Abstract
Purpose
To evaluate the efficacy of apalutamide before or after treatment with abiraterone acetate and prednisone (AAP) in patients with progressive metastatic castration-resistant prostate cancer (mCRPC).
Experimental Design
Two cohorts were studied: AAP-naïve and post-AAP patients who had received ≥6 months of AAP. Patients had progressive mCRPC per rising prostate-specific antigen (PSA) and/or imaging, without prior chemotherapy exposure. All received apalutamide 240 mg/day. Primary endpoint was ≥50% decline in 12-week PSA according to Prostate Cancer Working Group 2 criteria. Secondary endpoints included time to PSA progression and time on treatment.
Results
Forty-six patients enrolled in the AAP-naïve (n = 25) and post-AAP (n = 21) cohorts. The 12-week PSA response rate was 88% (22 of 25) and 22% (4 of 18), median time to PSA progression was 18.2 months (95% CI, 8.3 months–not reached) and 3.7 months (95% CI, 2.8–5.6 months), and median time on treatment 21 months (range, 2.6–37.5) and 4.9 months (range, 1.3–23.2), for the AAP-naïve and post-AAP cohorts, respectively. 80% (95% CI, 59–93) and 64% (95% CI, 43–82) of AAP-naïve and 43% (95% CI, 22–66) and 10% (95% CI, 1–30) of post-AAP patients remained on treatment for 6+ and 12+ months, respectively. Common treatment-emergent adverse events in both cohorts were grade 1 or 2 fatigue, diarrhea, nausea, and abdominal pain.
Conclusions
Apalutamide was safe, well tolerated, and demonstrated clinical activity in mCRPC with 80% of AAP-naïve and 43% of post-AAP patients remaining on treatment for 6 months or longer.
Keywords: Apalutamide, castration-resistant prostate cancer, safety, antitumor activity, abiraterone acetate
Introduction
Apalutamide (formerly ARN-509) is a next-generation androgen receptor (AR) antagonist (1) targeting known oncogenic changes in AR signaling that contribute to the lethal castration-resistant phenotype (2). The prostate cancer therapeutic landscape has changed significantly since the development of apalutamide as six life-prolonging therapies are now approved, including two AR-signaling–directed therapies, abiraterone and enzalutamide, which has broad indications in metastatic castration-resistant prostate cancer (mCRPC) (3–10). A major challenge in successful drug development for the benefit of CRPC patients is to identify active drugs and to learn how best to utilize them in sequence or in combination.
Apalutamide selectively and irreversibly binds with high affinity to the ligand-binding domain of the AR, inducing a conformational change in the AR that impairs translocation of the receptor complex into the nucleus of the cell and thereby prevents binding of androgen response elements. In mice CRPC xenograft models, apalutamide produced dose-dependent tumor regressions superior to those achieved with bicalutamide or enzalutamide (1). Additionally, maximal antitumor effects occurred in animals bearing CRPC tumors at 4-fold lower concentrations in the central nervous system, a 3-fold lower dose, and approximately 9-fold lower plasma levels of apalutamide relative to enzalutamide, indicative of higher tumor-to-plasma penetration. A first-in-human phase I open-label study (ARN-509-001; NCT01171898) of apalutamide confirmed proof of mechanism and safety of apalutamide, and identified an optimal biologic dose for further study (11). In the phase II portion of trial ARN-509-001 in nonmetastatic CRPC patients, apalutamide demonstrated significant antitumor effects in men, with a favorable safety profile (12).
In this report of mCRPC cohorts of trial ARN-509-001, we evaluate apalutamide activity in patients with and without prior abiraterone acetate and prednisone (AAP) therapy using Prostate Cancer Working Group 2 (PCWG2) criteria (13) including swim lanes that illustrate the duration of treatment as an additional indicator of meaningful clinical benefit that reflects the total patient experience on this trial (14).
Methods
Patients
Patients with mCRPC were enrolled in one of two cohorts: an AAP-naïve cohort (AAP-naïve) and an AAP-pretreated cohort (post-AAP). Results from a third cohort of patients with nonmetastatic CRPC were reported separately (12). All patients had pathologically proven prostate adenocarcinoma and had received ongoing androgen deprivation therapy with a gonadotropin-releasing hormone analog or orchiectomy, and had castration levels of serum testosterone of ≤50 ng/dL within 4 weeks of study enrollment; Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; a life expectancy of ≥3 months; corrected QT interval of ≤450 ms; and adequate cardiac, renal, hepatic, and bone marrow function.
