Abstract
Background
Although several studies have been conducted on the role of surgery in localized neuroblastoma, the impact of surgical timing and extent of primary tumor resection on outcome in high-risk patients remains controversial.
Methods
Patients from the German neuroblastoma trial NB97 with localized neuroblastoma INSS stage 1–3 age > 18 months were included for retrospective analysis. Imaging reports were reviewed by two independent physicians for Image Defined Risk Factors (IDRF). Operation notes and corresponding imaging reports were analyzed for surgical radicality. The extent of tumor resection was classified as complete resection (95–100%), gross total resection (90–95%), incomplete resection (50–90%), and biopsy (<50%) and correlated with local control rate and outcome. Patients were stratified according to the International Neuroblastoma Risk Group (INRG) staging system. Survival curves were estimated according to the method of Kaplan and Meier and compared by the log-rank test.
Results
A total of 179 patients were included in this study. 77 patients underwent more than one primary tumor operation. After best surgery, 68.7% of patients achieved complete resection of the primary tumor, 16.8% gross total resection, 14.0% incomplete surgery, and 0.5% biopsy only. The cumulative complication rate was 20.3% and the surgery associated mortality rate was 1.1%. Image defined risk factors (IDRF) predicted the extent of resection. Patients with complete resection had a better local-progression-free survival (LPFS), event-free survival (EFS) and OS (overall survival) than the other groups. Subgroup analyses showed better EFS, LPFS and OS for patients with complete resection in INRG high-risk patients. Multivariable analyses revealed resection (complete vs. other), and MYCN (non-amplified vs. amplified) as independent prognostic factors for EFS, LPFS and OS.
Conclusions
In patients with localized neuroblastoma age 18 months or older, especially in INRG high-risk patients harboring MYCN amplification, extended surgery of the primary tumor site improved local control rate and survival with an acceptable risk of complications.
Keywords: Surgical oncology, High-risk neuroblastoma, Localized neuroblastoma, Neuroblastoma surgery
Background
Neuroblastoma is the most common solid extracranial malignancy of childhood and the most common malignant tumor in infants [1]. Despite the advances in multidrug therapy, surgery still plays a major role in treatment of neuroblastoma (NB) [2]. Children presenting with localized disease have an overall better prognosis, mainly depending on the degree of tumor resection [3]. Indeed, completely resected tumors (INSS stage 1) rarely relapse and do not require postoperative chemotherapy [4, 5]. There is, however, still a controversy regarding the value of radical surgery for extended local disease [2]. Biological and clinical prognostic markers may help to stratify risks and guide therapy in these patients, but prospective randomized trials with surgical endpoints are still missing. In 2008, the INRG group implemented a pretreatment risk stratification considering age, MYCN amplification, histological tumor grade, Image Defined Risk Factors and 11q deletion to classify NB patients into very low-, low-, intermediate- and high-risk patients [6]. In this study, we analyzed the impact of the extent of tumor resection on outcome of patients older than 18 months with localized non-metastatic NB aged, who were treated according to the German prospective clinical trial NB97.
Methods
All patients registered by the German prospective clinical trial NB97 between November 1, 1996, and September 30, 2004, were included in this analysis when they met the following criteria: stage 1–3 neuroblastoma diagnosed according to the International Neuroblastoma Staging System criteria [7]; age at diagnosis >18 months but less than 21 years. Written informed consent was obtained from patients or their guardians for participation in the study design, data collection and treatment (Registration number: NCT00017225, ClinicalTrials.gov).
