Abstract
Background and Purpose
To report outcomes and toxicities of a single-institution Phase I/II study of stereotactic body radiotherapy (SBRT) in the treatment of unresectable hepatocellular cancer (HCC) and intrahepatic cholangiocarcinoma (IHC).
Materials and Methods
Patients with Child-Pugh score less than 8 were eligible. A total of 32 lesions in 26 patients were treated with SBRT. Kaplan-Meier survival analysis was performed. Toxicities were graded by CTCAEv4 criteria and response was scored by EASL guidelines.
Results
Median prescribed dose was 55 Gy (range 40 – 55 Gy) delivered in 5 fractions. Mean tumor diameter was 5.0 cm and mean GTV was 107 cc. Median follow-up was 8.8 months with a median survival of 11.1 months, and one-year overall survival was 45%. Overall response rate was 42% and one-year local control was 91%. Nine patients experienced a decline in Child-Pugh class following treatment, and two grade 5 hepatic failure toxicities occurred during study follow-up.
Conclusions
Primary hepatic malignancies not amenable to surgical resection portend a poor prognosis, despite available treatment options. Though radiation-induced liver disease (RILD) is rare following SBRT, this study demonstrates a risk of hepatic failure despite adherence to protocol constraints.
Keywords: SBRT, hepatocellular carcinoma, cholangiocarcinoma
Introduction
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (IHC) are the two most prevalent primary hepatic malignancies. HCC accounts for the majority of primary hepatic malignancies and ranks third in cancer-related death worldwide [1]. IHC comprises 5–10% of hepatic malignancies [2]. Therapies are guided by tumor stage and underlying hepatic function with an ideal treatment consisting of surgical resection or liver transplantation. The majority of patients are not candidates for surgical management [3], and a variety of locoregional and systemic options exist. Locoregional therapies include transarterial chemoembolization (TACE), radiofrequency ablation, cryoablation, percutaneous ethanol or acetic acid ablation, and radioembolization; however there are limitations to candidacy for these procedures. Systemic therapy includes sorafenib, which has shown a survival benefit in the randomized placebo-controlled SHARP trial for advanced HCC [4] or cytotoxic chemotherapy and targeted agents for IHC.
Hepatic irradiation was initially utilized infrequently because the intolerance of the normal liver to radiation prevented tumoricidal doses. Classic radiation induced liver disease (RILD), a clinical triad of anicteric hepatomegaly, ascites and elevated liver enzymes has been noted following liver radiation [5]. Non-classic RILD, hepatic toxicity that does not meet classis RILD parameters, can also occur and may manifest as extreme elevation in transaminases or jaundice. Analysis of the Normal Tissue Complication Probability (NTCP) model for hepatic irradiation has shown the importance of volume effect [6]. This model predicts that small volumes of liver may receive high radiation doses without clinical hepatic toxicity, shifting emphasis to focal radiotherapy to spare normal liver. Stereotactic body radiotherapy (SBRT), in which high dose hypofractionated (typically 5 or fewer fractions) conformal external-beam radiation is delivered to a tumor, is a noninvasive therapeutic option for patients with primary hepatic malignancies. Advances in image guidance and respiratory motion management have further enabled this technology. SBRT has been investigated prospectively for both HCC [7–11] and IHC [8, 12, 13] using a variety of dose and fractionation strategies with 1 year local control rates ranging from 65% to 100%. Though the incidence of grade 3 and higher hepatic toxicity is a rare complication (<5%) after liver SBRT [14], hepatotoxicity (including G5 liver failure) has been reported in some of these studies [7, 9]. In the absence of RILD, changes in Child-Pugh (CP) classification following liver SBRT have been noted [8, 9]. Retrospective studies have evaluated dosimetric parameters that are correlated with decline in CP class following SBRT [15,16].
A variety of treatment schedules have been used to evaluate safety and efficacy of SBRT for treatment of primary hepatic malignancies. In this prospective institutional study, a 5 fraction SBRT regimen of 55 Gy was selected with dose decrements to meet constraints to normal structures. The primary endpoint was toxicity evaluation, with a goal of fewer than 10% G4 or higher study related toxicity within 90 days (toxicity evaluation was not limited to hepatic toxicities). Secondary objectives included late toxicity, local recurrence, response rate, and overall survival (OS). The final results are reported in this manuscript.
