Abstract
While many studies have focused on identifying the neural and behavioral characteristics of decoding-based reading disorder (RD, aka developmental dyslexia), the etiology of RD remains largely unknown and understudied. Because the brain plays an intermediate role between genetic factors and behavioral outcomes, it is promising to address causality from a neural perspective. In the current, Part I of the two-part review, we discuss neuroimaging approaches to addressing the causality issue and review the results of studies that have employed these approaches. We assume that if a neural signature were associated with RD etiology, it would (a) manifest across comparisons in different languages, (b) be experience independent and appear in comparisons between RD and reading-matched controls, (c) be present both pre- and post-intervention, (d) be found in at-risk, pre-reading children and (e) be associated with genetic risk. We discuss each of these five characteristics in turn and summarize the studies that have examined each of them. The available literature provides evidence that anomalies in left temporo-parietal cortex, and possibly occipito-temporal cortex, may be closely related to the etiology of RD. Improved understanding of the etiology of RD can help improve the accuracy of early detection and enable targeted intervention of cognitive processes that are amenable to change, leading to improved outcomes in at-risk or affected populations.
Keywords: reading disorder, developmental dyslexia, etiology, causal inference, neuroimaging, cross-cultural, reading-matched design, behavioral intervention, preliterate, imaging genetics
1. INTRODUCTION
The etiology of decoding-based reading disorder (RD; aka developmental dyslexia) remains a largely open question. This situation is partly reflected in clinical and educational definitions that characterize RD at the symptomatic level of developmental behaviors. For example, the American Psychiatric Association (2013) currently defines RD as “a neurocognitive disorder characterized by non-fluent word identification and poor spelling performance, which are not the result of sensory impairments, impairments in intelligence, or inadequate educational experience”. While this and similar definitions allow a pragmatic approach to identifying RD based on standardized reading scores or the discrepancy between actual reading achievement and expected achievement given ability, they do little to support a deeper understanding of RD, particularly with regard to identifying causal mechanisms. A failure to accurately characterize the etiology of RD presents an obstacle to accurately identifying children who are at risk for RD before they suffer academically, a delay that impedes more effective interventions (Wanzek & Vaughn, 2007). On the other hand, these definitions, to some extent, reflect the state of RD research, which is still far from reaching a comprehensive understanding of the origins of this developmental disorder (Goswami, 2015).
As will be discussed in Part II of this two-part review, which will focus on the neural correlates of typical and atypical reading from a developmental perspective, we know that: (a) Fluent reading requires the hierarchical integration of multiple component processes, including orthographic recognition, orthographic-phonological mapping, and semantic access. (b) Fluent reading can be impaired when there is atypical local neural organization in relevant brain areas (e.g., the left temporo-parietal cortex (TPC), including the posterior superior temporal gyrus (STG) and the supramarginal gyrus; the left occipito-temporal cortex (OTC) including the fusiform and the inferior temporal gyri; and the left inferior frontal gyrus (IFG)) and disrupted connectivity between these area (e.g., the left arcuate fasciculus, which connects OTC with frontal areas through the TPC) (Catani, Jones, Donato, & Ffytche, 2003; Catani, Jones, & Ffytche, 2005). (c) RD presents with anomalies associated with both impairments (e.g., hypoactivation of TPC, OTC, and IFG) and compensatory mechanisms (e.g., hyperactivation of the left precentral gyrus (PreCG)) in multiple brain networks and there is not a one-to-one mapping between abnormalities in specific brain regions and impairment in component reading processes. Each component of reading requires a widespread network of multiple brain areas, and a given brain area may also be involved in multiple processes. For example, the left posterior fusiform gyrus plays a central role in both orthographic recognition and processing phonological information during reading (Martin, Kronbichler, & Richlan, 2016; Zhao et al., 2016). (d) The neural bases of reading are developmental and undergo changes in terms of how each region contributes to reading, with differing developmental trajectories between typically developing children and those with RD.
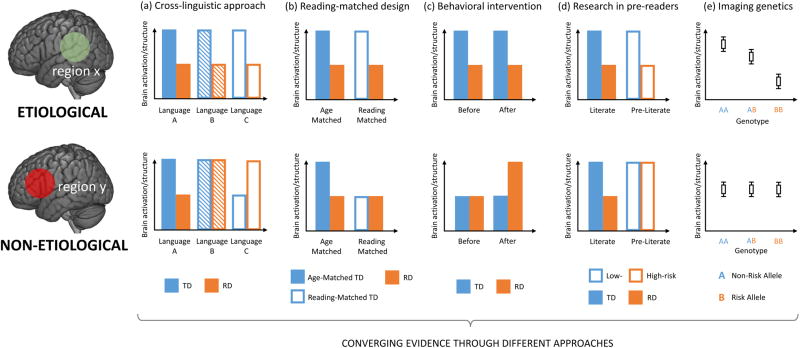
Against this background of anomalies associated with RD, we turn to another essential question in this review: the etiology of RD. We approach this question from a neuroconstructivist perspective (Karmiloff-Smith, 2009), considering the observed brain state of an individual with RD as the complex result of the ontogeny of neural organization operating under genetic and other biological constraints. Thus, the observed anomalies follow from both an etiological basis and a process of potentially normal development that may produce secondary anomalies (including both deficits and compensation) and special approaches are needed to dissociate these. In the following sections, we summarize five approaches that have been used to explore RD causality, and review the fruits of these approaches. Strictly establishing causality for complex human neurocognitive phenotypes is, in general, a challenging if not impossible goal with presently available techniques. We adopt realistically achievable criteria, partially overlapping with those proposed by Goswami (2015), for identifying causal neurobiological bases of RD as those persistently observed in RD (or preliterate children at risk for RD): (a) across languages, (b) regardless of development and experience, (c) both pre- and post-intervention, (d) before formal reading instruction, and (e) are genetically linked to RD (Figure 1). We discuss each of these criteria, the corresponding approaches, and results from functional and structural magnetic resonance imaging (MRI).
Figure 1.
Five main approaches that can support etiological inference of reading disorder (RD) are presented schematically, including: (a) comparing RD with controls in different languages; (b) comparing RD with both age-matched and reading level-matched normal controls; (c) comparing neural impairments in RD between pre- and post-intervention; (d) comparing pre-readers with high- and low-risk for developing RD; and (e) investigating associations between neural and genetic variations. For each approach, we give an example illustrating one of several possible outcomes when a neural anomaly is etiological (in the upper panel) or non-etiological (in the lower panel). Note: region x/y could be any part of brain; brain activation could be measured by blood-oxygen-level dependent signal during a specific task, or by other techniques such as event-related potentials; brain structure could be measured by for example, cortical thickness, volume or surface area for gray matter, or fractional anisotropy for white matter fibers; TD, typically developing control group; RD, reading disorder group; Age-Matched, comparing RD with chronological age-matched controls; Reading-Matched, comparing RD with reading level-matched controls; Low-risk, preliterate children without a family history for RD; High-risk, preliterate children with a family history for RD
2. CROSS-LINGUISTIC COMPARISONS
As one important step towards understanding RD causality, we first consider whether the etiology of RD is universal or language-specific (Frost, 2012; Richlan, 2014). Although controversy remains over the nature of the “reading network” across languages, there is evidence for a core “reading network” that is relatively conserved across languages, even between alphabetic and non-alphabetic languages (Krafnick et al., 2016; Zhu, Nie, Chang, Gao, & Niu, 2014) and languages of varying orthographic depth (Bolger, Perfetti, & Schneider, 2005; Rueckl et al., 2015). If the etiology of RD lies within this core “reading network”, then neurobiological variations associated with RD should be found cross-linguistically.
