Abstract
Bats (Order Chiroptera) are an abundant group of mammals with tremendous ecological value as insectivores and plant dispersers, but their role as reservoirs of zoonotic diseases has received more attention in the last decade. With the goal of managing disease in free-ranging bats, we tested modified vaccinia Ankara (MVA) and raccoon poxvirus (RCN) as potential vaccine vectors in the Brazilian Free-tailed bat (Tadarida brasiliensis), using biophotonic in vivo imaging and immunogenicity studies. Animals were administered recombinant poxviral vectors expressing the luciferase gene (MVA-luc, RCN-luc) through oronasal (ON) or intramuscular (IM) routes and subsequently monitored for bioluminescent signal indicative of viral infection. No clinical illness was noted after exposure to any of the vectors, and limited luciferase expression was observed. Higher and longer levels of expression were observed with the RCN-luc construct. When given IM, luciferase expression was limited to the site of injection, while ON exposure led to initial expression in the oral cavity, often followed by secondary replication at another location, likely the gastric mucosa or gastric associated lymphatic tissue. Viral DNA was detected in oral swabs up to 7 and 9 days post infection (dpi) for MVA and RCN, respectively. While no live virus was detected in oral swabs from MVA-infected bats, titers up to 3.88 x 104 PFU/ml were recovered from oral swabs of RCN-infected bats. Viral DNA was also detected in fecal samples from two bats inoculated IM with RCN, but no live virus was recovered. Finally, we examined the immunogenicity of a RCN based rabies vaccine (RCN-G) following ON administration. Significant rabies neutralizing antibody titers were detected in the serum of immunized bats using the rapid fluorescence focus inhibition test (RFFIT). These studies highlight the safety and immunogenicity of attenuated poxviruses and their potential use as vaccine vectors in bats.
Keywords: Poxvirus, Vaccine, Bat, Chiroptera, Rabies
1. Introduction
Over the last few decades, the importance of bats (order Chiroptera) in the maintenance and transmission of zoonotic diseases has become increasingly evident; bats are thought to harbor the most zoonotic agents per species [1]. The list of pathogens that infect bats includes the major mammalian paramyxoviruses [2], coronaviruses [3], [4], filoviruses [5], [6], [7], distinct influenza lineages [8], [9], hepadnaviruses [10], and hantaviruses [11], as well as lyssaviruses such as rabies virus [12], [13]. In the United States, bats are often the most common source of rabies infections in humans [14], and in Central and South America, rabies transmitted by vampire bats is a serious zoonotic and economic issue [15]. This association between bats and pathogens that significantly impacts human populations has increased public fear and misunderstanding of these animals and lead to culling campaigns [15], [16], [17], [18]. Unfortunately, culling campaigns often lead to the death of valuable non-target bat species [16] and appear ineffective in reducing disease incidence [17]. Alternatively, vaccination of other wildlife species has been successful in mitigating the public health impact of rabies with the development of efficient and practical distribution methods for mass immunization.
Poxviral vectors have been used extensively for oral vaccines to control infectious diseases in a variety of animal species over the last 25 years [19], [20]. Several advantages of poxviruses as vaccine vectors include: (1) their allowance of large insertions of foreign DNA; (2) ease of manufacturing; (3) thermal and genetic stability; (4) safety and infectivity for multiple target species; and (5) ability to infect via mucosal and dermal routes. For example, an oral rabies vaccine, constructed by inserting the rabies G glycoprotein into vaccinia virus and distributed via baits, has been used for many years to curtail rabies outbreaks in foxes, raccoons and other animals in North America and Europe [21]. Previous studies assessing a vaccinia-based rabies vaccine (VR-G) in Desmodus bats demonstrated the protective efficacy of that construct [22], [23], [24]. However, this vector has undesirable side-effects, especially in immunocompromised individuals [25], necessitating the development of attenuated virus strains. More recently, an oral sylvatic plague vaccine using another poxvirus (raccoon pox, RCN) was shown to protect prairie dogs and is currently being tested in large-scale field trials [26], [27]. RCN was first isolated from the upper respiratory tract of apparently healthy raccoons in North America [28]. It has since been shown to be safe and effective in a variety of species, including domestic cats, piglets, dogs, raccoons, skunks, foxes, bobcats, rabbits, sheep, prairie dogs, non-human primates, and chickens, with none of the immunized animals showing clinical side effects [29], [30], [31], [32]. Additionally, RCN has been shown to be immunogenic via non-parenteral routes in both domestic species [29] and free ranging wildlife [33], [34]. Another orthopoxvirus vector, modified vaccinia Ankara (MVA), is a highly attenuated form of vaccinia [35], [36] and has also been demonstrated to safely and effectively induce immunity [37], [38].
Based on the success of mucosal vaccination with poxvirus vectors in many other species, we hypothesized that poxviruses could be immunogenic and safe when given mucosally in chiropteran species. To test this, we assessed the infectivity and pathogenicity of MVA and RCN in T. brasiliensis via in vivo imaging studies. The immunogenicity of RCN given oronasally was also assessed using standard serologic techniques after vaccination with an RCN-based rabies vaccine (RCN-G).
2. Materials and methods
2.1. Ethics statement
The use of bats in this experiment was approved by (Protocol #EP111018) and conducted in accordance with the U.S. Geological Survey (USGS), National Wildlife Health Center (NWHC), Animal Care and Use Committee (ACUC).
