Abstract
Evaluation of functional outcome is widely used across species to assess the recovery process following various pathological conditions, including spinal cord injury, musculo-skeletal injury, mithochondrial disease, neuropathic cancer, Huntington's disease, chronic pain, cortical lesion, and olivocerebellar degeneration among others. The Stroke Therapy Academic Industry Roundtable (STAIR) recommends multiple endpoints for behavioral studies in pre-clinical stroke research, to demonstrate their clinical relevance. One of the more challenging tasks in experimental stroke research is measuring long-term functional outcome in mice. It is, however, becoming more important, since transgenic mice are increasingly used for modeling human neurological disorders. Using CatWalk, we characterized long-lasting gait/locomotion deficits following mouse distal middle cerebral artery occlusion (dMCAO). The post-dMCAO assessment was performed at 7, 14, 21, and 28 days after experimental ischemia. When compared to sham-operated mice, dMCAO animals displayed a statistically significant decrease in Spatial parameters (such as Paw Area), while the Temporal parameters (Stand, Initial and Terminal Dual Stances) were significantly increased for three weeks after surgery. Kinetic parameters were significantly decreased in dMCAO animals at 7 days after dMCAO. The Interlimb coordination group of parameters displayed the strongest deficits at 21 days. While CatWalk variables were altered in all paws, the degree of change was greatest for the parameters measured from the Right Front Paw (contralateral to the lesion). All parameters measured in dMCAO and Sham-operated groups reached similar levels at four weeks after the experimental insult, which reflects a spontaneous post-ischemic recovery. Based on our investigation, we conclude that CatWalk represents a relevant and sensitive analysis, which allows long-term characterization of animal functional recovery in the dMCAO model of experimental ischemia.
Keywords: Mouse dMCAO, Long-term functional outcome, Stroke, Gait analysis, CatWalk
1. Introduction
Stroke is one of the most serious pathological conditions with a high social and financial impact. Since stroke mortality is decreasing more rapidly than stroke incidence, approximately 70–85% of the patients suffer long-term disability [1]. Therefore, one of the major goals in the field of stroke research focuses on enhancing functional capability. In pre-clinical studies, assessment of functional deficits after experimental stroke has become increasingly important in order to overcome the roadblock in transition from bench to bedside [2]. Academic Industry Roundtable (STAIR) recommendations for preclinical studies comprises the measurement of stroke outcome in behavioral studies at least 2 or 3 weeks or longer after the experimental injury to demonstrate a sustained benefit and ensure sufficient clinical relevance [3]. Experimental models of stroke have frequently utilized rats because their size facilitates experimental manipulation of their head and neck vasculature. In addition, rats generally demonstrate good performance in behavioral testing. Mouse models of experimental ischemia are more challenging for reasons such as smaller body size and limited intelligence [4], making behavioral testing more demanding than in rats. Mice are also the best-characterized animal in genetics and molecular biology. Murine direct (distal) middle artery occlusion (dMCAO) is a widely utilized model of experimental cortical ischemia. Besides its high reproducibility and survival rate, the advantage of this model is that it produces brain damage analogous to human stroke. The localization of the vascular obstruction (middle cerebral artery) and relatively small size (14–18% of the damaged hemisphere) makes dMCAO model compatible to the vast majority of human ischemic strokes [5,6]. Cerebral ischemia results in development of heterogeneous pathological lesions, which include a necrotic core and a periinfart area (salvageable area at the edge of the core) in the hemisphere ipsilateral to the lesion. The dMCAO model induces an infarct core in the frontal and parietal cortex, evolving in adjacent temporal, frontal and cingulate cortex [6]. Different behavioral tests have been used to characterize behavioral deficits induced by MCAO in mice (dMCAO in Sun et al., 60 min MCAO Bouët et al., 30 min MCAO in Balkaya et al. [4,7,8]). Bouët and colleagues analyzed the long-term effects of 60 min transient MCAO (induced by an intraluminal approach) in mice, using a battery of sensorimotor (including adhesive removal, corner, pole, and staircase tests) and cognitive tests (Morris water maze). The study demonstrated that adhesive removal, staircase and pole tests detected behavioral deficits for up to one month after the insult [8]. dMCAO-induced brain damage is characterized by less severity, as compared to the intraluminal occlusion model. Therefore, analysis of the functional recovery after dMCAO performed by Freret and colleagues showed that dMCAO-induced sensorimotor deficits could not be detected by most of the behavioral tests, including corner, cylinder, pole, or accelerated rotarod tests. The authors demonstrated that the adhesive removal test was the only one sensitive enough to detect long-term (lasting for approximately 1 month) deficits [9].
CatWalk Automated Gait Analysis System has been proposed as one of the most sensitive tool for long-term (at least 3–4 weeks) evaluation of animal locomotion and gait impairment by Balkaya and colleagues compared to test such as Pole and Rotarod [10]. CatWalk was originally developed to assess locomotion in rats before and after spinal cord injury [11]. It was later used as a new approach in the investigation of functional impairment in different animal models of stroke, including mouse dMCAO [12].
Balkaya and colleagues evaluated the functional recovery for 4 weeks after 30 min of focal transient MCAO, using a battery of behavioral test including CatWalk [10]. The authors concluded that CatWalk and adhesive removal tests were the most suitable for conducting long-term recovery studies. They also encouraged other investigators to report long-term functional outcomes in their studies of cerebral ischemia. More recently, CatWalk has been used in different experimental stroke studies, including genetic influence on stroke recovery in mice [13], and in vivo regulation of miRNAs after mouse stroke [14]. In these studies, CatWalk has demonstrated high sensitivity to sensorimotor alterations associated with mild injury after experimental ischemia. Since the gait analysis has become a widely-used tool for the assessment of the behavioral deficits, a comprehensive characterization of this method is needed in order to achieve reproducibility and reliability of the results.
The main goal of the present study is to provide a detailed characterization of long-term functional deficits in the dMCAO model of mouse stroke, using the CatWalk Automated Gait Analysis System. Our results will contribute toward standardization of the methods and practices in the assessment of gait and motor function after experimental ischemia.
2. Material and methods
2.1. Animal groups
The experimental procedures were performed in accordance with the University Of New Mexico Office Of Animal Care Compliance. All institutional and national guidelines for the care and use of laboratory animals were followed. Two month-old male C57BL/6 mice were purchased from The Jackson Laboratory. Mice were housed in pairs with ad libitum access to water and food (standard chow). Each cage contained a cardboard box for environmental enrichment. Animals were divided into an ischemic group (dMCAO, N = 10) and a sham-operated group (Sham, N= 10), and daily monitored for weight and food intake.
2.2. Distal middle cerebral artery procedure
An experimental model of cortical ischemia, distal middle cerebral artery occlusion (dMCAO) was performed as described previously [5,14,15]. The advantages of this model are: high reproducibility, high survival rate, and the relatively small size, which makes dMCAO model compatible to human stroke [6]. dMCAO was performed on the left hemisphere, with the MCA exposed via a transtemporal approach [5,16]. The mice were anesthetized using isoflurane gas (induction dosage 2–3%; maintenance dosage 1.5–2%) and a mixture of O2:N2O gases in the ratio 2:1. A small burr hole was made (located 1 mm rostral to the fusion of zygoma and squamosal bone, and 3 mm ventral to the parietal bone), and the MCA was coagulated with low-heat electrocautery (Bovie Medical, FL). In sham-operated animals, the MCA was exposed but not coagulated.
2.3. MRI
dMCAO brain damage, affecting mostly the somatosensory cortex, was confirmed at 24 h after dMCAO, using T2-weighted MRI. A 4.7T Biospec® dedicated research MR scanner (Bruker Biospin; Billerica) equipped with a single tuned surface coil for mouse brain (RAPID Biomedical, Rimpar), was used for the acquisition. The mice were anesthetized as described in surgical procedure. Real-time monitoring of physiological parameters (heart rate and respiratory rate) was performed during the procedure. A tri-pilot scan using gradient echo sequence was used to acquire initial localizer images. T2-weighted MRI was performed with a fast spin-echo sequence (RARE), TR/TE= 5000/ 56 ms, FOV= 4 cm × 4 cm, Slice thickness = 1 mm, Interslice distance =1 mm, number of slices= 12, matrix =256 × 256, number of average = 3.
2.4. Immunohistochemistry
Twenty eight days after the surgery, animals were anesthetized and subsequently perfused with 10 ml of PBS, followed by 10 ml of 4% paraformaldehyde (PFA). Brains were removed, fixed in 4% PFA, and cryoprotected in 30% sucrose for 72 h at 4 °C. Coronal brain sections (16 µm thickness) were mounted and subjected to immunostaining. The slices were rehydrated in EtOH gradient from 100% to 75%, post-fixed with 4% PFA, and quenched with 50 mM NH4Cl. Antigen retrieval was performed with Vector Unmasking Solution, according to manufacturer’s instructions (Vector Laboratories; H-3301). 10% normal goat serum (NGS) was used as a blocking buffer. Sections were incubated overnight at 4 °C with Cy3-conjugated anti-NeuN antibody (Millipore, ABN78C3; 1:100 dilution). The incubation was performed in a humidity chamber.
2.5. Animal weight and food intake monitoring
All experimental animals were weighed daily, for 28 days after surgery (N =10 mice per group). Animals were grouped according to their weight at the day of surgery (prior to surgical procedure). Body weight in the dMCAO group ranged from 20.5 to 22 g (average: 21.5 g) and in the Sham group, from 20.5 to 24 g (average: 22.15 g). The weight was normalized to each animal’s body weight on day 0 recorded immediately prior to surgery (Day 0 =100%; Day 1= X1%). Mice had ad libitum access to food and water. Food intake was daily monitored by weighing the pellets and subtracting the weight of the remaining pellets on the following day (measured at 08:00 am). Subsequently, the weight of the pellets (g) was divided by sum of the weight of two animals hosted in the same cage. The food intake was expressed as mg food/g animal per day.
