Abstract
Background:
There is a lack of evidence-based therapies for the treatment of heart failure (HF) with preserved ejection fraction (HFpEF). Beta blockers may provide some benefit in HFpEF due to their proven role in HF with reduced ejection fraction.
Aim:
The main objective of the present study was to evaluate the efficacy of controlled-release metoprolol (metoprolol succinate) in HFpEF.
Materials and Methods:
This was an investigator-initiated, randomized, double-blind, placebo-controlled, 14-week pilot study with metoprolol succinate as a study drug. Dose titration protocol was used with optional upward titration of doses ranging from 25 to 100 mg. The end points included clinical, echocardiographic, biochemical (N-terminal pro-B-type natriuretic peptide and serum carboxy-terminal propeptide of procollagen type I), and quality of life (QoL) (SF-36) parameters.
Results:
Twenty patients were enrolled in each of the treatment arms. An improvement in New York Heart Association class and exercise capacity was seen in both treatment arms. The mean change in various echocardiographic and biochemical parameters between the two groups was statistically insignificant. A significant improvement in some QoL parameters was observed in both the groups. No serious adverse events were seen.
Conclusion:
Hence, this pilot study showed that metoprolol succinate possibly has some beneficial role in HFpEF as reflected by improvement in some parameters. The findings highlight the need of a larger study with longer follow-up to provide a definitive answer.
Keywords: Diastolic dysfunction, heart failure, metoprolol, tissue Doppler, treadmill test
INTRODUCTION
Heart failure (HF) with preserved ejection fraction (HFpEF) is a clinical syndrome of exercise intolerance and/or congestion, concentric left ventricular (LV) hypertrophy (LVH) or remodeling, and abnormalities in both active LV relaxation and LV stiffness indicating diastolic dysfunction in the presence of a LV ejection fraction (EF) within normal limits (i.e., LVEF >50%). Around half of HF patients have HFpEF. Although these patients have a better outcome than those with reduced EF, the morbidity and mortality associated with HFpEF is considerable.[1]
In contrast to systolic HF, evidence base for the treatment of HFpEF is weaker. The current management of patients with HFpEF is directed to symptomatic relief of congestion with diuretics and risk factor modification.[2] According to the American College of Cardiology American Heart Association 2013 guidelines for the management of HF in adults, Class I recommendations for pharmacological treatment of HFpEF include control of systolic and diastolic hypertension (HTN) (level of evidence B) and use of diuretics for relief of volume overload symptoms (level of evidence C). Other recommendations include the use of beta-blocking agents, angiotensin converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) in patients having HTN with HFpEF, control of ventricular rate in patients with HFpEF and atrial fibrillation, and restoration and maintenance of sinus rhythm in patients with atrial fibrillation (Class IIa, level of evidence C).[3] As most of the recommendations are based on expert opinion (level of evidence C), there is a need to generate data in this field.
Various therapies with established role in systolic HF have been tested in HFpEF. These include ARBs such as candesartan (the CHARM-PRESERVED trial),[4] irbesartan (the I-PRESERVED [Irbesartan in HFpEF Study]),[5] and valsartan (the VALsartan in Diastolic Dysfunction study);[6] ACE inhibitors such as perindopril (Perindopril for Elderly People With Chronic HF trial);[7] digoxin (Digitalis Intervention Group-Preserved EF);[8] aldosterone antagonists such as spironolactone (Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist[9] and Effect of Spironolactone on Diastolic Function and Exercise Capacity in Patients with HFpEF [ALDO-DHF])[10] and eplerenone (Randomized Aldosterone Antagonism in HFpEF;[11] and sildenafil (Effect of Phosphodiesterase 5 Inhibition on Exercise Capacity and Clinical Status in HFpEF trial).[12] However, none succeeded to provide survival benefit compared to placebo.