The NB97 trial was a randomized trial comparing ASCT (autologous stem cell transplantation) and oral maintenance chemotherapy in high-risk patients. According to the NB97 protocol, the patients with localized NB were prospectively stratified in NB97 high-risk, NB97 standard-risk or NB97 low-risk subgroups according to INSS stage, age, MYCN-Status and threatening symptoms. NB97 standard-risk patients received 4 cycles of chemotherapy after first surgery and a second resection when necessary whereas NB97 low-risk patients were observed for up to 12 months with examinations and staging every 6 weeks. NB97 high-risk patients were randomized and received ASCT or oral maintenance therapy. The trial protocol had been evaluated by the Institutional Ethical Boards of the University of Cologne and participating hospitals. All patients participated in the trial after informed consent and on voluntary basis. Trial protocol and results of the primary trial end point have been published before [8, 9]. The NB97 protocol also provided clear recommendations on timing and extent of tumor resection. For example, initial or delayed complete resection was advised when no vascular structures or adjacent organs were involved. Incomplete resection was acceptable to reduce the risk of acute complications and long-term organ impairments. Nephrectomy or insertion of vascular prostheses was discouraged. Therefore, complete initial resection was reserved only for patients with well-encapsulated primary tumors. All other patients were recommended to undergo second look operations after four to six cycles of induction chemotherapy. Moreover, radiation therapy of 40 Gy was advised for patients with unresectable residuals of the primary tumor present after induction chemotherapy. Finally, radiation doses less than 40 Gy were applied to protect adjacent structures with low radiation tolerance [10]. Data on extent and complications of resection were collected prospectively using case report forms.
For this analysis, imaging reports, operation notes and pathology reports were retrospectively reviewed by two independent experienced physicians, and discrepant results were clarified after repeated joint review of the patients’ files. In this study, we distinguished between two types of operations, as described before [11]. Briefly, first operation was the tumor operation performed before or within the first six cycles of induction chemotherapy, and best operation was the most extensive removal of primary tumor tissue done at any time during first-line therapy. For outcome analysis, the extent of resection was classified as follows: no operation or biopsy removing less than 50% of tumor tissue; incomplete resection of 50% to less than 90% of tumor volume present at the time of surgery; gross total resection removing more than 90% of the tumor; or complete resection without macroscopic postoperative tumor residuals. It was not possible to include the category of microscopic complete resection because neuroblastomas were rarely removed in toto, and therefore, the pathologist often received several tumor fragments, making confirmation of microscopic complete resection impossible. IDRF were not established in 1997, and therefore, they had to be assessed retrospectively by review of initial imaging and operation notes based on the definitions published by the European International Society of Pediatric Oncology Neuroblastoma Group [12] and the International Neuroblastoma Risk Group [13, 14]. During induction chemotherapy, re-staging, including magnetic resonance imaging of the primary tumor site, bone marrow assessment, and metaiodobenzylguanidine (MIBG) scintigraphy, was scheduled after four to six chemotherapy cycles. If progression or relapse was suspected, complete staging was necessary. Disease status and response to treatment were categorized as complete remission, very good partial remission, partial remission, stable disease, or progression according to the published International Neuroblastoma Response Criteria [7]. Pretreatment Risk Stratification was performed retrospectively according to INRG staging system [6] considering age, MYCN amplification, histological grade, IDRF and 11q deletion stratifying all patients into INRG very low-, INRG low-, INRG intermediate- and INRG high-risk. Histology was defined by INPC guidelines as described before [15].
Statistical analysis
For statistical analysis, the IBM SPSS software (Version 24.0.0; Armonk, NY, IBM Corp.) and R version 3.3.1 were used. Proportions were compared by Fisher’s exact test. Survival curves were estimated according to the method of Kaplan and Meier and compared by the log-rank test. Event-free survival (EFS) time was calculated as the time from diagnosis to event or last examination if the patient had no event. Relapse, progression, and death from disease or surgery-related were regarded as events. Local progression–free survival (LPFS) was calculated from diagnosis to relapse or progression of the primary tumor site or last examination if the patient had no local recurrence. This means that patients who died from metastatic disease without progression at the primary tumor site were censored at the time of death for LPFS analysis. Overall survival (OS) was calculated as the time from diagnosis to death from disease or surgery-related or last examination if the patient survived. Multivariable backward selected Cox regression analyses were performed to analyze the prognostic value of the following factors: stage (1 or 2 vs. 3), extent of best resection (complete vs. other), MYCN status (amplified vs. not amplified) and IDRF (no vs. >1). The likelihood ratio test p-value for inclusion was p ≤ 0.05.