Patients in the AAP-naïve cohort had disease progression, based on either a rising prostate-specific antigen (PSA) (≥2 ng/mL within 2 weeks of study enrollment) or measurable disease (new or progressive tissue disease based on computed tomography/magnetic resonance imaging scans) by modified Response Evaluation Criteria in Solid Tumors (RECIST 1.0) (15) or radiographic progression (≥2 new bone lesions, PCWG2 criteria) (13). Patients in both cohorts were excluded if they had been treated previously with enzalutamide, ketoconazole, or chemotherapy for mCRPC or had a history of seizure or conditions that predispose to seizures. Patients in the post-AAP cohort were also required to have received ≥6 months of abiraterone acetate treatment to ensure therapeutic benefit prior to disease progression.
Institutional review boards approved the study, which was conducted accordingly to the Declaration of Helsinki, the International Conference on Harmonisation, and the Guidelines for Good Clinical Practice. Data were anonymized to protect the identities of subjects involved in the research. All patients gave written informed consent.
Study design
Patients received apalutamide 240 mg/day (11) on a continuous daily dosing regimen, and remained on study treatment until either evidence of both PSA progression and radiographic progression or clinical progression alone, development of unacceptable toxicity, or withdrawal of consent (Supplementary Fig. S1A). Dose modifications (e.g., short treatment breaks or dose reduction) were allowed in case of treatment-related toxicities.
Endpoints
PSA response rate was assessed using the PCWG2 criteria (13), with slight modifications. The primary endpoint was a 50% or greater decline in PSA from baseline at 12 weeks (or earlier for those who discontinued therapy). The maximal PSA change at any time on the study was also reported for each patient. The secondary endpoints were time to PSA progression, measured from the start of treatment until the criteria for PSA progression were met, according to the modified PCWG2 (≥25% and ≥2 ng above nadir, confirmed ≥3 weeks later or ≥25% and ≥2 ng/mL after 12 weeks in the absence of decline); progression-free survival, measured from the start of the treatment until radiographic disease progression or death (based on investigator’s assessment); and objective response rate, measured as changes in target and nontarget lesions relative to baseline reported every 12 weeks using modified RECIST 1.0 (15) and PCWG2 criteria (13).
Tumor evaluations were performed every three cycles (12 weeks). Safety was assessed continuously from the first dose until 30 days after the last dose or until the resolution or stability of any National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0 treatment-emergent or treatment-emergent drug-related toxicity. Disease progression was defined as evidence of both PSA progression (≥25% and >2 ng/mL above PSA nadir confirmed ≥3 weeks later or >2 ng/mL above baseline PSA after 12 weeks) and radiographic progression (soft tissue metastases by modified RECIST) (15) seen on computed tomography/magnetic resonance imaging scans and/or bone metastases by 99mTc-methylene diphosphate bone scans by PCWG2 criteria, and clinically by the occurrence of a skeletal-related event, pain progression, or worsening of disease-related symptoms requiring new systemic anti–prostate cancer therapy. Per Prostate Cancer Working Group 3 (PCWG3) recommendations (14), swim lane plots were generated to track the time on apalutamide treatment for individual patients with duration of ≥6 months as a measure of clinical benefit.
Statistical analysis
The study was expected to enroll 20 patients in the AAP-naïve cohort. In the post-AAP cohort, enrollment was staged such that if at least 1 in 10 patients achieved ≥50% PSA decline at 12 weeks, expansion to 20 total patients was planned.
All patients who received at least one dose of apalutamide were included in the efficacy and safety analyses. In the post-AAP cohort, three patients were excluded (one without evidence of progressive mCRPC, two who did not receive prior abiraterone acetate for at least 6 months) from the efficacy analysis. Summary statistics were reported for demographics, baseline characteristics, adverse events, vital signs, and clinical laboratory evaluations. The change in PSA at 12 weeks relative to baseline (primary endpoint) and the maximal change in PSA at any time on study relative to baseline are presented in waterfall plots and descriptively summarized. The Kaplan-Meier method was used to estimate the median time to events and 95% confidence intervals.
Results
Patients
Patients were enrolled in the phase II portion of the study from November 2011 to June 2012. The cutoff date for the final data analysis was December 31, 2014. Patients had a median age of 68 years in the AAP-naïve cohort and 67 years in the post-AAP cohort; median times since initial diagnosis were 61 months and 107 months, respectively (Table 1). Roughly half of the patients had an ECOG PS of 0 (52% and 62% in the AAP-naïve and post-AAP cohorts, respectively). Gleason score ≤7 at initial diagnosis was observed in 28% and 67% of the AAP-naïve and post-AAP cohorts, respectively. Three patients were excluded from the efficacy analysis in the post-AAP cohort; one failed to meet criteria for progressive metastatic disease and two did not receive abiraterone acetate for ≥6 months (Supplementary Fig. S1B).
Table 1.
