Abstract
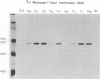
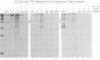
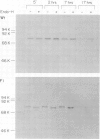
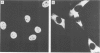
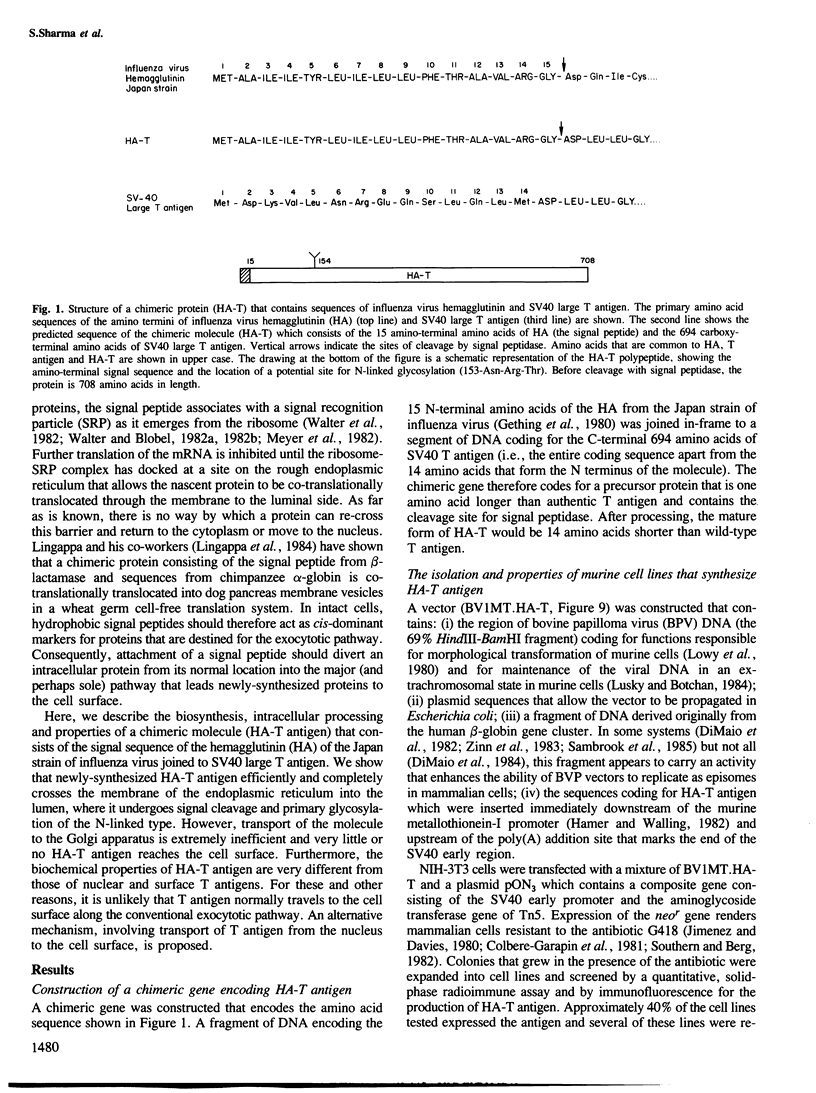
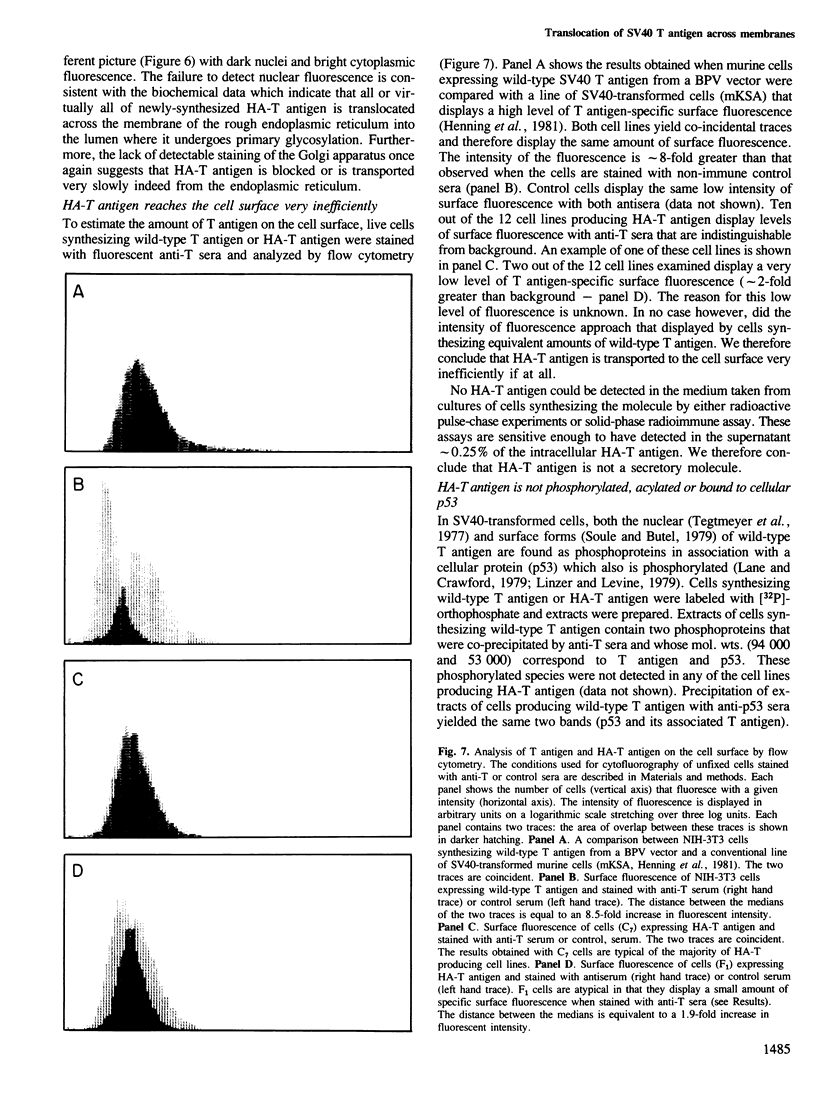
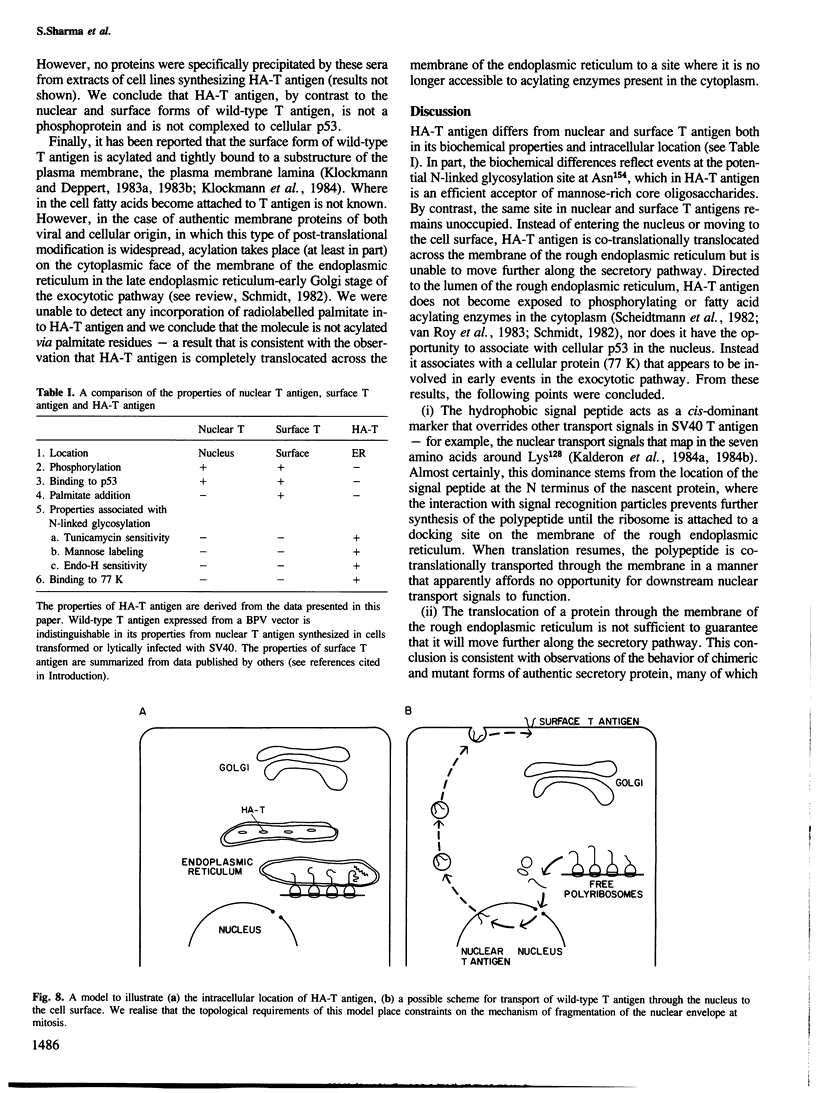
A chimeric gene consisting of DNA coding for the 15-amino acid signal peptide of influenza virus hemagglutinin and the C-terminal 694 amino acids of SV40 large T antigen was inserted into a bovine papilloma virus (BPV) expression vector and introduced into NIH-3T3 cells. Cell lines were obtained that express high levels (approximately 5 X 10(6) molecules/cell) of the chimeric protein (HA-T antigen). The biochemical properties and intracellular localization of HA-T antigens were compared with those of wild-type T antigen. Wild-type T antigen. Wild-type T antigen is located chiefly in the cell nucleus, although a small fraction is detected on the cell surface. By contrast, HA-T antigen is found exclusively in the endoplasmic reticulum (ER). During biosynthesis, HA-T antigen is co-translationally translocated across the membrane of the ER, the signal peptide is cleaved and a mannose-rich oligosaccharide is attached to the polypeptide (T antigen contains one potential N-linked glycosylation site at Asn154). HA-T antigen does not become terminally glycosylated or acylated and little or none reaches the cell surface. These results suggest that T antigen is incapable of being transported along the exocytotic pathway. To explain the presence of wild-type T antigen on the surface of SV40-transformed cells, an alternative route is proposed involving transport of T antigen from the nucleus to the cell surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Siegl B., Quellhorst S., Brandner G., Braun D. G. Monoclonal antibodies against simian virus 