2.6. CatWalk description
2.6.1. CatWalk
Gait analysis was performed using the CatWalk Automated Gait Analysis System (Noldus Technology Company; software version XT 10.5.505). Although the system and protocol are described in detail here, further technical details have been previously reported [17–19], and in the Noldus Company manual (version 10.5). CatWalk is an automated, computer-assisted system. The main part of the CatWalk system consists of a glass plate suspended 1.5 m above the floor. Perpendicular to the plate are two black panels creating a corridor, with a ceiling covering the upper part of the device. This structure forms a “walkway” that the mice traverse from one side to the other. The corridor walls are placed as close as possible, enabling the animal to walk without meandering, but freely. A green LED light illuminates the plate from the edge, and the camera continuously records the light. The light becomes more intense when contact is made with the glass plate. A red LED light permanently illuminates the corridor from the ceiling; consequently, a silhouette is created when the animal enters the corridor. Each Paw and the silhouette are captured by a high-speed video camera positioned underneath the walkway. For our studies, each individual footprint was digitalized and labeled into a discrete amount of pixels of digital brightness ranging from 0 to 255 (arbitrary units). This information was quantified by CatWalk XT 10.5.505 a specialized software to generate numerous parameters for qualitative and quantitative analysis of individual footprints and gait.
2.6.2. CatWalk settings
The walkway area was set according to the company’s recommendations. The specified setup was sufficient to record about 5 step cycles per run. The high-speed camera recorded at 100 frames per second (model: GP-2360C GEViCAM), and included a 6 mm lens set at the aperture of 1.5 (model: DFGHA–1B Fujifilm). The recording was done in a darkened room. Our previous testing showed that the best outcome (an optimal range between the maximum and minimum print intensity to filter out background) was obtained when camera gain was set to 20.00 (dB), and the green intensity threshold was set to 0.15. The software defined the walkway as a grid, the X coordinate along the length of the glass, and the Y coordinate along the width of the glass, and automatically assigns values to pixel intensity.
2.6.3. Animal training and testing criteria
No food or water restrictions were used in our experiments. A few food pellets were placed in the goal box, as a motivator cue for a successful run. The training and experimental procedures were performed during the same period of the day (from 8 am to 2 pm). The training started one week prior to surgery and lasted at least two days, until the animals learned how to cross the walkway without interruption. Wooley and colleagues investigated the effect of repeated trials on walking in mice and concluded that some parameters could progressively decrease over time. To minimize any effect of the repetition, only one trial (group of runs for one animal) was recorded per day (at least five completed runs) [20]. According to previously published studies on mouse MCAO, the animals that turn back or walk backwards receive a second try [18]. In our study, only one animal was not able to accomplish the trial during the first day. On training and testing days, the mice were brought into the testing room and left for 30 min prior to the experiment, for habituation to the experimental environment. Training and data acquisition were completed in a dark and silent room, which was properly cleaned and vented one hour before the experiment. Prior to training, the animals were allowed to walk freely for 1–2 min to become familiar with the apparatus. On training days, each animal was allowed to move at-will along the corridor and freely explore the walkway for approximately 25 min. No noise was used to motivate the animal to run. However, if the animal came out and walked around the walkway area, a noise (either rubbing the fingers or clapping) was used to motivate them to go back into the walkway. Although the manufacturer’s main criteria for recording is to record at least three accomplished runs per day (not necessarily consecutive runs), 50% of animals accomplished at least three consecutive runs without any difficulties during the training period, and 100% of the animals accomplished at least five runs per trial. The glass plate was cleaned after each trial, or as needed. It is important to work with mice of the same age, to rule out fundamental differences in test performance and define a clear baseline [4,20]. The average recording on preoperative training day 4, the last training day before the surgery, was defined as a baseline. At day 2 after the surgical procedure, a postoperative training was performed using the same criteria as in post-surgery testing days (data not shown, since only a training/reminder session was performed). Post-surgery testing was performed at 7, 14, 21, and 28 days after dMCAO. Based on the experimental stroke literature and our previous studies, these time points correspond to gradual changes in post-stroke recovery process [14,15]. During testing days, the animals were allowed to move freely back and forth on the walkway for 15–20 min, and a minimum of five completed runs were collected. Animals, which could not complete a trial in 20 min (enough time to transverse the walkway 15–20 times, and to complete at least five compliant runs), were allowed a second try the next day (to avoid stressing the animal). Compliant run criteria were defined as a run between 1 and 5 s duration (ensuring an average speed between 8 and 40 cm/s), and maximum speed variation of 60%. The training and experimental protocols were performed according to the manufacturer’s instructions, available literature, and previous experience in our laboratory [10,12,14,18,19].
2.6.4. Gait/locomotion analysis
Analysis was performed using catwalk XT 10.5.505 Software. The percentage of lost runs suggest that three runs per animal and time point may be insufficient for an accurate analysis; consequently, we collected at least 5 accomplished runs per animal and time point, in agreement with Batka and colleagues' observations [19]. Gait parameters were automatically generated after each footprint was labeled. Every footprint was manually inspected and respectively named LF (Left Front), LH (Left Hind), RF (Right Front) and RH (Right Hind) paws. Gait parameters were detected separately for each paw (Right Front, RF; Right Hind, RH; Left Front, LF; and Left Hind, LH). Since dMCAO was performed in the rostral part of the left hemisphere, the damage primarily impaired somatosensory function of the right extremities, with more prominent effect on the right front paw. Supplemental Table S1 provides a full list and description of the gait parameters studied. These parameters were categorized into five major groups: a) Run characterization, b) Temporal parameters, c) Spatial parameters, d) Kinetic parameters, and e) Interlimb coordination parameters. The P-values are listed in Supplemental Table S2. The analysis included 5 time points: day −4 (4 days before surgery, baseline), 7, 14, 21, and 28 days after the surgical procedure. Two of the 16 parameters within the Kinetic group displayed statistically significant differences at day −4 before surgery (Paw Angle Body Axis and Paw Angle Movement Vector). Consequently, they were not considered for analysis purposes; none of the 36 Temporal, the 52 Spatial, or the 75 Interlimb Coordination parameters showed differences. Under the null hypothesis of no population difference in these 179 parameters it is not surprising to observe a few false positives even after FDR correction due to minor measurement errors or trial performance variability for the mice.
2.6.5. Post-analysis criteria
Following each acquisition, the runs were examined and analyzed in slow motion. In post-acquisition analysis, the runs that displayed behavioral abnormalities (animal looking around, sniffing, stopping, hitching, or similar) were discarded. Out of the total 1067 runs, 519 runs were accomplished; after an additional post-analysis, only 401 (77.26% of the accomplished runs) were classified as suitable for statistical analysis. The number of obtained runs ranged from 5 to 9 per day and animal, but after manual analysis only 3–5 were selected and averaged per animal and time point for statistical purposes. Previous studies have used a Maximum Variation of 60% in the mouse MCAO model [10,14,18]. In agreement with these studies and recommendations from Noldus, we set a Maximum Speed Variation of 60% in our study. It is important to point out that only 12.61% of the analyzed runs had a Maximum Speed Variation between 51 and 60%. Therefore, based on the CatWalk manual and previously published literature [10,14,18,19], we believe that a Maximum Speed Variation of 60% is adequate to ensure consistent speed, as long as the training period was properly done. The accomplished runs were averaged for statistical purpose using the average per animal run and time point. Only one dMCAO animal was excluded during the post-analysis procedure because the animal could not accomplish the trial at 21 days. Parameters such as Toe Spread, Intermediate Toe Spread or Manual Print Length were manually classified in front and hind paws (when the full footprint was recorded).
2.7. Statistical analysis
Statistical analysis was performed using the R programming language (version 3.3.1). Each of the 192 catwalk parameter trajectories were modeled using a linear mixed-effects (LME) model (R package “nlme”) including a mouse-specific random intercept and a first-order autoregressive AR(1) error structure over time [21]. The LME model has more statistical power for longitudinal data analysis, particularly when there is correlation over time, relative to methods more commonly seen in similar analyses, such as Repeated Measures ANOVA, Analysis of Rates, or Percent Changes. To be robust to distributional model assumptions (typically it is assumed that the residuals are Gaussian and homoskedastic), we implemented a permutation test where treatment labels were permuted among mice to approximate the null distribution using 10,000 repetitions. Hypotheses of primary interest were mean differences between experimental groups [dMCAO− Sham] (days −4, 7, 14, 21, and 28). Hypothesis tests were considered statistically significant (difference between experimental groups exists) if the Benjamini-Hochberg (BH) false discovery rate (FDR) corrected p-value was less than the pre-specified Type-I error rate of 0.05 as described below.
The BH-FDR adjustment was applied over groups of associated variables, and this occurred in two ways. (1) For a set of variables measured in the same units and being related in a natural way, such as all the paw labels (RH/RF/LH/LF) over the Step Cycle variables, the adjustment was applied to all p-values at days −4, 7, 14, 21, and 28 (e.g., 5*4= 20 tests for Step Cycle), and there were 39 such groups of variables. (2) Alternatively, if there was no natural group for a variable, such as Duration or Cadence, then the adjustment was applied to all p-values at days −4, 7, 14, 21, 28 just within that particular variable (e.g., 5 tests for Duration), and there were 7 such variables.
Finally, to summarize each variable's relationship with Weight and Average Speed, Spearman correlation coefficients were calculated with each of the other 192 variables within each treatment group and day along with BH-FDR adjusted p-values for the null hypothesis that the correlation coefficient equals zero. Spearman correlation is a nonparametric method, which does not assume linearity as Pearson correlation does, thus it is robust to departures from linearity and bivariate Gaussianity.