Methods and Materials
Patients
This is a prospective trial approved by the institutional review board (NCT 01668134). Patients with HCC, IHC or biphenotypic hepatic malignancy who were not surgical candidates were eligible. Pathology was not required for patients with HCC if the lesion met characteristic imaging features. Patients had to be older than 18 years of age with a Karnofsky Performance Status greater than or equal to 60. Patients with four or fewer intrahepatic lesions were eligible. Limited extrahepatic disease (defined as volume less than hepatic tumor volume) and tumor vascular thrombosis were allowed. Prior local or systemic therapies were allowed, and decisions to proceed with SBRT were discussed at a multidisciplinary tumor board. Patients with CP score between 5 and 7 were eligible. Patients with prior abdominal radiation were ineligible. Pretreatment evaluation included history and physical exam and laboratory evaluation within 1 month of enrollment and abdominal imaging within two months of enrollment. Target accrual was enrollment of 40 patients over two years.
Treatment
Prior to simulation for radiation treatment planning, patients underwent placement of fiducial markers via a percutaneous or endoscopic approach. Patients were immobilized using an alpha cradle. Four-dimensional CT simulation with 1.5 – 3 mm slice thickness was performed using a Phillips Big Bore 16-slice CT simulator (Philips Healthcare, Cleveland, OH) with intravenous contrast. The end exhalation phase was used as the primary dataset for treatment planning. The gross tumor volume (GTV) consisted of the primary lesion delineated using the CT simulation image at end-exhalation and fused diagnostic images. Clinical target volume (CTV) expansions were not routinely performed. A 0.5 cm expansion of the GTV was used to generate a planning target volume (PTV). Both three-dimensional conformal radiation and intensity modulated radiation therapy were allowed.
The prescribed dose was 55 Gy in 5 fractions with adjustment based on mean liver dose. If necessary to meet this tissue constraint, dose was adjusted in 5 Gy decrements to a minimum of 40 Gy in 5 fractions. Ninety-five percent of the PTV was required to receive 95% of the prescription dose, and 99% of the PTV was required to receive 90% of the prescription dose. The global maximum was less than 140% of the prescription dose. At least 700 cc of normal liver was spared by 20 Gy and the mean liver dose was required to be less than20 Gy. Dose constraints to other organs at risk are summarized in Appendix A in Supplementary data.
Treatments were delivered every other day using a linear accelerator with photon energies ranging from 6 – 18MV. Daily cone-beam CT images were obtained to evaluate patient setup, and a radiation oncologist was present at each treatment. The RPM Respiratory Gating System (Varian, Palo Alto, CA) was utilized for expiratory respiratory gating on fiducial markers, pre-existing biliary stent or intrahepatic lipiodol from prior TACE. No concomitant medications were required during radiotherapy; anti-emetics and proton-pump inhibitors were prescribed as needed based on symptoms during and after radiotherapy. Adjuvant systemic therapy following RT was allowed, and was administered per the discretion of the medical oncologist.
Evaluation
Patients were evaluated weekly during SBRT, at 6–8 weeks following completion of therapy, every 3 months for the first 12 months, then every 6 months. Blood work and hepatic triphasic CT or MRI were obtained at each follow-up visit. Toxicities were scored base on the revised NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Tumor response to treatment was evaluated by European Association for the Study of the Liver (EASL) criteria.
Statistics
Overall survival (OS), progression-free survival (PFS) and local control rates were evaluated using the Kaplan-Meier method. Local control was defined as no evidence of progression in the treated lesion. All analyses were performed using SAS 9.3 (Cary, NC).
Results
Patients and Treatment
Between February 2012 and May 2014, 28 patients consented to participate in the study. Two patients were ineligible for treatment due to inability to meet treatment planning objectives related to extent of disease burden. Twenty-six patients were evaluable for the primary endpoint of incidence of G4+ toxicity within 90 days of treatment.