40 nuclear large T tumour antigen: epitope mapping, papova virus cross-reaction and cell surface staining. EMBO J. 1984 Jul;3(7):1485–1491. doi: 10.1002/j.1460-2075.1984.tb02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Little P. F., Annison G., Williamson R., Flavell R. A. Structure of the human G gamma-A gamma-delta-beta-globin gene locus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4827–4831. doi: 10.1073/pnas.76.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- De Robertis E. M. Nucleocytoplasmic segregation of proteins and RNAs. Cell. 1983 Apr;32(4):1021–1025. doi: 10.1016/0092-8674(83)90285-4. [DOI] [PubMed] [Google Scholar]
- Deppert W., Hanke K., Henning R. Simian virus 40 T-antigen-related cell surface antigen: serological demonstration on simian virus 40-transformed monolayer cells in situ. J Virol. 1980 Aug;35(2):505–518. doi: 10.1128/jvi.35.2.505-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Domains of simian virus 40 large T-antigen exposed on the cell surface. Virology. 1982 Oct 15;122(1):56–70. doi: 10.1016/0042-6822(82)90377-4. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Corbin V., Sibley E., Maniatis T. High-level expression of a cloned HLA heavy chain gene introduced into mouse cells on a bovine papillomavirus vector. Mol Cell Biol. 1984 Feb;4(2):340–350. doi: 10.1128/mcb.4.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Rose J. K. Conversion of a secretory protein into a transmembrane protein results in its transport to the Golgi complex but not to the cell surface. Cell. 1984 Jul;37(3):779–787. doi: 10.1016/0092-8674(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Walling M. Regulation in vivo of a cloned mammalian gene: cadmium induces the transcription of a mouse metallothionein gene in SV40 vectors. J Mol Appl Genet. 1982;1(4):273–288. [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. A., Lukanidin E., Fey G., Sambrook J. The structure and expression of two defective adenovirus 2/simian virus 40 hybrids. J Mol Biol. 1978 Apr 5;120(2):209–247. doi: 10.1016/0022-2836(78)90065-7. [DOI] [PubMed] [Google Scholar]