Results
Patients
Among 1121 patients in the NB97 trial, 191 patients with INSS stage 1–3 >18 months were eligible for analysis. Patients with INSS stage 4 disease (n = 383) and patients with non-stage 4 neuroblastoma <18 months of age (n = 547) did not meet inclusion criteria. Two patients with ganglioneuroma and 10 patients with an early progression within the first 40 days of chemotherapy were excluded from the study. 179 patients with localized neuroblastoma met all inclusion criteria (Fig. 1). The median age at diagnosis was 3.64 years (range, 1.5 to 20.8 years), and the median observation time of the surviving patients was 10.2 years (range, 0.5 to 17.3 years). 105 events were recorded. Among 30 relapses, there were 9 local relapses and in 35 patients with progression, we found 27 localized progressions of the primary tumor site. Thirty-three patients died. Table 1 lists details of the patients’ key characteristics.
Fig. 1.
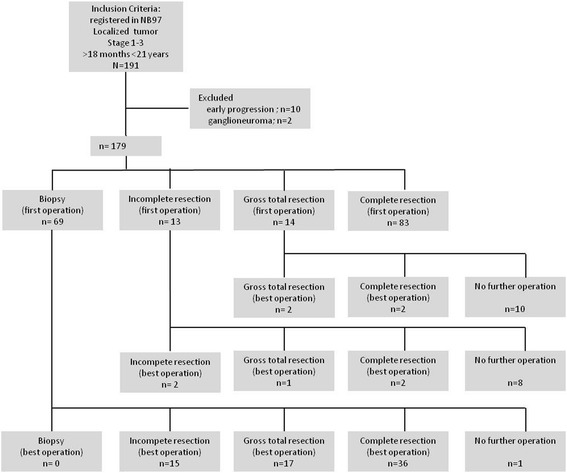
Patient flow diagram
Table 1.
Key demographic and clinical characteristics of 179 patients with localized NB >18 months
| Characteristic | No. | % |
|---|---|---|
| Sex | ||
| male | 86 | 48.0 |
| female | 93 | 52.0 |
| Primary tumor site | ||
| Neck | 4 | 2.2 |
| Chest | 38 | 21.2 |
| Abdomen | 47 | 26.3 |
| Adrenal | 80 | 44.7 |
| Pelvis | 7 | 3.9 |
| combined | 3 | 1.7 |
| MYCN status | ||
| non-amplified | 140 | 78.2 |
| amplified | 36 | 20.1 |
| not available | 3 | 1.7 |
| INSS stage | ||
| stage 1 | 68 | 38.0 |
| stage 2 | 46 | 25.7 |
| stage 3 | 65 | 36.3 |
| NB97 risk group | ||
| low | 96 | 53.6 |
| standard | 36 | 20.1 |
| high | 47 | 26.1 |
| IDRF | ||
| yes | 70 | 39.1 |
| no | 73 | 40.8 |
| not available | 36 | 20.1 |
| INRG risk group | ||
| very low | 88 | 49.2 |
| low | 4 | 2.2 |
| intermediate | 23 | 12.8 |
| high | 36 | 20.1 |
| not available | 28 | 15.6 |
| First operation | ||
| biopsy only | 69 | 38.5 |
| incomplete resection | 13 | 7.3 |
| gross total resection | 14 | 7.8 |
| complete resection | 83 | 46.4 |
| Last operation | ||
| biopsy only | 0 | 0 |
| incomplete resection | 17 | 9.5 |
| gross total resection | 20 | 11.2 |
| complete resection | 40 | 22.3 |
| Best operation | ||
| biopsy only | 1 | 0.6 |
| incomplete resection | 25 | 14.0 |
| gross total resection | 30 | 16.8 |
| complete resection | 123 | 68.7 |
| Chemotherapy | ||
| yes | 82 | 45.8 |
| no | 97 | 54.2 |
| RT of primary tumor site | ||
| yes | 7 | 3.9 |
| no | 172 | 96.1 |
| myeloablative Chemo with ASCT | ||
| yes | 23 | 12.8 |
| no | 156 | 87.2 |
Before or during induction chemotherapy, complete resection was achieved in 83 patients (46.4%), gross total resection in 14 patients (7.8%), incomplete resection in 13 patients (7.3%), and biopsy in 69 patients (38.5%). For 8 patients no imaging reports were available and extent of resection was based on the case report forms of the trial only. During treatment, 77 patients had at least one more surgical resection of primary tumor site. After best surgery, 68.7% of patients achieved complete resection of the primary tumor, 16.8% gross total resection, 14.0% incomplete surgery, and 0.5% biopsy only.
