Abstract
Almost all cases of human rabies result from dog bites, making the elimination of canine rabies a global priority. During recent decades, many countries in the Western Hemisphere have carried out large-scale dog vaccination campaigns, controlled their free-ranging dog populations and enforced legislation for responsible pet ownership. This article reviews progress in eliminating canine rabies from the Western Hemisphere. After briefly summarizing the history of control efforts and describing the approaches listed above, we note that programs in some countries have been hindered by societal attitudes and severe economic disparities, which underlines the need to discuss measures that will be required to complete the elimination of canine rabies throughout the region. We also note that there is a constant threat for dog-maintained epizootics to re-occur, so as long as dog-maintained rabies “hot spots” are still present, free-roaming dog populations remain large, herd immunity becomes low and dog-derived rabies lyssavirus (RABLV) variants continue to circulate in close proximity to rabies-naïve dog populations. The elimination of dog-maintained rabies will be only feasible if both dog-maintained and dog-derived RABLV lineages and variants are permanently eliminated. This may be possible by keeping dog herd immunity above 70% at all times, fostering sustained laboratory-based surveillance through reliable rabies diagnosis and RABLV genetic typing in dogs, domestic animals and wildlife, as well as continuing to educate the population on the risk of rabies transmission, prevention and responsible pet ownership. Complete elimination of canine rabies requires permanent funding, with governments and people committed to make it a reality. An accompanying article reviews the history and epidemiology of canine rabies in the Western Hemisphere, beginning with its introduction during the period of European colonization, and discusses how spillovers of viruses between dogs and various wild carnivores will affect future eradication efforts (Velasco-Villa et al., 2017).
1. Introduction
Nearly all cases of human rabies result from dog bites. During recent decades, many countries in the Western Hemisphere have markedly reduced the incidence of human rabies by carrying out large-scale dog vaccination campaigns, controlling free-ranging dog populations and enforcing legislation for responsible pet ownership, as well as providing rabies postexposure prophylaxis (Schneider et al., 2007). As a consequence, the annual incidence of human rabies has been reduced almost to zero in North America, and the number of cases in Central and South America and the Caribbean has markedly decreased (Vigilato et al., 2013).
In this article, we review progress in eliminating canine rabies from the Western Hemisphere. After briefly summarizing the history of canine rabies control and describing successful programs, we note that elimination efforts in a small number of countries continue to be hindered by societal attitudes and severe economic disparities. We then explain how the achievement of hemisphere-wide eradication will require supplementing the three strategies listed above with other mechanisms, including wider education of the public on the prevention of rabies transmission, the creation of decentralized networks for animal control and enhanced laboratory-based surveillance. An accompanying article reviews the history and epidemiology of canine rabies in the Western Hemisphere, beginning with its introduction during the period of European colonization, and discusses how spillovers of viruses between dogs and various wild carnivores will affect future eradication efforts (Velasco-Villa et al., 2017).
2. Background: rabies in the Western Hemisphere
Rabies is an acute encephalomyelitis caused by viruses in the genus Lyssavirus of the family Rhabdoviridae. Rabies lyssavirus (RABLV), the prototype species of the genus, causes disease in nearly all terrestrial mammals. Despite the worldwide distribution of RABLV, only a few animal species in the orders Carnivora, Chiroptera and Primates play a critical role in maintaining the circulation of the virus in nature. Most of these mammals harbor species-specific RABLV variants which circulate within discrete geographic boundaries (Table 1). However, because almost all cases of human rabies result from the bites of rabid dogs, dog-maintained viruses are by far the most important target of control measures.
Table 1.
Major rabies reservoir hosts in the Western Hemisphere.
| Reservoir host common name |
Virus variant common name (reservoir host species) | Geographic range | Frequency of human infections |
|---|---|---|---|
| Bat-maintained rabies virus variants | |||
| New World bats | Nearly 30 rabies virus variants in more than 20 different bat species | All Western Hemisphere | Rare |
| Bat-related rabies virus variants | |||
| Raccoons | Raccoon variant (Procyon lotor) | Eastern United States and Southeast and Central Canada | Rare |
| Skunks | South-central skunk variant (Mephitis mephitis) and North-central skunk Mexico variant (Spilogale putorious) | South-central U. S. and North-central Mexico | Rare |
| Marmoset | Sagui/marmoset variant (Callithrix jacchus) | Northeastern Brazil | Rare |
| Kinkaju | Chosna/Kinkajou variant (Potos flavus) | Peru (Amazon region) | Never reported |
| Dog-maintained rabies virus variants | |||
| Dogs | Dog lineages from specific countries | Marginal distribution across Latin America and the Caribbean | Common in rabies hot spots |
| Dog-related rabies virus variants | |||
| Grey fox | Texas gray fox variant (Urocyon cinereoargenteus) | Texas (extinct) | Never reported |
| Grey fox | Arizona gray fox variant (Urocyon cinereoargenteus) | Arizona, New Mexico, Texas and Northwest | Rare |
| Mexico | |||
| Crab-eating fox | Crab-eating fox variant (Cerdocyon thous) | Northeastern Brazil | Rare |
| Peruvian fox | Peruvian fox variant (Lycalopex sechurae) | Northwestern Peru | Never reported |
| Striped skunk | California skunk variant (Mephitis mephitis) | California U. S. | Never reported |
| Striped skunk | Sinaloa, Durango, Sonora skunk variant (Mephitis mephitis) | Northwestern Mexico | Rare |
| Spotted skunk | Baja California Sur Mexico (Spilogale putorious lucasana) | Baja California Sur Mexico | Rare |
| Coyote | Coyote/dog variant (Canis latrans) | South Eastern U. S (extinct), North Eastern | Once reported |
| Mexico (likely present) | |||
| Mongoose | Mongoose/dog variant (Herpestes javanicus) | Caribbean | Common |
| Arctic fox | Arctic fox variant (Vulpes lagopus) | Alaska, Canada, Greenland | Once reported |
As described in detail in the accompanying article (Velasco-Villa et al., 2017), historical records and phylogenetic analysis of viral sequences indicate that the introduction and establishment of rabies in domestic dogs and some wild mesocarnivores in the Western Hemisphere occurred after massive European colonization events (Smith et al., 1992; Lucas et al., 2008). Since then, different lineages of RABLV circulating within dog populations have recurrently spilled over and caused disease in a wide range of susceptible mammals. These sporadic epizootics have been the source for subsequent establishment of dog-maintained RABLV lineages in a number of species of wild terrestrial mesocarnivores such as skunks, foxes, coyotes and mongooses, giving rise to different dog-derived RABLV variants throughout the Americas (Carnieli et al., 2008; Smith, 1989; Velasco-Villa et al., 2008). The establishment of dog-derived RABLV lineages in wildlife seems to be reservoir-specific, and lineages are maintained in geographically defined enzootic regions (Table 1).
Current surveillance reports in the USA have identified the geographic distribution of major independent enzootics associated with these different terrestrial carnivore species and their respective RABLV variants (Monroe et al., 2016). Thus, a dog-maintained RABLV lineage is operationally defined as a virus that is perpetuated between ill and healthy dogs exclusively. In contrast, a dog-derived RABLV variant is a formerly dog-maintained lineage that has spilled over and become established within a specific population of wild terrestrial mesocarnivores, in which rabies transmission cycles become independently perpetuated.
As discussed in the companion paper, novel dog-derived RABLV variants may be sporadically implicated in spillover infections into other susceptible mammals, causing epizootics with limited duration and extension (Smith, 1989; Monroe et al., 2016). However, there is a continuing risk that these variants could become reestablished in unvaccinated dog populations, since spillovers from wildlife into unvaccinated dogs are common in rural areas (Velasco-Villa et al., 2008). Some current examples of dog-derived RABLV variants and their potential impact on the elimination of canine rabies will be addressed further in this review.
3. Regional control efforts and lessons learned
Efforts at rabies control and prevention in the Americas date back to the time of Louis Pasteur’s vaccine studies. In 1888, three years after the first human vaccination against rabies in France, brains of rabbits infected with RABLV were brought to Mexico City by Dr Eduardo Liceaga, who initiated the first efforts at human rabies prevention in Latin America (Table 2) (Rodríguez de Romo, 1996; Lucas et al., 2008).
Table 2.