The hallmark of HFpEF is increased sympathetic activity and LVH. Beta blockers have been proposed to offer potential benefits in HFpEF by causing changes in adrenergic receptor profiles, reducing risk of arrhythmias, and improving ventricular remodeling and metabolic efficiency.[13,14] However, evidence base for the use of beta blockers in this condition is limited. A number of cohort studies and meta-analyses reported a nonsignificant influence on mortality and rehospitalization risks with beta blockers in HFpEF.[15,16,17,18] Few researchers reported a beneficial effect of carvedilol, a combined alpha and beta blocker in diastolic HF with preserved systolic function.[19,20,21] Japanese diastolic HF study (J-DHF), however, failed to show a significant advantage of carvedilol over placebo on composite end point of cardiovascular death and hospitalization.[22] Aronow et al. showed that propranolol reduces mortality and nonfatal myocardial infarction (MI), improves LVEF, and reduces LV mass in older patients with CHF, prior MI, and LVEF ≥40% treated with diuretics plus ACE inhibitors.[23] However, this was an uncontrolled study. In another study, atenolol caused reduction in circulating levels of the procollagen peptide type 1 and improvement in diastolic function.[24] Nodari et al. observed an improvement in E: A ratio and hemodynamics with nebivolol in patients with HTN and LV diastolic dysfunction.[25] A subgroup analysis of the SENIORS trial (Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with HF)[26] in patients with EF >35% showed a nonsignificant reduction in the primary end point of all-cause mortality or cardiovascular hospitalizations. However, the patient population was >70 years of age, and the trial results showed that nebivolol was less effective as the age increased. Another study with nebivolol could not demonstrate improvement in exercise capacity in patients with HFpEF.[27]
Metoprolol is one of the most widely accepted drugs in HF, but its role in HFpEF is not established. β-PRESERVE study evaluating the long-term effects of metoprolol succinate on morbidity and mortality in HFpEF is ongoing.[28] Controlled release metoprolol (metoprolol succinate) provides additional advantage of providing sustained effects and hence once daily administration. It was decided to first conduct a pilot study to determine feasibility and incorporate any changes in the methodology of the full study based on the results obtained in this study. The data obtained in the pilot study will not be included in the final complete study.
MATERIALS AND METHODS
This was an investigator-initiated, randomized, double-blind, single center, placebo-controlled, pilot, 14-week parallel group study having 2 arms: HFPEF patients treated with metoprolol succinate or placebo. Patients with HFPEF visiting the Cardiology Outpatient Department and HTN clinic of Postgraduate Institute of Medical Education and Research, Chandigarh, India, were screened for inclusion in the study. They were included, if they were 18 years and above, had New York Heart Association (NYHA) functional Class II–III of at least 4 weeks' duration, LVEF ≥50% in a nondilated LV (LV end-diastolic volume < 97 ml/m2 measured by echocardiography), echocardiographic evidence of LV diastolic dysfunction, and were willing to give written informed consent. They were excluded if: (1) Clinically unstable as defined by any change in diuretic dose in the month before enrollment, (2) significant valvular heart disease, pericardial disease, hypertrophic or restrictive cardiomyopathy, (3) unstable angina or MI within past 4 weeks, (4) any previous LVEF below 40%, (5) any contraindication to metoprolol use, (6) patients already on beta blockers which cannot be withdrawn, (7) current participation (including prior 30 days) in any other therapeutic trial, and (8) any condition that, in the opinion of investigator, may prevent the participant from adhering to the trial protocol.
A single-blind placebo run in the period of 2 weeks was given for all eligible participants during which beta blockers were withdrawn, if a patient was receiving them but coexisting therapies were continued. After the run-in period, the participants were block randomized (constant block length of 4 throughout the study) in a 1:1 ratio to metoprolol succinate 25 mg or placebo. Randomization and allocation sequence generation were done by investigators not directly involved in the evaluation of outcomes. The randomization code was revealed to the investigators once recruitment, data collection, and analyses were complete. During the study, follow-up was done every 2 weeks for 12 weeks. A dose upward titration protocol with monitoring of blood pressure and heart rate (target blood pressure and heart rate as 120/80 mm Hg and 60 beats/min, respectively) was implemented for dose increments up to a maximum dose of 100 mg once daily. For patients not tolerating increased titration of drug, temporary reduction in dosage was done and decision on further escalation made on individual basis by the treating cardiologist. Clinical, biochemical, and echocardiographic parameters were assessed at baseline and after 12 weeks. Compliance was assessed by pill counting and questionnaire.