2.2. Animals
Adult male bats (T. brasiliensis; n = 22) were caught in Brazos County, Texas under Texas Parks and Wildlife Department permit number SPR-1104-610 and Texas A&M University ACUC approval number 2012-130 (courtesy of Mike Smotherman, Texas A&M University, USA). After acclimating to captivity, the bats were transferred to NWHC (Madison, Wisconsin, USA), where all bat studies were conducted under ABSL-3 conditions. Upon transfer to the NWHC, bats were maintained in flight cages for a quarantine period of 30 days. During this time blood samples were taken and bats were treated topically for parasites. Electronic microchip identification units (Avid Identification Systems, Inc., Folsom, Louisiana, USA) were inserted into each animal, between the scapulae, via subcutaneous injection. Bats were maintained on mealworms (Tenebrio molitor) supplemented with vitamins and an omega fatty acids mixture, and water was available ad libitum. Light cycles were set to 12 h of light per day inverted from the natural cycle to allow monitoring of bat activities during facility working hours.
2.3. Viruses and cells
The RCN-luc strain used in this study was previously described [32]. The MVA-GFP strain used to create the MVA-luc constructs was generously provided by Inviragen (Madison, WI), while RCN-G [34] was kindly provided by the Centers for Disease Control (Atlanta, GA). Recombinant viruses were generated and amplified on cell monolayers of rat embryonic fibroblasts (Rat-2, ATCC #CRL-1764), baby hamster kidney cells (BHK-21, ATCC #CRL-12072), African Green monkey kidney epithelial cells (Vero, ATCC #CCL-18), or primary chicken embryo fibroblasts (CEF, Charles River Laboratories, INC, Wilmington, WA, USA)). Cell cultures were maintained at 37 °C and 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM) or Opti-MEM® (Life technologies, Madison, WI 53719), supplemented with 2–5% fetal bovine serum (FBS). Viruses were titrated prior to use with plaque dilution assays in 6-well plates.
2.4. Construction of recombinant MVA-luc
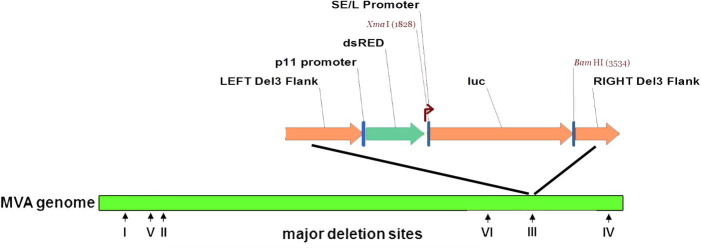
Recombinant MVA-luc viruses were constructed as described elsewhere [39]. Briefly, CEF cells were infected with MVA-GFP at a multiplicity of 0.05 PFU per cell; one hour later the cells were transfected using the FuGENE® reagent protocol (Promega, Fitchburg, WI) with a pI2 transfer plasmid containing (1) DNA flanking segments adjacent to deletion III within the HindIII A fragment of MVA, (2) the Red Fluorescent Protein (RFP) under the control of a p11 promoter, and (3) the firefly luciferase gene (luc) under the control of a strong synthetic early/late (SE/L) vaccinia virus promoter upstream (Fig. S1). At 48–72 h post transfection, the cell cultures were put through three freeze–thaw cycles, harvested, sonicated, and centrifuged at 500g for 5 min at 4 °C. The sonicated cell extracts were plated onto fresh BHK-21 cells and overlaid with 0.8% agarose. After 48–72 h, through the use of specific microscope filters, the recombinant viruses were detected by the presence of the RFP gene, which replaced the GFP gene during homologous recombination. Selected cell/virus samples were sonicated and plated again as described above. After four consecutive rounds of plaque isolation, recombinant MVA-luc virus was confirmed by PCR analysis using OneTaq® Quick-Load® 2X Master Mix with Standard Buffer (New England BioLabs Inc., Ipswich, MA 01938, USA) amplifying the insertion at the Del III flanks using ATGCGGCACCTCTCTTAA as a forward primer and CCAAAGCTTGCACATACATAAGTA as the reverse primer. The virus subsequently amplified in freshly prepared CEF cells.
2.5. Bioluminescent imaging
Biophotonic luminescent imaging (BLI) has been successfully used to assess the infectivity, infection course, and tissue tropism of viruses and candidate vaccine vectors [40]. Unlike traditional pathogenicity studies that require euthanasia of animals at different time points, BLI allows study of the course of infection over time in a single host, increasing the information gained while reducing the number of animals required.
Groups of 8 wild-caught T. brasiliensis were separated into two screened flight cages (33″W × 66″D × 84″H) approximately 5 m apart. Four bats from each group were given 109 plaque forming units (PFU) in 100 μl of either RCN-luc or MVA-luc by intramuscular (IM) injection, split into two 50 μl volumes injected into each thigh muscle. The remaining four bats in each group were given the same amount of virus in 70 μl sterile saline; using a micropipette with sterile tips, the volume split among the nostrils (10 μl given each nare) and mouth (50 μl) for oronasal (ON) exposure. Bats were monitored for 3 h post inoculation for signs of adverse effects. Animals were scanned using an IVIS 200 Biophotonic imager (PerkinElmer, Hopkinton, MA, USA) at one-day post infection (dpi) and every-other day thereafter until 2 consecutive images with less than 100 radiance units (comparable to background) were observed. Bats were scanned prior to infection and confirmed to have no auto-luminescence beyond background levels. Imaging was conducted at roughly the same time period each day, midway through the bats’ active period. On imaging days the bats were separated individually into paper lunch bags, weighed, and injected intraperitoneally (i.p) with d-luciferin (Potassium-Luciferin, Gold Biotechnology, St. Louis, MO 63132, dose: 150 mg/kg) at 14 min prior to imaging the ON group and 26 min prior to imaging the IM group, which were empirically determined to be the time of peak luminescence post substrate exposure on the first day of imaging. All bats were examined for signs of disease or discomfort at the time of substrate injection. For imaging, animals were anesthetized by chamber-delivered isoflurane and positioned in the imager in dorsal recumbency with wings extended laterally, where they were maintained on mask-delivered isoflurane. After imaging the anesthesia was ceased, bats were monitored and given thermal support during recovery. After 11 dpi the bats with remaining detectable luminescence were imaged every 3 days until luminescence was below detectable levels. Images were collected and analyzed using Living Image software (Caliper Life Sciences, Alameda, California, USA). A region of interest (ROI) was created which covered the entire body of the bat when analyzing the luminescence data.
