- and pesticide-inhibited acetylcholinesterase. J. Enzyme Inhib. Med. Chem. 2010;25(4):509–512. doi: 10.3109/14756360903357569. [http://dx.doi.org/10.3109/14756360903357569]. [PMID: 20192902]. [DOI] [PubMed] [Google Scholar]
- 118.Musilek K., Dolezal M., Gunn-Moore F., Kuca K. Design, evaluation and structure-activity relationship studies of the AChE reactivators against organophosphorus pesticides. Med. Res. Rev. 2011;31(4):548–575. doi: 10.1002/med.20192. [http://dx.doi.org/10.1002/med.20192]. [PMID: 20027669]. [DOI] [PubMed] [Google Scholar]
- 119.Liston D.R., Nielsen J.A., Villalobos A., Chapin D., Jones S.B., Hubbard S.T., Shalaby I.A., Ramirez A., Nason D., White W.F. Pharmacology of selective acetylcholinesterase inhibitors: implications for use in Alzheimers disease. Eur. J. Pharmacol. 2004;486(1):9–17. doi: 10.1016/j.ejphar.2003.11.080. [DOI] [PubMed] [Google Scholar]; Mehndiratta M.M., Pandey S., Kuntzer T. Acetylcholinesterase inhibitor treatment for myasthenia gravis. Cochrane Database Syst. Rev. 2014;10(10):CD006986. doi: 10.1002/14651858.CD006986.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mareova P., Mohelska H., Dolejs J., Kuca K. Socio-economic aspects of Alzheimers disease. Curr. Alzheimer Res. 2015;12(9):903–911. doi: 10.2174/156720501209151019111448. [http://dx.doi.org/10.2174/156720501209151019111448]. [PMID: 26510983]. [DOI] [PubMed] [Google Scholar]
- 121.Greig N.H., Utsuki T., Yu Q., Zhu X., Holloway H.W., Perry T., Lee B., Ingram D.K., Lahiri D.K. A new therapeutic target in Alzheimers disease treatment: attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001;17(3):159–165. doi: 10.1185/0300799039117057. [http://dx.doi.org/ 10.1185/03007990152673800]. [PMID: 11900310]. [DOI] [PubMed] [Google Scholar]
- 122.Bajgar J., Fusek J., Kuca K., Bartosova L., Jun D. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini Rev. Med. Chem. 2007;7(5):461–466. doi: 10.2174/138955707780619581. [http://dx.doi.org/10.2174/138955707780619581]. [PMID: 17504181]. [DOI] [PubMed] [Google Scholar]
- 123.Adem A. Putative Mechanisms of Action of Tacrine in Alzheimers-Disease. Acta Neurol. Scand. 1992;85:69–74. doi: 10.1111/j.1600-0404.1992.tb04458.x. [DOI] [PubMed] [Google Scholar]; Adem A., Mohammed A.K., Winblad B. Multiple effects of tetrahydroaminoacridine on the cholinergic system: biochemical and behavioural aspects. J. Neural Transm. Park. Dis. Dement. Sect. 1990;2(2):113–128. doi: 10.1007/BF02260899. [DOI] [PubMed] [Google Scholar]; Bajgar J., Skopec F., Herink J., Patocka J., Kvetina J. Effect of 7-methoxytacrine and L-carnitine on the activity of choline acetyltransferase. Gen. Physiol. Biophys. 1999;18(Spec No):3–6. [PubMed] [Google Scholar]
- 124.Lahiri D.K., Farlow M.R., Sambamurti K. The secretion of amyloid beta-peptides is inhibited in the tacrine-treated human neuroblastoma cells. Brain Res. Mol. Brain Res. 1998;62(2):131–140. doi: 10.1016/s0169-328x(98)00236-8. [DOI] [PubMed] [Google Scholar]; Lahiri D.K., Lewis S., Farlow M.R. Tacrine alters the secretion of the beta-amyloid precursor protein in cell lines. J. Neurosci. Res. 1994;37(6):777–787. doi: 10.1002/jnr.490370612. [DOI] [PubMed] [Google Scholar]