Major milestone events for the gradual elimination of dog-specific RABLV lineages from the Western Hemisphere.
| Period | Milestone events, in chronological order | Impact on confirmed rabies | |
|---|---|---|---|
|
|
|||
| Cases in dogs | Cases in humans | ||
| 1888-early 1900’s | Beginning of rabies vaccination in humans with Semple’s type vaccines. | ND | ND |
| 1960–1979 | Production of suckling-mouse-brain vaccine. | 1960s: ND | 1960s: ND |
| Availability of PEP for humans in large cities. | 1979: 23000 | 1979: 310 | |
| Vaccination campaigns of owned dogs in large cities. | |||
| Dog population management by removal of stray dogs in capital cities. | |||
| Introduction of rabies diagnostic units in major cities, based on histopathology (Seller’s staining). | |||
| Implementation of direct immunofluorescent antibody test (DFA) in national reference laboratories only. | |||
| 1980–1990 | Dog rabies is recognized as a public health problem | 1980: 24000 | 1980: 300 |
| PAHO/WHO advocates and coordinates rabies control and prevention initiatives throughout | 1990: 8500 | 1990: 270 | |
| REDIPRA | |||
| Creation of multifunctional anti-rabies centers in major cities. | |||
| 1991–2015 | Establishment of comprehensive national rabies control and prevention programs for dogs and humans, administered by Ministries of Health with federal budget. | 1991: 16250 | 1991: 240 |
| 2015: 316 | 2015: 3 | ||
| Acquisition of cell culture-derived rabies vaccines for humans and animals | |||
| Implementation of a revolving fund administered by PAHO to acquire rabies biologics a cover expenses of cold chain storage | |||
| Execution of massive dog vaccination campaigns with intersectoral and community cooperation twice a year. | |||
| Establishment of minimum potency policies for human and animal rabies vaccine manufactures to counteract adverse cold chain and field conditions | |||
| Implementation of regional financial strategies (PAHO revolving fund) for consolidated acquisition of rabies biologicals for humans and animals at more affordable prices | |||
| Creation of decentralized nationwide rabies diagnostic networks, using DFA as gold standard. Improvement of cold chain for human and animal vaccines at Federal, State, Municipal and local levels | |||
| Creation of decentralized nation-wide animal control Units | |||
| Legislation and enforcement of responsible pet ownership | |||
| Embrace policies for elimination and management of stray dog populations in collaboration with NGOs, humane societies and the community. | |||
| Communication outreach sponsored by Ministries of Health and the federal government, with intersectoral collaboration. | |||
| Technology transfer agreements with WHO collaborating centers on rabies to strengthen laboratory-based rabies surveillance. | |||
| Implementation of oral rabies vaccination for free-roaming dogs and wildlife species maintaining dog-specific and dog-related RABV. | |||
ND: not determined. Source for the numbers of humans and dogs infected with dog-specific RABLV variants: SIRVERA/SIEPI-PANAFTOSA/OPS-OMS (updated to 2015) http://siepi.panaftosa.org.br/. REDIPRA (Reunión de Directores de Programas de Rabia de las Américas), from its acronym in Spanish.
Canada and the USA were the first countries of the Americas to make significant advances in the control and elimination of dog-maintained RABLV variants from their dog populations. From the 1940s to the late 1950s, most of the sporadic dog rabies epizootics in Canada were related to arctic fox rabies that spilled over from the arctic fox RABLV variant into sled dogs, particularly in northern regions of the country (Johnson, 1971). During this time, control of rabies in the USA and Mexico became more problematic due to the greater diversity of dog-maintained and dog-derived RABLVs circulating in both countries and the implicit challenges associated with coordination of bi-national efforts (Velasco-Villa et al., 2008). The USA attained elimination of all dog-maintained lineages and the dog/coyote RABLV variant by 2007 (Sidwa et al., 2005). The dog/coyote RABLV variant was the first dog-derived RABLV eliminated from a territory in the Western Hemisphere by means of oral vaccination of coyotes (Slate et al., 2009).
In 1954, Eduardo Fuenzalida and Raul Palacios made possible low-cost domestic production of vaccines that were later used for both human rabies postexposure prophylaxis (PEP) and vaccination of dogs. This beta-propiolactone fully-inactivated vaccine with low myelin content was first licensed for use in humans in Chile in 1960, and later adopted by Uruguay for human PEP in 1963, followed by Argentina and Peru in 1964, Brazil and Venezuela in 1965, Cuba and Mexico in 1967, and Ecuador and Guatemala in 1969 (PAHO, 2010). Mexico was one of the first countries in Latin America to implement large-scale vaccination of dogs against rabies in preparation for the 1968 Olympic Games inMexico City. The Fuenzalida-Palacios vaccine and a live attenuated cell-culture vaccine of chick-embryo origin (CEO), commercially available at that time, were used for this campaign (Lucas et al., 2008; Steele, 1988; Secretaria de Salud, 2001).
As the commercial and domestic production of cell culture-derived live-attenuated and fully inactivated Fuenzalida-Palacios vaccines increased in the early 1970’s, sporadic efforts to carry out mass vaccination of dogs were expanded to other large Latin American capitals, such as Buenos Aires, Argentina, Lima, Peru and several major cities in Mexico. (Lucas et al., 2008; Navarro et al., 2007). In 1970, Mexico vaccinated more than 200,000 dogs, and by the end of that decade mass vaccinations of dogs, mostly with the CEO vaccine, reached over a million doses a year. However, despite these massive vaccination efforts, dog and human rabies cases did not decrease significantly during the period 1970–1989. In this 20-year period, Mexico reported a total of 1430 cases in humans and nearly 76,000 in dogs. The number of vaccine doses applied per year in the dog population continued to increase as the numbers of humans and dogs exploded and more commercially produced fully inactivated cell-culture derived vaccines became available.
In 1989, Mexico replaced the use of live-attenuated vaccines with more potent inactivated cell-culture-derived canine vaccines. In the same year, the number dog vaccinations in Mexico was above 5 million doses a year, which increased to 15 million doses by 2005. Consistency in dog vaccination coverage has been kept stable since 2005, reaching nearly 16 million doses administered during “the rabies vaccination week” in 2015. The shift to fully inactivated cell-culture vaccines, together with the dramatic increase in vaccination coverage (and some other important actions) translated into a steep decrease in the number of dog rabies cases, from 3049 in 1990 to 6 in 2015, and human infections went from 60 to 0 in the same period (Secretaria de Salud, 2001; Secretaria de Salud, 2016).
A critical milestone in the implementation of sustainable rabies control and prevention programs in Latin America was the political recognition of canine rabies as a human public health problem, which resulted in the transfer of all rabies control and prevention activities to ministries of health. Previously, rabies control in dogs had been strictly coordinated and funded by ministries of agriculture in most Latin American countries. The new initiative guaranteed sustained budgets for all activities related to rabies control and prevention in dogs, such as mass vaccination, creation and maintenance of a diagnostic infrastructure, construction of anti-rabies centers, support for dog population control activities and educational outreach (Table 2) (Schneider et al., 2007; Lucas et al., 2008). In 1983, this initiative was actively disseminated and later established as a political commitment through Pan American Health Organization/World Health Organization (PAHO/WHO) member countries. Periodic meetings coordinated by PAHO (called REDIPRA from its acronym in Spanish) gathered all Directors of National Rabies Programs to follow up on progress made and address ongoing challenges (Table 2) (Belotto et al., 2005; Schneider et al., 2007).
Early attempts at laboratory-based surveillance were made through the diagnosis of rabies in humans and animals by laboratory-based testing that began between 1960 and 1979. Seller’s staining of dog brain tissue predominated as the method of choice for rabies diagnosis during that period, but this test had low specificity, low sensitivity and required an experienced pathologist. The current gold-standard for rabies diagnosis worldwide is the direct immune-fluorescent antibody test (DFA), which was first employed in 1958 and has been widely used in Latin America since the early 1980s (Table 2) (Schneider et al., 2007; Orciari and Rupprecht, 2011). This period was also remarkable for the creation of multifunctional anti-rabies centers in major urban areas that were equipped with veterinarians and quarantine facilities, where suspicious rabid dogs were assessed and euthanized, and brain samples were taken for submission to rabies diagnostic labs. Vaccinations for owned dogs were also provided free of charge. These centers were mostly funded by local, state and municipal governments during this period (Table 2) (Belotto, 1988; Steele, 1988; Lucas et al., 2008; Navarro et al., 2007).