At 87 dpi, a random group of six bats, all initially exposed to RCN-luc by either the ON (n = 4) or IM (n = 2) route, was given a booster exposure to the RCN-luc vaccine via the ON route at the same dose. Bats from this group were imaged at 1, 3, and 5 dpi using the same protocol as described above.
2.6. Assessment of viral shedding
During anesthesia for each imaging time-point, oral swabs were collected (CLASSIQSwabs, Copan Flock Technologies, 25125 Brescia, Italy) and placed in a 1.5 ml tube containing 200 μl DMEM media. Fecal samples were also retrieved when available from the bags in which the individuals were contained during imaging, placed in a 1.5 ml tube without media. All samples were quickly stored at -80 °C until testing. Prior to testing, 100 μl DMEM media was added to fecal samples, vortexed thoroughly and sonicated four times in a bath sonicator for 15 s. DNA was extracted from 40 μl of the samples using the Zymo Quick-gDNA™ MiniPrep kit (Zymo Research, Irvine, CA 92614, U.S.A.), and a PCR assay was run to assess for presence of the inoculated virus. PCR was performed with OneTaq® Quick-Load® 2X Master Mix with Standard Buffer (New England BioLabs Inc., Ipswich, MA 01938, USA) and primers targeting the luc insert flanks [Supplemental info]. The limit of detection for this PCR protocol was determined to be 0.125 picograms of DNA, or 6.8x104 copies, as determined by serial dilution of a known quantity of viral DNA. Any samples positive by PCR were then assessed for levels of live virus by determining the median tissue culture infective dose (TCID50). For RCN-luc samples, positive wells were assessed by plaque observation, and the TCID50 was calculated and used to approximate plaque forming units (PFU)/ml by the Spearman & Kärber algorithm [41]. For MVA-luc samples, positive wells were assessed for viral titer by the luciferase marker using steadylite plus™ (PerkinElmer Inc, Waltham, MA 02451). Serial dilutions from 10−2 to 10−7 were made, and 100 μl from each was used to infect a 96 well plate with BHK-21 cells at ∼80% confluency. Due to minimal sample volume left, no smaller dilutions were possible. After 3 days of infection, 100 μl of the steadylite reagent was added to each well, mixed by pipetting, and after 15 min the plates were scanned in a luminometer (Veritas™ Microplate Luminometer, Turner BioSystems, Inc, Sunnyvale, CA 94085).
2.7. RCN-G immunization
An additional group of five T. brasiliensis were housed separately in the same type of caging and given RCN-G via the ON route to assess the immunogenicity of RCN-delivered rabies CVS strain glycoprotein. For this exposure the bats were anesthetized with isoflurane prior to exposure. A dose of 108 PFU of viral vaccine was given in 70 μl sterile saline, split between 50 μl orally and 10 μl in each nare. Bats were monitored through their anesthesia recovery for 3 h for potential adverse events. Serum samples were obtained prior to vaccination and at 30 and 60 dpi and tested for the presence of anti-rabies neutralizing antibodies by the rapid fluorescence focus inhibition test (RFFIT). Serum samples from bats given RCN-luc (used in the luminescence study) were also collected at 0 and 60 dpi and used for controls. Serum was collected by making a small lance in the interfemoral vein and collecting up to 200 μl of blood in a capillary tube (Microvette® CB 300 Blood Collection System, Sarstedt AG & Co., Nümbrecht, Germany), which was subsequently centrifuged at 10,000g for 10 min. A micropipette was used to collect the serum from the top of the blood container and transfer it to a separate tube for storage at −80°.
Testing was conducted at the CDC Poxvirus and Rabies Branch using standard RFFIT protocols [42], augmented for smaller volumes of serum as previously described [43]. The assay was run in triplicate for each sample, and the results reported represent average titers. Prior to the 60 dpi sample collection, two bats from the RCN-G group were lost from the study due to non-vaccine related mortalities.
2.8. Statistical analysis
Analysis of the data was performed using the R-commander software package [44]. Weight change was analyzed with repeated measures ANOVA, where ‘weight’ is a function of group, route, and time, plus all interactions, using individual bats as the repeated measures. Differences in luminescence were analyzed using a linear mixed-effects model fit by the restricted maximum likelihood approach (REML).