- 125.Tumiatti V., Minarini A., Bolognesi M.L., Milelli A., Rosini M., Melchiorre C. Tacrine derivatives and Alzheimers disease. Curr. Med. Chem. 2010;17(17):1825–1838. doi: 10.2174/092986710791111206. [DOI] [PubMed] [Google Scholar]; Luo W., Li Y.P., He Y., Huang S.L., Li D., Gu L.Q., Huang Z.S. Synthesis and evaluation of heterobivalent tacrine derivatives as potential multi-functional anti-Alzheimer agents. Eur. J. Med. Chem. 2011;46(6):2609–2616. doi: 10.1016/j.ejmech.2011.03.058. [DOI] [PubMed] [Google Scholar]; Hamulakova S., Janovec L., Hrabinova M., Spilovska K., Korabecny J., Kristian P., Kuca K., Imrich J. Synthesis and biological evaluation of novel tacrine derivatives and tacrine-coumarin hybrids as cholinesterase inhibitors. J. Med. Chem. 2014;57(16):7073–7084. doi: 10.1021/jm5008648. [DOI] [PubMed] [Google Scholar]; Nepovimova E., Uliassi E., Korabecny J., Pena-Altamira L.E., Samez S., Pesaresi A., Garcia G.E., Bartolini M., Andrisano V., Bergamini C., Fato R., Lamba D., Roberti M., Kuca K., Monti B., Bolognesi M.L. Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-Iβ aggregation and to exert anticholinesterase and antioxidant effects. . J. Med. Chem. 2014;57(20):8576–8589. doi: 10.1021/jm5010804. [DOI] [PubMed] [Google Scholar]; Spilovska K., Korabecny J., Kral J., Horova A., Musilek K., Soukup O., Drtinova L., Gazova Z., Siposova K., Kuca K. Methoxytacrine-adamantylamine heterodimers as cholinesterase inhibitors in Alzheimers disease treatmentsynthesis, biological evaluation and molecular modeling studies. Molecules. 2013;18(2):2397–2418. doi: 10.3390/molecules18022397. [DOI] [PMC free article] [PubMed] [Google Scholar]; Benchekroun M., Bartolini M., Egea J., Romero A., Soriano E., Pudlo M., Luzet V., Andrisano V., Jimeno M.L., LA3pez M.G., Wehle S., Gharbi T., Refouvelet B., de AndrA(c)s L., Herrera-Arozamena C., Monti B., Bolognesi M.L., Rodriguez-Franco M.I., Decker M., Marco-Contelles J., Ismaili L. Novel tacrine-grafted Ugi adducts as multipotent anti-Alzheimer drugs: a synthetic renewal in tacrineferulic acid hybrids. Chem.Med.Chem. 2015;10(3):523–539. doi: 10.1002/cmdc.201402409. [DOI] [PubMed] [Google Scholar]; Esquivias-Perez M., Maalej E., Romero A., Chabchoub F., Samadi A., Marco-Contelles J., Oset-Gasque M.J. Nontoxic and neuroprotective β-naphthotacrines for Alzheimers disease. Chem. Res. Toxicol. 2013;26(6):986–992. doi: 10.1021/tx400138s. [DOI] [PubMed] [Google Scholar]
- 126.Gottwald M.D., Rozanski R.I. Rivastigmine, a brain-region selective acetylcholinesterase inhibitor for treating Alzheimers disease: review and current status. Expert Opin. Investig. Drugs. 1999;8(10):1673–1682. doi: 10.1517/13543784.8.10.1673. [http://dx.doi.org/10.1517/13543784.8. 10.1673]. [PMID: 11139819]. [DOI] [PubMed] [Google Scholar]
- 127.Onor M.L., Trevisiol M., Aguglia E. Rivastigmine in the treatment of Alzheimers disease: an update. Clin. Interv. Aging. 2007;2(1):17–32. doi: 10.2147/ciia.2007.2.1.17. [http://dx.doi.org/10.2147/ciia.2007.2.1.17]. [PMID: 18044073]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Andin J., Enz A., Gentsch C., Marcusson J. Rivastigmine as a modulator of the neuronal glutamate transporter rEAAC1 mRNA expression. Dement. Geriatr. Cogn. Disord. 2005;19(1):18–23. doi: 10.1159/000080966. [http://dx.doi.org/10.1159/000080966]. [PMID: 15383741]. [DOI] [PubMed] [Google Scholar]