4. Current status of the elimination of dog-maintained RABLV variants from dog populations in Latin America
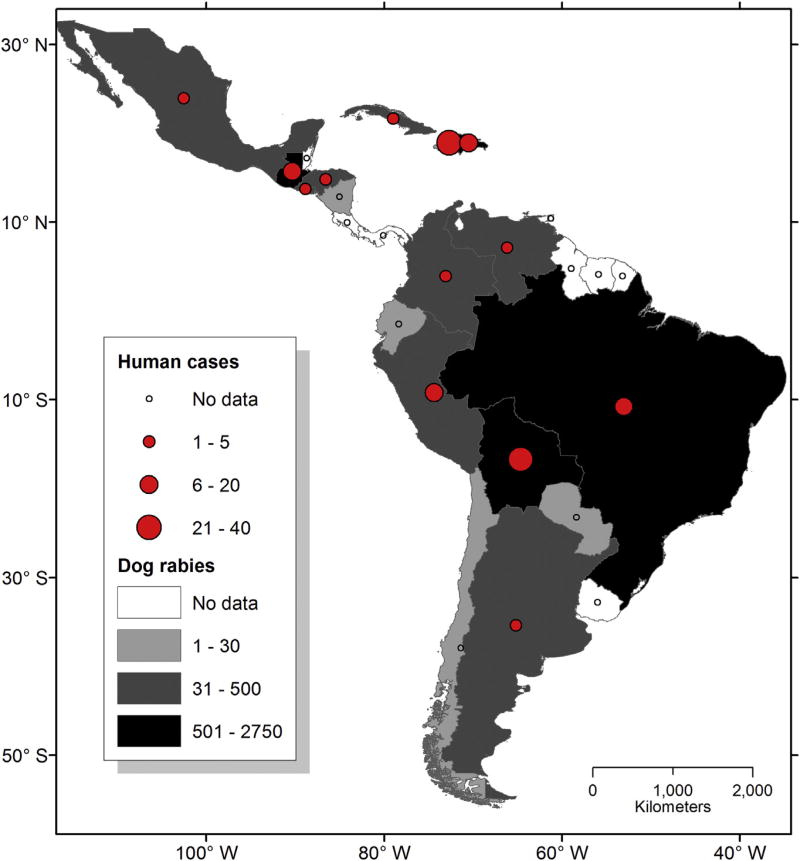
In the period from 2005 to 2015, a 98% reduction in rabies cases in dogs contributed to a 96% reduction in human rabies (Fig. 1, Table 3). These remarkable efforts have reduced the human rabies burden in Latin America, from 285 cases in 1970 to 3 in 2015 (Figs. 1 and 2, Table 3). During the decade 2005–2015, 139 human cases of rabies transmitted by dogs were reported in the region, with peaks in 2006 (30 cases) and 2011 (24 cases). Haiti and Bolivia reported the largest number of human rabies cases transmitted by dogs, with 38 and 29 cases respectively (Fig. 1). Nonetheless, rabies burden in humans in this countries may be likely underreported (Fig. 1, Table 3).
Fig. 1.
Distribution of dog-maintained rabies and its impact on rabies in humans transmitted from dogs in Latin America and the Caribbean. Solid red circles depict the total number of human rabies cases from 1993 to 2015; the size of each circle represents the number of cases according to the scale (lower left). Grayscale of countries represents the number of laboratory-confirmed rabid dogs carrying dog-maintained RABLV variants from 1993 to 2015. Scale for number of cases is depicted in the lower left. Countries with no cases or no data available are in white. Source, SIRVERA/SIEPI-PANAFTOSA/PAHO/WHO, http://new.paho.org/panaftosa. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Countries of Latin America and the Caribbean that have reported cases of human rabies transmitted by dogs and circulation of dog-maintained RABLV lineages, as reported to PAHO during 2005–2012.
| Country | Notes | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Argentina | A, B | 1 | 1 | ||||||||||
| Bolivia | A, B | 8 | 4 | 4 | 4 | 3 | 5 | 1 | 2 | 29 | |||
| Brazil | A, B | 1 | 6 | 1 | 2 | 1 | 2 | 2 | 3 | 1 | 19 | ||
| Colombia | A, B | 2 | 2 | 4 | |||||||||
| Cuba | A | 1 | 2 | 3 | |||||||||
| Dominican Republic | A | 1 | 3 | 3 | 2 | 2 | 2 | 13 | |||||
| El Salvador | A, C | 2 | 2 | 1 | 5 | ||||||||
| Guatemala | A, C | 1 | 1 | 1 | 3 | 3 | 3 | 12 | |||||
| Haiti | 1 | 11 | 6 | 4 | 1 | 13 | 2 | ? | 38 | ||||
| Honduras | A, C | 1 | 1 | 2 | |||||||||
| Mexico | A, B | 2 | 2 | ||||||||||
| Peru | A, B | 2 | 2 | 1 | 1 | 2 | 1 | 9 | |||||
| Venezuela | C | 1 | 1 | 2 |
A: Countries reporting laboratory-confirmed rabies cases in dogs, humans, livestock, and wildlife to PAHO on a monthly basis, this category also include Chile, Costa Rica, Ecuador, Panama, Paraguay, and Uruguay, which have reported no cases in the last decade. B: Countries with a decentralized laboratory-based rabies surveillance network, conducting RABLV variant typing on a regular basis, this category also includes Chile. Notes: 1. As for laboratory based surveillance coverage and viral typing Costa Rica, Ecuador, Nicaragua, Panama, Uruguay and Paraguay do not have enough laboratory-based evidence to objectively assessed the presence or absence of dog-maintained RABLV lineages. Furthermore, these countries have neighboring territories with confirmed enzootic dog-maintained RABLV lineages. 2. No information on rabies incidence was available for Belize, Guyana, and Suriname due to a lack of active laboratory based surveillance. 3. Trinidad & Tobago recently published a retrospective report supporting absence of canine rabies from the island till 2013. Source: SIRVERA/SIEPI-PANAFTOSA/OPS-OMS (2005–2015).
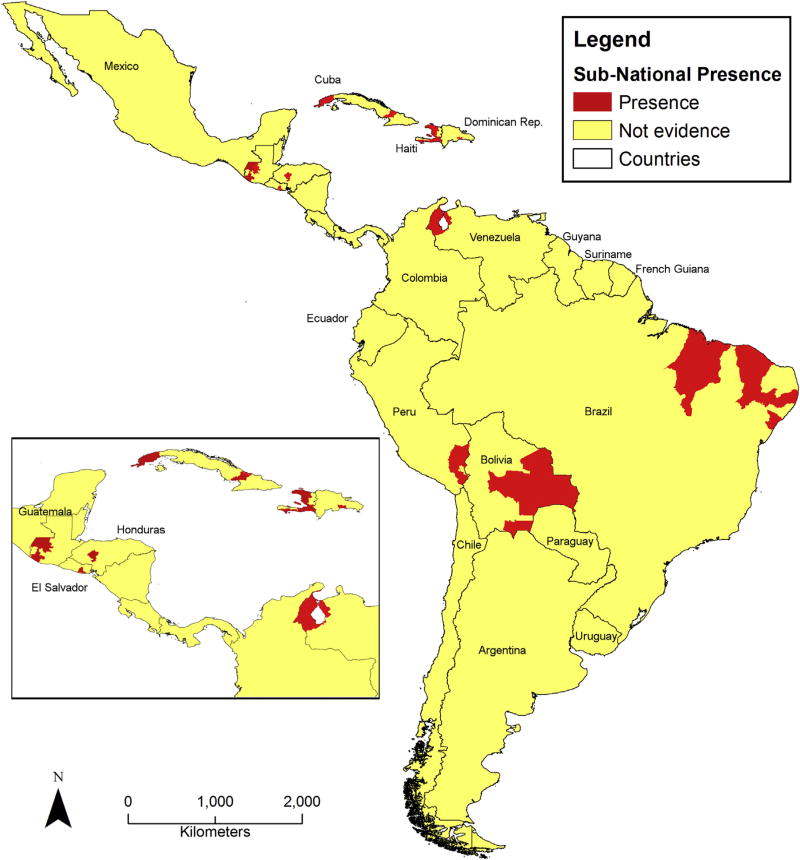
Fig. 2.