3. Results
3.1. Brain damage after dMCAO
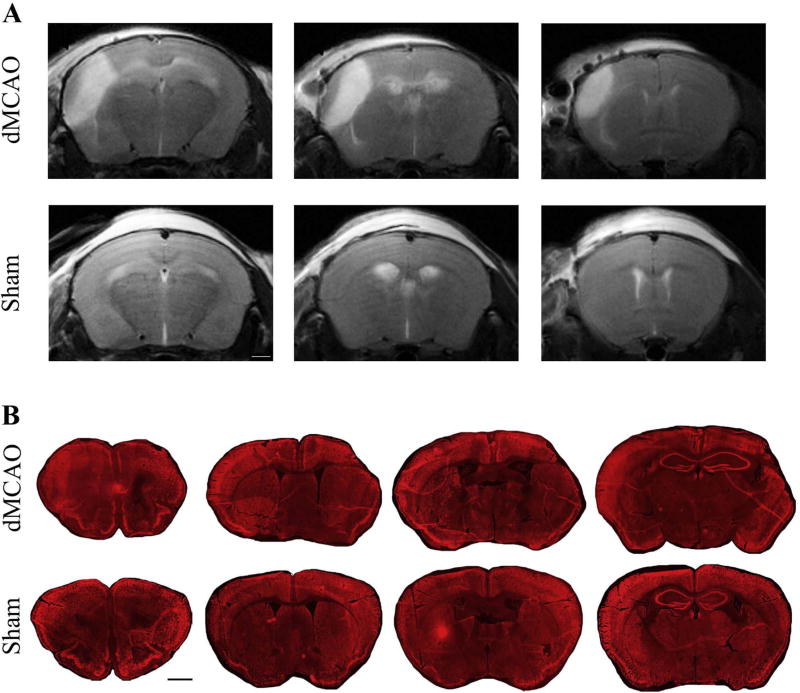
In the dMCAO model, brain damage progresses from vasogenic edema at 24 h to a substantial resorption of the necrotic tissue at later stages after surgery. Based on our previous studies using MRI and histology, dMCAO induces the infarct, which measures (relative to the intact hemisphere) in average 14% at 24 h, 18% at 7 days, 13% at 14 days, and 15% at 21 days after stroke [14,15]. In the present study, T2-weighted MRI was performed at 24 h after the surgical procedure, to confirm dMCAO-induced brain injury. MRI showed clear ischemic damage in the cortical area of the left hemisphere in the dMCAO group, while no visible damage was seen in the Sham animals (Fig. 1A). At 28 days after dMCAO, the animals were sacrificed, and the brains were subjected to histological evaluation. Histological analysis using immunofluorescence staining for neuronal marker Neun, showed substantial damage in dMCAO mice, reflected in visible tissue loss and shrinkage of the lesioned hemisphere at 28 days after the experimental ischemia (B).
Fig. 1. Brain damage after dMCAO.
A) Representative T2-weighted MRI-based images of the mouse brains at 24 h after dMCAO (upper panels) or sham surgery (lower panels). Three sections per mouse are shown to demonstrate the damage at different brain levels. Note significant damage in the cortical area of the left hemisphere in dMCAO group, as compared to the Sham group with no apparent cortical damage. Bar: 1 mm. B) Representative histological images from dMCAO and Sham animals, at 28 days after surgical procedures (4 sections per mouse). Coronal sections were inmunostained with Cy-3-conjugated anti-NeuN antibody. At 28 days, significant tissue loss and shrinkage of the lesioned hemisphere were observed in the dMCAO group. Bar: 1 mm.
3.2. Weight and food intake monitoring
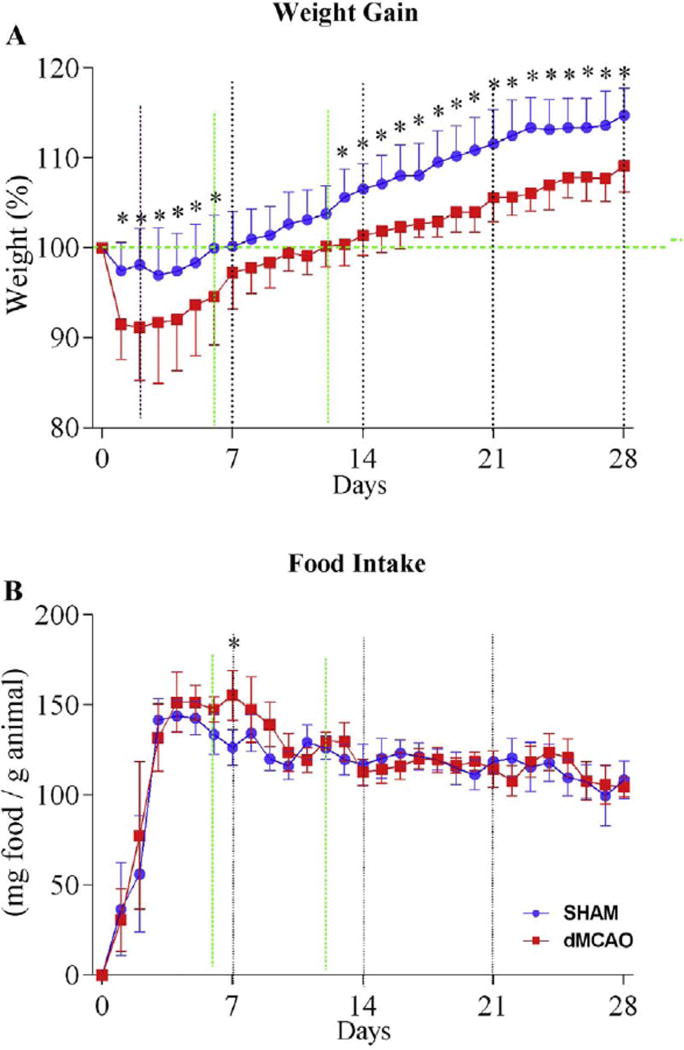
Animal weight and food intake were evaluated regularly, throughout the experiment. All animals lost body weight after surgical procedures. This is in agreement with previous observations in rats [22,23], and mice [24]. Parkkinen and colleagues describe the relationship between CatWalk parameters and body weight in rats [22]. Hezte et al., however, did not find a similar correlation at 10 days before and after the mouse cerebral ischemia (60 min MCAO) [18]. It is, therefore, important to monitor the weight and carefully analyze a possible relationship with CatWalk parameters in different animal models of stroke [25,26]. One day after stroke onset, dMCAO animals lost 8.5% of their weight, while Sham animals lost only 2.5%. In subsequent days, both groups tended to gain weight until day 28. The average weight of the ischemic animals was lower, compared to sham-operated controls throughout the experiment, although the weight difference was not statistically different between days 7 and 12 after dMCAO. Sham animals displayed a full weight recovery on day 6, while the weight of dMCAO animals recovered only on day 12 (Fig. 2A). Different results were obtained in the studies using 60 min MCA occlusion in mice, where the animals did not regain their original weight until six weeks after stroke [27].
Fig. 2. Experimental ischemia influences both body weight and food intake.
A) Body weight was monitored once a day, starting at day 0, immediately before surgery, for up to 28 days. Data are expressed as the % weight (expressing the daily weight with respect to day 0). B) Food intake was calculated by weighing the pellets and subtracting the weight of the remaining pellets on the following day (measured daily at 08:00 am), and expressed as mg food/g animal weight (per day). Values are represented as mean ± SD. T-test was performed to analyze differences (* p≤0.05).
Both dMCAO and Sham animals exhibited an acute hypophagic phase lasting for 3 days after surgery: the food intake on day one was 30.6 and 36.5 mg food/g animal in dMCAO and Sham groups, respectively (Fig. 2B). In agreement with our data, Arsenijevic and colleagues observed an acute hyphophagic response in permanent MCA-occluded mice, with subsequently regained appetite during one week after the experimental ischemia [26]. The compensatory hyperphagic phase lasted from day 4 to day 9, displaying a statistically significant difference between groups at day 7 (155 and 126 mg food/g animal intake in dMCAO and Shams, respectively, Fig. 2B). As mentioned above, on day 7, a minimal difference in the body weight between the groups was accompanied by a significant increase in food intake in the dMCAO group, which could reflect a recovery process in the ischemic animals.
3.3. Gait/locomotion analysis
3.3.1. Run characterization parameters
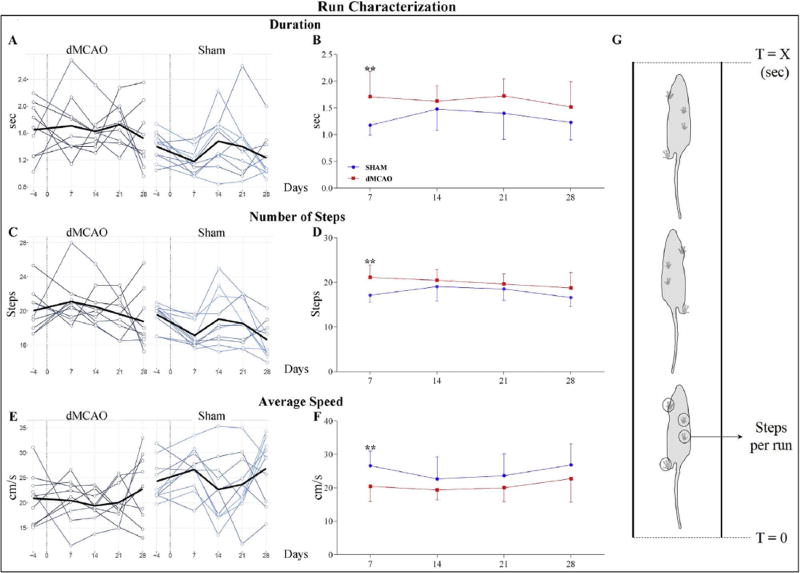
To evaluate the extent of gait impairment, we analyzed the run parameters in dMCAO and Sham groups, at 7, 14, 21, and 28 days after the injury. All the animals were able to easily traverse the walkway, consequently minimal variations were observed at all post-injury time points. Overall, both groups displayed time-dependent alteration in Run Characterization parameters, with more severe impairment at 7 days. At this time point, dMCAO animals displayed statistically significant differences from Shams in Duration, Average Speed and Number of Steps parameters (Fig. 3A–G). The dMCAO group displayed significantly lower Average Speed and, coherently, higher Run Duration and Number of Steps, compared to the Sham group. While the differences in the Run Characterization parameters between the two animal groups were statistically significant at 7 days post-dMCAO, the pattern remained similar at other post-injury time points, with minimal differences displayed at 28 days after the insult (Fig. 3A–G). These results are in agreement with other studies [24,28,29], and reflect a natural recovery of the animals after cerebral ischemia.
Fig. 3. Run Characterization parameters are altered in dMCAO animals.
