Abstract
It is now well accepted that receptors can regulate cellular signaling pathways in the absence of a stimulating ligand, and inverse agonists can reduce this ligand-independent or “constitutive” receptor activity. Both the serotonin 5-HT2A and 5-HT2C receptors have demonstrated constitutive receptor activity in vitro and in vivo. Each has been identified as a target for the treatment of schizophrenia. Further, most, if not all, atypical antipsychotic drugs have inverse agonist properties at both 5-HT2A and 5-HT2C receptors. This paper describes our current knowledge of inverse agonism of atypical antipsychotics at 5-HT2A/2C receptor subtypes in vitro and in vivo. Exploiting inverse agonist properties of antipsychotic drugs may provide new avenues for drug development.
Keywords: Antipsychotic drugs, atypical antipsychotic drugs, inverse agonism, constitutive activity, serotonin, 5-HT2A receptors, 5-HT2C receptors, schizophrenia
SEROTONIN RECEPTORS
Serotonin (5-hydroxytryptamine, 5-HT) is a biogenic monoamine with paracrine, neurocrine, and hormonal functions (for reviews see [1–3]). These effects are mediated by a variety of serotonin receptors within seven families (5-HT1 to 5-HT7), which are further divided into multiple subtypes. The 5-HT2 receptor family is comprised of three subtypes: 5-HT2A, 5-HT2B, and 5-HT2C. Drugs with affinity for 5-HT2A/2C receptors have been used as treatments for disorders such as schizophrenia [4–6], depression [7, 8], and more recently insomnia (SR46349B and M100, 907, see clinicaltrials.gov) [9]. Importantly, evidence has suggested that the effects of these medications are mediated through inverse agonism at 5-HT2A and/or 5-HT2C receptors [6, 8, 10].
Of the seven types of 5-HT receptors, all are G-protein coupled receptors (GPCRs), except for the ion channel-associated 5-HT3 receptors. Within each subfamily, 5-HT receptors can share pharmacological and biochemical characteristics while remaining distinct from one another. For example, 5-HT2A and 5-HT2C receptors have a high degree of amino acid homology and can regulate similar cellular signaling pathways (reviewed in [11, 12]), but differences between the two receptors have been reported [13–14]. In terms of similarities, both are GPCRs that function through an association with the G protein, Gq/11, among other transducing molecules. When an agonist, such as 5-HT, binds to 5-HT2A or 5-HT2C receptors, it leads to the activation of phospholipases such as phospholipase C [PLC] and phospholipase A2 [PLA2] and increases in inositol trisphosphate and intracellular Ca2+ and the release of free arachidonic acid [15–19]. In addition to these agonist-elicited effects via 5-HT2 receptors, studies have shown that similarities in the cellular and behavioral effects are produced in response to inverse agonists at 5-HT2A and 5-HT2C receptors. Indeed, much of the evidence in favor of 5-HT2A and 5-HT2C receptor inverse agonism derives from work with atypical antipsychotics (reviewed below), which has led to the hypothesis that atypical antipsychotics alleviate symptoms of schizophrenia as a consequence of their inverse agonist properties at 5-HT2 receptors.
CONSTITUTIVE RECEPTOR ACTIVITY AND INVERSE AGONIST EFFICACY
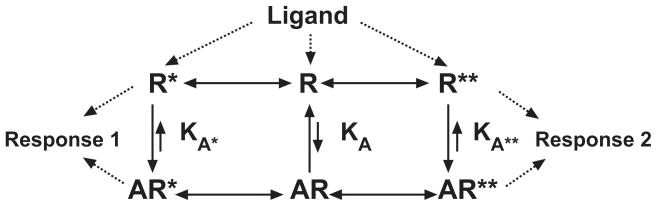
The discovery of inverse agonism was based on the pioneering work of Cerione and colleagues [20, 21] and further research by Costa and Herz [22]. These studies showed that in receptor systems, there is a spontaneous formation of active receptor conformations that produce measurable responses in the absence of a stimulating ligand (i.e., agonist), which is now referred to as constitutive or ligand-independent receptor activity. Ligands that were able to reduce this constitutive activity were defined as inverse agonists (for review see [23, 24].) It is now generally accepted that many receptor systems can be constitutively active [25]. Moreover, many ligands that were first characterized as receptor antagonists, including over 80% of the classical GPCR antagonists, exhibit inverse agonism [23, 24]. Although the early experiments of Cerione et al. and of Costa and Herz led to the development of the two-state model of receptor function, where receptors in a population were in equilibrium between an inactive and an active receptor conformation capable of eliciting a cellular response in the absence of a ligand, subsequent studies have resulted in the modification of the two-state model such that receptors can exist in more than one active conformation (multi-active state models). In these multi-active state models, such as the three-state model shown in Fig. 1, constitutive receptor activity, and inverse agonism, is dependent upon the response measured.
Fig. 1.
Three-state model of receptor function. Receptors can exist in an inactive conformation (R) or in multiple active conformations (two are shown: R* or R**). Active conformations can produce responses in the absence of a ligand (constitutive activity) or upon binding of a ligand (A). Agonists differentially stabilize an active conformation depending upon the value of the equilibrium dissociation constants KA* and KA** relative to the KA. Conversely, inverse agonists stabilize the inactive conformation of a receptor. For neutral antagonists, there is no receptor state selectivity and the value of the equilibrium dissociation constants KA* and KA** will be equal to that of KA. Berg et al. (2005) [36]**.