- 129.Bailey J.A., Ray B., Greig N.H., Lahiri D.K. Rivastigmine lowers AI and increases sAPPI± levels, which parallel elevated synaptic markers and metabolic activity in degenerating primary rat neurons. PLoS One. 2011;6(7):e21954. doi: 10.1371/journal.pone.0021954. [EP -.]. [http://dx.doi.org/ 10.1371/journal.pone.0021954]. [PMID: 21799757]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Albuquerque E.X., Alkondon M., Pereira E.F., Castro N.G., Schrattenholz A., Barbosa C.T., Bonfante-Cabarcas R., Aracava Y., Eisenberg H.M., Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J. Pharmacol. Exp. Ther. . 1997;280(3):1117–1136. [PubMed] [Google Scholar]; Schrattenholz A., Pereira E.F., Roth U., Weber K.H., Albuquerque E.X., Maelicke A. Agonist responses of neuronal nicotinic acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol. Pharmacol. 1996;49(1):1–6. [PubMed] [Google Scholar]
- 131.Coyle J., Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: effects on the course of Alzheimers disease. Biol. Psychiatry. 2001;49(3):289–299. doi: 10.1016/s0006-3223(00)01101-x. [http://dx.doi.org/10.1016/S0006-3223(00)01101-X]. [PMID: 11230880]. [DOI] [PubMed] [Google Scholar]
- 132.Kryger G., Silman I., Sussman J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure. 1999;7(3):297–307. doi: 10.1016/s0969-2126(99)80040-9. [http://dx.doi.org/10.1016/S0969-2126(99)80040-9]. [PMID: 10368299]. [DOI] [PubMed] [Google Scholar]
- 133.Inestrosa N.C., Alvarez A., PA(c)rez C.A., Moreno R.D., Vicente M., Linker C., Casanueva O.I., Soto C., Garrido J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimers fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. doi: 10.1016/s0896-6273(00)80108-7. [http://dx.doi.org/10. 1016/S0896-6273(00)80108-7]. [PMID: 8608006]. [DOI] [PubMed] [Google Scholar]
- 134.Birks J. Cholinesterase inhibitors for Alzheimers disease. Cochrane Database Syst. Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [PMID: 16437532]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anand R., Gill K.D., Mahdi A.A. Therapeutics of Alzheimer's disease: Past, present and future. Neuropharmacology. 2014. pp. 27–50. [DOI] [PubMed]
- 136.Phillips L.H., II The epidemiology of myasthenia gravis. Ann. N. Y. Acad. Sci. 2003;998:407–412. doi: 10.1196/annals.1254.053. [http://dx.doi.org/10.1196/ annals.1254.053]. [PMID: 14592908]. [DOI] [PubMed] [Google Scholar]
- 137.GarcA-a-Carrasco M., Escarcega R.O., Fuentes-Alexandro S., Riebeling C., Cervera R. Therapeutic options in autoimmune myasthenia gravis. Autoimmun. Rev. 2007;6(6):373–378. doi: 10.1016/j.autrev.2007.01.001. [http:// dx.doi.org/10.1016/j.autrev.2007.01.001]. [PMID: 17537383]. [DOI] [PubMed] [Google Scholar]