A) Duration parameter indicates time the animal needs to cross the walkway. Multiline plots represent the Run Duration for each animal (light plots) at different time points; the average values per group are shown with dark lines. B) Graph demonstrates statistical differences in the average Run Characterization parameter, between dMCAO (red plot) and Sham (blue plot) groups, at 7 days after surgery. C) Number of Steps parameter represents total number of selected steps per run. Multiline plots represent number of steps for each animal (light plots), and dark lines represents the average values per group. D) Graphs demonstrate statistical differences in the average Number of Steps parameter, between dMCAO (red plot) and Sham (blue plot) groups. E) Average Speed parameter represents average speed of the selected steps. Multiline plots: average speed for each animal (light plots), and mean of average speed per each group (dark line). F) Graphs demonstrate statistical differences in the Average Speed parameter, between dMCAO (red plot) and Sham (blue plot) groups. G) Schematic representation of selected steps in a run from T= 0 (start of the run) to T = X (end of the run). For plots B, D, and F: mean ± SD. Parameters were analyzed using a linear mixed-effects (LME) model, * p ≤ 0.05, ** p≤ 0.01. P-values were adjusted by Benjamini Hochberg (BH) correction.
3.3.2. Temporal parameters
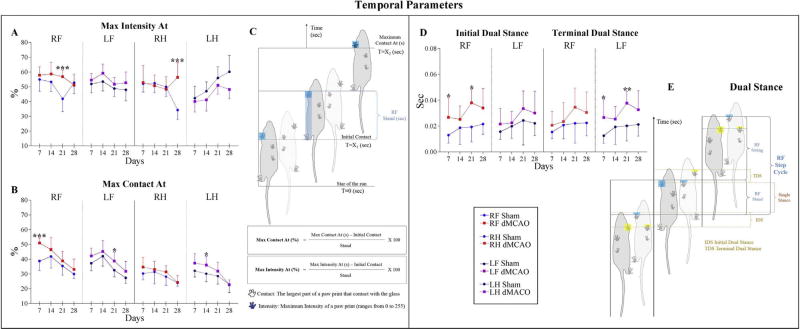
Significant differences between dMCAO and Sham mice were found in: Initial Dual Stance, Terminal Dual Stance, Max intensity At%, and Max Contact At%. Max Intensity At (%) is defined as the time in seconds since the start of the run when maximum intensity is measured, relative to stand. On the affected side, Max Intensity At (%) was increased at 21 days (post-dMCAO) in the Right Front Paw, and at 28 days in the Right Hind Paw (Fig. 4A and C). In contrast, no statistically significant differences were seen at 7 and 14 days. Concomitantly with Max Intensity At (%), Max Contact At (%) was increased in dMCAO animals at 7 days in RF Paw, at 14 days in LH Paw, and at 21 days in LF Paw (Fig. 4B and C). Dual Stance is defined as time (in seconds) of ground contact for either Hind or Front paws. CatWalk (10.5 version) distinguishes between Initial and Terminal Dual Stances (IDS, TDS). IDS is the time of the initial step in each Step Cycle, when both Hind or Front paws simultaneously make contact with the glass plate, and TDS is the similar time detected in the second step of each Step Cycle (Supplemental Table S1, Fig. 4E). Postoperatively, ischemic animals showed a longer IDS and TDS contact time, in all paws over the entire testing period. The sham group displayed a statistically significant difference only in IDS for the RF Paw at 7 and 21 days (Fig. 4D and E). TDS for each paw is, at the same time, the IDS for the following step cycle of the contralateral paw. In agreement with this definition, TDS for the LF Paw was statistically different between the groups, at 7 and 21 days after the ischemia (Fig. 4D and E). Our observations that dMCAO had a long-lasting effect on several Temporal parameters are in agreement with a previous study, reporting a higher percentage of time of Max Contact At% in ischemic animals [10].
Fig. 4. Effect of dMCAO on Temporal parameters.
Graphs represent Temporal parameters displaying significant differences between dMCAO and Sham animal groups. Parameters are represented for each paw separately. For dMCAO group: RF: Right Front (red line), LF: Left Front (purple line), RH: Right Hind (red dashed line), and LH: Left Hind (purple dashed line). For Sham animals: RF: Right Front (blue line), LF: Left Front (black line), RH: Right Hind (blue dashed line), and LH: Left Hind (black dashed line). Deficits were detected in different paws, but preferentially in the right front (RF) paw affected by left dMCAO lesion, and its contralateral left front (LF) paw. Max Intensity At (A) and Max Contact At (B) parameters display the time (from start of the run) when a Paw makes maximum intensity contact with the glass plate. C) Schematic representation of the Max Intensity At and Max Contact At parameters. D) Initial and Terminal Dual Stance parameters represent the duration of simultaneous ground contact with two paws. E) Schematic illustration of the Initial and Terminal Dual Stance parameters. Values are represented as mean ± SD. Parameters were analyzed using a linear mixed-effects (LME) model, * p≤ 0.05, ** p≤ 0.01, *** p ≤ 0.001. P-values were adjusted by Benjamini Hochberg (BH) correction.
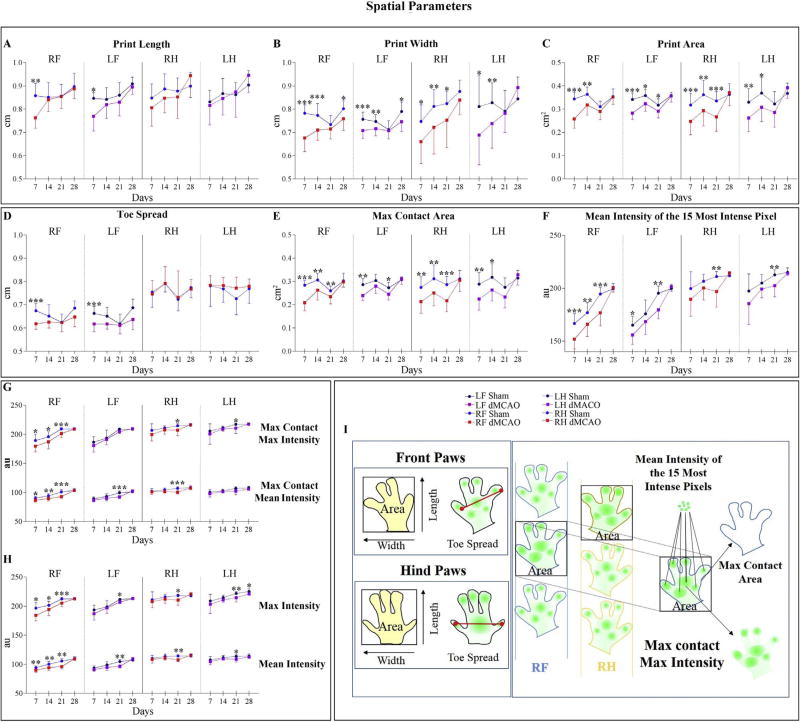
3.3.3. Spatial parameters
Spatial parameters were decreased following experimental injury during the entire postoperative testing period. Print Length for both Front paws was significantly decreased in ischemic mice at 7 days after dMCAO (Fig. 5A and I). In agreement with this result, Print Width was significantly affected at 7, 14, and 21 days in both Front and Hind paws. The difference in Print Width remained statistically significant over the entire 28 days of postoperative testing period (excluding RF and LF paws at 21 days, RH at 28 days and LH Paw at 21 and 28 days; Fig. 5B and I). Coherently, the measurements of Print Area were similar to those of Print Width, being significantly decreased in all paws of the dMCAO mice at 7 and 14 days, and significantly decreased in LF and RH paws at 21 days (Fig. 5C and I). In the dMCAO group, Toe Spread (fingertip distance) in Front Paw was significantly shorter at 7 days (Fig. 5D and I). The area of an individual Paw print at the moment of its maximal contact with the ground (Max Contact Area cm2), was decreased in Front and Hind paws of the ischemic mice over 21 days after the insult. Similar results were detected by Hetze et al. at 10 days after the experimental stroke [18]. All the differences between groups disappeared at 28 days (Fig. 5E and I). Shams exerted significantly more pressure on contact with the walkway, measured as light intensity. Pressure-related parameters including Mean Intensity of the 15 Most Intense Pixels, Max Contact Max Intensity, Max Contact Mean Intensity, Max Intensity, and Mean Intensity, are expressed in arbitrary units a.u. (Fig. 5F–I). Importantly, at 28 days all paws were seen to exert similar pressure, and only Max intensity in the LH Paw was significantly decreased at 28 days (Fig. 5H and I).
Fig. 5. Effect of dMCAO on Spatial parameters.
Graphs represent Spatial parameters displaying significant differences between dMCAO and Sham animal groups, at different time points after surgeries. Parameters are represented for each paw separately. For dMCAO group: RF: Right Front (red line), LF: Left Front (purple line), RH: Right Hind (red dashed line), and LH: Left Hind (purple dashed line). For Sham animals: RF: Right Front (blue line), LF: Left Front (black line), RH: Right Hind (blue dashed line), and LH: Left Hind (black dashed line). Parameters describing the Paw area, include Print Length (A), Print Width (B), and Print Area (C). D) Toe Spread, defined as the distance between the first and the fifth toe, was significantly decreased in Front paws of dMCAO mice, at 7 days post-surgery. E) Max Contact Area, the Area of an individual paw print during its maximal contact with the glass plate, was decreased in dMCAO mice for 21 days after injury. Coherently with this parameter, the Mean Intensity of the 15 Most Intense Pixel (F), Max Contact Max intensity and Max Contact Mean Intensity (G), Max Intensity, and Mean Intensity (H) were also affected for 21 days. I) Schematic explanation of spatial parameter calculations. Animals were tested once a week for 28 days. Values are represented as mean ± SD. Parameters were analyzed using a linear mixed-effects (LME) model, * p≤ 0.05, ** p≤ 0.01, *** p ≤ 0.001. P-values were adjusted by Benjamini Hochberg (BH) correction.