The primary outcome was proportion of patients showing improvement of ≥1 in NYHA class at 12 weeks with metoprolol succinate compared to placebo. The secondary outcomes included alteration in exercise capacity using exercise stress testing (treadmill test using Bruce protocol), change in tissue Doppler indices of diastolic dysfunction, change in LV wall thickness and LV mass on echocardiography, change in N-terminal pro-B-type natriuretic peptide (NT-proBNP) and propeptide of procollagen type I (PICP) levels (estimated by ELISA), and alteration in quality of life (QoL) using SF-36 questionnaire. The safety end point included the incidence and type of adverse events and a number of study drug withdrawals. To avoid interobserver variability, all the echocardiographic parameters were evaluated by a single cardiologist who was blinded to study medication and the order of assessment.
The study protocol was approved by the Institutional Ethics Committee, and all the participants signed a written informed consent before enrollment to the study. The study was conducted in accordance with ethical guidelines for biomedical research on human beings, Indian Council of Medical Research. The trial is registered with clinical trial registry-India (REFCTRI-2010 000438).
Statistical analysis
Considering an alpha error of 5% and power of 80% and hypothesizing that 40% patients receiving metoprolol CR with HFpEF will show an improvement in symptoms by 1 NYHA HF class as against the expected background response in 20% with placebo, the required sample size was 82 patients in each group. Expecting 80% compliance, 196 patients needed to be enrolled. Here, we report the results of the pilot study conducted on forty patients with the primary aim to bring forth the need to bring any amendment in the study protocol.
Data were expressed as mean ± standard deviation (95% confidence intervals), numbers (percentages), and median (interquartile range [IQR]). Analysis was carried out using both intention to treat (ITT) and per protocol methods. The proportions of patients showing improvement of ≥1 in NYHA class in the two groups were compared using Fisher's exact test. For other parameters, mean change from baseline in two groups was determined and between-group comparison was carried out by unpaired t-test/nonparametric test depending on their nature. P < 0.05 was considered statistically significant.
RESULTS
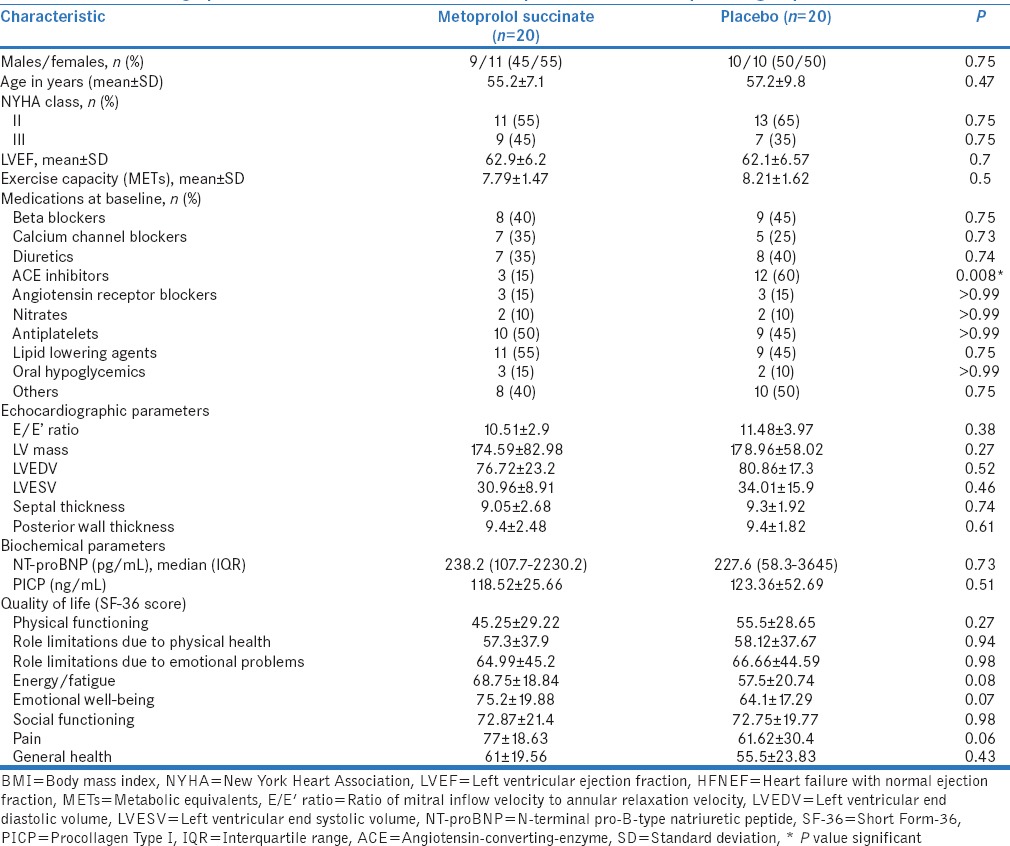
Seventy-three patients were screened for potential eligibility in the study. Twenty patients in each group were available for ITT analysis [Figure 1]. One patient in each group was lost to follow-up after randomization, whereas two patients were lost to follow-up after 2–8 weeks in each group. Hence, 17 patients in each group were evaluated for per protocol analysis. The two study arms were similar at baseline with respect to various demographic and clinical characteristics [Table 1]. However, the use of ACE inhibitors at baseline was significantly higher in placebo arm (P = 0.008). The final results for primary outcome were adjusted for this variable.