While some behavioral characteristics of RD, particularly rapid naming problems (e.g., Katzir, Shaul, Breznitz, & Wolf, 2004), vary across writing systems, others, including phonological processing deficits, have been argued to be similar across languages (Goswami et al., 2011). Similarly, neuroimaging research has found both language-specific and language-independent neural signatures (Martin et al., 2016; Richlan, 2014). Studies comparing alphabetic languages of varying orthographic transparency (English, French, and Italian) show similar dysfunctions in both brain function (Paulesu et al., 2001) and structure (gray and white matter volume; GMV & WMV) (Silani et al., 2005) in left temporal and occipito-temporal regions. Jednorog et al. (2015) found consistent cross-language (French, German and Polish) volumetric differences between typical and RD readers in the left thalamus and a correlation between GMV of the left supramarginal gyrus and reading ability (in typical readers) across languages. A consistent neural manifestation of RD across orthographies is also supported by a recent meta-analysis. Martin et al. (2016) compared functional MRI abnormalities in RD and confirmed the existence of similarities across orthographies (e.g., hypoactivation in the inferior parietal (IPL) and OTC across deep and shallow orthographies), although variability across orthographies was also observed (e.g., hypoactivation in the left IPL and pars triangularis and hyperactivation in the insula were found in deep orthographies, while hypoactivation in left TPC and hyperactivation in left PreCG were found in shallow orthographies).
Another important source of information comes from comparisons of findings in alphabetic languages (e.g., English) with those obtained in non-alphabetic writing systems (e.g., Chinese). The neural anomalies found in studies of RD in Chinese readers vary in terms of their consistency with those identified in alphabetic languages. For example, while some researchers found that Chinese RD children show atypical anatomy in left TPC (Xia, Hoeft, Zhang, & Shu, 2016) and the cerebellum (Yang, Yang, Chen, & Bi, 2016), similar to that reported in studies that have examined English RD, others report anomalies in areas such as the left middle frontal gyrus (Siok, Niu, Jin, Perfetti, & Tan, 2008), which are not associated with RD in studies of alphabetic languages. Notably, Chinese RD has been specifically associated with anomalies in the left middle frontal gyrus and the superior parietal lobule, which may reflect larger semantic and visuospatial demands, and heavier working memory load associated with the logographic system (Siok et al., 2008; Siok, Perfetti, Jin, & Tan, 2004; Siok, Spinks, Jin, & Tan, 2009; Xu, Yang, Siok, & Tan, 2015).
Although several studies have compared reading-related neural measures of Chinese and English readers (Krafnick et al., 2016; Mei et al., 2015; Tan et al., 2003; Zhu et al., 2014), only one study has compared RD in both languages (Hu et al., 2010). Hu et al. found that both English RD and Chinese RD showed reduced activation in the left middle frontal, posterior middle temporal, and angular gyri, and OTC, compared with their culturally matched, typically reading peers. Further, Hu et al. found no significant differences between these English-speaking and Chinese-speaking RD samples. This result supports a common neural basis for RD across writing systems. At the structural connectivity level, a recent multivariate pattern analysis of Chinese RD suggested both universal and language-specific anomalies (Cui, Xia, Su, Shu, & Gong, 2016). Supervised learning on measurements from 50 white matter fibers were used to classify Chinese children as RD or typical. Fibers repeatedly reported in alphabetic studies (e.g., left superior longitudinal fasciculus; Vandermosten, Boets, Wouters, & Ghesquiere, 2012) and those not reported in alphabetic studies, such as the cingulum and fornix, both contributed significantly to the classification model (Cui et al., 2016).
Cross-linguistic studies provide evidence for RD-related anomalies in both core reading-related areas and language-specific regions, leaving a fairly inconsistent picture of possible RD etiology. Current cross-linguistic studies of RD, which are almost all non-longitudinal studies of literate individuals, are limited in their ability to address the question of whether the etiology of RD may lie in these language-specific regions in some cases. This question can be better addressed when cross-linguistic studies are combined with other approaches, for example, longitudinal designs with preliterate at-risk children, which can help distinguish universal and language-specific etiological anomalies from those associated with language-specific development.
3. READING-MATCHED (DEVELOPMENTAL) DESIGN
The reading-matched design (Goswami, 2003) applies the neuroconstructivist approach (Karmiloff-Smith, 2009) to address causality in RD. This approach is motivated by the need to account for differential environmental effects on development (e.g., lack of reading exposure) before attempting to make etiological inferences. This design assumes that (a) etiology-related deficits will emerge between RD and both reading-matched and age-matched controls; and (b) ability-related deficits will emerge between RD and age-matched controls, but not between RD and reading-matched controls. This design has been widely used in behavioral research (Swan & Goswami, 1997) and has seen increasing use in neuroimaging studies (e.g., Cao et al., 2016; Hoeft et al., 2006; Hoeft et al., 2007; Anthony J Krafnick, Flowers, Luetje, Napoliello, & Eden, 2014; Olulade, Napoliello, & Eden, 2013; Xia et al., 2016).
Hoeft and colleagues were the first to employ reading-matched designs in neuroimaging to study the etiology of RD and found hypoactivation in left TPC during phonological processing in RD relative to both age- and reading-matched controls (Hoeft et al., 2006), as well as decreased GMV in the same region (Hoeft et al., 2007). In contrast, regions of hyperactivation in the left IFG in RD were found only in age-matched comparison, suggesting a distinction between secondary neural anomalies in the IFG and anomalies in TPC of possible etiological origin (i.e., primary impairments). Recently, Xia et al. (2016) found GMV decrease in left TPC in Chinese RD, further supporting anomalies in TPC as a developmentally and linguistically invariant characteristic of RD.
The interpretation of fMRI results is constrained by the task used in the scanner. As Hoeft et al. (2006) used a phonological task, we cannot exclude the possibility that other functional anomalies may be present in comparisons with reading-matched controls under different task demands. However, converging findings for structural anomalies of TPC in RD children from multiple language backgrounds (Hoeft et al., 2007; Xia et al., 2016) suggest that anomalous findings of TPC in reading-matched designs are not solely attributable to task demands. However, structural findings are not unequivocal as Krafnick et al. (2014) found RD-specific GMV decrease only in the right PreCG, but no significant differences in TPC, OTC or associated white matter. Reading-matched designs have also been used to provide evidence against possible etiologies, such as visual deficits (Olulade et al., 2013).
Comparisons of RD to both age-matched and reading-matched controls provide a valuable method for differentiating developmentally invariant and potentially causal anomalies from those related to development or experience, which reflect compensation or brain reorganization secondary to causal mechanisms. Although only a few neuroimaging studies have employed this design to date, such studies have provided converging evidence for structural and functional abnormalities in left TPC, suggesting this region is related to the etiology of RD.