Baseline patient characteristics are summarized in Table 1. The cohort included 12 patients with HCC, 12 patients with IHC, and 2 patients with a biphenotypic tumor. Eight patients with HCC had biopsy-proven disease. SBRT was the first line of treatment in 9 patients. Eight patients with HCC received TACE prior to SBRT. No patients with HCC received sorafenib prior to SBRT. Eight patients with biphenotypic tumors or IHC received systemic chemotherapy prior to SBRT. Fifteen patients had underlying cirrhosis and 3 were CP class B. Seven patients had tumor vascular thrombosis and no patients had extrahepatic disease.
Table 1.
Patient, Disease, and Prior Treatment Characteristics
| Characteristic | Number (%) |
|---|---|
| Age [median (range)] | 72 (51–95) |
| Gender | |
| Male | 12 (46%) |
| Female | 14 (54%) |
| Histology | |
| Hepatocellular carcinoma | 12 (46%) |
| Intrahepatic cholangiocarcinom | 12 (46%) |
| Biphenotypic hepatobiliary tumor | 2 (8%) |
| Reason for unresectability | |
| Patient factors | 11 (42%) |
| Tumor factors | 11 (42%) |
| Patient/Tumor factors | 4 (1 6 %) |
| Cirrhosis | 15 (58%) |
| Child Pugh Class A | |
| Child Pugh score 5 | 17 (65%) |
| Child Pugh score 6 | 6 (23%) |
| Child Pugh Class B (score 7) | 3 (12%) |
| Baseline laboratory values [median (range)] | |
| Bilirubin (mg/dl) | 0.6 (0.3 – 3) |
| Albumin (g/dl) | 3.9 (2.6 – 4.7) |
| INR | 1.12 (0.9 – 1.49) |
| AST (U/L) | 55 (24 – 123) |
| ALT (U/L) | 51 (14 – 167) |
| Creatinine (mg/dl) | 0.79 (0.46 – 1.87) |
| Platelets (1000/mm3) | 122 (52 – 3) |
| Alkaline Phosphatase (U/L) | 116 (70 – 420) |
| AFP (HCC only) | 48 (<5 – 4472) |
| ECOG performance status | |
| 0 | 1 (4%) |
| 1 | 21 (80%) |
| 2 | 2 (8%) |
| 3 | 1 (4%) |
| Prior therapies# | |
| Surgery | 2 (8%) |
| Radiofrequency ablation | 1 (4%) |
| TACE | 8 (31%) |
| Microwave ablation | 1 (4%) |
| Chemotherapy | 9 (35%) |
| AJCC T stage (IHC/biphenotypic) | |
| T1 | 7 (50%) |
| T2 | 5 (36%) |
| T3 | 1 (7%) |
| T4 | 1 (7%) |
| BCLC stage (HCC) | |
| A1 | 1 (8%) |
| A2 | 0 (0%) |
| A3 | 1 (8%) |
| A4 | 3 (25%) |
| B | 4 (33%) |
| C | 3 (25%) |
| Number of lesions | |
| 1 | 22 (84%) |
| 2 | 3 (12%) |
| 3 | 1 (4%) |
| Tumor vascular thrombosis | 7 (27%) |
| Tumor diameter [median (range)] (cm) | 5.0 (1.6 – 12.3) |
| ≤2 cm | 3 (12%) |
| 2–4 cm | 7 (27%) |
| 4–6 cm | 6 (23%) |
| 6–8 cm | 6 (23%) |
| 8–10 cm | 3 (12%) |
| >10 cm | 1 (4%) |
Some patients received multiple therapies prior to SBRT
Treatment was completed in 25 of 26 (96%) patients with a median prescription dose of 55 Gy (40–55 Gy). Twenty-one patients received 55 Gy in 5 fractions, while 5 patients had dose reduction per protocol (four patients received 50 Gy/5 fractions, one patient received 40 Gy/5 fractions) based on mean liver dose. Additional details of treatment dosimetry are shown in Appendix B in Supplemental data. One patient discontinued treatment after receiving 4 of 5 prescribed fractions, due to hospitalization and decline in health secondary to pre-existing gastrointestinal ulcers.
Twenty-three patients had solitary lesions treated with SBRT. In the patients with more than one lesion, the lesions were included a single treatment volume in 3 of the 4 patients. The fourth patient required 2 separate PTVs which were treated concurrently. Median lesion diameter was 5.0 cm (1.6 – 12.3 cm). Median GTV was 107 cc (16.9 – 625.9 cc), and median PTV was 231 cc (53.5 – 964 cc).