3.3.4. Kinetic parameters
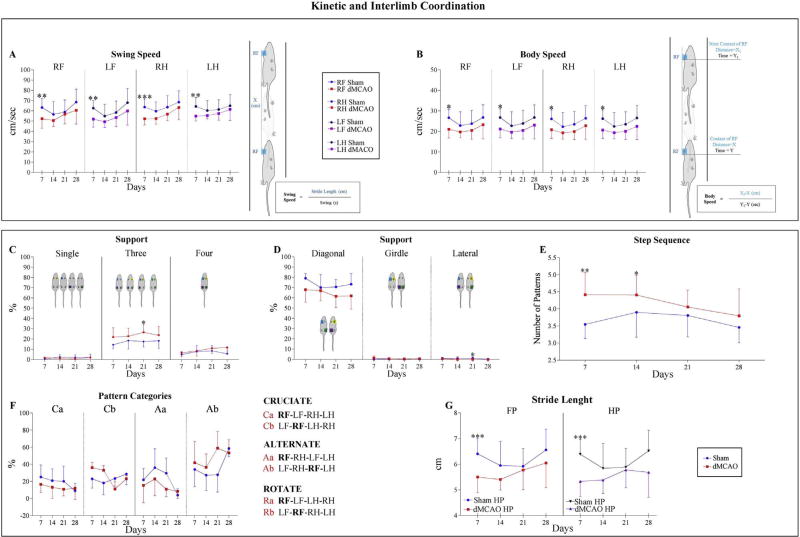
Swing Speed, the velocity (distance/time) when Paw is not in contact with the glass plate (Supplemental Table S1), was decreased over the entire 28 days of postoperative testing in the ischemic animals, as compared to Sham group (Fig. 6A), with more severe impairment at 7 days. At this time point, dMCAO animals exhibited statistically significant differences in swing speed for all paws, demonstrating that ischemia affected both Right and Left limbs. Complementarily, Body Speed (cm/s), defined as the speed of the body during a Step Cycle for a specific Paw, exhibited the same pattern. Body Speed in the ischemic animals was significantly decreased in all paws at 7 days, and remained lowered over the entire postoperative testing period (B).
Fig. 6. Kinetic and Interlimb Coordination parameters.
Kinetic and Interlimb Coordination parameters were largely affected by experimental ischemia at 7 days after surgery. Kinetic parameters are demonstrated on panels A and B. A) Swing Speed was significantly decreased at 7 days in dMCAO animals compared with the Sham animals. B) Body Speed was significantly affected at 7 days in the dMCAO group in contrast to the Sham group. For additional details for A and B, please see schematic explanation and legends. The animal is walking along the walkway, and blue boxes represent time when the Paw is in contact with the glass plate. Interlimb Coordination Parameters: Support parameter expresses the relative duration of contact with the glass plate for single, double (Diagonal, Girdle or Lateral), three of four paws simultaneously (see graphs and schematic explanations on C and D). E–F) Pattern Categories: Six normal step patterns have been described in rodents, which fall in three categories: Cruciate (Ca, Cb), Alternate (Aa, Ab), and Rotate (Ra, Rb). Please, see additional explanations on panel F legends. Step Sequence Number of Patterns reflects the number of patterns that fall into Cruciate, Alternate or Rotate categories. G) Stride Length is the distance between successive placements of one Paw. This parameter was significantly decreased at 7 days in dMCAO, compared to Sham animals. Front paws (FP) and Hind paws (HP) were combined by the CatWalk software to provide higher resolution. Values are represented as mean ± SD. Parameters were analyzed using a linear mixed-effects (LME) model. P-values were adjusted by Benjamini Hochberg (BH) correction. * p≤ 0.05, ** p ≤ 0.01, *** p≤ 0.001.
3.3.5. Interlimb coordination parameters
Our investigation revealed a clear impairment in Interlimb Coordination parameters at 21 days after the experimental ischemia. Since the mice received left MCAO occlusion, the Right side displayed significant alterations, while the left side did not show any significant differences throughout the experiment. Our analysis detected differences in the proportion of time in each Step Cycle, during which body weight was supported by one Paw only (Support Single), by two paws (Support Diagonal, Lateral, or Girdle), by three paws (Support Three), or by all paws (Support Four) (Fig. 6C–D). Coordination was based on Normal Step Sequence Patterns (Step Cycles representing sequential placement of paws). Six patterns have been described in rodents: Rotate (Ra, Rb), Cruciate (Ca, Cb), and Alternate (Aa, Ab) [30]. The Regularity Index expresses the number of Normal Step Sequence Patterns relative to the total number of Paw placements (Supplemental Table S1). In our study, Regularity Index was not significantly affected in dMCAO animals, compared with the Sham animals (Supplemental Table S2). Interestingly, the Number of Patterns was significantly higher in dMCAO animals at 7 and 14 days after stroke onset (Fig. 6E and F). Complete absence of Rotatory Pattern (Ra or Rb) is in agreement with our observations of the infrequent girdle and lateral support, which define Rotatory Pattern (Fig. 6C and D). Conversely, Cruciate (Ca and Cb) and Alternate (Aa and Ab) patterns, which require diagonal paw placement, were significantly decreased in dMCAO animals at 7 days (Fig. 6D and F). Consistent with Bakta and colleagues’ observations, at Average Speed of 20–25 cm/s, all mice displayed preferentially Cruciate and Alternate patterns [19]. Stride Length parameter displayed a significant difference at 7 days after the insult, in Front and Hind paws (Fig. 6G).
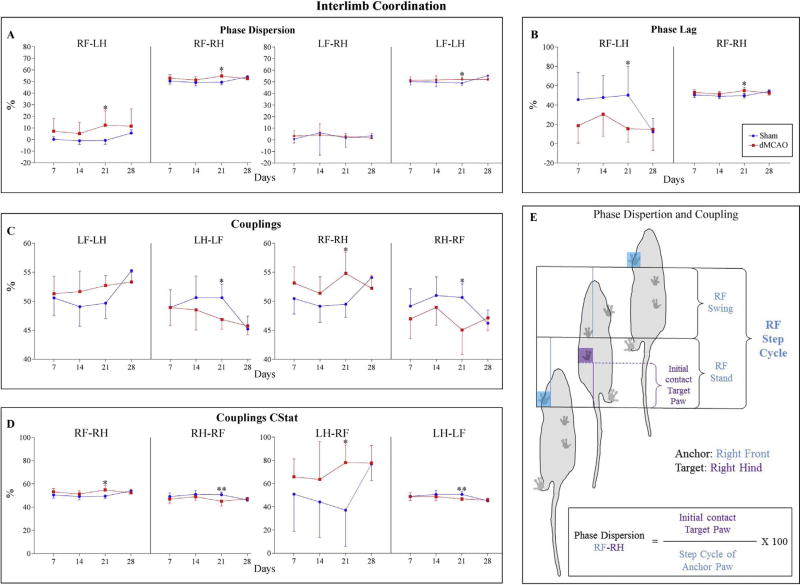
Phase dispersion is the moment of initial contact of a Target Paw expressed as the percentage of the Step Cycle for the Anchor Paw, ranging from −50% to 75% (Supplemental Table S1 and Fig. 7E). A negative value means that the Target Paw has been placed before the Anchor Paw, and a positive value means the Target Paw was placed after the Anchor Paw. In contrast, in Coupling, the Target Paw cannot be placed before the Anchor Paw and, therefore, it ranges from 0 to 100%. In agreement with our previous analysis for the individual paws, irregularities were observed in Phase Dispersion (Mean calculations, Fig. 7A and E). We detected that the Diagonal Limb pair Right Front Paw (RF)-Left Hind Paw (LH) was increased in ischemic animals compared with the controls, over the entire testing period. This is in agreement with Hetze and colleagues’ observations [18] at 10 days after the experimental stroke. Additionally, in contrast to the Sham group, the Lateral Limb pair RF-RH was slightly increased in the ischemic animals (Fig. 7A and E). Both temporal relationships, involving RF Paw as an Anchor Paw, reached a significant difference at 21 days after the ischemic injury. Complementarily, Lateral Limb pair LF-LH was significantly increased at 21 days, compared with the Sham group, showing that the left side is slightly altered in order to compensate decreased effectiveness in coordination. This is in agreement with previous observations describing Lateral Limb pair LF-LH after transient MCAO [10].
Fig. 7.
Temporal relationship between two paws within a Step Cycle is significantly affected in dMCAO animals compared with the Sham group. A) Graphs demonstrate either Right Front Paw (RF) or Left Front Paw (LF) acting as anchor paws in Phase Dispersion Parameter. B) Phase Dispersion calculated as a Circular Variable (CStat) is more accurately defined by the term Phase Lag. Graphs demonstrate Right Front Paw as the Anchor paw. C) Couplings describe the temporal relationship of two paws. Couplings are computed the same way as Phase Dispersion, but a Target Paw can never precede an Anchor. Graphs reveal statistically significant differences between dMCAO and Sham animals; the difference was more robust at 21 days, in the pairs involving either Right or Left Front paws (RF, LF). D) Similarly to Couplings, Circular Variable (CStat) in the dMCAO group was significantly altered at 21 days, when compared to Sham animals. E) Schematic representation of the calculations (expressed as%) of the Phase Dispersion and Coupling parameters for the pair RF-RH, in which RF acts as an Anchor Paw (blue) and RH as a Target paw (purple). Values are represented as mean ± SD. Parameters were analyzed using a linear mixed-effects (LME) model, * p≤ 0.05, ** p ≤ 0.01. P-values were adjusted by Benjamini Hochberg (BH) correction.
Because Phase Dispersion and Coupling have slightly different definitions, they can be mistakenly regarded as interchangeable parameters. Therefore, CatWalk software provides an additional statistical analysis CStat, which represents Phase Dispersion and Coupling using Circular Variables (see CatWalk 10.5.505 manual, Appendix C for details). In Circular Statistics, Phase Dispersion is more correctly described by the term Phase Lag, a variable that ranges from 0% to 100% (Supplemental Table S1). In CStat analysis, Phase Dispersion or Phase Lag displayed irregularities in Diagonal, and Lateral interlimb coordination. The values detected for Diagonal Limb pair RF-LH decreased in ischemic animals compared to sham animals. In addition, the Lateral Limb pair RF-RH was increased in dMCAO animals in contrast to the Sham group. Both limb pairs reached statistical significance at 21 days (Fig. 7B and E). Complementarily, some Coupling parameters in CStat analysis were also significantly affected at 21 days after ischemia. The values detected for Diagonal Limb pair LH-RF and the Lateral Limb pair RF-RH were significantly increased in dMCAO animals at 21 days. Correspondingly, the Lateral Limb pairs RH-RF and LH-LF were significantly decreased in dMCAO animals at 21 days, compared with the Sham group (Fig. 7D and E).