Figure 1.
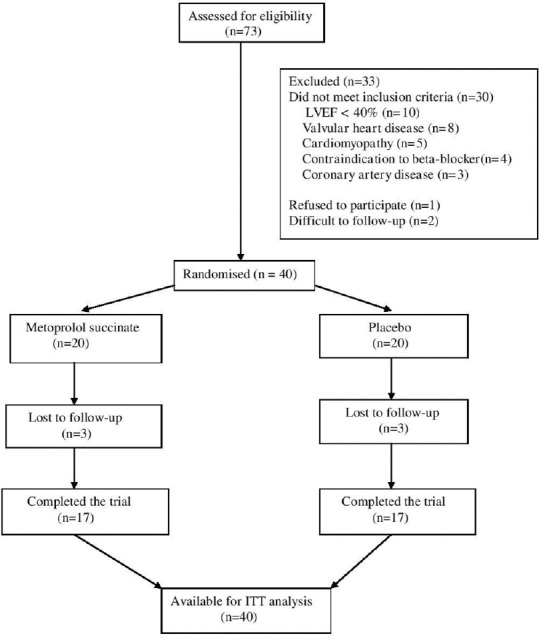
The CONSORT diagram for flow of study participants
Table 1.
Baseline demographic and clinical characteristics of metoprolol succinate and placebo groups
In the metoprolol group, dose was titrated to maximum of 100 mg in 65% patients (mean dose at 12 weeks: 86.7 mg). The mean heart rate at 12 weeks in metoprolol (73 ± 9 bpm) and placebo groups (77 ± 10 bpm) did not change significantly from baseline (P = 0.62 and 0.99 for metoprolol and placebo, respectively). In metoprolol group, the systolic and diastolic blood pressures at 12 weeks were 126 ± 15 and 81 ± 9 mmHg and were statistically similar to baseline values (P = 0.19 and 0.99 for systolic and diastolic, respectively). Furthermore, no significant change was observed in blood pressure values in placebo group (P = 0.09 and 0.2 for systolic and diastolic, respectively). During the study, calcium channel blockers were added in three patients (one in placebo and two in metoprolol group) due to high blood pressure records.
There was an improvement in clinical symptoms in both the groups [Figure 2]. In metoprolol group, the number of patients having Class II and Class III symptoms decreased from 11 to 9 and 9 to 1, respectively, while seven patients were able to attain Class I symptoms at 12 weeks. In the placebo group, the corresponding reductions in the number of patients with Class II and Class III symptoms were 13–7 and 7–2, respectively, with eight patients attaining Class I symptoms. However, there was no significant difference in the proportion of patients showing improvement of ≥1 in NYHA class at 12 weeks with metoprolol compared to placebo (13/20 vs. 11/20) (P = 0.75). The results remained statistically insignificant after adjustment for baseline variability in the ACE inhibitor use. There was an improvement in exercise capacity as demonstrated by increase in the number of metabolic equivalents achieved in both groups at 12 weeks (metabolic equivalents [METs] at 12 weeks: 8.44 ± 2.16 in metoprolol group; 8.28 ± 1.77 in placebo group) with metoprolol group showing a more favorable trend although not statistically significant (P value for change from baseline at 12 weeks: 0.09 in metoprolol and 0.65 in placebo group). However, the change was not statistically significant between two groups [Table 2].
Figure 2.