Two patients with IHC received adjuvant therapy following SBRT; one received capecitabine and the other received erlotinib. Four patients with HCC received adjuvant sorafenib. Cytotoxic chemotherapy could be initiated 4 weeks following radiotherapy, and sorafenib could be initiated 2 weeks following radiation.
Toxicity and Change in CP Score
Median follow-up time was 8.8 months (range 0.3 – 33 months). No complications were noted following placement of fiducial markers. During follow-up, 6 patients experienced G3+ acute toxicities (≤60 days from treatment), and G3+ late toxicities (>60 days from treatment) were observed in 5 patients. A total of 17 G3+ acute treatment-related toxicities and 18 G3+ late toxicities were noted and are summarized in Table 2. The most commonly noted treatment-related toxicity was lymphopenia, with 8 patients experiencing G3 acute toxicity, 1 patient experiencing G4 acute toxicity and 5 patients with G3 late toxicity. Lymphopenia existed prior to SBRT in 4 of these patients. No patients experienced any documented infections related to lymphopenia during follow-up. Acute G3 abdominal pain was noted in 4 patients, while elevated bilirubin was observed in 2 patients. Late toxicities included G3 abdominal pain and ascites in 2 patients each.
Table 2.
Study-related acute (≤ 60 days after SBRT) and late (> 60 days after SBRT) biochemical changes and toxicities.
| Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|
| Acute Toxicity (≤ 60 days)
| |||
| Lymphopenia | 6 (23%) | 1 (4%) | |
| Abdominal Pain | 4 (15%) | ||
| Bilirubin | 2 (8%) | ||
| Platelets | 1 (4%) | ||
| PTT | 1 (4%) | ||
| INR | 1 (4%) | ||
| Hepatic Failure | 1 (4%) | ||
|
Late Toxicity (> 60 days) | |||
| Lymphopenia | 5 (19%) | ||
| Thrombocytopenia | 1 (4%) | ||
| INR | 1 (4%) | ||
| PTT | 1 (4%) | ||
| ALT | 1 (4%) | ||
| Bilirubin | 1 (4%) | ||
| Biliary stricture | 1 (4%) | ||
| Abdominal pain | 2 (8%) | ||
| Ascites | 2 (8%) | ||
| Vomiting | 1 (4%) | ||
| Skin fibrosis | 1 (4%) | ||
| Hepatic Failure | 1 (4%) | ||
Nine of 26 patients experienced a decline in CP class following treatment all of which represented a change of more than two points in the CP score. The CP class decline was associated with progression of disease in one patient. Five patients declined from class A to B, 3 from class A to C and one from class B to C. Among the patients with CP class decline, there were two deaths from hepatic failure, prompting early interim analysis and closure of the study. Hepatic dosimetric analysis for all patients and patients with G5 toxicity appears in Table 3, including protocol specified dose constraints as well as other metrics that have been associated with hepatic toxicity following SBRT.
Table 3.
Liver dose volume histogram (DVH) data for all patient as well as the two patients who experienced G5 hepatic toxicity
| All patients Median (range) | Patient 1a | Patient 2b | Goal/Constraint | |
|---|---|---|---|---|
| Uninvolved liver volume | 1550 cc (917 –3556) | 1980 cc | 1400 cc | |
| GTV volume | 107 (16.9 – 625.9) | 32.4 cc | 111.1 cc | |
| Mean dose (liver –GTV) | 16.0 Gy (3.0 –23.5 Gy) | 15.5 Gy | 19.2 Gy | <20 Gy |
| V15 | 36% (7.5 –56) | 38% | 37% | |
| rV15cc | 947 cc (524 –2048) | 1221 cc | 883 cc | >700 cc |
| V18 | 33% (7 –49) | 35% | 33% | |
| rV18cc | 1019 cc (634 –2143) | 1289 cc | 941 cc | >800 cc |
| V20 | 31% (7 –49) | 33% | 30% | |
| rV20cc | 1095 cc (668 –2253) | 1324 cc | 977 cc | >700 cc |
| V25 | 25%(5 –40) | 25% | 25% | <36% |
| V30 | 21% (4 – 35) | 17.8% | 21% |
VXX – volume (%) of liver receiving XX Gy or greater. rVXXcc – volume (cc) of liver spared by XX Gy.