The most radical resection of the primary tumor was achieved during first operation in 106 patients (59.2%) and during delayed surgery in 73 patients (40.8%). According to the protocol guidelines, 3.9% of all patients underwent external-beam radiation therapy of unresectable residuals of the primary tumor, in which case a median dose of 36 Gy was applied.
Complications during first and best operation
A total of 56 patients experienced complications during 276 surgical interventions (20.3%) when undergoing first-line treatment for neuroblastoma. During first operation (n = 179) horner’s syndrome (3.9%), organ injury (3.4%) and intraoperative bleeding (2.8%) were the most frequent complications. No surgical related deaths occurred and the total complication rate was 19.6%. All other complications (infection, ileus, vessel or nerve injury) occurred in <1.7% of patients, respectively.
At best operation (n = 179) 50 complications in 40 patients occurred including horner’s syndrome with 5.6%, while organ injury and major bleeding/vessel injury account up to 4.4% and 3.9%, respectively. Two patients died due to surgery (1.1%). The complication rate at best surgery was 27.9%. Twenty-one patients (52.5%) out of 40 patients who experienced one or more complications had more than one operation.
The complication rate was not significantly different between patients with complete resection after best surgery (22.4%), patients with gross total resection (13.3%) and incomplete resection (32%; p = 0.395).
Outcome after first operation
Complete resection could be achieved in 83/179 patients (46.4%). The 5-year EFS rate of all patients with complete resection was 87.8% ± 3.6%, the 5-year LPFS rate was 93.6% ± 2.7%, and the 5-year OS rate was 92.3% ± 3.0%. For patients with gross total resection, 5-year EFS and LPFS rate were 78.6% ± 11.0%, and the 5-year OS rate was 92.9% ± 6.9%. After incomplete resection, 5-year EFS and LPFS rate were 66.7% ± 13.6%, and the 5-year OS rate was 83.3% ± 10.8%. Patients with biopsy only had a 5-year EFS rate of 61.5% ± 5.9%, a 5-year LPFS rate of 66.7% ± 6.0%, and a 5-year OS rate of 75.8% ± 5.3% after first operation. The extent of first operation had an impact on EFS (p = 0.000), LPFS (p = 0.000), and OS (p = 0.018; Fig. 2; Table 2). Of note, any surgical resection compared to biopsy improved patient’s outcome in EFS and LPFS (p = 0.001) and OS (p = 0.002).
Fig. 2.
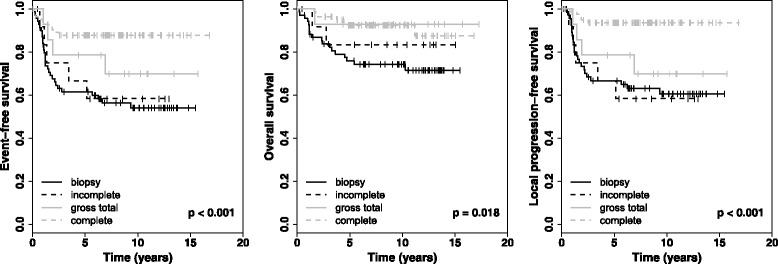
Kaplan-Meier-curves for all patients with localized NB (n = 179) after first operation
Table 2.