Canine rabies elimination efforts in Latin America and the Caribbean. Geographic location of dog-maintained rabies foci, as confirmed by PAHO collaborating center laboratories in the period 2010–15.
A more dramatic impact of these achievements can be best observed with cumulative figures published within the period 1970–1983, when 224,689 cases in dogs and 3662 cases in humans were reported for only the major cities of Mexico, Guatemala, Honduras, El Salvador, Colombia, Venezuela, Ecuador, Peru, Bolivia, Brazil and Argentina (Steele, 1988). The contemporary persistence of dog and human rabies in some countries of the region is caused by great economic disparities that hinder the regular acquisition of dog and human rabies vaccines, as well as by severe logistic constraints to deliver such vaccines to target populations (Figs. 1 and 2, Table 3) (Schneider et al., 2007; PAHO, 2010).
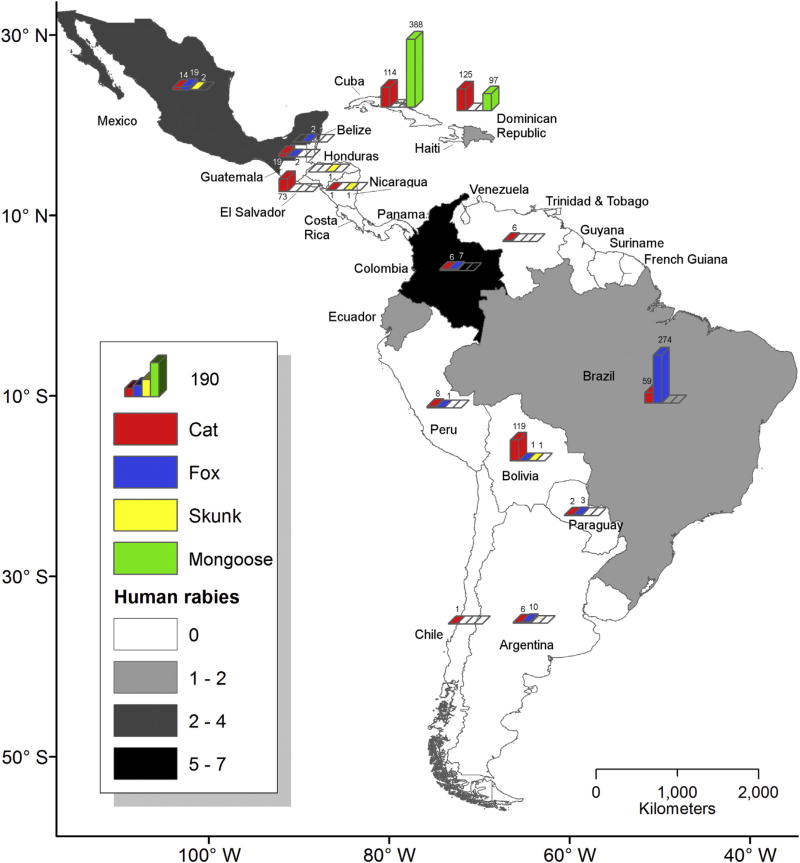
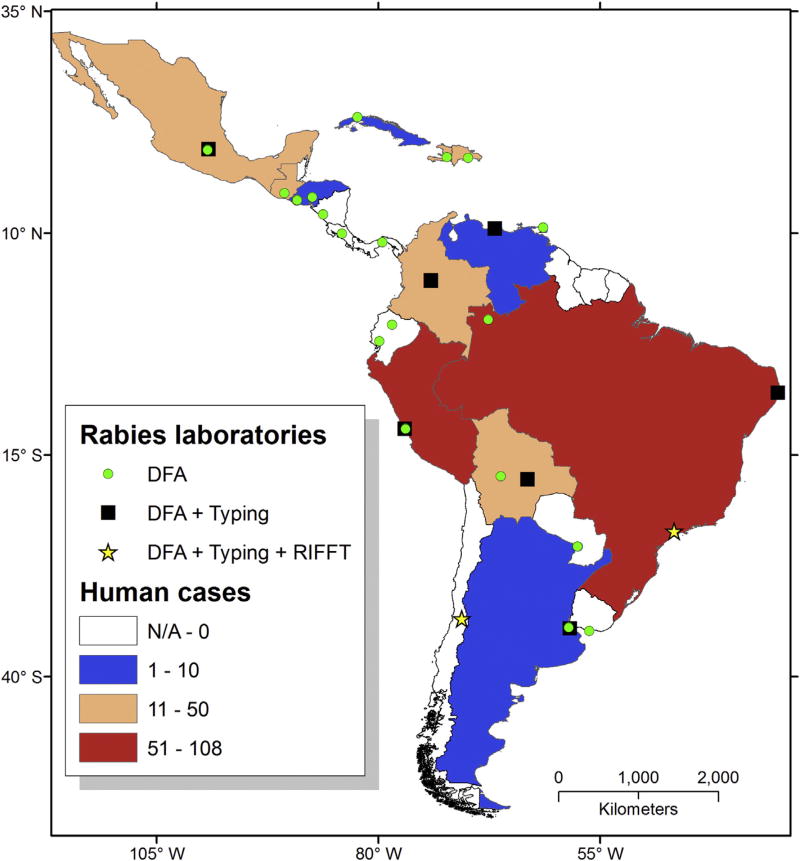
The significant reduction of human rabies cases associated with dogs is causing a shift in laboratory-based rabies surveillance to wildlife (bats and mesocarnivores), which is revealing long-term RABLV circulation in previously unnoticed wild reservoir hosts (Fig. 3) (Schneider et al., 2007; Vigilato et al., 2013). Nonetheless, under current laboratory-based rabies surveillance conditions, it remains challenging to ascertain the elimination status of dog-maintained RABLV variants in some countries of Latin America (Fig. 4). Several countries continue to report rabies transmitted by dog exposures or the occurrence of rabies in dogs, but without the laboratory capacity to perform RABLV variant typing (Table 3). Variant typing is necessary to understand the origin of the virus and its associated reservoir host in any particular case (Table 3, Fig. 3). If an alternative variant is not identified or demonstrated, the presence or absence of dog-maintained variants cannot be verified and elimination status cannot be determined. This problem underscores the need for enhanced laboratory-based surveillance networks with viral typing capabilities (Figs. 3 and 4).
Fig. 3.
Laboratory-confirmed infections with dog-derived RABLV variants in humans and animals that were acquired from carnivore species other than dogs from 2005 to 15. Grayscale reflects the number of confirmed human cases in which dog-derived variants transmitted by cats and wild carnivore hosts were identified. (Cats are not reservoir hosts, but can be involved as secondary transmitters of rabies.) Countries that did not report cases during this time period (Chile, Argentina, Guatemala, Costa Rica, El Salvador and Panama) and those with no data available or that do not report to PAHO (Trinidad and Tobago, Guyana, Surinam, and French Guiana) are shown in white. Colored bars within countries depict the most frequently positive species and major transmitters to humans, with cats (red), foxes (blue), skunks (yellow), and mongoose (green). Source, SIRVERA/SIEPI-PANAFTOSA/PAHO/WHO, http://new.paho.org/panaftosa. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Distribution of rabies diagnostic laboratories in Latin America and the Caribbean, their capacity and their affiliation to a Ministry of Health or Ministry of Agriculture. Color-coded countries represent total human rabies cases reported, as a reflection of testing capacity. Countries with low numbers of cases are in blue, medium in pink, and high in red. Countries with no cases reported or no data available are shown in white. Laboratory diagnostic capacity is depicted as follows: green dots for countries performing the “gold standard” direct fluorescent antibody test (DFA), filled black squares for countries performing DFA and viral typing by monoclonal antibodies and/or sequencing techniques (typing), and gold stars for countries performing DFA, viral typing and antibody titration via rapid immune fluorescent foci inhibition test (RIFFT). Countries with two icons (dot and square) represent independent diagnostic capacities for laboratories of the Ministry of Health and the Ministry of Agriculture. The locations of the icons represent the actual geographic locations of the labs. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The continued circulation of dog-maintained RABLV variants in the dog populations of semirural and irregular settings threatens progress toward elimination, and has caused sporadic outbreaks in dogs, humans, and wildlife in border areas of Argentina, Bolivia, Brazil, Colombia, Cuba, Dominican Republic, El Salvador, Ecuador, Guatemala, Haiti, Honduras, Mexico, Nicaragua, Peru and Venezuela, where elimination had apparently been achieved (Fig. 2, Table 3). Delimited areas within these countries have been identified by PAHO as rabies “hot-spots”, due to the persistence of dog-maintained RABLV cases related with continued viral circulation over the past 6 years. As noted, these apparent setbacks are the result of great economic, health and cultural disparities, which continue to pose major challenges to the control and eventual elimination of dog-maintained rabies. These persistent hot spots are geographically remote with difficult access by land, living conditions are extremely poor, free-roaming dog populations are large, rabies herd immunity is low and national mass vaccination campaigns do not reach these locations regularly (Schneider et al., 2007; Vigilato et al., 2013).