Patient 1 had a diagnosis of IHC with Child-Pugh class A (score 5) cirrhosis arising from primary sclerosing cholangitis.
Patient 2 had a diagnosis of HCC (treated with prior TACE) with Child-Pugh class B (score of 7 secondary to hypoalbuminemia and medication-controlled ascites) cirrhosis in the setting of Hepatitis C.
Response
The one-year local control rate was 91% for all patients. The overall response rate by EASL criteria was 42%. Radiographic response was seen in 6/12 patients with HCC, 4/12 with IHC and in 1/2 patients with a biphenotypic tumors. Five patients had a complete radiographic response (4 with HCC and 1 with IHC). Time to radiographic response ranged from 1.6 to 9.0 months after treatment. One patient with 2 HCC lesions treated with SBRT was successfully downstaged to meet Milan criteria and underwent orthotopic liver transplantation 6 months following SBRT. Two patients received subsequent local therapies (one with TACE and one with cryoembolization) after progression of hepatic disease.
Overall Survival and Time to Progression
The median survival for all patients was 11.1 months, and the one-year OS was 45% (95% CI 25–64%), shown in Figure 1A. In patients with HCC, the one-year OS was 38% (95% CI 12–64%) and the median survival was 9.8 months. For patients with IHC/biphenotypic tumors, the one-year OS was 51% (95%CI 22–75%) and median survival was 13.2 months.
Figure 1.
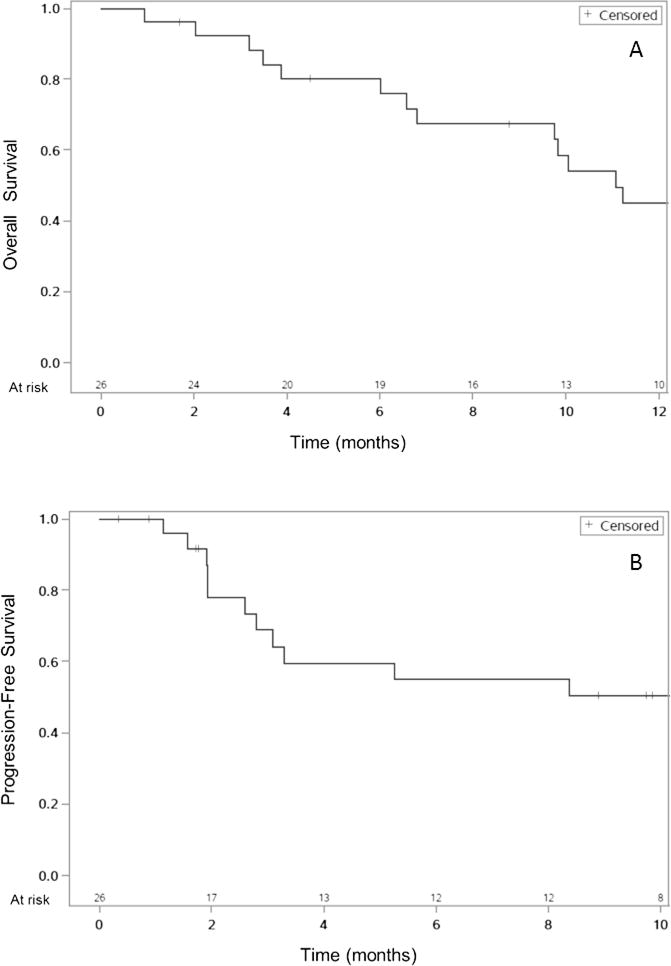
A. Overall survival (OS) from time of SBRT start. Median OS 11.1 months, one-year OS 45%. B. Progression-free survival (PFS) from time of SBRT start. Median PFS 15.2 months, one-year PFS 50%.