4. BEHAVIORAL INTERVENTION
Behavioral intervention is a complementary approach that can be used to indirectly address the etiology question in RD research, allowing longitudinal investigation of environmental manipulations. Intervention studies can help distinguish etiology-related impairments that are likely to be (but not always) invariant to intervention from experience-related normalization and compensation. With respect to causality, the intervention approach assumes that neural etiological anomalies will be generally unresponsive to intervention, while secondary anomalies arising from atypical developmental trajectories may show normalization towards typical neural patterns following intervention. Additionally, intervention studies can identify compensatory mechanisms – neural changes outside the canonical reading network that are associated with behavioral improvement. However, intervention research has focused more on the neural mechanisms of normalization and compensation rather than causal deficits (Barquero, Davis, & Cutting, 2014).
A coordinate-based meta-analysis of neuroimaging studies of reading interventions identified post-intervention activation increases in bilateral frontal language regions and in additional regions outside canonical reading and language networks (Barquero et al., 2014). The results suggest that intervention leads to normalization or compensatory recruitment of frontal language regions, suggesting that these regions may not be etiological. This is in line with the finding that activation in the right IFG is related to performance in RD (Bach et al., 2010; but see Ingvar et al., 2002) and that hyperactivation in the left IFG is associated with developmental experience (Hoeft et al., 2007; but see Richlan, Kronbichler, & Wimmer (2011) for evidence of hypoactivation in adults with RD). Structural neuroimaging studies also demonstrate that specific brain regions can be normalized by behavioral intervention, for example, GMV in the left anterior fusiform gyrus, precuneus, and right cerebellum increases with behavioral intervention (Krafnick, Flowers, Napoliello, & Eden, 2011). Functional and structural connectivity studies also report post-intervention normalization, for example, of functional connectivity between the left middle frontal gyrus and intraparietal sulcus in resting state fMRI (Koyama et al., 2013) and fractional anisotropy (FA) in a region of the left anterior centrum semiovale (Keller & Just, 2009).
Notably, fewer studies have found intervention-related changes in left TPC, a region frequently associated with RD. This is in line with the idea that anomalies in TPC may be associated with the etiology of RD. However, the lack of statistically significant results should not be taken as evidence, and some studies found intervention-related change in neighboring regions such as the dorsal IPL and intraparietal sulcus (e.g., Eden et al., 2004; Heim, Pape-Neumann, van Ermingen-Marbach, Brinkhaus, & Grande, 2015). On the other hand, training-driven normalization in the left OTC has been found at the functional level, including activation during overt single word reading (Heim et al., 2015) and intrinsic functional connectivity at rest (Koyama et al., 2013). This is in contrast to cortical thickness abnormalities in the same region that persist even after intervention (Ma et al., 2015), which suggest a possible causal impairment in the ventral pathway, at the neuroanatomical rather than neurofunctional level.
Finally, intervention can be combined with other strategies, for example, reading level-matched designs, to better distinguish normalization of experience-related anomalies from those that are potentially causal. Using such combination, Olulade et al. (2013) demonstrated that children with RD show hypoactivation in bilateral V5/MT compared with age-matched controls, but have similar activation compared to reading level-matched controls. Moreover, this anomaly can be normalized by a phonological-based reading intervention. These two findings provide strong evidence that motion perception deficits and corresponding hypoactivation in V5/MT are not casual to RD, but a secondary characteristic associated with reduced reading experience.
5. MRI RESEARCH WITH PRELITERATE CHILDREN AT RISK FOR RD
A key step in investigating the etiology of RD is to distinguish etiological anomalies from those that emerge developmentally due to differences in reading exposure and experience. Approaches that identify anomalies prior to formal literacy instruction can minimize the influence of developmental experience that confounds studies of older readers and provide complementary evidence to reading-matched and intervention designs. One such approach is to study preliterate children classified as at-risk for developing RD (hereafter: at-risk children) based on the presence or absence of familial history of RD (e.g., Hosseini et al., 2013), or combined with other early measures, including health, laterality, motor skills, language ability and special needs education (Helland, Plante, & Hugdahl, 2011).
At the neuroanatomical level, at-risk children show reduced gray matter volume in bilateral TPC, left OTC and the right lingual gyrus (Raschle, Chang, & Gaab, 2011). Interestingly, Black et al. (2012) found that temporo-parietal grey matter reductions, driven by cortical surface area, were more strongly related to maternal than paternal reading history, suggesting a parent-of-origin effect on the etiology of RD. Anomalies within the reading network of at-risk children have also been supported by studies using other measures of cortical structure, including asymmetry (Vanderauwera et al., 2016), topological properties (Hosseini et al., 2013) and sulcal pattern (Im, Raschle, Smith, Ellen Grant, & Gaab, 2015). Anomalies in at-risk children have also been found in left hemisphere white matter pathways, although there is inconsistency in which pathways are affected. Langer et al. (2015) found reduced FA in the left arcuate fasciculus in at-risk infants, which was associated with lower expressive language scores, suggesting an anomaly of phonological pathways. This finding is further supported by recent studies using tract-specific analysis (Wang et al., 2016) and T1 intensities in specific fibers (Kraft et al., 2016). On the other hand, Vandermosten et al. (2015) found decreased average FA in the left inferior fronto-occipital fasciculus, rather than arcuate fasciculus, in at-risk children. Furthermore, FA in these two ventral fiber tracts was associated with phonological awareness, suggesting a possible role for ventral areas in reading development, phonological processing and RD etiology. In line with structural evidence focusing on gray and white matter, at-risk children are characterized by decreased activation in left TPC and bilateral OTC during phonological processing (Debska et al., 2016; Raschle, Zuk, & Gaab, 2012). Hypoactivation in left superior/middle/inferior frontal gyri and precentral gyri in RD during rapid auditory processing has also been reported (Raschle, Stering, Meissner, & Gaab, 2014).
One major limitation of much of the current research in preliterate samples is the lack of follow-up data. Not all at-risk children will later be identified as having RD, and longitudinal studies are needed to distinguish anomalies that might causally precede RD from those that might be associated with at-risk status. To our knowledge, there are only three published MRI studies that followed at-risk children from preschool through formal reading instruction, when RD can be identified (Clark et al., 2014; Morken, Helland, Hugdahl, & Specht, 2016; Wang et al., 2016). In a prospective study of pre-reading high- and low-risk children (age 6), Clark et al. (2014) found that children who would later develop RD (at age 12) had thinner cortex in regions associated with sensory processing and executive function such as Heschl’s, lingual, and middle cingulate gyri compared to those who did not develop RD. The difference in Heschl's gyrus, but not other regions, persisted through development. In contrast, cortical thickness differences between RD and non-RD children within “reading network” regions (e.g., IFG, TPC and OTC) were evident after years of reading instruction, but not in pre-readers. These findings suggest that the etiology of RD may be related to the primary auditory cortex, rather than the traditional “reading network”, in line with sensory deficit theories. However, a lack of significant differences on a single brain measure does necessarily mean that the corresponding system is intact; the system may be impaired in other respects. For example, in the same Norwegian sample as in Clark et al. (2014), Morken et al. (2016) examined effective connectivity within the "reading network" (i.e., left IPL, STG, OTC, IFG and PreCG). This analysis revealed altered effective connectivity from OTC to STG and IFG in pre-reading, future RD children, which normalized following reading instruction. RD and non-RD children also had transient differences in effective connectivity from IFG to PreCG and IPL after the onset of reading instruction, suggesting a compensatory mechanism. By age 12, RD and non-RD children had similar effective connectivity. These patterns suggest that unknown etiological factors differentially affect the development of the reading network between RD and non-RD children.