Results of univariate outcome analysis of 179 patients with localized NB >18 months
| Biopsy (<50%) | Incomplete resection (50–90%) | Gross total resection (90–95%) | Complete resection (>95%) | P (comparing four groups) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Survival | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| First operation | |||||||||
| No. of Patients | 69 | 13 | 14 | 83 | |||||
| 5-year EFS | 61.5 | 5.9 | 66.7 | 13.6 | 78.6 | 11.0 | 87.8 | 3.6 | 0.000 |
| 5-year OS | 75.8 | 5.3 | 83.3 | 10.8 | 92.9 | 6.9 | 92.3 | 3.1 | 0.018 |
| 5-year LPFS | 66.7 | 6.0 | 66.7 | 13.6 | 78.6 | 11.0 | 93.6 | 2.8 | 0.000 |
| Best operation | |||||||||
| No. of Patients | 1 | 25 | 30 | 123 | |||||
| 5-year EFS | censored | censored | 58.0 | 10.2 | 59.8 | 9.0 | 82.8 | 3.4 | 0.001 |
| 5-year OS | censored | censored | 70.5 | 9.4 | 75.4 | 8.1 | 90.8 | 2.7 | 0.020 |
| 5-year LPFS | censored | censored | 66.2 | 10.4 | 62.7 | 9.0 | 87.1 | 3.1 | 0.001 |
Outcome after best operation
Complete resection could be achieved in 123/179 patients (68.7%). The extent of resection at the best operation was associated with better EFS (p = 0.001), better LPFS (p = 0.001), and better OS (p = 0.020). The 5-year EFS rate of patients with complete resection was 82.8.0% ± 3.4%, the 5-year LPFS rate was 87.1% ± 3.1%, and the 5-year OS rate was 90.8% ± 2.7%. For patients with gross total resection, 5-year EFS rate was 59.8% ± 9.0%, 5-year LPFS rate was 62.7% ± 9.0%, and the 5-year OS rate was 75.4% ± 8.1%. After incomplete resection, 5-year EFS rate was 58.0% ± 10.2%, 5-year LPFS rate was 66.2% ± 10.5%, and the 5-year OS rate was 70.5% ± 9.4%. One patient had a biopsy only and did not undergo a second operation, because he died eight months after diagnosis while undergoing ASCT. The 5-year survival rates are listed in Table 2, and life-tables are shown in Fig. 3. Multivariable analysis revealed resection (complete vs. other), and MYCN (non-amplified vs. amplified) as independent prognostic factors for EFS and OS.
Fig. 3.

Kaplan-Meier-curves for all patients with localized NB (n = 179) after best operation
INSS stage and MYCN amplification
Subgroup analysis according to INSS stage after best operation confirmed all patients with stage 1 disease underwent complete surgical resection. In patients with INSS stage 2, there was no significant difference between incomplete resection, gross total resection and complete resection in EFS (p = 0.901), LPFS (p = 0.520) and OS (p = 0.446). But patients with INSS stage 3 disease showed a statistical significance according to the extent of resection in EFS (p = 0.032) and LPFS (p = 0.034), but not in OS (p = 0.053). Analyzing stage 3 patients with MYCN amplification (n = 29) and without MYCN amplification (n = 35) separately, MYCN-amplified patients showed a better outcome correlated to the extent of resection at best operation (EFS p = 0.001; LPFS p = 0.001; OS p = 0.002) whereas non-MYCN-amplified patients did not (EFS p = 0.411; LPFS p = 0.177; OS p = 0.905).
INRG
According to INRG pretreatment risk classification 151 patients could be analyzed retrospectively. All patients with MYCN amplification belong to the high-risk group, resulting in 36 patients (20.1%). Patients with a histological category of GNB intermixed without MYCN amplification or IDRF (L1) are categorized as very low-risk (n = 88, 49.2%). Any patients with NB or nodular GNB harboring more than one IDRF (L2) may belong to low- (n = 4, 2.2%) or intermediate- risk (n = 23, 12.8%) depending on grade of tumor differentiation and 11 q aberration.
High-risk patients (n = 36) show a better EFS (p = 0.001), LPFS (p = 0.001) and OS (p = 0.001) after best surgery when tumor resection was complete (Fig. 4). Patients belonging to intermediate-risk did not benefit from complete surgery (EFS p = 0.411; LPFS p = 0.177; OS p = 0.905).
Fig. 4.