Thirteen patients (50%) experienced disease recurrence during study follow-up. Two patients had in-field local failure. Eight patients had regional failure within the liver outside of the treatment field. Seven patients developed distant failure. Median PFS in all patients was 15.2 months, shown in Figure 1B. Median PFS was 5.3 months in patients with HCC and 24.7 months in patients with IHC/biphenotypic tumors. One-year PFS was 50% (95%CI 29–69%) in all patients, 48% (95%CI 12–67%) in patients with HCC, and 68% (95% CI 27–80%) in patients with IHC/biphenotypic tumors.
Discussion
In this study, 26 patients with primary hepatic malignancies were treated with SBRT (55 Gy/5 fractions). The majority (81%) of patients did not require de-escalation of prescription dose to meet dose constraints. Despite adherence to protocol constraints, two patients had G5 hepatic failure toxicities that were likely related to radiotherapy meeting pre-determined early stopping criteria for the trial. One patient was a non-cirrhotic with IHC who primary sclerosing cholangitis (PSC) and renal transplant on tacrolimus. This patient developed hepatomegaly, ascites and elevated bilirubin and liver enzymes within 1 month of completion of radiotherapy and progressed to hepatic failure and death. There is no known association of PSC or tacrolimus with sensitization to radiation. Tacrolimus has been rarely related with hepatic veno-occlusive disease in renal transplant patients [17] however this patient showed no hepatic dysfunction (other than baseline elevation in alkaline phosphatase) prior to SBRT. The second patient was a long-standing hepatitis C cirrhotic (CP class B) who developed similar symptoms and succumbed hepatic failure in the setting of a gastrointestinal bleed within 1 month following SBRT. With elevated bilirubin at presentation, neither case is consistent with classic RILD although radiotherapy must be considered as a cause for toxicity give the temporal relationship. In retrospect, the patient may have downplayed prior history of ascites and encephalopathy that may reflect a higher CP class than noted.
Dosimetric correlations with decline in CP class following SBRT have been analyzed as a marker for treatment-related hepatotoxicity. Son and colleagues evaluated 47 patients with HCC treated with SBRT (36 Gy/3 fractions)[15]. They elicited a relationship between the volume of liver spared by 18 Gy (rV18) and decline in CP class with a recommendation of a rV18 Gy greater than 800 cc. Dyk et al have associated the volume of liver receiving 25 Gy (V25) with decline in CP class in patients receiving hepatic SBRT [16]. In the patients with primary liver cancers, a V25 greater than 36% was correlated with CP class decline.
Underlying cirrhosis or baseline liver dysfunction are known to increase hepatic susceptibility to radiation toxicities. In an initial evaluation of liver SBRT, Mendez-Romero and colleagues evaluated 11 CP class A/B patients with HCC [7] who were treated to 25–37.5 Gy in 3–5 fractions with one observed G5 RILD toxicity. Nonfatal classic RILD following SBRT has been noted as a dose-limiting toxicity in 3/11 CP class B patients in another series [18] with a conclusion that CP class B patients warrant treatment with a lower dose and fraction size. In an analysis of HCC patients with CP class B/C cirrhosis, Culleton and colleagues describe a decline of at least 2 points in CP score in 63% of patients [11]. Median OS was 7.9 months in this group, with a lower CP score being associated with improved survival. One patient in this analysis suffered fatal liver failure 3 months following SBRT. In concordance with these findings, patients with a CP score greater than 7 are frequently excluded from liver SBRT trials.
Andolino et al have evaluated SBRT for HCC for both CP class B (40 Gy/5 fractions) and CP class A (44 Gy/3 fractions) [19]. 50% of CP class B patients developed progressive liver failure following treatment. Kang and colleagues evaluated 47 patients with HCC treated with SBRT (60 Gy/3 fractions) following incomplete TACE and noted decline from CP class A to B in 6 patients and development of ascites in 4 patients without evidence of classic RILD [20]. In contrast to the small median GTV in these two studies (29 and 14.9 cc), our median GTV was significantly larger at 107 cc.