3. Results
3.1. In vivo imaging studies
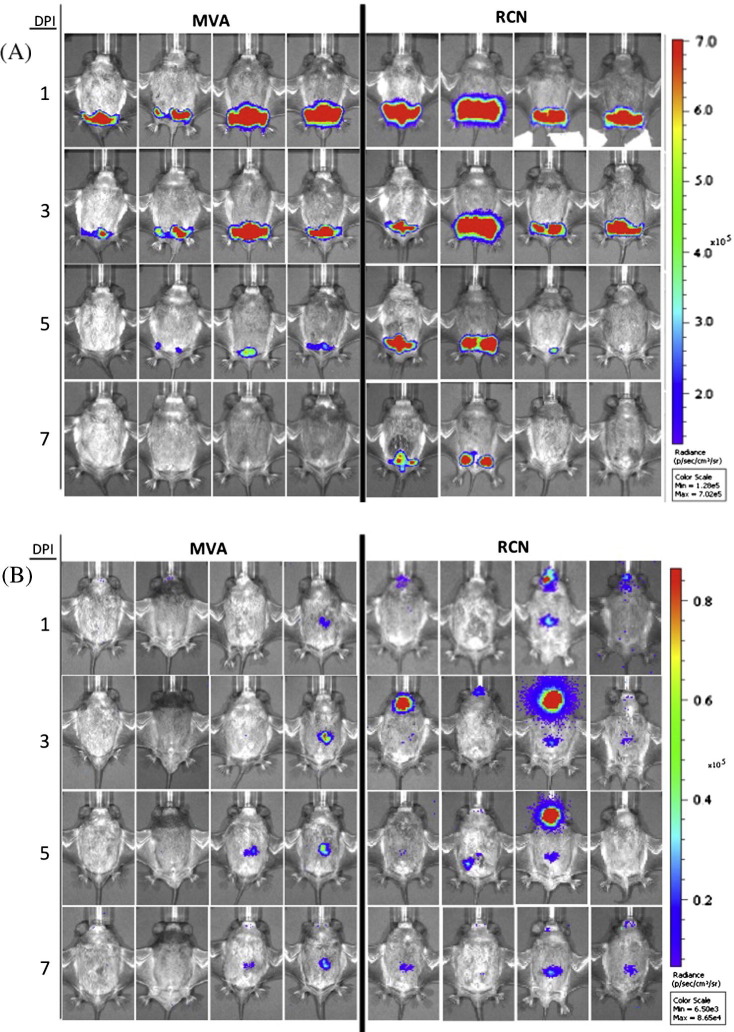
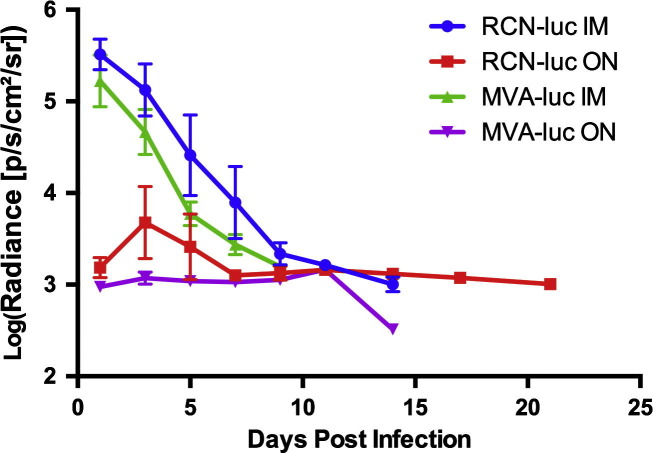
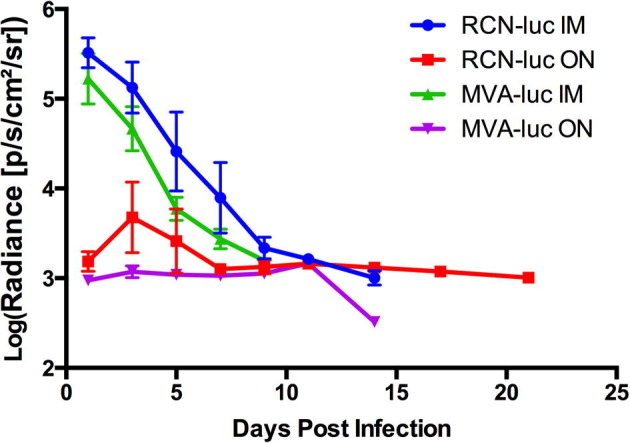
To assess the infectivity, tissue tropism, and course of infection of RCN and MVA in T. brasiliensis, bats were infected with recombinant virus expressing the firefly luciferase gene (luc). Two routes were assessed; the IM route and the ON route, which is most biologically relevant for wildlife vaccination. Throughout the study, no clinical signs of disease, lesions, or significant weight loss were observed after administration of viral vectors (Fig. S2, Table S3). All bats infected with luc-expressing poxvirus vectors had detectable expression of luminescence by 1 dpi, and peak levels were observed at 1 and 3 dpi for the IM and ON routes, respectively (Fig. 1, Fig. 2 ). Viral infection via IM exposure was cleared within 7 days for MVA and 9 days for RCN, while infection after ON exposure was cleared within 9 days for MVA and 21 days for RCN (final images for RCN given ON not shown). Statistical analysis revealed that luminescence was significantly higher (P = 0.028) in bats that received RCN compared to MVA and significantly higher (P = 0.032) for those administered virus by the IM route compared to the ON route. In the IM injected groups, significant viral spread to other areas was not evident. In contrast, an initial site of viral replication was evident in the oral cavity after ON exposure, often followed by a secondary site of expression further down the gastrointestinal tract. All luminescence measurements are listed in Table S4.
Fig. 1.
Luminescent images for bats given attenuated poxviral vectors, raccoon poxvirus (RCN) or modified vaccinia Ankara (MVA) via intramuscular (IM) (A) or oronasal (ON) routes (B). Images were taken with the IVIS 200 Biophotonic imager and analyzed using the Living Image software. For each vector group, the scale of luminescence is given in photons/second/cm2/steridian (p/s/cm2/sr) which has been standardized to compare individuals over time in days post infection (DPI).
Fig. 2.