- 138.Thanvi B.R., Lo T.C. Update on myasthenia gravis. Postgrad. Med. J. 2004;80(950):690–700. doi: 10.1136/pgmj.2004.018903. [http://dx.doi.org/10.1136/ pgmj.2004.018903]. [PMID: 15579606]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoch W., McConville J., Helms S., Newsom-Davis J., Melms A., Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med. 2001;7(3):365–368. doi: 10.1038/85520. [http://dx.doi.org/10.1038/85520]. [PMID: 11231638]. [DOI] [PubMed] [Google Scholar]
- 140.Punga A.R., Stalberg E. Acetylcholinesterase inhibitors in MG: to be or not to be? Muscle Nerve. 2009;39(6):724–728. doi: 10.1002/mus.21319. [http://dx. doi.org/10.1002/mus.21319]. [PMID: 19260048]. [DOI] [PubMed] [Google Scholar]
- 141.Richman D.P., Agius M.A. Treatment principles in the management of autoimmune myasthenia gravis. Ann. N. Y. Acad. Sci. 2003;998:457–472. doi: 10.1196/annals.1254.060. [http://dx.doi.org/10.1196/annals.1254. 060]. [PMID: 14592915]. [DOI] [PubMed] [Google Scholar]
- 142.Mehndiratta M.M., Pandey S., Kuntzer T. Acetylcholinesterase inhibitor treatment for myasthenia gravis. Cochrane Database Syst. Rev. 2011;(2):CD006986. doi: 10.1002/14651858.CD006986.pub2. [PMID: 21328290]. [DOI] [PubMed] [Google Scholar]
- 143.Garcia-Carrasco M., Escarcega R.O., Fuentes-Alexandro S., Riebeling C., Cervera R. Therapeutic options in autoimmune myasthenia gravis. Autoimmun. Rev. 2007;6(6):373–378. doi: 10.1016/j.autrev.2007.01.001. [http:// dx.doi.org/10.1016/j.autrev.2007.01.001]. [PMID: 17537383]. [DOI] [PubMed] [Google Scholar]
- 144.McNamara R., Mihalakis M.J. Acute colonic pseudo-obstruction: rapid correction with neostigmine in the emergency department. J. Emerg. Med. 2008;35(2):167–170. doi: 10.1016/j.jemermed.2007.06.043. [http://dx.doi.org/10.1016/ j.jemermed.2007.06.043]. [PMID: 18242923]. [DOI] [PubMed] [Google Scholar]
- 145.Hodge A.S., Humphrey D.R., Rosenberry T.L. Ambenonium is a rapidly reversible noncovalent inhibitor of acetylcholinesterase, with one of the highest known affinities. Mol. Pharmacol. 1992;41(5):937–942. [PMID: 1588924]. [PubMed] [Google Scholar]
- 146.Bolognesi M.L., Cavalli A., Andrisano V., Bartolini M., Banzi R., Antonello A., Rosini M., Melchiorre C. Design, synthesis and biological evaluation of ambenonium derivatives as AChE inhibitors. FARMACO. 2003;58(9):917–928. doi: 10.1016/S0014-827X(03)00150-2. [http://dx.doi.org/ 10.1016/S0014-827X(03)00150-2]. [PMID: 13679187]. [DOI] [PubMed] [Google Scholar]
- 147.Harada T., Fushimi K., Kato A., Ito Y., Nishijima S., Sugaya K., Yamada S. Demonstration of muscarinic and nicotinic receptor binding activities of distigmine to treat detrusor underactivity. Biol. Pharm. Bull. 2010;33(4):653–658. doi: 10.1248/bpb.33.653. [http://dx.doi.org/10.1248/ bpb.33.653]. [PMID: 20410601]. [DOI] [PubMed] [Google Scholar]
- 148.Komloova M., Musilek K., Dolezal M., Gunn-Moore F., Kuca K. Structure-activity relationship of quaternary acetylcholinesterase inhibitors - outlook for early myasthenia gravis treatment. Curr. Med. Chem. 2010;17(17):1810–1824. doi: 10.2174/092986710791111198. [http://dx.doi.org/10.2174/ 092986710791111198]. [PMID: 20345342]. [DOI] [PubMed] [Google Scholar]