Two prospective studies out of Princess Margaret Hospital have evaluated SBRT with larger treatments volumes using individualized dose allocation with a 6-fraction regimen ranging from 24–54 Gy. Tse et al included HCC and IHC (CP class A) with a median GTV of 173 cc [8]. While there were no G4/5 toxicities, 17% of patients experienced a decline in CP class. Bujold et al assessed 102 patients with HCC (CP class A) and a median GTV size of 117 cc [9]. Seven G5 toxicities (5 liver failures, 1 cholangitis, 1 gastrointestinal bleed) were noted without classic RILD. A change in CP score occurred in 46% of patients and a change in CP class occurred in 29% of patients, with recovery of hepatic function in a majority of patients after 12 months. The authors of these two studies discuss that individualized dose allocation enabled treatment of larger tumors that would have otherwise been unable to meet dose constraints, and note that a disadvantage is that patients with a larger burden of disease would receive lower prescription doses.
Treatment of cholangiocarcinoma by SBRT is less well studied than HCC. Kopek and colleagues have evaluated 27 patients treated with 45 Gy/3 fractions. Duodenal toxicities were more prevalent than hepatic toxicities, and median OS was 10.6 months. Two other prospective studies have included patients with IHC along with HCC [13] or with HCC and metastases [8]. Tse et al report transient biliary obstruction and decline in CP class in two of 10 patients with IHC treated with SBRT [8]. The role of radiotherapy in IHC should be further elucidated by the NRG-GI001 study which randomizes patients with localized, unresectable intrahepatic cholangiocarcinoma to chemotherapy versus chemotherapy followed by hypofractionated radiation.
In the current study, which was closed to accrual due to meeting early stopping criteria after the second G5 hepatic failure toxicity, the 1-year OS of 45% and median survival of 11.1 months remain favorable in comparison with best supportive care (33% and 7.9 months, respectively) and similar to sorafenib (44% and 10.7 months)[4]. 35% of our patients experienced decline in CP score following SBRT treatment, which is within the range of expected toxicity based on other evaluations. Large lesion size (median GTV 107 cc), presence of tumor vascular thrombosis in 27% of patients, and heavy pretreatment with other modalities are patient risk factors in this study. This study has several limitations including enrollment at a single-institution and small patient numbers (which limits further assessment of factors that predisposed patients to toxicities). Underlying cirrhosis and CP class are factors that decrease hepatic reserve and limit radiation tolerance; however other intrinsic patient factors play a role. Tumor volume is another factor, and often dose must be decreased for larger tumors to spare uninvolved liver. More conservative hepatic dose constraints are also evolving. The protocol constraints were based on a historic biologic equivalent dose with α/β = 3 (BED3) constraint of 49.5 Gy (33 Gy in 22 fractions) [21], and with 700 cc of liver receiving less than 20 Gy in 5 fractions, the BED3 of the spared liver is 46.7 Gy at maximum. In comparison, 18 Gy in 3 fractions allowed by Son et al for 800 cc of normal liver can be reported as a BED3 of 54 Gy [15]. Despite our initial constrant falling within the range of standard constraints, our institution is now sparing 700 cc of liver by 15 Gy (BED3 40Gy), while other parameters considered during treatment planning are V25 and rV18cc. The treatment plans of both patients with G5 toxicity in this study meet these new constraints and guidelines (Table 3), supporting a conclusion that additional patient factors are involved in hepatic toxicity. Sanuki et al have recently described toxicities that predict for non-classic RILD in patients with HCC receiving SBRT [22]. They have found G3 elevation in transaminases, CP score of 8 or more and/or G3 thrombocytopenia to be predictive for fatal hepatic failure. Some of these finding correlate with toxicities found in our data, one patient with fatal hepatic failure had G3 elevation in transaminases (ALT), ten patients had post-treatment CP scores of greater than 8 (including the two G5 toxicities) and one patient had G3 thrombocytopenia without hepatic failure.
In addition to hepatic toxicity and changes in CP class, it is important to consider the overall incidence of G3+ toxicities. In this study, 17 acute G3+ toxicities were observed in 6 patients, with lymphopenia being the most common toxicity. Lymphopenia occurs following radiotherapy by direct destruction of mature circulating lymphocytes and has been noted with a variety of treatment sites including lung [23] and brain [24]. In addition, splenic pooling and defects in thymopoesis are postulated to result in lymphopenia in cirrhotic patients [25]. Our overall rates of G3+ toxicity are within the ranges reported in comparable studies. In patients with HCC, Bujold reported 30% G3+ toxicities (predominantly elevation in transaminases [9], while Sanuki reports 24% G3+ toxicities (primarily thrombocytopenia) [22]. The reported G3+ toxities are somewhat lower in Kang et al (11%) (predominantly GI ulceration and thrombocytopenia) [20]. Of these reports, only Bujold assesses post-treatment leukocyte counts, and demonstrated minimal toxicity.