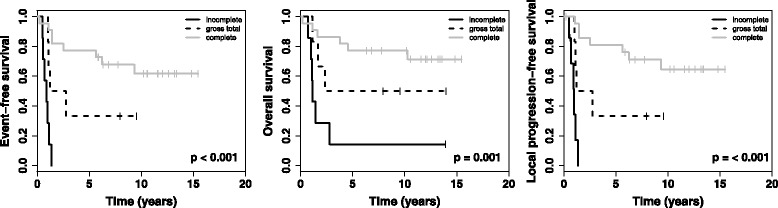
Kaplan-Meier-curves for INRG high-risk patients after best operation
Discussion
In our cohort, extended surgery of the primary tumor site improved local control rate and outcome in patients older than 18 months with localized neuroblastoma. After complete resection during treatment the patients had a better local-progression-free survival (LPFS), event-free survival (EFS) and OS than the other groups. The wide range of clinical manifestations of neuroblastomas, their localization and their resectability leads to a high heterogeneity of our cohort. The NB97 protocol clearly recommended less aggressive surgical approach particularly during first operation in an effort to avoid surgical complications. Most surgeons choose a less aggressive approach when the disease was considered to be unresectable during operation. Thus, the question whether patients did not undergo complete resection because of less aggressive surgical approaches, or simply due to unresectable disease cannot be answered retrospectively. Moreover, less aggressive surgeons are more likely to classify patients as unresectable and vice versa. Prospective studies with a statement of the surgeon’s decision in his/hers operation notes are needed to address different approaches.
However, subgroup analyses showed better EFS and OS for patients with complete resection in INSS stage 3 and MYCN amplification. Patients with MYCN amplification belong to the INRG high-risk stratification and are therefore of special interest since the role of surgery in high-risk patients remains controversial. Other studies of high-risk patients especially evaluating stage 4 patients show no advantage of complete resection on patient’s outcome [11, 16], while lower local recurrence rates and better OS are reported after extended surgery by La Quaglia et al. [17, 18]. But analyzing localized neuroblastoma in patients >1 year, radical surgery is recommended which is in line with our findings [2]. A meta-analysis of 2599 patients with stage 3 and 4 neuroblastomas from 33 studies demonstrated that relative risk of mortality was decreased in patients who underwent >90% resection [19]. Our study indicates that complete surgical resection of the primary tumor site should be attempted in INRG high-risk patients.
Risk of complications at first and best operation is comparable to previously published data [2]. Since the presence of IDRF predicts the risk of complications and the extent of surgery, the impact of IDRF should become an integrated part of therapy planning as shown before [20–22], but cannot be safely used as an independent risk factor for outcome. Therefore, INRG classification considering IDRF, histological category, MYCN amplification, grade of tumor differentiation and chromosomal aberrations, was applied in our study and showed a significant difference in high-risk patients with complete surgical resection of the tumor. Future trials should encourage a complete resection of the primary tumor in INRG high-risk patients with a more aggressive approach to improve outcome with an acceptable risk of complication, but probable life-threatening complications should still be avoided.
In this study postoperative staging to assess the presence and extent of possible residual tumors was carried out by computed tomography, magnetic resonance imaging or ultrasound, but MRI was recommended as gold standard for follow-up. The interval between surgery and imaging varied between one and three months postoperatively, which can result in differences of the extent of resection described by imaging studies and by operation notes. Whenever there was inconsistency (26.7% of all cases), joint review with a radiologist was performed.
Even in patients with localized neuroblastoma older than 18 months, spontaneous regression or differentiation can occur at any time throughout therapy. This should be considered in patients with incomplete resection or gross total resection achieved at first operation. In this study, only few patients with incomplete/gross total resection after first operation underwent second operations to achieve complete resection, whereas most patients did not (9 vs. 18). Due to the small number of patients, a benefit of further surgery in these cases remains unclear. Possibly, molecular characterization of the primary tumors will be able to answer these questions in the future.
Conclusion
In patients with localized neuroblastoma >18 months, especially with INRG high-risk classification, extended surgery improves EFS, LPFS and OS and should therefore be attempted.