Average luciferase expression per vector by route over time for the four groups of bats given luciferase-expressing constructs. Luminescence is given in photons/second/cm2/steridian (p/s/cm2/sr). Luminescence was significantly higher (P = 0.028) for those receiving raccoon poxvirus (RCN) than modified vaccinia Ankara (MVA) and also higher (P = 0.032) for those administered virus by the intramuscular (IM) route compared to the oronasal (ON) route.
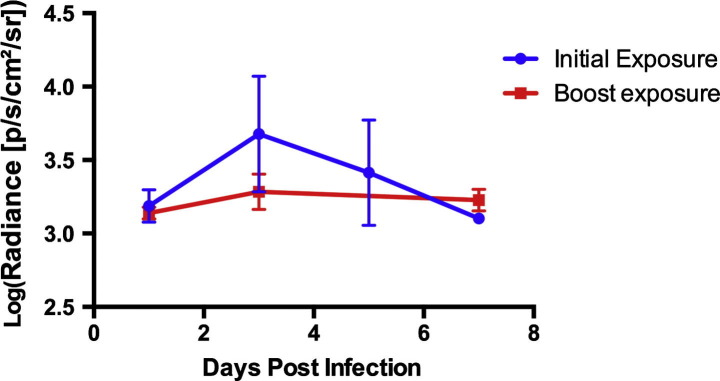
In the group of bats re-exposed to RCN-luc to assess whether prior exposure would affect the infectivity of the viral vectors, no significant difference (P = 0.33) was detected in luminescence when compared to initial exposure through 5 dpi (Fig. 3 ).
Fig. 3.
Average luciferase expression after oronasal (ON) exposure to raccoon poxvirus (RCN) initially and after re-exposure. Luminescence is given in photons/second/cm2/steridian (p/s/cm2/sr). No significant difference (P = 0.33) was detected in luminescence when compared to initial exposure through 5 days post infection.
3.2. Assessment of viral shedding
PCR analysis of oral swabs revealed the presence of MVA-luc DNA in 5 out of 8 bats up to 7 dpi (3 infected IM, 2 infected ON), however no live virus was recovered. RCN-luc DNA was present in 4 out of 8 bats up to 9 dpi (2 infected IM, 2 infected ON), with low levels of live virus detected (6.33 × 103 PFU/ml average, with a median of 9.20 PFU/ml in those with detectable virus). Two bats from the RCN-luc group that had live virus detected by oral swabs also had PCR positive fecal samples at 7 and 9 dpi, however no live virus was recoverable from these samples in titration. Viral shedding appears to occur at very low levels independent of route of exposure, and with no evidence of shedding viable MVA.
3.3. Antibody responses to RCN-G
To assess the immunogenicity of RCN, an additional group of bats (n = 5) was infected via the ON route with RCN expressing rabies virus surface glycoprotein (RCN-G). Again, there was no evidence of clinical disease in any of the vaccinated bats in this experiment. The rabies virus G is very well characterized and known to induce protective humoral immunity to rabies virus [30], [45], [46]. All bats assessed by RFFIT had negligible Ab titers prior to vaccination (Table S5). As a control, bats vaccinated with luc-expressing virus for the imaging study (N = 7) were bled at 0 and 60 dpi and assessed by RFFIT as well. By 30 dpi all rabies vaccinated bats (5/5) developed Ab titers greater than 0.2 IU/ml (0.20–11.46, with a mean of 5.14), while 7/7 bats that received RCN-luc had titers ⩽0.09 IU/ml (Table S5). While there is no “protective” level of rabies virus neutralizing antibodies (RVNA), there is a positive correlation between RVNA titers and the level of protection after virus challenge [22], [47], [48], [49], [50]. Titers between 0.1 and 3.0 IU have been protective for other mammalian species [22], [47], [48], [49], [50].
4. Discussion
Despite the association between bats and zoonotic diseases, there is currently no significant effort to decrease the incidence of important infectious diseases in these animals. Instead, efforts are more focused on culling of populations (e.g. vampire bats in Latin America) or controlling disease after spill-over into other animal hosts. The continued spillover of rabies virus strains to humans and domestic animals from terrestrial carnivores has led to the development of successful oral rabies vaccination (ORV) programs in Europe and North America. These campaigns often utilize recombinant viral vectors that stimulate immunity to the surface glycoprotein of rabies when ingested orally. When distributed in baits targeted toward certain rabies-carrying species, these vaccines lead to protection from the virus and reduction in local incidence of disease, and even local eradication of some strains of rabies [20], [51]. ORV programs are continually evolving and making use of the best available vaccine technology and disease modeling studies. In this study we assessed whether two attenuated poxviruses might be viable vaccine vectors in a bat species.
Through in vivo imaging studies we have demonstrated that attenuated MVA and RCN are able to infect T. brasiliensis via the ON route for a limited time, without causing disease. Our studies demonstrate the tissue tropism and course of infection after exposure of a new animal model, T. brasiliensis, to attenuated orthopoxviruses. We show evidence of limited autologous spread of the viruses after ON exposure, although there is no evidence that the virus spreads outside of GI-associated tissues. MVA was cleared faster and resulted in less detectable luminescence compared to RCN, which may be expected due to the highly attenuated nature of MVA. The lack of significant difference in the levels of viral encoded protein (luciferase) production upon re-infection with RCN, as shown in the booster study (Fig. 3), may be important if bats had been previously exposed to RCN, or if boost inoculations of RCN-vectored vaccine are necessary. While neither vector caused clinical illness, the fact that RCN produced more viral encoded protein (luciferase) over a longer period suggests it is a more immunogenic vector when expressing heterologous antigens. Due to limitations of this study, we were not able to compare the immunogenicity of the vectors directly.