As distant progression and out-of-field hepatic progression remain problematic in the patients receiving SBRT, the role of systemic therapy is being evaluated in the current phase III randomized trial for HCC assessing sorafenib versus SBRT followed by sorafenib (RTOG 1112). The radiation portion of this study consists of a prescription dose of 50 Gy in 5 fractions (lower than the 55 Gy in 5 fractions used in our study), with de-escalation to meet protocol-specified dose constraints in hopes of providing local control benefits with minimization of toxicity. This study shows that there is some fragility of this patient population that needs to be addressed as more patients are treated with this promising modality. Hopefully further studies, with well-defined dose constraints and clear mechanisms for dose de-escalation can increase safety and perhaps even further define the population who is more at risk for toxicity.
Supplementary Material
Acknowledgments
We would like to thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri, for the use of the Clinical Trials Core which provided funding for this study. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The following authors report the following potential conflicts of interest (authors not listed reported no potential conflicts): Dr. Olsen reports grants, travel expenses and speaker’s fees from ViewRay, Inc. Dr. Parikh reports grants from Philips Healthcare and Varian Medical Systems, ownership in Holaira, Inc, and acts as a consultant for Medtronic/Covidien.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep. 2013;29:1259–67. doi: 10.3892/or.2013.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–48. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 6.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–21. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 7.Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJM, Nowak PCJM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase iii study. Acta Oncol. 2006;45:831–7. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 8.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–64. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 9.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RKS, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–9. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 10.Scorsetti M, Comito T, Cozzi L, Clerici E, Tozzi A, Franzese C, et al. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT) J Cancer Res Clin Oncol. 2015 doi: 10.1007/s00432-015-1929-y. [DOI] [PubMed] [Google Scholar]
- 11.Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, et al. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412–7. doi: 10.1016/j.radonc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Kopek N, Holt MI, Hansen AT, Høyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94:47–52. doi: 10.1016/j.radonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–93. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Feng M, Ben-Josef E. Radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21:271–7. doi: 10.1016/j.semradonc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Son SH, Choi BO, Ryu MR, Kang YN, Jang JS, Bae SH, et al. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys. 2010;78:1073–80. doi: 10.1016/j.ijrobp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Dyk P, Weiner A, Badiyan S, Myerson R, Parikh P, Olsen J. Effect of high-dose stereotactic body radiation therapy on liver function in the treatment of primary and metastatic liver malignancies using the Child-Pugh score classification system. Pract Radiat Oncol. 2015;5:176–82. doi: 10.1016/j.prro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Vallet-Pichard A, Rerolle J-P, Fontaine H, Larousserie F, Peraldi M-N, Kreis H, et al. Veno-occlusive disease of the liver in renal transplant patients. Nephrol Dial Transplant. 2003;18:1663–6. doi: 10.1093/ndt/gfg222. [DOI] [PubMed] [Google Scholar]
- 18.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–25. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 19.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–53. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Kang J-K, Kim M-S, Cho CK, Yang KM, Yoo HJ, Kim JH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–31. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 21.Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) fo liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Sanuki N, Takeda A, Oku Y, Eriguchi T, Nishimura S, Aoki Y, et al. Influence of liver toxicities on prognosis after stereotactic body radiotherapy for hepatocellular carcinoma. Hep Res. 2015;45:540–547. doi: 10.1111/hepr.12383. [DOI] [PubMed] [Google Scholar]
- 23.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremicheal RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–91. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, DeWees TA, Badiyan SN, Speirs CK, Mullen DF, Fergus S, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolamike for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92:1000–7. doi: 10.1016/j.ijrobp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Lario M, Munoz L, Ubeda M, Borrero MJ, Martinez J, Monserrat J, et al. Defective thymopoeisis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723–30. doi: 10.1016/j.jhep.2013.05.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