- 149.Maruyama S., Oki T., Otsuka A., Shinbo H., Ozono S., Kageyama S., Mikami Y., Araki I., Takeda M., Masuyama K., Yamada S. Human muscarinic receptor binding characteristics of antimuscarinic agents to treat overactive bladder. J. Urol. 2006;175(1):365–369. doi: 10.1016/S0022-5347(05)00017-0. [http://dx.doi.org/10.1016/S0022-5347(05)00017-0]. [PMID: 16406943]. [DOI] [PubMed] [Google Scholar]
- 150.Kobayashi S., Ikeda K., Miyata K. Comparison of in vitro selectivity profiles of solifenacin succinate (YM905) and current antimuscarinic drugs in bladder and salivary glands: a Ca2+ mobilization study in monkey cells. Life Sci. 2004;74(7):843–853. doi: 10.1016/j.lfs.2003.07.019. [http://dx.doi.org/10.1016/j.lfs.2003.07.019]. [PMID: 14659973]. [DOI] [PubMed] [Google Scholar]
- 151.Kiwamoto H., Ma F.H., Higashira H., Park Y.C., Kurita T. Identification of muscarinic receptor subtypes of cultured smooth muscle cells and tissue of human bladder body. Int. J. Urol. 2001;8(10):557–563. doi: 10.1046/j.1442-2042.2001.00370.x. [http://dx.doi.org/10.1046/j.1442-2042.2001.00370.x]. [PMID: 11737484]. [DOI] [PubMed] [Google Scholar]
- 152.Mansfield K.J., Chandran J.J., Vaux K.J., Millard R.J., Christopoulos A., Mitchelson F.J., Burcher E. Comparison of receptor binding characteristics of commonly used muscarinic antagonists in human bladder detrusor and mucosa. J. Pharmacol. Exp. Ther. 2009;328(3):893–899. doi: 10.1124/jpet.108.145508. [http://dx.doi.org/10.1124/ jpet.108.145508]. [PMID: 19029429]. [DOI] [PubMed] [Google Scholar]
- 153.Sinha S., Gupta S., Malhotra S., Krishna N.S., Meru A.V., Babu V., Bansal V., Garg M., Kumar N., Chugh A., Ray A. AE9C90CB: a novel, bladder-selective muscarinic receptor antagonist for the treatment of overactive bladder. Br. J. Pharmacol. 2010;160(5):1119–1127. doi: 10.1111/j.1476-5381.2010.00752.x. [http://dx.doi.org/10.1111/ j.1476-5381.2010.00752.x]. [PMID: 20590605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Winter M., Wille T., Musilek K., Kuca K., Thiermann H., Worek F. Investigation of the reactivation kinetics of a large series of bispyridinium oximes with organophosphate-inhibited human acetylcholinesterase. Toxicol. Lett. 2015;244:136–142. doi: 10.1016/j.toxlet.2015.07.007. [PMID: 26210933]. [DOI] [PubMed] [Google Scholar]
- 155.Horn G., Wille T., Musilek K., Kuca K., Thiermann H. [DOI] [PubMed]; Worek F. Reactivation kinetics of 31 structurally different bispyridinium oximes with organophosphate-inhibited human butyrylcholinesterase. Arch. Toxicol. 2015;89(3):405–414. doi: 10.1007/s00204-014-1288-5. [http://dx.doi.org/10.1007/s00204-014-1288-5]. [PMID: 24912784]. [DOI] [PubMed] [Google Scholar]
- 156.Musilek K., Komloova M., Holas O., Hrabinova M., Pohanka M., Dohnal V., Nachon F., Dolezal M., Kuca K. Preparation and in vitro screening of symmetrical bis-isoquinolinium cholinesterase inhibitors bearing various connecting linkageimplications for early Myasthenia gravis treatment. Eur. J. Med. Chem. 2011;46(2):811–818. doi: 10.1016/j.ejmech.2010.12.011. [http://dx.doi.org/10.1016/j.ejmech.2010.12.011]. [PMID: 21236521]. [DOI] [PubMed] [Google Scholar]