Most recently, Wang et al. (2016) found a region in the left arcuate fasciculus that showed lower FA cross-sectionally in at-risk children from pre-reading through age 12. These findings suggest that altered structural connectivity in the left arcuate fasciculus exists at the preliteracy stage and may persist well after formal reading instruction, a possible indicator of RD etiology. In a longitudinal analysis, FA increased more rapidly in both low-risk children compared to high-risk children, and in children who became good readers compared to those who became poor readers, suggestive of an etiological developmental trajectory. In the right superior longitudinal fasciculus, the rate of change in FA was greater in high-risk typical readers compared to high-risk poor readers, indicating that at-risk children with positive outcomes may atypically recruit this right hemisphere tract as reading skills develop.
Recent studies using longitudinal methods extend prior comparisons between at-risk and low-risk pre-readers, providing important information about the developmental trajectories of functional and structural anomalies in regions associated with phonological and orthographic processing. Such designs are necessary for a comprehensive understanding of the etiology of RD in the appropriate developmental context. However, few studies have reported longitudinal data to date. Another limitation of studies of preliterate children is that differences in development and incidental print exposure cannot be eliminated as a source of neural differences. Nevertheless, studies of at-risk children have potential for identifying causal anomalies and distinguishing these from secondary anomalies associated with development. Regions identified in these studies provide promising intermediate phenotypes for early identification of children at risk for RD (Ozernov-Palchik & Gaab, 2016) and candidate neural endophenotypes for genetic association studies.
6. IMAGING GENETICS AND RD RISK GENES
Reading disorder has a partially genetic basis, with heritability estimates around 0.5—0.7 for reading-related processes (Grigorenko, 2004; Hart et al., 2013) and several risk genes (e.g., DCDC2, KIAA0319, DYX1C1, and ROBO1) have been associated with RD (Paracchini, Scerri, & Monaco, 2007). However, it is challenging to address the etiology of RD through gene-behavior associations alone, given the phenotypic variability of RD and complexity of reading. An alternative approach is to establish links between genetic variation, candidate neural endophenotypes, and behavior by using imaging genetics and interpret such findings in conjunction with results from the complementary approaches we have discussed. Because RD results from genetic, environmental and gene-environment interactions (G × E), identifying a link from genes to neurobiology to RD is not necessarily sufficient to establish an etiological basis, as the effect of genetic factors must be interpreted in the environment in which they are expressed. As environmental factors are frequently difficult to quantify, we primarily focus on imaging genetic studies here, while noting their limitations and the need to consider environmental and G × E effects.
Genes such as DCDC2 are highly expressed within the reading network (Meng et al., 2005) and associated with GMV throughout bilateral inferior temporal, temporo-parietal and inferior frontal regions (Meda et al., 2008). In animal models, knockdown and knockout of Dyx1c1, Kiaa0319 or Dcdc2 (Centanni et al., 2016; Currier, Etchegaray, Haight, Galaburda, & Rosen, 2011; Meng et al., 2005; Paracchini et al., 2006; Wang et al., 2006) disrupts neuronal migration, consistent with the association between single nucleotide polymorphisms (SNPs) on these genes and cortical structure in humans (e.g., Meda et al., 2008). These genes are also associated with relevant behavioral phenotypes, possibly linking disrupted neuronal migration to behavior and cognition. For example, Kiaa0319 knockdown reduces the fidelity of temporal auditory coding at the neural level (Centanni et al., 2014; Szalkowski et al., 2013). Notably, Kiaa0319 and Dcdc2 knockdowns produce distinct deficits in auditory processing, suggesting that genetic risk variants for RD lead to slightly different neural etiologies (Centanni et al., 2016). In humans, these risk genes have been linked to WMV in left TPC, cingulum and the posterior corpus callosum, and WMV mediates the association between single nucleotide polymorphisms and reading ability in typical readers (Darki, Peyrard-Janvid, Matsson, Kere, & Klingberg, 2012; Scerri et al., 2012). The DCDC2 intron 2 deletion has also been associated with altered FA in both RD and normal controls (Marino et al., 2014).
Although most research focuses on DYX1C1, DCDC2 and KIAA0319, other loci have also been linked to RD and neural phenotypes, for example, a polymorphism thought to regulate SLC2A3 (Roeske et al., 2011). In general, there is a need for larger samples to replicate genetic associations in RD (particularly DYX1C1, Marino et al., 2005) and establish evidence that the effect of genetic risk variants on behavioral phenotypes is mediated by intermediate neural phenotypes. As RD is a complex trait, environmental factors and G × E interactions also contribute to the etiology of RD (Pennington et al., 2009). Currently, behavioral genetic research in this area is limited and will remain methodologically challenging because the environment cannot be randomly assigned in the human research, making it difficult to isolate gene-specific effects, environment-specific effects, and the interaction between them (e.g., Dilnot, Hamilton, Maughan, & Snowling, 2016). The majority of behavioral findings in RD indicate that heritability is higher in enriched environments (e.g., Friend, DeFries, & Olson, 2008), although there is also evidence consistent with the diathesis-stress model of increased genetic susceptibility in poor environments (Mascheretti et al., 2013). A clear understanding of how gene and environment together impact brain networks that support reading development is an open question and an important step towards fully understanding the cause of RD. To date, only one neuroimaging study has investigated this interaction (Powers, Wang, Beach, Sideridis, & Gaab, 2016), though several studies have been done to examine gene-brain relationships (e.g., Darki et al., 2012) and environment-brain relationships (e.g., Noble et al., 2015) separately. Specifically, Powers et al. (2016) found that familial risk for RD activity during phonological processing in left IFG and the right fusiform gyrus correlated with home literacy environment more strongly in pre-readers without familial risk than in at-risk children. This suggests that familial risk, a measure of genetic liability for RD, modulates brain-environment associations.
In addition to further research on G × E interactions, it is also important to combine imaging genetics studies with the approaches discussed above to distinguish the genetic bases for RD etiology from genetic constraints on cortical development that may emerge through developmental exposure to print or compensation (Galaburda, LoTurco, Ramus, Fitch, & Rosen, 2006; Hashimoto et al., 2016).
7. GENERAL DISCUSSION
To date, several approaches have been employed to differentiate neural impairments associated with the etiology of RD from those associated with subsequent atypical development. In this review, we discussed five of these approaches and the results of relevant studies. Even though there remains considerable variability among findings, anomalies in brain regions such as TPC and OTC are consistently observed across languages, robust to reading intervention, and associated with familial risk in preliterate children and genetic risk factors within the general population, suggesting they are relevant to the etiology of RD. The TPC and OTC findings are notably distinguished by the finding that functional differences between RD children and reading-matched controls have been found more consistently in left TPC, and these functional differences also correspond to structural anomalies in reading-matched comparisons. On the other hand, differences between RD and reading-matching controls have been found less consistently in OTC and evidence suggests that impairments in OTC may be in part normalized by experience.