Patient characteristics
| AAP-naïve mCRPC (n = 25) | Post-AAP mCRPC (n = 21) | |
|---|---|---|
| Age, years, median (range) | 68 (53–91) | 67 (48–83) |
| Baseline PSA, ng/mL | ||
| n | 25 | 21 |
| Median (range) | 14.7 (1.1–2552.1) | 58.4 (1.1–6074.3) |
| Baseline LDH, U/L | ||
| n | 18 | 18 |
| Median (range) | 192.5 (89–518) | 233.0 (149–1075) |
| Baseline ALK-P, U/L | ||
| n | 25 | 20 |
| Median (range) | 78.0 (44–802) | 96.5 (33–737) |
| ECOG PS, n (%) | ||
| 0 | 13 (52) | 13 (62) |
| 1 | 12 (48) | 8 (38) |
| Gleason score at initial diagnosis, n (%) | ||
| ≤7 | 7 (28) | 14 (67) |
| 8–10 | 18 (72) | 6 (29) |
| Missing | 0 | 1 (5) |
| Time since initial diagnosis, months, median (range) | 61 (10–191) | 107 (16–236) |
| Primary treatment, n (%) | ||
| Prostatectomy +/− salvage radiation | 10 (40) | 13 (62) |
| Primary radiation | 11 (44) | 12 (57) |
| No primary or salvage radiation | 13 (52) | 5 (24) |
| Metastases | ||
| Bone | 11 (44) | 8 (38) |
| Soft tissue | 9 (36) | 5 (24) |
Abbreviations: ALK-P, alkaline phosphatase; LDH, lactate dehydrogenase.
PSA responses
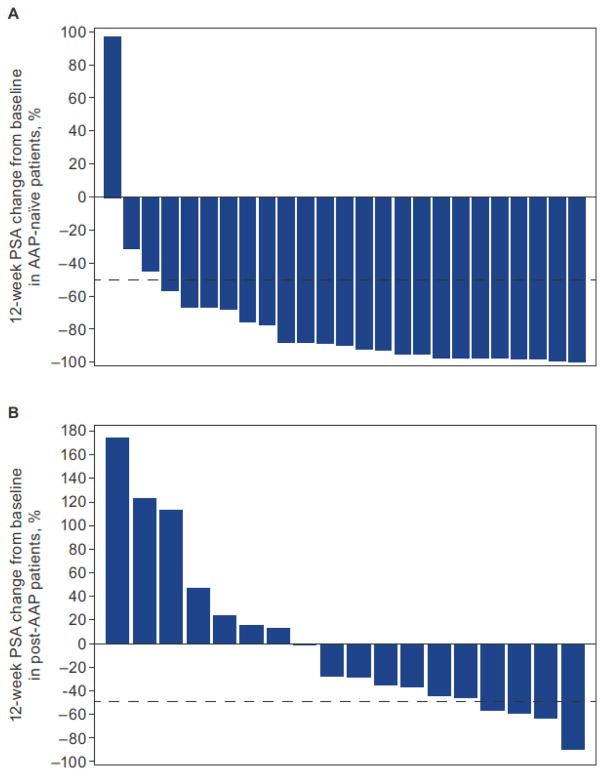
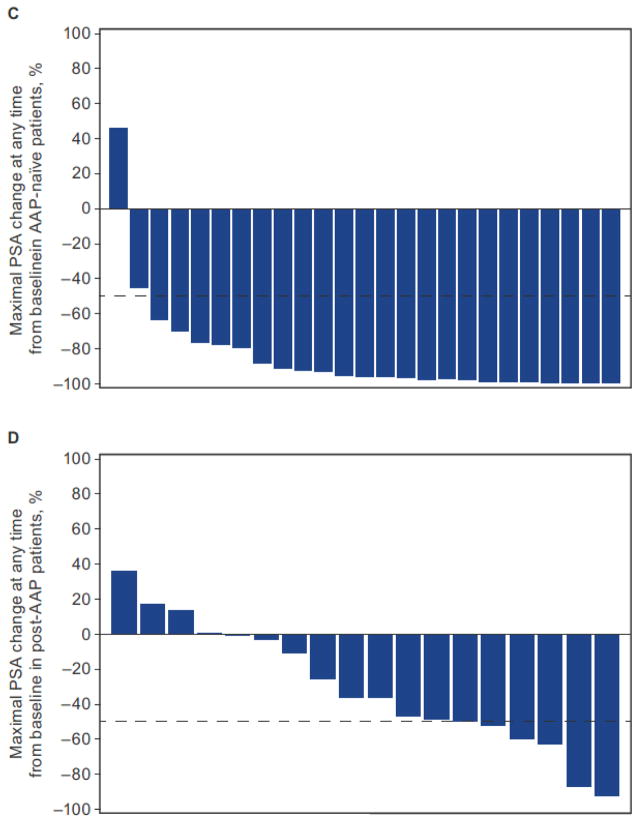
The 12-week PSA response rate was 88% (22 of 25) (95% CI, 69–97) for AAP-naïve patients and 22% (4 of 18) (95% CI, 6–48) for post-AAP patients (Table 2, Fig. 1A and 1B). The maximal percent PSA decline from baseline for patients (i.e., ≥50% decline at any time) was 92% (23 of 25) for AAP-naïve patients and 28% (5 of 18) for post-AAP patients (Fig. 1C and 1D).