In the three-state model (as developed by Kenakin [23] and Leff [26]) and shown in Fig. 1, the receptor can exist in either an inactive conformation (R) or in one of two active conformations (R* or R**). These active forms can produce a response in the absence of an agonist (e.g. the constitutive activity), and the proportion of receptors in these states is determined by allosteric transition constants, L and M. Since L and M can differ, the magnitude of constitutive receptor activity can differ depending upon the response measured. When a ligand (A) is introduced, it binds to the receptor conformations according to the magnitude of the various affinity constants, KA, KA* and KA**. If the ligand has a higher affinity for one, or both, of the active conformations, it will enrich the proportion of the receptor population in that active conformation and thus increase the magnitude of response, acting as an agonist. If, on the other hand, the ligand has preferential affinity for the inactive conformation (R), it will enrich the population of inactive receptor, by depleting one or both of the active conformation. In this case, by depleting the quantity of an active receptor conformation, the response associated with that conformation will decrease and the ligand will be an inverse agonist for that response. In this model, ligand efficacy can be defined as the ratio of the affinity constant for the inactive receptor conformation (KA) to that of either of the active conformations (KA* or KA**). If the KA/KA* or KA/KA** ratio is less than one, the ligand will behave as an agonist (enriching the proportion of the active receptor conformation) for that response. On the other hand, if the KA/KA* or KA/KA** ratio is greater than 1, the ligand will behave as an inverse agonist for that response. The magnitude of efficacy (as an agonist or an inverse agonist) is based upon the magnitude of the difference in affinity constant. In this model of receptor function, drugs that are antagonists are relatively rare, as they must have equal affinity for all receptor conformations (KA/KA* and KA/KA** ratios of 1) and therefore do not alter the quantity of R* or R** and thus do not change the level of ongoing response associated with either receptor conformation.
As shown in Fig. 1, the three-state model of receptor function also takes into account the fact that a single receptor with different active receptor conformations can regulate more than one response pathway (represented as “Response 1” and “Response 2”). Thus, in receptor systems, there can be preferential activity toward a given response depending on the proportion of receptors in the corresponding active conformations. This would affect not only basal activity of the system (constitutive activity of response 1 greater than that of response 2) but also ligand-dependent activation or inactivation. Notably, the relative efficacy of ligands to regulate the multiple signaling pathways can differ. For example, a ligand could be an agonist for one response, but an inverse agonist for another. This phenomenon of response-dependent relative efficacy has been described using a number of terms including “stimulus-trafficking” “biased agonism” and “functional selectivity” (reviewed in [27]).
Functional selectivity is based upon ligands having differential efficacy for different signaling pathways coupled to a receptor in cells, therefore measurement of response-dependent ligand efficacy allows for assessment of functional selectivity properties of ligands. In certain circumstances where potency is influenced by efficacy, functional selectivity can be revealed as response-dependent potency differences. However, this situation occurs only for agonist ligands and only for those agonists for which there is a high efficiency of receptor-effector coupling (i.e. full agonists with “receptor reserve”). For weaker partial agonists, maximal response (Emax) reflects efficacy and therefore response-dependent Emax values reflect functional selectivity. For inverse agonists, “efficacy” is based upon their ability to promote receptor inactivation (e.g., to stabilize inactive conformations) and thereby reduce ligand-independent receptor-mediated signaling. Since potency of inverse agonists is not affected by efficacy, functional selectivity is reflected solely by response-dependent Emax values. Importantly, this allows for the use of relative efficacy comparison between signaling pathways using maximal responses as a measure of inverse agonist functional selectivity. In this manner, a recent study showed differences in the signaling profiles of two antipsychotic drugs (risperidone and paliperidone), which displayed inverse agonist properties for some (but not all) 5-HT2 receptor –mediated signaling pathways [28].
The degree of ligand-independent or constitutive receptor activity for a given receptor-effector pathway can most easily be measured as the magnitude of the reduction of basal effector activity produced by a full inverse agonist. The initial methods to detect inverse agonism in GPCR systems incorporated measures of reduction in basal [35S]GTPγS activity to assess constitutive receptor activity toward G protein activation [29–31], as well as various functional assays of changes in downstream basal effector signaling (e.g., PLC) [32]. Using these techniques, ligands could be classified as receptor agonists, inverse agonists, or neutral antagonists, for the measured pathway. As described above, agonists and inverse agonists are ligands that either increase or decrease basal effector responses, respectively, whereas antagonists are ligands that, on their own, do not alter the basal response, but block the effects of either an agonist or an inverse agonist. Measurements of inverse agonist-mediated reduction in basal effector activity depend on the level of constitutive activity of the system and thus are infl-uenced directly by the level of receptor expression and efficiency of receptor-effector coupling [33–35]. To observe inverse agonism, it is necessary that the receptor system produces a measurable basal effector response and therefore is not useful in systems with little to no basal activity. Given this, the majority of the foundational inverse agonist research has involved studies using transfected or mutated cells with a high expression of receptors to produce a high basal activity in vitro to allow visualization of inverse agonist properties of ligands. Using this approach, 5-HT2C receptors have been shown to have constitutive activity in transfected cell lines, but it has proven more difficult to show constitutive receptor activity in native tissue or in a behavioral assay (for review of 5-HT2 receptor inverse agonism see [10] and [36]). Interestingly, the reverse is true for detecting constitutive receptor activity of 5-HT2A receptors as inverse agonism at 5-HT2A receptors has been more readily detected in vivo [37]. Importantly, as basal activity is not as pronounced in some receptor systems, a particularly sensitive method for detecting constitutive receptor activity and inverse agonist efficacy is by determining the effects of prolonged inverse agonist treatment on a given receptor-effector response.
Similarly to agonist stimulation, prolonged ligand-independent receptor activity can lead to a reduction of effector activity. Thus receptor signaling systems can exist in a state of constitutive, partial desensitization as a result ligand-independent receptor activity toward desensitization mechanisms. Prolonged (e.g. >4 h) treatment with an inverse agonist can promote re-sensitization of the receptor-effector response that may be visualized by enhanced responsiveness to agonist stimulation following washout of the inverse agonist. This second and very sensitive method for detection of inverse agonism has been used to study inverse agonist functional selectivity at 5-HT2 receptors [28, 32, 36, 38].