Acknowledgements
We thank all patients and their parents for consent and participation. We thank all staff of the NB97 study, especially Monika Schmitz and Martina Breuer for their help with data collection.
Funding
The study NB 97 was funded by the German Cancer Aid (grant no. 70–2290-Be) to FB. The funding body was neither involved in the design of the study nor collection, analysis, interpretation of data or in writing the manuscript.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASCT
autologous stem cell transplantation
- EFS
event-free survival
- GNB
Ganglioneuroblastoma
- Gy
Gray
- IDRF
image defined risk factor
- INPC
International Neuroblastoma Pathology Classification
- INRG
International Neuroblastoma Risk Group
- INSS
International Neuroblastoma Staging System
- LPFS
local-progression-free survival
- MIBG
metaiodobenzylguanidine scintigraphy
- MRI
magnetic resonance imaging
- MYCN
oncogene
- NB
neuroblastoma
- OS
overall survival
- RT
radiotherapy
- vs.
versus
Authors’ contributions
Conception and design: JF, TS, BH, FB, DS. Administrative support: FB, DS. Collection and assembly of data: JF, AP, GC, MD, Data analysis and interpretation: JF, AP, RV, TS, BH. Manuscript writing: All authors. All authors have read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The trial was approved by Ethics Committee of the University Hospital of Cologne (no. 9764). Written informed consent was obtained from patients or their guardians for participation in the study design, data collection and treatment (Registration number: NCT00017225, ClinicalTrials.gov).
Consent for publication
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Janina Fischer, Phone: 0049-152-28925871, Email: Janina.fischer_@uk-koeln.de.
Alexandra Pohl, Email: Alexandra.Pohl@med.uni-muenchen.de.
Ruth Volland, Email: ruth.volland@uk-koeln.de.
Barbara Hero, Email: Barbara.hero@uk-koeln.de.
Martin Dübbers, Email: martin.duebbers@uk-koeln.de.
Grigore Cernaianu, Email: grigorec@hotmail.com.
Frank Berthold, Email: frank.berthold@uk-koeln.de.
Dietrich von Schweinitz, Email: Dietrich.Schweinitz@med.uni-muenchen.de.
Thorsten Simon, Email: Thorsten.simon@uk-koeln.de.
References
- 1.Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24(1):65–86. doi: 10.1016/j.hoc.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 2.von Schweinitz D, Hero B, Berthold F. The impact of surgical radicality on outcome in childhood neuroblastoma. Eur J Pediatr Surg. 2002;12(6):402–409. doi: 10.1055/s-2002-36952. [DOI] [PubMed] [Google Scholar]
- 3.De Bernardi B, Mosseri V, Rubie H, Castel V, Foot A, Ladenstein R, Laureys G, Beck-Popovic M, de Lacerda AF, Pearson AD, et al. Treatment of localised resectable neuroblastoma. Results of the LNESG1 study by the SIOP Europe neuroblastoma group. Br J Cancer. 2008;99(7):1027–1033. doi: 10.1038/sj.bjc.6604640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushner BH, Cheung NK, LaQuaglia MP, Ambros PF, Ambros IM, Bonilla MA, Ladanyi M, Gerald WL. International neuroblastoma staging system stage 1 neuroblastoma: a prospective study and literature review. J Clin Oncol. 1996;14(7):2174–2180. doi: 10.1200/JCO.1996.14.7.2174. [DOI] [PubMed] [Google Scholar]
- 5.Alvarado CS, London WB, Look AT, Brodeur GM, Altmiller DH, Thorner PS, Joshi VV, Rowe ST, Nash MB, Smith EI, et al. Natural history and biology of stage a neuroblastoma: a pediatric oncology group study. J Pediatr Hematol Oncol. 2000;22(3):197–205. doi: 10.1097/00043426-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, et al. The international neuroblastoma risk group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 8.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(9):649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 9.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, Berthold F. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21. doi: 10.1186/1471-2407-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon T, Hero B, Bongartz R, Schmidt M, Muller RP, Berthold F. Intensified external-beam radiation therapy improves the outcome of stage 4 neuroblastoma in children > 1 year with residual local disease. Strahlenther Onkol. 2006;182(7):389–394. doi: 10.1007/s00066-006-1498-8. [DOI] [PubMed] [Google Scholar]