Surveillance approaches in countries such as Costa Rica, Belize, Nicaragua, Panama, Paraguay, Surinam and Uruguay, do not allow for the accurate assessment of the status of enzootic dog-maintained RABLV elimination (Figs. 1 and 3, Table 3). These countries frequently report rabies in livestock, but do not conduct RABLV typing on a regular basis, despite having neighboring countries with enzootic dog-maintained RABLV (Figs. 1 and 2). In contrast, the situation on island countries such as Trinidad and Tobago that have not reported circulation of enzootic dog-maintained RABLV variants since the 1950’s may be radically different. To stress the importance of viral typing, recent studies have implicated enzootic vampire bat-specific RABLV variants in spillover events occurring in susceptible animals (including dogs) of these islands (Seetahal et al., 2013; Wright et al., 2002).
Canada and the USA, through their PAHO/WHO rabies research reference centers [the Canadian Food Inspection Agency (CFIA) and the U. S. Centers for Disease Control and Prevention (CDC)] and in conjunction with other government institutions, collaborate with countries of Latin America and the Caribbean to support regional capacities for laboratory-based surveillance, to expedite rabies detection, and to support reference activities such as antigenic and genetic typing of RABLV for identification of variants and most likely transmission sources. In addition, regional political agreements such as the North American Rabies Management Plan among Canada, the USA and Mexico, signed in 2008, which provide continuity for canine rabies elimination efforts in the region (Slate et al., 2009). This plan fosters the transfer of diagnostic and typing technology, expedites typing results through CDC and CFIA, provides regular training on existing or novel diagnostic and typing technologies, supplies and replenishes diagnostic/virus typing kits and reagents on a regular basis, and promotes the periodic exchange of relevant rabies epidemiological and epizootiological information (Slate et al., 2009).
The implementation and refinement of rabies control and prevention strategies in Latin America have been remarkably enhanced by introducing objective indicators to monitor circulation of dog-maintained RABLV, such as typing technologies that include sequencing, coupled with phylogenetic analysis and antigenic typing with panels of monoclonal antibodies (Orciari and Rupprecht, 2011). These techniques provide information on viral variants or lineages linked with specific reservoir hosts (Smith, 1989; Smith et al., 1992). The identification of a reservoir host helps to implement specific control and prevention measures, as well as allowing the expeditious detection of importation events (Rupprecht et al., 2008).
5. Foundation of a national program of rabies control and prevention
The strategic designation of human health ministries as the administrative entities responsible for rabies control and prevention programs constituted a key political breakthrough to move forward the elimination of dog-maintained rabies in Latin America (Schneider et al., 2007). This initiative provided the funding to:
maintain adequate vaccine stocks for the immunization of humans and animals at no cost;
construct a decentralized network for animal control, surveillance and vaccination, which will be also responsible for collecting and submitting samples to diagnostic laboratories;
build a decentralized rdiagnostic laboratory network to support surveillance and expedite local control and prevention;
organize educational outreach programs to promote awareness for rabies control and prevention.
Deficiencies in any of these four areas may reduce the effectiveness of a national program (Belotto et al., 2005; Lucas et al., 2008).
6. Implementation of nationwide dog vaccination campaigns
In the early 1990’s, mass dog vaccination campaigns were implemented across the continental Americas and the Caribbean in countries including Mexico, Guatemala, Honduras, Dominican Republic, Cuba, Colombia, Ecuador, Peru, Chile, Argentina and Brazil (Belotto, 1988; Chomel et al., 1988; Navarro et al., 2007; Schneider et al., 2007; Lucas et al., 2008; Suzuki et al., 2008; PAHO, 2010). These campaigns varied in the extent of geographic coverage, the proportion of the dog population vaccinated, the types of vaccines used (cell culture-derived vaccines being significantly more potent than nerve tissue-derived vaccines), and the assessment of their impact (Table 2, Fig. 2).
The different approaches had a direct effect on the pace at which the incidence of rabies was reduced in dogs and indirectly in humans (Belotto et al., 2005; Schneider et al., 2007). Through 2015, Brazil and the Dominican Republic reported dog-maintained RABLV variant in humans (1 and 2 cases, respectively). The data do not include Haiti, because it does not have an adequate laboratory-based surveillance system; however, there is known to be an ongoing dog rabies epizootic, and living conditions are of extreme poverty (Table 3, Fig. 2) (PAHO, 2010). The struggles of these three countries reiterate substantial differences in economic capacities, size, cultural idiosyncrasies, rabies control and prevention strategies (types of vaccines used, government entity that runs the rabies program, and available overall infrastructure to obtain and deliver samples to diagnostic labs), human and dog population demographics, circulation of dog-maintained lineages in rural dog populations and public health priorities (Belotto et al., 2005; Schneider et al., 2007).
To ensure vaccine availability and affordability for both dogs and humans, several countries have engaged in a revolving fund, in which PAHO serves as manager and negotiator of consolidated purchases of rabies biologics with manufacturers (Schneider et al., 2007; Vigilato et al., 2013). All participating countries contribute 3.5% of their projected net purchase price for vaccine to create a common fund that will serve as negotiation capital with manufacturer companies of rabies biologics. Three percent of the fund is used entirely as working capital to offer a line of credit to participating countries that may require it, and 0.5% is used to cover the administrative costs of purchasing activities. Such lines of credit enable member countries that lack liquidity at the time of the purchase to acquire vaccine or rabies biologics and pay the revolving fund within 60 days of receipt. The revolving fund has been an essential factor in making the Americas a global role model for the success of immunization programs, the introduction of new vaccines and the significant reduction of health disparities within the region (PAHO and WHO, 2014).
7. Supplementary strategies to increase coverage of mass dog vaccination campaigns
Dog population management critically impacts efforts to achieve sustained rabies herd immunity within a population (Lembo et al., 2012). Current practices in Latin America predominantly employ surgical spaying and neutering in animal control units or itinerant surgeries during mass vaccination campaigns. Culling has been practiced occasionally as a desperate measure to stop epizootics involving high numbers of human exposures.
Alternatively, hormone-based vaccine-induced contraception methods seem to be promising choices for stabilizing free-roaming dog populations in Latin American countries (Lucas et al., 2008; Smith et al., 2011; Wu et al., 2009). Parenteral or hand-out delivery methods for these types of formulations seem to be safe choices to reach owned-dog populations, but may be impractical in the medium- and long-term to reach free-roaming dogs. Oral vaccines with contraceptive activity need to be further characterized to ensure that non-target species will not be affected by their use, especially if they are distributed in oral formulations and delivered in the same fashion as for wildlife and their contraceptive component works for a wide spectrum of species. The concomitant administration of rabies parenteral vaccine and an immune-contraceptive vaccine has also been attempted in an effort to both manage populations and immunize against rabies. More research is needed to prove the efficacy, safety and practicality of such approaches in the field (Vargas-Pino et al., 2013; Wu et al., 2009).
Utilization of more potent canine vaccines, such as cell culture-derived vaccines (with 2 or more international units per dose), has provided an additional means of expanding dog vaccination coverage. An optimal vaccine for delivery under field conditions should be potent and stable, to withstand transportation to remote locations where an efficient cold chain is lacking (Lucas et al., 2008). Recently, most countries of Latin America have transitioned from nerve tissue-derived vaccines to cell culture-derived vaccines for both dogs and humans, because of side effects affecting the central nervous system of the target species, and because fully inactivated cell culture-derived preparations are significantly more potent (National Association of State Public Health Veterinarians Committee, 2008; Smith et al., 2011). Alternatively, some countries have instituted minimum potency requirements in their purchasing and acquisition policies, as a means to ensure that the vaccine will adequately immunize dogs when used in the field, even if cold-chain conditions are not ideal. Recently, thermotolerant and thermostable vaccine formulations seem to be an excellent alternative to overcome cold-chain constraints and to expand coverage with acceptable immunization results (Lankester et al., 2016; Smith et al., 2015).