5-HT2 RECEPTOR CONSTITUTIVE ACTIVITY AND INVERSE AGONISM
In Vitro studies
In vitro heterologous expression systems have proven instrumental for the study of inverse agonism and constitutive activity for a variety of receptors including 5-HT2 receptors. Moreover, they have shown that measures of inverse agonist efficacy and constitutive receptor activity are not only dependent on the signaling pathway but also on the cell background in which the receptors are expressed. Multiple researchers have reported agonist-independent receptor activity toward PLC activity for 5-HT2C receptors [32, 39–43] as well as toward PLA2 [19, 28, 32, 36]. The high degree of 5-HT2C constitutive receptor activity toward PLC has provided a system for further characterization of functional selectivity and constitutive desensitization of the 5-HT2C receptor system. As mentioned above, inverse agonists have the ability to reduce constitutive desensitization. Berg and colleagues [32] showed that in CHO cells expressing 5-HT2C receptors, prolonged treatment with 5-HT2C inverse agonists differentially increased receptor responsiveness for PLC, but not PLA2 which indicates that constitutive receptor activities toward desensitization pathways can also differ, depending upon the response measured. Further, these studies were the first to show that the 5-HT2C receptor does not exhibit a high degree of constitutive activation for all downstream pathways. For example, the constitutive 5-HT2C receptor activity for the PLA2 pathway is much less than that of PLC [32], subsequently, inverse agonist efficacy at the 5-HT2C receptor is also greater for PLC compared to PLA2 responses [32, 36].
As mentioned above, constitutive activity of the 5-HT2A receptors toward PLC activity has not been readily detected unless the receptor was mutated [44–46] or measured in systems with overexpression of associated G proteins [6]. Although constitutive 5-HT2A receptor activity for PLC is weak, higher constitutive activity has been reported for a reporter gene assay (Receptor Selection and Amplification Technology (R-SAT)) [6, 45] which suggests that, like the 5-HT2C receptor, constitutive activity of 5-HT2A receptors also differs with the signaling pathway studied.
In Vivo Studies
Among the first supporting evidence for 5-HT2 receptor inverse agonism in vivo came from studies demonstrating the varying effects of 5-HT2A receptor agonists, neutral antagonists, and inverse agonists on learning (for review see [37]). These drugs could be classified on the basis of whether they enhanced (agonists), had no effect (antagonists), or inhibited (inverse agonists) conditioned responses in the rabbit eyeblink model. The results of these experiments confirmed that native (non-mutated) 5-HT2A receptors were indeed constitutively active and identified previously well-characterized antagonists as inverse agonists, including ritanserin, MDL11939 and M100907. Moreover, these studies established the rabbit eyeblink model as a tool for monitoring inverse agonism at a systems level. Further studies by the Harvey group characterized the effects of chronic inverse agonist treatment on 5-HT2A receptor density [47, 48]. When rabbits were repeatedly administered the inverse agonists MDL11939 and M100, 907, there was a resultant increase in 5-HT2A receptor expression as measured by radioligand binding [47, 48]. Moreover, there were corresponding behavioral effects as a consequence of the receptor up-regulation. Following repeated treatment with the inverse agonists, there was an increase in the rate of learning response [47].
Additional evidence for effects of 5-HT2 receptor inverse agonism in vivo involves the regulation of dopamine release. Both 5-HT2A and 5-HT2C receptors have been shown to modulate dopamine release in the brain [49–53]. For example, the well-characterized 5-HT2C receptor inverse agonist, SB206553, increased dopamine levels in the nucleus accumbens that was blocked by the neutral antagonist, SB 242084 [53]. Similarly, the 5-HT2A receptor inverse agonists, M100907 and SR46349B, have been shown to increase the release of dopamine in mesolimbic and mesocortical brain regions [51–52, 54–56]. Moreover, inverse agonist action at 5-HT2C and 5-HT2A receptors may converge downstream at the level of dopamine release. In studies with atypical antipsychotics, mesocortical dopamine release was enhanced [57]. Given that most if not all atypical antipsychotics have inverse agonist properties at 5-HT2C and 5-HT2A receptors (see discussion below), it has been proposed that inverse agonist activity at both these receptors contribute to alleviate the negative symptoms and cognitive deficits observed in schizophrenia [58].
SCHIZOPHRENIA AND 5-HT2 RECEPTORS
First Generation Antipsychotics
Schizophrenia is a severe mental disorder characterized by delusions, hallucinations, disorganized speech, cognitive deficits and affective symptoms. Although schizophrenia was first described over a century ago, in the intervening years, treatments for schizophrenia still have not progressed to a point where all symptoms are controlled. Furthermore, despite the number of treatment options available, schizophrenia remains one of the leading causes of disability in the world [59]. Schizophrenia is defined by indications that can be divided into four groups: positive, negative, cognitive, and mood-related. Positive and negative symptoms are described based on the idea that “positive” refers to extra behaviors observed in the patient population whereas “negative” refers to behaviors or emotions that are “missing.” Positive symptoms are among those most commonly associated with schizophrenia and can include hallucinations, delusions, and paranoia. Negative symptoms include flat affect, ambivalence, social withdraw, and anhedonia. The cognitive effects generally involve deficits in both learning and memory. Finally, mood-related symptoms include anxiety, depression, agitation, and/or suicidality. It is important that even with this diverse symptomatology, all antipsychotics currently on the market mainly target the psychosis associated with schizophrenia [60].
Hallucinations and delusions are only two symptoms of schizophrenia, but they are representative of the characteristic change in perception exhibited in patients. The first drugs approved to treat schizophrenia and diminish psychosis were traditionally dopamine antagonists. Of these “typical” or “first generation” antipsychotics, haloperidol was the most efficacious. Unfortunately, there were significant problems associated with the use of haloperidol, such as cardiac events, tardive dyskinesia or extrapyramidal side effects including tremor, akathisia (inability to sit still), dystonia (twisting or repetitive motions), and slurred speech (for review see [61]). As these drugs were dopamine D2 receptor antagonists, it was thought that their actions at D2 receptors contributed to both the benefits and adverse motor effects. Subsequently, because of the adverse effects associated with typical antipsychotics, second-generation or “atypical” antipsychotics were developed.