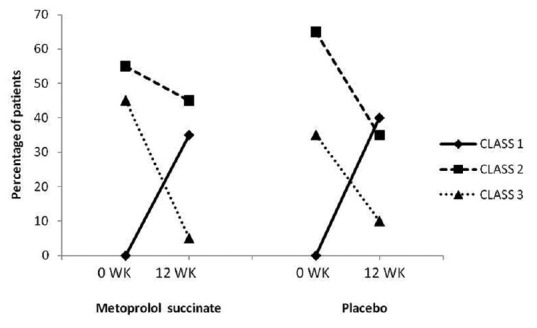
Percentage of patients belonging to different New York Heart Association classes at baseline and 12 weeks in metoprolol succinate and placebo groups
Table 2.
Comparison of changes in exercise capacity, echocardiographic, and biochemical parameters between metoprolol succinate and placebo groups
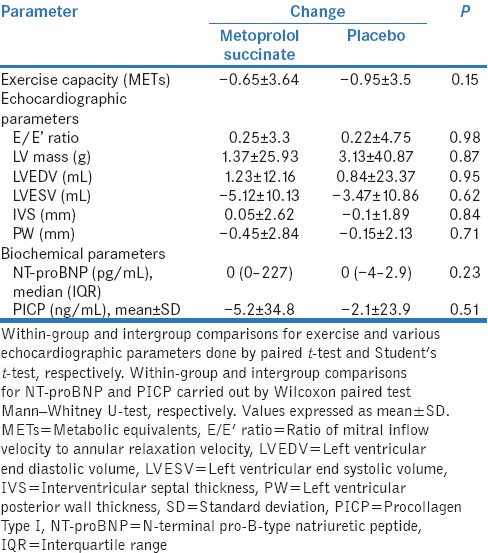
The mean change in various echocardiographic parameters was not significantly different between two groups [Table 2]. In metoprolol group, there was a significant decline in LV end systolic volume (LVESV) at 12 weeks (25.84 ± 7.74; P = 0.03). An increase in stroke volume (LV end diastolic volume-LVESV) at 12 weeks was also observed in both metoprolol (51 ml from 45 ml at baseline) and placebo (51 ml from 46 ml at baseline) arms. No statistically significant difference was observed for mean change in NT-proBNP levels between two groups. Although in metoprolol group, a significant increase in values was noted at 12 weeks (median [IQR]: 649.8 [200–2936]; P = 0.04), it could be explained due to few outlier values and no particular trend was seen. In the metoprolol group, the levels of PICP decreased at 12 weeks from baseline indicating reversal of myocardial fibrosis, but the changes cannot be deemed important in the absence of statistical significance (113.32 ± 24.95; P = 0.43).
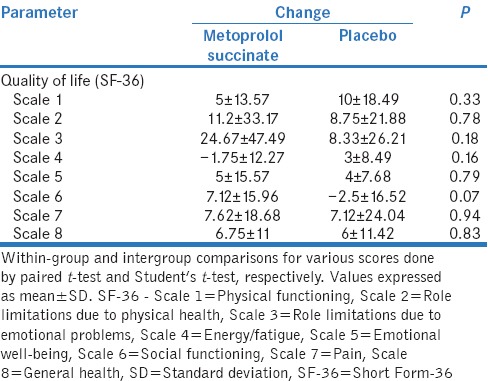
There was a significant improvement in general health (67.75 ± 18.38; P = 0.01) and role limitations due to emotional problems subscales (89.67 ± 18.41; P = 0.03) of SF-36 score at 12 weeks in the metoprolol group. However, there was no significant difference in any of the subscales when mean change from baseline was compared between two groups [Table 3].
Table 3.
Comparison of changes in various quality of life parameters between metoprolol succinate and placebo groups
One patient in metoprolol group presented with low blood pressure (100/70 mm Hg) on 50 mg daily dose at third follow-up visit which was asymptomatic. Hence, the dose was reduced to 25 mg following which the blood pressure normalized and the dose was maintained at 25 mg later on. The study medications were otherwise well tolerated, and there were no adverse event-related drug withdrawals.
The results of per protocol analysis were similar to ITT for all the parameters.
DISCUSSION
Our study evaluated the role of metoprolol in patients having HF with normal EF. Although metoprolol was not significantly better than placebo with respect to improvement in functional NYHA class, its use led to a significant decline in LVESV. A favorable trend toward increase in exercise capacity was also observed in metoprolol group. The study also showed that more than 50% patients in both treatment arms showed improvement in functional NYHA class after 12 weeks. A significant increase in NT-proBNP levels and a trend toward reduction in serum procollagen propeptide levels was also seen in the metoprolol group. There also was a significant improvement in general health and role limitations due to emotional problems subscales of SF-36 score with metoprolol. There were no serious adverse events and treatment discontinuations among any study participant.