Table 2.
Efficacy outcomes
| AAP-naïve mCRPC (n = 25) | Post-AAP mCRPC (n = 18) | |
|---|---|---|
| PSA response ratea, n (%) | ||
| 12 weeks | 22 (88) | 4 (22) |
| 24 weeks | 20 (80) | 1 (6) |
| 36 weeks | 17 (68) | 0 |
| Maximal PSA responseb, n (%) | 23 (92) | 5 (28) |
| Median time to PSA progression, months (95% CI) | 18.2 (8.3–NR) | 3.7 (2.8–5.6) |
| Median PFSc, months (95% CI) | NR (16.7–NR) | NR (NR–NR) |
| ORRd, n/N (%) | 4/8 (50) | 0/10 (0) |
Abbreviations: ORR, objective response rate; PFS, progression-free survival.
≥50% decline in PSA from baseline from PCWG2 criteria.
Maximal PSA response is the maximal percent reduction post baseline for the individual patient at any time point.
Per protocol, patients who had progressive disease that was not confirmed prior to subsequent therapy were censored back to their last assessment prior to subsequent therapy.
Eight patients in the AA-naïve cohort and 10 patients in the post-AAP mCRPC cohort had measurable disease at baseline.
Figure 1.
Waterfall plots showing 12-week PSA response in patients with AA-naïve mCRPC (A) and post-AAP mCRPC (B), and maximal PSA response at any time point in patients with AAP-naïve mCRPC (C) and post-AAP mCRPC (D). PK, pharmacokinetics.
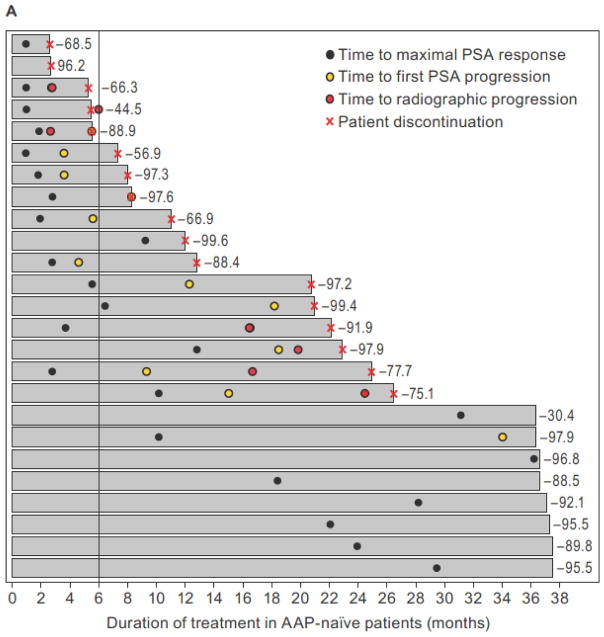
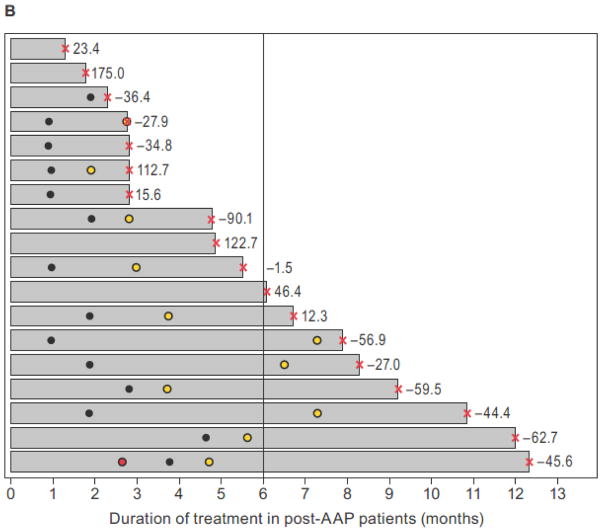
The median duration of apalutamide treatment was 21 months (range: 2.6–37.5) for AAP-naïve and 4.9 months (range: 1.3–23.2) for post-AAP cohorts. The duration of apalutamide treatment in individual patients ordered sequentially from lowest to highest 12-week PSA response rate is shown in swim lane plots for AAP-naïve patients (Fig. 2A) and post-AAP patients (Fig. 2B) (14). These plots illustrate the individual patient experience over time. Notable is the disconnect between the degree of PSA decline and time on therapy in many patients. By cohort, a total of 80% (20 of 25) (95% CI, 59–93) and 64% (16 of 25) (95% CI, 43–82) of AAP-naïve patients and 43% (9 of 21) (95% CI, 22–66) and 10% (2 of 21) (95% CI, 1–30) of post-AAP patients remained on treatment with apalutamide for 6+ and 12+ months, respectively.