Second Generation Antipsychotics
The second generation of antipsychotics were found to have lower risk of extrapyramidal side effects and tardive dyskinesias and thus are referred to as “atypical”. Although antagonism or weak efficacy at dopamine D2 receptors appears essential for antipsychotic activity, the complete molecular mechanism that underlies the therapeutic efficacy of atypical antipsychotic drugs is still unknown. Several theories have been postulated to account for atypicality, including 1) higher affinity for 5-HT2A receptors than for dopamine D2 receptors (i.e., the Meltzer hypothesis), 2) faster off-rate of binding to D2 receptors, and 3) agonism at presynaptic versus antagonism at post-synaptic D2 receptors (for example aripiprazole) [62]. Although these drugs produce fewer extrapyramidal side effects, some, if not most, were found to exhibit significant metabolic side-effects including weight gain, hyperglycemia, dyslipidemia [63], and in the case of clozapine, an increased risk of agranulocytosis (reviewed in [61]). Since in addition to binding D2 and 5-HT2 receptors, these drugs bind to multiple receptor subtypes, including alpha 1 adrenergic and H1 histamine receptors, it is possible that some adverse effects are a result of “off-target” signaling of these receptor systems [64].
Atypical antipsychotics used to treat schizophrenia have been shown to increase the release of dopamine in the prefrontal cortex, and 5-HT2A, 5-HT2C, and 5-HT1A receptors have been shown to mediate this effect [65]. Furthermore, clozapine, olanzapine, quetiapine (see Table 1), and other atypical antipsychotics have high affinities not only for 5-HT2A, but also 5-HT2C receptors [66]. Thus, an alternative approach may be to indirectly modulate dopamine levels through 5-HT2A/2C receptor inverse agonism (discussed below) that may effectively manage the positive symptoms of schizophrenia leading to a more effective therapeutic strategy.
Table 1.
Atypical Antipsychotic Drugs.
| Generic Name | Trade Name | FDA Approval (Year) | 5-HT2A/5-HT 2c Inverse Agonism |
|---|---|---|---|
| Clozapine | Clozaril | 1990 | [32, 43] |
| Risperidone | Risperdal | 1993 | [28] |
| Olanzapine | Zyprexa | 1996 | [43, 69] |
| Quetiapine | Seroquel | 1997 | [89]** |
| Ziprasidone | Geodon | 2001 | [43] |
| Aripiprazole | Abilify | 2002 | [69] |
| Paliperidone | Invega | 2006 | [28] |
| Asenapine | Saphris | 2009 | NR |
| Iloperidone | Fanapt | 2009 | NR |
Sources: National Institute of Mental Health (NIMH), Food and Drug Administration (FDA). Arranged in order of approval date.
quetiapine is a dopamine D2 receptor inverse agonist [89]. NR= not reported.
ATYPICAL ANTIPSYCHOTICS AS 5-HT2 RECEPTOR INVERSE AGONISTS
As described above, many studies have shown inverse agonism for 5-HT2 receptor ligands. Once discovered, researchers were interested in examining whether atypical antipsychotics, which had known binding affinity for 5-HT2 receptors, also had inverse agonist effects at those receptors. Clozapine was the first FDA approved atypical antipsychotic that was distinct from the typical antipsychotics in that it had high affinity for 5-HT2 receptors and was classified as an antagonist at these receptors. Upon further investigation, Westphal and Sanders-Bush [67] were among the first to present evidence of functional inverse agonism at the 5-HT2C with clozapine in a heterologous system (NIH/3T3 fibroblasts). They demonstrated that as an inverse agonist, clozapine had functional effects and receptor binding characteristics that were opposite that of 5-HT2 agonists. For example, clozapine, as an inverse agonist, bound the inactive (uncoupled) form of 5-HT2C with a high affinity; by contrast, agonists had a higher affinity for the active (G-protein coupled) form of the 5-HT2C receptor. Additional studies that examined the effects of the atypical antipsychotics clozapine, olanzapine, and risperidone also found that they had inverse agonist properties at 5-HT2C receptors in vitro. Interestingly, all atypical antipsychotic drugs tested displayed inverse agonist activity at 5-HT2C receptors, whereas almost all of the typical antipsychotics only displayed antagonist properties in this system [43]. Inverse agonism of clozapine in vivo has also been reported using microdialysis techniques in a manner similar to the initial studies that described 5-HT2 inverse agonism. Specifically, clozapine increased dopamine release in the nucleus accumbens and striatum, which indicates that clozapine has inverse agonist activity at the 5-HT2C receptor in vivo [68]. Combined, these data lead to the hypothesis that 5-HT2C inverse agonism may play a role in the therapeutic effects of atypical antipsychotics.
As mentioned above, olanzapine, an atypical antipsychotic approved in 1996 (six years after clozapine), has also been shown to have inverse agonist activity at 5-HT2 receptors. Again, these studies utilized a heterologous system of cells expressing a human isoform of 5-HT2 receptors. In CHO cells, Zhang and colleagues [69] report that olanzapine exhibited inverse agonism at the INI isoform of the 5-HT2C receptor as defined by a decrease in 5-HT2C receptor-mediated calcium signaling. However, conflicting results have been reported when the 5-HT2C receptor is expressed in a different cell background (HEK-293 cells). Rauser and colleagues [70] found that several drugs without antipsychotic properties and many typical antipsychotics were inverse agonists at the human 5-HT2C receptors. These variations across experimental approaches confirm that observations of inverse agonism can be dependent on both the signaling pathway measured and the cell phenotype in which the receptors are expressed.