Couplings, the temporal relationship between the placements of two paws within a Step Cycle, were also impaired after dMCAO. The Coupling Lateral Limb pair RF-RH was increased in ischemic animals, compared to the Sham animals, reaching statistical significance at 21 days. Contrastingly, the values for Lateral Limb pairs RH-RF and LHLF were statistically decreased at 21 days, when compared with the Sham group. These data imply that in contrast to Shams, dMCAO animals displayed an impaired Lateral interlimb coordination in right and left sides. Interestingly, in Couplings parameters Girdle (RH-LH, LH-RH) and Diagonal (LF-RH, RH-LF), coordination was not significantly affected by ischemia over the entire 28 day postoperative testing period, while demonstrating higher impairment in the pair involving RF Paw (Fig. 7C and E) (Supplemental Table S2).
In conclusion, our data revealed that at 21 days after dMCAO, Diagonal and Lateral interlimb coordination was impaired in ischemic animals, compared to the shams. Since experimental ischemia affected the left hemisphere, the Right Front Paw displayed the higher differences, and consequently, due to compensatory effects, Left Front Paw (LF) was also affected. Coherently, at 21 days, we observed that dMCAO had a significant effect on Phase Dispersion and Coupling for almost all the limb pairs involving Right Front (RF) or Left Front (LF) paws (both by normal and CStat calculations). In Phase Dispersion parameters (Mean and CStat), we observed changes in the Lateral and Diagonal pairs. This is in agreement with previous observations [18]. Statistical differences in parameters such as Phase Dispersion or Couplings are not always meaningful by themselves due to the minimal differences in each parameter. To display the differences between dMCAO and Sham animals in Coupling and Phase Dispersion more clearly, we generated a heatmap demonstrating the actual magnitude of differences between the groups (Supplemental Fig. S1).
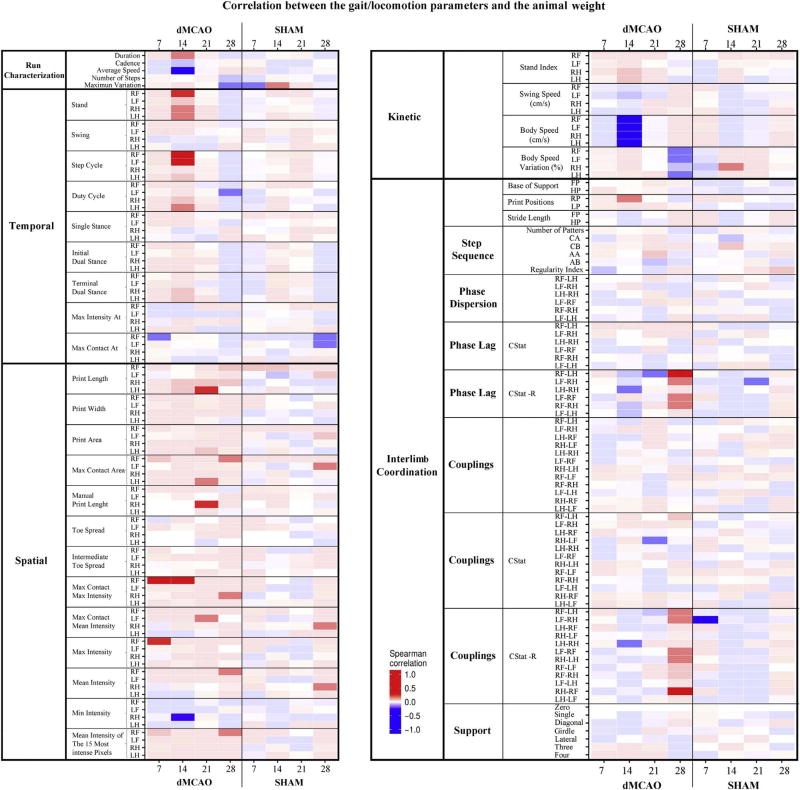
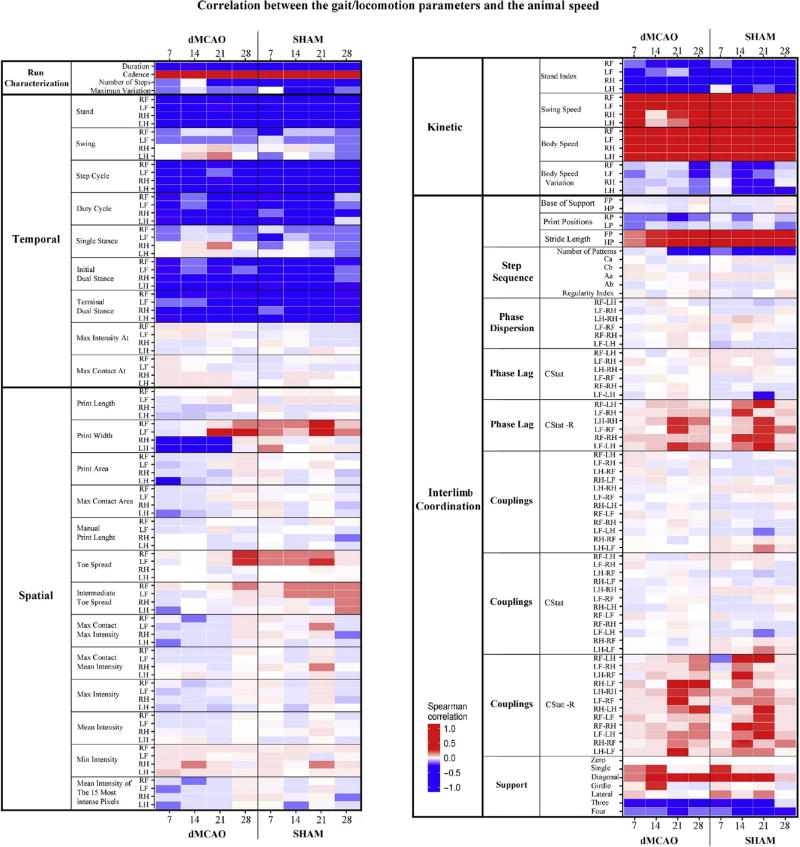
3.3.6. Correlation between gait/locomotion parameters and animal weight and speed
In the dMCAO group, body weight had a strong positive correlation (Fig. 8, bright red) with a few parameters such as Stand (RF Paw at 14 days), Step Cycle (both for RF and LF paws at 14 days), Print Length (LH at 21 days), Max Contact (RF at 7 and 14 days), and Max Intensity (RF at 7 days). There was a strong negative correlation between the body weight and parameters, such as Average Speed at 14 days, and Body Speed for all paws at 14 days (Fig. 8, bright blue). Interestingly, no such correlation was seen in the Sham group (Fig. 8). In contrast, we found a large number of parameters that correlated with speed. Among the parameters that negatively correlated with speed (Fig. 9, bright blue) were the majority of Temporal parameters (for all paws at all time points). Strong positive correlation was among Kinetic parameters, such as Swing and Body Speed (Fig. 9, bright red). Additionally, some parameters from the Spatial and Interlimb coordination groups displayed certain correlations with animal speed. Speed correlation was similar in the dMCAO and Sham groups (Fig. 9). These observations are in line with previous studies [19].
Fig. 8. Correlation between the animal weight and speed with CatWalk parameters.
Weight altered a small number of CatWalk parameters in dMCAO animals for 21 days, and scarcely at 28 days. Transparency of colors specifies significance of correlation: more saturated colors are associated with more significant correlation. Color bar represents Spearman correlation coefficient values ranging from +1 (dark red, strong positive correlation) to −1 (dark blue, strong negative correlation). Spearman correlation coefficients (R) were calculated for each variable within each treatment group and day, along with BH-FDR adjusted p-values for the null hypothesis that the correlation coefficient equals zero. CStat R is the strength of the directedness, and therefore a measure of the variation in the CStat.
Fig. 9. Correlation between the gait/locomotion parameters and the animal speed.
Heatmap demonstrates the influence of animal speed on the mean values of CatWalk parameters, measured in dMCAO and Sham animals at 7, 14, 21 and 28 days after surgeries. Speed negatively correlated with Temporal parameters and mainly positively correlated with Kinetic parameters. Colors define the correlation coefficient for each variable with Average Speed (cm/s). The level of color transparency specifies significance: more saturated colors relate to more significant correlation. Spearman correlation coefficients (R) were calculated with each of the variables within each treatment group and day. CStat R is the strength of the directedness, and therefore a measure of the variation in the CStat.
3.3.7. Heatmap of the altered parameters in ischemic mice
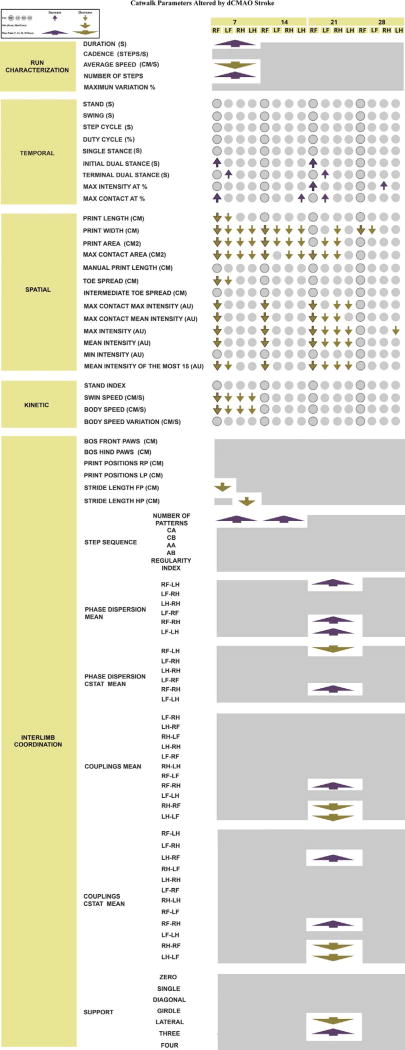
A primary focus of this study was to obtain a general characterization of the gait abnormalities in mice after dMCAO (Fig. 10). Overall, ischemic mice exhibited a time-dependent impairment, as compared with the sham-operated group. dMCAO animals exhibited more severe impairment at 7 days after the experimental stroke, when a higher number of affected parameters was detected. Interestingly, throughout the experiment, the differences were narrowed, demonstrating a natural recovery after cerebral ischemia. Differences in parameters of interlimb coordination were intensified at 21 days, and almost dissipated at 28 days after dMCAO.