For the assessment of efficacy of a medicinal product in HF, clinical symptoms comprising alteration in NYHA class are recommended as a primary end point.[29] Hence, we used change in NYHA class as primary end point. There was a significant improvement in NYHA class in both the groups and thus between group differences were attenuated. This could be due to several reasons. The reporting of NYHA class is somewhat subjective. Although the subjectivity was taken care of by blinding, the fact remains that patients were receiving some treatment. Therefore, we suspect a large placebo effect complexed with the fact that patients in a clinical trial receive extra care, the well-known Hawthorne effect, which led to a situation where there was improvement in both the groups. Another possibility is that the favorable effect of beta blockers may take some time to peak later than 12 weeks and prolonging of trial can lead to greater effects.
We also assessed exercise capacity, another efficacy end point in HF trials.[29] The advantage of exercise testing over NYHA class is its nonsubjective nature and hence freedom from assessment bias. An improvement in exercise capacity was noted in both the groups and correlated with improvement in NYHA class. The reasons for lack of statistical significance in intergroup comparisons are similar as with NYHA class because both are symptom-based parameters.
Tissue Doppler imaging is the most sensitive and widely available echocardiographic technique for the assessment of LV diastolic function,[30] and it is used to measure the ratio of early mitral valve flow velocity (E) to early diastolic lengthening velocity (E'). In LV diastolic dysfunction, there is a continuous decline in tissue Doppler-derived intramyocardial velocities. As a consequence, E' decreases and the E/E' ratio continuously increases with advanced LV diastolic dysfunction. A small increase in E/E' ratio was observed in metoprolol group not significantly different from placebo arm. There was a significant decline in LVESV in metoprolol group accompanied by an increase in stroke volume. These findings are encouraging and merit further exploration in larger, longer duration trials.
NT-proBNP has been characterized as a surrogate marker of LV dysfunction, NYHA class, and prognosis.[31,32] In our study, rise in NT-proBNP levels in the metoprolol group contrary to improvements in other parameters was observed. This could partially be explained by wide variability in the levels of NT-proBNP, varied spectra of disease presentation and influence of other comorbidities. Furthermore, findings in a previous study[33] concluding the nonbeneficial role of NT-proBNP-guided therapy in HFPEF in contrast to HF with reduced EF, raise possible speculations on the confirmatory prognostic role of this biomarker in HFPEF per se circulating levels of the carboxy-terminal PICP, an index of collagen type I synthesis, correlate with the extent of myocardial fibrosis. A linear relationship between PICP levels and indices of diastolic function and filling pressure has been proposed.[24] In our study, there was a reduction in serum procollagen propeptide levels at 12 weeks as compared to the baseline levels in metoprolol group, possibly reflecting the partial reversal of myocardial fibrosis although statistical significance can be expected only with longer follow-up.
There are a few limitations in the present study. This is a pilot study conducted in a smaller population as against the calculated larger sample. The duration of follow-up was 12 weeks while a longer duration would have been more informative owing to the observed trends seen in exercise capacity and echocardiographic parameters in the metoprolol group. Hence, considering these results, we have prolonged the duration of follow-up to 6 months instead of 3 months in our further ongoing complete trial. Besides, the observed improvement in QoL scores in both the groups can be explained by the presence of a large placebo effect and needs to be tested in real life setting outside a clinical trial. Despite the limitations, the study provides important insights into the efficacy of metoprolol in HFpEF and provides background information for multicentric clinical trial using hard end points.
CONCLUSION
Our study demonstrated efficacy and safety of metoprolol succinate in HFpEF using soft end points. This is, however, a pilot study and the results of the complete ongoing trial with larger patient population and longer follow-up are expected to provide a definitive evidence.