Clozapine was also reported to be an inverse agonist at the 5-HT2A receptor [44, 71]. These studies utilized mutated 5-HT2A receptors and measured stimulation of the PLC pathway. Certain mutations produced 5-HT2A receptors with a high degree of constitutive receptor activity. The antipsychotic clozapine was able to decrease this basal response indicating its inverse agonist properties at the 5-HT2A receptor. The atypical antipsychotic, risperidone, has also been characterized as a 5-HT2A receptor inverse agonist. Similar to clozapine, in vitro risperidone produced a significant reduction in basal PLC activity in a system with a high level of constitutively active 5-HT2A receptors [44].
Despite having similar inverse agonist properties at 5-HT2 receptors, the atypical antipsychotics also produce distinct cellular signaling profiles. As discussed above, inverse agonists can also display functional selectivity. Interestingly, differences between atypical antipsychotics have been recently described when comparing risperidone and paliperidone [28]. Risperidone is an atypical antipsychotic approved for use in the clinic in 1993. Paliperidone (approved in 2006) is the major active metabolite of risperidone, differing by only a single hydroxyl group. Both paliperidone and risperidone display simple, competitive antagonism in radioligand binding assays with similar affinities for their target receptors [72–74]; however, there have been some reports of differences in therapeutic effects between the two drugs [75–77], In a variety of heterologous systems expressing 5-HT2A or 5-HT2C receptors, it was determined that there were distinct differences in the efficacy of these two drugs for a number of signaling responses [28]. Since the two drugs have different therapeutic effects, differences at the signaling level could have important implications for variations observed in the clinic. Further, these results are consistent with the idea that inverse agonist properties at 5-HT2 receptors may contribute to therapeutic efficacy of atypical antipsychotics.
More recently, a potent 5-HT2 receptor inverse agonist, pimavanserin (ACP-103) was developed as a lead compound for a new avenue of potential antipsychotic treatments. In 2006, in vitro studies utilizing a heterologous system expressing human 5-HT2 receptors first described inverse agonism of pimavanserin at both 5-HT2A and 5-HT2C receptors [78]. In behavioral studies, pimavanserin also reduced 5-HT2-mediated behaviors suggesting it could be acting as either an antagonist or inverse agonist at 5-HT2 receptors [78]. Using the R-SAT technique, the researchers were able to observe inverse agonism of basal 5-HT2 responses and they concluded that the in vivo and in vitro effects were consistent with 5-HT2 inverse agonism as a mechanism for antipsychotic-like efficacy [78]. Subsequent studies confirmed the effects of pimavanserin and reported a better side-effect profile in animal models predictive of antipsychotic activity [79–82] suggesting that inverse agonists targeting 5-HT2 receptors may have improved antipsychotic efficacy and tolerability. However, although pimavanserin is reported to enhance the efficacy and tolerability of the atypical antipsychotic, risperidone, but not the typical antipsychotic, haloperidol [83], clinical trials conducted with pimavanserin monotherapy have been disappointing. By contrast, recent studies suggest that pimavanserin may be effective for treating the secondary psychosis associated with Parkinson’s disease which is thought to be due to 5-HT2A receptor activity [86, 87]. Currently, pimavanserin is in clinical trials for treatment of psychosis associated with Parkinson’s disease [88 and see www.clinicaltrials.gov].
In addition to inverse agonism, it has been suggested that 5-HT2C receptor agonists may be effective antipsychotics for treatment of schizophrenia and other psychiatric disorders [90]. For example, the preclinical profile of the selective 5-HT2C receptor agonist, vabicaserin, indicated antipsychotic-like efficacy [91]. However, results of a Phase II trial with vabicaserin in comparison to olanzapine and placebo indicated that although the PANSS (positive and negative symptom scale) total scores for vabicaserin were improved at the low dose (200 mg) but not the higher dose (400 mg), the improvement was much lower than that of olanzapine. Further, the overall site ratings were not suggestive of clinical efficacy for vabicaserin [92]. Interestingly, using an updated quantitative systems pharmacology model to predict steady state clinical efficacy of vabicaserin as monotherapy, Liu et al, [92] reported that vabicaserin had limited clinical benefit for treatment of schizophrenia, consistent with the results of the Phase II clinical trial.
SUMMARY AND CONCLUSION
In summary, the potential for development of 5-HT2 receptor inverse agonists as either front-line approaches for the treatment of psychosis or as adjuvant therapies for schizophrenia has been documented. However, although evidence suggests that therapeutic efficacy may be due to an inverse agonist property of a given ligand, in general, evidence supporting therapeutic relevance of inverse agonism is lacking in clinical settings (for review [84]) and the relevance of inverse agonist properties of atypical antipsychotics needs to be addressed further. Based on the lack of clinical efficacy for treatment of schizophrenia by selective 5-HT2A receptor inverse agonists (e.g., pimavanserin) and the selective 5-HT2C receptor agonist, vabicaserin, its intriguing to speculate that ligands that are selective for a single receptor subtype regardless of the drug property (i.e., agonist or inverse agonist) may not be therapeutically effective for treatment of diseases with multiple etiologies such as schizophrenia. Interestingly, applications of 5-HT2 receptor inverse agonists have recently extended beyond schizophrenia. For example, in addition to psychosis associated with Parkinson’s disease [86], 5-HT2 receptor inverse agonists are also being evaluated as treatments for psychosis related to Alzheimer’s disease [85] and for treatment of movement disorders related to Parkinson’s disease [87]. Overall, a better understanding of inverse agonism at 5-HT2 receptor systems could have far-reaching implications for the development of novel therapeutics.
Acknowledgments
The authors wish to acknowledge support from Janssen Scientific Affairs, LLC and the National Institutes of Health, USPHS grant T32DA031115.