Figure 2.
Swim lane plots showing duration of apalutamide treatment (x-axis) aligned sequentially in individual patients from shortest (top lane) to longest (bottom lane) treatment duration (y-axis) in the AAP-naïve mCRPC (A) and post-AAP mCRPC (B) cohorts. Respective 12-week PSA response rates are shown to the right of the lanes. Vertical line demarcates patients on treatment for ≥6 months as a measure of clinical benefit.
Secondary outcomes
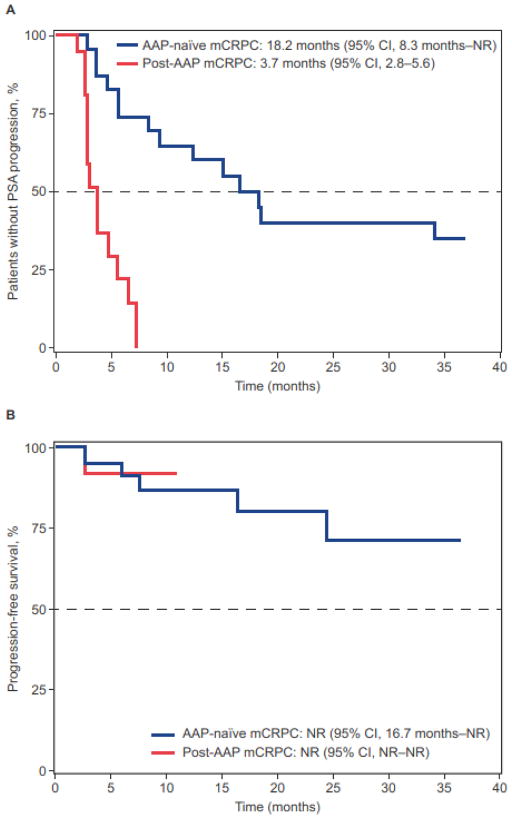
The median time to PSA progression was 18.2 months (95% CI, 8.3 months–not reached [NR]) for AAP-naïve patients and 3.7 months (95% CI, 2.8–5.6 months) for post-AAP patients (Table 2, Fig. 3A). The median progression-free survival was NR (95% CI, 16.7 months–NR) for AAP-naïve patients after 22.1 months of follow-up, and NR (95% CI, NR–NR) for post-AAP patients after 5.6 months of follow-up, respectively (Fig. 3B).
Figure 3.
Secondary outcomes. Time to PSA progression (A), and progression-free survival (B). Per protocol, patients were allowed to discontinue treatment for PSA progression.
Based on RECIST, of the eight AAP-naïve patients with measurable target lesions at baseline, tumor regression to a partial response was observed in four and stable disease in two for 14 months and 2.8 months. There were no tumor regressions in the 10 post-AAP patients, although four had stable disease for 2.5, 3.8, 5.1, and 5.6 months, respectively.
Safety
The most common treatment-emergent adverse events in AAP-naïve patients were fatigue, nausea, and abdominal pain; fatigue, diarrhea, and nausea were the most common treatment-emergent adverse events in post-AAP patients (Table 3). Most treatment-emergent adverse events were grade 1 or 2. The only grade 3 treatment-emergent adverse events reported in more than one patient in each cohort was anemia in two AAP-naïve patients (8%) and back pain in two post-AAP patients (10%). The most common drug-related treatment-emergent adverse events were fatigue (48% and 52%, respectively), diarrhea (32% and 19%, respectively), and nausea (32% and 24%). Serious adverse events were reported by eight (32%) AAP-naïve patients and six (29%) post-AAP patients, but none were assessed as drug related. No adverse events of fall were reported as serious, and no seizures were reported. Dose modifications (reduction and/or interruption) occurred in seven (28%) AAP-naïve patients and eight (38%) post-AAP patients; one dose reduction occurred in three (12%) AAP-naïve patients and one (5%) post-AAP patient, and at least one dose interruption occurred in five (20%) AAP-naïve patients and eight (38%) post-AAP patients. The median number of days with treatment interruption was 18 (range: 3–29) for AAP-naïve patients and 11 (range: 5–27) for post-AAP patients. Most dose reductions and interruptions were because of adverse events. The adverse events that led to permanent treatment discontinuation were abdominal pain (n = 1 [4%]), gastrointestinal hemorrhage (n = 1 [4%]), large intestinal hemorrhage (n = 1 [4%]), and arthralgia (n = 1 [4%]) in the AAP-naïve cohort (in 4 of 25 patients), and fatigue (n = 1 [5%]) and muscle disorder (n = 1 [5%]) in the post-AAP patient cohort (in 1 of 21 patients). Disease progression was the most common reason for treatment discontinuation. Discontinuation due to adverse events was observed in 12% (3 of 25 patients) of AAP-naïve patients and in 5% (1 of 21 patients) of post-AAP patients. Two patients in the AAP-naïve cohort died from disease progression.