Financial support and sponsorship
The study was supported by Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abbate A, Arena R, Abouzaki N, Van Tassell BW, Canada J, Shah K, et al. Heart failure with preserved ejection fraction: Refocusing on diastole. Int J Cardiol. 2015;179:430–40. doi: 10.1016/j.ijcard.2014.11.106. [DOI] [PubMed] [Google Scholar]
- 2.Nativi-Nicolau J, Ryan JJ, Fang JC. Current therapeutic approach in heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:525–38. doi: 10.1016/j.hfc.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-preserved trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 5.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: A randomised trial. Lancet. 2007;369:2079–87. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: The ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 10.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–91. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 11.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (RAAM-PEF) J Card Fail. 2011;17:634–42. doi: 10.1016/j.cardfail.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Oghlakian GO, Sipahi I, Fang JC. Treatment of heart failure with preserved ejection fraction: Have we been pursuing the wrong paradigm? Mayo Clin Proc. 2011;86:531–9. doi: 10.4065/mcp.2010.0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of ß-blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014;312:2008–18. doi: 10.1001/jama.2014.15241. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J. Effects of beta-blockers on heart failure with preserved ejection fraction: A meta-analysis. PLoS One. 2014;9:e90555. doi: 10.1371/journal.pone.0090555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: Findings from the OPTIMIZE-HF registry. J Am Coll Cardiol. 2009;53:184–92. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Arora A, Shafiq N, Reddy S, Pandhi P, Mittal N, et al. Pharmacotherapy of heart failure with normal ejection fraction (HFNEF) – A systematic review. Br J Clin Pharmacol. 2011;72:369–80. doi: 10.1111/j.1365-2125.2011.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergström A, Andersson B, Edner M, Nylander E, Persson H, Dahlström U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC) Eur J Heart Fail. 2004;6:453–61. doi: 10.1016/j.ejheart.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Takeda Y, Fukutomi T, Suzuki S, Yamamoto K, Ogata M, Kondo H, et al. Effects of carvedilol on plasma B-type natriuretic peptide concentration and symptoms in patients with heart failure and preserved ejection fraction. Am J Cardiol. 2004;94:448–53. doi: 10.1016/j.amjcard.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Massie BM, Nelson JJ, Lukas MA, Greenberg B, Fowler MB, Gilbert EM, et al. Comparison of outcomes and usefulness of carvedilol across a spectrum of left ventricular ejection fractions in patients with heart failure in clinical practice. Am J Cardiol. 2007;99:1263–8. doi: 10.1016/j.amjcard.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto K, Origasa H, Hori M. J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: The Japanese Diastolic Heart Failure Study (J-DHF) Eur J Heart Fail. 2013;15:110–8. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 23.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction > or = 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80:207–9. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]
- 24.Brunottea RM, Kahana T, Lopez B, Edner M, Gonzalez A, Dıez J, et al. Myocardial fibrosis and diastolic dysfunction in patients with hypertension: Results from the Swedish irbesartan left ventricular hypertrophy investigation versus atenolol (SILVHIA) J Hypertens. 2007;25:1958–66. doi: 10.1097/HJH.0b013e3282170ada. [DOI] [PubMed] [Google Scholar]
- 25.Nodari S, Metra M, Dei Cas L. Beta-blocker treatment of patients with diastolic heart failure and arterial hypertension. A prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol. Eur J Heart Fail. 2003;5:621–7. doi: 10.1016/s1388-9842(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 26.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 27.Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: Results of the ELANDD study. Eur J Heart Fail. 2012;14:219–25. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Shi H, Zhang J, Lu Y, Fu M, Ge J. Beta-PRESERVE Study Investigators. Rationale and design of the beta-blocker in heart failure with normal left ventricular ejection fraction (beta-PRESERVE) study. Eur J Heart Fail. 2010;12:181–5. doi: 10.1093/eurjhf/hfp193. [DOI] [PubMed] [Google Scholar]
- 29.Guideline on clinical investigation of medicinal products for the treatment of acute heart failure. Committee for Medicinal Products for Human Use (CHMP) 2015 May 21; CPMP/EWP/2986/03 Rev.1. European Medicines Agency. [Google Scholar]
- 30.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–18. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 31.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61:1498–506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 32.Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J. 2012;164:763–70.e3. doi: 10.1016/j.ahj.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeder MT, Rickenbacher P, Rickli H, Abbühl H, Gutmann M, Erne P, et al. N-terminal pro brain natriuretic peptide-guided management in patients with heart failure and preserved ejection fraction: Findings from the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) Eur J Heart Fail. 2013;15:1148–56. doi: 10.1093/eurjhf/hft076. [DOI] [PubMed] [Google Scholar]