Fig. 10. Catwalk parameters altered by dMCAO stroke.
A complete list of CatWalk parameters, which were significantly increased (purple) or decreased (golden) across all post-injury time points, in dMCAO animals as compared to Sham controls. Figure summarizes the analysis based on linear mixed-effects (LME) model. Parameters such as Phase Lag (CStat −R) or Couplings (CStat-R) were not included since the LME model did not reveal differences when groups were compared. Rectangles represent each of the time points (7, 14, 21 or 28 days per condition, dMCAO or Sham). Circles represent an individual Paw per time point (each of the paws RF, RH, LF, LH, at 7, 14, 21, 28 days). Arrows indicate increase or decrease of an individual Paw or a time point parameter. Solid line outlines circles and arrows representing Right Front Paw parameters since this Paw is directly affected by left dMCAO (please see the legend on the Figure for more details). Parameters were analyzed using a linear mixed-effects (LME) model. P-values were adjusted by Benjamini Hochberg (BH-FDR) correction
4. Discussion
To the best of our knowledge, this is the first study to extensively characterize a long-term functional outcome following distal MCA occlusion in C57/BL6 mice, using the CatWalk gait/locomotion analysis system. Functional outcome assessment after mouse dMCAO using CatWalk (in comparison to DigiGait) was reported in [12], but the evaluation was performed at day 1 after the ischemic insult. Several other groups, including ours, used this gait analysis after mouse dMCAO, but only some of the CatWalk parameters were characterized in these studies [7,14]. Mild brain injury makes dMCAO compatible to human stroke, but at the same time, sets up limitations for continuous behavioral assessment. In our study, we demonstrate a usefulness of CatWalk for long-term assessment of post-stroke recovery in mice. We demonstrated that CatWalk gait analysis represents a sensitive tool, which allows identification of a considerable number of gait and locomotion parameters that are significantly affected by dMCAO at different times following cerebral ischemia. A complete summary of our results is schematically shown in Fig. 10, and is briefly discussed below.
4.1. Run characterization parameters
The Run Characterization parameters showed a consistent impairment in Ischemic animals compared to Sham animals, with significant differences in Duration, Average Speed, and Number of Steps. Differences between the two animal groups were statistically significant at 7 days post-dMCAO. Wang et al. did not observe any statistically significant difference in Cadence in rats at 4 days or 5 weeks (in 60 min MCAO rat model) [25]; yet, Parkinen and colleagues showed that Cadence and Run Duration were altered in ischemic rats [22]. In agreement with our observations in mice, other researchers describe significant differences in Run Duration, [10,18]. A decrease in walking Speed in dMCAO animals (when compared with Sham controls) is consistent with observations in stroke patients [31,32].
4.2. Temporal parameters
In our study, Ischemic animals did not display changes in Stand (Stance Phase), which is in disagreement with Hetze et al. [18], and Lubjuhn et al. [12]. In dMCAO animals, at 7 and 21 days, the Initial Dual Stance (IDS) parameter was significantly increased for RF Paw, while Terminal Dual Stance (TDS) was significantly increased for the LF Paw. This could reflect a compensation by the Left Front Paw for the abnormal limb movements. As components of walking velocity, these parameters are relevant to post-stroke recovery. These observations are in line with our previously described results [14]. Since gait coordination requires communication between different parts of the locomotor system, our observation supports the concept described in [17]. Our data revealed that Max Intensity At% and Max Contact At% were slightly affected throughout the experiment. Max Intensity At% increase in ischemic animals is in agreement with results by Hetze et al. [18].
4.3. Spatial parameters
Decreased Spatial Parameters were observed in the dMCAO group for 21 days after the ischemic insult. These results are in line with previous studies in ischemic rats [25]. Unlike Wang et al., and our own observations, Parkkinen and colleagues [22] did not find statistical differences between Sham and dMCAO groups in Max Contact Area, Max Intensity, Print Width, Print Length, and Toe Spread. This could be explained by the low number of animals used (7 MCAO, 6 Sham), vs Wang et al. (22 MCAO, 18 Sham). Opposite to our observations, Balkaya and colleagues described that Front Paw parameters were significantly larger in MCAO animals compared to Sham animals [10]. Our data, however, clearly demonstrate statistically significant reduction in Paw Width, Paw Length and, consequently, in the Paw Area, for 21 days after the ischemic insult.
A decreased intensity was observed in ischemic animals. In addition, print intensity parameters were decreased at 7, 14 and 21 days, with subsequent recovery at 28 days. These spatial changes in walk, propulsion, and weight bearing could reflect a natural recovery process in post-ischemic mice. These observations are in agreement with other studies in rodents [18,25]. Interestingly, decreased intensity has been observed in previous research in hemiparetic patients [33,34].
4.4. Kinetic parameters
Ischemic animals displayed a statistically significant decrease in Swing Speed and Body Speed parameters for all paws, at 7 days after dMCAO. This demonstrates that at this time point, ischemia-induced brain damage alters well-coordinated kinetics interplay between the four limbs. This is in agreement with other studies using transient MCAO models [10,18].
4.5. Interlimb coordination parameters
Our investigation revealed a clear impairment of Interlimb Coordination Parameters, with a remarkably significant increase at 21 days after stroke, which is in agreement with reports by other researchers [10,18]. Hence, we conclude that in the dMCAO group, the coordination was impaired for both Front and Hind paws. In contrast to our observations, Lubjuhn and coworkers showed an increase in Stride Length in MCAO mice, on day one after the permanent occlusion [12]. This difference could, however, be related to differences in the reported average speed between groups (23.0 cm/s Sham vs 39.6 cm/s pdMC-AO). Batka and colleagues have demonstrated that Stride Length has a linear relationship in the Left and Right sides with speed [19]. In agreement with their observations, our investigation establishes that Stride Length correlated with speed in both dMCAO and Sham animals. The Number of Patterns that the animals used during the run was increased in ischemic animals when compared with controls, at 7 and 14 days. This is in agreement with previous studies [10,25].
In Phase Dispersion and Coupling, significant differences are not always meaningful because Phase Dispersion ranged from −50 to 75 (range of 125) and Coupling ranged from 0 to 100 (range of 100), so a difference in 5 units does not necessary imply a qualitative difference. To emphasize the statistical differences and to improve the clarity of Phase Dispersion and Coupling parameters (normal and CStat calculation), we showed the magnitude of changes in the original units (Supplemental Fig. S1). The main differences in percentage as well as in order of magnitude decreased at 21 days. Moreover, in Phase Dispersion and Coupling parameters involving RF paw the greatest differences in the order of magnitude are exhibited (supplemental Fig. S1), as well as in Couplings and Phase Dispersion parameters (CStat calculation). The Linear Mixed Model (LMM) statistic and order of magnitude exhibit the largest differences at the same time point.
We noticed the inconsistency in the average values of several parameters in the Sham group at 28 days after surgery, including Max Intensity At RH paw, Print length RH paw, Max Contact Area LH paw, and Print Length LH. This could be explained by the changes in behavioral patterns associated with prolonged training in mice. Nonetheless, the majority of the parameters remained stable. To overcome any inconsistency, we utilized the LME analysis with more statistical power for longitudinal analysis, as described in Methods.
Interlimb Coordination parameters have been used as an estimation of interlimb coordination recovery in various animal models [18,35]. Gait impairment in humans is characterized by asymmetry. Here, we identified long-term asymmetries in the Interlimb Coordination parameters in mice, which is in agreement with previous observations in rodents [18,23,25]. Importantly, in our study, the majority of the Interlimb Coordination parameters were significantly impaired at 21 days after ischemia. This is in agreement with our previous studies, demonstrating a delayed neuronal necrosis in the peri-infarct area of stroke at 21 days after dMCAO [14]. Neuronal injury results in additional damage of the brain in the vicinity of the initial infarct, and could lead to additional deficits in animal locomotion and coordination. We also demonstrated that at 21 days, there was a considerable number of damaged and necrotic neurons in the nonlesioned hemisphere, which points to a propagation of ischemic damage from the lesioned to the intact hemisphere [14]. This process, together with delayed neuronal damage and death, may contribute to the observed delayed deficits in the interlimb coordination.
4.6. Analysis of the possible influence of weight and speed on CatWalk parameters
In accordance with recently published articles by Batka et al. and de Haas et al. [19,36], we evaluated a possible correlation of every parameter with speed and weight. In addition, based on findings by Batka and colleagues that CatWalk parameters vary with speed, we decreased this variation by selecting the runs with similar speed for the subsequent analysis. The average speed variation in our study ranged approximately between 20 and 25 cm/s.
Changes in animal weight represent an indirect consequence of stroke and reflect post-stroke regeneration. We, therefore, performed a separate correlation analysis (shown in Fig. 9), in order to demonstrate a possible effect of this variable on gait parameters. We believe that these separate correlation analyses involving speed and weight allowed us to emphasize primary (speed) and secondary (weight) influence of cerebral ischemia on the animal gait and locomotion. The correlation between animal weight and some of the CatWalk parameters was more pronounced in the dMCAO group, suggesting that either the weight loss itself or the damage in the cortical area slightly influenced the weight bearing during the walk. Therefore, possible correlations should be calculated in order to rule out a possible weight effect, especially, if an experimental stroke is used in combination with obesity models such as ob/ob, db/db, or High Fat Diet (HFD). Interestingly, speed correlated with a very large number of parameters, which is in agreement with Batka and colleagues’ observations [19]. In line with Hetze et al., we either recommend making corrections to speed, or to use parameters independent from walking speed [18].
4.7. Relevance to human stroke
The alterations in several gait/locomotion parameters after dMCAO showed some similarity with the deficits recorded in stroke patients. These include Run Characterization parameters such as Average Speed, and Spatial parameter such as Max Contact Max Intensity, characterizing walk, propulsion, and weight bearing [31–34]. These observations demonstrate that CatWalk provides a relevant tool for evaluation of stroke outcome measures in pre-clinical animal studies.