The development of significant levels of rabies neutralizing antibodies after ON exposure to RCN-G demonstrates that the vaccine is highly immunogenic in T. brasiliensis. While previous studies have demonstrated the effectiveness of injectable vaccines in this species [52], our study is the first to demonstrate effective mucosal vaccination. An average anti-rabies G titer of 5.14 IU at 60 dpi was detected in bats orally administered RCN-G, which is higher than the levels obtained after vaccination with the VR-G construct in any previous studies, including oral, IM, and scarification routes of exposure (28–30). While these differences may be due to the animal models used, this is additional evidence of the superiority of the RCN vector over vaccinia in bat species. Additionally, the previous studies failed to address the infectivity of vaccinia in the bat host, relying on the lack of clinical disease and development of protective immunity to assess the virus-host interaction. We were unable to assess the duration of immunity past 60 days, but this would be useful information to collect in future studies.
Limited oral shedding of the RCN virus was detected in bats by both PCR and culture of live virus up to 9 dpi, but at very low levels. While there was no evidence of shedding of live MVA virus, there was PCR evidence of vector DNA in oral swabs through 7 dpi; detection of live virus may have been limited due to the method used and the necessary lack of lower dilutions resulting from low sample volumes. One of the advantages of in vivo imaging studies is that it is not necessary to sacrifice animals to obtain data, and the bats used in this study went on to be used in a different investigation. Because of this, we were unable to assess organ tissue for evidence of pathology during the infection trials. However, the lack of any apparent morbidity in RCN-treated bats, along with RCN’s natural history, record of success in various domestic and non-domestic species, and ability to induce immunity via the oral route, make it a very attractive candidate for use in free-ranging bats.
The results of our experiments provide proof-of-principle that oral vaccination is possible in free-ranging bats. In future work, topical vehicles will be developed that could be used to deliver oral vaccines to the fur coat of bats as they roost or are otherwise congregated. Bats are fastidious animals and spend a large proportion of their time self-grooming [53], which could lead to significant oral exposure to topically applied vaccines. A vaccine that would broadly protect free-ranging bats from rabies virus infection may reduce local incidence of rabies in certain bat populations, limiting the amount of spill-over into humans and other species. T. brasiliensis represents a species that roosts in dense colonies and is exposed to significant levels of circulating rabies virus in their population that may result in human exposures [54]. Therefore, a topically delivered rabies vaccine may be directly applicable to this species, but only if a method for mass application (such as a spray) could be developed. Further research is required to assess the viral vector in other species, such as vampire bats (Desmodus spp.), for which topically distributed poison protocols are well developed for culling of colonies [15], [55]. While culling has been successful in reducing overall numbers of Desmodus at a local level, it has not reduced the incidence of rabies in bat populations and may indeed be counterproductive [17]. Development of topical poxvirus vectored vaccines could potentially lead to effective, applicable, and practical means for reducing disease burden in some free ranging bat populations.
Acknowledgments
We would like to acknowledge the technical assistance of Jennifer Brunner, Elizabeth Falendysz, Nicole Ward, and the other employees at the NWHC that helped with bat care. Additionally, we are grateful to Robin Russell and Katie Richgels for assistance in statistical analysis, Erik Hofmeister for reviewing the manuscript, and David Blehert and Dave Redell for providing their knowledge and expertise. This work was supported by the USGS and the National Institutes of Health, Ruth L. Kirschstein National Research Service Award Institutional Training Grant T32 RR023916, from the National Center for Research Resources. The findings and conclusions in this report are those of the authors and do not represent the views of the Centers for Disease Control. The use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.08.088.
Appendix A. Supplementary material
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
References
- 1.Luis A.D., Hayman D.T.S., O’shea T.J., Cryan P.M., Gilbert A.T. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. Royal Soc. B: Biol. Sci. 2013;280(1756):20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drexler J.F., Corman V.M., Muller M.A., Maganga G.D., Vallo P., Binger T. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005 Oct;310(5):676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 4.Memish Z.A., Mishra N., Olival K.J., Fagbo S.F., Kapoor V., Epstein J.H. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect Dis. 2013 Nov;19(11):1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leroy E.M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P. Fruit bats as reservoirs of Ebola virus. Nature. 2005 Dec 1;438(7068):575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 6.Pourrut X., Souris M., Towner J.S., Rollin P.E., Nichol S.T., Gonzalez J.P. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect. Dis. 2009;9:159. doi: 10.1186/1471-2334-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amman B.R., Carroll S.A., Reed Z.D., Sealy T.K., Balinandi S., Swanepoel R. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012;8(10):e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong S., Li Y., Rivailler P., Conrardy C., Castillo D.A., Chen L.M. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U S A. 2012;109(11):4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Tefsen B., Shi Y., Gao G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014 Apr;22(4):183–191. doi: 10.1016/j.tim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler J.F., Geipel A., König A., Corman V.M., van Riel D., Leijten L.M. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc. Natl. Acad. Sci. U S A. 2013;110(40):16151–16156. doi: 10.1073/pnas.1308049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W.-P., Lin X.-D., Wang W., Tian J.-H., Cong M.-L., Zhang H.-L. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013;9(2):e1003159. doi: 10.1371/journal.ppat.1003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badrane H., Tordo N. Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. J. Virol. 2001 Sep;75(17):8096–8104. doi: 10.1128/JVI.75.17.8096-8104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banyard A.C., Evans J.S., Luo T.R., Fooks A.R. Lyssaviruses and bats: emergence and zoonotic threat. Viruses. 2014 Aug;6(8):2974–2990. doi: 10.3390/v6082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyer J.L., Yager P., Orciari L., Greenberg L., Wallace R., Hanlon C.A. Rabies surveillance in the United States during 2013. J. Am. Vet. Med. Assoc. 2014 Nov 15;245(10):1111–1123. doi: 10.2460/javma.245.10.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson N., Aréchiga-Ceballos N., Aguilar-Setien A. Vampire bat rabies: ecology, epidemiology and control. Viruses. 2014:1911–1928. doi: 10.3390/v6051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayen F. Haematophagous bats in Brazil, their role in rabies transmission, impact on public health, livestock industry and alternatives to an indiscriminate reduction of bat population. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2003;50(10):469–472. doi: 10.1046/j.1439-0450.2003.00713.x. [DOI] [PubMed] [Google Scholar]
- 17.Streicker D.G., Recuenco S., Valderrama W., Gomez Benavides J., Vargas I., Pacheco V. Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proc. Royal Soc. B: Biol. Sci. 2012 Jul 26;279(1742):3384–3392. doi: 10.1098/rspb.2012.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoner-Duncan B., Streicker D.G., Tedeschi C.M. Vampire bats and rabies: toward an ecological solution to a public health problem. PLoS Negl. Trop. Dis. 2014;8(6):e2867. doi: 10.1371/journal.pntd.0002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller T., Freuling C.M., Wysocki P., Roumiantzeff M., Freney J., Mettenleiter T.C. Terrestrial rabies control in the European Union: historical achievements and challenges ahead. Vet. J. 2015;203(1):10–17. doi: 10.1016/j.tvjl.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Slate D., Algeo T.P., Nelson K.M., Chipman R.B., Donovan D., Blanton J.D. Oral rabies vaccination in North America: opportunities, complexities, and challenges. PLoS Negl. Trop. Diseases. 2009;3(12):e549-e. doi: 10.1371/journal.pntd.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weyer J., Rupprecht C.E., Nel L.H. Poxvirus-vectored vaccines for rabies–a review. Vaccine. 2009 Nov;27(51):7198–7201. doi: 10.1016/j.vaccine.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Almeida M.F., Martorelli L.F., Aires C.C., Barros R.F., Massad E. Vaccinating the vampire bat Desmodus rotundus against rabies. Virus Res. 2008;137(2):275–277. doi: 10.1016/j.virusres.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Aguilar-Setién A., Leon Y.C., Tesoro E.C., Kretschmer R., Brochier B., Pastoret P.-P. Vaccination of vampire bats using recombinant vaccinia-rabies virus. J. Wildl. Dis. 2002;38(3):539–544. doi: 10.7589/0090-3558-38.3.539. [DOI] [PubMed] [Google Scholar]
- 24.Setien A.A., Brochier B., Tordo N., De Paz O., Desmettre P., Peharpre D. Experimental rabies infection and oral vaccination in vampire bats (Desmodus rotundus) Vaccine. 1998;16(11–12):1122–1126. doi: 10.1016/s0264-410x(98)80108-4. [DOI] [PubMed] [Google Scholar]
- 25.Baxby D. Safety of recombinant vaccinia vaccines. Lancet. 1991;337(8746):913. doi: 10.1016/0140-6736(91)90241-g. [DOI] [PubMed] [Google Scholar]
- 26.Rocke T.E., Kingstad-Bakke B., Berlier W., Osorio J.E. A recombinant raccoon poxvirus vaccine expressing both yersinia pestis F1 and truncated V antigens protects animals against lethal plague. Vaccines (Basel) 2014:772–784. doi: 10.3390/vaccines2040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tripp D.W., Rocke T.E., Streich S.P., Abbott R.C., Osorio J.E., Miller M.W. Apparent field safety of a raccoon poxvirus-vectored plague vaccine in free-ranging prairie dogs (Cynomys spp.), Colorado, USA. J. Wildl. Dis. 2015;51(2):401–410. doi: 10.7589/2014-02-051. [DOI] [PubMed] [Google Scholar]
- 28.Herman Y. Isolation and characterization of a naturally occuring poxvirus of raccoons. In: Kallio R.E., editor. Bacteriological Proceedings of the 64th Annual Meeting of the American Society for Microbiology; 1964 May 3–7. American Society for Microbiology; Washington, D.C.: 1964. p. 117. [Google Scholar]
- 29.Osorio J.E., Frank R.S., Moss K., Taraska T., Powell T., Stinchcomb D.T. Raccoon poxvirus as a mucosal vaccine vector for domestic cats. J. Drug Target. 2003;11(8–10):463–470. doi: 10.1080/10611860410001670062. [DOI] [PubMed] [Google Scholar]