Table 3.
Safety
| AAP-naïve mCRPC (n = 25) | Post-AAP mCRPC (n = 21) | |||
|---|---|---|---|---|
|
| ||||
| Treatment-emergent adverse eventsa | All grades | Grade ≥3 | All grades | Grade ≥3 |
| Fatigue | 15 (60) | 0 | 11 (52) | 1 (5) |
| Nausea | 14 (56) | 0 | 7 (33) | 0 |
| Abdominal pain | 12 (48) | 1 (4) | 2 (10) | 0 |
| Diarrhea | 11 (44) | 0 | 8 (38) | 0 |
| Dyspnea | 7 (28) | 1 (4) | 3 (14) | 0 |
| Rash | 7 (28) | 0 | 0 | 0 |
| Arthralgia | 6 (24) | 0 | 6 (29) | 0 |
| Back pain | 6 (24) | 0 | 4 (19) | 2 (10) |
| Cough | 6 (24) | 0 | 2 (10) | 0 |
| Anemia | 5 (20) | 2 (8) | 3 (14) | 0 |
| Hot flush | 5 (20) | 0 | 0 | 0 |
| Decreased appetite | 4 (16) | 0 | 5 (24) | 0 |
| Dizziness | 4 (16) | 0 | 2 (10) | 0 |
| Insomnia | 4 (16) | 0 | 1 (5) | 0 |
| Peripheral edema | 4 (16) | 0 | 1 (5) | 0 |
| Upper respiratory tract infection | 4 (16) | 0 | 1 (5) | 0 |
| Musculoskeletal chest pain | 4 (16) | 0 | 3 (14) | 1 (5) |
| Vomiting | 4 (16) | 0 | 4 (19) | 1 (5) |
| Headache | 3 (12) | 0 | 4 (19) | 0 |
| Constipation | 2 (8) | 1 (4) | 5 (24) | 1 (5) |
| Flatulence | 4 (16) | 0 | 0 | 0 |
| Musculoskeletal pain | 2 (8) | 0 | 6 (29) | 1 (5) |
Based on National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0 reported in >15% of patients in either cohort.
Discussion
This is the first study to prospectively show the impact of prior AAP exposure on outcome, and underscores the limitations in relying solely on PSA response to assess treatment benefit. For AAP-naïve and AAP-exposed patients, the PSA response rates after 12 weeks on study following treatment with apalutamide were 88% (22 of 25) and 22% (4 of 18), and median time to PSA progression 18.5 and 3.8 months, respectively, suggesting that the drug was effective in the former and had limited efficacy for the latter. Arguably more important for the post-AAP group, however, was that forty-three percent (9 of 21) of patients were still on treatment for 6 months or longer (95% CI, 22–66), including five who had a PSA decline of >50%. The results show that simple reliance on the ≥50% decline in PSA as the “indicator of a favorable treatment effect” for a defined clinical cohort can underestimate the proportion of patients who may benefit. The recognized heterogeneity of mCRPC can result in progression in single lesions as opposed to systemic progression that is not always reflected by PSA response (16). It is for this reason that PCWG3 defined the outcome measure of “no longer clinically benefitting” to ensure that drugs from which patients are benefitting are not stopped prematurely (14).
Overall, the median duration of drug exposure in the AAP-naïve cohort (21 months) compared favorably with the median duration of exposure observed in those with chemotherapy-naïve mCRPC treated with other AR-targeted therapies such as abiraterone acetate (13.8 months) (8) and enzalutamide (16.6 months) in the first-line setting (3). Direct comparisons, however, are limited by the small sample size enrolled. The most common adverse events (primarily grade 1 or 2) were fatigue, diarrhea, nausea, and abdominal pain. Grade 3 and 4 adverse events were infrequent. Most adverse events of rash or pruritus were grade 1 and 2 and did not lead to dose modifications. One patient in the post-AAP cohort had a grade 3 erythematous rash that led to dose interruption.