The lack of consensus in identifying TPC or OTC by using these five approaches may reflect the inherent heterogeneity in RD and complexity of the underlying etiology. Indeed, it is likely to be inadequate to characterize the etiology of RD in terms of a specific regional anomaly; more sophisticated, systems-level models of RD are needed. Other anomalies associated with RD, particularly in the dorsal IFG/PreCG, do not appear to be consistently related to the etiology of RD using these approaches, and instead likely to reflect the result of compensation or other processes secondary to any cause of RD (Hancock, Richlan & Hoeft, 2017). This distinction highlights the promise of these approaches and the importance of applying them jointly when investigating the etiology of RD and other neurodevelopmental disorders.
8. FUTURE DIRECTIONS
Our understanding of the etiology of RD is still nascent. The majority of neuroimaging studies of RD have employed two group cross-sectional designs in literate readers, which are of limited utility in improving our understanding of RD etiology. As summarized in this review, more informative approaches, such as including reading-matched controls, studying pre-literate, at-risk individuals, and imaging genetics are becoming more common. However, most studies are still limited by small sample sizes and limited language environments. Meta-analyses and multi-site analyses can help initially address these issues and efficiently integrate existing studies, particularly imaging genetics studies, which require high statistical power (Eckert et al., 2016; Thompson et al., 2015).
In addition to continued use of the approaches we have reviewed here, three emerging research directions also hold promise for improving our understanding of RD etiology. First, studies should consider the comorbidity of RD. RD is phenotypically heterogeneous, with evidence for deficits in multiple domains (Pennington, 2006) and high comorbidity with other developmental disorders, such as specific language impairment (Ramus, Marshall, Rosen, & van der Lely, 2013; Talli, Sprenger-Charolles, & Stavrakaki, 2016), attention-deficit/hyperactivity disorder (Moreau & Waldie, 2015), and speech-sound disorder (Sices, Taylor, Freebairn, Hansen, & Lewis, 2007). These disorders might have shared and distinctive etiologies with RD (Butterworth & Kovas, 2013). Therefore, future studies need to take RD subtypes and comorbidity into consideration. For example, while we have focused on the linguistic aspects of RD, there is also a mixed body of evidence for deficits in the visual system in RD, possibly related to magnocellular dysfunction (Gori, Seitz, Ronconi, Franceschini, & Facoetti, 2015), in statistical learning (Lum, Ullman, & Conti-Ramsden, 2013) and other non-linguistic domains (Vidyasagar & Pammer, 2010). It is an open question whether such deficits are reliably present in some or all individuals with RD and how these deficits might be related to the etiology of RD.
Secondly, most functional neuroimaging studies of RD have employed fMRI or ERP techniques to investigate reading, auditory or language processing at a relatively high level of analysis. As an important supplement, recent research has begun to improve our understanding of neural function at multiple, hierarchal timescales during language and sensory processing (Giraud & Poeppel, 2012), with evidence for anomalous neural oscillations and temporal processing at both slow (Cutini, Szucs, Mead, Huss, & Goswami, 2016; De Vos, Vanvooren, Vanderauwera, Ghesquiere, & Wouters, 2016; Goswami, 2011; Molinaro, Lizarazu, Lallier, Bourguignon, & Carreiras, 2016; Power, Colling, Mead, Barnes, & Goswami, 2016) and fast (Lehongre, Morillon, Giraud, & Ramus, 2013; Lehongre, Ramus, Villiermet, Schwartz, & Giraud, 2011) timescales in RD. Mechanistic models are available for linking these processes to neural coding (Hyafil, Fontolan, Kabdebon, Gutkin, & Giraud, 2015; Shamir, Ghitza, Epstein, & Kopell, 2009) and the role of excitatory-inhibitory neural interactions in regulating these oscillations (Muthukumaraswamy, Edden, Jones, Swettenham, & Singh, 2009; Wang, 2010). Currently little is known regarding either normal or atypical development of this oscillatory hierarchy, although there is clear evidence for maturational changes (Cho et al., 2015). Future studies of RD—using the approaches we have discussed here—at the level of neural oscillations, in conjunction with measures of neurometabolite concentrations (e.g., Pugh et al., 2014) and growing understanding of the spatial and temporal characteristics of gene expression in the human brain (Kang et al., 2011) may be highly informative for understanding the etiology of RD at a more detailed biological level (Hancock, Pugh, & Hoeft, in press).
Finally, well-established paradigms for investigating neural function at a relatively low level are seeing novel application in RD. Recently Perrachione et al. (2016) used neural adaptation paradigms to investigate neural function in response to a range of auditory and visual linguistic and non-linguistic stimuli in both children and adults with RD. They found that individuals with RD had a consistently reduced neural adaptation response. This result suggests that the etiology of RD is not in stimulus-specific cortical processing, but a widespread cortical dysfunction that manifests behaviorally in a restricted domain, that is, reading. Although this study did not disentangle the effects of development and experience, the results suggest intriguing new research directions that examine the fundamental neurophysiology of RD.
Greater understanding of the etiological bases for RD can help improve early detection of reading-related problems and allow cognitive processes that are amenable to change in intervention to be precisely targeted, leading to better outcomes in at-risk or affected populations. In this review, we summarized five main approaches that have been used in addressing causality issue in RD research, as well as future directions for applying these approaches. To advance our understanding of the etiology of RD, we must keep in mind that causality cannot be fully addressed by a single paradigm, but requires the integration of multiple lines of interdisciplinary evidence.