- 30.Esposito J.J., Chandler F.W., Baer G.M. Oral immunization of animals with raccoon poxvirus expressing rabies virus glycoprotein. Spring Harbor Laboratory Press; Cold Spring Harbor: 1989. pp. 403–408. (Vaccines). [Google Scholar]
- 31.DeMartini J.C., Bickle H.M., Brodie S.J., He B.X., Esposito J.J. Raccoon poxvirus rabies virus glycoprotein recombinant vaccine in sheep. Arch. Virol. 1993;133(1–2):211–222. doi: 10.1007/BF01309757. [DOI] [PubMed] [Google Scholar]
- 32.Hwa S.-H., Iams K.P., Hall J.S., Kingstad B.A., Osorio J.E. Characterization of recombinant raccoonpox vaccine vectors in chickens. Avian Dis. 2010;54(4):1157–1165. doi: 10.1637/9315-032410-Reg.1. [DOI] [PubMed] [Google Scholar]
- 33.Rocke T.E., Smith S.R., Stinchcomb D.T., Osorio J.E. Immunization of black-tailed prairie dog against plague through consumption of vaccine-laden baits. J. Wildl. Dis. 2008;44(4):930. doi: 10.7589/0090-3558-44.4.930. [DOI] [PubMed] [Google Scholar]
- 34.Esposito J.J., Knight J.C., Shaddock J.H., Novembre F.J., Baer G.M. Successful oral rabies vaccination of raccoons with raccoon poxvirus recombinants expressing rabies virus glycoprotein. Virology. 1988 Jul 1;165(1):313–316. doi: 10.1016/0042-6822(88)90692-7. [DOI] [PubMed] [Google Scholar]
- 35.Mayr A., Stickl H., Müller H.K., Danner K., Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author’s transl) Zentralbl Bakteriol B. 1978 Dec;167(5–6):375–390. [PubMed] [Google Scholar]
- 36.Antoine G., Scheiflinger F., Dorner F., Falkner F.G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244(2):365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert S.C. Clinical development of Modified Vaccinia virus Ankara vaccines. Vaccine. 2013;31(39):4241–4246. doi: 10.1016/j.vaccine.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Gherardi M.M. Recombinant poxviruses as mucosal vaccine vectors. J. Gen. Virol. 2005 Nov 1;86(11):2925–2936. doi: 10.1099/vir.0.81181-0. [DOI] [PubMed] [Google Scholar]
- 39.Earl P.L., Moss B., Wyatt L.S., Carroll M.W. Generation of recombinant vaccinia viruses. In: Ausubel F.M., editor. Current protocols in molecular biology. 2001. [DOI] [PubMed] [Google Scholar]
- 40.Contag C.H., Spilman S.D., Contag P.R., Oshiro M., Eames B., Dennery P. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem. Photobiol. 1997;66(4):523–531. doi: 10.1111/j.1751-1097.1997.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 41.Killington H. Virol. Meth. Manual. 1996 [Google Scholar]
- 42.Smith J.S., Yager P.A., Baer G.M. A rapid reproducible test for determining rabies neutralizing antibody. Bull. World Health Organ. 1973;48(5):535–541. [PMC free article] [PubMed] [Google Scholar]
- 43.Kuzmin I.V., Niezgoda M., Franka R., Agwanda B., Markotter W., Beagley J.C. Lagos bat virus in Kenya. J. Clin. Microbiol. 2008;46(4):1451–1461. doi: 10.1128/JCM.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox J. The R Commander: a basic statistics graphical user interface to R. J. Stat. Softw. 2005:1–42. [Google Scholar]
- 45.Wiktor T.J., Macfarlan R.I., Reagan K.J., Dietzschold B., Curtis P.J., Wunner W.H. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc. Natl. Acad. Sci. U S A. 1984;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henderson H., Jackson F., Bean K., Panasuk B., Niezgoda M., Slate D. Oral immunization of raccoons and skunks with a canine adenovirus recombinant rabies vaccine. Vaccine. 2009;27(51):7194–7197. doi: 10.1016/j.vaccine.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 47.Rupprecht C.E., Wiktor T.J., Johnston D.H., Hamir A.N., Dietzschold B., Wunner W.H. Oral immunization and protection of raccoons (Procyon lotor) with a vaccinia-rabies glycoprotein recombinant virus vaccine. Proc. Natl. Acad. Sci. U S A. 1986;83(20):7947–7950. doi: 10.1073/pnas.83.20.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupprecht C.E., Dietzschold B., Cox J.H., Schneider L.G. Oral vaccination of raccoons (Procyon lotor) with an attenuated (SAD-B19) rabies virus vaccine. J. Wildl. Dis. 1989 Oct;25(4):548–554. doi: 10.7589/0090-3558-25.4.548. [DOI] [PubMed] [Google Scholar]
- 49.Follmann E., Ritter D., Swor R., Dunbar M., Hueffer K. Preliminary evaluation of Raboral V-RG(R) oral rabies vaccine in Arctic foxes (Vulpes lagopus) J. Wildl. Dis. 2011;47(4):1032–1035. doi: 10.7589/0090-3558-47.4.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown L.J., Rosatte R.C., Fehlner-Gardiner C., Bachmann P., Ellison J.A., Jackson F.R. Oral vaccination and protection of red foxes (Vulpes vulpes) against rabies using ONRAB, an adenovirus-rabies recombinant vaccine. Vaccine. 2014;32(8):984–989. doi: 10.1016/j.vaccine.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Vitasek J. A review of rabies elimination in Europe. Vet. Med. - Czech. 2004;5:171–185. [Google Scholar]
- 52.Turmelle A.S., Allen L.C., Schmidt-French B.A., Jackson F.R., Kunz T.H., McCracken G.F. Response to vaccination with a commercial inactivated rabies vaccine in a captive colony of Brazilian free-tailed bats (Tadarida brasiliensis) J. Zoo. Wildl. Med. 2010;41(1):140–143. doi: 10.1638/2008-0161.1. [DOI] [PubMed] [Google Scholar]
- 53.Carter G., Leffer L. Social grooming in bats: are vampire bats exceptional? PLoS ONE. 2015;10:e0138430. doi: 10.1371/journal.pone.0138430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayes B.C., Wilson P.J., Oertli E.H., Hunt P.R., Rohde R.E. Epidemiology of rabies in bats in Texas (2001−2010) J. Am. Vet. Med. Assoc. 2013;243(8):1129–1137. doi: 10.2460/javma.243.8.1129. [DOI] [PubMed] [Google Scholar]
- 55.Linhart S.B., Flores Crespo R., Mitchell G.C. Control of vampire bats by means of an anticoagulant. Bol. Oficina Sanit. Panam. 1972;73(2):100–109. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.