- Henning R., Lange-Mutschler J., Deppert W. SV40-transformed cells express SV40 T antigen-related antigens on the cell surface. Virology. 1981 Jan 30;108(2):325–337. doi: 10.1016/0042-6822(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Hsiung N., Fitts R., Wilson S., Milne A., Hamer D. Efficient production of hepatitis B surface antigen using a bovine papilloma virus-metallothionein vector. J Mol Appl Genet. 1984;2(5):497–506. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Jimenez A., Davies J. Expression of a transposable antibiotic resistance element in Saccharomyces. Nature. 1980 Oct 30;287(5785):869–871. doi: 10.1038/287869a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Karin M., Slater E. P., Herschman H. R. Regulation of metallothionein synthesis in HeLa cells by heavy metals and glucocorticoids. J Cell Physiol. 1981 Jan;106(1):63–74. doi: 10.1002/jcp.1041060108. [DOI] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 1983;2(7):1151–1157. doi: 10.1002/j.1460-2075.1983.tb01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylation: a new post-translational modification specific for plasma membrane-associated simian virus 40 large T-antigen. FEBS Lett. 1983 Jan 24;151(2):257–259. doi: 10.1016/0014-5793(83)80081-7. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Antigenic relationship of SV40 early proteins to purified large T polypeptide. Virology. 1979 Sep;97(2):295–306. doi: 10.1016/0042-6822(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Lange-Mutschler J., Deppert W., Hanke K., Henning R. Detection of simian virus 40 T-antigen-related antigens by a 125I-protein A-binding assay and by immunofluorescence microscopy on the surface of SV40-transformed monolayer cells. J Gen Virol. 