- 157.Soukup O., Jun D., Zdarova-Karasova J., Patocka J., Musilek K., Korabecny J., Krusek J., Kaniakova M., Sepsova V., Mandikova J., Trejtnar F., Pohanka M., Drtinova L., Pavlik M., Tobin G., Kuca K. A resurrection of 7-MEOTA: a comparison with tacrine. Curr. Alzheimer Res. 2013;10(8):893–906. doi: 10.2174/1567205011310080011. [http://dx. doi.org/10.2174/1567205011310080011]. [PMID: 24093535]. [DOI] [PubMed] [Google Scholar]
- 158.Zemek F., Drtinova L., Nepovimova E., Sepsova V., Korabecny J., Klimes J., Kuca K. Outcomes of Alzheimers disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin. Drug Saf. 2014;13(6):759–774. doi: 10.1517/14740338.2014.914168. [PMID: 24845946]. [DOI] [PubMed] [Google Scholar]
- 159.Luo W., Yu Q.S., Kulkarni S.S., Parrish D.A., Holloway H.W., Tweedie D., Shafferman A., Lahiri D.K., Brossi A., Greig N.H. Inhibition of human acetyl- and butyrylcholinesterase by novel carbamates of (-)- and (+)-tetrahydrofurobenzofuran and methanobenzodioxepine. J. Med. Chem. 2006;49(7):2174–2185. doi: 10.1021/jm050578p. [http://dx.doi.org/10.1021/jm050578p]. [PMID: 16570913]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Triggle D.J., Filler R. The pharmacology of physostigmine. CNS Drug Rev. 1998;4:87–136. [http://dx.doi.org/10.1111/j.1527-3458.1998.tb00059.x]. [Google Scholar]
- 161.Nakayama K., Katsu H., Kitazumi K. Effect of distigmine bromide on the central cholinergic system. J. Psychopharmacol. (Oxford) 2009;23(2):190–193. doi: 10.1177/0269881108089817. [http://dx.doi.org/10.1177/ 0269881108089817]. [PMID: 18515453]. [DOI] [PubMed] [Google Scholar]
- 162.Soukup O., Kumar U.K., Proska J., Bratova L., Adem A., Jun D., Fusek J., Kuca K., Tobin G. The effect of oxime reactivators on muscarinic receptors: functional and binding examinations. Environ. Toxicol. Pharmacol. 2011;31(3):364–370. doi: 10.1016/j.etap.2011.01.003. [http://dx. doi.org/10.1016/j.etap.2011.01.003]. [PMID: 21787706]. [DOI] [PubMed] [Google Scholar]
- 163.Soukup O., Krusek J., Kaniakova M., Kumar U.K., Oz M., Jun D., Fusek J., Kuca K., Tobin G. Oxime reactivators and their in vivo and in vitro effects on nicotinic receptors. Physiol. Res. 2011;60(4):679–686. doi: 10.33549/physiolres.932105. [PMID: 21574759]. [DOI] [PubMed] [Google Scholar]
- 164.Sepsova V., Krusek J., Zdarova K.J., Zemek F., Musilek K., Kuca K., Soukup O. The interaction of quaternary reversible acetylcholinesterase inhibitors with the nicotinic receptor. Physiol. Res. 2014;63(6):771–777. doi: 10.33549/physiolres.932768. [PMID: 25157661]. [DOI] [PubMed] [Google Scholar]
- 165.Woodruff-Pak D.S., Lander C., Geerts H. Nicotinic cholinergic modulation: galantamine as a prototype. CNS Drug Rev. 2002;8(4):405–426. doi: 10.1111/j.1527-3458.2002.tb00237.x. [http://dx.doi.org/10.1111/j.1527-3458.2002.tb00237. x]. [PMID: 12481195]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ago Y., Koda K., Ota Y., Kita Y., Fukada A., Takuma K., Matsuda T. Donepezil, but not galantamine, blocks muscarinic receptor-mediated in vitro and in vivo responses. Synapse. 2011;65(12):1373–1377. doi: 10.1002/syn.20969. [http://dx.doi.org/10.1002/syn.20969]. [PMID: 21780184]. [DOI] [PubMed] [Google Scholar]