Given the potential for seizures due to off-target effects of AR antagonists on GABAA receptors (1, 17, 18), safety was an important component of this study. In preclinical models, apalutamide demonstrated a promising profile, with relatively low brain penetration and a high therapeutic index. Importantly, no seizure activity was observed in this clinical study, which enrolled a total of ~100 patients (12). This not only suggests the potential to use apalutamide as a single agent, but supports development of future combination therapies in which the tolerability as well as the safety profile of a drug are important aspects to take into consideration when combining drugs.
Until 2010, docetaxel was the only approved treatment to show an overall survival benefit in mCRPC (19, 20). Now, several new therapeutic approaches have been shown to prolong life and are approved by the US Food and Drug Administration. (3–10). Given the rapid pace of drug development and drug approvals that continue to expand the range of treatment options available to patients with mCRPC, the questions of sequencing and testing combinatorial strategies have become paramount. Since the biologic factors contributing to intrinsic or early resistance to abiraterone acetate may differ from acquired resistance after an initial response and predict limited efficacy for subsequent treatment with AR-targeted agents (21), a minimum of 6 months of prior abiraterone acetate was an eligibility requirement in the post-AAP cohort, thereby ensuring that the patients had experienced prior therapeutic benefit to the first AR-targeted drug. The efficacy of apalutamide in patients who had shorter exposure times to AAP (3–6 months) remains an open question.
Although both groups had some degree of response to treatment, the different times on treatment for the two cohorts highlight the importance of characterizing patients based on prior exposure and response to prior therapy (14). Also unknown is whether outcomes would be better if combinations of agents that target different points on the AR-signaling axis, such as abiraterone acetate and apalutamide, were utilized, relative to sequencing the drugs as was done in this trial.
Overall, apalutamide was safe and well tolerated in patients with mCRPC. The safety profile is consistent with that of the phase I trial in mCRPC as well as the phase II data in nonmetastatic CRPC (12). Evidence of clinical benefit in patients with mCRPC was demonstrated in both AAP-naïve (80%) and post-AAP (43%) patients who remained on treatment for 6 months or longer. These results support further clinical development of apalutamide, which is ongoing across the spectrum of advanced prostate disease, including randomized phase III trials in nonmetastatic CRPC (NCT01946204) (22) and mCRPC (NCT02257736) (23).
Supplementary Material
Translational Relevance.
This study addressed the effect of activity of apalutamide in metastatic castration-resistant prostate cancer patients who were naïve to prior abiraterone plus prednisone (AAP) therapy as well as in patients who had received prior exposure to AAP. As expected, the apalutamide response was less in post-AAP versus AAP-naïve patients—22% versus 80% using a ≥50% prostate-specific antigen decline end point. Using newly defined Prostate Cancer Working Group 3–recommended swim plots, which illustrate time on treatment, the outcomes are radically different as the proportion of patients who remained on treatment for ≥6 months with apalutamide was 80% in the AAP-naïve cohort versus 43% in the post-AAP cohort. This represents a new standard that demonstrates the patient experience, useful for physicians when considering options to continue treatment until the patient is no longer clinically benefitting.
Acknowledgments
Funding/Support and Role of Funder/Sponsor:
This study was funded by Aragon Pharmaceuticals, Inc. Janssen Research & Development, LLC, is performing work on behalf of Aragon. This research was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The sponsor was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript.
Writing assistance was provided by Ira Mills, PhD, and Hajira Koeller, PhD, of PAREXEL, and was funded by Janssen Global Services, LLC. The current affiliation of Edna Chow Maneval is Ignyta, Inc., San Diego, CA.