Oral vaccination may become an alternative to vaccinate free-roaming dog populations to increase coverage in places that are virtually impossible to reach through mass parenteral vaccination campaigns. However, no country in Latin America has engaged consistently in such activity, because it requires a more complex logistical apparatus and a more expensive vaccine (Corn et al., 2003). More recent vaccine developments implicate the use of genetically modified (reverse genetics), highly immunogenic and attenuated fixed rabies virus constructs that are also capable of expressing a contraceptive component. What these new generation vaccines are pursuing is a drastic reduction in production and delivery costs (for their single dose efficacy) as well as making more affordable, accessible and efficient dog population control. More investment, research and policy changes are needed to make feasible the application of these novel tools (Huang et al., 2015; Li et al., 2012; Wu et al., 2009).
8. Legislation and law enforcement of responsible dog ownership
Local and nationwide laws for responsible pet ownership and their adequate enforcement are paramount for maintaining a healthy dog population, which consequently reduces the risk of transmission of zoonotic diseases to humans (National Association of State Public Health Veterinarians Committee, 2008). Enforcement requires cooperation and action among all levels of government in Latin American countries, together with nonprofit organizations, local humane societies and pet owners.
9. Evaluating the impact of rabies vaccination
Current practices to assess rabies vaccination coverage in Latin America compare the number of vaccinated dogs (based on vaccination certificates issued, vaccination collars/tags used or verbal confirmation by owners after surveys) to estimate dog populations (Alves et al., 2005; Flores-Ibarra and Estrella-Valenzuela, 2004; Suzuki et al., 2008). However, this approach has some implicit biases if free-roaming or feral dog populations are not counted, particularly because they constitute the most important segment of the dog population in the maintenance of dog-maintained RABLV (Wandeler et al., 1988).
More effective methods that have not been fully implemented in Latin America include random samplings with mark-and-release strategies, in which serum samples are taken from some dogs to measure rabies-specific antibodies as evidence of vaccination (Chomel et al., 1988; Suzuki et al., 2008; Wandeler et al., 1988). A mark-resight survey of dogs temporarily marked during vaccination campaigns is another way of estimating vaccination coverage and a rough estimate of the dog population (Conan et al., 2015). Although such methods are more expensive to implement, their long-term benefits for improved vaccination strategies and better administration of available resources make them more cost-effective (Lembo et al., 2012).
10. Role of laboratory-based surveillance networks and animal control units
To enhance laboratory-based surveillance coverage and harmonize the standardized use of diagnostic techniques in the Americas, representatives of rabies reference laboratories from the Ministries of Agriculture and Health of Argentina, Bolivia, Brazil, Colombia, Chile, Costa Rica, Cuba, Dominican Republic, Ecuador, El Salvador, Guatemala, Haiti, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Trinidad and Tobago, Uruguay and Venezuela met in September, 2011, for the first joint conference of human and animal health laboratories to strengthen national capacities for the diagnosis and surveillance of urban and wildlife rabies. Based on the extent of territorial coverage of their diagnostic laboratory networks and the annual number of samples processed, the countries with the greatest number of samples tested in one year are Argentina, Brazil, Colombia, Mexico and Peru (Table 4) (CDC, SIRVERA/SIEPI-PANAFTOSA/OPS/OMS, 2012).
Table 4.
Enhanced laboratory-based surveillance: countries of the Americas with the largest number of samples processed in a year.
| Country | Number of laboratories in the network |
Number of samples processed |
|---|---|---|
| Argentina | 11 | 2600 |
| Brazil | 37 | 30000 |
| Colombia | 12 | 2700 |
| Mexico | 29 | 11000 |
| Peru | 21 | 10000 |
| United States | 125 | 125000 |
CDC, SIRVERA/SIEPI-PANAFTOSA/OPS/OMS, 2012.
The significance of the number of laboratories and the total numbers of samples processed represents to some extent the level of confidence for a country to validate its rabies-free status, as regards the circulation of dog-maintained RABLV lineages. Moreover, these indicators may be also linked with laboratory-based sensitive approaches for the detection of imported dog-maintained RABLV lineages that may jeopardize rabies-free status (Fig. 4). A suitable laboratory based-surveillance system should provide comprehensive territorial coverage (including all rabies “hot spots”) and be based on standardized laboratory diagnostic techniques for detection of RABLV antigen, including the “gold-standard” DFA, antibody titration by the rapid fluorescent-focus inhibition test (RFFIT), indirect fluorescent antibody test (IFA) and RABLV RNA detection via reverse transcription PCR assays (Orciari and Rupprecht, 2011). Particular emphasis should be given to genetic typing technologies that have been shown to be very informative to assess the impact of control and prevention strategies on the elimination of dog-maintained RABLV lineages. Typing is also essential for the early detection of imported dog-maintained and dog-derived RABLV variants, the characterization and distribution of variants established in wildlife (mainly terrestrial mesocarnivores and bats) and the detection of novel RABLV linked to the rise of new potential reservoir hosts (Smith, 1989 and Smith et al., 1992; Velasco-Villa et al., 2008).
Genetic typing has not yet been fully implemented across Latin America. Adequate sampling will be critical to obtain reliable information on elimination status and monitoring of emerging rabies public health threats. Robust genetic typing results obtained through adequate sampling should encompass year-round and geographically comprehensive monitoring of:
all rabid dogs in regions where dog-maintained rabies has become rare;
all outbreaks in mesocarnivores in countries or regions where dog-maintained rabies has been enzootic for decades;
recurrent routbreaks in mesocarnivores where bat-maintained rabies is common or has not previously been detected;
recurrent outbreaks in livestock in regions where vampire bat-transmitted rabies is not common; and
all dead bats encountered in peri-domestic areas.
Government-run animal control units play a critical role in rabies surveillance in Latin America, because they are the main providers of samples tested by diagnostic laboratories in the long-term monitoring of free-roaming dogs and wildlife. They also participate in vaccination of dogs on a daily basis and during mass vaccination campaigns. They also participate in the removal of stray dogs from public places, the quarantine of dogs that have bitten humans, and euthanasia of suspect rabid animals to obtain central nervous system samples for testing. Animal control units also perform on-site spaying and neutering of dogs and cats, and they may also serve as adoption centers (Lucas et al., 2008; National Association of State Public Health Veterinarians Committee, 2008). These units are subsidized by local governments, and either provide most services free of charge or charge low, symbolic fees.
11. Ecological challenges in the elimination of dog-maintained and dog-derived RABLV
Dog-derived RABLV variants that have become established in meso-carnivore populations of the Americas present serious challenges for the elimination of enzootic rabies in dogs and the subsequent reduction of the rabies burden of humans (Fig. 2, Table 3). It is clear that maintenance of thorough laboratory-based surveillance (including genetic typing), coupled with ideal herd immunity (above 70% of the total population) and lowered free-ranging dog population densities are key elements in preventing the return of enzootic dog-derived variants into the dog population (Figs. 3 and 4) (PAHO, 2010; Wandeler et al., 1988).
Cuba, Puerto Rico, the Dominican Republic and Haiti in the Caribbean region present special illustrations of the latter concern, as dog and mongoose populations maintain the same dog-derived RABLV variant (Fig. 3) (Nadin-Davis et al., 2006, 2008). High reproductive rates of both animal species and low vaccination coverage in free-roaming dogs make it difficult to achieve the needed levels of herd immunity to definitively eliminate rabies from both the dog and mongoose populations (Figs. 2 and 3). A customized plan of action will be required to simultaneously target both species for sustained disruption of the maintenance cycle. In this particular case, because both vulnerable populations are free-roaming, the implementation of an oral vaccination program for both dogs and mongooses may be the only feasible means of eradicating dog-derived RABLV variants from these island countries.