- 167.Di Angelantonio S., Bernardi G., Mercuri N.B. Donepezil modulates nicotinic receptors of substantia nigra dopaminergic neurones. Br. J. Pharmacol. 2004;141(4):644–652. doi: 10.1038/sj.bjp.0705660. [http://dx.doi. org/10.1038/sj.bjp.0705660]. [PMID: 14744806]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moretti R., Torre P., Vilotti C., Antonello R.M., Pizzolato G. Rivastigmine and Parkinson dementia complex. Expert Opin. Pharmacother. 2007;8(6):817–829. doi: 10.1517/14656566.8.6.817. [http://dx.doi.org/10.1517/ 14656566.8.6.817]. [PMID: 17425477]. [DOI] [PubMed] [Google Scholar]
- 169.Smulders C.J., Zwart R., Bermudez I., van Kleef R.G., Groot-Kormelink P.J., Vijverberg H.P. Cholinergic drugs potentiate human nicotinic alpha4beta2 acetylcholine receptors by a competitive mechanism. Eur. J. Pharmacol. 2005;509(2-3):97–108. doi: 10.1016/j.ejphar.2004.12.037. [http:// dx.doi.org/10.1016/j.ejphar.2004.12.037]. [PMID: 15733544]. [DOI] [PubMed] [Google Scholar]
- 170.Perry E.K., Smith C.J., Court J.A., Bonham J.R., Rodway M., Atack J.R. Interaction of 9-amino-1,2,3,4-tetrahydroaminoacridine (THA) with human cortical nicotinic and muscarinic receptor binding in vitro. Neurosci. Lett. 1988;91(2):211–216. doi: 10.1016/0304-3940(88)90770-7. [http://dx. doi.org/10.1016/0304-3940(88)90770-7]. [PMID: 3185960]. [DOI] [PubMed] [Google Scholar]
- 171.Volpe L.S., Biagioni T.M., Marquis J.K. In vitro modulation of bovine caudate muscarinic receptor number by organophosphates and carbamates. Toxicol. Appl. Pharmacol. 1985;78(2):226–234. doi: 10.1016/0041-008x(85)90286-8. [http://dx.doi.org/10.1016/0041-008X(85)90286-8]. [PMID: 4035678]. [DOI] [PubMed] [Google Scholar]
- 172.Svobodova L., Krusek J., Hendrych T., Vyskocil F. Physostigmine modulation of acetylcholine currents in COS cells transfected with mouse muscle nicotinic receptor. Neurosci. Lett. 2006;401(1-2):20–24. doi: 10.1016/j.neulet.2006.02.065. [http://dx.doi.org/10.1016/j.neulet.2006.02. 065]. [PMID: 16530961]. [DOI] [PubMed] [Google Scholar]
- 173.Clarke P.B., Reuben M., el-Bizri H. Blockade of nicotinic responses by physostigmine, tacrine and other cholinesterase inhibitors in rat striatum. Br. J. Pharmacol. 1994;111(3):695–702. doi: 10.1111/j.1476-5381.1994.tb14793.x. [http://dx.doi.org/10.1111/j.1476-5381.1994.tb14793.x]. [PMID: 8019748]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lockhart B., Closier M., Howard K., Steward C., Lestage P. Differential inhibition of [3H]-oxotremorine-M and [3H]-quinuclinidyl benzilate binding to muscarinic receptors in rat brain membranes with acetylcholinesterase inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363(4):429–438. doi: 10.1007/s002100000382. [http:// dx.doi.org/10.1007/s002100000382]. [PMID: 11330337]. [DOI] [PubMed] [Google Scholar]
- 175.Craig C.R., Stitzel R.E. Modern pharmacology with clinical applications. Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 176.Pascuzzo G.J., Akaike A., Maleque M.A., Shaw K.P., Aronstam R.S., Rickett D.L., Albuquerque E.X. The nature of the inter- actions of pyridostigmine with the nicotinic acetylcholine receptor-ionic channel complex. I. Agonist, desensitizing, and binding properties. Mol. Pharmacol. 1984;25(1):92–101. [PMID: 6323955]. [PubMed] [Google Scholar]