LIST OF ABBREVIATIONS
- 5-HT2A
serotonin 2A receptor subtype
- 5-HT2C
serotonin 2C receptor subtype
- G protein
guanine nucleotide binding protein
- PLA2
phospholipase A2
- PLC
phospholipase C
Footnotes
Reprinted from Trends Pharmacol Sci vol 26, pg 625-30; Berg KA, Harvey KA, Spampinato U, Clarke WP. “Physiological relevance of constitutive activity of 5-HT2A and 5-HT2C receptors” with permission from Elsevier.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71(4):533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 2.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44(3):151–62. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 3.Van Oekelen D, Luyten WH, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72(22):2429–49. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- 4.Di Pietro NC, Seamans JK. Dopamine and serotonin interactions in the prefrontal cortex: insights on antipsychotic drugs and their mechanism of action. Pharmacopsychiatry. 2007;40(Suppl 1):S27–33. doi: 10.1055/s-2007-992133. [DOI] [PubMed] [Google Scholar]
- 5.Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology. 2007;32(8):1715–26. doi: 10.1038/sj.npp.1301305. [DOI] [PubMed] [Google Scholar]
- 6.Weiner DM, Burstein ES, Nash N, et al. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299(1):268–76. [PubMed] [Google Scholar]
- 7.Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology. 2003;28(2):402–12. doi: 10.1038/sj.npp.1300057. [DOI] [PubMed] [Google Scholar]
- 8.Berg KA, Harvey JA, Spampinato U, Clarke WP. Physiological and therapeutic relevance of constitutive activity of 5-HT 2A and 5-HT 2C receptors for the treatment of depression. Prog Brain Res. 2008;172:287–305. doi: 10.1016/S0079-6123(08)00914-X. [DOI] [PubMed] [Google Scholar]
- 9.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. 2011;15(4):269–81. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther. 2009;121(2):160–73. doi: 10.1016/j.pharmthera.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108(5):1614–41. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 12.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 13.Berg KA, Stout BD, Maayani S, Clarke WP. Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytrypta-mine2C receptor-mediated phospholipase C activation. Pharmacol Exp Ther. 2001;299(2):593–602. [PubMed] [Google Scholar]
- 14.Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharmacol. 1994;46(3):477–84. [PubMed] [Google Scholar]
- 15.Raymond JR, Mukhin YV, Gelasco A, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92(2–3):179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 16.Noda M, Higashida H, Aoki S, Wada K. Multiple signal transduction pathways mediated by 5-HT receptors. Mol Neurobiol. 2004;29(1):31–9. doi: 10.1385/MN:29:1:31. [DOI] [PubMed] [Google Scholar]
- 17.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195(1):198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71(4):533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 19.Berg KA, Maayani S, Goldfarb J, Clarke WP. Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann N Y Acad Sci. 1998;861:104–10. doi: 10.1111/j.1749-6632.1998.tb10180.x. [DOI] [PubMed] [Google Scholar]
- 20.Cerione RA, Sibley DR, Codina J, et al. Reconstitution of a hormone-sensitive adenylate cyclase system. The pure beta-adrenergic receptor and guanine nucleotide regulatory protein confer hormone responsiveness on the resolved catalytic unit. J Biol Chem. 1984;259(16):9979–82. [PubMed] [Google Scholar]
- 21.Cerione RA, Codina J, Benovic JL, Lefkowitz RJ, Birnbaumer L, Caron MG. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984;23(20):4519–25. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- 22.Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci. 1989;86(19):7321–5. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenakin T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15(3):598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 24.Kenakin T. Efficacy as a vector: the relative prevalence and paucity of inverse agonism. Mol Pharmacol. 2004;65(1):2–11. doi: 10.1124/mol.65.1.2. [DOI] [PubMed] [Google Scholar]
- 25.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366(5):381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 26.Leff P, Scaramellini C, Law C, McKechnie K. A three-state receptor model of agonist action. Trends Pharmacol Sci. 1997;18(10):355–62. doi: 10.1016/s0165-6147(97)01105-x. [DOI] [PubMed] [Google Scholar]
- 27.Urban JD, Clarke WP, von Zastrow M, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 28.Clarke WP, Chavera TA, Silva M, Sullivan LC, Berg KA. Signalling profile differences: paliperidone versus risperidone. Br J Pharmacol. 2013;170(3):532–45. doi: 10.1111/bph.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian WN, Duzic E, Lanier SM, Deth RC. Determinants of alpha 2-adrenergic receptor activation of G proteins: evidence for a precoupled receptor/G protein state. Mol Pharmacol. 1994;45:524–31. [PubMed] [Google Scholar]
- 30.McLoughlin DJ, Strange PG. Mechanisms of agonism and inverse agonism at serotonin 5-HT1A receptors. J Neurochem. 2000;74:347–57. doi: 10.1046/j.1471-4159.2000.0740347.x. [DOI] [PubMed] [Google Scholar]
- 31.Bouaboula M, Perrachon S, Milligan L, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–9. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 32.Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP. Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol. 1999;55(5):863–72. [PubMed] [Google Scholar]
- 33.Black JW, Leff P, Shankley NP, Wood J. An operational model of pharmacological agonism: the effect of E/[A] curve shape on agonist dissociation constant estimation. Br J Pharmacol. 1985;84(2):561–71. doi: 10.1111/j.1476-5381.1985.tb12941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyer D, Boddeke HW. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol Sci. 1993;14(7):270–5. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- 35.Kenakin T. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol Rev. 1996;48(3):413–63. [PubMed] [Google Scholar]