Acknowledgments
Funding Information: National Institutes of Health, Grant/Award Number: K23HD054720, R01HD078351, R01HD086168, R01HD067254/R01HD044073, R01HD065794, P01HD001994, R01MH104438, R01MH103371; National Science Foundation Grant/Award Number: NSF1540854, University of California Office of the President (UCOP) Grant/Award Number: MRP-17-454925, Oak Foundation, Grant/Award Number: ORIO-16-012; the Potter Family; China Scholarship Council; Fundamental Research Fund for the Central University; National Natural Science Foundation of China, Grant/Award Number: 31271082
Abbreviations
- ERP
event-related potential
- FA
fractional anisotropy
- G × E
gene-environment interactions
- GMV
gray matter volume
- IFG
inferior frontal gyrus
- IPL
inferior parietal lobule
- MRI
magnetic resonance imaging
- OTC
occipito-parietal cortex
- PreCG
precentral gyrus
- RD
reading disorder
- STG
superior temporal gyrus
- TPC
temporo-parietal cortex
- WMV
white matter volume
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Bach S, Brandeis D, Hofstetter C, Martin E, Richardson U, Brem S. Early emergence of deviant frontal fMRI activity for phonological processes in poor beginning readers. Neuroimage. 2010;53(2):682–693. doi: 10.1016/j.neuroimage.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Barquero LA, Davis N, Cutting LE. Neuroimaging of reading intervention: a systematic review and activation likelihood estimate meta-analysis. PLoS One. 2014;9(1):e83668. doi: 10.1371/journal.pone.0083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, Hoeft F. Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. Neuroimage. 2012;59(3):3021–3032. doi: 10.1016/j.neuroimage.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Human Brain Mapping. 2005;25(1):92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B, Kovas Y. Understanding Neurocognitive Developmental Disorders Can Improve Education for All. Science. 2013;340(6130):300–305. doi: 10.1126/science.1231022. [DOI] [PubMed] [Google Scholar]
- Cao F, Yan X, Wang Z, Liu Y, Wang J, Spray GJ, Deng Y. Neural signatures of phonological deficits in Chinese developmental dyslexia. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.11.051. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Centanni TM, Booker AB, Chen F, Sloan AM, Carraway RS, Rennaker RL, Kilgard MP. Knockdown of Dyslexia-Gene Dcdc2 Interferes with Speech Sound Discrimination in Continuous Streams. J Neurosci. 2016;36(17):4895–4906. doi: 10.1523/JNEUROSCI.4202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Booker AB, Sloan AM, Chen F, Maher BJ, Carraway RS, Kilgard MP. Knockdown of the dyslexia-associated gene Kiaa0319 impairs temporal responses to speech stimuli in rat primary auditory cortex. Cereb Cortex. 2014;24(7):1753–1766. doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Walker CP, Polizzotto NR, Wozny TA, Fissell C, Chen CM, Lewis DA. Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb Cortex. 2015;25(6):1509–1518. doi: 10.1093/cercor/bht341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW, Hugdahl K. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 2014;137(Pt 12):3136–3141. doi: 10.1093/brain/awu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Xia Z, Su M, Shu H, Gong G. Disrupted white matter connectivity underlying developmental dyslexia: A machine learning approach. Human Brain Mapping. 2016;37(4):1443–1458. doi: 10.1002/hbm.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier TA, Etchegaray MA, Haight JL, Galaburda AM, Rosen GD. The effects of embryonic knockdown of the candidate dyslexia susceptibility gene homologue Dyx1c1 on the distribution of GABAergic neurons in the cerebral cortex. Neuroscience. 2011;172:535–546. doi: 10.1016/j.neuroscience.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutini S, Szucs D, Mead N, Huss M, Goswami U. Atypical right hemisphere response to slow temporal modulations in children with developmental dyslexia. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki F, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biol Psychiatry. 2012;72(8):671–676. doi: 10.1016/j.biopsych.2012.05.008. [DOI] [PubMed] [Google Scholar]
- De Vos A, Vanvooren S, Vanderauwera J, Ghesquiere P, Wouters J. Atypical neural synchronization to speech envelope modulations in dyslexia. Brain and Language. 2016;164:106–117. doi: 10.1016/j.bandl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Debska A, Luniewska M, Chyl K, Banaszkiewicz A, Zelechowska A, Wypych M, Jednorog K. Neural basis of phonological awareness in beginning readers with familial risk of dyslexia-Results from shallow orthography. Neuroimage. 2016;132:406–416. doi: 10.1016/j.neuroimage.2016.02.063. [DOI] [PubMed] [Google Scholar]
- Dilnot J, Hamilton L, Maughan B, Snowling MJ. Child and environmental risk factors predicting readiness for learning in children at high risk of dyslexia. Development and psychopathology. 2016:1–10. doi: 10.1017/S0954579416000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Berninger VW, Vaden KI, Gebregziabher M, Tsu L, Consortium DD. Gray Matter Features of Reading Disability: A Combined Meta-Analytic and Direct Analysis Approach. eneuro. 2016 doi: 10.1523/ENEURO.0103-15.2015. ENEURO. 0103-0115.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44(3):411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Friend A, DeFries JC, Olson RK. Parental education moderates genetic influences on reading disability. Psychol Sci. 2008;19(11):1124–1130. doi: 10.1111/j.1467-9280.2008.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R. Towards a universal model of reading. Behav Brain Sci. 2012;35(5):263–279. doi: 10.1017/S0140525X11001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nat Neurosci. 2006;9(10):1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15(4):511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori S, Seitz AR, Ronconi L, Franceschini S, Facoetti A. Multiple Causal Links Between Magnocellular–Dorsal Pathway Deficit and Developmental Dyslexia. Cereb Cortex. 2015:bhv206. doi: 10.1093/cercor/bhv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. Why theories about developmental dyslexia require developmental designs. Trends Cogn Sci. 2003;7(12):534–540. doi: 10.1016/j.tics.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends Cogn Sci. 2011;15(1):3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Goswami U. Sensory theories of developmental dyslexia: three challenges for research. Nat Rev Neurosci. 2015;16(1):43–54. doi: 10.1038/nrn3836. [DOI] [PubMed] [Google Scholar]
- Goswami U, Wang HL, Cruz A, Fosker T, Mead N, Huss M. Language-universal sensory deficits in developmental dyslexia: English, Spanish, and Chinese. J Cogn Neurosci. 2011;23(2):325–337. doi: 10.1162/jocn.2010.21453. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL. Genetic bases of developmental dyslexia: A capsule review of heritability estimates. Enfance. 2004;56(3):273–288. doi: 10.3917/enf.563.0273. [DOI] [Google Scholar]
- Hart SA, Logan JA, Soden-Hensler B, Kershaw S, Taylor J, Schatschneider C. Exploring how nature and nurture affect the development of reading: an analysis of the Florida Twin Project on reading. Dev Psychol. 2013;49(10):1971. doi: 10.1037/a0031348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R, Pugh K, Hoeft F. The neural noise hypothesis of developmental dyslexia. Trends in Cognitive Sciences. doi: 10.1016/j.tics.2017.03.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R, Richlan F, Hoeft F. Possible roles for frontostriatal circuits in reading disorder. Neuroscience and Biobehavoral Reviews. 2017;72:243–260. doi: 10.1016/j.neubiorev.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Fukui K, Takeuchi H, Yokota S, Kikuchi Y, Tomita H, Kawashima R. Effects of the BDNF Val66Met Polymorphism on Gray Matter Volume in Typically Developing Children and Adolescents. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Pape-Neumann J, van Ermingen-Marbach M, Brinkhaus M, Grande M. Shared vs. specific brain activation changes in dyslexia after training of phonology, attention, or reading. Brain Struct Funct. 2015;220(4):2191–2207. doi: 10.1007/s00429-014-0784-y. [DOI] [PubMed] [Google Scholar]