- 11.Simon T, Haberle B, Hero B, von Schweinitz D, Berthold F. Role of surgery in the treatment of patients with stage 4 neuroblastoma age 18 months or older at diagnosis. J Clin Oncol. 2013;31(6):752–758. doi: 10.1200/JCO.2012.45.9339. [DOI] [PubMed] [Google Scholar]
- 12.Cecchetto G, Mosseri V, De Bernardi B, Helardot P, Monclair T, Costa E, Horcher E, Neuenschwander S, Toma P, Rizzo A, et al. Surgical risk factors in primary surgery for localized neuroblastoma: the LNESG1 study of the European International Society of Pediatric Oncology Neuroblastoma Group. J Clin Oncol. 2005;23(33):8483–8489. doi: 10.1200/JCO.2005.02.4661. [DOI] [PubMed] [Google Scholar]
- 13.Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK, Nuchtern JG, et al. The international neuroblastoma risk group (INRG) staging system: an INRG task force report. J Clin Oncol. 2009;27(2):298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brisse HJ, McCarville MB, Granata C, Krug KB, Wootton-Gorges SL, Kanegawa K, Giammarile F, Schmidt M, Shulkin BL, Matthay KK, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the international neuroblastoma risk group project. Radiology. 2011;261(1):243–257. doi: 10.1148/radiol.11101352. [DOI] [PubMed] [Google Scholar]
- 15.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, et al. The international neuroblastoma pathology classification (the Shimada system) Cancer. 1999;86(2):364–372. doi: 10.1002/(SICI)1097-0142(19990715)86:2<364::AID-CNCR21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.McGregor LM, Rao BN, Davidoff AM, Billups CA, Hongeng S, Santana VM, Hill DA, Fuller C, Furman WL. The impact of early resection of primary neuroblastoma on the survival of children older than 1 year of age with stage 4 disease: the St. Jude Children's Research Hospital Experience. Cancer. 2005;104(12):2837–2846. doi: 10.1002/cncr.21566. [DOI] [PubMed] [Google Scholar]
- 17.La Quaglia MP, Kushner BH, Heller G, Bonilla MA, Lindsley KL, Cheung NK. Stage 4 neuroblastoma diagnosed at more than 1 year of age: gross total resection and clinical outcome. J Pediatr Surg. 1994;29(8):1162–1165. doi: 10.1016/0022-3468(94)90301-8. [DOI] [PubMed] [Google Scholar]
- 18.La Quaglia MP, Kushner BH, Su W, Heller G, Kramer K, Abramson S, Rosen N, Wolden S, Cheung NK. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004;39(3):412–417. doi: 10.1016/j.jpedsurg.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 19.La Quaglia MP. State of the art in oncology: high risk neuroblastoma, alveolar rhabdomyosarcoma, desmoplastic small round cell tumor, and POST-TEXT 3 and 4 hepatoblastoma. J Pediatr Surg. 2014;49(2):233–240. doi: 10.1016/j.jpedsurg.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 20.La Quaglia MP. The role of primary tumor resection in neuroblastoma: when and how much? Pediatr Blood Cancer. 2015;62(9):1516–1517. doi: 10.1002/pbc.25585. [DOI] [PubMed] [Google Scholar]
- 21.Simon T, Hero B, Benz-Bohm G, von Schweinitz D, Berthold F. Review of image defined risk factors in localized neuroblastoma patients: results of the GPOH NB97 trial. Pediatr Blood Cancer. 2008;50(5):965–969. doi: 10.1002/pbc.21343. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda A, Nishikawa M, Uehara S, Oue T, Usui N, Inoue M, Fukuzawa M, Okuyama H. Can image-defined risk factors predict surgical complications in localized neuroblastoma? Eur J Pediatr Surg. 2016;26(1):117–122. doi: 10.1055/s-0035-1566100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