- 36.Berg KA, Harvey JA, Spampinato U, Clarke WP. Physiological relevance of constitutive activity of 5-HT2A and 5-HT2C receptors. Trends Pharmacol Sci. 2005;26(12):625–30. doi: 10.1016/j.tips.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Harvey JA. Role of the serotonin 5-HT(2A) receptor in learning. Learn Mem. 2003;10(5):355–62. doi: 10.1101/lm.60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilbanks AM, Laporte SA, Bohn LM, Barak LS, Caron MG. Apparent loss-of-function mutant GPCRs revealed as constitutively desensitized receptors. Biochemistry. 2002;41(40):11981–9. doi: 10.1021/bi020275m. [DOI] [PubMed] [Google Scholar]
- 39.Barker EL, Westphal RS, Schmidt D, Sanders-Bush E. Constitutively active 5-hydroxytryptamine2C receptors reveal novel inverse agonist activity of receptor ligands. J Biol Chem. 1994;269(16):11687–90. [PubMed] [Google Scholar]
- 40.Herrick-Davis K, Grinde E, Niswender CM. Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J Neurochem. 1999;73(4):1711–7. doi: 10.1046/j.1471-4159.1999.731711.x. [DOI] [PubMed] [Google Scholar]
- 41.Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem. 1999;274(14):9472–8. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- 42.Westphal RS, Backstrom JR, Sanders-Bush E. Increased basal phosphorylation of the constitutively active serotonin 2C receptor accompanies agonist-mediated desensitization. Mol Pharmacol. 1995;48(2):200–5. [PubMed] [Google Scholar]
- 43.Herrick-Davis K, Grinde E, Teitler M. Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther. 2000;295(1):226–32. [PubMed] [Google Scholar]
- 44.Egan C, Herrick-Davis K, Teitler M. Creation of a constitutively activated state of the 5-HT2A receptor by site-directed mutagenesis: revelation of inverse agonist activity of antagonists. Ann N Y Acad Sci. 1998;861:136–9. doi: 10.1111/j.1749-6632.1998.tb10184.x. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro DA, Kristiansen K, Weiner DM, Kroeze WK, Roth BL. Evidence for a model of agonist-induced activation of 5-hydroxytryptamine 2A serotonin receptors that involves the disruption of a strong ionic interaction between helices 3 and 6. J Biol Chem. 2002;277(13):11441–9. doi: 10.1074/jbc.M111675200. [DOI] [PubMed] [Google Scholar]
- 46.Teitler M, Herrick-Davis K, Purohit A. Constitutive activity of G-protein coupled receptors: emphasis on serotonin receptors. Curr Top Med Chem. 2002;2(6):529–38. doi: 10.2174/1568026023393859. [DOI] [PubMed] [Google Scholar]
- 47.Aloyo VJ, Dave KD, Rahman T, Harvey JA. Selective and divergent regulation of cortical 5-HT(2A) receptors in rabbit. J Pharmacol Exp Ther. 2001;299(3):1066–72. [PubMed] [Google Scholar]
- 48.Dave KD, Harvey JA, Aloyo VJ. The time-course for up- and down-regulation of the cortical 5-hydroxytryptamine (5-HT)2A receptor density predicts 5-HT2A receptor-mediated behavior in the rabbit. J Pharmacol Exp Ther. 2007;323(1):327–35. doi: 10.1124/jpet.107.121707. [DOI] [PubMed] [Google Scholar]
- 49.Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37(7):953–5. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 50.Di Giovanni G, De Deurwaerdére P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U. Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience. 1999;91(2):587–97. doi: 10.1016/s0306-4522(98)00655-1. [DOI] [PubMed] [Google Scholar]
- 51.Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38(8):1195–205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 52.Gobert A, Rivet JM, Lejeune F, et al. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36(3):205–21. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 53.De Deurwaerdère P, Navailles S, Berg KA, Clarke WP, Spampinato U. Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci. 2004;24(13):3235–41. doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt CJ, Fadayel GM. The selective 5-HT2A receptor antagonist, MDL 100, 907, increases dopamine efflux in the prefrontal cortex of the rat. Eur J Pharmacol. 1995;273(3):273–9. doi: 10.1016/0014-2999(94)00698-7. [DOI] [PubMed] [Google Scholar]
- 55.Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31(2):265–77. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- 56.Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog Brain Res. 2008;172:7–44. doi: 10.1016/S0079-6123(08)00902-3. [DOI] [PubMed] [Google Scholar]
- 58.Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog Brain Res. 2008;172:177–97. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- 59.Theodoridou A, Rössler W. Disease burden and disability-adjusted life years due to schizophrenia and psychotic disorders. In: Preedy VR, Watson RR, editors. Handbook of Disease Burdens and Quality of Life Measures. New York: Springer Science and Business Media; 2010. pp. 1493–1507. Pt. 2, 2.6. [Google Scholar]
- 60.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10(1):79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 61.Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81(5):617–22. [PubMed] [Google Scholar]
- 62.MacDonald GJ, Bartolomé JM. A decade of progress in the discovery and development of ‘atypical’ antipsychotics. Prog Med Chem. 2010;49:37–80. doi: 10.1016/S0079-6468(10)49002-5. [DOI] [PubMed] [Google Scholar]
- 63.Shirzadi AA, Ghaemi SN. Side effects of atypical antipsychotics: extrapyramidal symptoms and the metabolic syndrome. Harv Rev Psychiatry. 2006;14(3):152–64. doi: 10.1080/10673220600748486. [DOI] [PubMed] [Google Scholar]
- 64.Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13(1):27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- 65.Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76(5):1521–31. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 66.Cussac D, Newman-Tancredi A, Nicolas JP, Boutin JA, Millan MJ. Antagonist properties of the novel antipsychotic, S16924, at cloned, human serotonin 5-HT2C receptors: a parallel phosphatidylinositol and calcium accumulation comparison with clozapine and haloperidol. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(5):549–54. doi: 10.1007/s002100000221. [DOI] [PubMed] [Google Scholar]