- Helland T, Plante E, Hugdahl K. Predicting Dyslexia at Age 11 from a Risk Index Questionnaire at Age 5. Dyslexia. 2011;17(3):207–226. doi: 10.1002/dys.432. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Gabrieli JD. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26(42):10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, Gabrieli JD. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci U S A. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SM, Black JM, Soriano T, Bugescu N, Martinez R, Raman MM, Hoeft F. Topological properties of large-scale structural brain networks in children with familial risk for reading difficulties. Neuroimage. 2013;71:260–274. doi: 10.1016/j.neuroimage.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lee HL, Zhang Q, Liu T, Geng LB, Seghier ML, Price CJ. Developmental dyslexia in Chinese and English populations: dissociating the effect of dyslexia from language differences. Brain. 2010;133(Pt 6):1694–1706. doi: 10.1093/brain/awq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil A, Fontolan L, Kabdebon C, Gutkin B, Giraud AL. Speech encoding by coupled cortical theta and gamma oscillations. eLife. 2015;4:e06213. doi: 10.7554/eLife.06213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Ellen Grant P, Gaab N. Atypical Sulcal Pattern in Children with Developmental Dyslexia and At-Risk Kindergarteners. Cereb Cortex. 2015 doi: 10.1093/cercor/bhu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar M, af Trampe P, Greitz T, Eriksson L, Stone-Elander S, von Euler C. Residual differences in language processing in compensated dyslexics revealed in simple word reading tasks. Brain and Language. 2002;83(2):249–267. doi: 10.1016/s0093-934x(02)00055-x. [DOI] [PubMed] [Google Scholar]
- Jednorog K, Marchewka A, Altarelli I, Monzalvo Lopez AK, van Ermingen-Marbach M, Grande M, Ramus F. How reliable are gray matter disruptions in specific reading disability across multiple countries and languages? Insights from a large-scale voxel-based morphometry study. Human Brain Mapping. 2015;36(5):1741–1754. doi: 10.1002/hbm.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Nativism versus neuroconstructivism: rethinking the study of developmental disorders. Dev Psychol. 2009;45(1):56–63. doi: 10.1037/a0014506. [DOI] [PubMed] [Google Scholar]
- Katzir T, Shaul S, Breznitz Z, Wolf M. The universal and the unique in dyslexia: A cross-linguistic investigation of reading and reading fluency in Hebrew-and English-speaking children with reading disorders. Reading and Writing. 2004;17(7–8):739–768. doi: 10.1007/s11145-004-2655-z. [DOI] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, Milham MP. Cortical signatures of dyslexia and remediation: an intrinsic functional connectivity approach. PLoS One. 2013;8(2):e55454. doi: 10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, Eden GF. An investigation into the origin of anatomical differences in dyslexia. The Journal of Neuroscience. 2014;34(3):901–908. doi: 10.1523/JNEUROSCI.2092-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Napoliello EM, Eden GF. Gray matter volume changes following reading intervention in dyslexic children. Neuroimage. 2011;57(3):733–741. doi: 10.1016/j.neuroimage.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick AJ, Tan LH, Flowers DL, Luetje MM, Napoliello EM, Siok WT, Eden GF. Chinese Character and English Word processing in children's ventral occipitotemporal cortex: fMRI evidence for script invariance. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft I, Schreiber J, Cafiero R, Metere R, Schaadt G, Brauer J, Wilcke A. Predicting early signs of dyslexia at a preliterate age by combining behavioral assessment with structural MRI. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Langer N, Peysakhovich B, Zuk J, Drottar M, Sliva DD, Smith S, Gaab N. White Matter Alterations in Infants at Risk for Developmental Dyslexia. Cereb Cortex. 2015:bhv281. doi: 10.1093/cercor/bhv281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehongre K, Morillon B, Giraud A-L, Ramus F. Impaired auditory sampling in dyslexia: further evidence from combined fMRI and EEG. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehongre K, Ramus F, Villiermet N, Schwartz D, Giraud AL. Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron. 2011;72(6):1080–1090. doi: 10.1016/j.neuron.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lum JA, Ullman MT, Conti-Ramsden G. Procedural learning is impaired in dyslexia: Evidence from a meta-analysis of serial reaction time studies. Res Dev Disabil. 2013;34(10):3460–3476. doi: 10.1016/j.ridd.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Koyama MS, Milham MP, Castellanos FX, Quinn BT, Pardoe H, Blackmon K. Cortical thickness abnormalities associated with dyslexia, independent of remediation status. Neuroimage Clin. 2015;7:177–186. doi: 10.1016/j.nicl.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino C, Giorda R, Lorusso ML, Vanzin L, Salandi N, Nobile M, Battaglia M. A family-based association study does not support DYX1C1 on 15q21. 3 as a candidate gene in developmental dyslexia. European Journal of Human Genetics. 2005;13(4):491–499. doi: 10.1038/sj.ejhg.5201356. [DOI] [PubMed] [Google Scholar]
- Marino C, Scifo P, Della Rosa PA, Mascheretti S, Facoetti A, Lorusso ML, Perani D. The DCDC2/intron 2 deletion and white matter disorganization: Focus on developmental dyslexia. Cortex. 2014;57:227–243. doi: 10.1016/j.cortex.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Kronbichler M, Richlan F. Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Human Brain Mapping. 2016 doi: 10.1002/hbm.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascheretti S, Bureau A, Battaglia M, Simone D, Quadrelli E, Croteau J, Marino C. An assessment of gene-by-environment interactions in developmental dyslexia-related phenotypes. Genes Brain and Behavior. 2013;12(1):47–55. doi: 10.1111/gbb.12000. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gelernter J, Gruen JR, Calhoun VD, Meng H, Cope NA, Pearlson GD. Polymorphism of DCDC2 Reveals Differences in Cortical Morphology of Healthy Individuals-A Preliminary Voxel Based Morphometry Study. Brain Imaging Behav. 2008;2(1):21–26. doi: 10.1007/s11682-007-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xue G, Lu ZL, He Q, Wei M, Zhang M, Chen C. Native language experience shapes neural basis of addressed and assembled phonologies. Neuroimage. 2015;114:38–48. doi: 10.1016/j.neuroimage.2015.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, Olson RK, O'Reilly-Pol T. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc Natl Acad Sci U S A. 2005;102(47):17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro N, Lizarazu M, Lallier M, Bourguignon M, Carreiras M. Out-of-synchrony speech entrainment in developmental dyslexia. Human Brain Mapping. 2016 doi: 10.1002/hbm.23206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau D, Waldie KE. Developmental Learning Disorders: From Generic Interventions to Individualized Remediation. Front Psychol. 2015;6:2053. doi: 10.3389/fpsyg.2015.02053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morken F, Helland T, Hugdahl K, Specht K. Reading in Dyslexia across Literacy Development: A Longitudinal Study of Effective Connectivity. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.09.060. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106(20):8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Napoliello EM, Eden GF. Abnormal visual motion processing is not a cause of dyslexia. Neuron. 2013;79(1):180–190. doi: 10.1016/j.neuron.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov-Palchik O, Gaab N. Tackling the 'dyslexia paradox': reading brain and behavior for early markers of developmental dyslexiax. Wiley Interdiscip Rev Cogn Sci. 2016;7(2):156–176. doi: 10.1002/wcs.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paracchini S, Scerri T, Monaco AP. The genetic lexicon of dyslexia. Annu Rev Genomics Hum Genet. 2007;8:57–79. doi: 10.1146/annurev.genom.8.080706.092312. [DOI] [PubMed] [Google Scholar]
- Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Monaco AP. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 2006;15(10):1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Demonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Frith U. Dyslexia: cultural diversity and biological unity. Science. 2001;291(5511):2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Pennington BF. From single to multiple deficit models of developmental disorders. Cognition. 2006;101(2):385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Pennington BF, McGrath LM, Rosenberg J, Barnard H, Smith SD, Willcutt EG, Olson RK. Gene × environment interactions in reading disability and attention-deficit/hyperactivity disorder. Dev Psychol. 2009;45(1):77–89. doi: 10.1037/a0014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrachione TK, Del Tufo SN, Winter R, Murtagh J, Cyr A, Chang P, Gabrieli JD. Dysfunction of Rapid Neural Adaptation in Dyslexia. Neuron. 2016;92(6):1383–1397. doi: 10.1016/j.neuron.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AJ, Colling LJ, Mead N, Barnes L, Goswami U. Neural encoding of the speech envelope by children with developmental dyslexia. Brain and Language. 2016;160:1–10. doi: 10.1016/j.bandl.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SJ, Wang Y, Beach SD, Sideridis GD, Gaab N. Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: an fMRI study. Annals of Dyslexia. 2016:1–24. doi: 10.1007/s11881-016-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Frost SJ, Rothman DL, Hoeft F, Del Tufo SN, Mason GF, Fulbright RK. Glutamate and choline levels predict individual differences in reading ability in emergent readers. J Neurosci. 2014;34(11):4082–4089. doi: 10.1523/JNEUROSCI.3907-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F, Marshall CR, Rosen S, van der Lely HK. Phonological deficits in specific language impairment and developmental dyslexia: towards a multidimensional model. Brain. 2013;136(Pt 2):630–645. doi: 10.1093/brain/aws356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2011;57(3):742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Stering PL, Meissner SN, Gaab N. Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cereb Cortex. 2014;24(9):2489–2501. doi: 10.1093/cercor/bht104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci U S A. 2012;109(6):2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. Functional neuroanatomy of developmental dyslexia: the role of orthographic depth. Front Hum Neurosci. 2014;8:347. doi: 10.3389/fnhum.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 2011;56(3):1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Roeske D, Ludwig KU, Neuhoff N, Becker J, Bartling J, Bruder J, Schulte-Korne G. First genome-wide association scan on neurophysiological endophenotypes points to trans-regulation effects on SLC2A3 in dyslexic children. Mol Psychiatry. 2011;16(1):97–107. doi: 10.1038/mp.2009.102. [DOI] [PubMed] [Google Scholar]
- Rueckl JG, Paz-Alonso PM, Molfese PJ, Kuo WJ, Bick A, Frost SJ, Frost R. Universal brain signature of proficient reading: Evidence from four contrasting languages. Proc Natl Acad Sci U S A. 2015;112(50):15510–15515. doi: 10.1073/pnas.1509321112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri TS, Darki F, Newbury DF, Whitehouse AJ, Peyrard-Janvid M, Matsson H, Paracchini S. The dyslexia candidate locus on 2p12 is associated with general cognitive ability and white matter structure. PLoS One. 2012;7(11):e50321. doi: 10.1371/journal.pone.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir M, Ghitza O, Epstein S, Kopell N. Representation of time-varying stimuli by a network exhibiting oscillations on a faster time scale. PLoS Comput Biol. 2009;5(5):e1000370. doi: 10.1371/journal.pcbi.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sices L, Taylor HG, Freebairn L, Hansen A, Lewis B. Relationship between speech-sound disorders and early literacy skills in preschool-age children: Impact of comorbid language impairment. Journal of developmental and behavioral pediatrics: JDBP. 2007;28(6):438. doi: 10.1097/DBP.0b013e31811ff8ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Paulesu E. Brain abnormalities underlying altered activation in dyslexia: a voxel based morphometry study. Brain. 2005;128(Pt 10):2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci U S A. 2008;105(14):5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431(7004):71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Siok WT, Spinks JA, Jin Z, Tan LH. Developmental dyslexia is characterized by the co-existence of visuospatial and phonological disorders in Chinese children. Current Biology. 2009;19(19):R890–892. doi: 10.1016/j.cub.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Swan D, Goswami U. Phonological awareness deficits in developmental dyslexia and the phonological representations hypothesis. J Exp Child Psychol. 1997;66(1):18–41. doi: 10.1006/jecp.1997.2375. [DOI] [PubMed] [Google Scholar]
- Szalkowski CE, Fiondella CF, Truong DT, Rosen GD, LoTurco JJ, Fitch RH. The effects of Kiaa0319 knockdown on cortical and subcortical anatomy in male rats. Int J Dev Neurosci. 2013;31(2):116–122. doi: 10.1016/j.ijdevneu.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talli I, Sprenger-Charolles L, Stavrakaki S. Specific language impairment and developmental dyslexia: What are the boundaries? Data from Greek children. Res Dev Disabil. 2016;49:339–353. doi: 10.1016/j.ridd.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong JH, Gao JH. Neural systems of second language reading are shaped by native language. Human Brain Mapping. 2003;18(3):158–166. doi: 10.1002/hbm.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera J, Altarelli I, Vandermosten M, De Vos A, Wouters J, Ghesquière P. Atypical Structural Asymmetry of the Planum Temporale is Related to Family History of Dyslexia. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw348. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci Biobehav Rev. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Vanderauwera J, Theys C, De Vos A, Vanvooren S, Sunaert S, Ghesquiere P. A DTI tractography study in pre-readers at risk for dyslexia. Dev Cogn Neurosci. 2015;14:8–15. doi: 10.1016/j.dcn.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagar TR, Pammer K. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends Cogn Sci. 2010;14(2):57–63. doi: 10.1016/j.tics.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mauer MV, Raney T, Peysakhovich B, Becker BL, Sliva DD, Gaab N. Development of Tract-Specific White Matter Pathways During Early Reading Development in At-Risk Children and Typical Controls. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Paramasivam M, Thomas A, Bai J, Kaminen-Ahola N, Kere J, Loturco JJ. DYX1C1 functions in neuronal migration in developing neocortex. Neuroscience. 2006;143(2):515–522. doi: 10.1016/j.neuroscience.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Wanzek J, Vaughn S. Research-based implications from extensive early reading interventions. School Psychology Review. 2007;36(4):541. [Google Scholar]
- Xia Z, Hoeft F, Zhang L, Shu H. Neuroanatomical anomalies of dyslexia: Disambiguating the effects of disorder, performance, and maturation. Neuropsychologia. 2016;81:68–78. doi: 10.1016/j.neuropsychologia.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Yang J, Siok WT, Tan LH. Atypical lateralization of phonological working memory in developmental dyslexia. Journal of Neurolinguistics. 2015;33:67–77. doi: 10.1016/j.jneuroling.2014.07.004. [DOI] [Google Scholar]
- Yang Y-H, Yang Y, Chen B, Bi H-Y. Anomalous cerebellar anatomy in Chinese children with dyslexia. Front Psychol. 2016;7:324. doi: 10.3389/fpsyg.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen C, Shao L, Wang Y, Xiao X, Chen C, Xue G. Orthographic and Phonological Representations in the Fusiform Cortex. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw300. [DOI] [PubMed] [Google Scholar]
- Zhu L, Nie Y, Chang C, Gao JH, Niu Z. Different patterns and development characteristics of processing written logographic characters and alphabetic words: an ALE meta-analysis. Human Brain Mapping. 2014;35(6):2607–2618. doi: 10.1002/hbm.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]