1981 Feb;52(Pt 2):301–312. doi: 10.1099/0022-1317-52-2-301. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Chaidez J., Yost C. S., Hedgpeth J. Determinants for protein localization: beta-lactamase signal sequence directs globin across microsomal membranes. Proc Natl Acad Sci U S A. 1984 Jan;81(2):456–460. doi: 10.1073/pnas.81.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Dvoretzky I., Shober R., Law M. F., Engel L., Howley P. M. In vitro tumorigenic transformation by a defined sub-genomic fragment of bovine papilloma virus DNA. Nature. 1980 Sep 4;287(5777):72–74. doi: 10.1038/287072a0. [DOI] [PubMed] [Google Scholar]
- Luborsky S. W., Chandrasekaran K. Subcellular distribution of simian virus 40 T antigen species in various cell lines: the 56K protein. Int J Cancer. 1980 Apr 15;25(4):517–527. doi: 10.1002/ijc.2910250414. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
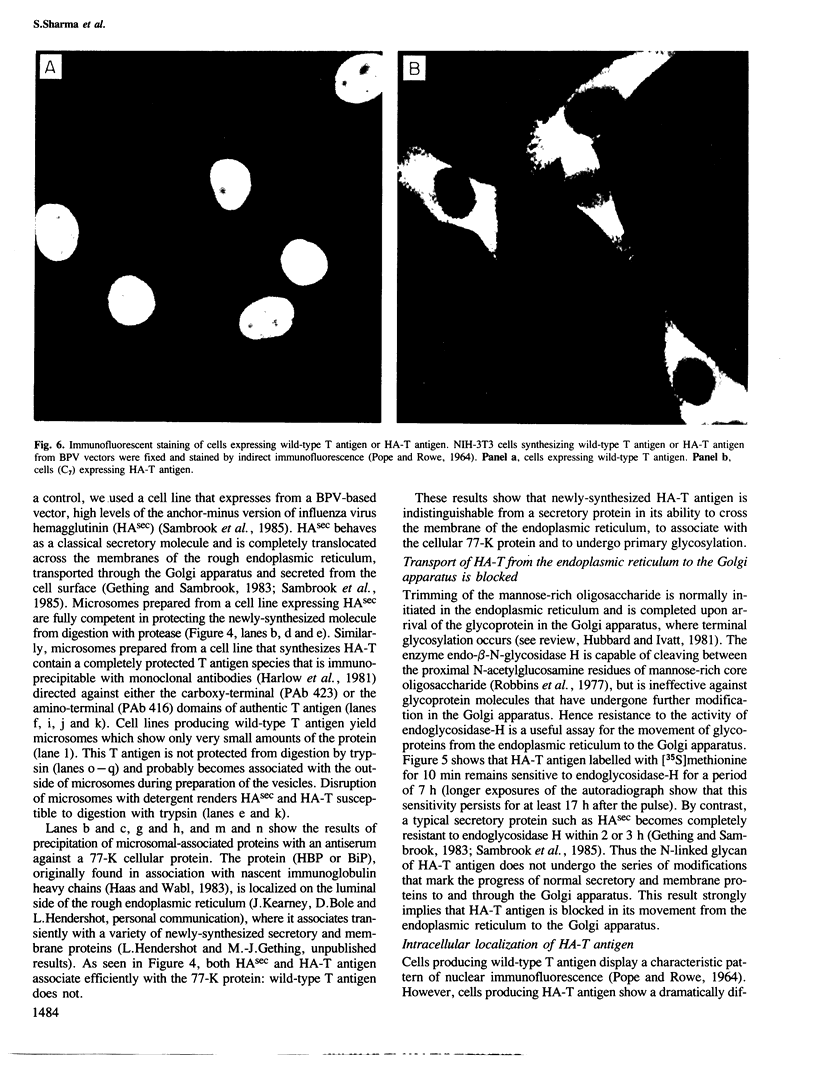
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis G. N., Hamer D. H. Regulation of a metallothionein-growth hormone hybrid gene in bovine papilloma virus. Proc Natl Acad Sci U S A. 1983 Jan;80(2):397–401. doi: 10.1073/pnas.80.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Rodgers L., White J., Gething M. J. Lines of BPV-transformed murine cells that constitutively express influenza virus hemagglutinin. EMBO J. 1985 Jan;4(1):91–103. doi: 10.1002/j.1460-2075.1985.tb02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Butel J. S. Detection of a complex of SV40 large tumor antigen and 53K cellular protein on the surface of SV40-transformed mouse cells. J Cell Biochem. 1982;19(2):127–144. doi: 10.1002/jcb.240190204. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F. Acylation of viral spike glycoproteins: a feature of enveloped RNA viruses. Virology. 1982 Jan 15;116(1):327–338. doi: 10.1016/0042-6822(82)90424-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P., Watts C., Wickner W. Membrane assembly from purified components. I. Isolated M13 procoat does not require ribosomes or soluble proteins for processing by membranes. Cell. 1981 Aug;25(2):341–345. doi: 10.1016/0092-8674(81)90052-0. [DOI] [PubMed] [Google Scholar]
- Soule H. R., Butel J. S. Subcellular Localization of simian virus 40 large tumor antigen. J Virol. 1979 May;30(2):523–532. doi: 10.1128/jvi.30.2.523-532.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. R., Lanford R. E., Butel J. S. Antigenic and immunogenic characteristics of nuclear and membrane-associated simian virus 40 tumor antigen. J Virol. 1980 Feb;33(2):887–901. doi: 10.1128/jvi.33.2.887-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevethia S. S. Cytolytic T lymphocyte response to SV40. Surv Immunol Res. 1983;2(3):312–314. doi: 10.1007/BF02918441. [DOI] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Hu S. L., Grodzicker T. Translational control of SV40 T antigen expressed from the adenovirus late promoter. Cell. 1983 Jun;33(2):455–464. doi: 10.1016/0092-8674(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., McMichael A. J., Carter N. P., Huddleston J. A., Brownlee G. G. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984 Nov;39(1):13–25. doi: 10.1016/0092-8674(84)90187-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J Exp Med. 1984 Aug 1;160(2):552–563. doi: 10.1084/jem.160.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Metabolic turnover of phosphorylation sites in simian virus 40 large T antigen. J Virol. 1983 Jan;45(1):442–446. doi: 10.1128/jvi.45.1.442-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]