- 67.Westphal RS, Sanders-Bush E. Reciprocal binding properties of 5-hydroxytryptamine type 2C receptor agonists and inverse agonists. Mol Pharmacol. 1994;46(5):937–42. [PubMed] [Google Scholar]
- 68.Navailles S, De Deurwaerdère P, Spampinato U. Clozapine and haloperidol differentially alter the constitutive activity of central serotonin2C receptors in vivo. Biol Psychiatry. 2006;59(6):568–75. doi: 10.1016/j.biopsych.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 69.Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J. Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms. Biochem Pharmacol. 2006;71(4):521–9. doi: 10.1016/j.bcp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Rauser L, Savage JE, Meltzer HY, Roth BL. Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxy-tryptamine(2C) receptor. J Pharmacol Exp Ther. 2001;299(1):83–9. [PubMed] [Google Scholar]
- 71.Vanover KE, Harvey SC, Son T, et al. Pharmacological characterization of AC-90179 [2-(4-methoxyphenyl)-N-(4-methyl-benzyl)-N-(1-methyl-piperidin-4-yl)-acetamide hydrochloride]: a selective serotonin 2A receptor inverse agonist. J Pharmacol Exp Ther. 2004;310(3):943–51. doi: 10.1124/jpet.104.066688. [DOI] [PubMed] [Google Scholar]
- 72.Dolder C, Nelson M, Deyo Z. Paliperidone for schizophrenia. Am J Health Syst Pharm. 2008;65(5):403–13. doi: 10.2146/ajhp070261. [DOI] [PubMed] [Google Scholar]
- 73.Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33(5):1100–19. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schotte A, Janssen P, Gommeren W, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124(1–2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 75.Turkoz I, Bossie C, Lindenmayer J, Scooler N, Canuso C. Paliperidone ER and oral risperidone in patients with schizophrenia: a compoarative database analysis. BMC Psychiatry. 2011;11:21. doi: 10.1186/1471-244X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;35:218–26. doi: 10.1016/j.pnpbp.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 77.de Leon J, Wynn G, Sandson NB. The pharmacokinetics of paliperidone versus risperidone. Psychosomatics. 2010;51(1):80–8. doi: 10.1176/appi.psy.51.1.80. [DOI] [PubMed] [Google Scholar]
- 78.Vanover KE, Weiner DM, Makhay M, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperi-din-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R, 3R)-dihydroxybutanedioate (2: 1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2006;317(2):910–18. doi: 10.1124/jpet.105.097006. [DOI] [PubMed] [Google Scholar]
- 79.Snigdha S, Horiguchi M, Huang M, et al. Attenuation of phencyclidine-induced object recognition deficits by the combination of atypical antipsychotic drugs and pimavanserin (ACP 103), a 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2010;332(2):622–31. doi: 10.1124/jpet.109.156349. [DOI] [PubMed] [Google Scholar]
- 80.Gardell LR, Vanover KE, Pounds L, et al. ACP-103, a 5-hydroxytryptamine 2A receptor inverse agonist, improves the antipsychotic efficacy and side-effect profile of haloperidol and risperidone in experimental models. J Pharmacol Exp Ther. 2007;322(2):862–70. doi: 10.1124/jpet.107.121715. [DOI] [PubMed] [Google Scholar]
- 81.Abbas A, Roth BL. Pimavanserin tartrate: a 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opin Pharmacother. 2008;9(18):3251–9. doi: 10.1517/14656560802532707. [DOI] [PubMed] [Google Scholar]
- 82.Roberts C. ACP-103, a 5-HT2A receptor inverse agonist. Curr Opin Investig Drugs. 2006;7(7):653–60. [PubMed] [Google Scholar]
- 83.Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2–3):144–52. doi: 10.1016/j.schres.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 84.Parra S, Bond RA. Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant? Curr Opin Pharmacol. 2007;7(2):146–50. doi: 10.1016/j.coph.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Price DL, Bonhaus DW, McFarland K. Pimavanserin, a 5-HT2A receptor inverse agonist, reverses psychosis-like behaviors in a rodent model of Alzheimer’s disease. Behav Pharmacol. 2012;23(4):426–33. doi: 10.1097/FBP.0b013e3283566082. [DOI] [PubMed] [Google Scholar]
- 86.McFarland K, Price DL, Bonhaus DW. Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson’s disease. Behav Pharmacol. 2011;22(7):681–92. doi: 10.1097/FBP.0b013e32834aff98. [DOI] [PubMed] [Google Scholar]
- 87.Navailles S, Lagière M, Roumegous A, et al. Serotonin2C ligands exhibiting full negative and positive intrinsic activity elicit purposeless oral movements in rats: distinct effects of agonists and inverse agonists in a rat model of Parkinson’s disease. Int J Neuropsychopharmacol. 2013;16(3):593–606. doi: 10.1017/S1461145712000417. [DOI] [PubMed] [Google Scholar]
- 88.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomized, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–40. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 89.Akam E, Strange PG. Inverse agonist properties of atypical antipsychotic drugs. Biochem Pharmacol. 2004;67(11):2039–45. doi: 10.1016/j.bcp.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 90.Rosenzweig-Lipson S, Comery TA, Marquis KL, Gross J, Dunlop J. 5-HT2C agonists as therapeutics for the treatment of schizophrenia. Hanb Exp Pharmacol. 2012;213:147–65. doi: 10.1007/978-3-642-25758-2_6. [DOI] [PubMed] [Google Scholar]
- 91.Shen JH, Zhao Y, Rosenzweig-Lipson S, et al. A 6-week randomized, double-blind, placebo-controlled, comparator referenced trial of vabicaserin in acute schizophrenia. J Psychiatr Res. 2014;53:14–22. doi: 10.1016/j.jpsychires.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 92.Liu J, Ogden A, Comery TA, Spiros A, Roberts P, Geerts H. Prediction of efficacy of vabixaserin, a 5-HT2C agonist, for the treatment of schizophrenia using a quantitative systems pharmacology model. CPT Pharmacometrics Syst Pharmacol. 2014;3:e111. doi: 10.1038/psp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]