Footnotes
Data Access, Responsibility, and Analysis:
Dana E. Rathkopf had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions:
Conception and Design: Dana E. Rathkopf, Edna Chow Maneval, Howard I. Scher
Collection and assembly of data: Dana E. Rathkopf, Emmanuel S. Antonarakis, Ronald F. Tutrone, Joshi J. Alumkal, Charles J. Ryan, Mansoor Saleh, Ralph J. Hauke, Edna Chow Maneval
Data analysis and interpretation: Dana E. Rathkopf, Emmanuel S. Antonarakis, Neal D. Shore, Joshi J. Alumkal, Charles J. Ryan, Mansoor Saleh, Ralph J. Hauke, Rajesh Bandekar, Edna Chow Maneval, Carla J. de Boer, Margaret K. Yu, Howard I. Scher
Provision of study material or patients: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Conflict of Interest Disclosures:
Dana E. Rathkopf reports a consultant or advisory role at Janssen Oncology and research funding from Janssen Oncology, Medivation, Celgene, Takeda, Millennium, Ferring, and Novartis; Emmanuel S. Antonarakis reports a consultant or advisory role at Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, and Astellas Pharma; honoraria from Sanofi, Dendreon, Medivation, Janssen Biotech, ESSA, and Astellas Pharma; research funding from Janssen Biotech, Johnson & Johnson, Sanofi, Dendreon, Aragon Pharmaceuticals, Exelixis, Millennium, Genentech, Novartis, Astellas Pharma, and Tokai Pharmaceuticals; and receipt of travel, accommodations, and expenses from Sanofi, Dendreon, and Medivation; Neal D. Shore reports a consultant or advisory role at Astellas Pharma, Bayer, Janssen Scientific Affairs, Dendreon, Sanofi, Takeda, Tolmar, and Ferring; Ronald F. Tutrone reports a consultant or advisory role at Medivation/Astellas; stock ownership in Nymox and Sophiris Bio Inc.; honoraria from Nymox; research funding from Nymox, Medivation/Astellas, Janssen Oncology, Sophiris Bio Inc., Bayer, American Medical Systems, Boston Scientific, Advaxis, MDxHealth, and Genomic Health; and has served on speakers’ bureaus for Medivation/Astellas and Dendreon; Joshi J. Alumkal reports institutional research funding from Aragon Pharmaceuticals, Janssen Oncology, Astellas Pharma, Millenium, Novartis, and Zenith Epigenetics; income for consulting with Astellas Pharma and for educational sessions with Bayer HealthCare Pharmaceuticals; Charles J. Ryan reports a consultant or advisory role at Bayer and Millennium; honoraria from Janssen Oncology and Astellas Pharma; and research funding from BIND Biosciences, Karyopharm Therapeutics, and Novartis; Mansoor Saleh reports no conflicts of interest; Ralph J. Hauke reports stock ownership in Aethlon; honoraria from Best Doctors, Inc; research funding from US Oncology, Bavarian Nordic, Bristol-Myers Squibb, Merck, Amgen; remuneration for an ABIM Subspecialty Board; and has a patent pending on an immunotherapeutic agent; Rajesh Bandekar reports employment or leadership position at Janssen Research & Development and stock ownership in Johnson & Johnson; Edna Chow Maneval reports employment or leadership position at Aragon Pharmaceuticals (past); Carla J. de Boer reports employment or leadership position at Janssen Biologics and stock ownership in Janssen Biologics; Margaret K. Yu reports employment or leadership position at Janssen Research & Development and stock ownership in Johnson & Johnson; Howard I. Scher reports institutional research funding from Astellas Pharma, BIND, Exelixis, Innocrin, Medivation, and Janssen Illumina; a consultant or advisory role (compensated) at Astellas Pharma, Sanofi, and WCG Oncology (uncompensated), Medivation, and Janssen; and receipt of travel, accommodations, and expenses from Astellas, Ferring, Medivation, and Sanofi.
References
- 1.Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24:251–7. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 6.Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de SP, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–25. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de SP, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 9.Sartor AO, Heinrich D, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno PJ, et al. Radium-223 chloride impact on skeletal-related events in patients with castration-resistant prostate cancer (CRPC) with bone metastases: a phase III randomized trial (ALSYMPCA) J Clin Oncol. 2012;30 (suppl 5; abstr 9) [Google Scholar]
- 10.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 11.Rathkopf DE, Morris MJ, Fox JJ, Danila DC, Slovin SF, Hager JH, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31:3525–30. doi: 10.1200/JCO.2013.50.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MR, Antonarakis ES, Ryan CJ, Berry WR, Shore ND, Liu G, et al. Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol. 2016;70:963–70. doi: 10.1016/j.eururo.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011;29:3695–704. doi: 10.1200/JCO.2011.35.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster WR, Car BD, Shi H, Levesque PC, Obermeier MT, Gan J, et al. Drug safety is a barrier to the discovery and development of new androgen receptor antagonists. Prostate. 2011;71:480–8. doi: 10.1002/pros.21263. [DOI] [PubMed] [Google Scholar]
- 18.Jung ME, Ouk S, Yoo D, Sawyers CL, Chen C, Tran C, et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC) J Med Chem. 2010;53:2779–96. doi: 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 20.Tannock IF, de WR, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 21.Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol. 2015;67:23–9. doi: 10.1016/j.eururo.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 22.Tombal B, Jassem J, Shreeve SM, Trudel GC, Sosa A, Kheoh T, et al. SPARTAN - a randomized double-blind, comparative study of ARN-509 plus androgen deprivation therapy (ADT) vs ADT alone in nonmetastatic castration-resistant prostate cancer (M0-CRPC) Ann Oncol. 2014;25(suppl 4):iv279. [Google Scholar]
- 23.Rathkopf D, Attard G, Efstathiou E, Yu M, Griffin T, Todd M, et al. A phase 3 randomized, placebo-controlled double-blind study of ARN-509 plus abiraterone acetate versus abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer. J Clin Oncol. 2015;33(suppl abstr):TPS5071. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.