A more recent instance of a dog-derived variant returning to a dog population with low herd immunity due to inadequate vaccine coverage occurred in Mexico in 2011, within 80 km of the USA border. A dog-derived coyote RABLV variant was first detected in southern Texas along the USA-Mexico border in 1988 (CDC, 1995), but retrospective studies showed that it was actually circulating in the 1970s (Velasco-Villa et al., 2008). An oral vaccination program implemented in Texas in 1995 contributed to a dramatic reduction in its circulation in coyotes by 2004 (Sidwa et al., 2005), and its eventual elimination from USA dog and coyote populations by 2007 (Slate et al., 2009). The virus was also thought to have been eliminated from dogs in northeastern Mexico, but in fact it continued to circulate in the coyote population, and was reintroduced into dogs through coyote-dog contact. The 2011 re-introduction makes it clear that long-term efforts will be needed to eliminate this dog-derived variant from the coyote population. Latin American countries may benefit from instituting similar oral rabies vaccination programs in wildlife, especially to prevent the re-introduction of dog-derived variants into dog populations (Fig. 3).
12. Conclusions
The dramatic decrease in the incidence of dog-maintained rabies in the Western Hemisphere over the past decade and its reduction in humans to almost zero may tend to create a false sense of security in public health officials and governments, to the point that canine rabies may no longer be considered a public health problem, leading to budget cuts or the eventual elimination of strategic national control activities. The danger with this notion is that there is a constant threat for epizootics to re-occur, so as long as dog-maintained rabies “hot spots” are still present and whenever free-roaming dog populations remain large, herd immunity becomes low and dog-derived variants continue to circulate in close proximity to naïve dog populations.
The elimination of canine rabies from the Western Hemisphere will only be feasible if both dog-maintained and dog-derived RABLV lineages and variants are permanently eliminated, particularly in settings in which dog-maintained viruses are equally fit to be maintained by wildlife, as is the case of dog/mongoose rabies in the Caribbean. However, canine rabies elimination may be possible by keeping dog herd immunity above 70% at all times, fostering sustained laboratory-based surveillance through reliable diagnosis and RABLV genetic typing in dogs, domestic animals and wildlife (terrestrial mesocarnivores and bats), and continuing to educate the public on the risk of rabies transmission, prevention methods and responsible pet ownership. The complete elimination of canine rabies, especially in “hot spots,” requires permanent funding, with governments and people committed to bringing resources and elimination activities to those remote localities where the problem persists.
Acknowledgments
The authors acknowledge editorial comments from Kim Hummel, which were critical during the preparation of this document. The authors did not receive any financial remuneration for writing this article and declare that no conflict of interest exists. The opinions expressed and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or the US Department of Health and Human Services.
References
- Alves GP, Matos MRd, Reichmann MdL, Dominguez MH. Estimation of the dog and cat population in the State of São Paulo. Rev. De. Saúde Pub. 2005;39:891–897. doi: 10.1590/s0034-89102005000600004. [DOI] [PubMed] [Google Scholar]
- Belotto AJ. Organization of mass vaccination for dogs in Brazil. Rev. Infect. Dis. 1988;10:S693–S696. doi: 10.1093/clinids/10.supplement_4.s693. [DOI] [PubMed] [Google Scholar]
- Belotto A, Leanes LF, Schneider MC, Tamayo H, Correa E. Overview of rabies in the Americas. Virus Res. 2005;111:5–12. doi: 10.1016/j.virusres.2005.03.006. http://dx.doi.org/10.1016/j.virusres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Carnieli P, Jr, Fahl, Wde O, Castilho JG, Oliveira, Rde N, Macedo CI, Durymanova E, Jorge RS, Morato RG, Spíndola RO, Machado LM, Ungar, de Sa JE, Carrieri ML, Kotait I. Characterization of Rabies virus isolated from canids and identification of the main wild canid host in Northeastern Brazil. Virus Res. 2008;131:33–46. doi: 10.1016/j.virusres.2007.08.007. http://dx.doi.org/10.1016/j.virusres.2007.08.007. [DOI] [PubMed] [Google Scholar]
- CDC. Translocation of Coyote Rabies-Florida, 1994. MMWR. 1995;44:580–581. 587. [PubMed] [Google Scholar]
- Chomel B, Chappuis G, Bullon F, Cardenas E, David de Beublain T, Lombard M, Giambruno E. Mass vaccination campaign against rabies: are dogs correctly protected? Rev. Infect. Dis. 1988;10:S697–S702. doi: 10.1093/clinids/10.supplement_4.s697. [DOI] [PubMed] [Google Scholar]
- Conan A, Kent A, Koman K, Konink S, Knobel D. Evaluation of methods for short-term marking of domestic dogs for rabies control. Prev. Vet. Med. 2015;121(1–2):179–182. doi: 10.1016/j.prevetmed.2015.05.008. http://dx.doi.org/10.1016/j.prevetmed.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Corn JL, Méndez JR, Catalán EE. Evaluation of baits for delivery of oral rabies vaccine to dogs in Guatemala. Am. J. Trop. Med. Hyg. 2003;69:155–158. [PubMed] [Google Scholar]
- Flores-Ibarra M, Estrella-Valenzuela G. Canine ecology and socioeconomic factors associated with dogs unvaccinated against rabies in a Mexican city across the US-Mexico border. Prev. Veterinary Med. 2004;62:79–87. doi: 10.1016/j.prevetmed.2003.10.002. http://dx.doi.org/10.1016/j.prevetmed.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Huang F, Ahmad W, Duan M, Liu Z, Guan Z, Zhang M, Qiao B, Li Y, Song Y, Song Y, Chen Y, Amjad, Ali M. Efficiency of live attenuated and inactivated rabies viruses in prophylactic and post exposure vaccination against the street virus strain. Acta Virol. 2015;59:117–124. doi: 10.4149/av_2015_02_117. [DOI] [PubMed] [Google Scholar]
- Johnson HN. General epizootiology of rabies. In: Nagano Y, Davenport FM, editors. Rabies; Proceedings of Working Conference on Rabies Sponsored by Japan-United States Cooperative Medical Science, Program; University of Tokyo Press; 1971. pp. 237–251. [Google Scholar]
- Lankester FJ, Wouters PA, Czupryna A, Palmer GH, Mzimbiri I, Cleaveland S, Francis MJ, Sutton DJ, Sonnemans DG. Thermotolerance of an inactivated rabies vaccine for dogs. Vaccine. 2016;34:5504–5511. doi: 10.1016/j.vaccine.2016.10.015. http://dx.doi.org/10.1016/j.vaccine.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Lembo T on behalf of the Partners for Rabies Prevention. The blue print for rabies prevention and control: a novel operational toolkit for rabies elimination. PLoS Negl. Trop. Dis. 2012;6:e1388. doi: 10.1371/journal.pntd.0001388. http://dx.doi.org/10.1371/journal.pntd.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ertel A, Portocarrero C, Barkhouse DA, Dietzschold B, Hooper DC, Faber M. Postexposure treatment with the live-attenuated rabies virus (RV) vaccine TriGAS triggers the clearance of wild-type RV from the Central Nervous System (CNS) through the rapid induction of genes relevant to adaptive immunity in CNS tissues. J. Virol. 2012;86:3200–3210. doi: 10.1128/JVI.06699-11. http://dx.doi.org/10.1128/JVI.06699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CH, Pino FV, Baer G, Morales PK, Cedillo VG, Blanco MA, Avila MH. Rabies control in Mexico. Dev. Biol. Basel. 2008;131:167–175. [PubMed] [Google Scholar]
- Monroe BP, Yager P, Blanton J, Birhane MG, Wadhwa A, Orciari L, Petersen B, Wallace R. Rabies surveillance in the United States during 2014. J. Am. Vet. Med. Assoc. 2016 Apr 1;248(7):777–788. doi: 10.2460/javma.248.7.777. http://dx.doi.org/10.2460/javma.248.7.777 2016. [DOI] [PubMed] [Google Scholar]
- Nadin-Davis SA, Torres G, Ribas M, de L, Guzman M, De La Paz RC, Morales M, Wandeler AI. A molecular epidemiological study of rabies in Cuba. Epidemiol. Infect. 2006;134:1313–1324. doi: 10.1017/S0950268806006297. http://dx.doi.org/10.1017/S0950268806006297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin-Davis SA, Velez J, Malaga C, Wandeler AI. A molecular epidemiological study of rabies in Puerto Rico. Virus Res. 2008;131:8–15. doi: 10.1016/j.virusres.2007.08.002. http://dx.doi.org/10.1016/j.virusres.2007.08.002. [DOI] [PubMed] [Google Scholar]
- National Association of State Public Health Veterinarians Committee. Compendium of animal rabies prevention and control. J. Am. Vet. Med. Assoc. 2008;232:1478–1486. doi: 10.2460/javma.232.10.1478. [DOI] [PubMed] [Google Scholar]
- Navarro AM, Bustamante J, Sato A. Situación actual y control de la rabia en el Perú. Rev. Peru. Med. Exp. Salud Pub. 2007;24:46–50. [Google Scholar]
- Orciari LA, Rupprecht CE. Rabies virus. In: Landry ML, Caliendo AM, Ginocchio CC, Tang YW, Valsamakis A, editors. Manual of Clinical Microbiology. Vol. 2. ASM Press; Washington, United States of America: 2011. pp. 1470–1478. [Google Scholar]
- PAHO. [13 Meeting of Latin American National Rabies Control Program Directors] 13 Reunión de Directores de los Programas Nacionales de Control de la Rabia en América Latina. Pan-American Health Organization, B; uenos Aires: 2010. [Google Scholar]
- PAHO. World Health Organization. About PAHO Revolving Fund. 2014 http://www.paho.org/hq/index.php?option=com_content&view=article&id=9562&Itemid=40717&lang=en.