5. Conclusions
Altogether, our results demonstrate that CatWalk provides a sensitive analysis, which enables detection of the high number of gait/locomotion parameters significantly affected by dMCAO in mice. Impairment of these parameters was time-dependent, and mainly resolved at 28 days after the experimental ischemia, adequately reflecting severity and extension of the brain damage. In addition, our data demonstrate some similarity in alteration of several parameters, between mouse and human stroke.
6. Recommendations for other researchers
Valid and reliable assessment of functional deficits associated with experimental ischemia is especially important for testing potential stroke treatments. We successfully used CatWalk in our studies for in vivo inhibition of microRNA miR-155 during post-stroke recovery. We demonstrated that CatWalk gait analysis was sensitive enough to detect significant differences between treated and control experimental groups [14]. Based on the present investigation, we offer several recommendations for researchers who plan to evaluate the post-stroke recovery process using CatWalk:
There is a debate over the number of runs that should be collected per trial (as a rule, only 3–5 runs per trial are collected). Because of the large post-acquisition loss, we recommend initially collecting approximately 5–9 runs.
Detection settings must be optimized to achieve an optimal intensity to avoid background with the paw prints. The most important settings to be adjusted manually are Camera Gain and Green Intensity Threshold.
The CatWalk software and manual provide instructions and recommendations to achieve an optimal range, including a profile to work with rats or mice. Rats will exert much more pressure than mice, and some mouse models will exert more pressure consistently with the age and weight, thus, these parameters must be considered and adjusted during statistical analysis.
Speed range should be defined prior to the study, and the runs within 20–25 cm/s speed range should be selected for further analysis. This recommendation is based on the findings of Batka et al., that speed influences 90% of parameters, including Swing, Stand, and interlimb coordination parameters.
As with many other behavioral tests, CatWalk is a very investigator-sensitive system, and thus the animals are influenced by the investigator’s way of handling the animals. Therefore, the same researcher should work during the whole experiment with the same group of animals.
Basic rules, including: ventilating the room one hour prior to the experiment, cleaning of the CatWalk device with alcohol, and performing the testing at the same period of the day, are critical for testing.
In future studies, we recommend including all successful runs in the analysis of differences between cohorts, as averaging the values might hide some barely detectable differences
Finally, the detailed description of methods in published articles will contribute to development of reproducible protocols for pre-clinical studies on post-stroke recovery.
Supplementary Material
Acknowledgments
We would like to thank Isabel Cano Perez for her assistance in developing the graphical summary of the CatWalk parameters altered by dMCAO. We are grateful to Dr. Kevin R. Smith for the extensive manuscript editing. This work was supported by the National Institutes of Health [NIH/NINDS R01NS082225].
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbr.2017.05.042.
References
- 1.DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci. Biobehav. Rev. 2001;25(4):325–342. doi: 10.1016/s0149-7634(01)00017-3. [DOI] [PubMed] [Google Scholar]
- 2.Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, Planas A, Rothwell N, Schwaninger M, Schwab ME, Vivien D, Wieloch T, Dirnagl U. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc. Dis. 2008;25(3):268–278. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group S. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkaya M, Krober JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J. Cereb. Blood Flow Metab. 2013;33(3):330–338. doi: 10.1038/jcbfm.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuraoka M, Furuta T, Matsuwaki T, Omatsu T, Ishii Y, Kyuwa S, Yoshikawa Y. Direct experimental occlusion of the distal middle cerebral artery induces high reproducibility of brain ischemia in mice. Exp. Anim. 2009;58(1):19–29. doi: 10.1538/expanim.58.19. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2(3):396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun C, Sun H, Wu S, Lee CC, Akamatsu Y, Wang RK, Kernie SG, Liu J. Conditional ablation of neuroprogenitor cells in adult mice impedes recovery of poststroke cognitive function and reduces synaptic connectivity in the perforant pathway. J. Neurosci. 2013;33(44):17314–17325. doi: 10.1523/JNEUROSCI.2129-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp. Neurol. 2007;203(2):555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, Schumann- Bard P, Boulouard M. Behavioral deficits after distal focal cerebral ischemia in mice: usefulness of adhesive removal test. Behav. Neurosci. 2009;123(1):224–230. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- 10.Balkaya M, Krober J, Gertz K, Peruzzaro S, Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J. Neurosci. Methods. 2013;213(2):179–187. doi: 10.1016/j.jneumeth.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma. 2001;18(2):187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- 12.Lubjuhn J, Gastens A, von Wilpert G, Bargiotas P, Herrmann O, Murikinati S, Rabie T, Marti HH, Amende I, Hampton TG, Schwaninger M. Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J. Neurosci. Methods. 2009;184(1):95–103. doi: 10.1016/j.jneumeth.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Qin L, Jing D, Parauda S, Carmel J, Ratan RR, Lee FS, Cho S. An adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic stroke. J. Neurosci. 2014;34(7):2493–2502. doi: 10.1523/JNEUROSCI.4140-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, Bragin D, Yang Y, Erhardt EB, Roitbak T. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J. Neurosci. 2015;35(36):12446–12464. doi: 10.1523/JNEUROSCI.1641-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pena-Philippides JC, Yang Y, Bragina O, Hagberg S, Nemoto E, Roitbak T. Effect of pulsed electromagnetic field (PEMF) on infarct size and inflammation after cerebral ischemia in mice. Transl. Stroke Res. 2014;5(4):491–500. doi: 10.1007/s12975-014-0334-1. [DOI] [PubMed] [Google Scholar]
- 16.Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, Schallert T, Jones TA. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J. Neurosci. Methods. 2008;170(2):229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamers FP, Koopmans GC, Joosten EA. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23(3–4):537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 18.Hetze S, Romer C, Teufelhart C, Meisel A, Engel O. Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. J. Neurosci. Methods. 2012;206(1):7–14. doi: 10.1016/j.jneumeth.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Batka RJ, Brown TJ, McMillan KP, Meadows RM, Jones KJ, Haulcomb MM. The need for speed in rodent locomotion analyses. Anat. Rec. 2014;297(10):1839–1864. doi: 10.1002/ar.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wooley CM, Xing S, Burgess RW, Cox GA, Seburn KL. experience and genetic background influence treadmill walking in mice. Physiol. Behav. 2009;96(2):350–361. doi: 10.1016/j.physbeh.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gałecki A, Burzykowski T. Linear Mixed-Effects Models Using R. Springer; New York: 2013. [Google Scholar]
- 22.Parkkinen S, Ortega FJ, Kuptsova K, Huttunen J, Tarkka I, Jolkkonen J. Gait impairment in a rat model of focal cerebral ischemia. Stroke Res. Treat. 2013;2013:410972. doi: 10.1155/2013/410972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Encarnacion A, Horie N, Keren-Gill H, Bliss TM, Steinberg GK, Shamloo M. Long-term behavioral assessment of function in an experimental model for ischemic stroke. J. Neurosci. Methods. 2011;196(2):247–257. doi: 10.1016/j.jneumeth.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SY, Marasini S, Kim GH, Ku T, Choi C, Park MY, Kim EH, Lee YD, Suh-Kim H, Kim SS. A method for generating a mouse model of stroke: evaluation of parameters for blood flow, behavior, and survival [corrected] Exp. Neurobiol. 2014;23(1):104–114. doi: 10.5607/en.2014.23.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, Abrams GM, Liu J. A comprehensive analysis of gait impairment after experimental stroke and the therapeutic effect of environmental enrichment in rats. J, Cereb. Blood Flow Metab. 2008;28(12):1936–1950. doi: 10.1038/jcbfm.2008.82. [DOI] [PubMed] [Google Scholar]
- 26.Arsenijevic D, de Bilbao F, Plamondon J, Paradis E, Vallet P, Richard D, Langhans W, Giannakopoulos P. Increased infarct size and lack of hyperphagic response after focal cerebral ischemia in peroxisome proliferator-activated receptor beta-deficient mice. J. Cereb. Blood Flow Metab. 2006;26(3):433–445. doi: 10.1038/sj.jcbfm.9600200. [DOI] [PubMed] [Google Scholar]
- 27.Verma R, Friedler BD, Harris NM, McCullough LD. Pair housing reverses post-stroke depressive behavior in mice. Behav. Brain Res. 2014;269:155–163. doi: 10.1016/j.bbr.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28(4):365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Connolly ES, Jr, Winfree CJ, Stern DM, Solomon RA, Pinsky DJ. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery. 1996;38(3):523–531. doi: 10.1097/00006123-199603000-00021. discussion 532. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Almstrom S, Gimenez-Llort L, Chang R, Ove Ogren S, Hoffer B, Olson L. Gait analysis of adult paraplegic rats after spinal cord repair. Exp. Neurol. 1997;148(2):544–557. doi: 10.1006/exnr.1997.6708. [DOI] [PubMed] [Google Scholar]
- 31.Stokic DS, Horn TS, Ramshur JM, Chow JW. Agreement between temporospatial gait parameters of an electronic walkway and a motion capture system in healthy and chronic stroke populations. Am. J. Phys. Med. Rehabil. 2009;88(6):437–444. doi: 10.1097/PHM.0b013e3181a5b1ec. [DOI] [PubMed] [Google Scholar]
- 32.Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait & Posture. 1996;4:136–148. [Google Scholar]
- 33.Hall AL, Peterson CL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin. Biomech. 2011;26(5):509–515. doi: 10.1016/j.clinbiomech.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokic DS, Horn TS, Ramshur JM, Chow JW. Agreement between temporospatial gait parameters of an electronic walkway and a motion capture system in healthy and chronic stroke populations. Am. J. Phys. Med. Rehabil. 2009;88(6):437–444. doi: 10.1097/PHM.0b013e3181a5b1ec. [DOI] [PubMed] [Google Scholar]
- 35.Mountney A, Leung LY, Pedersen R, Shear D, Tortella F. Longitudinal assessment of gait abnormalities following penetrating ballistic-like brain injury in rats. J. Neurosci. Methods. 2013;212(1):1–16. doi: 10.1016/j.jneumeth.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Ria de Haas FGR, Smeitink Jan A. Gait analysis in a mouse model resembling Leigh disease. Behav. Brain Res. 2016;296:191–198. doi: 10.1016/j.bbr.2015.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.