- Rodríguez de Romo AC. La ciencia pasteuriana a través de la vacuna anti-rrábica: caso de Mexico. DYNAMIS. Acta Hisp. Sci. Med. Hist. Illus. 1996;16:291–316. [PubMed] [Google Scholar]
- Rupprecht CE, Barrett J, Briggs D, Cliquet F, Fooks AR, Lumlertdacha B, Meslin FX, Müler T, Nel LH, Schneider C, Tordo N, Wandeler AI. Can rabies be eradicated? Dev. Biol. Basell. 2008;131:95–121. [PubMed] [Google Scholar]
- Schneider MC, Belotto A, Adé MP, Hendrickx S, Leanes LF, Freitas Rodrigues MJ, Medina G, Correa E. Current status of human rabies transmitted by dogs in Latin America. Cad. Saúde Pub. 2007;23:2049–2063. doi: 10.1590/s0102-311x2007000900013. [DOI] [PubMed] [Google Scholar]
- Secretaria de Salud. Programa de acción: rabia. Secretaria de Salud México. 2001:9–44. http://www.salud.gob.mx/unidades/cdi/documentos/rabia.pdf.
- Secretaria de Salud. Situación de los casos de rabia canina. 2016 http://www.gob.mx/salud/acciones-y-programas/situacion-de-los-casos-de-rabia-canina.
- Seetahal JF, Velasco-Villa A, Allicock OM, Adesiyun AA, Bissessar J, Amour K, Phillip-Hosein A, Marston DA, McElhinney LM, Shi M, Wharwood CA, Fooks AR, Carrington CV. Evolutionary history and phylogeography of rabies viruses associated with outbreaks in Trinidad. PLoS Negl. Trop. Dis. 2013;7:e2365. doi: 10.1371/journal.pntd.0002365. http://dx.doi.org/10.1371/journal.pntd.0002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwa TJ, Wilson PJ, Moore GM, Oertli EH, Hicks BN, Rohde RE, Johnston DH. Evaluation of oral rabies vaccination programs for control of rabies epizootics in coyotes and gray foxes: 1995–2003. J. Am. Vet. Med. Assoc. 2005;227:785–792. doi: 10.2460/javma.2005.227.785. http://dx.doi.org/10.2460/javma.2005.227.785. [DOI] [PubMed] [Google Scholar]
- Slate D, Algeo TP, Nelson KM, Chipman RB, Donovan D, Blanton JD, Niezgoda M, Rupprecht CE. Oral rabies vaccination in North America: opportunities, complexities, and challenges. PLoS Negl. Trop. Dis. 2009;3:e549. doi: 10.1371/journal.pntd.0000549. http://dx.doi.org/10.1371/journal.pntd.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS. Rabies virus epitopic variation: use in ecologic studies. Adv. Virus Res. 1989;36:215–253. doi: 10.1016/s0065-3527(08)60586-2. [DOI] [PubMed] [Google Scholar]
- Smith JS, Orciari LA, Yager PA, Seidel DH, Warner CK. Epidemiologic and historical relationships among 87 rabies virus samples as determined by limited sequence analysis. J. Infect. Dis. 1992;166:296–307. doi: 10.1093/infdis/166.2.296. http://dx.doi.org/10.1093/infdis/166.2.296. [DOI] [PubMed] [Google Scholar]
- Smith TG, Wu X, Franka R, Rupprecht CE. Design of future rabies biologics and antiviral drugs. Adv. Virus Res. 2011;79:345–363. doi: 10.1016/B978-0-12-387040-7.00016-0. http://dx.doi.org/10.1016/B978-0-12-387040-7.00016-0. [DOI] [PubMed] [Google Scholar]
- Smith TG, Siirin M, Wu X, Hanlon CA, Bronshtein V. Rabies vaccine preserved by vaporization is thermostable and immunogenic. Vaccine. 2015;33:2203–2206. doi: 10.1016/j.vaccine.2015.03.025. http://dx.doi.org/10.1016/j.vaccine.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JH. Rabies in the Americas and remarks on global aspects. Rev. Infect. Dis. 1988;10:S585–S597. doi: 10.1093/clinids/10.supplement_4.s585. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Pereira JA, Frias LA, Lopez R, Mutinelli LE, López R, Mutinelli LE, Pons ER. Rabies-vaccination coverage and profiles of the owned-dog population in Santa Cruz de la Sierra, Bolivia. Zoonoses Public Health. 2008;55:177–183. doi: 10.1111/j.1863-2378.2008.01114.x. http://dx.doi.org/10.1111/j.1863-2378.2008.01114.x. [DOI] [PubMed] [Google Scholar]
- Vargas-Pino F, Gutiérrez-Cedillo V, Canales-Vargas EJ, Gress-Ortega LR, Miller LA, Rupprecht CE, Bender SC, García-Reyna P, Ocampo-López J, Slate D. Concomitant administration of GonaCon™ and rabies vaccine in female dogs (Canis familiaris) in Mexico. Vaccine. 2013;31:4442–4447. doi: 10.1016/j.vaccine.2013.06.061. http://dx.doi.org/10.1016/j.vaccine.2013.06.061. [DOI] [PubMed] [Google Scholar]
- Velasco-Villa Andres, Mauldin Matthew R, Shi Mang, Escobar Luis E, Gallardo-Romero Nadia F, Damon Inger, Olson Victoria A, Streicker Daniel G, Emerson Ginny. The History of Rabies in the Western Hemisphere (in press) 2017 doi: 10.1016/j.antiviral.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Villa A, Reeder SA, Orciari LA, Yager PA, Franka R, Blanton JD, Zuckero L, Hunt P, Oertli EH, Robinson LE, Rupprecht CE. Enzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United States. Emerg. Infect. Dis. 2008;14:1849–1854. doi: 10.3201/eid1412.080876. http://dx.doi.org/10.3201/eid1412.080876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigilato MAN, Cosivi O, Knöbl T, Clavijo A, Silva HMT. Rabies update for Latin America and the Caribbean. EID. 2013;19:678–679. doi: 10.3201/eid1904.121482. http://dx.doi.org/10.3201/eid1904.121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandeler AI, Budde A, Capt S, Kappeler A, Matter H. Dog ecology and dog rabies control. Rev. Infect. Dis. 1988;10:S684–S688. doi: 10.1093/clinids/10.supplement_4.s684. [DOI] [PubMed] [Google Scholar]
- Wright A, Rampersad J, Ryan J, Ammons D. Molecular characterization of rabies virus isolates from Trinidad. Vet. Microbiol. 2002;87:95–102. doi: 10.1016/s0378-1135(02)00045-7. http://dx.doi.org/10.1016/S03378-1135(02)00045-7. [DOI] [PubMed] [Google Scholar]
- Wu X, Franka R, Svoboda P, Pohl J, Rupprecht CE. Development of combined vaccines for rabies and immunocontraception. Vaccine. 2009;27:7202–7209. doi: 10.1016/j.vaccine.2009.09.025. http://dx.doi.org/10.1016/j.vaccine.2009.09.025. [DOI] [PubMed] [Google Scholar]